Abstract
Tissue transmission optical absorption spectroscopy provides dynamic information on metabolism and function. Murine genetic malleability makes it a major model for heart research. The diminutive size of the mouse heart makes optical transmission studies challenging. Using a perfused murine heart center mounted in an integrating sphere for light collection with a ventricular cavity optical catheter as an internal light source provided an effective method of optical data collection in this model. This approach provided high signal to noise optical spectra which when fit with model spectra provided information on tissue oxygenation and redox state. This technique was applied to the study of cardiac ischemia and ischemia reperfusion which generates extreme heart motion, especially during the ischemic contracture. The integrating sphere reduced motion artifacts associated with a fixed optical pickup and methods were developed to compensate for changes in tissue thickness. During ischemia, rapid decreases in myoglobin oxygenation occurred along with increases in cytochrome reduction levels. Surprisingly, when ischemic contracture occurred, myoglobin remained fully deoxygenated, while the cytochromes became more reduced consistent with a further, and critical, reduction of mitochondrial oxygen tension during ischemic contraction. This optical arrangement is an effective method of monitoring murine heart metabolism.
Keywords: myoglobin, cytochromes, linear least squares fitting, oxidative phosphorylation, oxygen, mitochondria membrane potential, optical pathlength
Introduction
Optical spectroscopy provides real-time, non-destructive measurement of the redox state of the chromophores associated with the mitochondrial oxidative phosphorylation complexes (MOPC) that were first recognized by Keilin[1] as well as the cytosolic oxygen tension from myoglobin[2]. This approach has been applied to many tissues systems including heart[3–9], brain[10] and liver[9]. With regard to the heart and heart mitochondria, recent transmission optical spectroscopy visible light studies[6, 11–13] have demonstrated the detection of numerous aspects of oxidative phosphorylation, in addition to the monitoring of cytosolic oxygen via myoglobin. The initial reducing equivalent entry into the cytochrome chain can be observed via the absorbance of FMN that is reduced in Complex I, The next stage for the reducing equivalent cascade is cytochrome b of Complex III with two redox chromophores, high (cyt bH) and low (cyt bL) b hemes. The distribution of these species providing an estimate of the mitochondrial membrane potential under some conditions[14]. The reducing equivalents are passed within complex III to cytochrome c1 (cyt c1) and then on to the intermediate redox agent between Complex III and Complex IV (or cytochrome oxidase (COX)), which is cytochrome c (Cyt c). Both Cyt c and Cyt c1 have been observed in the isolated perfused heart[6, 12]. Finally the terminal oxidase of oxidative phosphorylation, COX, using reduced Cyt c to reduce molecular oxygen, can be observed in several states in mitochondria including the fully reduced from at 605 nm (Cyt a605) as well as the two electron reduction state at 607 nm (Cyt a607) and three electron reduction state at 580nm (Cyt a580)[13] as characterized by a series of papers from Wikstrom and others[15–17]. This high signal to noise information on the mitochondrial redox state and cytosolic oxygenation provided by this technology[6] has already been useful in studying the oxygen delivery to the perfused heart[12] as well as the regulation of cardiac coronary vascular tone[11].
The mouse is an important experimental tool in cardiology research due to the ability to perform genetic manipulations to test specific hypotheses. We developed a small side firing fiber optic that has been effective in the perfused mouse heart[11, 18]. Though the small fiber optic provided an excellent source of light for transmural illumination, we noted using a fixed position fiber optic for light collection, as shown in Figure 1, was problematical during even modest translational motions of the heart. With motion, the transmitted light could be inadequately detected by the fixed pickup resulting in large amplitude changes in light collection. We reasoned that placing the perfused heart in the center of an integrating sphere to sample all the transmitted light from the heart would minimize the errors generated by a fixed fiber optic detector and translational motion of the heart. The small size of the murine heart was actually an advantage in placing the sample in the center of the integrating sphere. This approach is similar to the integrating sphere system we use for isolated mitochondria suspensions [13, 19].
Figure 1:
Transmission spectroscopy of the perfused murine heart with fixed fiber. Ventricular cavity optical side firing light source with fixed fiber detector collecting light from a fixed position on the beating heart.
The purpose of this study was to evaluate the use of a custom designed mouse heart perfusion chamber that uses a light catheter in the ventricle cavity as the light source and an integrating sphere to sample all of the transmitted light from the heart. The spectroscopic analysis of the data was performed as previously published with a linear least squares analysis using individual reference spectra[6]. We then applied this technique to an important set of conditions in the perfused heart that involve ischemia, ischemic contraction and reperfusion which generate large motions of the heart to test the light detection configuration but also provide unique information on the cytosolic and mitochondrial redox state through this clinically relevant protocol in this model system[20, 21].
Methods
System Design
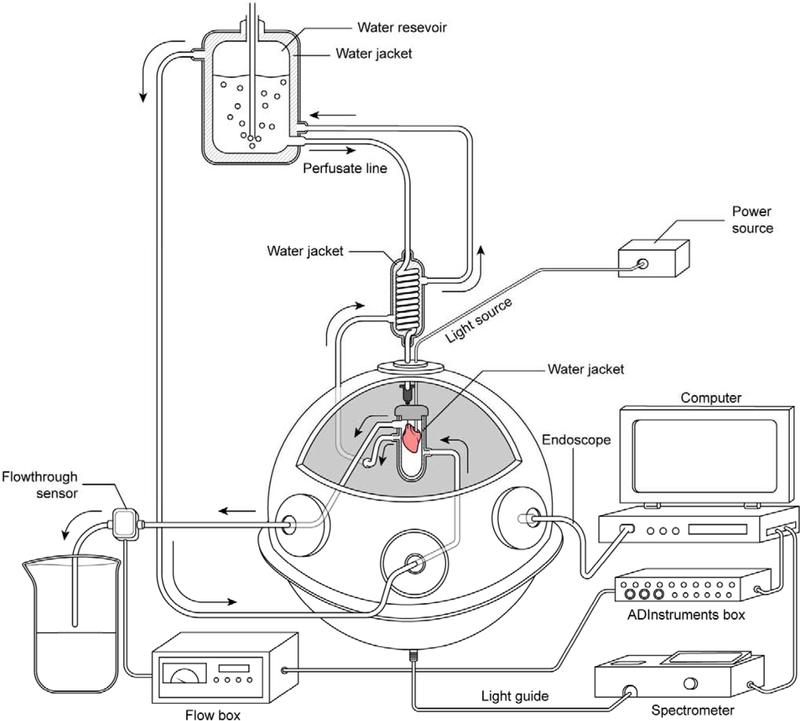
A schematic diagram of the system is presented in Figure 2a. All optical measurements were made in a 6-inch diameter integrating sphere with Spectraflect reflectance coating (model RTC-060-SF, LabSphere, Inc., North Sutton, NH, USA) identical to our previous reports on isolated mitochondria [13, 19]. The basic preparation was a retrograde aortic perfusion, also called Langendorff preparation [22], at constant perfusion pressure described in detail below. A side firing fiber optic catheter (Polymicro Technologies FIP100110125, 125 micron diameter) was inserted into the left ventricle as previously published [11, 18]. The perfused heart was placed in a specially designed water jacketed glass chamber maintaining temperature at 37+1°C, similar to what is used in NMR experiments of intact hearts [23, 24]. It is important to note that this chamber was optically transparent except for an optical baffle on the bottom of the chamber. This self-baffle was created by painting the bottom of the chamber with an acrylic white reflective paint to prevent the sphere’s detector, located at the bottom of the sphere, from viewing directly the first strike light from the sample. The water circulation for the water jacket was provided through side ports in the integrating sphere, as previously reported for mitochondrial preparations [13]. Air was voided from the perfusion chamber and the heart was lowered into the integrating sphere residing approximately at the center of the sphere, also called a center mounted sphere condition[25] (Figure 2B). Effluent flow was collected from the reservoir and exited the integrating sphere via a side port. The flow from the effluent tract was used to collect the coronary flow using a 1PXN flow-through sensor coupled to a TS410 Tubing Flow Module (Transonic).
Figure 2:
Integrating sphere light collection system for perfused heart. A) Schematic representation of transmural spectroscopy in the integrating sphere. The side firing catheter is placed in the heart, which is bathed in a sealed water jacketed all glass chamber. The chamber is then placed in the center of an integrating sphere with the light detector on the bottom of the sphere. The bottom of the water jacketed glass chamber is made white to prevent light from striking the detector at the bottom of the sphere directly. The perfusion pressure is maintained at 70 mm Hg by regulated gas pressure over the water jacketed perfusate chamber. The light collected from the heart is directed to a rapid scanning spectrophotometer coupled to a data collecting computer. Coronary flow is monitored as the outflow from the glass chamber and recorded in a computer. An endoscope is inserted in one of the sampling ports of the sphere to permit online visual monitoring of the heart during an experimental procedure. Note the circulating thermoregulated water was maintained at 37 C ° with a temperature regulated circulating bath (not shown for simplicity). B) Image of perfused murine heart with ventricular cavity light source in center mounted position of integrating sphere. The light detector is at the bottom of the integrating sphere collecting all the light transmitted through the heart with little or no geometric selection preference. See Figure 2 for schematic. For the purposes of this picture one of the ports was removed to permit the photography, under normal conditions the sphere is closed to minimize non-reflective surfaces.
A white light source was impinged on the endocardium of the heart via the light catheter from an external light source. The light passed through the heart wall (see Figure 2B) and reflected off of the integrating sphere resulting in a near uniform sampling of the transmitted light at the bottom of the sphere with appropriately positioned light baffles. The spectral properties of the transmitted light were determined using a rapid scanning spectrophotometer interfaced with a computer using a custom designed Labview program and open source spectrometer drivers as previously described [6].
Heart Excision and Perfusion
All animal protocols were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee and performed in accordance with the guidelines described in the Animal Care and Welfare Act (7 USC 2142 § 13). All experiments were conducted on C57 BL/6N mice between 12 and 16 weeks of age (Taconic Farms). The mice were anesthetized using sodium pentobarbitol (50mg/kg) and anticoagulated with heparin (50 USP) via intraperitoneal injection. Through a trans-diaphragmatic approach, the heart was excised and submerged in ice-cold Krebs-Henseleit (KH) buffer. The KH buffer was composed of (in mM) 120 NaCl, 25 NaHCO3, 4.7 KCl, 1.75 CaCl2, 1.2 MgSO4, 1.2 KH2PO4 and 11 glucose and passed through a 0.22 µm filter before use. The aorta was cannulated with a 26 gauge needle and retrograde perfused on a constant pressure Langendorff perfusion system[26, 27]. A side firing fiber optic was inserted through an incision in left atrial appendage and passed through the mitral valve into the left ventricular cavity. In selected experiments, the use of a latex balloon was employed to measure left ventricular developed pressure (LVDP). In this case, the down-firing fiber optic was placed inside the latex balloon, and both were placed in the left ventricle through the left atrial appendage. Hemodynamic measurements from the latex balloon were made by use of a pressure transducer coupled to a bridge amp (ADInstruments, Sydney, Australia).
Ischemia/Reperfusion Protocol
The murine hearts were allowed to equilibrate to retrograde perfusion for 20 minutes. 20 minutes of global ischemia ensued, followed by 90 minutes of reperfusion. To confirm ischemic damage TCC staining was performed at the end of the study. At the conclusion of the experiment, the hearts were perfused with 0.1 % TTC and incubated at 37°C for 30 minutes, and fixed in formalin.
Optical Absorbance Spectroscopy
Spectra data were collected via a cooled rapid-scanning spectrometer (QE65PRO, Ocean Optics). A 2mm fiber optic light guide (Thor laboratories) was fixed to the bottom of the integrating sphere. Light intensity was recorded at 1,044 points between 349 – 742 nm. Spectra were collected at 1 sample per second using a custom LabVIEW-based program [6, 11]. The software permitted real-time monitoring of the raw transmitted light, absorbance, as well as the absorbance difference between a dynamically defined control period and the current acquisition. The data was fit with reference spectra of the cardiac metabolically active chromophores in real-time using a linear-least squares regression model as previously described[6, 11, 12] in both difference and absolute spectra. Reference spectra included the multiple states of complex IV (a580, a605, and a607), complex III (cyt bH,bL and c1), as well as cyt c, and I0 correction for optical sieving [6]. New in this study was the use of oxygenated (MbO) and deoxygenated myoglobin (MbDO) absolute spectra, rather than the difference spectrum between these species. This was done to detect total changes in myoglobin detected as described below. As suggested in the literature [28], there are slight differences between species in the spectral properties of myoglobin, thus we used mouse myoglobin standards obtained from homogenates of hearts after assuring chemical reduction of the myoglobin. In real time, the data was fit on a time course displaying the change in optical density of each reference as compared to a control point set at the beginning of the experiment providing feedback to the experimentalist concerning the establishment of steady states and data quality. During spectral post-processing, blocks of 100 spectra (100 seconds) were generally averaged from steady state data at different states with a spectral window of 535 to 630nm to focus on the chromophores of interest in this study.
Statistical Methods
Goodness-of-fit statistics for the spectral fitting were established using the LabVIEW weighted mean square error in the General Linear Fit function. All summary data are reported as means ± SE of the data series. Student t tests were applied to test the null hypothesis for difference spectra or directly with control conditions where appropriate.
Results
Optical spectroscopy in the murine perfused heart in an integrating sphere
Light scattering and motion artifact can limit optical measurements in beating hearts, especially when metabolically challenged. While transmural spectroscopy has been successful in large perfused hearts without the use of an integrating sphere and a fixed optical fiber detector [6, 11, 12, 18] the application of this technique to the small murine heart was found to be problematic, especially under severe metabolic conditions associated with ischemic contraction and reperfusion (I/R). The motion of the heart in front of the fixed fiber optic (Figure 1) caused excessive optical artifacts associated with the tissue alignment with the detection fiber. To overcome this limitation we used an integrating sphere coupled to a ventricular light source to minimize the impact of tissue motion on the detection of transmitted light (Figure 2A and B). Furthermore, in prior experiments with large hearts the temperature of the preparation was primarily maintained by high flow of the prewarmed perfusion media. During no-flow ischemia in these high surface to volume ratio small hearts, the temperature would plummet requiring the heart to be bathed in a temperature regulated medium. A water jacketed perfusion chamber would be difficult to couple to a fixed fiber detection system. However, the integrating sphere allowed the use of a glass sealed water jacketed perfusion chamber for temperature regulation during ischemia that prevented many of the geometric and motion issues generated by a fixed fiber detector of the transmitted light discussed above.
Spectral Fitting of Ischemia/Reperfusion: Use of Myoglobin Absorbance for Pathlength Correction.
The integrating sphere system is effective at minimizing translational motion errors, however, it does not correct for tissue thickness or changes in the optical characteristics of the tissue. In general, the light is mostly reflected inside the integrating sphere without impinging on the tissue after traversing the ventricular wall. Thus the “first pass” of the light through the tissue dominates the observed absorbance. This first pass of light through the heart could be influenced by the thickness of the heart, for example because of contracture or an edematous state that could change the scattering and photon migration through the tissue. In any event, any change in pathlength due to thickness or edema needs to be corrected for to interpret the light transmission data. Herein, we will refer to alterations in tissue sampling as changes in pathlength without necessarily ascribing this to cardiac wall thickness or edema as it is beyond the scope of this current technical study.
A representative time course of light transmittance at 700nm of the I/R protocol is shown in Fig. 3a. The four conditions are control, ischemia, ischemic contraction and reperfusion. In general, ischemia induced very small variable changes in the 700 nm absorbance as observed in prior work on the rabbit heart [6]. Ischemic contracture, occurring later in the ischemia period, consistently resulted in a large decrease in 700 nm transmittance indicative of an increase in tissue pathlength, specifically due to an increase in cardiac wall thickness with contracture. This assumption was confirmed by placing a pressurized balloon in the ventricle to prevent the physical contraction of the tissue, which greatly diminished the reduction of transmittance at 700 nm with contraction. With reperfusion the heart transiently increased its 700 nm transmittance. This was followed by a return to near control levels and a slow increase in transmission over the time course of the recovery. These data are consistent with a transient reduction of pathlength with reperfusion, followed by a transient recovery and a long term decrease in heart optical tissue sampling during the reperfusion period. TCC staining indicated a 30 to 40% damaged region in the heart consistent with previous protocols from this lab[29].
Figure 3:
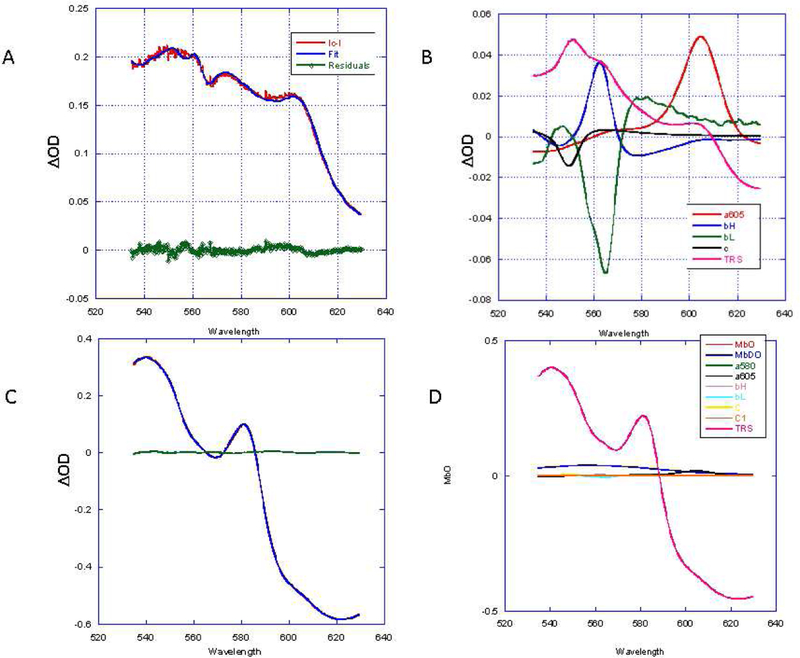
Spectral analysis of ischemia reperfusion protocol. A) Time course of light transmittance at 700 nm (red) and change in total myoglobin (TM) absorbance (blue) during the ischemia reperfusion protocol. Periods labeled: control (C), ischemia (I), Ischemic Contraction (IC) and Ischemic Reperfusion (IR). IR is divided into an early (e) and late (L) stage. The ΔTM was determined from summing the MbO and MbDO in difference spectra relative to control (C). Examples of the fitting analysis for 4 difference spectra are shown in panels B-E. In each case the overall fitting and residuals is shown in an insert at the top right of the figure. The reference spectral contributions to the fit are presented in the main panel. The color coding of the reference spectra is presented in a key at the upper right corner of the Figure. B) The difference spectrum between C and I. C) The difference spectrum between I and IC. D) The difference spectrum between IC and IRe. E) Difference spectrum between IRe and IRL. Note that the spectral fitting residuals with the individual MbO and MbDO spectra versus the MbO/MbDO difference spectra used in prior studies were slightly different since the myoglobin difference spectrum was not forced on the linear fit and MbO and MbDO spectra were used independently.
Transmittance at 700nm wavelength occurs in a region of the spectrum where metabolically active chromophores contribute minimally relative to the visible light, thereby providing a general indication of the tissue sampling. However, some absorbance by chromophores could interfere even at these infrared wavelengths [30, 31], thus a more specific and quantitative approach would be desirable. To quantify changes in optical pathlength, we explored the use of total tissue myoglobin as a measure of tissue sampled or pathlength. Murine cardiac myoglobin concentration has been determined to be 200–220μM [28, 32] and is relatively homogeneously distributed over the heart tissue [33]. There are basically three states of myoglobin in heart tissue, oxygenated (MbO), deoxygenated (MbDO) and chemically oxidized myoglobin (Mbmet). However, we have not detected Mbmet under normal physiological or even ischemic reperfusion conditions, which is likely due to the high activity of myoglobin reductase in heart tissue. Thus, without major tissue leakage, we have assumed that the total myoglobin content is constant at 280μM [28] either in the MbO or MbDO form that can be differentiated optically in the intact heart. Thus, by measuring the change in total detected myoglobin content we can estimate the differences in tissue pathlength in our protocols. If Mbmet were formed, it would have shown a unique and easily detected optical signature [34]. However, as already stated, we found no evidence of Mbmet formation in these studies.
To determine the myoglobin content using MbO and MbDO the relative extinction coefficients for these species need to be known. The literature is not consistent with the extinction values for myoglobin, especially between species [28]. We have found based on our own experiments that the ratio of MbO (581nm) to MbDO (552 nm) was 1.14 in mouse heart by removing oxygen from fully oxygenated, chemically reduced, mouse myoglobin. This ratio is consistent with the literature values ranging from 1.13 to 1.15 [34, 35], with molar extinction coefficients of 15000 M−1 cm−1 for MbO581 and 13000 M−1 cm−1 for MbDO552 [34]. These data would imply that with any change in myoglobin oxygenation, the MbO and MbDO absorbance should maintain a ratio of 1.14 if no changes in sampled tissue volume occur. The change in total myoglobin absorbance was calculated from the following formula: Total Change in Myoglobin(ΔTM)= Absorbance MbO (581 nm) + (1.14xAbsorbance MbDO (552nm). If the path length, or amount of tissue sampled, changed during the perturbation, the ΔTM determined from the changes in MbDO and MbO could be used to quantitate the relative changes in the amount of tissue sampled.
We also used another approach measuring the absorbance of MbO and MbDO in the raw absorbance spectrum of the tissue during the experimental steady state periods. This approach assumed that the absorbance of the tissue was dominated by the high concentration of myoglobin in the mouse heart [28]. The small contribution of the cytochromes, especially under reduced states in ischemia, could be represented by the optical difference spectrum for these chromophores since cyto c[31], cyto c1[32], cyto bH, cyto bL[33] and COX [34] have very weak, relatively non-specific absorbances in the 500 to 700nm region in their oxidized state. Clearly, this approach is an estimate without using the actual absolute spectra of these other chromophores, but serves to confirm the difference method for determining tissue pathlength with ΔTM. This analysis of absolute spectra, however, provided the distinct advantage of permitting the estimation of the redox or oxygenation state of each chromophore within each condition, rather than just the difference. This is useful in order to compensate for pathlength changes when quantitating redox changes, which requires the initial redox or oxygenation state of the chromophores to be known.
A third post-processing method was also applied to differentiate pathlength from biochemical changes. This involved recording the initial condition, used in the difference spectrum, and using it a reference spectrum (called Tissue State Reference (TRS)) to fit the resulting difference spectrum. The rationale here was that if a simple change in pathlength occurred, more or less tissue would be in the field with the same spectral properties as the initial TRS, This would result in the fitting routine using only TRS to fit the difference spectrum. However, if redox changes occurred during this period, the TRS would not be on its own to fit the difference spectra. The different post-processing methods described above provided controls for the spectral analysis and should be internally consistent when reporting spectral differences. The difference spectra were analyzed using the previously published linear least squares approach with the myoglobin difference spectrum replaced by MbO and MbDO to permit the calculation of ΔTM. If myoglobin only changes its oxygenation state then the TΔM should be zero as any MbO would be replaced by MbDO and vice versa, corrected for extinction coefficients. Representative spectral fits of the IR time course data are shown for a single experiment in Figure 3 (B–E) while the 700 nm transmittance and ΔTM time course are shown in Figure 3A. These spectra (completely unfiltered in the frequency domain) typically provided signal to noise values for fully reduced COX605 in excess of 6:1 over 1s of data collection. The summary of the myoglobin data from this differential approach is presented in Table 1a. It is important to note that the changes in 700nm transmission and ΔTM are highly correlated suggesting that both are sampling a similar process, as well as the difference spectra showed no evidence of translation motion revealed by large full spectrum changes in signal amplitude detected when a fixed fiber optic collection scheme was used.
Table 1A.
Effect of IR protocol of the myoglobin species change in absorbance. ((SEM) n=5))
| Chromophore | I-C | I-IC | IC-IRe | IRe-IRL |
|---|---|---|---|---|
| ΔMbO | −0.7(0.2)* | −0.05(.02) | 0.8(0.04)* | −0.2(.07)* |
| ΔМbDO | 0.6(.07)* | 0.8(.07)* | −1(.06)* | −0.07(.01) |
| ΔTM | −0.07(.06) | 0.2(.05)* | −0.2(0.03)* | −0.2(.05)* |
p<.05 versus null
Focusing on ΔTM and pathlength effects, in the example of Figure 3A, ischemia was associated with small increase in ΔTM, consistent with an increase in pathlength. Considering all the experiment results (see Table 1A) the effect of ischemia on ΔTM was relatively small and variable across the hearts studied. With ischemic contraction a large reproducible increase in ΔTM occurred (~0.2 OD), detected as a larger increase in MbDO when compared to MbO. Again, it is not possible to create MbDO without an concomitant decrease in MbO, thus the pathlength must have increased to result in more detected myoglobin. This result is consistent with myoglobin being primarily in the MbDO state after ischemia and the pathlength increasing, likely due to wall thickening in contraction, in the absence of MbO.
With reperfusion, complex but reproducible alterations in ΔTM occurred. Initially, ΔTM decreased rapidly with the initiation of perfusion, implying a decrease in pathlength, followed by a short recovery of optical pathlength over a 500 to 800 sec period. After this transient recovery of ΔTM, a slow decline in ΔTM occurred consistent with a long term decrease in pathlength.
We compared ΔTM analysis with direct fitting of the individual absorption spectra from the heart. Examples of these fits from the same experiment as represented in Figure 3 are shown in Figure 4. Again, the absolute spectrum of the tissue was fit with the dominant absolute MbO and MbDO and the reduced difference cytochrome spectral models. The ΔTM measurement provides an estimate of the tissue pathlength. However, using this data to compensate for the effects of pathlength on the mitochondrial chromophores depends on the initial absorption state, the pathlength change and the final absorption state. Regrettably, difference spectroscopy only provides the change in pathlength, which makes correcting the absorbance for this change difficult without characterizing the initial absorbance condition. In contrast to myoglobin, which exhibits distinct spectral signatures for its different oxygenated and redox states, the oxidized cytochromes do not generally have strong spectral signatures in the 535 to 700 nm region. Thus, this obviates the need to deconvolve the different oxidized states of the cytochromes, which would be a formidable task in this accessible bandwidth. As discussed above, with the low absorbance and broad spectral properties of the oxidized spectra of the cytochromes, we explored the use of the difference reduced spectrum to fit the steady state absorption spectra to determine the reduced chromophore content under different conditions along with the dominant MbO and MbDO signals of the mouse heart. If successful, this would permit an estimate of the steady state levels of MbO and MbDO along with the redox state of the individual mitochondrial chromophores that will be helpful in compensating for pathlength changes.
Figure 4:
Fitting of absolute spectrum of steady states in the IR protocol. The fitting solutions for the reference spectra are presented with the raw data and residuals as an insert in the upper left hand of the plot. A: Control. B:Ischemia. C: Ischemic Contraction. D: Reperfusion early. E:Reperfusion Late.
The spectra and linear least square fits of the absorbance for the C, I, IC, IRe, and IRL absolute spectra are shown in Figure 4. The myoglobin data summary for this analysis is presented in Table 1b. The residuals suggested that even without the oxidized spectral references for the cytochromes that good spectral approximations were being provided using this approach especially under ischemic conditions were the reduced form of the cytochromes dominate the spectrum. There is good qualitative correlation between the difference approach presented in Figure 3 and the fitting of absolute spectra in Figure 4, as reported in Table 1a and b. The total absorbance of the control mouse heart myoglobin is 1 OD (Table 1b), consistent with prior studies [11], and corresponds to an optical pathlength of 2.4 millimeters (using a 15000 M−1 cm−1 extinction coefficient and 280 μM for myoglobin concentration) larger than the physical dimensions of the mouse heart wall[36] (~1.2 millimeters) due to light scattering [6]. Importantly, the absolute spectra provides information on the state of all species during each experimental condition; for example, it shows that there is no oxygenated myoglobin in the ischemic states, I or IC. While deoxygenated myoglobin (~14%) and reduced cytochromes are detected in the control conditions consistent with the previously described hypoxia in saline perfused hearts likely due to the dysregulation of vascular tone[11]. With regard to the estimate of pathlength changes, the absolute spectral analysis provides very similar data to the ΔTM approach, with modest changes during ischemia and ~20% increase in pathlength associated with IC reversed early in reperfusion and then a long decline in pathlength resulting in a ~30% decrease in pathlength.
Table 1B.
Effect of IR protocol on absolute myoglobin absorbance.((SEM) n=5)
| Chromophore | C | I | IC | IRe | IRL |
|---|---|---|---|---|---|
| MbO | 0.8(.03) | 0* | 0* | 0.8(0.1) | 0.6(0.08)* |
| MbDO | 0.1(0.1) | 0.9(0.06)* | 1.2(0.06)* | 0.2(0.3) | 0.2(0.02) |
| TM | 1.0(0.3) | 0.9(0.06) | 1.2(0.06)* | 0.94(0.04 | 0.7(0.07)* |
| %MbDO | 14(1.0) | 100* | 100* | 15(2.0) | 23(4) |
p<0.05 versus control condition
The third approach was to use the reference tissue TRS in the fitting routine to separate pathlength differences from biochemical changes. We focused this approach on the two large changes in pathlength in the IR protocol, the transition from I to IC and IRe to IRL. Examples of this approach are shown in Figure 5. Figure 5a presents the difference spectrum of I versus IC with the fit using TRS for I. As seen in Figure 5b, this fit reported a decrease in bL absorbance together with an increase in bH and a605 (see summary in Table 2). These data are consistent with the further reduction of COX with contraction along with a depolarization of the mitochondrial membrane, which oxidizes bL and reduces bH [14]. Figure 5c presents the difference spectrum of IRe and IRL with the fit using TRS for IRe. In contrast the to I-IC transition, the IRe TRS (20% mean decrease) was adequate to fit the large optical absorbance change with little or no change in the other chromophores. These data imply that the optical changes between IRe and IRL were simply due to the amount of tissue in the optical path. This could be attributed to edema or other alterations in wall thickness. In addition, the fact that TRS was adequate to fit the changes associated with the IRe to IRL transition, it is unlikely that any significant selective loss of chromophores such as cytosolic myoglobin or cyt c occurred during this period. Suggesting that the assumption that total tissue myoglobin concentration remained constant in this protocol.
Figure 5.
Fitting of difference spectra using Tissue Reference Spectrum. A: Difference spectrum of I versus IC with fit and residuals using I TRS. B: Reference spectra contributions to the fit including I TRS. C: Difference spectrum of IRe versus IRL with fit and residuals. D: Reference spectra contributions to the fit including IRe TRS.
Table 2.
TSR Analysis of Chromophore changes during I to IC and IRe to IRL transitions (SEM)(n=5)
| Chromophore (ΔOD) | I-IC | IRe-IRL |
|---|---|---|
| MbO | −0.00(0.02) | 0.00(0.00) |
| MbDO | 0.04(0.02) | −0.01(0.01) |
| A580 | 0.00(0.01) | 0.00(0.00) |
| A605 | 0.04(0.00)* | 0.00 (0.01) |
| bH | 0.04(0.00)* | −0.00(0.01) |
| bL | −0.05(0.00)* | 0.00(0.00) |
| c | 0.00(0.01) | 0.00(0.00) |
| C1 | 0.00(0.00) | 0.00(0.01) |
| TSR | 8%(0.8)* | −20%(4.2)* |
p<0.05 versus null
Discussion
Herein we demonstrate an approach to collect transmission optical spectra from the isolated perfused mouse heart, a major model system in the evaluation of myocardial function and metabolism [37]. These optical data provide insight into the cytosolic oxygen as well as mitochondrial redox state, degree of oxygenation as well as membrane potential in the intact beating heart [6, 11]. All of these parameters are believed to play critical roles in the function of the heart under normal and pathophysiological conditions. The diminutive size of the mouse heart was a challenge for these studies using a fixed optical fiber optical to collect transmitted light. We placed the perfused heart, with an optical fiber within the ventricle as a light source, at the center of an integrating sphere to sample all the transmitted light without geometric concerns. Combined with a spectral fitting approach [6, 11], adequate signal to noise data for the myocardial cytosolic myoglobin and mitochondrial cytochromes with minimal translational motion artifacts were obtained with excellent temporal resolution. However, methods for compensating for optical pathlength changes through the tissue needed to be developed even with the use of an integrating sphere.
Technically, the approach used is similar to preparing a perfused heart for study in an NMR magnetic chamber for MRS or MRI studies [24, 37–39]. Once the optical fiber is inserted into the ventricle as the light source, the heart is placed in a glass chamber that is center mounted in the integrating sphere. Coronary flow and oxygen extraction can be monitored from the fluid exiting the chamber after traversing the coronary circulation. A simple video camera permits the monitoring of the heart in the sphere to assure fiber placement and overall function of the heart. The transmitted light from the heart was adequate for the camera detection and in some cases needed to be attenuated to prevent saturation. Using this approach, the difficulties associated with alignment of the collecting fiber (Figure 1) with the transmitted light from the heart as well as the effect of translational motion of the heart moving relative to the fiber optic detector are eliminated based on the spectral evidence collected throughout this protocol. The integrating sphere samples the light from the center mounted sample with little or no bias with respect to the geometry of the source. Again, the light detector is located at the bottom of the sphere to avoid any direct illumination effects. We originally used a similar integrating sphere to minimize scattering effects on mitochondria suspensions with good results [13].
Though the integrating sphere provided a robust collection system for the transmitted light, it does not correct for changes in the pathlength of the tissue. In this highly scattering medium, the physical dimensions alone do not determine pathlength as the light is highly scattered when traversing the tissue, as can be seen in Figure 1a and 2b. This results in the light pathlength to be generally longer than the physical thickness of the tissue in transmission mode [6]. In this system, we found the control pathlength, based on the absorbance of myoglobin, to be ~2.4 mm or roughly twice the average mouse heart wall thickness, which varies from 1 to 1.8 mm through the cardiac cycle [36]. Indeed, the optical pathlength is a function not only of the physical dimensions of the tissue, but also of the edematous state that impacts tissue scattering [40] and that is one of the many outcomes of cardiac ischemia [41], as occurred in this study. As all absorption measures depend on pathlength to determine the concentration of a given chromophore, a robust method to determine optical pathlength is critical for the interpretation of transmission optical data. In this study, we provided three complementary methods of determining or compensating for the optical pathlength through the heart wall.
The first two methods rely on the assumption of a constant content and uniform distribution of myoglobin in the heart. If myoglobin is constant, then the sum of MbO and MbDO, corrected for extinction coefficients, should also remain constant if no change in pathlength occurred. However, if pathlength increases or decreases during a perturbation, then the ΔTM should accurately reflect this change. In the perfused mouse heart, the ischemic contraction clearly increased ΔTM as primarily evidenced by an increasing MbDO, which was the dominant form of myoglobin in the ischemic heart that is contracting. With reperfusion ΔTM decreased by 30%, suggesting the optical pathlength through the tissue decreased during the full course of the reperfusion either due to edema or thickness of the heart wall.
The second method relied on directly detecting the absorption of MbO and MbDO in the absolute spectrum. It was surprising that even with no correction for non-metabolically active chromophores or tissue scattering effects, usually minimized by difference analysis, that good spectral fits of the data were obtained using only the myoglobin species and the reduced forms of the cytochromes. Though this method should be refined to also include the absolute spectral references of the oxidized and reduced cytochromes, which in some cases difficult to achieve, the dominance of the myoglobin and the reduced form of the cytochromes results in a good fit of the absolute spectra under most conditions, but particularly in the ischemic states where the cytochromes are in their fully reduced state. Using this approach, we confirmed that there is MbDO and partially reduced cytochromes in the control perfused heart, as established in an earlier study using vasodilators [11]. This approach also determines very similar pathlength changes in IC and reperfusion as the ΔTM approach, again with the assumption that myoglobin content is constant in these studies.
The third method was used to identify biochemical redox changes in the presence of changes in pathlength. In this approach, a TSR was taken under the initial conditions of a transition and used in the fitting routine of the difference between the start and end of the transition. If all optical changes were dominated by the pathlength, then the appropriately scaled TSR should fit the data adequately. Indeed, with the large pathlength change from IRe to IRL, this analysis revealed no redox or myoglobin changes, given that a 30% reduction in TSR was enough to fit the difference spectrum. This pathlength change agreed well with the ΔTM and absolute spectral analysis discussed above. In contrast, the pathlength increase observed with the transition from I to IC was not fit well by the I TSR on its own, as increases in bH and a605 were detected while a proportional decrease in bL absorbance occurred (Table 2, Figure 5). Having these chromophores change in opposite directions is also inconsistent with a simple pathlength change. These data are interpreted as contraction either inducing or being caused by the removal of adequate oxygen to generate a more fully reduced COX, completely blocking oxidative phosphorylation with a subsequent decrease in the mitochondrial membrane potential that oxidizes bL with a corresponding bH reduction. These results suggest a novel finding that the full metabolic impact of ischemia is not reached until contraction occurs. At this point, the cause and effect of these events, contraction versus compromise of mitochondrial membrane potential and reduction of COX, are unclear. The contraction could occur independently of the metabolic changes as the increased metabolic requirements of contraction drive down oxygen and result in the redox effects; alternatively, the background tissue metabolic rate could have decreased oxygen to the sub-saturating values for COX resulting in a further metabolic failure that resulted in contraction. Further experimental will be required to resolve this relationship between contraction and mitochondrial metabolic state. It is also important to point out that the fact that TSR was all that was required to fit the transition from IRe to IRL, where a large change in tissue absorbance occurred, implies that the ratio of all of the chromophores essentially constant over this period, consistent with the concentration of cytosolic myoglobin and the relatively mobile cytochrome c all remained constant, relative to the “fixed” COX and cyt b elements, over this reperfusion period, in the intact heart.
In summary, a robust method of collecting transmission spectra from the intact beating mouse heart is presented using an optical catheter as a light source and an integrating sphere for light detection. This system provides high signal to noise optical data of the myocardial chromophores providing essentially real-time non-destructive metabolic information together with the classical functional information available from this intact organ model system. The combination of a ventricular cavity light source coupled to an integrating sphere light collection reduces the impact of translational motion in this small heart. Several new approaches were provided to compensate for optical pathlength in this complex tissue, which aid in the interpretation of the optical transmission data. These different post-processing approaches were internally consistent and provided different advantages depending on the questions be addressed. This methodology can be combined with fluorescence or absorption dyes with appropriate excitation and emission properties to provide additional information such as pH, intracellular calcium concentration and other optical reporters of cellular milieu or function.
Tyler et al Highlights:
A system is described for optical spectroscopy of the intact murine heart
Integrating sphere for light collection minimizes motion artifacts
Dynamic Information on cardiac oxygenation and redox state provided
Ischemia and reperfusion impact tissue oxygenation and redox state
Acknowledgments
Financial Remuneration to Authors: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimers: None
REFERENCES
- [1].Keilin D, On cytochrome, a respiratory pigment, common to animals, yeast, and higher plants, Proc.R.Soc.Lond B Biol.Sci, 98 (1925) 312–339. [Google Scholar]
- [2].Wittenberg BA, Wittenberg JB, Transport of oxygen in musle, Anu.Rev.Physiol, 51 (1989) 857–878. [DOI] [PubMed] [Google Scholar]
- [3].Tamura M, Oshino N, Chance B, Silver IA, Optical measurements of intracellular oxygen concentrations of rat heart in vitro, Arch.Biochem.Biophys, 191 (1978) 18–22. [DOI] [PubMed] [Google Scholar]
- [4].Arai AE, Kasserra CE, Territo PR, Gandjbakhche AH, Balaban RS, Myocardial oxygenation in vivo: optical spectroscopy of cytoplasmic myoglobin and mitochondrial cytochromes, Am.J.Physiol, 277 (1999) H683–H697. [DOI] [PubMed] [Google Scholar]
- [5].Heineman FW, Kupriyanov VV, Marshall R, Fralix TA, Balaban RS, Myocardial oxygenation in the isolated working rabbit heart as a function of work, Am.J.Physiol, 262 (1992) H255–H267. [DOI] [PubMed] [Google Scholar]
- [6].Femnou AN, Kuzmiak-Glancy S, Covian R, Giles AV, Kay MW, Balaban RS, Intracardiac light catheter for rapid scanning transmural absorbance spectroscopy of perfused myocardium: measurement of myoglobin oxygenation and mitochondria redox state, Am J Physiol Heart Circ Physiol, 313 (2017) H1199–H1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hassinen IE, Hiltunen JK, Takala TES, Reflectance spectrophotometric monitoring of the isolated perfused heart as a method of measuring the oxidation-reduction state of cytochromes and oxygenation of myoglobin, Cardio-vasc.Res, 15 (1981) 86–91. [DOI] [PubMed] [Google Scholar]
- [8].Snow TR, Kleinman LH, Lamanna J.c., Wichsler AS, Jobsis FF, Response of cytochrome a,a3 in the in situ canine heart to transient ischemic episodes, Basic Res.Cardiol, 76 (1981) 289–304. [DOI] [PubMed] [Google Scholar]
- [9].Ti Y, Lin W-C, Effects of probe contact pressure on in vivo optical spectroscopy, Optics Express, 16 (2008) 4250–4262. [DOI] [PubMed] [Google Scholar]
- [10].Sakata Y, Abajian M, Ripple MO, Springett R, Measurement of the oxidation state of mitochondrial cytochrome c from the neocortex of the mammalian brain, Biomedical optics express, 3 (2012) 1933–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giles AV, Sun J, Femnou AN, Kuzmiak-Glancy S, Taylor JF, Covian R, Murphy E, Balaban RS, Paradoxical Arteriole Constriction Compromises Cytosolic and Mitochondrial Oxygen Delivery in the Isolated Saline-Perfused Heart, Am J Physiol Heart Circ Physiol, (2018). [DOI] [PMC free article] [PubMed]
- [12].Kuzmiak-Glancy S, Covian R, Femnou AN, Glancy B, Jaimes R 3rd, Wengrowski AM, Garrott K, French SA, Balaban RS, Kay MW, Cardiac performance is limited by oxygen delivery to the mitochondria in the crystalloid-perfused working heart, Am J Physiol Heart Circ Physiol, 314 (2018) H704–H715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chess DJ, Billings E, Covian R, Glancy B, French S, Taylor J, de BH, Murphy E, Balaban RS, Optical spectroscopy in turbid media using an integrating sphere: mitochondrial chromophore analysis during metabolic transitions, Anal Biochem, 439 (2013) 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kim N, Ripple MO, Springett R, Measurement of the mitochondrial membrane potential and pH gradient from the redox poise of the hemes of the bc1 complex, Biophysical journal, 102 (2012) 1194–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wikstrom M, Morgan JE, The dioxygen cycle. Spectral, kinetic, and thermodynamic characteristics of ferryl and peroxy intermediates observed by reversal of the cytochrome oxidase reaction, J.Biol.Chem, 267 (1992) 10266–10273. [PubMed] [Google Scholar]
- [16].Wikstrom M, Energy-dependent reversal of the cytochrome oxidase reaction, Proc.Natl.Acad.Sci.U.S.A, 78 (1981) 4051–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Varotsis C, Zhang Y, Appelman EH, Babcock GT, Resolution of the reaction sequence during the reduction of O2 by cytochrome oxidase, Proc.Natl.Acad.Sci.U.S.A, 90 (1993) 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Femnou AN, Giles A, Balaban RS, Intra-cardiac Side-Firing Light Catheter for Monitoring Cellular Metabolism using Transmural Absorbance Spectroscopy of Perfused Mammalian Hearts, J Vis Exp, (2019). [DOI] [PMC free article] [PubMed]
- [19].Glancy B, Willis WT, Chess DJ, Balaban RS, Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria, Biochemistry, 52 (2013) 2793–2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Quarrie R, Lee DS, Steinbaugh G, Cramer B, Erdahl W, Pfeiffer DR, Zweier JL, Crestanello JA, Ischemic preconditioning preserves mitochondrial membrane potential and limits reactive oxygen species production, Journal of Surgical Research, 178 (2012) 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steenbergen C, Murphy E, Watts JA, London RE, Correlation between cytosolic free calcium, contracture, ATP, and irreversible ischemic injury in perfused rat heart, Circulation Research, 66 (1990) 135–146. [DOI] [PubMed] [Google Scholar]
- [22].Langendorff O, Untersuchungen am überlebenden Säugethierherzen, Archiv für die gesamte Physiologie des Menschen und der Tiere, 61 (1895) 291–332. [Google Scholar]
- [23].Cross HR, Murphy E, Bolli R, Ping P, Steenbergen C, Expression of activated PKC epsilon (PKC epsilon) protects the ischemic heart, without attenuating ischemic H(+) production, J Mol Cell Cardiol, 34 (2002) 361–367. [DOI] [PubMed] [Google Scholar]
- [24].Matthews PM, Williams SR, Seymour AM, Schwartz A, Dube G, Gadian DG, Radda GK, A 31P-NMR study of some metabolic and functional effects of the inotropic agents epinephrine and ouabain, and the ionophore R02–2985 (X537A) in the isolated, perfused rat heart, Biochimica et Biophysica Acta, 720 (1982) 163–171. [DOI] [PubMed] [Google Scholar]
- [25].Safwat HH, Effect of Centrally Located Samples in Integrating Sphere, J Opt Soc Am, 60 (1970) 534–&. [Google Scholar]
- [26].Bell RM, Mocanu MM, Yellon DM, Retrograde heart perfusion: The Langendorff technique of isolated heart perfusion, Journal of Molecular and Cellular Cardiology, 50 (2011) 940–950. [DOI] [PubMed] [Google Scholar]
- [27].Liao R, Podesser BK, Lim CC, The continuing evolution of the Langendorff and ejecting murine heart: new advances in cardiac phenotyping, American journal of physiology. Heart and circulatory physiology, 303 (2012) H156–H167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Masuda K, Truscott K, Lin PC, Kreutzer U, Chung Y, Sriram R, Jue T, Determination of myoglobin concentration in blood-perfused tissue, Eur J Appl Physiol, 104 (2008) 41–48. [DOI] [PubMed] [Google Scholar]
- [29].Boylston JA, Sun J, Chen Y, Gucek M, Sack MN, Murphy E, Characterization of the cardiac succinylome and its role in ischemia–reperfusion injury, Journal of Molecular and Cellular Cardiology, 88 (2015) 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jobsis FF, Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters, Science, 198 (1977) 1264–1267. [DOI] [PubMed] [Google Scholar]
- [31].Schenkman KA, Marble DR, Feigl EO, Burns DH, Near-infrared spectroscopic measurement of myoglobin oxygen saturation in the presence of hemoglobin using partial least-squares analysis, Appl Spectrosc, 53 (1999) 325–331. [Google Scholar]
- [32].Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J, Disruption of myoglobin in mice induces multiple compensatory mechanisms, Proc Natl Acad Sci U S A, 96 (1999) 10495–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Phillips D, Aponte AM, Covian R, Neufeld E, Yu ZX, Balaban RS, Homogenous protein programming in the mammalian left and right ventricle free walls, Physiol Genomics, 43 (2011) 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bowen WJ, The absorption spectra and extinction coefficients of myoglobin, J Biol Chem, 179 (1949) 235–245. [PubMed] [Google Scholar]
- [35].Theorell H, de DC, Crystalline human myoglobin from heart-muscle and urine, Arch Biochem, 12 (1947) 113–124. [PubMed] [Google Scholar]
- [36].Saito S, Masuda K, Mori Y, Nakatani S, Yoshioka Y, Murase K, Mapping of left ventricle wall thickness in mice using 11.7-T magnetic resonance imaging, Magnetic Resonance Imaging, 36 (2017) 128–134. [DOI] [PubMed] [Google Scholar]
- [37].Akki A, Gupta A, Weiss RG, Magnetic resonance imaging and spectroscopy of the murine cardiovascular system, American journal of physiology. Heart and circulatory physiology, 304 (2013) H633–H648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Katz LA, Koretsky AP, Balaban RS, Activation of dehydrogenase activity and cardiac respiration: a 31P-NMR study, The American journal of physiology, 255 (1988) H185–188. [DOI] [PubMed] [Google Scholar]
- [39].Camitta MGW, Gabel SA, Chulada P, Bradbury JA, Langenbach R, Zeldin DC, Murphy E, Cyclooxygenase-1 and -2 Knockout Mice Demonstrate Increased Cardiac Ischemia/Reperfusion Injury but Are Protected by Acute Preconditioning, Circulation, 104 (2001) 2453–2458. [DOI] [PubMed] [Google Scholar]
- [40].Xie J, Qian Z, Yang T, Li W, Hu G, Minimally invasive assessment of the effect of mannitol and hypertonic saline therapy on traumatic brain edema using measurements of reduced scattering coefficient (μs′), Appl. Opt, 49 (2010) 5407–5414. [DOI] [PubMed] [Google Scholar]
- [41].Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG, Edema as a Very Early Marker for Acute Myocardial Ischemia: A Cardiovascular Magnetic Resonance Study, Journal of the American College of Cardiology, 53 (2009) 1194–1201. [DOI] [PubMed] [Google Scholar]