Abstract
Background:
Methicillin-resistant Staphylococcus aureus bacteremia (MRSA-B) may fail to improve with standard monotherapy, particularly in patients with multifocal infection, incomplete source control, or persistent bacteremia. Synergy observed in vitro between ceftaroline (CPT) and daptomycin (DAP) or vancomycin (VAN) may translate into clinical benefit. Here, we describe our experience with DAP/CPT and VAN/CPT for complicated MRSA-B after monotherapy failure.
Methods:
Single-center, retrospective review of consecutive patients treated with DAP/CPT or VAN/CPT for MRSA-B after monotherapy failure from 1 January 2016 to 30 November 2018.
Results:
We identified 11 instances of combination therapy in 10 patients (DAP/CPT = 6, VAN/CPT = 5) with 1 patient receiving VAN/CPT followed by DAP/CPT. Rates of multifocal infection, incomplete source control, persistent bacteremia, and infective endocarditis were high (100%, 80%, 60%, and 60%, respectively). Combination therapy was initiated most commonly for persistent bacteremia (60%). When patients were persistently bacteremic, median preceding duration was 13 days and median time to clearance was 3 days. Total microbiologic cure rate was 100%. There were zero instances of bacteremia relapse at 30 days (30D) or 60 days (60D). All-cause 30D and 60D mortality rates were 11.1% and 33.3%, respectively.
Conclusions:
Combination therapy demonstrated success in diverse cases of refractory MRSA-B, including instances of persistent bacteremia paired with incomplete source control. Optimal timing and therapeutic cadence for combination therapy remain unclear. Our findings suggest that DAP/CPT and VAN/CPT can be considered for complicated MRSA bacteremia when other treatment options fail or are unavailable. We propose persistent bacteremia with incomplete source control to be a clinical niche particularly worthy of further investigation.
Keywords: antibiotic combination therapy, antibiotic salvage, antimicrobial synergy, ceftaroline, daptomycin, methicillin-resistant Staphylococcus aureus, MRSA bacteremia, persistent bacteremia, vancomycin
Introduction
Staphylococcus aureus bacteremia has demonstrated mortality rates as high as 30–40%.1,2 Complicated infections, persistent bacteremia, monotherapy failure, and incomplete source control are all independently associated with poor outcomes.3 Risk factors for persistent staphylococcal bacteremia include methicillin resistance, multifocal infectious foci, and delayed removal of eradicable foci.4 Methicillin-resistant S. aureus (MRSA) infections are associated with increased mortality rates and healthcare burdens compared with methicillin-sensitive isolates.5 The cornerstone of antimicrobial therapy for MRSA bacteremia (MRSA-B) remains intravenous vancomycin (VAN) with target dosing ideally directed by the ratio of area under the curve (AUC) to minimum inhibitory concentration (MIC) with a goal of AUC/MIC ⩾ 400.6,7 Daptomycin (DAP) represents an alternative first-line option typically reserved for MRSA-B that has relapsed or persisted despite VAN treatment, or for MRSA strains with VAN MIC ⩾ 2 µg/mL.6 These two drugs are the only agents approved for MRSA-B by the United States Food and Drug Administration.6
Patients may fail to improve and infections may persist despite optimal antibiotic selection, even when drug susceptibility is evident in vitro. No standard ‘third-line’ regimen for MRSA-B has yet been established. For instances of persistent bacteremia with VAN failure, the most recent MRSA clinical practice guidelines prepared by the Infectious Diseases Society of America do provide some guidance, suggesting DAP in combination with another antibiotic, including gentamicin, rifampin, linezolid, trimethoprim-sulfamethoxazole, or a beta-lactam.8 Evidence suggests that addition of an anti-staphylococcal beta-lactam to DAP or VAN offers multiple potential synergistic effects which may translate into clinical utility.9–15 Synergy with DAP has been reported with various beta-lactams including nafcillin, oxacillin, and ceftaroline (CPT).9–12
The novel cephalosporin CPT is a recent entrant into the antibiotic armamentarium, and was approved in 2010 for community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections.16 Amongst beta-lactams, it is noteworthy for its anti-MRSA bactericidal activity afforded by affinity for the mecA-encoded penicillin-binding protein 2a (which confers methicillin-resistance).16,17 As such, CPT may be particularly attractive when selecting a beta-lactam adjunct. Clinical success has been reported with CPT in combination with both DAP and VAN for treating MRSA-B; however, no comparative studies are available or describe a single center’s experience with both regimens.12–14,18–22 We are interested in the potential for these regimens to improve clinical outcomes in patients with MRSA-B. Here, we report on our single-center experience using DAP/CPT or VAN/CPT as treatment options for complicated MRSA bacteremia following monotherapy failure. We hope that our findings can inform clinicians given the relative lack of randomized controlled trials in this area.
Methods
Study design and patients
This is a retrospective review of patients conducted at The University of Texas Medical Branch, Galveston, Texas. The study protocol was approved by the same university’s Institutional Review Board (#18-0300) including a waiver for informed consent. Potential candidates were identified through a search of our institutional medication administration record, and confirmed with manual review of electronic medical charts. Data reviewed included patient demographics, comorbid conditions, laboratory and radiographic findings, clinical notes, and microbiologic data. Patients were eligible for inclusion if they met the following criteria: age of 18 years or older at the time of admission, had at least two positive blood cultures with MRSA growth, received initial appropriate monotherapy (i.e. DAP or VAN or both), and received combination therapy for ⩾24 h with either DAP/CPT or VAN/CPT. To minimize confounding effects, patients were excluded if they received other relevant antibiotics with MRSA activity within 72 h of initiation of combination therapy (i.e. clindamycin, linezolid, tetracyclines, tigecycline, trimethoprim-sulfamethoxazole).
Definitions
Multifocal infections were exemplified by the presence of one or more infectious foci beyond the bloodstream itself (e.g. endocarditis, osteomyelitis, septic arthritis, cellulitis, pneumonia, empyema, septic pulmonary emboli, CNS abscess, or meningitis). Source control was considered indicated if such recommendation was present in an infectious disease consultant’s note(s). Preceding bacteremia was timed in days from sentinel blood culture collection until initiation of combination therapy. Ongoing bacteremia duration was calculated in number of calendar days from combination therapy initiation to the first day of microbiologic cure, which was satisfied by at least two sterile sets of blood cultures. Persistent bacteremia was defined as lasting four or more calendar days.23 Bacteremia relapse was defined as new MRSA blood culture growth after previous sterilization. Finally, all-cause mortality rates were determined by the number of patients deceased during 30-day and 60-day periods following initial positive blood culture collection.
Statistics and calculations
We analyzed continuous variables with median plus range for skewed data. Categorical variables were described by frequency and percentages. Calculations and statistical analyses were performed with Microsoft Excel 2013 software.
Results
We identified 10 patients who satisfied the prespecified inclusion and exclusion criteria; 4 patients received VAN/CPT alone, 5 received DAP/CPT alone, and 1 patient received VAN/CPT followed by DAP/CPT. The baseline demographic and clinical characteristics of these patients are displayed in Table 1.
Table 1.
Patient population.
| Patient characteristics | n = 10 (%) |
|---|---|
| Age in years, median (range) | 61.5 (27–88) |
| Male sex | 6 (60) |
| Non-White ethnicity | 3 (30) |
| Comorbid conditions present | 8 (80) |
| Cardiovascular disease | 5 (50) |
| Impaired fasting glucose or diabetes | 5 (50) |
| Chronic kidney disease | 3 (30) |
| Liver disease | 3 (30) |
| Other immunocompromising condition | 2 (20) |
| Prosthetic or foreign material | 2 (20) |
| Active malignancy | 1 (10) |
| Receipt of antibiotics in the preceding 90 days | 4 (40) |
| Penicillin allergy reported | 2 (20) |
| History of endocarditis | 1 (10) |
| PBS, median (range) | 3 (0–4) |
| CCI, median (range) | 4.5 (0–11) |
CCI, Charlson comorbidity score; PBS, Pitt bacteremia score.
Study population
Overall median patient age was 61.5 years (range 27–88) and a majority of patients were male (60%) and White (70%). Cardiovascular disease and impaired fasting glucose were the most-common comorbidities, seen in five (50%) patients each. Four (40%) patients had documented receipt of antibiotics in the preceding 90 days and one (10%) had history of endocarditis. All 10 (100%) patients were admitted from the general community. There were no documented cases of intravenous drug use in our population.
Clinical and microbiological features
All 10 (100%) patients received initial VAN therapy, underwent transesophageal echocardiography, and received infectious diseases consultation. There were zero documented instances of intermediate susceptibility or resistance to either VAN or DAP, with MIC for each drug ⩽2 µg/dl in every case. CPT MICs were available for six patients and demonstrated preserved susceptibility (⩽2 µg/dl), with a median of 0.5 µg/dl (range 0.25–1). All 10 (100%) patients had multifocal infections, with endocarditis being the most common observed focus (60%, n = 6). Eight (80%) patients had two or more infectious foci, three (30%) had intravascular catheters or devices at bacteremia onset, and two (20%) had orthopedic infections. Complete source control was indicated in every case and achieved in two (20%). Persistent bacteremia was the most common qualification for monotherapy failure (60%, n = 6), with a median preceding duration of bacteremia of 13 days despite adequate monotherapy (range 6–16). Details of the individual cases can be found in Table 2.
Table 2.
Details of individual cases.
| Case # | Age, sex, and comorbid conditions | Infectious focus | Complete source control? | Prior therapy (drug, duration, MIC)a | Preceding duration of bacteremia before combo therapy | Reason for monotherapy failure | Combination therapy regimen and total duration | Ceftaroline dose and MICa | Ongoing bacteremia duration with combo therapy | Subsequent regimen | Microbiologic cure | Alive at 30 days?b | Alive at 60 days?b | Adverse effects |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27 M; none | Facial cellulitis, septic pulmonary emboli | No | VAN (5 days, MIC ⩽0.5) | Bacteremia cleared | Sub-therapeutic VAN troughs | VAN/CPT, 7 days | 600 mg q8h, MIC 0.5 | Cleared prior | VAN, 6 weeks | Resolved | Yes | Yes | None |
| 2 | 85 F; CAD, ESRD, diabetes, pacemaker | Septic arthritis, mitral endocarditis | Yes | VAN (8 days, MIC 1) | 8 days | Persistent bacteremia | VAN/CPT, 7 days | 200 mg q12h, MIC 0.5 | 3 days | VAN, 6 weeks | Resolved | Yes | No | None |
| 3 | 61 M; CNL, CLD | Leg cellulitis, cerebral abscesses | No | VAN (27 days, MIC 1) | Bacteremia cleared | Cerebral abscess formation | VAN/CPT, 13 days | 600 mg q8h | Cleared prior | MIN, indefinite | Resolved | lost to follow-up | lost to follow-up | Thrombocytopenia |
| 4 | 42 F; none | C5 osteomyelitis, retropharyngeal abscess | No | VAN (1 day, MIC 1) | 1 day | Spinal abscess formation | VAN/CPT, 9 days | 600 mg q8h, MIC 0.5 | 5 days | VAN/RIF, 6 weeks | Resolved | Yes | Yes | Rash |
| 5 | 88 F; ESRD, CHF, hx of MSSA endocarditis | Mitral endocarditis, AVF infection | No | VAN (6 days, MIC 1) | 6 days | Persistent bacteremia | VAN/CPT, 2 days | 200 mg q8h | Failed | None | ||||
| VAN/CPT | DAP/CPT, 7 days | 400 mg q8h | 4 days | VAN, 6 weeks | Resolved | Yes | Yes | None | ||||||
| 6 | 64 M; CAD, AS, ICD | Septic thrombophlebitis, ICD-related endocarditis | No | VAN (7 days, MIC 1), DAP (9 days, MIC 0.5) | 16 days | Persistent bacteremia | DAP/CPT, 20 days | 600 mg q8h | 1 day | DAP, 3 weeks | Resolved | Yes | Yes | None |
| 7 | 58 F; TTP, lupus, prediabetes | CLABSI, aortic endocarditis | No | VAN (7 days, MIC 0.5), DAP (7 days, MIC 0.5) | 14 days | Persistent bacteremia | DAP/CPT, 24 days | 600 mg q12h, MIC 0.5 | 1 day | DAP, 3 weeks | Resolved | Yes | No | None |
| 8 | 63 M; CAD, CHF, COPD, diabetes | CLABSI, IABP infection | No | VAN (6 days, MIC 1) | 6 days | Persistent bacteremia | DAP/CPT, 6 days | 600 mg q12h | 3 days | n/a | Resolved | No | No | None |
| 9 | 62 M; CAD, CLD, diabetes | Aortic endocarditis | Yes | VAN (10 days, MIC 0.5), DAP (9 days, MIC 0.5) | Bacteremia cleared | Worsening leukocytosis | DAP/CPT, 6 days | 600 mg q12h, MIC 0.25 | Cleared prior | DAP/RIF, 4 weeks | Resolved | Yes | Yes | Eosinophilia |
| 10 | 55 M; CLD, foot osteomyelitis, diabetes | Aortic endocarditis, prostate abscess, septic renal & splenic emboli | No | VAN (9 days, MIC 2), DAP (4 days, MIC 0.5) | 13 days | Persistent bacteremia | DAP/CPT, 13 days | 600 mg q12h, MIC 1 | 9 days | DAP/RIF, 6 weeks | Resolved | Yes | Yes | None |
Expressed MICs are highest reported during therapy with respective antibiotic.
30 and 60 day mortality are calculated from time of initial MRSA blood culture.
AS, aortic stenosis; AVF, arteriovenous fistula; CAD, coronary artery disease; CHF, congestive heart failure; CLASBI, central line-associated bloodstream infection; CLD, chronic liver disease; CNL, chronic neutrophilic leukemia; COPD, chronic obstructive pulmonary disease; CPT, ceftaroline; DAP, daptomycin; ESRD, end-stage renal disease; ICD, implantable cardioverter defibrillator; MIC, minimum inhibitory concentration; MIN, minocycline; MSSA, methicillin-sensitive Staphylococcus aureus; RIF, rifampin; TTP, thrombotic thrombocytopenic purpura; VAN, vancomycin.
Patient outcomes
Microbiologic cure was observed in 100% (n = 10) of patients treated with CPT combination therapy, 90% with the first regimen. The single instance of microbiologic treatment failure, seen in a case of persistent bacteremia treated with VAN/CPT, achieved subsequent blood culture sterility 2 days later on DAP/CPT. When patients who were persistently bacteremic (median preceding duration 13 days, range 6–16) were switched to CPT-containing regimens, median subsequent time to clearance was 3 days (range 1–9). All of these patients had incomplete source control when blood culture sterility was realized. In one patient achieving full source control, they cleared their bacteremia 2 days postprocedure (aortic valve replacement). In their case, CPT was added to DAP for progressive, postoperative leukocytosis and not for persistent bacteremia. The second patient who achieved full source control (after chest tube placement for exudative pleural effusion) had already cleared their bacteremia 5 days previously (while receiving VAN/CPT). Median total duration of combination therapy was 9 days (range 6–24). In patients surviving to hospital discharge (90%, n = 9), all CPT-containing regimens were modified, either as step-down to monotherapy with DAP (22.2%, n = 2) or VAN (33.3%, n = 3), rifampin-containing combination therapy (33.3%, n = 3), or palliative suppression with minocycline monotherapy (10%, n = 1). No patients were discharged on CPT. Mention of potential adverse effects from either combination regimen occurred three times, with rash, eosinophilia, and thrombocytopenia each cited once. There were no known episodes of bacteremia relapse at 30 days or 60 days. All-cause 30- and 60-day mortality in our series were 11.1% and 33.3%, respectively. One patient (on DAP/CPT) was palliatively extubated and died in the hospital due to numerous medical issues upon which MRSA infection was superimposed. Two patients died after hospital discharge within 60 days of bacteremia onset due to unknown circumstances. One patient was lost to follow up and thus not included in mortality analysis. A summary of these outcomes can be viewed in Table 3.
Table 3.
Patient outcomes.
| Outcome | n = 10 (%) |
|---|---|
| Microbiologic cure | 10 (100) |
| >>on first combination regimen | 9 (90) |
| Relapse at 30 days | 0 (0) |
| Relapse at 60 days | 0 (0) |
| In-hospital mortality | 1 (10) |
| 30-day mortalitya | 1 (11.1) |
| 60-day mortalitya | 3 (33.3) |
| Persistent bacteremia subgroup | n = 6 (%) |
| Ongoing bacteremia duration in days, median (range) | 3 (1–9) |
| In-hospital mortality | 1 (16.7) |
| 30-day mortality | 1 (16.7) |
| 60-day mortality | 3 (50) |
n = 9 as one patient was lost to follow-up.
Discussion
CPT appears to hold value as an adjunctive agent for MRSA bacteremia after monotherapy failure. This includes cases complicated by multiple infectious foci, persistent infection, or incomplete source control. Although persistent bacteremia prompted most of the combination therapy utilization in our series, we did not restrict patient selection solely to that indication. This cohort provides a snapshot of some of the most refractory cases of MRSA bacteremia. We believe our experience offers valuable perspective into real-world applications of this evolving treatment paradigm.
Even when antibiotic susceptibility is preserved in vitro, infections may fail to improve on appropriate monotherapy. This is particularly true in high inoculum infections (e.g. those with numerous infectious foci, retained intravascular hardware, poor source control) which may overwhelm an antibiotic’s ability to exhibit sufficient bactericidal activity for satisfactory effect. Considering the high frequency of multifocal infections and incomplete source control, all of our cases probably qualify as having high-inocula. With S. aureus, this ‘inoculum effect’ has been shown in vitro to impair VAN activity more than DAP.24,25 In one murine model, the inoculum effect was not observed with CPT.26 Thus, one could posit advantage of DAP/CPT over VAN/CPT for such infections, although data are currently lacking. One study suggested sustained activity of DAP/CPT in a simulated model of high-inoculum infective endocarditis (a comparative VAN/CPT assay was not available).27 Achieving indicated source control is often impossible due to limiting clinical factors, including high operative risk or patient preference. Our series was characterized by a high rate of multifocal infections with incomplete source control yet combination therapy remained successful. Additional studies focusing on the role of source control, or lack thereof, would be instructive, as we believe this area to hold particular promise as a potential niche for CPT combination therapy.
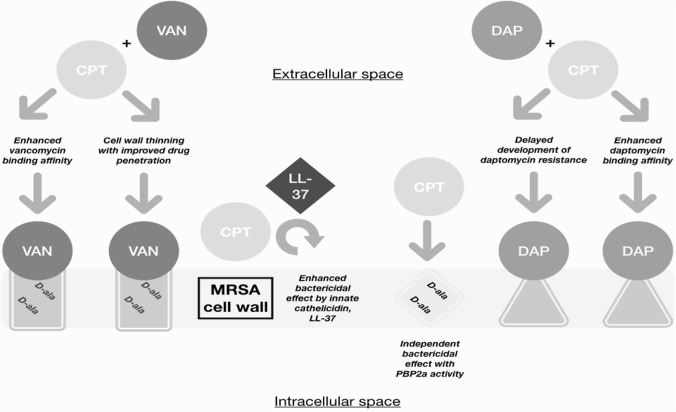
Proposed synergy between beta-lactams and DAP is predicated on various observations made in vitro, including enhanced bactericidal activity, improved DAP-binding affinity, recruitment of immune system-derived antimicrobial peptides, and delay or reversal of DAP resistance.9–15 Similar mechanisms have been proposed to account for observed synergy with VAN, including beta-lactam-induced cell wall thinning and enhanced target-specific VAN binding, with superior synergism proposed with CPT.15 Synergy has been demonstrated in vitro with both DAP/CPT and VAN/CPT, with groups employing various assays including time-kill analyses and modified E-test techniques.15,21,28,29 Figure 1 provides an illustrated summary of the proposed synergy mechanisms present with DAP/CPT and VAN/CPT.
Figure 1.
Proposed synergy mechanisms with CPT and DAP or VAN in MRSA infection.
CPT, ceftaroline; DAP, daptomycin; MRSA, methicillin-resistant Staphylococcus aureus; VAN, vancomycin.
Our findings suggest augmented bactericidal activity when CPT is added to DAP or VAN. This is evidenced by an overall microbiologic cure rate of 100% across the entire patient series. This includes the persistent bacteremia subgroup, who saw a 3-day median time-to-eradication, trailing a median preceding duration of 13 days. These results compare favorably with the largest case series analyzing CPT-containing combination therapy for staphylococcal bacteremia, in which 26 patients on DAP/CPT realized a 2-day median time-to-eradication following a median 10 days of bacteremia.12 That same study also observed similarly high rates of infective endocarditis (54%) and clinical cure (96%). Another large series of 12 patients treated with DAP/CPT saw a significantly higher mortality rate of 50%.20 Regarding experience with VAN-based therapy, our results are similar to that reported in the largest available case series of patients treated with VAN/CPT.18 In that group, all patients had multifocal infections (100%, n = 5), most had persistent bacteremia (80%, n = 4), endocarditis was common (40%, n = 2), and clinical success was realized in the majority (80%, n = 4). Our experience is highlighted by two instances of rapid microbiologic cure within approximately 24 h of adding CPT, trailing 14 and 16 days of bacteremia, respectively. It is possible that these observations represent late effects of the preceding monotherapy, however. Considering the historically high mortality and morbidity of staphylococcal bacteremia, our results, and those of others, suggest that CPT combination therapy could improve the therapeutic landscape of this serious condition.
Our study has several limitations due to its small, single-center, retrospective nature, which may hinder generalizability. Our results do not allow us to propose superiority of one regimen, though both have demonstrated clinical merit. Decisions to initiate (or withhold) combination therapy were at the discretion of the individual treating clinicians and allocation bias may be present. Unanswered is the optimal time to begin a combination regimen, and the appropriate cadence in therapy (i.e. second-line, third-line). We cannot predict if these patients would have cleared their bloodstream infections had their previous antibiotic regimens been continued for additional time. Conversely, starting CPT-combination regimens earlier may have prevented additional morbidity or mortality through timelier clearance. Importantly, combination regimens containing other beta-lactams (and nonbeta-lactams) deserve attention.30,31 The role of CPT in staphylococcal bacteremia may be limited by concerns regarding its relatively high cost and the development of drug resistance. For instance, the cost of CPT administration at our institution equals US $326 per day. This is substantially higher than with VAN ($25), nafcillin ($77), or DAP ($132). Potential total costs could be higher with combination therapy, yet the converse may also be true if overall treatment times could be significantly reduced. Albeit currently rare, CPT-resistant clinical isolates have been described.32 CPT resistance rates can be predicted to rise with increased use. Institutions might be wise to curtail costs and drug resistance by requiring oversight of CPT use by infectious disease specialists or antimicrobial stewardship programs. At our hospital, CPT use is restricted as such. Lastly, although we propose that our observed effects were due to antimicrobial synergy, it is not possible to accurately assess the contribution of CPT acting alone within our analysis. Additive effects remain biologically plausible, as multiple clinical reports illustrate success with CPT monotherapy for MRSA-B.33,34 Likely the most compelling evidence for synergism lies with those reports describing clinical success and accompanying in vitro evidence of improvement or restoration of drug susceptibility in the presence of CPT.21,22,29
Conclusion
Our results depict successful patient outcomes with CPT combination therapy and illustrate its potential value in treating MRSA bacteremia. This includes persistent and high inoculum infections hindered by incomplete source control, significant burdens of comorbid disease, and failure of standard therapies. Prospective, randomized, controlled studies will better define CPT combination therapy’s full potential. Meanwhile, we suggest our colleagues to consider DAP/CPT or VAN/CPT when patients afflicted with MRSA bacteremia have failed other treatment options.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Joseph Patrik Hornak  https://orcid.org/0000-0002-7644-6880
https://orcid.org/0000-0002-7644-6880
Contributor Information
Joseph Patrik Hornak, Department of Internal Medicine, The University of Texas Medical Branch, Galveston, TX, USA.
Seher Anjum, National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA.
David Reynoso, The University of Texas Medical Branch, Division of Infectious Diseases, 301 University Blvd., Rte. 0435, Marvin Graves Building 4.210H, Galveston, TX, 77555-0435, USA.
References
- 1. Mylotte JM, McDermott C, Spooner JA. Prospective study of 114 consecutive episodes of Staphylococcus aureus bacteremia. Rev Infect Dis 1987; 9: 891–907. [DOI] [PubMed] [Google Scholar]
- 2. Wyllie DH, Crook DW, Peto TE. Mortality after Staphylococcus aureus bacteraemia in two hospitals in Oxfordshire, 1997-2003: cohort study. BMJ 2006; 333: 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25: 362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chong YP, Park SJ, Kim HS, et al. Persistent Staphylococcus aureus bacteremia: a prospective analysis of risk factors, outcomes, and microbiologic and genotypic characteristics of isolates. Medicine 2013; 92: 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36: 53–59. [DOI] [PubMed] [Google Scholar]
- 6. Holland TL, Arnold C, Fowler VG. Clinical management of Staphylococcus aureus bacteremia. JAMA 2014; 312: 1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am J Health Syst Pharm 2009; 66: 82–98. [DOI] [PubMed] [Google Scholar]
- 8. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52: 285–292. [DOI] [PubMed] [Google Scholar]
- 9. Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphyloccocal β-lactam to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Infect Dis 2011; 53: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sakoulas G, Okumura CY, Thienphrapa W, et al. Nafcillin enhances innate immune mediated-killing of methicillin-resistant Staphylococcus aureus. J Mol Med (Berl) 2014; 92: 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehta S, Singh C, Plata KB, et al. Beta-lactams increase the antibacterial activity of daptomycin against clinical methicillin-resistant Staphylococcus aureus strains and prevent selection of daptomycin-resistant derivatives. Antimicrob Agents Chemother 2012; 56: 6192–6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sakoulas G, Moise PA, Casapao AM, et al. Antimicrobial salvage therapy for persistent staphylococcal bacteremia using daptomycin plus ceftaroline. Clin Ther 2014; 36: 1317–1333. [DOI] [PubMed] [Google Scholar]
- 13. Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2019; 63: e02483–e02518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hand A, Sakoulas G. Daptomycin in combination with other antibiotics for the treatment of complicated methicillin-resistant Staphylococcus aureus bacteremia. Clin Ther 2014; 36: 1303–1316. [DOI] [PubMed] [Google Scholar]
- 15. Tran KN, Rybak MJ. β-Lactam combinations with vancomycin show synergistic activity against vancomycin-susceptible Staphylococcus aureus, vancomycin-intermediate S. aureus (VISA), and heterogeneous VISA. Antimicrob Agents Chemother 2018; 62: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. TEFLARO®(ceftaroline fosamil)[package insert]. Forest Pharmaceuticals Inc., St. Louis, MO, https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/200327s000lbl.pdf (2010)
- 17. Kosowska-Shick K, McGhee PL, Appelbaum PC. Affinity of ceftaroline and other β-lactams for penicillin-binding proteins from Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 2010; 54: 1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gritsenko D, Federenko M, Ruhe JJ, et al. Combination therapy with vancomycin and ceftaroline for refractory methicillin-resistant Staphylococcus aureus bacteremia: a case series. Clin Ther 2017; 39: 212–218. [DOI] [PubMed] [Google Scholar]
- 19. Dilworth TJ, Ibrahim O, Hall P, et al. β-Lactams enhance vancomycin activity against methicillin-resistant Staphylococcus aureus bacteremia compared to vancomycin alone. Antimicrob Agents Chemother 2014; 58: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cortes-Penfield N, Oliver NT, Hunter A, et al. Daptomycin and combination daptomycin-ceftaroline as salvage therapy for persistent methicillin-resistant Staphylococcus aureus bacteremia. Infect Dis (Lond) 2018; 50: 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rose WE, Schulz LT, Andes D, et al. Addition of ceftaroline to daptomycin after emergence of daptomycin-nonsusceptible Staphylococcus aureus during therapy improves antibacterial activity. Antimicrob Agents Chemother 2012; 56: 5296–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shafiq I, Bulman ZP, Spitznogle SL, et al. A combination of ceftaroline and daptomycin has synergistic and bactericidal activity in vitro against daptomycin nonsusceptible methicillin-resistant Staphylococcus aureus (MRSA). Infect Dis (London) 2017; 49: 410–416. [DOI] [PubMed] [Google Scholar]
- 23. Gilbert DN, Chambers HF, Eliopoulas GM, et al. The Sanford Guide to Antimicrobial Therapy, 46th ed Sperryville, VA: Antimicrobial Therapy, Inc, 2016. [Google Scholar]
- 24. LaPlante KL, Rybak MJ. Impact of high-inoculum Staphylococcus aureus on the activities of nafcillin, vancomycin, linezolid, and daptomycin, alone and in combination with gentamicin, in an in vitro pharmacodynamic model. Antimicrob Agents Chemother 2004; 48: 4665–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee DG, Murakami Y, Andes DR, et al. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 10(5) and 10(7) CFU injected into opposite thighs of neutropenic mice. Antimicrob Agents Chemother 2013; 57: 1434–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. So W, Crandon JL, Zhanel GG, et al. Comparison of in vivo and in vitro pharmacodynamics of a humanized regimen of 600 milligrams of ceftaroline fosamil every 12 hours against Staphylococcus aureus at initial inocula of 106 and 108 CFU per milliliter. Antimicrob Agents Chemother 2014; 58: 6931–6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Werth BJ, Barber KE, Ireland CE, et al. Evaluation of ceftaroline, vancomycin, daptomycin, or ceftaroline plus daptomycin against daptomycin-nonsusceptible methicillin-resistant Staphylococcus aureus in an in vitro pharmacokinetic/pharmacodynamic model of simulated endocardial vegetations. Antimicrob Agents Chemother 2014; 58: 3177–3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barber KE, Werth BJ, McRoberts JP, et al. A novel approach utilizing biofilm time-kill curves to assess the bactericidal activity of ceftaroline combinations against biofilm-producing methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2014; 58: 2989–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nigo M, Diaz L, Carvajal LP, et al. Ceftaroline-resistant, daptomycin-tolerant, and heterogeneous vancomycin-intermediate methicillin-resistant Staphylococcus aureus causing infective endocarditis. Antimicrob Agents Chemother 2017; 61: e01235–e01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jang HC, Kim SH, Kim KH, et al. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis 2009; 49: 395–401. [DOI] [PubMed] [Google Scholar]
- 31. Shaddix G, Patel K, Simmons M, et al. Successful clearance of persistent methicillin-resistant Staphylococcus aureus bacteremia with daptomycin, linezolid, and meropenem salvage therapy. Case Rep Infect Dis 2019; 2019: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alm RA, McLaughlin RE, Kos VN, et al. Analysis of Staphylococcus aureus clinical isolates with reduced susceptibility to ceftaroline: an epidemiological and structural perspective. J Antimicrob Chemother 2014; 69: 2065–2075. [DOI] [PubMed] [Google Scholar]
- 33. Ho TT, Cadena J, Childs LM, et al. Methicillin-resistant Staphylococcus aureus bacteraemia and endocarditis treated with ceftaroline salvage therapy. J Antimicrob Chemother 2012; 67: 1267–1270. [DOI] [PubMed] [Google Scholar]
- 34. Arshad S, Huang V, Hartman P, et al. Ceftaroline fosamil monotherapy for methicillin-resistant Staphylococcus aureus bacteremia: a comparative clinical outcomes study. Int J Infect Dis 2017; 57: 27–31. [DOI] [PubMed] [Google Scholar]