Abstract
Background
Tendinopathy is a chronic disorder that affects a huge population, and is causing high socioeconomical impacts worldwide. Tendinopathy was reported to be more prevalent in diabetic patients, and chronic inflammation was proposed to play an important role in its development. It was also known that diabetic patients present in a pro-inflammatory state. There is a possibility that the high glucose environment in diabetic patients lead to chronic inflammation in the tendon, and eventually the development of tendinopathy. In this study, we would simulate the diabetic environment in an in vitro setup, to assess the effect of a high glucose level on cultured tendinopathic and healthy tendon derived stem cells (TDSCs) under inflammatory stress. We would first like to assess whether there are differences between the inflammatory response in tendinopathic and healthy TDSCs. We would then investigate whether a high glucose level may lead to changes in the inflammatory response in healthy tendon cells.
Methods
Tendinopathic TDSCs were cultured from 2 torn rotator cuff tendons and 1 ruptured patellar tendon. Healthy TDSCs were cultured from 3 gender matched healthy hamstring tendons. Cells were stimulated by either 2ng/ml IL-1B for 24 hours, 11.1 mmol/L glucose for 24 hours, or both. mRNA was collected and processed for qPCR targeting B-actin, ALOX12, ALOX15, FPR1, FPR2, ChemR23, and COX2.
Results
Upregulation of FPR1 (p=0.050) ChemR23 (p=0.050), ALOX15 (p=0.050) was significantly weakened when comparing tendinopathic and healthy TDSCs stimulated with IL-1b. The upregulation of ALOX15 (p=0.050), was significantly lower in stimulated healthy TDSCs in a high glucose environment when comparing with those stimulated under a regular glucose level. A high glucose level also induced upregulation of COX2 (p=0.046) in healthy TDSCs and tendinopathic TDSCs (p=0.050).
Conclusion
The results of this study provide a possible explanation to the increased risk to develop tendinopathy in diabetic patients. Chronic inflammation observed in tendinopathy may be due to the weakening of pro-resolving responses in tendinopathic TDSCs, and a high glucose environment may lead to chronic inflammation and ultimately tendinopathy by persistent stimulation and weakening of pro-resolving response in healthy TDSCs.
Introduction
Tendinopathy refers to the pain, swelling, and impaired performance of the tendon.1 This chronic disorder is causing great socioeconomical impacts to our society.2 For example, the prevalence of tendinopathy was reported to affect as much as 50% of the population by the age of 60%.3 Anti-inflammatory drugs including, NSAIDs and glucocorticoids, are the first line treatment for the conservative management of tendinopathy. However, there have been claims that these drugs may negatively affect the structural healing of tendons.4 Despite the presence of various modalities of conservative and surgical treatments, more than one third of the patients do not respond and continue to present with persistent pain and disability.3,5 There is an urgent need further understand its character and pathogenesis, in search for more effective treatments to this chronic disease.
The pathogenesis of tendinopathy is yet to be well understood. Opposing to the previous concept that chronic tendinopathy presents as an inflammation free degenerative disease,1 there is a rise in evidence showing that inflammatory cells are present, and chronic inflammation is a feature of tendinopathy.6,7 Degenerative changes in the tendon could be due to chronic exposure to non-selective digestive enzymes produced from the inflammatory process. As suggested by a study in 2018, local tendon cells play an important role in the regulation of inflammation within the tendon.6 Various factors lead to changes in the local tendon cells, “priming” these mediators into a pro-inflammatory state of enhancement of inflammation, and dysregulation of the resolution of inflammation. However, characteristics of “priming” and factors leading to these changes are yet to be described.
Cyclooxygenase 2 (COX2) is an enzyme responsible for the production of prostaglandins, which leads to enhancement of inflammation via vasodilation and chemoattraction to inflammatory cells.8,9 Specific lipid pro-resolving mediators (SPMs) are molecules that play a major role in the resolution of inflammation.10 Enzymes such as, ALOX12, and ALOX15 are responsible for the production of these lipids, and they act upon specific protein receptors including FPR2 for lipoxin A1, and also ChemR23 for resolvin D3. Activated receptors then provides anti-inflammatory mediators, including IL-10, to resolve inflammation.10,11
According to a systematic review published in 2016, Diabetes mellitus (DM) is a risk factor of the development of tendinopathy. There is a 3.67 fold increase in the development of tendinopathy in DM patients.12 Previous in vitro studies have reported that a high glucose level induces negative impacts on viability and function of tendon cells.13,14 It was also reported that high glucose enhances an inflammatory response in endothelial cells.15 There is a possibility that the direct effect of a high glucose level on local tendon cells lead to dysregulation of the resolution of inflammation, leading to chronic inflammation and the increased risk in development of tendinopathy in DM patients. In this study, we would simulate the diabetic environment in an in vitro setup, to assess the effect of a high glucose level on cultured tendinopathic and healthy tendon derived stem cells (TDSCs). We would like to assess the role of a high glucose level on the development of tendinopathy, and to discuss the possibility whether controlling glucose levels could be beneficial in the management of tendinopathy.
Materials and method
Overview
To investigate the effect of glucose on the mRNA expression of healthy and tendinopathic TDSCs, cells were isolated from human specimens as primary culture. Cells were then expanded and seeded into 6-well plates, where treatments were performed. After a pilot study verifying the parameters, TDSCs were treated with 2 ng/ml IL-1B for 24 h as inflammatory stimulation. In a separate group, A high glucose environment of 11.1 mmol/L glucose was pre-incubated before inflammatory stimulation for healthy TDSCs. This specific glucose level have been adapted to represent a high glucose environment in previous in vitro studies. It represents cut off for 2hr oral glucose tolerance test in the diagnosis of DM,16 or in other words a high glucose level that should not be reached in the DM free population. Treated cells were lysed with mRNA extracted and reverse transcribed. The completed cDNA samples were diluted and measured for gene expression of the target genes including FPR1, FPR2, ALOX12, ALOX15, ChemR23, and COX2. Statistical analysis was used to detect significant differences between the groups.
Patient recruitment
The experiments in this study were approved by the Joint Chinese University of Hong Kong – New Territories East Cluster Clinical Research Ethics Committee (CREC) (Reference number 2013.479). In this study, tendon specimens of tendinopathic patients were collected. Inclusion criteria include a gender of either male or female, age of 18–60 years old. Age and gender matched healthy controls were also recruited.
Tendinopathic patients were recruited from patients undergoing surgical repair of a spontaneously ruptured tendon. It was previously suggested that healthy tendons are extremely unlikely to experience spontaneous ruptures since they can withstand very high tensile loads.17 We therefore assume that cases of spontaneous ruptures free from high energy trauma originate from only tendinopathic, degenerative tendons. Debridement of the ruptured tendon were collected during the surgery. Healthy controls were recruited from patients undergoing Anterior Cruciate Ligament Reconstruction. The stump of the autologous hamstring tendon graft used in the surgery, a healthy gracilis or the semi-tendinosis tendon, was collected during the surgery.
Primary culture of TDSCs
Human TDSCs were cultured according to a well established protocol. From a previous study from our group, cells cultured with this method consistently presented with characteristics of TDSCs.18 Immediately after collection of tendon specimen. The tendon specimen was rinsed with 10% Penicillin-Streptomycin-Neomycin (PSN), followed by 5 min of incubation with 0.25% trypsin in low glucose Dulbecco’s Modified Eagle Medium (LG DMEM). The specimen was minced in into less than 2 × 2 mm pieces, then incubated in 1 mg/ml collagenase type 1 (Sigma Aldrich) overnight.
On the following day, the remaining tendon digest was filtrated with a 70 μm cell strainer. Filtrate was centrifuged with 1500 rpm for 5 min. Supernatant was discarded, and cells were resuspended in 1 ml LG DMEM, then seeded into cell culture flasks with a density of less than 2000 cells/cm.2 After isolation, cells were cultured in LG DMEM with 10% PSN and 10% fetal bovine serum (FBS). Culture medium were changed 2 times a week to allow optimal growth. Cells were placed in an incubator at 37 °C and 5% CO2.
Pre-incubation with a high glucose environment
6 wells with 80% confluency were pre-treated with a high glucose levels for healthy TDSCs in this study. LG DMEM contains 1 g/L glucose originally. To induce a high glucose environment, additional glucose was added to LG DMEM with a glucose solution up to 11.1 mmol/L, immediately following IL-1B stimulation.
Stimulating an inflammatory response with IL-1B
6 wells of both healthy and tendinopathic TDSCs at 80% confluency was be treated with IL-1B infused medium LG DMEM 10% PSN, 10% FBS, with a concentration of 2 ng/ml for 24 h. These parameters were determined in a pilot study from our group, suggesting that this design is capable of successfully stimulating the TDSCs. 6 more wells of both healthy and tendinopathic TDSCs were un-treated, incubated in LG DMEM supplemented with 10% FBS and 10% PSN for 24 h, serving as a control to the IL-1B stimulation.
Real time polymerase chain reaction
Cells in 6 wells plates had mRNA extracted by the ThermoFisher PureLink RNA mini kit according to the developer’s protocol. RNA yield and quality was then analyzed, followed by reverse transcription of extracted mRNA using Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) enzyme, according to developer’s protocol.
rtPCR was performed on product cDNA, using the following primer sequences (Table 1).
Table 1.
Primer sequences used in this study.
| Primer | Sequence |
|---|---|
| FPR1 Forward | CTGAGTCACTCTCCCCAGGA |
| FPR1 Reverse | CCAGGAAGAGATAGCCAGCA |
| FPR2 Forward | GAAGCACACAGGAAAAGGAG |
| FPR2 Reverse | GACAAAGGTGACCCCAAG |
| ChemR23 Forward | CCCTACCACACACTCAACCT |
| ChemR23 Reverse | GGGCCACCTTGAACTTCTTG |
| ALOX12 Forward | GAAGATGTGACGATGGCCAC |
| ALOX12 Reverse | ATAGGGCCAGTCAAGTTGCT |
| ALOX15 Forward | GTGGAAAACAGTGTGGCCAT |
| ALOX15 Reverse | AGTAAGGTCCCAGGTGATGC |
| COX2 Forward | TTCAAATGAGATTGTGGGAAAAT |
| COX2 Reverse | AGATCATCTCTGCCTGAGTATCTT |
| B-Actin Forward | TGGAACGGTGAAGGTGACAG |
| B-Actin Reverse | GGCTTTTAGGATGGCAAGGG |
rtPCR was performed using the following program. At the hold stage, samples were first heated to 95 °C at a rate of 1.6 °C/second to be prepared for the cycling phase. The samples will then be subjected to 40 cycles of heating to 95 °C for 15 s, and data collection at 60 °C for 30 s and 72 °C for 30 s. After that, the program will enter the melt curve stage. In the melt curve stage, samples were again heated to 95 °C for 15 s, temperature will then be dropped to 60 °C for 1 min, followed by dissociation and data collection by heating to 95 °C for 15 s.
Statistical analysis
Data Collected from qPCR were in the form of cycle threshold value (ct value). Values of the target genes were subtracted with the ct value of the house keeping gene b-actin. Transformation using the function y= (2^-(target gene ct value – b-actin ct value)) was done, showing the relative expression of each target gene in comparison with the house keeping gene, or the dct value. dct values of treatments groups were further normalized to the control group for the ddct value. Log transformation was performed on dct and ddct values for presentation.
Using Statistical Product and Service Solutions (SPSS) version 20, comparisons were made between groups for every target gene including FPR1, FPR2, ChemR23, ALOX12, ALOX15, and COX2. The Mann–Whitney U test was used to determine significance in the gene expressions. Significant difference was defined as p < 0.05.
Results
Patient recruitment
With the protocol mentioned above, 1 specimen of a torn patellar tendon and 2 specimens from a torn rotator cuff was obtained from 1 male and 2 female patients (age 21–54). 3 specimens of healthy hamstring tendons were obtained from 3 gender matched patients (age 20–28).
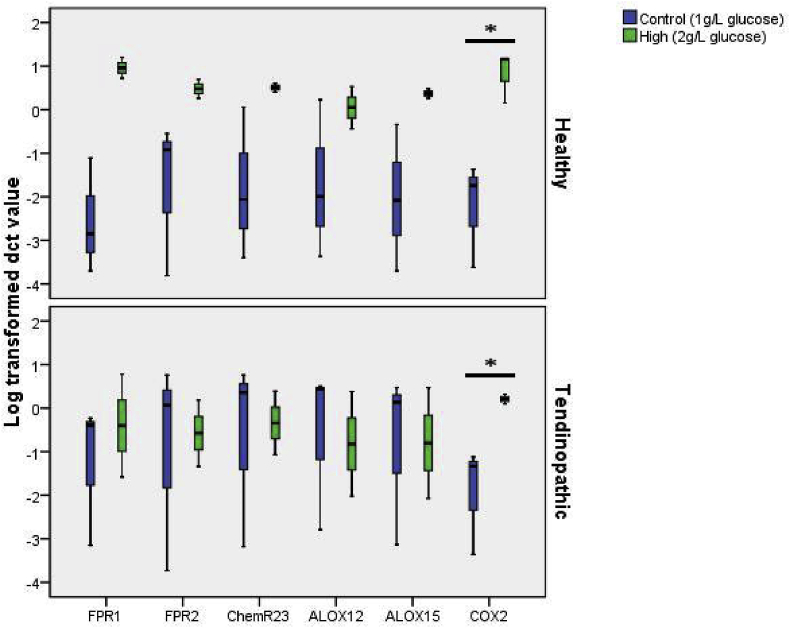
A high glucose level increases the mRNA expression of COX2 in both healthy and tendinopathic TDSCs
TDSCs were treated with a high glucose environment of 11.1 mmol/L for 24 h. The mRNA expression of COX2 was significantly increased after treatment in both healthy (p = 0.046) and tendinopathic TDSCs (p = 0.050) There were no significant changes in expression of other selected genes (Fig. 1).
Fig. 1.
Log transformed dCT value of ALOX12, ALOX15, FPR1, FPR2, ChemR23, COX2 normalized to B-actin. Comparison between TDSCs treated with 11.1 mmol/L glucose for 24 h and control under 1 g/L glucose. * indicate significant difference of p < 0.05.
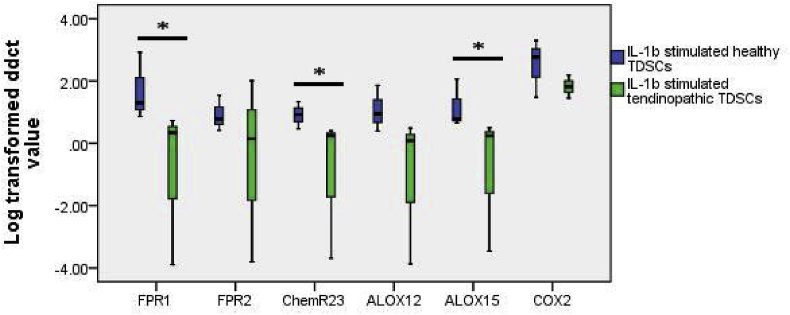
Upregulation of FPR1, ChemR23 and ALOX15 mRNA was weakened in tendinopathic TDSCs upon IL-1b stimulation
Healthy and tendinopathic TDSCs were stimulated with 2 ng/ml IL-1b for 24 h. Up-regulation of FPR1 (p = 0.050), ChemR23 (p = 0.050), and ALOX15 (p = 0.050) were significantly weakened in tendinopathic TDSCs in comparison with healthy TDSCs. There were no significant differences in the gene expression of all selected genes between healthy and tendinopathic TDSCs prior to stimulation (Fig. 2).
Fig. 2.
Log transformed ddCT value of ALOX12, ALOX15, FPR1, FPR2, ChemR23, COX2 normalized to b-actin and control group. Comparison between healthy and tendinopathic TDSCs stimulated with 2 ng/ml IL-1b for 24 h * indicate significant difference of p < 0.05.
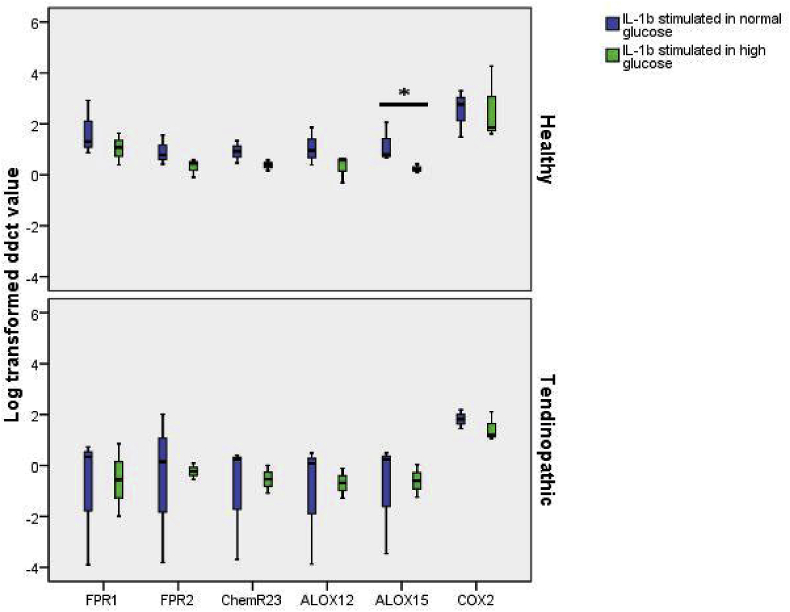
Upregulation of ALOX15 mRNA was weakened in IL-1b stimulated healthy TDSCs under a high glucose environment but no changes were observed in IL-1b stimulated tendinopathic TDSCs
TDSCs were stimulated with 2 ng/ml IL-1b for 24 h after pre-incubation under high glucose environment of 11.1 mmol/L for 24 h. The upregulation of ALOX15 (p = 0.050) was significantly weakened in healthy TDSCs stimulated under a high glucose environment. Stimulated Tendinopathic TDSCs did not show significant changes after pre-incubation in a high glucose environment (Fig. 3).
Fig. 3.
Log transformed ddCT value of ALOX12, ALOX15, FPR1, FPR2, ChemR23, COX2 normalized to b-actin and control group. Comparison between TDSCs stimulated with 2 ng/ml IL-1b for 24 h and TDSCs pre-incubated with 11.1 mmol/L glucose for 24 h prior to stimulation. * indicate significant difference of p < 0.05.
Discussion
Limitations
One major limitation of this study is that tendinopathic specimens were obtained from more elder patients in comparison to healthy tendons. Age related changes may act confounding factor, challenging the reliability of the results.
Another limitation is that tendinopathic specimens were obtained from surgical debridement of torn tendons. It was assumed that only tendinopathic tendons are susceptible to spontaneous ruptures, but the acute response from tendon rupture may also be presented in the tendinopathic specimens obtained. The time between tendon rupture and surgery was also not assessed, hence there is a possibility that the obtained tendon specimens do not represent typical chronic tendinopathy.
A third limitation is that an in vitro study with a high glucose environment may not accurately represent the in vivo impact of DM. DM is a global metabolic disturbance affecting many cell types around the body, leading to long term effects including but not limited to angiopathy and neuropathy. The current in vitro study only included the assessment of short term effects of glucose on tendon cells. Secondly, The increase in level of glucose around the tendon may not be directly proportional to increased blood glucose. Although it was previously reported that hyperglycemia may increase glucose uptake and consumption in tendons,19,20 exact level of glucose that can reach the tendon may be unclear. Further in vivo studies are required to deduce the appropriate glucose concentration in the tendon environment in cases of hyperglycemia of different severity.
A weakened pro-resolving response from local tendon cells may be a cause of chronic inflammation in tendinopathic tendons
It was previously mentioned that chronic inflammation is present in chronic tendinopathy, and also may plays an important role in its development.7 According to the findings of this study, tendinopathic tendons express a weakened pro-resolving response, in terms of a significant lower upregulation of both receptors of SPMs and the key enzymes of SPMs synthesis upon IL-1b stimulation. Microtraumas in the tendon tissue are inevitable from everyday use, hence tendon healing, including inflammation,21 plays a crucial role in maintaining the integrity of tendon tissues.22 However, a weakened pro-resolving response from tendon cells may lead to prolonged exposure of the tendon tissues to non-selective digestive enzymes from inflammation, causing excessive damage and degenerative changes.23
It was previously reported that other than DM, systemic diseases including hyperlipidemia,24 chronic gout,24 and hyperthyroidism25 may also lead to an increase risk in the development of tendinopathy. The mechanism in each featured systemic disease lead to tendinopathy may be different, but can be explained by the involvement of chronic inflammation and failed healing. Hyperlipidemia lead to adiposity and adipokine dysregulation, contributing to a pro-inflammatory state. Chronic gout lead to the deposition of urate crystals in soft tissue, contributing as a persistent stimulus for chronic inflammation. Hyperthyroidism leads to inhibited collagen synthesis and matrix metabolism, building weak matrices that are more prone to future injuries and inflammation. Persistent or recurrent inflammation appear to be a common feature of the mechanism of systemic diseases leading to tendinopathy, and results from this study suggests that weakening of the pro-resolving pathway may be a presentation in tendinopathic tendons. However, further support to this statement is required with future investigations on the pro-resolving response of tendons harvested from cases of different systemic diseases.
A high glucose level lead to a weakened pro-resolving response in healthy tendon cells
It was mentioned in previous paragraphs that tendinopathic tendons present with a weakened pro-resolving response upon inflammatory stimulation. It was further demonstrated that the weakening in pro-resolving response can be observed in healthy tendon cells under a high glucose environment.
Results suggest that a high glucose environment may initiate cellular changes weakening the pro-resolving pathway in tendon cells. This finding provides evidence that a high glucose may lead to tendinopathic changes in healthy tendon cells, suggesting an explanation to the increased risk of tendinopathy observed in diabetic patients.
It is notable that the weakened pro-resolving response observed in tendinopathic cells involved the genes FPR1, ChemR23, and ALOX15. On the other hand, the weakened response observed in healthy cells in a high glucose only involved ALOX15. The inconsistency between the involved genes in these two group may be explained by that the weakened pro-resolving response happen in different stages, of which a 24 h incubation in a high glucose environment can only lead to a stage involving less genes. However, to further support this hypothesis, investigations on longer time points is required.
Glucose level of 11 mM stimulates an inflammatory response on tendon cells in 24 h
In this study, healthy and tendinopathic TDSCs were treated with a high glucose environment of 11.1 mmol/L for 24 h. For both cell types, an up-regulation of pro-inflammatory marker COX2 was observed.
The results suggest that an elevated glucose level of 11 mM is enough to induce inflammatory stimulation to tendon cells. It is currently unclear if inflammatory stimulation occur in earlier time points, or if the stimulation could be reversed once glucose levels decrease. Nevertheless, this result raises the awareness that a strict control of glucose levels of 11 mM or less may be beneficial to control inflammation. Considering that chronic inflammation was proposed to play an important role in the development of tendinopathy from a healthy tendon, efforts in controlling glucose levels may contribute to the prevention of the development of tendinopathy.
Clinical relevance – glucose control may contribute to the prevention of tendinopathy in patients with systemic diseases
Anti-inflammatory agents such as NSAIDs and glucocorticoids are a large part of the conservative management to patients with tendinopathy.26 However, these treatments do not show long term efficacy, and glucocorticoids were also associated with tendon ruptures.27 One way to decrease the burden from tendinopathy is to improve the prevention of this disorder.
Results from this study have implications to patients with various systemic diseases, who are at risk of the development of tendinopathy. A high glucose level may trigger an inflammatory response and also weaken the pro-resolving pathway in healthy tendon cells. Both factors may lead to chronic inflammation, raising the risk of the development of tendinopathy.
According to the American diabetes association, an HbA1c level of 8 corresponds to a mean plasma glucose level of 8.1 to 12.116. Glucose control of an A1c goal of less than 8 may lower the risk of the development of tendinopathy in high risk patients.
Consistency and inconsistency with existing literature
From the results of this study, chronic inflammation is a feature presented in tendinopathic tendons. This finding does not agree with the previous concept that tendinopathy is an inflammation free, degenerative disease,1 indicating an absence of inflammation in the typical presentation of chronic tendinopathy. However, it was acknowledged that this study was not the first to suggest the presence of inflammation. A recent systematic review by our group also concluded that signs of chronic inflammation is presented in the majority of tendinopathic tendons reported in existing literature. A similar finding was also suggested in another systematic review in 2016.28
The concept of “priming” in tendinopathic tendon cells was suggested in 2018.6 Cellular changes in local tendon cells lead to dysregulation in inflammation, leading to tendinopathy. In this study, results are compatible with this concept, and dynamic character of “priming” was also demonstrated by assessing pro-resolving mediators in tendon cells under inflammatory stress.
The observed effect of glucose on tendon cells or inflammatory responses is also supported by other in vitro studies. In a previous study, an enhanced upregulation of inflammatory markers was observed in stimulated endothelial cells under a high glucose environment.15 It was also previously reported that a high glucose environment may induce apoptosis, inhibit proliferation, and suppress the expression of tendon related markers in TDSCs.14 Another study also reported that a high glucose environment would increase the expression of metalloproteinases in TDSCs.13 It can be observed that the negative impact of a high glucose environment on tendon cells is well appreciated, and this impact may be caused by inflammation.
Future studies
Validation of the current findings can be done with an increased sample size with age and gender matched patients. Downstream markers of pro-resolving response such as IL-10 or protein analysis of the selected genes could also provide validation to the current findings.
It was found that glucose affect the regulation of inflammation in tendon cells. However, it is currently unknown about the threshold of glucose level that would lead to this change, how this change is associated with different glucose levels, or whether it could be reversed once glucose levels return to normal. To further build up on the topic, more time points can be assessed regarding the effects of a high glucose environment on healthy and tendinopathic tendon cells. Identification of the threshold of glucose level and time which inflammatory changes occur may provide more evidence on practical guidelines on how glucose levels should be controlled for managing tendinopathy.
The investigation of circulating inflammatory cells in tendinopathic patients is another potentially rewarding area of research. It was demonstrated that pro-resolving responses of tendinopathic tendon cells were weakened. Considering the possibility that the same changes may be observed in circulating inflammatory cells, sampling of tendinopathic patients can be done less invasively with blood samples instead of tendon specimens.
Conclusion
The results of this study provide a possible explanation to the increased risk to develop tendinopathy in diabetic patients. Chronic inflammation observed in tendinopathy may be due to the weakening of pro-resolving responses in tendinopathic TDSCs, and a high glucose environment may lead to chronic inflammation and ultimately tendinopathy by persistent stimulation and weakening of pro-resolving response in healthy TDSCs.
Funding/support
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of competing interest
The authors have no conflicts of interest relevant to this article.
Acknowledgements
No financial or material support of any kind was received for the work described in this article.
References
- 1.Maffulli N., Khan K.M., Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. doi: 10.1016/s0749-8063(98)70021-0. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins C., Fu S.-C., Chua E. Critical review on the socio-economic impact of tendinopathy. Asia Pac J Sport Med Arthrosc Rehabil Technol. 2016;4:9–20. doi: 10.1016/j.asmart.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torstensen E.T., Bray R.C., Wiley J.P. Patellar tendinitis: a review of current concepts and treatment. Clin J Sport Med. 1994;4(2):77. [Google Scholar]
- 4.Chan K.-M., Fu S.-C. Anti-inflammatory management for tendon injuries - friends or foes? Sport Med Arthrosc Rehabil Ther Technol. 2009;1(1):23. doi: 10.1186/1758-2555-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seitz A.L., McClure P.W., Finucane S., Boardman N.D., Michener L.A. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech. 2011;26(1):1–12. doi: 10.1016/j.clinbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Dakin S.G., Newton J., Martinez F.O. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. 2018;52(6):359–367. doi: 10.1136/bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dakin S.G., Newton J., Martinez F.O. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med. November 2017 doi: 10.1136/bjsports-2017-098161. bjsports-2017-098161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang C., Chen Y., Huang J. The roles of inflammatory mediators and immunocytes in tendinopathy. J Orthop Translat. 2018;14:23–33. doi: 10.1016/j.jot.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 10.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serhan C.N. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25(1):101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 12.Ranger T.A., Wong A.M.Y., Cook J.L., Gaida J.E. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sports Med. 2016;50(16):982–989. doi: 10.1136/bjsports-2015-094735. [DOI] [PubMed] [Google Scholar]
- 13.Tsai W.-C., Liang F.-C., Cheng J.-W. High glucose concentration up-regulates the expression of matrix metalloproteinase-9 and -13 in tendon cells. BMC Muscoskelet Disord. 2013;14(1) doi: 10.1186/1471-2474-14-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Y.-C., Li Y.-J., Rui Y.-F. The effects of high glucose on tendon-derived stem cells: implications of the pathogenesis of diabetic tendon disorders. Oncotarget. 2017;8(11):17518–17528. doi: 10.18632/oncotarget.15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiro A., Ferrandis R., Garcia L., Alcazar E. Simultaneous and spontaneous bilateral rupture of the patellar tendon in rheumatoid arthritis. A case report. Acta Orthop Scand. 1975;46(4):700–703. doi: 10.3109/17453677508989253. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Supplement 1):S55–S64. doi: 10.2337/dc18-S006. [DOI] [PubMed] [Google Scholar]
- 17.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology. 2004;43(2):131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 18.Lui P.P.Y., Chan K.M. Tendon-derived stem cells (TDSCs): from basic science to potential roles in tendon pathology and tissue engineering applications. Stem Cell Rev and Rep. 2011;7(4):883–897. doi: 10.1007/s12015-011-9276-0. [DOI] [PubMed] [Google Scholar]
- 19.Hannukainen J., Kalliokoski K.K., Nuutila P. In vivo measurements of glucose uptake in human Achilles tendon during different exercise intensities. Int J Sports Med. 2005;26(9):727–731. doi: 10.1055/s-2005-837458. [DOI] [PubMed] [Google Scholar]
- 20.Masood T., Kalliokoski K., Bojsen-Møller J., Finni T. Muscle-tendon glucose uptake in Achilles tendon rupture and tendinopathy before and after eccentric rehabilitation: comparative case reports. Phys Ther Sport. 2016;21:14–19. doi: 10.1016/j.ptsp.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Fu S.-C., Rolf C., Cheuk Y.-C., Lui P.P., Chan K.-M. Deciphering the pathogenesis of tendinopathy: a three-stages process. Sport Med Arthrosc Rehabil Ther Technol. 2010;2:30. doi: 10.1186/1758-2555-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snedeker J.G. How high glucose levels affect tendon homeostasis. In: Ackermann P.W., Hart D.A., editors. Metabolic Influences On Risk For Tendon Disorders. Advances in Experimental Medicine and Biology. Springer International Publishing; Cham: 2016. pp. 191–198. [DOI] [PubMed] [Google Scholar]
- 23.Tabas I., Glass C.K. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abate M., Schiavone C., Salini V., Andia I. Occurrence of tendon pathologies in metabolic disorders. Rheumatology. 2013;52(4):599–608. doi: 10.1093/rheumatology/kes395. [DOI] [PubMed] [Google Scholar]
- 25.Oliva F., Berardi A.C., Misiti S., Verga Falzacappa C., Iacone A., Maffulli N. Thyroid hormones enhance growth and counteract apoptosis in human tenocytes isolated from rotator cuff tendons. Cell Death Dis. 2013;4:e705. doi: 10.1038/cddis.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andres B.M., Murrell G.A.C. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008;466(7):1539–1554. doi: 10.1007/s11999-008-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaux J.F., Croisier J.L., Forthomme B., Crielaard J.M. Critical analysis of classical conservative treatments of tendinopathies. Rev Med Liege. 2015;70(9):456–460. [PubMed] [Google Scholar]
- 28.Dean B.J.F., Gettings P., Dakin S.G., Carr A.J. Are inflammatory cells increased in painful human tendinopathy? A systematic review. Br J Sports Med. 2016;50(4):216–220. doi: 10.1136/bjsports-2015-094754. [DOI] [PubMed] [Google Scholar]