Summary
The generation of brain region-specific progenitors from human embryonic stem cells (hESCs) is critical for their application. However, transcriptional regulation of neural regionalization in humans is poorly understood. Here, we applied a rostrocaudal patterning system from hESCs to dissect global transcriptional networks controlling early neural regionalization. We found that SOX21 is required for rostral forebrain fate specification. SOX21 knockout led to activation of Wnt signaling, resulting in caudalization of regional identity of rostral forebrain neural progenitor cells. Moreover, we identified WNT8B as a SOX21 direct target. Deletion of WNT8B or inhibition of Wnt signaling in SOX21 knockout neural progenitor cells restored rostral forebrain identity. Furthermore, SOX21 interacted with β-catenin, interfering with the binding of TCF4/β-catenin complex to the WNT8B enhancer. Collectively, these results unveil the unknown role of SOX21 and shed light on how a transcriptional factor modulates early neural regionalization through crosstalk with a key component of Wnt signaling.
Key words: SOX21, forebrain, human embryonic stem cells, neural regionalization, Wnt signaling, WNT8B
Highlights
-
•
The transcriptomic analysis of rostrocaudal patterning of hESC-derived NPCs
-
•
SOX21 KO leads to caudalized regional identity in rostral forebrain progenitors
-
•
SOX21 represses Wnt signaling to ensure the rostral forebrain identity
-
•
WNT8B is a major downstream target of SOX21
Jin and colleagues identify SOX21 as a key player in neural regionalization from human embryonic stem cells. SOX21 ensures the rostral forebrain identity of neural progenitors by repressing WNT8B expression.
Introduction
During early mammalian embryogenesis, the central nervous system is patterned to acquire regional identities and divides into dorsoventral and rostrocaudal compartments (Rubenstein and Rakic, 2013). Along the rostral-caudal (R-C) axis, the neural tube developes into the forebrain, midbrain, hindbrain, and the spinal cord at the early phase of R-C specification (Itasaki, 2015). Each region will give rise to specific subtypes of neural progenitor cells (NPCs) and neurons (Kiecker and Lumsden, 2012, Wurst and Bally-Cuif, 2001). Among these regions, the forebrain is the most complex, containing the telencephalon and diencephalon. The precise patterning of the forebrain is critically important for the proper wiring of the cerebrum.
Investigations of how neural regionalization is controlled during the early stages of development have been conducted primarily in model animals. Although many signaling molecules, including fibroblast growth factor, Wnt, and retinoic acid (RA), are involved in rostrocaudal patterning (Irioka et al., 2005, Tuazon and Mullins, 2015), Wnt is perhaps the most crucial (Andoniadou and Martinez-Barbera, 2013, Twyman, 2009). A morphogen gradient of Wnt signaling is important for rostrocaudal patterning, and low levels of Wnt/β-catenin activities are required to specify the telencephalic fate (Fossat et al., 2011, Fossat et al., 2012, Kiecker and Niehrs, 2001, Nordstrom et al., 2002). Furthermore, RA plays a central role in inducing the posterior hindbrain and spinal cord (Glover et al., 2006). Despite these advances, the question of how neural cells acquire regional identities by the Wnt gradient is far from completely understood. Several Wnt ligands are expressed in the neural tube, such as Wnt1, Wnt3a, and Wnt8b (Ciani and Salinas, 2005). Among Wnt ligands, Wnt8b has been found to localize in the dorsal midline of the caudal forebrain at the eight-somite stage (Liu et al., 2010). The wedge-shaped expression domain of Wnt8b in the diencephalon has been suggested to indicate the location of the zona limitans intrathalamica (Puelles and Martinez, 2013). Moreover, Wnt8b has been reported to regulate the diencephalic versus telencephalic fate in zebrafish (Houart et al., 2002). However, how WNT8B expression is spatiotemporally regulated in the forebrain remains largely unknown.
Difficulties in obtaining and manipulating human embryonic neural tissues have severely hindered the investigation of human neural development. Highly robust methods for neural differentiation from human pluripotent stem cells (hPSCs) have been developed (Chambers et al., 2009). However, the protocol to generate regionally specified NPCs from human PSCs was not available until recent years (Imaizumi et al., 2015, Kirkeby et al., 2012, Kriks et al., 2011). Similar to neural development in vivo, the rostrocaudal identity (ranging from the telencephalon to the spinal cord) can be induced by the dose-dependent control of Wnt and RA signaling, and the dorsoventral identity can be modulated by Shh signaling. Rostrocaudally patterned NPCs have been obtained by application of the porcupine inhibitor IWP-2 (IWP2, a Wnt signaling inhibitor), different dosages of the glycogen synthetase kinase 3 (GSK3) inhibitor CT99021 (CT, a Wnt signaling activator), or CT combined with RA (Imaizumi et al., 2015, Kirkeby et al., 2012). The system provides a useful tool to address the question of how Wnt signaling is precisely modulated for the establishment and maintenance of the rostrocaudal identity in early human neural development.
In this study, we adopted a robust protocol to control the regional identities of human embryonic stem cell (hESC)-derived NPCs and focused on the role of transcriptional regulation for Wnt signaling and cell identity in positional patterning. Through conducting genome-wide transcriptional analyses for rostrocaudally specified NPCs ranging from the telencephalic forebrain to the spinal cord, we revealed that the transcriptional factor SOX21 plays an important role in forebrain regionalization. SOX21 is a member of the B2 group of SOX family proteins, harboring a high-mobility group DNA binding domain (Kamachi and Kondoh, 2013, Uchikawa et al., 1999). The expression of SOX21 initiates in the anterior neural plate of mouse embryos at the embryonic day 8.0 (E8.0) (Uchikawa et al., 2011). Previous studies have suggested roles of Sox21 in the progression of neurogenesis in the mouse, chicken, and Xenopus (Matsuda et al., 2012, Sandberg et al., 2005, Whittington et al., 2015). However, the role of SOX21 in neural patterning, particularly in human neural development, remains unclear. Here, we reveal that SOX21 represses WNT8B expression, participating in the control of forebrain regionalization. This study unveils a previously unappreciated role of SOX21 and sheds light on the transcriptional control of rostrocaudal patterning during early neural differentiation of hESCs.
Results
The Rostrocaudal Patterning of hESC-Derived NPCs
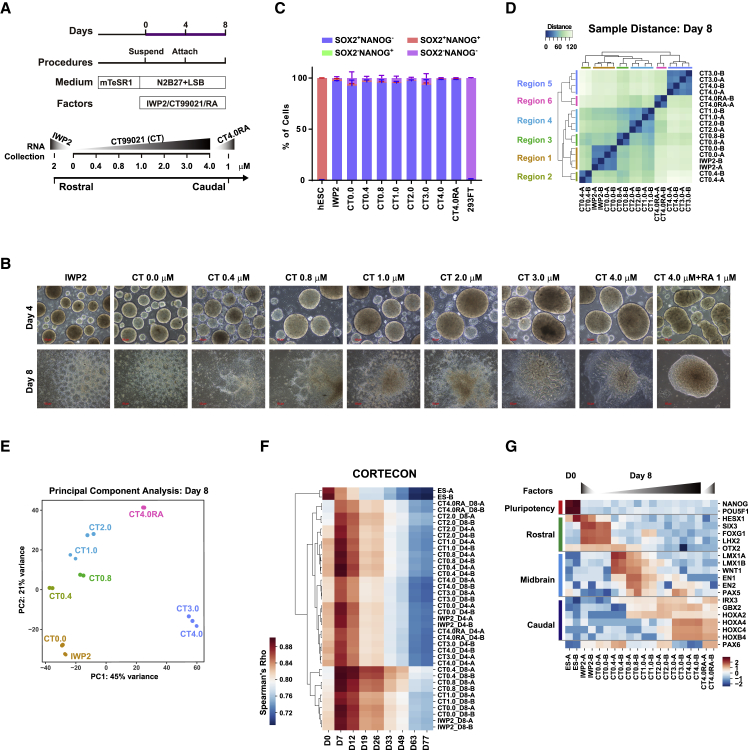
To dissect the transcriptional network controlling the rostrocaudal specification of early neural differentiation, we generated NPCs from hESCs of the H9 line (Thomson et al., 1998) with defined regional identities ranging from the rostral forebrain to the spinal cord based on published protocols (Imaizumi et al., 2015, Kirkeby et al., 2012, Kriks et al., 2011). Initially, hESCs were suspended in N2B27 medium containing dual SMAD inhibitors (LSB: 50 nM LDN193189 and 5 μM SB431542) and patterning factors, including IWP2, CT, and RA, for 4 days. At day 4, aggregates were attached to culture dishes and cultured for a further 4 days to yield NPCs with more specific regional identities along the R-C axis (Figure 1A). Generally, rostrocaudal patterning was established by the dose-dependent control of WNT signaling. Inhibition of Wnt signaling by IWP2 (2 μM) induced the rostral forebrain fate, while activation of Wnt signaling by CT induced the diencephalic, mesencephalic, and rhombencephalic fate, depending on CT concentrations ranging from 0.4 to 4.0 μM. RA (1.0 μM) coupled with 4.0 μM CT (CT4.0RA) was used to induce the spinal cord fate. The ventralization factor was not added in the current differentiation system because we mainly focused on the transcriptional regulation of rostrocaudal patterning.
Figure 1.
Transcriptome Analysis of the Rostrocaudal Patterning System
(A) A schematic illustration of an aggregation-based rostrocaudal neural differentiation protocol. Differentiation was induced by LSB (50 nM LDN193189 and 5 μM SB431542). The following patterning factors were used for the generation of region-specific neural progenitor cells along the rostrocaudal axis: 2 μM IWP-2 (IWP2), 0–4.0 μM CT99021 (CT), and 1 μM RA with 4.0 μM CT (CT4.0RA) from days 0 to 8.
(B) Bright-field images of neural aggregates at day 4 and rosettes at day 8. The RNA samples for RNA-seq were collected at days 4 and 8 from all groups. Scale bars, 100 μm.
(C) The percentage of SOX2+NANOG− cells quantified by flow cytometric analysis at day 8. 293FT cells and undifferentiated WT hESCs were used as controls. Data are shown as means ± SEM; n = 3.
(D) Unsupervised hierarchical clustering of 18 samples at day 8.
(E) Principal-component analysis of 18 samples at day 8.
(F) Comparative analysis of our RNA-seq data with the CORTECON neural differentiation system. Spearman correlations with different days of CORTECON samples are shown.
(G) A heatmap of RNA-seq results for region-specific marker genes at day 8. Suffixes A and B represent biological replicates.
See also Figure S1.
We first examined the morphology of aggregates at days 4 and 8 (Figure 1B). Consistent with a report by Imaizumi et al. (2015), the size of the aggregates increased with higher CT concentrations. The proportion of SOX2+NANOG− NPCs was very high (>97%) under all differentiation conditions used (Figure 1C), suggesting the successful neural induction. To validate the regional features of these NPCs, we then carried out RNA sequencing (RNA-seq) for cell samples collected from 19 groups (each with 2 biological replicates), covering 3 time points (days 0, 4, 8) and 9 differentiation conditions (IWP2, CT0.0, CT0.4, CT0.8, CT1.0, CT2.0, CT3.0, CT4.0, CT4.0RA) (Figures 1A and 1B). To analyze transcriptomes of the positionally patterned NPCs, we applied unsupervised hierarchical clustering and found six major regions along the R-C axis at day 8, designated as region 1 (IWP2 and CT0.0), region 2 (CT0.4), region 3 (CT0.8), region 4 (CT1.0 and CT2.0), region 5 (CT3.0 and CT4.0), and region 6 (CT4.0RA) (Figure 1D). The six regions could already be classified at day 4 (Figure S1A). To better characterize gene expression patterns along the R-C axis, we applied principal-component analysis (Figures 1E and S1B). The second principal component accounted for the trajectory of rostrocaudal patterning, while the first principal component separated region 5 (NPCs treated with CT 3.0–4.0 μM at day 8) from the rest, indicating that these NPCs might have unique characteristics (Figure 1E). In addition, we compared our RNA-seq data with the CORTECON system (van de Leemput et al., 2014). Spearman correlation analysis revealed that all of our positionally patterned NPCs highly correlated with the neural differentiation stage (day 7), suggesting the progenitor nature of our NPCs (Figure 1F).
To systematically investigate the coexpression relationships among genes in positionally patterned NPCs, we performed weighted gene coexpression network analysis (WGCNA) and identified 26 distinct coexpression modules corresponding to clusters of correlated genes (Figure S1C; Table S1). Notably, seven modules showed region-specific expression patterns and might represent core gene networks along the R-C axis (Figure S1D). Functional enrichment analysis was conducted to characterize these modules (Figure S1E). The Darkgrey module (region 1) was enriched for terms such as anteroposterior axis specification and embryonic axis specification, including genes such as SIX3, LHX2, HESX1, and FEZF1, which started to be expressed as early as day 4. Terms enriched in the Darkgreen module (region 1) were extracellular organization and metabolic process, including genes such as FOXG1 and DLX5, which were induced at day 8. Although the Pink module (region 2) was enriched for terms highly correlated with midbrain development, such as LMX1A, LMX1B, and WNT1, some genes that are known to also be expressed in the caudal forebrain were present, such as WNT8B, EMX2, OTX1, and DLX2. Caudal region modules, including the Lightgreen, Lightcyan, Black, and Grey60, were enriched for terms such as nervous system development, regionalization, and morphogenesis. In addition, the Turquoise module (day 0, undifferentiated hESCs) was enriched for pluripotency-related terms (Figure S1E). Taken together, these analyses provide rich information for unveiling gene coexpression networks controlling positional patterning.
With these RNA-seq data, we examined the relative gene expression levels of known regional markers (Figure 1G). The simultaneously high expression of HESX1, SIX3, FOXG1, and LHX2 in NPCs from the IWP2 or 0.0 μM CT group (region 1) indicated a rostral forebrain identity. Some midbrain markers (LMX1A, LMX1B, and WNT1) were detected with CT concentrations at 0.4 to 1.0 μM, whereas other midbrain markers (EN1, EN2, PAX5) were highly expressed in NPCs treated with 0.8 μM CT (region 3). NPCs from groups treated with CT concentrations at and above 2.0 μM highly expressed markers of the hindbrain, including GBX2, HOXA2, HOXA4, HOXC4, and HOXB4. Notably, NPCs from the CT4.0RA group (region 6) highly expressed PAX6, HOXB4, and HOXC4, representing the spinal cord identity. Altogether, these results verified that patterning factors (IWP2/CT/RA) efficiently induced the rostrocaudal identities of NPCs, recapitulating gene expression patterns in vivo to some extent.
SOX21 Is Required for Rostral Forebrain Specification from hESCs
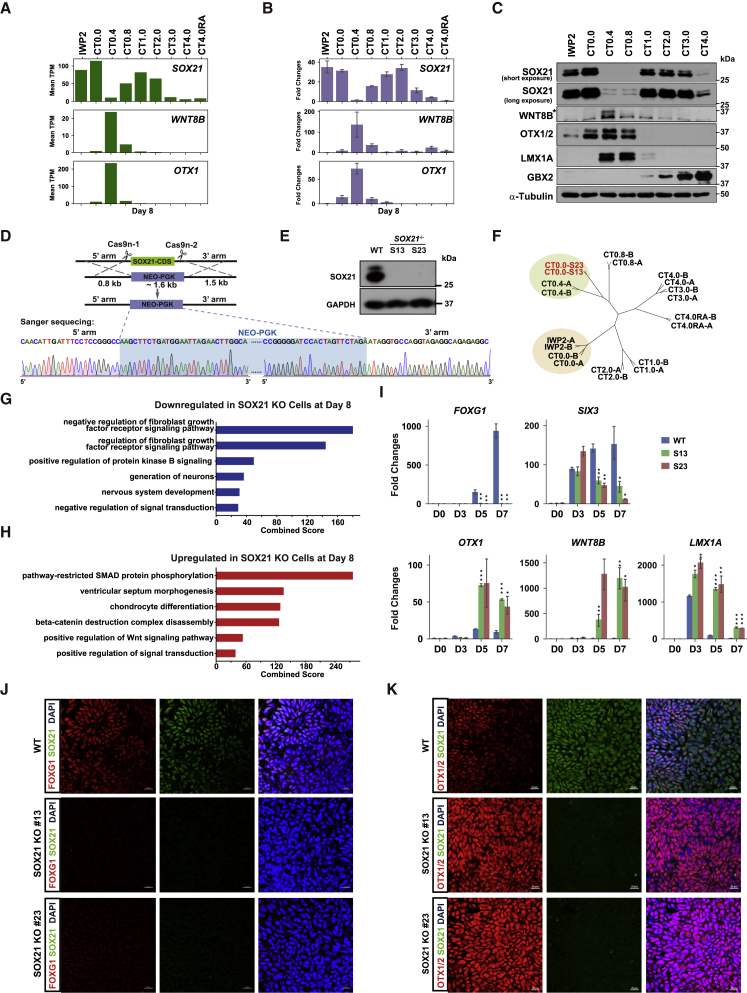
The aforementioned WGCNA analysis revealed that WNT8B was specifically expressed in NPCs of the 0.4 μM CT group (region 2) (Figure S1D). Interestingly, our RNA-seq results showed that the mRNA of SOX21 was very low in NPCs of the 0.4 μM CT group (Figure 2A). The expression patterns of SOX21, WNT8B, and OTX1 at both the mRNA and protein levels were verified by real-time qPCR and western blot analysis (Figures 2B and 2C). A reverse relationship appeared between the expression levels of SOX21 and WNT8B in NPCs of the 0.4 μM CT group. Previously, Sox21 expression has been reported to be very low in the dorsal diencephalon at the 11- to 12-somite stage of mouse embryos (Uchikawa et al., 2011), whereas Wnt8b has been detected at the diencephalic-telencephalic boundary of mouse embryos at E10.5 (Fotaki et al., 2010) and at that of human embryos at Carnegie stages 15 and 16 (Lako et al., 1998). Given the unique expression pattern of Sox21 and Wnt8b as well as the implications of Wnt8b in forebrain patterning (Houart et al., 2002), we anticipated that SOX21 might have a role in the control of WNT8B expression and forebrain patterning. To test this hypothesis, we knocked out SOX21 in hESCs of the H9 line (Thomson et al., 1998) using the CRISPR/Cas9 nickase system via homology-directed repair (Figure 2D). Two clones (S13 and S23) harboring homozygous SOX21 deletions were established, and the absence of SOX21 in NPCs was verified by western blot and Sanger sequencing analyses (Figures 2D and 2E).
Figure 2.
SOX21 Participates in Forebrain Regionalization
(A) Expression levels of SOX21, WNT8B, and OTX1 at day 8 of rostrocaudal differentiation obtained from our RNA-seq data.
(B) Real-time qPCR validation of SOX21, WNT8B, and OTX1 expression levels at day 8. Data are represented as fold changes relative to undifferentiated WT hESCs (day 0) and shown as means ± SEM; n = 3.
(C) Representative western blot analysis of SOX21, WNT8B, OTX1/2, LMX1A, and GBX2 at day 8. Antibodies of OTX1/2 recognize both OTX1 and OTX2. A star indicates the specific band of WNT8B proteins.
(D) The strategy to establish SOX21 KO clones using the CRISPR/Cas9 nickase with four gRNAs. A map of Sanger sequencing results shows the homology recombination site in SOX21 KO hESCs. The PGK-NEO cassette is highlighted with light blue. CDS, coding sequence.
(E) Representative western blot analysis of SOX21 protein level in hESC-derived NPCs. WT, wild-type cells; S13 and S23 are two SOX21 KO clones.
(F) Hierarchical clustering between SOX21 KO samples (S13 and S23) and WT rostrocaudal differentiation samples at day 8.
(G and H) GO terms enriched for downregulated (G) and upregulated (H) genes in SOX21 KO cells. Only the top 6 GO terms with FDR <0.05 and combined score >15 are listed.
(I) Real-time qPCR validation of DEGs at days 0, 3, 5, and 7 (CT = 0.0 μM). Data are represented as fold changes relative to undifferentiated WT hESCs (day 0) and shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3).
(J and K) Typical immunofluorescence images of FOXG1, OTX1/OTX2, and SOX21 at day 7. Antibodies against OTX1/OTX2 recognize both OTX1 and OTX2. Scale bars, 20 μm.
See also Figure S2.
To obtain evidence for a role of SOX21 in early forebrain patterning, we generated rostral forebrain NPCs from hESCs based on the protocol described in Figure 1A (CT = 0.0 μM) (Kirkeby et al., 2012). This protocol was used hereafter in all neural differentiation experiments of this study. Between wild-type (WT) and SOX21 knockout (KO) NPCs, the percentage of SOX2+NANOG− cells was similar (Figure S2A), and there was no detectable difference in the NPC proliferation rate as quantified by EdU+ cells (Figure S2B). Next, to uncover genes regulated by SOX21 at a genome-wide scale, RNA was collected from NPCs at differentiation days 0, 4, and 8, with two biological replicates for RNA-seq. The Pearson's correlation coefficient was greater than 0.98 between the S13 and S23 clones, providing evidence for the specific effect of SOX21 KO generated by the CRSPR/Cas9 approach (Figure S2C). Analysis of differentially expressed genes (DEGs) identified 363 upregulated and 367 downregulated genes (fold changes >2 and false discovery rate [FDR] <0.05) in SOX21 KO NPCs at day 8 (Figure S2D). Notably, clustering analysis of DEGs revealed that the regional identity of SOX21 KO NPCs was closer to that of NPCs treated with 0.4 μM CT at day 8 (Figure 2F), indicating that the regional identity of rostral forebrain NPCs was caudalized in the absence of SOX21. Furthermore, gene ontology (GO) analysis of DEGs showed that downregulated genes were enriched for the term of nervous system development, including rostral forebrain markers, such as FOXG1 and SIX3, whereas upregulated genes were enriched for the term of positive regulation of Wnt signaling pathway, including WNT8B (Figures 2G, 2H, and S2D). Real-time qPCR analysis verified significant downregulation of FOXG1 and SIX3 as well as upregulation of OTX1, LMX1A, and WNT8B in SOX21 KO cells compared with WT cells (Figure 2I). Immunofluorescence staining results also revealed a substantial reduction in FOXG1 and SIX3 expression (Figures 2J and S2E) but an increase in OTX1/OTX2 expression in SOX21 KO NPCs (Figure 2K). Western blot analysis also showed higher protein levels of WNT8B and OTX1/OTX2 in SOX21 KO NPCs than in WT NPCs (see results in Figure 5B). To confirm the function of SOX21 in early neural differentiation and avoid the cell line bias, we generated SOX21 KO hESCs (clone ES8-S11) in another hESC line, SHhES8 (Zhu et al., 2017) using the same gene editing strategy described above. Alterations in gene expression similar to those in NPCs derived from SOX21 KO H9 hESCs were observed (Figure S2F).
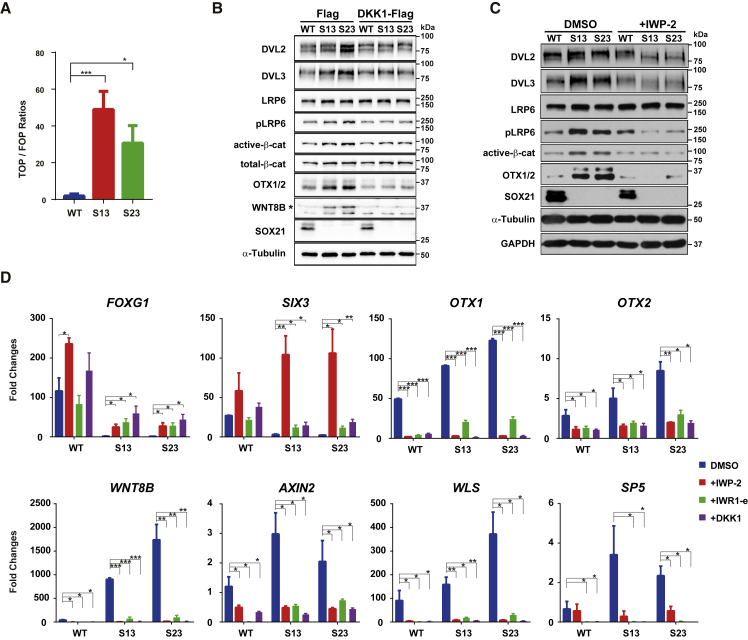
Figure 5.
SOX21 Represses Wnt Signaling to Ensure Rostral Forebrain Specification
(A) Examination of Wnt signaling activities by TOP/FOPFlash reporter assays in WT and SOX21 KO NPCs at day 5. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3).
(B) Representative western blot analysis of Wnt signaling components and regional markers in WT and SOX21 KO NPCs when DKK1-Flag expression was induced by doxycycline (100 ng/mL) from days 0 to 6. The Flag-tagged vector was used as a control. Antibodies against OTX1/OTX2 recognize both OTX1 and OTX2. A star indicates the specific band of WNT8B proteins.
(C) Representative western blot analysis of levels of various proteins in WT and SOX21 KO NPCs in the absence or presence of the Wnt signaling inhibitor IWP-2 (2 μM) for 6 days.
(D) Real-time qPCR analysis of relative expression levels for regional markers as well as Wnt signaling-associated genes in WT and SOX21 KO NPCs treated with or without Wnt signaling inhibitors, including DKK1 (100 ng/mL), IWP-2 (2 μM), and IWR1-e (10 μM), for 5 days. Data are represented as fold changes relative to undifferentiated WT hESCs (day 0) and shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3). DMSO is the vehicle used to resolve the inhibitors.
See also Figure S4.
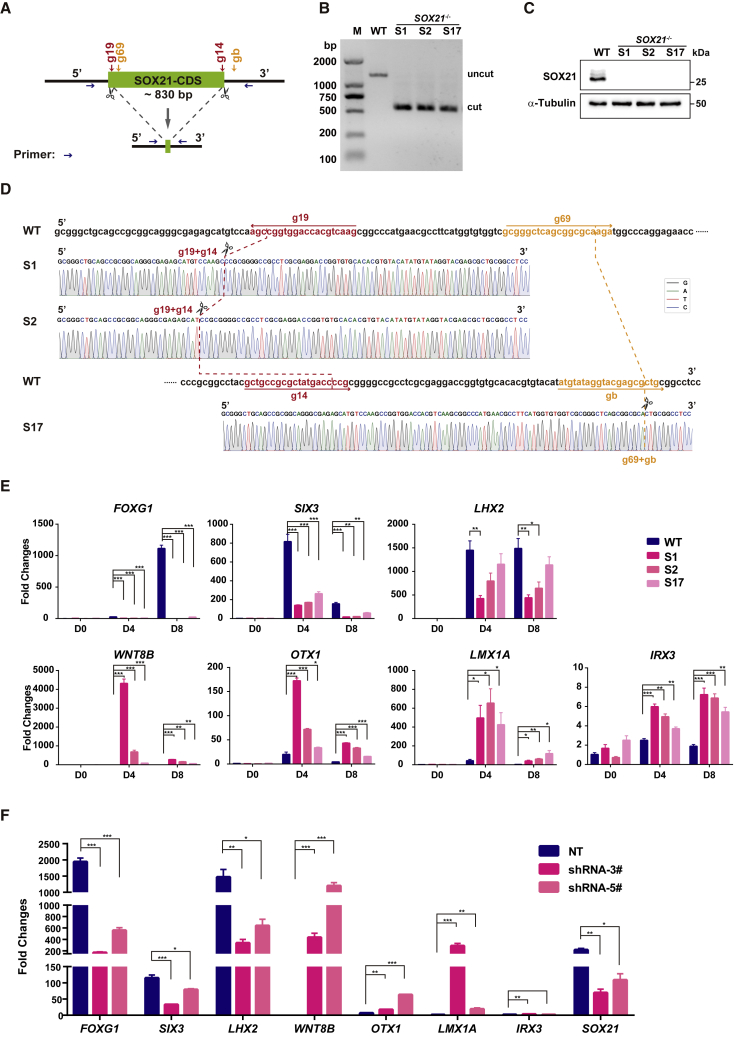
In addition, to further rule out potential off-target effects in the CRISPR/Cas9 nickase-mediated SOX21 KO cells, we generated three additional SOX21 KO clones (S1, S2, S17) using a donor-free paired gRNA-guided CRISPR/Cas9 KO strategy (paired-KO) (Liu et al., 2016) and two new sets of paired gRNAs (g19 + g14 and g69 + gb) (Figure 3A). PCR analysis suggested that the three SOX21 KO clones had biallelic deletion of SOX21 genomic DNA (Figure 3B). Western blot analysis and Sanger sequencing results validated the lack of SOX21 (Figures 3C and 3D). Consistently, FOXG1, SIX3, and LHX2 were all repressed, while WNT8B, OTX1, LMX1A, and IRX3 were significantly upregulated in SOX21 KO NPCs (Figure 3E). Furthermore, we knocked down SOX21 using two shRNAs, and similar alterations in gene expression were found in SOX21 knockdown NPCs (Figure 3F). These results clearly show that the alterations observed in SOX21 KO NPCs were specifically caused by the absence of SOX21 and that SOX21 is required for the specification of the rostral forebrain NPCs from hESCs.
Figure 3.
Generation of Additional SOX21 Knockout hESC Clones
(A) Schematic representation of the paired-KO strategy for SOX21 KO in H9 hESCs. Four different gRNAs targeting different loci were designed (g19 is paired with g14, and g69 is paired with gb). Primers for amplification of genomic DNA are indicated by blue arrows. CDS, coding sequence.
(B) Representative genomic DNA PCR results show the biallelic deletion of SOX21 in hESCs.
(C) Representative western blot analysis of SOX21 protein level in hESC-derived NPCs. WT, wild-type cells; S1, S2 and S17 are three SOX21 KO clones.
(D) Sanger sequencing results show ligation sites (dashed line) in two gRNA pairs (g19 + g14 and g69 + gb).
(E) Results from real-time qPCR analysis of regional marker expression in WT and SOX21−/− NPCs at days 0, 4, and 8. Data are represented as fold changes relative to undifferentiated WT hESCs (day 0) and shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3).
(F) Results from real-time qPCR analysis of regional marker expression in NT NPCs and SOX21 knockdown NPCs at day 8. For inducible knockdown of SOX21, cells were treated with doxycycline (100 ng/mL) from days 0 to 8. Data are represented as fold changes relative to undifferentiated WT ESCs (day 0) and shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 3). NT, nontarget control.
Identification of SOX21 Putative Target Genes
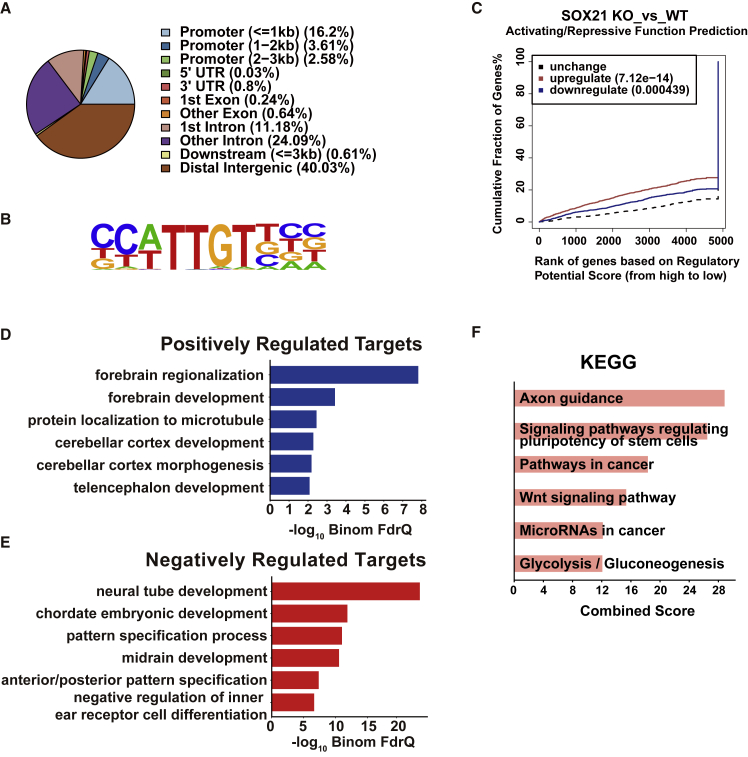
To understand how SOX21 participates in the control of forebrain regional identity, we carried out chromatin immunoprecipitation assays coupled to high-throughput DNA sequencing (ChIP-seq) in NPCs at day 5 and identified 3,765 confident (FDR q <0.01) binding peaks of SOX21 with a high signal-to-noise ratio (Figure S3A). Associations of SOX21 with some of these genes were verified by ChIP-qPCR (Figure S3B). Approximately 22% of SOX21 peaks were in promoters, and 40% were in distal intergenic regions (Figure 4A). De novo DNA motif analysis revealed that 66.16% of the peaks contained a C[AT]TTGT enriched sequence (p < 1 × 10−1032), consistent with the common consensus motif of SOX proteins (Figure 4B) (Harley et al., 1994). To identify putative target genes (PTG) of SOX21, we overlaid our transcriptomic dataset with that of SOX21 ChIP-seq and found that 358 and 490 PTGs were significantly downregulated (p < 4.39 × 10−4, Table S2) and upregulated (p < 7.12 × 10−14, Table S3), respectively (Figure 4C), in SOX21 KO cells. GO analysis of biological processes showed that terms of forebrain regionalization and telencephalon development were enriched by SOX21-positively regulated PTGs (Figure 4D), whereas PTGs negatively regulated by SOX21 were enriched for terms of pattern specification and midbrain development (Figure 4E). Moreover, disease ontology of mouse phenotype analysis showed that the positively regulated PTGs were associated with abnormal telencephalon development (Figure S3C), while the negatively regulated PTGs were associated with severe disease, including abnormal white matter morphology, abnormal neural tube, and exencephaly (Figure S3D). Moreover, our SOX21 function prediction results suggested that upregulated PTGs had much higher regulatory scores than the downregulated and unchanged ones in SOX21 KO NPCs (Figure 4C), suggesting that SOX21 tends to have more repressive than activating activities. Therefore, we examined the signaling pathways related to upregulated PTGs and found that the Wnt signaling pathway was enriched (Figure 4F), implying that SOX21 might have a repressive role in Wnt signaling. Taken together, these results further confirm that SOX21 contributes to rostral forebrain regional specification during early neural differentiation of hESCs.
Figure 4.
Genome-wide Analyses of SOX21 Binding Peaks
(A) A pie chart to show the distribution of SOX21-bound regions.
(B) The top-ranked DNA motif conserved in SOX21-bound regions revealed by the de novo motif discovery analysis.
(C) The BETA analysis of SOX21 functions by the integration of the ChIP-seq and RNA-seq datasets from SOX21 KO versus WT NPCs. Cumulative proportions of genes either downregulated (positively, blue), upregulated (negatively, red), or unchanged (black) increased over different regulatory potential score cutoffs. p values that represent the significance of the up- or down-group distributions were compared with those of the unchanged group by the Kolmogorov-Smirnov test.
(D and E) GO analysis of SOX21 positively (D) and negatively (E) regulated targets enriched by GREAT software. BinomFdrQ, the binomial FDR q value.
(F) KEGG signaling pathway analysis of SOX21 negatively regulated targets. The top six terms are shown.
See also Figure S3.
SOX21 Represses Wnt Signaling to Ensure Rostral Forebrain Identity
To validate the role of SOX21 in repressing Wnt signaling, we compared Wnt signaling activities between WT and SOX21 KO NPCs using the β-catenin/TCF reporter (TOP/FOPFlash) luciferase assay. Consistent with our RNA-seq data, SOX21 KO significantly elevated the reporter activity by approximately 30- to 50-fold (Figure 5A), supporting a repressive effect of SOX21 on the transcriptional activity of β-catenin. Moreover, SOX21 KO increased the protein levels of OTX1/OTX2 and WNT8B as well as active β-catenin, phosphorylated LRP6, DVL2, and DVL3 (Figure 5B). Importantly, overexpression of DKK1 (an antagonist of Wnt signaling) abrogated the increase in those protein levels caused by SOX21 KO (Figure 5B). Similar results were obtained when cells were treated with IWP-2 (2 μM) (Figure 5C). Furthermore, real-time qPCR results showed that DKK1 overexpression restored the expression of FOXG1 and SIX3 and suppressed the expression of OTX1, LMX1A, and Wnt signaling genes (AXIN2, WNT8B, WLS), while SOX21 expression was not altered by DKK1 overexpression (Figure S4A). In addition, three Wnt signaling inhibitors, IWP-2 (2 μM), IWR1-e (10 μM), and DKK1 (100 ng/mL), all efficiently corrected alterations in the expression of FOXG1 and SIX3 as well as OTX1, OTX2, and WNT8B (Figure 5D). As positive controls, Wnt signaling genes, such as AXIN2, WLS, and SP5, were all downregulated by the inhibitors (Figure 5D). These results clearly show that aberrant activation of Wnt signaling could account for most, if not all, SOX21 KO-caused changes in gene expression. Therefore, we propose that SOX21 represses Wnt signaling to ensure the rostral forebrain identity of hESC-derived NPCs.
WNT8B Is a Major Downstream Target of SOX21
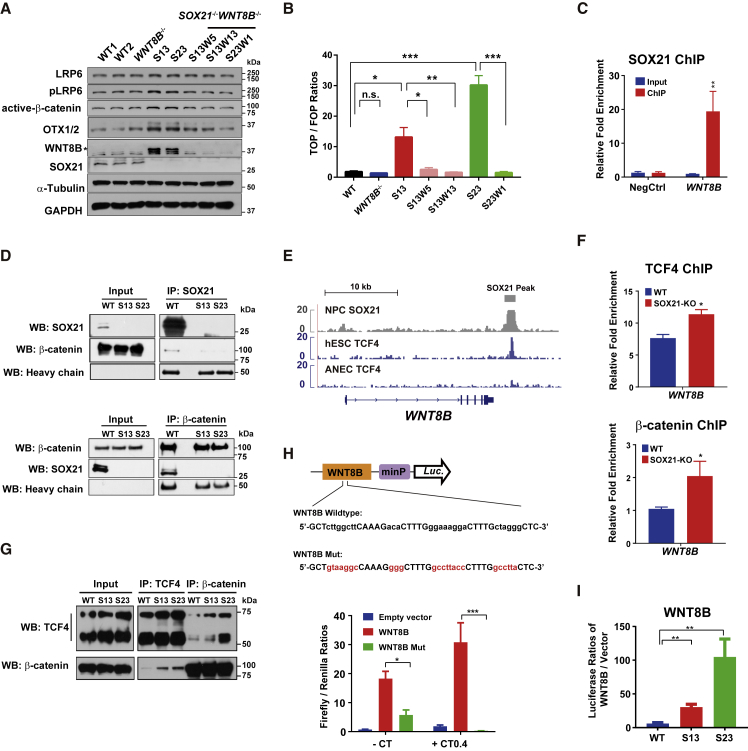
To dissect how SOX21 represses canonical Wnt signaling, we focused on WNT8B, which ranked first among significantly upregulated SOX21 PTGs in SOX21 KO cells (Figure S5A). To functionally test the hypothesis that WNT8B is a major target of SOX21 in the control of rostral forebrain identity, we knocked out WNT8B by targeting the second exon of WNT8B using the CRISPR/Cas9 system in WT and SOX21 KO cells (Figure S5B). Notably, double KO of WNT8B and SOX21 (named S13W5, S13W13, and S23W1) largely blocked the aberrant upregulation of WNT8B and OTX1/OTX2, as well as pLRP6 and active β-catenin, at the protein level (Figure 6A). Real-time qPCR results revealed that alterations in the expression of regional markers and Wnt signaling genes in SOX21 KO NPCs were corrected in WNT8B−/−SOX21−/− NPCs to different extents (Figure S5C). Moreover, TOP/FOPFlash luciferase reporter assays showed that the activation of Wnt signaling caused by SOX21 KO was abolished when SOX21 and WNT8B were both absent in NPCs (Figure 6B). Taken together, these results suggest that SOX21 ensures the rostral forebrain identity primarily by repressing WNT8B expression.
Figure 6.
WNT8B Is a Major Downstream Target of SOX21
(A) Representative western blot analysis of regional markers and Wnt signaling proteins in WT, SOX21−/−, WNT8B−/−, and SOX21−/−WNT8B−/− NPCs at day 6. WT, wild type; SOX21 and WNT8B double KO clones: S13W5 (SOX21−/− no. 13, WNT8B−/− no. 5), S13W13 (SOX21−/− no. 13, WNT8B−/− no. 13), S23W1 (SOX21−/− no. 23, WNT8B−/− no. 1).
(B) Examination of Wnt signaling activity by TOP/FOPFlash reporter assays in WT, SOX21−/−, WNT8B−/−, and SOX21−/−WNT8B−/− NPCs at day 5. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 4).
(C) ChIP-qPCR results of SOX21 at the WNT8B locus in NPCs at day 5. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 (n = 3). NegCtrl stands for the negative control, a gene desert region (chr21:25509072 + 25509220, GRCh37/hg19).
(D) Representative coimmunoprecipitation results for interactions between endogenous SOX21 and β-catenin in WT and SOX21 KO NPCs at day 7. IP: immunoprecipitation; WB: western blot.
(E) ChIP-seq signal profiles of SOX21 at the WNT8B locus in WT NPCs at day 5. ChIP-seq signal profiles of TCF4 in hESCs and anterior neural ectoderm cells (ANECs) were obtained from published data. The gray box above the signal tracks indicates the SOX21 binding site at the WNT8B locus.
(F) ChIP-qPCR analysis of TCF4 and β-catenin binding at the enhancer of WNT8B in WT and SOX21 KO NPCs at day 5. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01 (n = 3).
(G) Representative coimmunoprecipitation results of interactions between endogenous TCF4 and β-catenin in WT and SOX21 KO NPCs at day 7. IP: immunoprecipitation; WB: western blot.
(H) Results of luciferase reporter assays using an empty vector containing only a minimal promoter (minP) and vectors containing either the WT WNT8B enhancer sequence or the enhancer sequence with mutated TCF4 binding sites (WNT8B Mut), respectively, in response to Wnt signaling activation by CT99021 (0.4 μM) at day 6. Mutations at the TCF4 binding sites are indicated by red letters. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 4).
(I) Examination of WNT8B enhancer luciferase reporter activity in WT and SOX21 KO NPCs at day 6. Data are represented as ratios of luciferase activity relative to that with an empty vector containing a minP only. Data are shown as means ± SEM. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (n = 4).
See also Figure S5.
To understand how SOX21 repressed WNT8B expression, we first examined whether SOX21 could be recruited to the WNT8B enhancer. ChIP-qPCR results validated the association of SOX21 with the enhancer of WNT8B in NPCs (Figure 6C), suggesting a potentially direct control of WNT8B expression by SOX21. Next, we searched for SOX21 protein partners by coimmunoprecipitation coupled with a mass spectrometric analysis using specific antibodies against SOX21 in both WT and SOX21 KO NPCs. Among the protein partners identified (Table S4), we were most interested in β-catenin, as it usually forms complexes with TCF transcription factors to activate Wnt target genes (Nusse and Clevers, 2017). The specific interaction between SOX21 and β-catenin was verified by reciprocal coimmunoprecipitation assays (Figure 6D). Moreover, we analyzed published genome-wide DNA binding profiles of TCF4 in hESCs and anterior neural ectoderm cells (ANECs) (Tsankov et al., 2015) and found that TCF4 was associated with the enhancer of WNT8B in hESCs, but not in the ANECs (Figure 6E), suggesting that TCF4 might be excluded from the enhancer of WNT8B in NPCs. Furthermore, our ChIP-qPCR results showed that the recruitment of β-catenin and TCF4 to the WNT8B enhancer was greater in SOX21 KO NPCs than in WT NPCs (Figure 6F), further supporting the notion that SOX21 has an inhibitory effect on TCF4/β-catenin recruitment to the WNT8B locus. In addition, SOX21 KO enhanced the interaction intensity between TCF4 and β-catenin (Figure 6G). These results suggest that SOX21 may inhibit WNT8B expression via interference with the formation of TCF4/β-catenin complexes and their recruitment to the WNT8B locus.
Finally, to specifically show the relationship between TCF4/β-catenin and SOX21 in the regulation of WNT8B expression, we conducted reporter assays using a reporter vector containing either the WT WNT8B enhancer sequence or the enhancer sequence with mutated TCF4 binding motifs (Figure 6H). An increase in WT WNT8B reporter activity by CT (0.4 μM) treatment verified the activation of WNT8B expression by Wnt signaling (Figure 6H). Mutation of the TCF4 binding sequence in the WNT8B enhancer dramatically reduced reporter activity even under CT treatment (Figure 6H), suggesting that TCF4 recruitment plays a key role in WNT8B expression. Notably, WNT8B reporter activity was significantly higher in SOX21 KO NPCs than in WT NPCs (Figure 6I), in line with the increased WNT8B mRNA levels in SOX21 KO NPCs (Figures 2I, 3E, and S2F). Collectively, these results further support our conclusion that SOX21 interferes with the formation of TCF4/β-catenin complexes and their recruitment to the WNT8B enhancer to repress Wnt signaling in NPCs during early neural differentiation.
Discussion
In this study, we applied a rostrocaudal patterning system from hPSCs to mimic early human neural regionalization. The expression patterns of regional markers indicate that the Wnt gradient could induce rostrocaudally specified NPCs from hESCs in vitro. Transcriptomic analysis divided regional identities along the R-C axis into six regions as follows: region 1 (IWP2 and CT0.0), region 2 (CT0.4), region 3 (CT0.8), region 4 (CT1.0 and CT2.0), region 5 (CT3.0 and CT4.0), and region 6 (CT4.0RA). Gene coexpression network analysis further revealed that seven region-specific modules might represent the core regulatory network along the R-C axis. The global gene regulatory networks in regionally defined hESC-derived NPCs gained in this study will help future efforts aimed at the efficient generation of region-specific NPCs.
We revealed that SOX21 is required for rostral forebrain specification through repressing Wnt signaling in NPCs. In SOX21 KO NPCs, rostral forebrain genes were significantly downregulated, while caudal forebrain genes were upregulated. Importantly, our clustering analysis revealed that the SOX21 KO NPCs were most closely associated with NPCs of region 2 (CT0.4 group) with diencephalic-mesencephalic features. Therefore, the regional identity of rostral forebrain NPCs was caudalized in the absence of SOX21. Forebrain patterning requires the inhibition of posteriorizing Wnt signaling. Consistently, SOX21 KO substantially elevated Wnt signaling activity. Functionally, inhibition of Wnt signaling in SOX21 KO NPCs restored the rostral forebrain identity, providing direct evidence that SOX21 suppresses Wnt signaling to ensure rostral forebrain identity and prevent the caudal fate. Nevertheless, Sox proteins function dependently on developmental stages and cellular contexts, and the question of how SOX21 activates genes in the rostral forebrain needs further investigation. In addition to the rostral forebrain, SOX21 proteins were also detected in NPCs treated with 1.0–4.0 μM CT, suggesting that SOX21 has other functions in neural differentiation of hESCs and might act through different mechanisms. Sox21 KO mice are born normally, although they display cyclic alopecia and postnatal growth deficiency (Cheung et al., 2017, Kiso et al., 2009). This relatively minor phenotype could be explained by the functional redundancy among members of the Sox family, as they display overlaps of expression domains in the developing neural tissue (Uchikawa et al., 2011).
A few transcription factors have been known to repress Wnt signaling for forebrain specification in vertebrate development. For example, Six3 has been reported to inhibit the expression of Wnt1 and Wnt8b directly in the mouse anterior neural ectoderm (Lagutin et al., 2003, Liu et al., 2010), and Hesx1 has been shown to antagonize Wnt/β-catenin signaling in the mouse and zebrafish forebrain (Andoniadou et al., 2011). However, how these factors inhibit the transcription of Wnt/β-catenin targets and how canonical Wnt signaling is repressed in human forebrain development remain unclear. Many Sox proteins are known to suppress Wnt signaling via different mechanisms (Kormish et al., 2010, Wegner, 2010). Here, we found that SOX21 KO enhanced the recruitment of TCF4/β-catenin to the WNT8B enhancer. Thus, we propose that SOX21 might interfere with the binding of TCF4/β-catenin complexes to their target genes, repressing the activation of Wnt target genes. In addition, SOX21 formed protein complexes with β-catenin, and SOX21 KO increased the interaction between TCF4 and β-catenin. Because SOX21 KO also resulted in higher protein levels of TCF4, the increased interaction between TCF4 and β-catenin in SOX21 KO NPCs could be a result of elevated TCF4 protein level or the absence of a competitor.
Among SOX21 PTGs, we focused on WNT8B. WNT8B KO largely abrogated the defect of rostral forebrain specification caused by SOX21 KO, supporting the hypothesis that WNT8B is a key target of SOX21. Taken together, these results suggest that SOX21 prevents TCF4/β-catenin complexes from binding to the WNT8B enhancer in hESC-derived rostral forebrain NPCs. In the absence of SOX21, more TCF4/β-catenin complexes bind to and activate WNT8B, leading to the caudalization of regional identity of rostral forebrain NPCs. The transcriptional repression of WNT8B by SOX21 is required to generate rostral forebrain NPCs. The discovery of the new function of SOX21 will facilitate our understanding of the transcriptional control of early human forebrain patterning.
Experimental Procedures
Experimental methods are briefly summarized. See Supplemental Information for details.
Culture and Differentiation of hESCs
hESCs of the H9 (karyotype, XX) and SHhES8 (karyotype, XX) lines were maintained in mTeSR1 medium. For neural differentiation, hESC colonies were detached with dispase (Gibco) and resuspended in neural differentiation medium. The differentiation medium was prepared as follows: DMEM/F12:Neurobasal (1:1, Gibco), N2 (Gibco), B27 without vitamin A (Gibco), 2 mM glutamine, 0.1 mM nonessential amino acid, 5 μM SB431542 (Stemgent), and 50 nM LDN193189 (Stemgent). Rock inhibitor (Y-27632, 10 μM, Selleck) was present from days 0 to 2. On day 4, aggregates were plated onto Matrigel-coated six-well plates. From days 0 to 8, IWP-2 (2 μM, Millipore), or CT99021 (0–4.0 μM, STEMCELL), or RA (1 μM, Sigma) were included in the differentiation medium for region-specific neural differentiation.
Generation of KO hESC Lines
Homology arms were amplified from genomic DNA and inserted into the PGK-NEO-DTA targeting vector. The CRISPR/Cas9 plasmid was digested with Bbs I and ligated to the designed gRNAs. hESCs were digested into single cells and electroporated with appropriate combinations of CRISPR/Cas9 plasmids, with or without a donor plasmid. After a week of drug selection, colonies were collected for genotyping analyses.
RNA Extraction, cDNA Synthesis, and Real-Time qPCR
Total RNA was extracted using TRIzol reagent, and 2 μg total RNA was reverse transcribed into cDNA. Real-time qPCR was performed on the ABI ViiA7 Real-Time PCR system using SYBR Premix Ex Taq II (Takara). A list of primers used is provided in Table S5.
Western Blotting
Cells were lysed in the coimmunoprecipitation buffer, and protein concentrations were measured using the Pierce BCA Protein Assay Kit (Thermo Scientific). Twenty micrograms of total protein was separated by 10% SDS-PAGE, transferred to nitrocellulose blotting membranes, and blotted using primary and secondary antibodies. The antibodies used are listed in Table S6. All western blot analyses were conducted in at least three independent experiments, unless otherwise indicated.
Luciferase Reporter Assays
The WT or mutated WNT8B enhancer was cloned and inserted into the pGL4.22 vector (Promega). Then, 500 ng of the enhancer reporter plasmid or an empty vector and 10 ng of a pRL-TK internal control plasmid (Promega) were used for transfection. For Wnt signaling reporter assays, 500 ng of the 8xTOPFlash (Addgene, no. 12456) or 8xFOPFlash (Addgene, no. 12457) plasmid together with 50 ng of the pRL-TK internal control plasmid (Promega) were used for transfection. Luciferase activities were examined with the Dual-Glo Luciferase Assay System (Promega).
Flow Cytometric Analysis and Cell Proliferation Assays
Cells were fixed, permeabilized (Cytofix/Cytoperm Kit, BD Biosciences) and stained using antibodies against SOX2 and NANOG. For cell proliferation analysis, cells were incubated with 10 μM EdU (BD Biosciences) for 1 h and labeled using a Cell-Light EdU Apollo488 In Vitro Flow Cytometry Kit (RIBO). The data for stained samples were acquired on an Accuri C6 flow cytometer.
ChIP Assays
ChIP assays were performed as described previously with modifications (Zhu et al., 2017). See detailed methods in Supplemental Information. All primers used in ChIP-qPCR assays are listed in Table S7.
GO Analysis
GO analysis for DEGs and region-specific expressed genes was conducted using Enrichr (Kuleshov et al., 2016). Combined score (c) is the multiplication of the log of p value (p) with Z score (z); c = log(p) × z.
Statistical Analysis
The unpaired Student's t test was used for statistical tests unless stated otherwise, and data are presented as the mean ± SEM. Numbers of biological replicates relevant for individual experiments are stated in figure legends.
Author Contributions
Z.F. and X.L. designed the project, performed major experiments, analyzed data, and wrote the manuscript. J.W. and F.T. helped with immunostaining. Y.Z. participated in the initiation of the project. N.J. discussed the project and edited the manuscript. Y.J. directed the project and wrote the manuscript. Z.F. and X.L. contributed equally to this study.
Acknowledgments
This work was supported by the National Natural Science Foundation of China grants 31801224, 31730055, and 31871373; Ministry of Science and Technology of China grant 2016YFA0100100; Strategic Priority Research Program of the Chinese Academy of Sciences grant XDB19020100; and the Innovative Research Team of High-level Local Universities in Shanghai. The authors declare that they have no conflicts of interest with the contents of this article.
Published: November 21, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.10.013.
Accession Numbers
The RNA-seq and ChIP-seq data presented in this manuscript are accessible through GEO series accession number GEO: GSE110506.
Supplemental Information
References
- Andoniadou C.L., Martinez-Barbera J.P. Developmental mechanisms directing early anterior forebrain specification in vertebrates. Cell Mol. Life Sci. 2013;70:3739–3752. doi: 10.1007/s00018-013-1269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoniadou C.L., Signore M., Young R.M., Gaston-Massuet C., Wilson S.W., Fuchs E., Martinez-Barbera J.P. HESX1- and TCF3-mediated repression of Wnt/beta-catenin targets is required for normal development of the anterior forebrain. Development. 2011;138:4931–4942. doi: 10.1242/dev.066597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung L.Y., Okano H., Camper S.A. Sox21 deletion in mice causes postnatal growth deficiency without physiological disruption of hypothalamic-pituitary endocrine axes. Mol. Cell Endocrinol. 2017;439:213–223. doi: 10.1016/j.mce.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani L., Salinas P.C. WNTs in the vertebrate nervous system: from patterning to neuronal connectivity. Nat. Rev. Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- Fossat N., Jones V., Khoo P.L., Bogani D., Hardy A., Steiner K., Mukhopadhyay M., Westphal H., Nolan P.M., Arkell R. Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development. 2011;138:667–676. doi: 10.1242/dev.052803. [DOI] [PubMed] [Google Scholar]
- Fossat N., Jones V., Garcia-Garcia M.J., Tam P.P.L. Modulation of WNT signaling activity is key to the formation of the embryonic head. Cell Cycle. 2012;11:26–32. doi: 10.4161/cc.11.1.18700. [DOI] [PubMed] [Google Scholar]
- Fotaki V., Larralde O., Zeng S., McLaughlin D., Nichols J., Price D.J., Theil T., Mason J.O. Loss of Wnt8b has no overt effect on hippocampus development but leads to altered Wnt gene expression levels in dorsomedial telencephalon. Dev. Dyn. 2010;239:284–296. doi: 10.1002/dvdy.22137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover J.C., Renaud J.S., Lampe X., Rijli F.M. Hindbrain development and retinoids. Adv. Dev. Biol. 2006;16:145–180. [Google Scholar]
- Harley V.R., Lovell-Badge R., Goodfellow P.N. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994;22:1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houart C., Caneparo L., Heisenberg C., Barth K., Take-Uchi M., Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Imaizumi K., Sone T., Ibata K., Fujimori K., Yuzaki M., Akamatsu W., Okano H. Controlling the regional identity of hPSC-derived neurons to uncover neuronal subtype specificity of neurological disease phenotypes. Stem Cell Reports. 2015;5:1010–1022. doi: 10.1016/j.stemcr.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irioka T., Watanabe K., Mizusawa H., Mizuseki K., Sasai Y. Distinct effects of caudalizing factors on regional specification of embryonic stem cell-derived neural precursors. Brain Res. Dev. Brain Res. 2005;154:63–70. doi: 10.1016/j.devbrainres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Itasaki N. eLS. John Wiley; 2015. Vertebrate embryo: neural patterning. [Google Scholar]
- Kamachi Y., Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. The role of organizers in patterning the nervous system. Annu. Rev. Neurosci. 2012;35:347–367. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- Kiecker C., Niehrs C. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kirkeby A., Grealish S., Wolf D.A., Nelander J., Wood J., Lundblad M., Lindvall O., Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Kiso M., Tanaka S., Saba R., Matsuda S., Shimizu A., Ohyama M., Okano H.J., Shiroishi T., Okano H., Saga Y. The disruption of Sox21-mediated hair shaft cuticle differentiation causes cyclic alopecia in mice. Proc. Natl. Acad. Sci. U S A. 2009;106:9292–9297. doi: 10.1073/pnas.0808324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormish J.D., Sinner D., Zorn A.M. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev. Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuleshov M.V., Jones M.R., Rouillard A.D., Fernandez N.F., Duan Q.N., Wang Z.C., Koplev S., Jenkins S.L., Jagodnik K.M., Lachmann A. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–W97. doi: 10.1093/nar/gkw377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagutin O.V., Zhu C.C., Kobayashi D., Topczewski J., Shimamura K., Puelles L., Russell H.R., McKinnon P.J., Solnica-Krezel L., Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–379. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lako M., Lindsay S., Bullen P., Wilson D.I., Robson S.C., Strachan T. A novel mammalian wnt gene, WNT8B, shows brain-restricted expression in early development, with sharply delimited expression boundaries in the developing forebrain. Hum. Mol. Genet. 1998;7:813–822. doi: 10.1093/hmg/7.5.813. [DOI] [PubMed] [Google Scholar]
- Liu W., Lagutin O., Swindell E., Jamrich M., Oliver G. Neuroretina specification in mouse embryos requires Six3-mediated suppression of Wnt8b in the anterior neural plate. J. Clin. Invest. 2010;120:3568–3577. doi: 10.1172/JCI43219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z., Hui Y., Shi L., Chen Z., Xu X., Chi L., Fan B., Fang Y., Liu Y., Ma L. Efficient CRISPR/Cas9-mediated versatile, predictable, and donor-free gene knockout in human pluripotent stem cells. Stem Cell Reports. 2016;7:496–507. doi: 10.1016/j.stemcr.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda S., Kuwako K., Okano H.J., Tsutsumi S., Aburatani H., Saga Y., Matsuzaki Y., Akaike A., Sugimoto H., Okano H. Sox21 promotes hippocampal adult neurogenesis via the transcriptional repression of the Hes5 gene. J. Neurosci. 2012;32:12543–12557. doi: 10.1523/JNEUROSCI.5803-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom U., Jessell T.M., Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Nusse R., Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Puelles L., Martinez S. Chapter 8-patterning of the diencephalon. In: Rubenstein J.L.R., Rakic P., editors. Patterning and Cell Type Specification in the Developing CNS and PNS. Academic Press; 2013. pp. 151–172. [Google Scholar]
- Rubenstein J.L.R., Rakic P. Elsevier/Academic Press; 2013. Comprehensive developmental neuroscience: patterning and cell type specification in the developing CNS and PNS. [Google Scholar]
- Sandberg M., Kallstrom M., Muhr J. Sox21 promotes the progression of vertebrate neurogenesis. Nat. Neurosci. 2005;8:995–1001. doi: 10.1038/nn1493. [DOI] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tsankov A.M., Gu H., Akopian V., Ziller M.J., Donaghey J., Amit I., Gnirke A., Meissner A. Transcription factor binding dynamics during human ES cell differentiation. Nature. 2015;518:344–349. doi: 10.1038/nature14233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuazon F.B., Mullins M.C. Temporally coordinated signals progressively pattern the anteroposterior and dorsoventral body axes. Semin. Cell Dev. Biol. 2015;42:118–133. doi: 10.1016/j.semcdb.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twyman R.M. Wnt pathway and neural patterning A2-squire. In: Larry R., editor. Encyclopedia of Neuroscience. Academic Press; 2009. pp. 497–502. [Google Scholar]
- Uchikawa M., Kamachi Y., Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech. Dev. 1999;84:103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Uchikawa M., Yoshida M., Iwafuchi-Doi M., Matsuda K., Ishida Y., Takemoto T., Kondoh H. B1 and B2 Sox gene expression during neural plate development in chicken and mouse embryos: universal versus species-dependent features. Dev. Growth Differ. 2011;53:761–771. doi: 10.1111/j.1440-169X.2011.01286.x. [DOI] [PubMed] [Google Scholar]
- van de Leemput J., Boles N.C., Kiehl T.R., Corneo B., Lederman P., Menon V., Lee C., Martinez R.A., Levi B.P., Thompson C.L. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Wegner M. All purpose Sox: the many roles of Sox proteins in gene expression. Int. J. Biochem. Cell Biol. 2010;42:381–390. doi: 10.1016/j.biocel.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Whittington N., Cunningham D., Le T.K., De Maria D., Silva E.M. Sox21 regulates the progression of neuronal differentiation in a dose-dependent manner. Dev. Biol. 2015;397:237–247. doi: 10.1016/j.ydbio.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurst W., Bally-Cuif L. Neural plate patterning: upstream and downstream of the isthmic organizer. Nat. Rev. Neurosci. 2001;2:99–108. doi: 10.1038/35053516. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Li C., Zeng Y., Ding J., Qu Z., Gu J., Ge L., Tang F., Huang X., Zhou C. PHB associates with the HIRA complex to control an epigenetic-metabolic circuit in human ESCs. Cell Stem Cell. 2017;20:274–289.e7. doi: 10.1016/j.stem.2016.11.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.