Summary
Alzheimer's disease (AD) is characterized by memory impairments in its earliest clinical phase. The synaptic loss and dysfunction leading to failures of synaptic networks in AD brain directly cause cognitive deficits of patient. However, it remains unclear whether the synaptic networks in AD brain could be repaired. In this study, we generated functional human induced neural progenitor/stem cells (iNPCs) that had been transplanted into the hippocampus of immunodeficient wild-type and AD mice. The grafted human iNPCs efficiently differentiated into neurons that displayed long-term survival, progressively acquired mature membrane properties, formed graft-host synaptic connections with mouse neurons and functionally integrated into local synaptic circuits, which eventually reinforced and repaired the neural networks of host hippocampus. Consequently, AD mice with human iNPCs exhibited enhanced synaptic plasticity and improved cognitive abilities. Together, our results suggest that restoring synaptic failures by stem cells might provide new directions for the development of novel treatments for human AD.
Keywords: Alzheimer's disease, human induced neural progenitor cells, functional integration, synaptic networks, cognitive improvement
Highlights
-
•
Human iNPCs from immobilized peripheral blood display functional properties
-
•
Human iNPCs differentiate into mature neurons in AD brain without tumor formation
-
•
The functional integration of grafted human neurons reinforces synaptic circuits
-
•
Human iNPCs enhance synaptic plasticity and repair cognitive deficits of AD mouse
Synaptic failures in AD brain directly cause cognitive deficits of patient. Jing and colleagues reported that the functional integration of human peripheral blood-derived neural progenitor cells reinforced the host neural circuity, improved the synaptic plasticity and rescued the cognitive deficits of AD model mice, indicating that restoring synaptic failures might provide new directions for the development of novel treatments for AD.
Introduction
Alzheimer's disease (AD) is the most prevalent form of dementia and currently remains incurable (Hurd et al., 2013). AD is pathologically characterized by progressively emerged amyloid-β (Aβ) aggregation, tau-rich neurofibrillary tangles and extensive synaptic disruption and neuronal loss in the patient brain. The leading theory in the field postulated that Aβ accumulation in brain regions important for memory and cognition initiates AD (Hardy and Selkoe, 2002), which provides clues for drug discovery for the treatment of AD. Over three decades, pharmaceutical companies have focused on Aβ plaques or tau tangles and developed drugs to remove plaques or stop them from forming. However, several promising drugs targeting Aβ or tau failed to rescue the cognitive deficits of AD patients or delay the progression of the disease in recent clinical trials (Cacabelos, 2018, Doody et al., 2014).
The Aβ-induced synaptic dysfunction and loss occur at an early stage of AD and form a fundamental part of the pathological process of the disease (Palop and Mucke, 2010). Evidence implicates that the synaptic failures directly affected the synaptic and neural circuits, which in turn caused irreversible impairments of cognitive function emerging in the earliest clinic phase of AD patients (Davies et al., 1987, Palop and Mucke, 2010, Selkoe, 2002). Then, it is therapeutically attractive to repair the disrupted synapse and reinforce synaptic networks, which might reverse the cognitive deficits of AD and provide a meaningful clinical benefit. The global neural degeneration in the brain of AD makes people believe that it is challenging to treat AD using cell replacement strategies. Efforts to explore the possibilities of repairing synaptic damages in AD brain by cell replacement have been limited. Previous studies suggested that the transplantation of mesenchymal stem cells or fetal brain tissue-derived human neural stem cells rescued memory deficits by reducing neuronal apoptosis (Lee et al., 2010) or promoting expression of synaptic markers of AD animals (Ager et al., 2015). Several other studies reported the improvement of cognitive abilities of AD animals upon transplantation of human or mouse neural stem cells from embryonic stem cells (ESCs) or iPSCs but did not measure the neurochemical changes of synapses in host brains (Liu et al., 2013, Moghadam et al., 2009). Our previous study suggested that the grafted mouse ESC-derived basal forebrain cholinergic neurons (BFCNs) formed synapses with host neural cells, displayed electrophysiological activity in the host brains and improved the cognitive ability of AD mice (Yue et al., 2015). These observations direct our attention to detect whether human neural progenitors or neurons could functionally integrate into local neural circuitry and reinforce synaptic connectivity in AD brain.
Human neural stem cells provide a potentially unlimited source of different neural cell types on demands to repair the degenerated or injured brain. However, human adult neural stem cells persist into special niches of brain and are inaccessible (Gage, 2000). Hence, the in vitro generation and possible therapeutic applications of human induced neural stem/progenitor cells (iNPCs) are of special interest. The reprogramming from human somatic cells into human iNPCs resembling brain neural stem cells has been achieved in recent years (Brand and Livesey, 2011). However, the potential therapeutic use of the resulting human iNPCs has remained to be explored.
In this study, functional human iNPCs were produced from immobilized human peripheral blood cells and displayed typical properties of brain NPCs. After transplantation into the hippocampus of immunodeficient wild-type (WT) and AD mice, the human iNPCs rapidly differentiated into neurons and astrocytes that survived well up to 12 months. The human iNPC-derived neurons gradually possessed the mature membrane properties, received synaptic inputs and formed synaptic connections with mouse hippocampal neurons. Moreover, the AD mice exhibited enhanced synaptic plasticity and improved cognitive abilities upon human iNPC transplantation.
Results
Functional Human iNPCs Were Generated from a Small Volume of Peripheral Blood
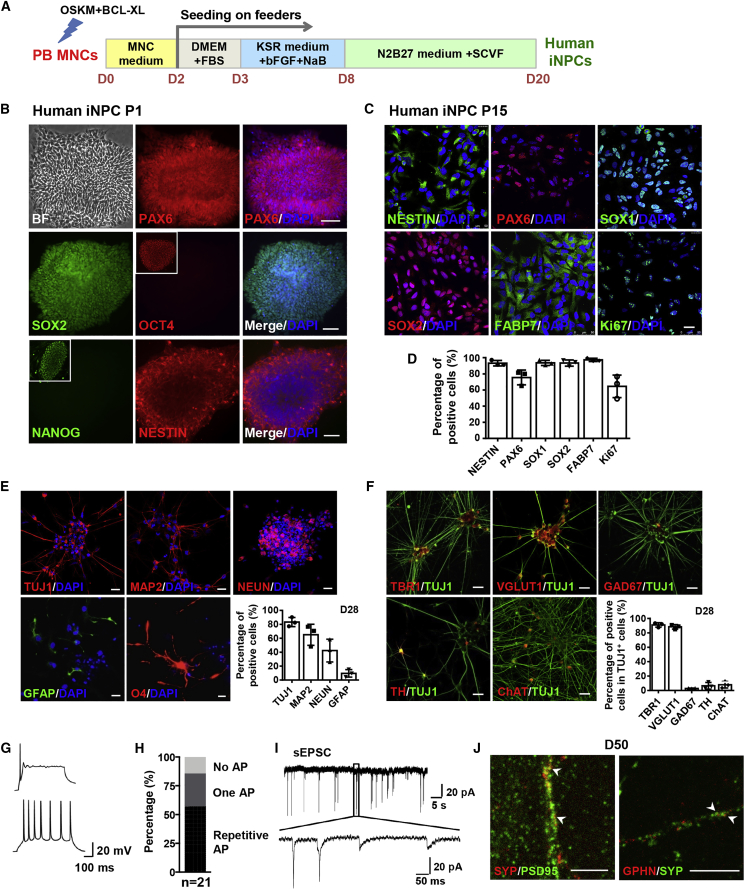
The approach used to generate iNPCs from immobilized adult peripheral blood mononuclear cells (PB MNCs) in this study is based on overexpression of four iPS factors (OCT4, SOX2, c-MYC, and KLF4) in combination with small molecules as shown in Figure 1A. In brief, erythroblasts in PB MNCs from 3 to 8 mL peripheral blood were expanded, transfected by episomal vectors containing four iPS factors and an anti-apoptotic factor BCL-XL, and then sequentially cultured in three different types of media for 8 days to initiate reprogramming of PB MNCs. Subsequently, cells were treated with a cocktail of four chemicals (SB431542, CHIR99021, VPA and Forskolin, SCVF) in N2B27 medium for neural fate conversion (Figure 1A). Finally, NPC-like colonies with distinct morphology appeared within 3 weeks (Figure S1A). These colonies homogeneously expressed the NPC markers PAX6, SOX2, and NESTIN but not the pluripotency markers OCT4 and NANOG at passage 1, indicating that the PB MNCs rapidly acquired a neural progenitor identity and converted into iNPCs (Figure 1B). The chemicals played critical roles during neural fate conversion and the generated NPC-like colonies rapidly lost their self-renewal ability and went into spontaneous differentiation without chemicals (Figure S1A). In contrast, the chemical-induced iNPCs remained stable during prolonged culture and sustained the homogeneous expression of NESTIN, PAX6, SOX1, SOX2, FABP7, and the proliferation marker Ki67 at passage 15 (Figures 1C and 1D). PCR analysis at passage 5 confirmed that the exogenous genes in episomal vectors were not inserted into the genome of iNPCs and the iNPCs were integration free (Figure S1B). The established iNPC lines have been expanded and serially passaged as single cells for over 25 passages with a normal karyotype and maintained the capacity to form neurosphere, indicative of the self-renewal ability of iNPCs (Figures S1C–S1E).
Figure 1.
The Characterization of Human iNPCs Converted from a Small Volume of Peripheral Blood
(A) Schematic representation of the approach used to direct the conversion of PB MNCs into iNPCs.
(B) Immunofluorescence analysis of human iNPCs at passage 1. Note the representative OCT4+ and NANOG+ iPSC colonies in outlined regions as positive controls.
(C) Immunofluorescence analysis of human iNPCs at passage 15.
(D) Quantification of the results shown in (C).
(E) Immunofluorescence analysis of human iNPC-derived neurons and astrocytes as at day 28, and oligodendrocytes at day 35, respectively, and corresponding differentiation efficiency.
(F) Immunofluorescence analysis of the subtypes of human iNPC-derived neurons and corresponding differentiation efficiency at day 28.
(G) Representative traces of single AP (top) and repetitive AP firing (bottom) of human iNPC-derived neurons at day 50 in response to step current injection.
(H) Percentages of human iNPC-derived cells with no AP, single AP or repetitive firing.
(I) Representative traces of spontaneous EPSCs received at a holding potential of −70 mV by human iNPC-derived neurons at day 50.
(J) Immunofluorescence analysis of SYNAPTOPHYSIN (SYP) co-labeling with PSD95 or GEPHYRIN (GPHN) in human iNPC-derived neurons at day 50. Arrowheads indicate the co-localization of pre- and postsynaptic dots.
Cell nuclei were counterstained with DAPI. Scale bars, 100 μm (B), 25 μm (C, E, F), 5 μm (J). n = 3 independent experiments. Data are represented as scatterplots with mean ± SD. Related to Figures S1 and S2.
To gain further insights into the transcriptional profile and cellular identity of human iNPCs, global gene expression of iNPCs was determined by bulk RNA sequencing of two different iNPC lines at passages 15 and 25, respectively. The top 1,000 upregulated and downregulated differentially expressed genes in human iNPCs revealed a clear difference between the transcriptomes of PB MNCs and iNPCs, while a high similarity was observed among all iNPC lines at different passages (Figures S2A–S2C). Gene ontology analysis showed that upregulated genes in iNPCs were mainly associated with neuronal differentiation and development (Figure S2C). These analyses confirmed that bona fide human iNPCs had been established and stably maintained in vitro. To further support this conclusion, we compared the global gene expression of human iNPCs with a published temporal transcriptome dataset of hESC-derived NPCs with prefrontal cortex identity (van de Leemput et al., 2014) and found that human iNPCs closely resembled hESC-derived NPCs at day 19 and 26 (Figure S2D). Hierarchical clustering analysis and scatterplots reflected that human iNPCs diverged from PB MNCs and closely clustered with hESC-derived NPCs (Figures S2E and S2F). In line with these findings, a comparative analysis with the database from BrainSpan Atlas of the Developing Human Brain showed that iNPCs mostly correlated with frontal cortex and ventral forebrain in fetal brain at 8 to 9 weeks (Figure S2G).
The potential of human iNPCs was assessed by their capacity to generate the three major neural lineages. Monolayer neural differentiation of human iNPCs gave rise robustly within 1 month of culture to neurons expressing neuronal marker genes TUJ1, MAP2, and NEUN as well as to astrocytes expressing GFAP, with larger numbers of neurons (Figure 1E). Differentiation toward oligodendrocytes was less efficient but some oligodendrocyte marker O4+ cells with a typical oligodendrocytic morphology were observed (Figure 1E). Among the iNPC-derived TUJ1+ neurons, over 90% were VGlut1-positive glutamatergic neurons expressing the cortical marker TBR1, but other subtypes of neurons, such as GAD67+ GABAergic neurons, TH+ dopaminergic neurons and ChAT+ cholinergic neurons, were also found (Figure 1F). The whole-cell patch-clamp recordings revealed that iNPC-derived neurons possessed functional membrane properties by firing either one or repetitive action potentials (APs) (Figures 1G and 1H) and expressing excitatory postsynaptic currents (Figure 1I). The co-localization of presynaptic marker SYNAPTOPHYSIN with postsynaptic marker PSD95 revealed the existence of glutamatergic synapses or with GEPHYRIN for GABAergic synapses (Figure 1J).
Collectively, these results suggest a rapid conversion from PB MNCs to stable, homogeneous and self-renewing human iNPCs with a forebrain identity and the capacity to three major neural lineages.
The Human iNPCs Efficiently Differentiated into Neurons and Survived for Long-Term in the Brain of Host Mice
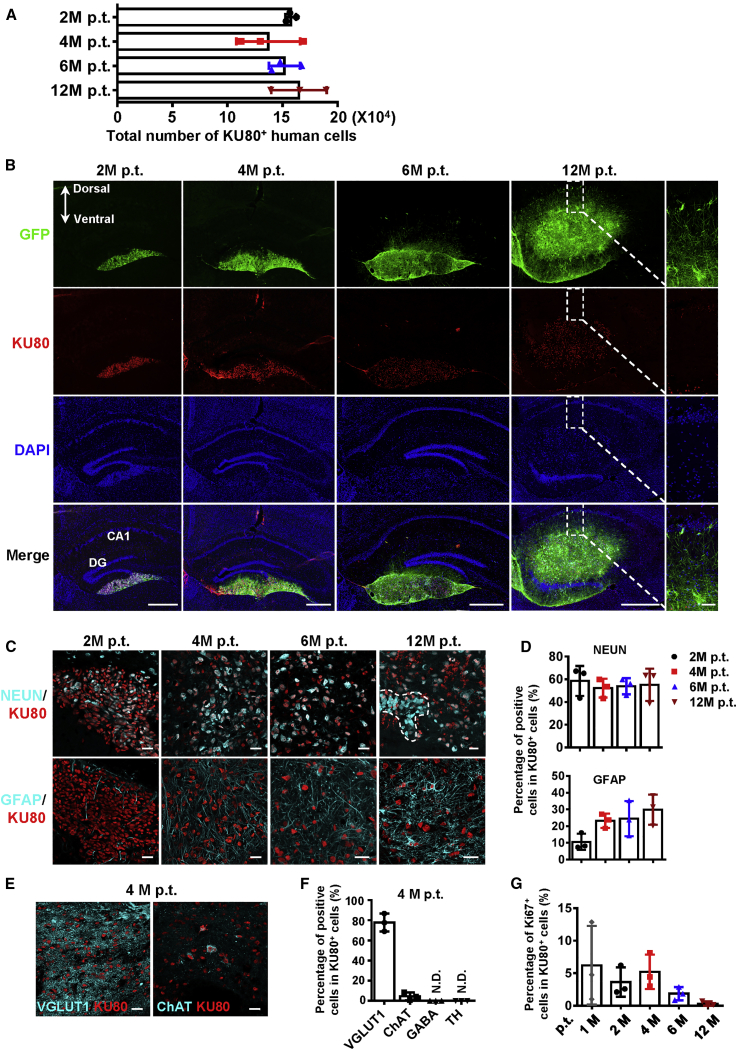
Next, we set out to investigate how human iNPCs function in vivo after transplantation. To this end, human iNPCs from donor 1# labeled with GFP at passage 15 were bilaterally transplanted into the hippocampus (105 cells per side, 2 × 105 cells per mouse) of immunodeficient Foxn1−/− mice (n = 39) by 2 months of age, which served as the WT control. The survival, migratory, differentiation and proliferation capacity of grafted iNPCs were determined 2, 4, 6, and 12 months after transplantation. The total number of grafted cells at each time point detected was around 1.5 × 105 (Figure 2A). GFP-positive grafted cells mainly located in hippocampus and extended long and abundant axons that projected into host dentate gyrus (DG) and CA1 region at 6 and 12 months (Figure 2B). All GFP+ cells expressed KU80, a human-specific nuclear marker, and the KU80+ human cells became more and more scattered from 2 to 12 months after transplantation (Figure S3A). One month after transplantation, majority of the KU80+ iNPCs rapidly differentiated into TUJ1+ neurons and NESTIN+ NPCs were seldom detected (Figures S3B and S3C). Then, KU80+NEUN+ human neurons gradually migrated and mixed with the KU80−NEUN+ endogenous hippocampal granular cells (Figure 2C, top panel). The fraction of iNPC-derived mature neurons was relatively stable over time, accounting for approximately 60% of grafted KU80+ cells (Figures 2C and 2D, top panels). In contrast, the differentiation of human iNPCs to astrocytes was slower. Double labeled KU80+GFAP+ astrocytes were detectable 2 months after transplantation and became a little abundant at 6 and 12 months (Figures 2C and 2D, bottom panels). Further analysis showed that the grafted human iNPCs predominantly gave rise to VGlut1+ glutamatergic neurons as well as a few ChAT+ cholinergic neurons and no other subtypes were detected (Figures 2E and 2F). Due to the quick differentiation of grafted iNPCs, the proliferation ability of human iNPCs dramatically decreased by lost expression of Ki67 and the graft overgrowth was not observed in the host brain. Only 5% of cells among grafted cells kept proliferating at 1 month after transplantation and the number decreased to 1% at 12 months (Figures 2G and S3D). These data suggest that human iNPCs rapidly stop proliferation and robustly differentiate into neurons and astrocytes in the host hippocampus. More importantly, the iNPC-derived human neurons have maintained viability for at least 12 months, gradually migrated and projected long and abundant axons into host hippocampus.
Figure 2.
Differentiation of Grafted Human iNPCs in Hippocampus of Immunodeficient Mice
(A) Total numbers of KU80+ human cells in the hippocampus of immunodeficient mice 2, 4, 6, and 12 months post transplantation (M p.t.).
(B) The coronal brain section harboring grafted GFP+ human iNPCs at 2, 4, 6, and 12 M p.t. (Top) Immunofluorescence analysis of KU80 expression among GFP+ cells (second panel). Cell nuclei were counterstained with DAPI.
(C) Immunofluorescence analysis of NEUN and GFAP expression in KU80+ cells. The dashed line indicates the KU80−NEUN+ host neurons mixing with grafted cells.
(D) Percentages of NEUN+ mature neurons and GFAP+ astrocytes among KU80+ grafted human cells shown in (C).
(E) Immunofluorescence analysis of subtypes of human iNPC-derived neurons among KU80+ cells at 4 M p.t.
(F) Quantification of the results shown in (E).
(G) Percentages of Ki67+ cells among KU80+ grafted cells at 1, 2, 4, 6, and 12 M p.t. shown in Figure S3D.
Scale bars, 500 μm (B), 50 μm (magnified image in B) and 25 μm (C and E). n = 3 mice per time point. Data are represented as scatterplots with mean ± SD. Related to Figure S3.
Human iNPC-Derived Neurons Gradually Matured in the Brain of Host Mice
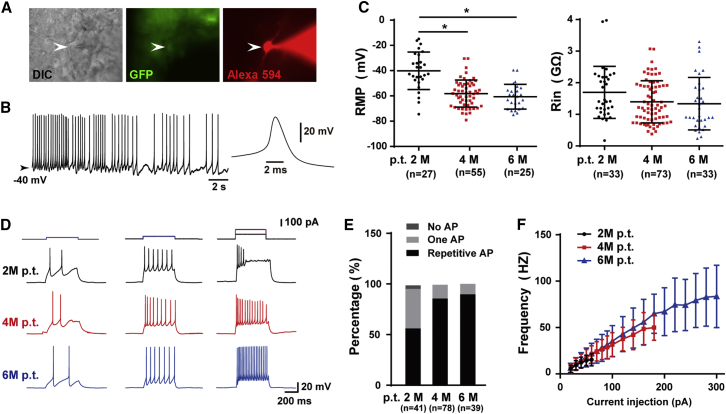
We then examined whether transplanted human iNPC-derived neurons became functionally mature. To address this question, we performed whole-cell recording in brains obtained from host mice 2, 4, and 6 months after transplantation. GFP-positive grafted cells in acute slices were identified under a fluorescence microscope and recorded by patch pipettes with Alexa Fluor 594 (Figure 3A). Some of the recorded human iNPC-derived neurons generated spontaneous APs due to the depolarized resting membrane potential, displaying typical neuronal properties (Figure 3B). The resting membrane potential of the GFP+ neurons gradually hyperpolarized and the input resistance progressively decreased in a time-dependent manner (Figure 3C). Larger current injections in neurons from 4- and 6-month, but not 2-month grafted brains induced repetitive firing without failure (Figure 3D), indicating that longer differentiating time promoted acquisition of mature membrane properties by human neurons in the host hippocampus. Consistently, 100% of the 4- and 6-month neurons recorded could generate APs and most of them discharged repetitively, while only ∼50% of recorded 2-month neurons showed repetitive firing in response to step current injections (Figure 3E). The F-I curves of 4- and 6-month neurons exhibit gradual increases in AP frequency with increasing current injections from 20 to 180 pA and to 300 pA, respectively (Figure 3F). Currents greater than 180 pA for 4-month neurons and 300 pA for 6-month neurons caused inactivation of voltage-gated sodium channels and thus failure of repetitive firing. In sharp contrast, 2-month neurons could only generate repetitive APs within a small current range, from 20 to 60 pA (Figure 3F). Collectively, the electrophysiological profiles displayed by human iNPC-derived neurons showed that grafted human neurons gradually mature in the host hippocampus and acquire functional membrane properties around 6 months after transplantation.
Figure 3.
The Maturation of Grafted Human iNPC-Derived Neurons in Mice Hippocampus
(A) Representative human iNPC-derived neurons (arrowhead) in hippocampus on acute slice from brain of an immunodeficient mouse 2 M p.t. Left, DIC image; middle, GFP; right, filling the cell with Alexa Fluor 594 (100 μM) during whole-cell recording.
(B) Spontaneous APs recorded in some iNPC-derived neurons on acute slices. Inset, an expanded AP for clarity.
(C) The resting membrane potential of human neuronal cells gradually hyperpolarized after transplantation and the input resistance decreased in a time-dependent manner. Data are represented as scatterplots with mean ± SD. One-way ANOVA followed by Tukey's post-hoc test. ∗p < 0.05.
(D) AP generation in grafted human neuronal cells in responses to step current injection.
(E) Percentage of human neuronal cells with no AP, single AP, or repetitive AP at 2, 4, and 6 M p.t.
(F) Comparison of F-I curves from human neuronal cells at 2, 4, and 6 M p.t. Data are represented as scatterplots with mean ± SD.
Sample size: 3 mice for 2 M p.t. and 6 M p.t. group, respectively; 6 mice for 4 M p.t. group. Numbers of recorded cells (n) were shown on the graphs in (C) and (E).
Human iNPC-Derived Neurons Functionally Integrated into the Synaptic Network of the Host Hippocampus
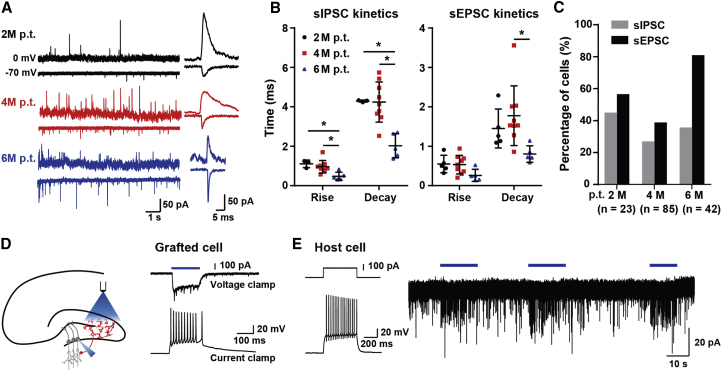
The finding that human iNPC-derived neurons functionally matured in vivo prompted us to further examine whether they integrated into the synaptic circuitry of host hippocampus. In acute slices, all three groups of neurons (2, 4, and 6 months after transplantation) were recorded both spontaneous excitatory postsynaptic currents (sEPSCs) and inhibitory postsynaptic currents (sIPSCs) (Figure 4A), indicating that the grafted human neurons received synaptic inputs from other neurons, either other grafted cells or host cells. In addition, the decreases of rise time or decay time constant were in line with the maturation of neurons (Figures 4A and 4B) and the percentage of 6-month neurons exhibiting EPSCs was much higher than those of 2- or 4-month neurons (Figure 4C), indicative of increased synaptic transmission. To further investigate functional synaptic connections from grafted cells to host cells, we employed an optogenetic approach and transduced grafted human iNPCs with channelrhodopsin-2 (ChR2) tagged with mCherry (Figure 4D), so that grafted cells could be identified by their expression of mCherry. In voltage-clamp mode, we could obtain inward currents in these cells in response to blue light stimulation at the perisomatic region. As shown in Figure 4D, fast synaptic events occurred together with the relatively slow light-evoked current, indicating synaptic inputs from other grafted cells. These inward currents could induce trains of APs when cells were recorded in current-clamp mode (Figure 4D). Similar light stimulation could not evoke AP firing in neighboring host granular cells with unique firing pattern, but was able to cause an increase in the frequency of EPSCs of granule cells (7/21), showing that grafted cells synapse onto host cells (Figure 4E). Together, the detection of synapse transmission between grafted human neurons and host hippocampal neurons indicates the functional integration of grafted human neurons into the synaptic networks of the host hippocampus.
Figure 4.
The Functional Integration of Human iNPC-Derived Neurons into Local Synaptic Networks of the Host Hippocampus
(A) Representative traces from human neuronal cells on acute slices from mice brains 2, 4, and 6 M p.t. showing sEPSCs at a holding potential of −70 mV and sIPSCs at 0 mV. Single synaptic events were shown for comparison.
(B) The kinetics of sEPSC and sIPSC from human neuronal cells at 2, 4, and 6 M p.t. Data are represented as scatterplots with mean ± SD. One-way ANOVA followed by Tukey's post-hoc test. ∗p < 0.05.
(C) The percentage of grafted human neuronal cells exhibiting sEPSCs and sIPSCs. Numbers of recorded cells (N) were shown on the graphs.
(D) Left, schematic drawing of the optogenetic experiment on acute slices. Right, blue light activated grafted human cells with ChR2 expression in voltage-clamp and current-clamp mode.
(E) Left, firing pattern of a neighbor host granular cell (GC). Right, light stimulation induced an increase in synaptic inputs in GC recorded in voltage-clamp mode at a holding potential of −70 mV. Sample size: 3 mice for 2 M p.t. and 6 M p.t. group, respectively; 6 mice for 4 M p.t. group; 3 mice for optogenetic assay.
The Functional Integration of Human iNPC-Derived Neurons Reinforced the Synaptic Circuits of Hippocampus in the Brain of AD Mice
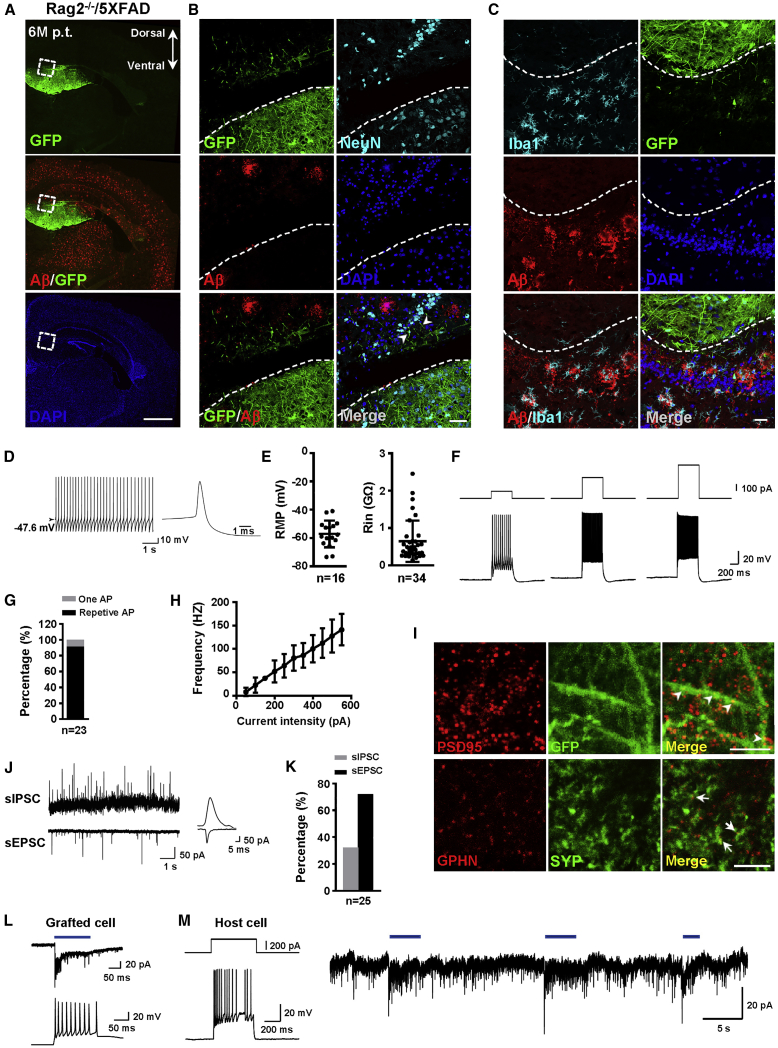
Given the functional integration of human iNPCs in WT immunodeficient mice, we next sought to determine whether they can also integrate in the brain of AD mice. To increase the survival of human iNPCs in the brain of AD mice, we crossed the well-established transgenic AD model 5XFAD mice with Rag2−/− mice, generating an immunodeficient AD mouse line, Rag2−/−/5XFAD. GFP-labeled iNPCs (passage 15) were bilaterally transplanted into hippocampus (105 cells per side) of 4-month-old Rag2−/−/5XFAD mice with abundant Aβ plaques. Six months after transplantation, abundant GFP+ grafted cells ([13.2 ± 2.8] × 104, n = 3) survived in the host hippocampus without any detectable graft overgrowth in the hippocampus or ectopic cell clusters in other regions of brain in Rag2−/−/5XFAD mice examined (n = 22) (Figure 5A). Immunostaining analysis showed that the green grafted cells were surrounded by abundant Aβ plaques, but there were no detectable Aβ plaques among GFP+ human cells (Figures 5A and 5B). The KU80+ human iNPCs were found to differentiate into NEUN+ mature neurons and in smaller numbers into GFAP+ astrocytes (Figures 5B and S4A). The long green neurites from grafted human neurons projected into the host neural cells (Figure 5B). Iba1+ microglia were exclusively associated with Aβ plaques but hardly detectable among GFP+ human cells (Figure 5C). Whole-cell recording was performed in acute slices from AD brain 6 months after transplantation and spontaneous AP firing was detected in human iNPC-derived neurons (Figure 5D). The resting membrane potential of the GFP+ neurons was around −60 mV and the input resistance was as low as 0.5 GΩ, resembling those of mature neurons (Figure 5E). Over 90% of recorded human neurons fired repetitive APs (Figures 5F and 5G). The F-I curve of human neurons exhibited gradual increases in AP frequency with current injections up to 550 pA (Figure 5H). Similar to those in WT mice, the human iNPC-derived neurons displayed mature membrane properties in the AD brain 6 months after transplantation.
Figure 5.
The Mature and Functional Integration of Human iNPC-Derived Neurons into Local Synaptic Networks of AD Hippocampus
(A) Top, the GFP+ grafted human cells in dentate gyrus on the coronal brain section of Rag2−/−/5XFAD mouse 6 M p.t. Bottom, Aβ deposits on the same brain section.
(B) The close-ups of GFP+ grafted human cells shown in (A). Arrow heads indicate that the green long fibers of human neurons in the grafts projected into the host neurons. Dashed lines indicate the borders between human cell grafts and host neurons.
(C) Immunofluorescence analysis of Iba1 in hippocampal region loaded with Aβ plaques and in GFP+ human cell grafts. Dashed lines indicate the borders between human cell grafts and host neurons.
(D) Spontaneous APs recorded in some iNPC-derived neurons. Inset, an expanded AP for clarity.
(E) The resting membrane potential (left) and input resistance (right) of human neuronal cells at 6 M p.t.
(F) AP generation in grafted human neuronal cells in response to step current injection.
(G) Percentage of human neuronal cells with one AP, or repetitive AP at 6 M p.t.
(H) The F-I curve of human neuronal cells at 6 M p.t.
(I) The green neurites from grafted iNPC-derived neurons expressing PSD95 and distributing among host hippocampal neurons at 6 M p.t. (top). Double immunofluorescence analysis of SYP and GPHN (bottom). Arrowheads indicate PSD95 dots in GFP+ human neurites. Arrows indicate co-localization of SYP and GPHN dots.
(J) Representative traces from human neuronal cells at 6 M p.t. showing sEPSCs at a holding potential of −70 mV and sIPSCs at 0 mV.
(K) The percentage of grafted human neuronal cells exhibiting sEPSCs and sIPSCs.
(L) Representative light-induced responses of grafted human cells with ChR2 expression in voltage-clamp (top) and current-clamp mode (bottom).
(M) Left, firing pattern of a neighbor host granular cell (GC). Right, light stimulation induced an increase in synaptic inputs in GC recorded in voltage-clamp mode at a holding potential of −70 mV.
Scale bars, 1 mm (A), 50 μm (B), 25 μm (C), and 5 μm (I). Sample size: 3 mice. Numbers of recorded cells (n) were shown on the graphs in (E), (G), and (K). Data are represented as means ± SD in (E) and (H). Related to Figure S4.
In addition, the grafted human neurons extensively expressed presynaptic markers SYNAPSIN1, SYNAPTOPHYSIN as well as the postsynaptic marker PSD95 (Figure S4B). Abundant green neurites with PSD95+ dots and co-localization of SYNAPTOPHYSIN+ and GEPHYRIN+ dots were detected (Figure 5I). Consistently, human neurons in AD mice received synaptic inputs from neighboring neurons by exhibiting both sEPSCs and sIPSCs (Figures 5J and 5K). Remarkably, the optogenetic assay revealed that the host hippocampal neurons received synaptic inputs when stimulating the grafted human neurons by blue light (Figures 5L and 5M), which directly indicated the synaptic transmission between grafted human neurons and host hippocampal neurons and confirmed the functional integration of human neurons into neural networks of AD mice.
All together, these results demonstrate that the grafted human iNPCs efficiently differentiate into mature neurons forming functionally synaptic connections with hippocampal neurons of the AD mice, suggesting a reinforcement of local neural circuitry upon human iNPC transplantation.
The Grafted Human iNPCs Rescued Cognitive Deficits of AD Mice
It was well documented that long-term potentiation (LTP) was severely impaired in the hippocampus of 5XFAD mice, as in numerous other AD model mice (Crouzin et al., 2013, Kimura and Ohno, 2009). LTP is considered to be an electrophysiological correlate of synaptic plasticity and LTP deficits in AD animals is associated with impaired performance in learning and memory (Chapman et al., 1999, Stephan et al., 2001). Then, we wonder whether the reinforcement in synaptic circuits upon human iNPC transplantation could have an impact on either synaptic or cognitive deficits of AD mice.
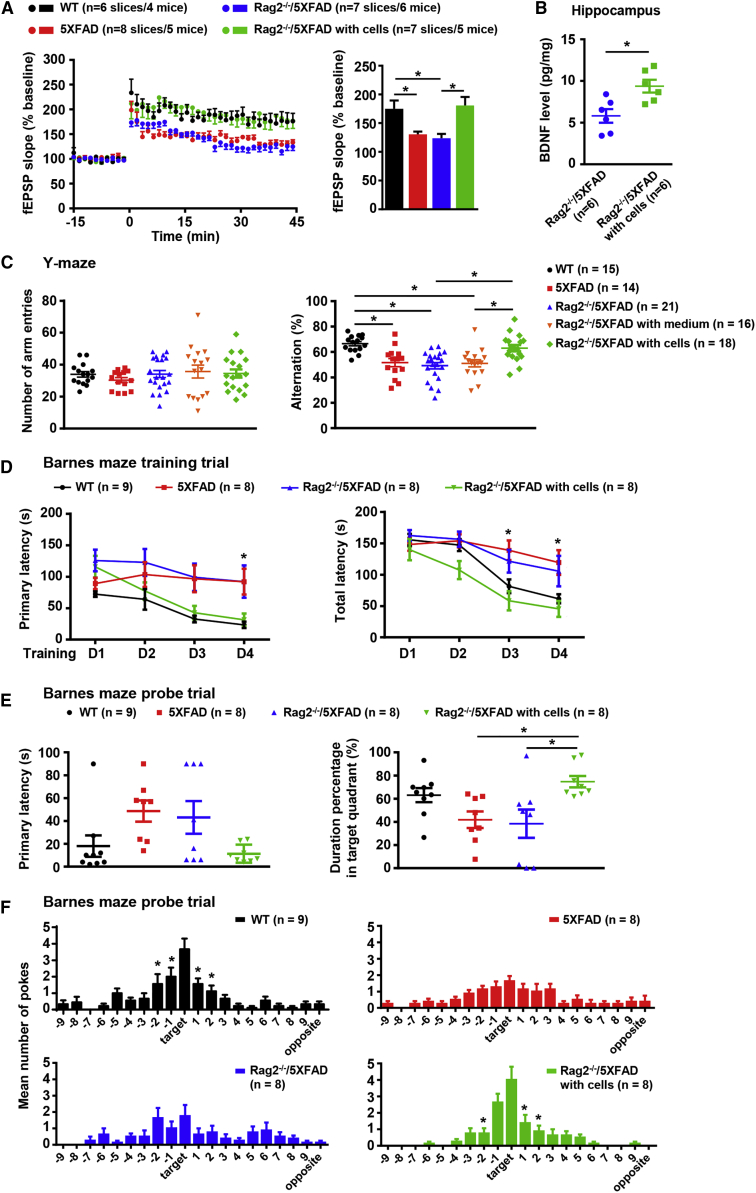
To test the synaptic plasticity of AD mice, we measured the LTP in CA3-CA1 pathway of hippocampus on brain slice from Rag2−/−/5XFAD mice with grafted human iNPCs 6 months after transplantation together with age-matched Rag2−/−/5XFAD littermates without grafted cells, 5XFAD and WT mice (Figure S5A). Grafted GFP+ human cells could be observed in the hippocampus of the coronal slices from Rag2−/−/5XFAD mice for LTP measurements (Figure S5B). Compared with WT mice, LTP induction by theta-burst stimulation in both 5XFAD and Rag2−/−/5XFAD mice was impaired, while Rag2−/−/5XFAD mice with grafted human iNPCs showed an increased LTP as high as WT mice (Figure 6A, left). One-way ANOVA comparing the average magnitude of LTP from 35 to 45 min after stimulation confirmed that both 5XFAD and Rag2−/−/5XFAD mice had an apparently disrupted LTP, whereas Rag2−/−/5XFAD mice with human iNPCs displayed an enhanced LTP comparable with that of WT mice (Figure 6A, right), indicative of improved synaptic plasticity upon transplantation.
Figure 6.
Grafted Human iNPCs Enhanced Hippocampal LTP and Rescued Cognitive Deficits of AD Mice
(A) Field potential recording at hippocampus CA3-CA1 pathway showed time course of LTP induced by Tris-buffered saline (left) and average fEPSP amplitudes (% baseline fEPSP) during the last 10 min of recording (right).
(B) The concentration of secreted BDNF by ELISA analyses in hippocampus from Rag2−/−/5XFAD mice with and without human iNPCs at 6 M p.t. Student's t test (two-tailed). ∗p < 0.05.
(C) Y-maze task was performed in five groups of mice, including WT controls, 5XFAD mice, Rag2−/−/5XFAD mice, Rag2−/−/5XFAD mice with vehicle as sham controls and Rag2−/−/5XFAD mice with human iNPCs. Left, the total numbers of arm entries. Right, percentage alternation.
(D) Training trials of Barnes maze revealed that Rag2−/−/5XFAD mice with grafted human iNPCs displayed significantly shorter escape latencies than AD mice without iNPCs.
(E) Probe trials of Barnes maze. Rag2−/−/5XFAD mice with grafted human iNPCs spent longer time in the target quadrant (right) than AD mice without cells.
(F) Number of pokes in each hole for each group of mice during the probe trial in Barnes maze.
Data are presented as the means ± SEM. One- or two-way ANOVA followed by Tukey's post-hoc test. ∗p < 0.05. Related to Figure S5.
The previous studies reported that the secretion of neural trophic factor increased in the brain of AD mice upon transplantation of neural stem cells (Blurton-Jones et al., 2009, Yue et al., 2015). Thus, we measured the secreted brain-derived neurotrophic factor (BDNF) 6 months after transplantation and found that the levels of BDNF in the hippocampus, but not whole brain, were markedly higher in Rag2−/−/5XFAD mice with human iNPCs than those without (Figures 6B and S5C), which might contribute to the enhancement of synaptic connections between grafted and host neurons.
We finally assessed the cognitive performance of grafted Rag2−/−/5XFAD mice 5 to 6 months after transplantation. Hippocampus-dependent spatial learning and reference memory abilities of WT and AD mice were measured by two behavioral tests, Y-maze spontaneous alternation task and Barnes maze (Figure S5A). In the Y-maze task, the number of arm entries during the test period was not different among groups and all groups of mice actively explored in maze (Figure 6C, left). 5XFAD mice, Rag2−/−/5XFAD mice and Rag2−/−/5XFAD with vehicle control displayed a significantly decreased alternation frequency compared with WT mice, whereas Rag2−/−/5XFAD with human iNPCs exhibited a markedly improved alternation performance reaching the level of WT mice (Figure 6C, right). To further confirm the restored cognitive abilities of AD mice, Barnes maze, a dry-land maze similar to Morris water maze, was performed subsequently. Barnes maze takes advantage of the superior abilities of rodents to find and escape through small holes and avoids strong aversive stimuli, such as water (Barnes, 1979). In Barnes maze test, spatial learning abilities were assessed by latency to reach the target hole during training trials. Grafted Rag2−/−/5XFAD mice exhibited gradually and significantly shorter latencies, including both primary and total latency, than AD controls and showed a steady improvement in learning comparable with WT mice (Figure 6D). The probe trials were performed on day 5 to assess spatial memory retention. Grafted Rag2−/−/5XFAD mice exhibited decreased primary latency (Figure 6E, left), spent more time searching in the target quadrant (Figure 6E, right) and displayed a recovery in memory abilities comparable with WT mice. In addition, the number of pokes in each hole directly showed the preference of grafted Rag2−/−/5XFAD as well as WT mice for the target hole (Figure 6F), reflecting the rescued memory abilities of grafted AD mice.
Together, these results suggest that AD mice exhibited enhancement of synaptic plasticity and evident improvement in cognitive abilities upon transplantation of human iNPCs.
Discussion
In this study, integration-free human iNPCs were generated from peripheral blood cells, which resembled brain NPCs both molecularly and physiologically. After transplantation, the human iNPCs differentiated into mature neurons in the hippocampus of adult mice, including AD mice. The functional integration of iNPC-derived neurons into the host synaptic networks contributed to enhance hippocampal plasticity and repair neural circuits of the host brain, which led to improved cognitive abilities of AD mice.
The Possible Application of Human iNPCs to Repair AD Brain
NPCs are multipotent stem cells with limited self-renewal capacity that are found in specific brain regions of mammals (Gage, 2000). Upon stimulation, brain NPCs have the potential to repair neurons lost after disease or injury, but the repairing capacity of NPCs in vivo is limited and the mechanisms underlying the activation, proliferation and differentiation of adult NPCs are poorly understood (Gage, 2000). Great efforts have been made in vitro to convert human somatic cells into iNPCs that are assumed to have invaluable potential in cell replacement therapies. With the advance of the iPSC technology and a better understanding of the reprogramming process, scientists have started to generate iNPCs from mouse or human fibroblasts via an iPSC intermediate by using transcription factors, such as four core iPSC factors alone or combined with neural lineage-specific factors, such as FoxG1, Zic3, Brn2 (Han et al., 2012, Kumar et al., 2012, Lujan et al., 2012) and with small molecules (Lu et al., 2013, Zhu et al., 2014). In parallel, attempts have been made to direct the reprogramming of human cord blood cells into iNPCs (Castano et al., 2014, Liao et al., 2015). PB MNCs, especially erythroblasts, are more favorable than fibroblasts or cord blood cells for reprogramming due to their easier accessibility, lower mutation burden and suitable epigenetic signature (Dowey et al., 2012). Recently, a study reported the generation of human iNPCs from PB MNCs of one donor using six factors (four iPS factors plus Nanog and Lin28) and two chemicals (Tang et al., 2016). Obviously, the previous studies have primarily focused on developing effective approaches to direct the neural fate conversion of somatic cells. Although the generation of functional iNPCs from human somatic cells has been proposed to be an important step toward regenerative medicine, none of the resulted iNPCs has been tested whether they can function well in vivo as in vitro.
In this study, we refined the approaches and used episomal vector-based approach to stably generate integration-free human iNPCs from a small volume (as low as 3 mL) of immobilized peripheral blood from several adults (Figures 1A and 1B). The generation of human iNPCs from PB MNCs was easy and rapid and could be in a patient-specific way if necessary. Therefore, iNPCs was more suitable as donor cells for the treatment of neurodegenerative disorders than human mesenchymal stem cells or neural stem cells from fetal brain (Lee et al., 2010, Ager et al., 2015). During iNPC generation, we found that the Yamanaka factors initiated the reprogramming of blood cells, but the four additional chemicals played vital and essential roles in the fully neural fate conversion from PB MNCs to iNPCs (Figure S1A). This observation provides clues to further modify the current approaches for iNPC generation and to uncover the molecular events underlying the switch from PB MNCs to iNPCs. Besides the systematical characterization of the human iNPCs in vitro as what previous studies did in general (Figures 1, S1, and S2), we thoroughly investigated the efficacy of human iNPCs in vivo. We found that the human iNPCs functioned properly in the host hippocampus of both WT and AD mouse (Figure 2, Figure 3, Figure 4, Figure 5). The AD mice benefited from human iNPCs transplantation and displayed improved hippocampus-dependent learning and memory abilities in two behavioral tests, the Y-maze and Barnes maze, suggesting that human iNPCs might be applicable as donor cells for treating AD (Figure 6). Thus, we not only generated the homogeneous, expandable and functional human iNPCs in a more efficient and stable manner, but also evaluated the therapeutic potential of human iNPCs in AD animals. Our results supported the notion that human iNPCs might represent a safer and more reliable material with distinct therapeutic perspective in regenerative medicine for the treatment of AD.
The Reinforcement of Synaptic and Neural Circuits by Grafted Human iNPCs in AD Brain
A better understanding of the neuropathology of AD has led to the realization that a single therapeutic strategy or intervention might not be sufficient for the recovery of cognitive impairments in AD patients. Great efforts have been made by pharmaceutical companies to develop drugs for preventing the deposition or clearing amyloid deposits in AD brain using Aβ vaccine and antibodies, but most therapeutic drugs targeting Aβ in clinic or under development have failed to rescue or slow AD symptoms. Deficits in neural circuit stabilization or integrity and synaptic plasticity at early stages of AD directly lead to cognitive dysfunction (Palop and Mucke, 2010, Selkoe, 2002). It is thought that restoring the integrity of synaptic and neural circuit might provide new directions for memory recovery in AD (Canter et al., 2016). Because of the tremendous therapeutic potential, different types of neural stem cells have been tested in AD animals for the possible therapeutic use in AD over the last decade (Ager et al., 2015, Lee et al., 2010, Liu et al., 2013, Moghadam et al., 2009). These studies have consistently reported that grafted NSCs improved to some degree the cognitive performances of AD animal models, but none of these studies have interrogated the changes in synaptic and neural circuits upon transplantation. The only exception published recently has failed to demonstrate the safety and efficacy of fetal brain-derived human NSCs (STEMCELL; HuCNS-SCs) in the Rag-5XFAD immune-deficient mouse 5 months after transplantation (Marsh et al., 2017). Thus, how the NSCs rescued the cognitive deficits of AD mouse or even whether the NSCs could be suitable for replacement therapy remain somewhat ambiguous.
In our previous study, we transplanted mouse ESC-derived BFCN progenitors into the basal forebrain of AD model mice. Two months after transplantation, the grafted BFCN progenitors differentiated into functional cholinergic neurons and the AD mice exhibited improvements in learning and memory performances (Yue et al., 2015). Further investigation showed that the grafted BFCNs expressed postsynaptic potentials and formed synapses with host neurons, which might contribute to ameliorate cognitive deficits of AD mice (Yue et al., 2015). To test different types of donor cells in different brain regions of AD mice, we performed a follow-up study here to systematically evaluate the safety of human iNPCs and address the potential of human iNPCs in repairing the disrupted synaptic networks of AD mice. First, the human iNPCs were transplanted into the hippocampus of WT and AD mice and evaluated for both short-term (1 and 2 months) and long-term (4, 6, and 12 months) observations. The Oct4 expression could not be detected among human iNPCs (Figure 1), which is critical for the safety concerns of transplantation. In addition, the human iNPCs are a homogeneous population (Figures 1, S1, and S2). The neural progenitors from ESCs or iPSCs were usually mixed and were hardly generated in a controlled way owing to the nonsynchronous differentiation of pluripotent stem cells (Liu et al., 2013, Moghadam et al., 2009). The grafted human iNPCs stopped proliferation in vivo and rapidly underwent terminal differentiation to give rise to mature neurons (Figures 2 and 5). No detectable graft overgrowth in the hippocampus or ectopic cell clusters in other regions of brain were detected at all the time points measured (39 WT mice and 22 AD mice) (Figures 2, 5, and S3), suggesting that iNPCs could safely modify the long-term progression of AD. Second, we confirmed the functional integration of grafted human iNPC-derived neurons, which in turn strengthened the impaired synaptic networks and reinforced the neural circuits in the AD brains (Figures 3, 4, 5, and 6). The human iNPC grafts were not invaded by Aβ plaques and neuroinflammation (Figure 5). Then, the iNPC-derived neurons had remained healthy for a long period of time (at least 12 months) in the hippocampus of AD mice (Figure 5). The grafted human neurons displayed high synaptic activities and received synaptic inputs from neighboring cells and functioned properly in vivo (Figures 4 and 5). In addition, the optogenetic approach in WT and AD mice revealed the graft-to-host synaptic transmission between human and murine neurons, directly elucidating that grafted human neurons functionally integrated into the synaptic circuitry (Figure 5). Consequently, grafted AD mice exhibited increased LTP and enhanced synaptic plasticity (Figure 6), suggesting that the integration of human neurons could repair the synaptic dysfunction of AD brain. These observations partially explained why grafted NPCs could alleviate the cognitive deficits of AD mice.
Unlike Parkinson's disease, the AD brain displayed global neuron loss. Thus, a single-pronged transplantation approach by using one type of donor cells to the treatment of AD may be insufficient. For that matter, the cell replacement for AD might need simultaneous transplantation of multiple types of neural cells and even with astrocytes into different brain regions, particular the cognition-relevant brain regions. So far, we had transplanted the BFCN progenitors into basal forebrain (Yue et al., 2015) and iNPCs, the glutamatergic neuron progenitors, into hippocampus of AD mice, respectively. Both types of grafted neural progenitors gave rise to mature and functional neurons that integrated into the host neural circuits and reinforced the synaptic networks. These attempts indicated that one type of grafted neural cells could target one brain region and repair the dysfunction of local neural networks of AD brain. Therefore, we deduced that various grafted NPCs could simultaneously repair multiple brain regions and ultimately alleviated the cognitive deficits of AD patients. The future study will address the establishment of transplantation strategies combining different NPCs in AD mouse and test whether different types of grafted neurons could function synergistically and improve the cognitive abilities of AD mouse better.
Together, our findings demonstrate that iNPCs derived from human peripheral blood differentiate into mature neurons in the hippocampus of host mice. These grafted human neurons functionally integrate into the host hippocampus, increase the synaptic connectivity and synaptic plasticity, reinforce the local neural circuit integrity and finally lead to the cognitive recovery of AD mice. Our study provides evidence supporting a possible therapeutic target for stem cell-based therapies, suggesting that iNPCs derived from somatic cells of individuals could be developed to restore the synaptic failures and functional impairments of AD.
Experimental Procedures
Isolation and Expansion of Human Adult PB MNCs
The use of human adult peripheral blood was approved by the Biomedical Research Ethics Committee, SIBS, CAS, and Ruijin Hospital Ethics Committee, Shanghai JiaoTong University School of Medicine, with written informed consent from the donors. Three participants were recruited in the study and designated as donor 1, 2, and 3, respectively. Isolation and expansion of PB MNCs was performed as described previously (Dowey et al., 2012). The additional details are included in the Supplemental Experimental Procedures.
Generation of iNPCs from Human Adult PB MNCs by Episomal Vector Transfection
The oriP/EBNA1-based episomal vectors EV SFFV-OCT4-2A-SOX2 (SFFV-OS), EV SFFV-MYC-2A-KLF4 (SFFV-MK), and EV SFFV-BCL-XL have been described previously (Su et al., 2013). The process for human iNPC generation is schematically summarized in Figure 1A. Two million PB MNCs were nucleofected with a mixture of the episomal vectors following the Amaxa 4D-Nucleofector Protocols for Unstimulated Human CD34+ Cells (Lonza). Subsequent steps in iNPC generation are detailed in the Supplemental Experimental Procedures.
Cell Transplantation
The animal experiments were performed following protocols approved by the Animal Ethics Committee of the Shanghai Institutes for Biological Sciences. The immunodeficient Foxn1−/− mice were purchased from Shanghai SLAC Laboratory Animal Company (Shanghai, China). The AD model mice 5XFAD (Jackson Laboratory, no. 006554) was purchased from Jackson Laboratory. Rag2−/−/5XFAD immunodeficient AD model mice were generated by crossing 5XFAD mice with Rag2−/− mice (a gift from Dr. Lijian Hui, SIBCB, China). Male mice were used in the study. GFP+ or ChR2-mCherry+ human iNPCs at passage 15 were targeted to the hippocampus DG region following coordinates relative to bregma: AP, −1.06 mm; ML, ±1.0 mm; DV, −2.5 mm. The cell transplantation was performed in immunodeficient Foxn1−/− mice at 2 months of age and Rag2−/−/5XFAD mice at 4 months of age. Details of the cell transplantation procedure are included in the Supplemental Experimental Procedures.
Electrophysiological Recording
Whole-cell patch-clamp recording was performed in human iNPC-derived neurons on differentiating day 50 (in vitro) and in EGFP+ or mCherry+ human iNPC-derived neurons in the coronal brain slices of immunodeficient mice (in vivo). The whole-cell recordings were performed with a MultiClamp 700B (Molecular Devices). The cultured cells were patched as described previously (Yue et al., 2015). The additional details of acute brain slices preparation, electrophysiological recording, optogenetic experiment and field potential recording are included in the Supplemental Experimental Procedures.
Behavioral Tests (Y-Maze and Barnes Maze)
The behavioral tests were performed in Rag2−/−/5XFAD with or without grafted human iNPCs at 9–10 months of age (5–6 months after transplantation) and age-matched WT and 5XFAD mice. Y-maze spontaneous alternation task was performed as described previously (Ohno et al., 2006). Barnes maze behavior test was performed as described previously (Sunyer et al., 2007). The additional details are included in the Supplemental Experimental Procedures.
Statistical Analysis
All statistical analyses were performed in GraphPad Prism software (GraphPad 7.0). Cell counting and electrophysiological data were presented as mean ± SD, while BDNF ELISA, behavior test, and LTP data were presented as mean ± SEM. Student's t test (two-tailed) was performed for statistical analysis between two groups. One- or two-way ANOVA with Tukey's multiple comparison post-hoc test was used when three or more groups were compared. Sample size (n) values were provided in the relevant text, figures, and figure legends. The statistical analyses were obtained from three independent experiments. Statistical significance was set at ∗p < 0.05.
Author Contributions
N.J., X.Zhang, and C.Y. initiated the study. T.Z. and C.Y. designed and performed the experiments. W.K. and Y.S. performed electrophysiological experiments. X.Zhou, Y.D., and X.C. performed behavioral test and LTP measurements. Y.Q. contributed mouse strain generation and performed cell transplantation experiments. S.F., R.W., G.C., R.T., and W.G. performed other experiments. N.J., C.Y., Y.S., and T.Z. conceived the study and interpreted results. C.Y. and Y.S. wrote the manuscript. N.J. supervised the study.
Acknowledgments
This work was supported in part by the "Strategic Priority Research Program" of the Chinese Academy of Sciences, grant no. (XDA16020501, XDA16020404), National Key Basic Research and Development Program of China (2018YFA0108000, 2018YFA0107200, 2017YFA0102700, 2015CB964500, 2014CB964804), National Natural Science Foundation of China (81671224, 31661143042, 91519314, 31630043, 31601185, 31571513, 31430058, 31471077). We are grateful to T. Li, D.M. Lai at Shanghai Jiao Tong University School of Medicine for their supporting in karyotype analysis. We would like to thank Dr. Francois Guillemot (The Francis Crick Institute, London) and Dr. Harold Gainer (NIH, Maryland) for their critical reading of the manuscript.
Published: November 21, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.10.012.
Contributor Information
Chunmei Yue, Email: cmyue@sibcb.ac.cn.
Naihe Jing, Email: njing@sibcb.ac.cn.
Accession Numbers
All RNA sequencing data are available at the GEO under accession number GSE107806.
Supplemental Information
References
- Ager R.R., Davis J.L., Agazaryan A., Benavente F., Poon W.W., LaFerla F.M., Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss. Hippocampus. 2015;25:813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C.A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M., Kitazawa M., Martinez-Coria H., Castello N.A., Muller F.J., Loring J.F., Yamasaki T.R., Poon W.W., Green K.N., LaFerla F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. U S A. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A.H., Livesey F.J. Neural stem cell biology in vertebrates and invertebrates: more alike than different? Neuron. 2011;70:719–729. doi: 10.1016/j.neuron.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Cacabelos R. Have there been improvements in Alzheimer's disease drug discovery over the past 5 years? Expert Opin. Drug Discov. 2018;13:523–538. doi: 10.1080/17460441.2018.1457645. [DOI] [PubMed] [Google Scholar]
- Canter R.G., Penney J., Tsai L.H. The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature. 2016;539:187–196. doi: 10.1038/nature20412. [DOI] [PubMed] [Google Scholar]
- Castano J., Menendez P., Bruzos-Cidon C., Straccia M., Sousa A., Zabaleta L., Vazquez N., Zubiarrain A., Sonntag K.C., Ugedo L. Fast and efficient neural conversion of human hematopoietic cells. Stem Cell Reports. 2014;3:1118–1131. doi: 10.1016/j.stemcr.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P.F., White G.L., Jones M.W., Cooper-Blacketer D., Marshall V.J., Irizarry M., Younkin L., Good M.A., Bliss T.V., Hyman B.T. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- Crouzin N., Baranger K., Cavalier M., Marchalant Y., Cohen-Solal C., Roman F.S., Khrestchatisky M., Rivera S., Feron F., Vignes M. Area-specific alterations of synaptic plasticity in the 5XFAD mouse model of Alzheimer's disease: dissociation between somatosensory cortex and hippocampus. PLoS One. 2013;8:e74667. doi: 10.1371/journal.pone.0074667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C.A., Mann D.M., Sumpter P.Q., Yates P.O. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J. Neurol. Sci. 1987;78:151–164. doi: 10.1016/0022-510x(87)90057-8. [DOI] [PubMed] [Google Scholar]
- Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- Dowey S.N., Huang X., Chou B.K., Ye Z., Cheng L. Generation of integration-free human induced pluripotent stem cells from postnatal blood mononuclear cells by plasmid vector expression. Nat. Protoc. 2012;7:2013–2021. doi: 10.1038/nprot.2012.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F.H. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Han D.W., Tapia N., Hermann A., Hemmer K., Hoing S., Arauzo-Bravo M.J., Zaehres H., Wu G., Frank S., Moritz S. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell. 2012;10:465–472. doi: 10.1016/j.stem.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hurd M.D., Martorell P., Delavande A., Mullen K.J., Langa K.M. Monetary costs of dementia in the United States. N. Engl. J. Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R., Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol. Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Declercq J., Eggermont K., Agirre X., Prosper F., Verfaillie C.M. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J. Mol. Cell Biol. 2012;4:252–255. doi: 10.1093/jmcb/mjs015. [DOI] [PubMed] [Google Scholar]
- Lee H.J., Lee J.K., Lee H., Shin J.W., Carter J.E., Sakamoto T., Jin H.K., Bae J.S. The therapeutic potential of human umbilical cord blood-derived mesenchymal stem cells in Alzheimer's disease. Neurosci. Lett. 2010;481:30–35. doi: 10.1016/j.neulet.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Liao W., Huang N., Yu J., Jares A., Yang J., Zieve G., Avila C., Jiang X., Zhang X.B., Ma Y. Direct conversion of cord blood CD34+ cells into neural stem cells by OCT4. Stem Cell Transl. Med. 2015;4:755–763. doi: 10.5966/sctm.2014-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Weick J.P., Liu H., Krencik R., Zhang X., Ma L., Zhou G.M., Ayala M., Zhang S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013;31:440–447. doi: 10.1038/nbt.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Liu H., Huang C.T., Chen H., Du Z., Liu Y., Sherafat M.A., Zhang S.C. Generation of integration-free and region-specific neural progenitors from primate fibroblasts. Cell Rep. 2013;3:1580–1591. doi: 10.1016/j.celrep.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan E., Chanda S., Ahlenius H., Sudhof T.C., Wernig M. Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc. Natl. Acad. Sci. U S A. 2012;109:2527–2532. doi: 10.1073/pnas.1121003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S.E., Yeung S.T., Torres M., Lau L., Davis J.L., Monuki E.S., Poon W.W., Blurton-Jones M. HuCNS-sc human NSCs fail to differentiate, form ectopic clusters, and provide no cognitive benefits in a transgenic model of Alzheimer's disease. Stem Cell Reports. 2017;8:235–248. doi: 10.1016/j.stemcr.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghadam F.H., Alaie H., Karbalaie K., Tanhaei S., Nasr Esfahani M.H., Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. doi: 10.1016/j.diff.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Ohno M., Chang L., Tseng W., Oakley H., Citron M., Klein W.L., Vassar R., Disterhoft J.F. Temporal memory deficits in Alzheimer's mouse models: rescue by genetic deletion of BACE1. Eur. J. Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- Palop J.J., Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat. Neurosci. 2010;13:812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe D.J. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Stephan A., Laroche S., Davis S. Generation of aggregated beta-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J. Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su R.J., Baylink D.J., Neises A., Kiroyan J.B., Meng X., Payne K.J., Tschudy-Seney B., Duan Y., Appleby N., Kearns-Jonker M. Efficient generation of integration-free iPS cells from human adult peripheral blood using BCL-XL together with Yamanaka factors. PLoS One. 2013;8:e64496. doi: 10.1371/journal.pone.0064496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer B., Patil S., Höger H., Lubec G. Barnes maze, a useful task to assess spatial reference memory in the mice. Protoc. Exch. 2007 [Google Scholar]
- Tang X., Wang S., Bai Y., Wu J., Fu L., Li M., Xu Q., Xu Z.Q., Alex Zhang Y., Chen Z. Conversion of adult human peripheral blood mononuclear cells into induced neural stem cell by using episomal vectors. Stem Cell Res. 2016;16:236–242. doi: 10.1016/j.scr.2016.01.016. [DOI] [PubMed] [Google Scholar]
- van de Leemput J., Boles N.C., Kiehl T.R., Corneo B., Lederman P., Menon V., Lee C., Martinez R.A., Levi B.P., Thompson C.L. CORTECON: a temporal transcriptome analysis of in vitro human cerebral cortex development from human embryonic stem cells. Neuron. 2014;83:51–68. doi: 10.1016/j.neuron.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Yue W., Li Y., Zhang T., Jiang M., Qian Y., Zhang M., Sheng N., Feng S., Tang K., Yu X. ESC-derived basal forebrain cholinergic neurons ameliorate the cognitive symptoms associated with Alzheimer's disease in mouse models. Stem Cell Reports. 2015;5:776–790. doi: 10.1016/j.stemcr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Ambasudhan R., Sun W., Kim H.J., Talantova M., Wang X., Zhang M., Zhang Y., Laurent T., Parker J. Small molecules enable OCT4-mediated direct reprogramming into expandable human neural stem cells. Cell Res. 2014;24:126–129. doi: 10.1038/cr.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.