Abstract
The Centers for Disease Control and Prevention, National Birth Defects Study suggests that environmental exposures including maternal thyroid diseases, maternal nicotine use, and use of selective serotonin reuptake inhibitors (SSRIs) may exacerbate incidence and or severity of craniofacial abnormalities including craniosynostosis. Premature fusion of a suture(s) of the skull defines the birth defect craniosynostosis which occurs in 1:1800–2500 births. A proposed mechanism of craniosynostosis is the disruption of proliferation and differentiation of cells in the perisutural area. Here, we hypothesize that pharmacological exposures including excess thyroid hormone, nicotine, and SSRIs lead to an alteration of stem cells within the sutures resulting in premature fusion. In utero exposure to nicotine and citalopram (SSRI) increased the risk of premature suture fusion in a wild-type murine model. Gli1+ stem cells were reduced, stem cell populations were depleted, and homeostasis of the suture mesenchyme was altered with exposure. Thus, although these pharmacological exposures can deplete calvarial stem cell populations leading to craniosynostosis, depletion of stem cells is not a unifying mechanism for pharmacological exposure associated craniosynostosis.
Keywords: Craniosynostosis, Stem cells, Gli1, Teratogens
1. Introduction
The Centers for Disease Control and Prevention (CDC), National Birth Defects Study has published data suggesting that “environmental” exposures including maternal thyroid diseases, maternal nicotine exposure, and use of selective serotonin reuptake inhibitors (SSRIs) by pregnant mothers may exacerbate incidence and or severity of craniofacial anomalies including craniosynostosis (Browne et al., 2011; Carmichael et al., 2008; Grewal et al., 2008). Thyrotoxicosis, (often transient) maternal hyperthyroidism occurs in 1 in every 500 pregnancies and has been associated with sagittal and coronal craniosynostosis in the fetus or infant (Cray Jr. et al., 2013). Despite the link between adverse birth outcomes of pre- and perinatal nicotine exposure, research suggests 11% of women in the United States continue to smoke or use alternative nicotine products through the third trimester of pregnancy (Hall, 2014; Schaal and Chellappan, 2014; Thun et al., 2013; Cardinale et al., 2012; Durham et al., 2019). Approximately 25% of depressed women continue SSRI therapy after becoming pregnant (accounting for up to 1–8% of pregnancies) (El Marroun et al., 2012; Malm et al., 2011; Olivier et al., 2011). Together these environmental exposures create an at-risk population particularly susceptible to birth defects including craniosynostosis and its associated comorbidities.
Craniosynostosis is a birth defect defined as the premature fusion of the suture(s) of the skull occurring in 1:1800–2500 births. This premature fusion of cranial sutures occurs before cessation of brain growth. When craniosynostosis is part of a syndrome, it is usually associated with facial, limb, ear, or heart malformations (Jabs, 2002), but most often occurs in isolation (nonsyndromic) (Boyadjiev, 2007). There is significant morbidity for craniosynostosis resulting from secondary effects of fusion including altered intracranial pressure and volume (Campbell et al., 1995), dilation of the subarachnoid spaces (Chadduck et al., 1992), optic nerve compression, papilledema, blindness (Miller, 2000), cognitive disabilities, and mental retardation (Miller, 2000; Arnaud et al., 1995). These severe abnormalities pose extensive, costly, and recurrent clinical/surgical management problems imposing significant emotional and financial burdens upon patients and families (Chatterjee et al., 2009; Esparza and Hinojosa, 2008). In the overwhelming majority of craniosynostosis cases, the specific etiology is unknown as it can result from intrinsic genetic (i.e. mutations in FGFR, TWIST, TGFβ), extrinsic environmental factors as indicated above, or gene-environment interactions (Robin, 1999; Durham et al., 2017a).
A proposed mechanism of craniosynostosis is the disruption of the balance of proliferation and differentiation of the osteogenic precursors in the perisutural area leading to bone growth within cranial sutures (Johnson and Wilkie, 2011; Agresti and Gosain, 2005; Fong et al., 2004; Opperman et al., 2000; Opperman and Rawlins, 2005; Passos-Bueno et al., 2008; Rawlins and Opperman, 2008; Yokota et al., 2014). The balance of cell types within the perisutural space is tightly controlled to maintain the fibrous tissue (capsular layer) between bone fronts (cambium layer) that is vital for proper brain and skull growth and development. Recently, undifferentiated cells identified as Gli1+ CD44+ Sca1+ have been identified in craniofacial bones and sutures (Zhao et al., 2015). Further, ablation of these undifferentiated cells has been correlated with fusion of the sutures (craniosynostosis). Since the population of undifferentiated cells in this area has been found to decrease with age, and suture fusion may occur naturally after growth is complete, it is possible that maintenance of this population is necessary for suture patency (Zhao et al., 2015; Maruyama et al., 2016; Wilk et al., 2017).
Here we sought to determine if environmental exposure to pharmacological agents known to increase the risk of craniosynostosis including those used to treat maternal thyroid disorder (levothyroxine), mimic maternal nicotine use, and maternal use of SSRI (citalopram) can specifically affect the stem cells resident to the newly defined suture stem cell niche. Our hypothesis, based on the CDC data, was that these pharmacological exposures would deplete stem cells resident to the calvarial sutures, precipitating premature suture fusion and abnormal craniofacial form.
2. Materials and methods
2.1. Pharmacological exposure animal model
Adult wild type, C57BL6 (Mus musculus, Jackson Laboratories, Bar Harbor, ME) male and female mice were utilized to produce in utero exposed litters. Control litters were produced from breeding pairs that were not exposed to any pharmacological agents (n=49 pups). Levothyroxine (Synthroid, 1 μg/day) was added to the drinking water of pregnant dams from E13-E20, a critical period for craniofacial development (n=19 pups) (Rasmussen et al., 2008; Krause et al., 2015; Capuco et al., 1999; Thordarson et al., 1992; Capelo et al., 2008; Darnerud et al., 1996; Lamb et al., 1986; Lamberg et al., 1986; Morriss-Kay and Wilkie, 2005; Maxson and Ishii, 2008; Holmes et al., 2015). Nicotine (Sigma Aldrich, St. Louis, MO, N3876, 200μg/ml) was diluted in drinking water per published methodology throughout pregnancy until birth (n=36 pups) (Durham et al., 2019; Alkam et al., 2013; Dodmane et al., 2014; Renda and Nashmi, 2014; Chistyakov et al., 2010; Klein et al., 2003). Citalopram (500μg/day) was also diluted in drinking water from E13-E20 (n=25 pups) (Holmes et al., 2015; Strekalova et al., 2006; Rantamaki et al., 2007; Jiao et al., 2011; Morrison and Spradling, 2008). All treatments were removed at birth of pups. Dosage was identified as midrange for preclinical studies in murine models, were replaced as needed to provide ad libitum access, and are based upon an average consumption of 4 ml per day for a pregnant dam. Resulting pups were grown to postnatal day 15 when they were sacrificed, and skulls were fixed with 4% paraformaldehyde, then switched to 70% Ethanol for micro-computed tomography (μCT) and finally processed for paraffin-based histology. Animal use protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee (AR#3510). All breeding procedures were carried out in an Association for Assessment and Accreditation of Laboratory Animal Care International accredited facility where all husbandry and related services are provided by the Division of Laboratory Animal Resources. Dams were exposed to only one of the pharmacological agents. All procedures and the reporting thereof are in compliance with the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines (Kilkenny et al., 2010).
2.2. Micro-computed tomography
μCT images were obtained on postnatal day 15 mouse skulls with a SkyScan 1174 (Kontich, Belgium) at a 22.57 μm voxel resolution. Scans were obtained on 129 animals. Mouse skulls were reconstructed with NRecon v1.6.4.8 (BrukermicroCT, Kontich, Belgium) as previously described (Parsons et al., 2014; Howie et al., 2016). Threshold settings were then set to only visualize bone volume within the skull. The width of the coronal and posterior interfrontal sutures were measured per published methodology at 25, 50, and 75% of its length as previously described (Howie et al., 2016). Width was defined as the distance between bony fronts.
2.3. Immunohistochemistry
For immunohistochemistry, representative samples (n=3) from each group (Control, levothyroxine, nicotine, citalopram) were decalcified in 0.25 M EDTA at pH 7.4 for 10 days. Skulls were then dehydrated in a graded series of ethyl alcohol (70–100%), cleared in xylene, and embedded in paraffin. Prior to embedding, the calvaria was removed from the cranial base and bisected into anterior and posterior portions anterior to the coronal suture. The anterior section was embedded to facilitate sagittal sectioning of the posterior interfrontal suture. The posterior portion was bisected along the sagittal suture to facilitate cutting through the coronal suture. Samples were sectioned on a rotary microtome and 6 μm sections were mounted on Superfrost Plus slides (ThermoFisher Scientific, Waltham, MA). Slides were subjected to an endogenous peroxidase activity block with 3% hydrogen peroxide and then washed sections were blocked in 1% goat serum or 1% donkey serum with 1% bovine serum albumin. Sections were incubated with the following primary antibodies at 4 °C overnight: Gli1 (Novus Biologicals, Centennial CO, USA, NBP1–78259, 1:500), CD44 (AbCam, Cambridge, MA, USA, ab157107, 1:100), Twist (ab50581, 1:500), Vimentin (ab11256, 1:250), Alkaline Phosphatase (ALP) (ab108337, 1:250), Active Caspase 3 (Caspase) (Ab2302, 1:75), Proliferating Cell Nuclear Antigen (PCNA) (ab18197, 1:3000). Sections were washed and incubated with HRP conjugated secondary antibody for one hour (ab6721, ab6885). Diaminobenzidine (DAB) (Vector Laboratories, Bulingame, CA) chromagen was used to identify immunoreactive structures. Suture area was digitally isolated for direct comparison between control and exposed individuals. At least 3 sections 30 μm apart per individual per treatment for each target were analyzed using Image J Software and the IHC Profiler Open Source Plugin for automated scoring of percent positivity as a means of normalizing to differing suture areas between control and exposed (Durham et al., 2019; Howie et al., 2016; Varghese et al., 2014; Durham et al., 2017b).
2.4. In Vitro cell treatment
Primary, wild type suture cells were isolated as previously described (Durham et al., 2019; Durham et al., 2016), plated at a density of 65,000 cells per well in 6 well plates and treated for 7 days with control media (DMEM, supplemented with 10% FBS, 1% penstrep, and 0.2% amphotericin) or media supplemented with pharmacological agents (levothyroxine (780 ng/ml), nicotine (25 ng/ml), or citalopram (250 ng/ml)) at levels mimicking circulating levels for 7 days (Durham et al., 2019; Durham et al., 2015). Subsequently, total RNA was isolated using the Qiagen RNEasy mini kit (Qiagen, Valenica, CA, USA) according to manufacturer’s protocol. Quantity and quality of RNA was assessed using a Synergy H1 Microplate reader and a Take3 Microvolume Plate (BioTek, Winooski, VT, USA). Complimentary DNA Synthesis was performed using Superscript II Reverse Transcriptase and random hexamer primer following manufacturers protocol (ThermoFisher Scientific). cDNA was subjected to quantitative PCR using Applied Biosystems TaqMan Gene Expression Master Mix and targeted TaqMan gene expression assays for stem cell related targets: CD44 (Mm01277161_m1), Ly6a (Mm00726568_s1), Gli1 (Mm00494654_m1), Nanog (Mm02019550_s1), Twist (Mm00442036_m1), Notch1 (Mm00627185_m1), Notch2 (Mm00803077_m1), Notch3 (Mm01345646_m1), Fut4 (Mm00487448_s1), Fzd9 (Mm01206511_s1), Stgal (Mm00486123_m1) (Maguire et al., 2013). Data were normalized to 18S (Mm03928990_g1) ribosomal RNA expression by ΔCT. Quantitative data were compared to control (n=16) for gene expression change due to treatment (levothyroxine n=14, nicotine n=13, citalopram n=10) by AACT methodology. We used statistical analyses for qrt-PCR data as previously published to determine statistical differences for gene expression after pharmacological exposures for targets of interest (Yuan et al., 2006). Differences were considered significant if p≤.05.
For flow cytometry, cell populations were split at passage 2 or 3 and treated for 7 days with control media, or media supplemented as above with pharmacological agents (levothyroxine n=13 coronal, n=8 posterior interfrontal, nicotine n=15 coronal, n=8 posterior interfrontal, citalopram n=11 coronal, n=8 posterior interfrontal). Cells were stained with the following antibodies: Sca1 (Ly6A, eBioscience, Waltham, MA, USA 25–5981-81, 1:2000), CD44 (BD Pharmingen, San Jose, CA, USA, 561859, 1:1000), CD45 (eBioscence, 61–0451-80,1:4000), CD34 (eBiosceince, 48–0341-80, 1:500). Zombie Violet Viability Dye (Biolegend, San Diego, CA, USA, 423114, 1:500) was used for counter staining. Cells were analyzed using the BD LSRFORTESSA flow cytometer (BD Biosciences, San Jose, CA, USA,). FlowJo v10 software was used to quantify the cell population of interest (CD44+, Sca1+, CD45−, CD34−) by both absolute number of cells, percentage of population, and median fluorescent intensity comparing to unstained control cell populations. All resources have been identified within the key resources table.
Key resources table.
| Reagent or resource | Source | Identifier |
|---|---|---|
| Antibodies | ||
| Rabbit Polyclonal Anti-Gli1 | Novus biological | NBP1–78259 |
| Rabbit Polyclonal Anti-CD44 | AbCam | Ab157107 |
| Rabbit Polyclonal Anti-Twist | AbCam | Ab50581 |
| Goat Polyclonal Anti-Vimentin | AbCam | Ab11256 |
| Rabbit Recombinant Monoclonal Anti-Alkaline Phosphatase, Tissue Non-Specific | AbCam | Ab108337 |
| Rabbit Polyclonal An6ti-Cleaved Caspase-3 | AbCam | Ab2302 |
| Rabbit Polyclonal Anti-Proliferating Cell Nuclear Antigen | AbCam | Ab18197 |
| Goat Anti-Rabbit IgG (HRP) | AbCam | Ab6721 |
| Donkey Anti-Goat IgG (HRP) | AbCam | Ab6885 |
| Ly6A/E (Sca1) Monoclonal Antiboidy (D7) PE-Cyanine7 | eBioscience | 25–5981–81 |
| CD44 Monoclonal Antibody FITC | BD Pharmingen | 561859 |
| CD45 Monoclonal Antibody (30-F11) PE-eFluor 610 | eBioscience | 61–0451–80 |
| CD34 Monoclonal Anitbody (RAM34) eFluor 450 | eBioscience | 48–0341–80 |
| Zombie Violet Viability Dye | Biolegend | 423,114 |
| Bacterial and virus strains | ||
| Biological samples | ||
| Chemicals, peptides, and recombinant proteins | ||
| Levothyroxine | Synthroid | |
| Nicotine | Sigma aldrich | N3876 |
| Citalopram | Celexa | |
| Critical commercial assays | ||
| TaqMan Gene Expression Assay CD44 | Applied biosystems | (Mm01277161_m1) |
| TaqMan Gene Expression Assay Ly6a | (Mm00726568_s1) | |
| TaqMan Gene Expression Assay Gli1 | (Mm00494654_m1) | |
| TaqMan Gene Expression Assay Nanog | (Mm02019550_s1) | |
| TaqMan Gene Expression Assay Twist | (Mm00442036_m1) | |
| TaqMan Gene Expression Assay Notch1 | (Mm00627185_m1) | |
| TaqMan Gene Expression Assay Notch2 | (Mm00803077_m1) | |
| TaqMan Gene Expression Assay Notch3 | (Mm01345646_m1) | |
| TaqMan Gene Expression Assay Fzd9 | (Mm01206511_s1) | |
| TaqMan Gene Expression Assay Stgal | (Mm00486123_m1) | |
| TaqMan Gene Expression Assay 18 s | (Mm03928990_g1) | |
| Deposited data | ||
| Experimental models: cell lines | ||
| Experimental models: organisms/strains | ||
| Mouse: C57BL6 | Jackson laboratories | Stock # 000664 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| CTAN | SkyScan | |
| NRecon v1.6.4.8 | SkyScan | |
| LSRFORTESSA Fow cytometer | BD | |
| FlowJo v10 | FlowJo | |
| SPSS 23.0 | IBM | |
| Other |
2.5. Statistics
Previous pharmacological studies in our laboratory suggested an n=18 per group to achieve sufficient power for our ex vivo measures (α=0.05, β=0.80, r > 0.40) (Cray et al., 2014). Measures were screened for normality and homogeneity of variance and subjected to Student’s t-test to compare effects by exposure; p≤.05 was considered significant. All statistical analyses were completed using SPSS 23.0 (IBM, Armonk, NY, USA). Data are represented as mean ± standard error of the mean.
3. Results
3.1. Pharmacological exposures alter craniofacial development
Representative μCT reconstructions of post-natal day 15 skulls from mouse pups exposed in utero to levothyroxine (~1 μg/day), nicotine (~800 μg/day), and citalopram (~500 μg/day) demonstrate dysmorphology as compared to control (Fig. 1A). While there was no increase in suture fusion risk associated with the levothyroxine exposure, posterior interfrontal suture width was reduced (Table 1, Fig. 1B). Nicotine exposure significantly increased the risk of premature posterior interfrontal suture fusion (Odds Ratio (OR)=3.08) (p=.014), however no significant change in either suture width was observed. As indicated by the dramatic dysmorphology of the citalopram exposed individual (Fig. 1A), suture fusion risk was significantly increased for both the coronal (OR=72) and posterior interfrontal sutures (OR=6.11) (p < .001). This increased fusion risk was correlated with significant narrowing of both sutures (p < .001).
Fig. 1.
Pharmacological exposures alter craniofacial development.
A) Representative μCT reconstructions of skulls, control (no exposure), levothyroxine (1 μg/day), nicotine (800 μg/day), and citalopram (500 μg/day) demonstrate gross dysmorphology with in utero pharmacological exposure. Vertical arrows indicate aberrant coronal sutures and horizontal arrows indicate posterior interfrontal sutures. B) Suture width measured on 2D slices of μCT scans at 25, 50 and 75% of each suture length indicates narrowing of the posterior interfrontal suture for levothyroxine exposed, and for both sutures in individuals exposed to citalopram. **=p < .01, ***=p < .001.
Table 1.
Aberrant suture fusion risk with pharmacological exposure.
| Coronal | Posterior interfrontal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Fused | Total | % | p-value | Relative risk | Fused | Total | % | p-value | Relative risk | |
| Control | 1 | 49 | 2.04 | 19 | 48 | 39.6 | ||||
| Levothyroxine | 0 | 19 | 0 | 6 | 19 | 31.6 | 0.732 | |||
| Nicotine | 0 | 36 | 0 | 24 | 36 | 66.7 | 0.014 | 1.907 | ||
| Citalopram | 15 | 25 | 60 | <0.001 | 5.44 | 20 | 25 | 80 | <0.001 | 3.48 |
3.2. Pharmacological exposures can decrease stem cells presence within calvarial sutures
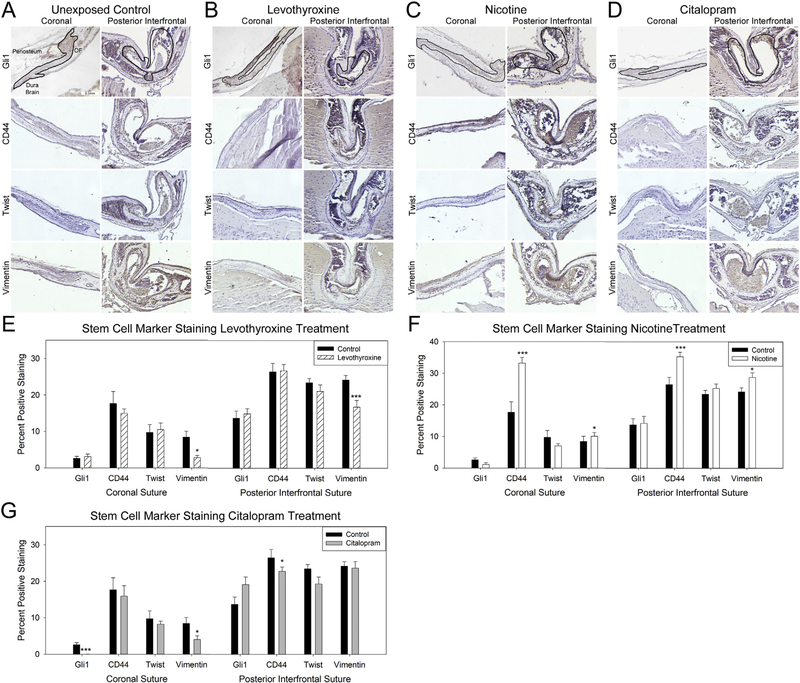
Staining for markers associated with stem cells including Gli1, CD44, Twist and Vimentin allowed for a suture specific assessment of stem cells. Staining indicated a reduction in vimentin within the suture mesenchyme of both the coronal (p=.037) and posterior interfrontal sutures (p < .001) exposed to levothyroxine compared to control (Fig. 2A–B,E). Conversely, nicotine exposure increased presence of CD44 (coronal p < .001, posterior interfrontal p < .001) and vimentin (coronal p=.043, posterior interfrontal p=.024) positivity within both sutures as compared to control (Fig. 2 A, C, F). Quantification of stem cell marker staining in sutures from individuals exposed in útero to citalopram indicated a significant decrease in stem markers in both sutures. Specifically, Gli1 (P < .001) and vimentin (p=.032) were reduced in the coronal suture, and CD44 (p < .001) was reduced in the posterior interfrontal suture (Fig. 2 A, D, G).
Fig. 2.
Pharmacological exposures can decrease stem cell presence within calvarial sutures.
Representative coronal (left) and posterior interfrontal (right) sutures from control (no exposure) (A), levothyroxine (1 μg/day) (B), nicotine (800 μg/day) (C), and citalopram (500 μg/day) (D) in utero exposed post-natal day 15 mouse pups. Sutures have been outlined in the Gli1 stained (top row) and CD44 (second row), Twist (third row), and Vimentin (bottom row) are all displayed with periosteum above, dura, and brain below with osteogenic fronts (OF) on either side. Quantification of positive staining for levothyroxine (E), nicotine (F), and citalopram (G) exposed as compared to control indicates variation of stem cell marker presence with exposure. n=3 specimen × 3 sections / exposure / target *p < .05, **p < .01, ***p < .001.
3.3. Pharmacological exposures can alter cell activity within calvarial sutures
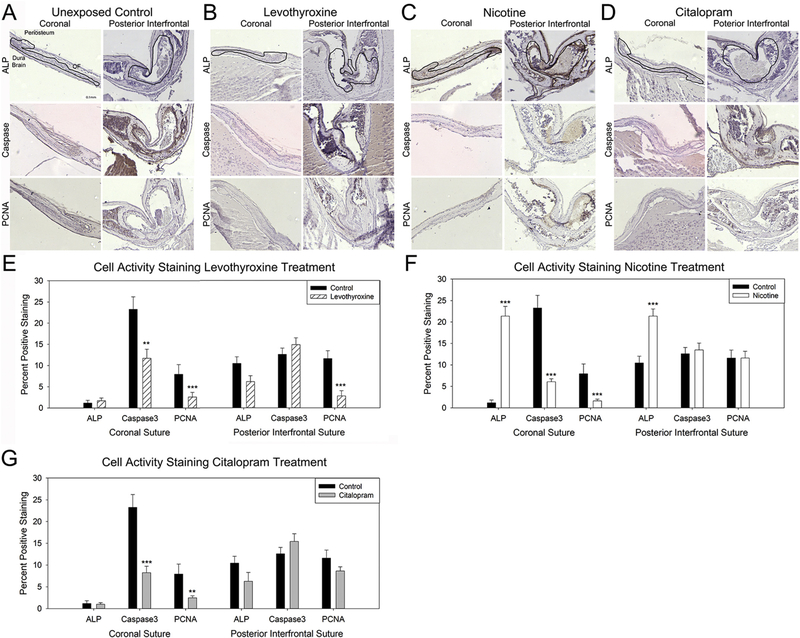
Staining for markers associated with cell activity including osteogenic differentiation via ALP, apoptosis (Caspase), and proliferation (PCNA) allowed for an assessment of activity within the sutures. Staining indicated a reduction in both apoptosis within the coronal suture (p=.003) and proliferation within both the coronal (p < .001) and posterior interfrontal (p < .001) sutures with in utero exposure to levothyroxine (Fig. 3A–B,E). Osteogenic differentiation as indicated by ALP staining was not affected by levothyroxine exposure. Interestingly, exposure in utero to nicotine did result in a dramatic increase in ALP staining for both the coronal (p < .001) and posterior interfrontal (p < .001) sutures. Apoptotic (p < .001) and proliferative (p < .001) activity decreased in the coronal sutures of individuals exposed in utero to nicotine as compared to unexposed controls (Fig. 3 A, C, F). For the citalopram exposure, cell differentiation, apoptosis, and proliferation were not changed in the posterior interfrontal suture with exposure, however both apoptotic (p < .001) and proliferative (p=.008) activities were reduced in the coronal suture with citalopram exposure (Fig. 4 A, D, G).
Fig. 3.
Pharmacological exposures can alter cell activity within calvarial sutures.
Representative coronal (left) and posterior interfrontal (right) sutures from control (no exposure) (A), levothyroxine (1 μg/day) (B), nicotine (800 μg/day) (C), and citalopram (500 μg/day) (D) in utero exposed post-natal day 15 mouse pups. Sutures have been outlined in the alkaline phosphatase (ALP) stained (top row) and active caspase-3 (caspase, second row), and proliferating cell nuclear antigen (PCNA, bottom row) are all displayed with periosteum above, dura, and brain below with osteogenic fronts (OF) on either side. Quantification of positive staining for levothyroxine (E), nicotine (F), and citalopram (G) exposed as compared to control indicates variation of cell activity markers with exposure. n=3 specimen × 3 sections / exposure / target **p < .01, ***p < .001.
Fig. 4.
Pharmacological Treatment can Decrease Expression of Stem Cell Related mRNA.
Heterogeneous cells isolated from calvarial sutures treated in vitro with levothyroxine (780ng/ml) (A), Nicotine (25ng/ml) (B), and citalopram (250ng/ml) demonstrate differential stem cell related gene expression when compared to cells treated with control media. Note the down-regulation of CD44 and Nanog mRNA with nicotine treatment and CD44 and Twist mRNA with citalopram treatment as compared to control. Control n=16, levothyroxine n=14, nicotine n=13, citalopram n=10. *p < .05, **p < .01.
3.4. Pharmacological treatment can decrease expression of stem cell related mRNA
Cells isolated from the calvarial sutures demonstrate down-regulation of stem cell related mRNA expression with in vitro treatment with nicotine (25ng/ml), and citalopram (250ng/ml). Expression of stem cell related genes including the calvarial stem cell specific marker Gli1 were unchanged with levothyroxine (780ng/ml) treatment (Fig. 4A). CD44 and Nanog gene expression were significantly reduced with nicotine treatment (p=.0055, p=.037 respectively) (Fig. 4B). Similarly, treatment with citalopram reduced CD44 and Twist1 gene expression significantly (p=.012, p=.0207 respectively) (Fig. 4C).
3.5. Pharmacological treatment can decrease CD44+, Sca1+, CD34−, CD45− stem cells
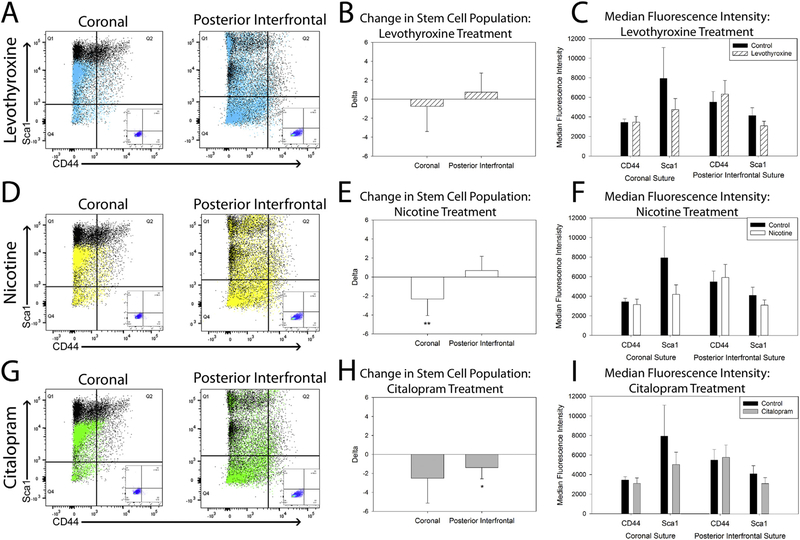
Heterogeneous cells isolated from the coronal and posterior interfrontal sutures exposed in vitro to teratogens demonstrate a reduction of cells with the stem phenotype CD44+, Sca1+, CD34−, CD45−. Levothyroxine treatment did not significantly alter the cell population of interest as compared to control untreated cells (Fig. 5 A–C). Nicotine treatment reduced the stem cell population in isolates from the coronal suture (p=.006) (Fig. 5 D–F). Citalopram treatment reduced the stem population of interest in isolates from the posterior interfrontal suture (p=.04) (Fig. 5 G–I). Overall, there was a trend towards reduction of median fluorescent intensity with each exposure particularly for the Sca1 positive population of cells (Fig. 5 C, F, I).
Fig. 5.
Pharmacological Treatment can decrease CD44+, Sca1+, CD34−, CD45−stem cells.
Representative plots of the stem cell population of interest (CD44 horizontal axis, Sca1 Vertical axis) from coronal (left) and posterior interfrontal (right) suture derived cells (Black=Control) overlaid with cells treated in vitro with levothyroxine (780 ng/ml) (A), nicotine (25 ng/ml) (D), and citalopram (250 ng/ml) (G) with unstained control (inset) for comparison (A, D, G). Stem cell populations remain unchanged after 7 days of in vitro treatment with levothyroxine (B). Coronal suture derived stem cells are reduced with nicotine treatment (E) and posterior interfrontal derived stem cells are reduced with citalopram treatment (H). Median fluorescent intensity for Sca1 trends down with exposure as indicated by the shift in the populations on the representative plots (C, F, I). Levothyroxine n=13 coronal, n=8 posterior interfrontal, nicotine n=15 coronal, n=8 posterior interfrontal, citalopram n=11 coronal, n=8 posterior interfrontal, *p < .05, **p < .01.
4. Discussion
Our assessment of this model of environmental exposure to pharmacological agents used to treat maternal thyroid disorder (levothyroxine), mimic maternal nicotine use, and maternal use of antidepressant SSRIs (citalopram) indicates that exposure to these agents can dramatically alter craniofacial development and stem cells. Similarities between human and murine craniofacial development make the wild-type model used here appropriate for this investigation. Without the complicating genetic predisposition to premature suture fusion, we are able to more specifically interrogate the effects of these pharmacological agents on suture maintenance (Durham et al., 2016). As it is possible that thyroid disorder and depression could spontaneously occur during pregnancy while nicotine use is more likely to occur before, after, and throughout gestation we have modeled clinically relevant conditions though our dosing schematics were different depending on the teratogen of interest. Further, a more specific assessment of the suture histologically may have revealed that rather than being fused, the suture was merely narrowed and thus not restricting craniofacial growth. This is a limitation of the thresholding and pixel size used for this μCT assessment that may reflect a need for higher resolution scanning in humans as histological investigation is inappropriate in this population. With higher resolution scanning, surgical teams can be more confident that suture growth restriction is occurring before neurosurgical intervention (Howie et al., 2016). Since the coronal suture normally remains patent in mice, the dramatic fusion rate identified with the citalopram exposure highlights a potential danger of using this medication during the period of pregnancy most closely associated with craniofacial development.
Narrowing the histological assessment of these two calvarial sutures to the suture mesenchyme specifically allowed for a characterization of cells within the fibrous space between osteogenic fronts, the capsular layer. Additionally, investigating the posterior interfrontal suture, which undergoes natural fusion around the second week of murine life provided a contrast to the normally patent coronal suture, and is the major reason for assessing these sutures at post-natal day 15 (Bradley et al., 1996; Grova et al., 2012). Further, these sutures are derived from different embryological origins; posterior interfrontal is neural crest derived while the coronal suture is head mesoderm derived (Opperman, 2000). Thus, differences between these sutures may be driven by embryological origin, natural reduction in stem cell presence due to fusion, or a multitude of other factors. Our analysis confirmed the presence of cells with a stem phenotype within the suture mesenchyme of both the coronal and posterior interfrontal sutures in correlation with the initial characterization of the calvarial sutures as a niche for stem cells (Zhao et al., 2015). Quantification of percent of positive staining within the suture mesenchyme allowed normalization of the data across multiple sizes of suture as indicated by the μCT assessment.
Vimentin, a marker associated with epithelial to mesenchymal transition and neural derived stem cells is reduced with levothyroxine exposure (Zhang and Jiao, 2015). This reduction may signal early transition of cells from a stem phenotype and may perhaps indicate early quiescence of cells within the fibrous sutures (Howie et al., 2016; Durham et al., 2017b). Vimentin and CD44 were enriched in the nicotine exposed sutures which may reflect the noted stimulatory effect of nicotine (Berrettini and Lerman, 2005; Giovino et al., 2012). Interestingly, only the citalopram exposure, which is associated with the most dramatic craniofacial abnormalities in this study, resulted in a reduction of Gli1, a defining characteristic of stem cells resident to the calvarial suture niche (Zhao et al., 2015). Additionally, vimentin and CD44 were reduced with the citalopram exposure indicating that cells within the suture mesenchyme may be a heterogeneous population presenting a spectrum of stem related phenotypes. Niches are defined by their ability to maintain a stem cell population within a local tissue. Signaling between cells within and without of a particular niche is vital to the maintenance of the microenvironment (Morrison and Spradling, 2008). Perhaps by affecting multiple stem related markers, citalopram exposure precipitated the most dramatic abnormalities.
Homeostasis of cells within the suture mesenchyme is vital to maintenance of the fibrous suture space between osteogenic fronts. Since nicotine is a known stimulant (Schaal and Chellappan, 2014; Thun et al., 2013; Cardinale et al., 2012), levothyroxine is known to preferentially affect cartilage cells (Howie et al., 2016; Durham et al., 2017b; Durham et al., 2016), citalopram is known to affect bone cells (Howie et al., 2018), understanding the cell activity with regard to osteogenic differentiation, programmed cell death, and proliferation is important to understanding how these pharmacological agents affect cells within the calvarial sutures. Immunohistochemical assessment of cell activity within the suture mesenchyme after levothyroxine exposure indicated a decrease in proliferation across both sutures, and a reduction in apoptotic activity compared to control in the coronal suture. Several hypotheses for the pathogenesis of craniosynostosis focus on imbalances between the processes of cell proliferation and apoptosis of the cells that comprise the suture mesenchyme (Johnson and Wilkie, 2011; Agresti and Gosain, 2005; Fong et al., 2004; Opperman et al., 2000; Opperman and Rawlins, 2005; Passos-Bueno et al., 2008; Rawlins and Opperman, 2008; Yokota et al., 2014). In a normal developing skull, a balance exists between cell proliferation and apoptosis; whereas, in the craniosynostotic skull, cell proliferation is believed to be a dominant and disruptive force.
Nicotine exposure also reduced apoptotic and proliferative activity in the coronal suture, but increased osteogenic differentiation as measured by alkaline phosphatase activity in both sutures. This increase in osteogenic differentiation is consistent with an increase in bone in these areas as suggested by the increased risk of posterior interfrontal suture fusion observed with this exposure. Citalopram exposure had an expected effect of reducing both proliferation and apoptosis in the coronal suture (Durham et al., 2015), but not in the posterior interfrontal suture. The tight coordination of cellular proliferation and differentiation are known to be important for maintenance of the patent cranial sutures and eventual or pathological fusion. It is also important to note that calvarial sutures are 3-dimensional structures. As fusion may occur along the length and depth of a suture, a disruption of homeostasis within just a small part of this vital growth site may result in premature fusion and eventual disruption of normal growth and development. Overall, each of these pharmacological agents affect both craniofacial form, and structure and function of the calvarial sutures.
To assess whether these pharmacological exposures targeted stem related gene expression in addition to antigen (protein) presence, we assessed expression of a variety of classically stem associated genes in heterogeneous cells isolated from murine calvarial sutures (Maguire et al., 2013). As no differences in expression profile were noted between populations of cells isolated from differing sutures, the data were collapsed to better assess gene expression in this heterogeneous population. Again, the levothyroxine exposure indicated no change as compared to control further validating that the effects of levothyroxine on murine calvarial growth may be time and region specific as well as being less dramatic than the other exposures investigated here (Howie et al., 2016; Durham et al., 2017b). Though the nicotine exposure drove an increase in presence of CD44 within the calvarial sutures, expression of CD44 and other genes associated with stem phenotypes were reduced. This may indicate that nicotine drives proliferation of all phenotypes within the suture leading to altered signaling for stem cell maintenance, perhaps due to hyperplasia. The proliferative effects of nicotine may also act on the diverse cell types within the suture mesenchyme to varying degrees causing altered cell to cell signaling. Citalopram exposure reduced expression of CD44, a classical mesenchymal stem cell marker, and Twist. Reduction of Twist, as in the Twist+/− murine model of craniosynostosis, is associated with dramatic craniofacial abnormalities including suture fusion, and has already been correlated with a reduction in suture specific stem cells (Zhao et al., 2015).
To date, suture stem cells have been characterized by the presence of a single marker (Gli1, Axin2, Prx1) rather than by a more definitive phenotype encompassing multiple factors (Zhao et al., 2015; Maruyama et al., 2016; Wilk et al., 2017). Though overlap between Gli1, Axin2 and Prx1 populations of cells resident to the suture mesenchyme has been identified, the specific phenotype of stem cells in the suture niche remains unclear. We sought to more specifically define stem cells within the calvarial sutures, and to determine if this specifically defined population was sensitive to pharmacological exposure. Based upon the initial characterization of this population, we chose Sca1 (Ly6a) and CD44 as our positive markers of stem cells and CD45 and CD31 as exclusionary markers to exclude hematopoietic and immune cells from the population of calvarial derived stem cells that have been identified as mesenchymal (Zhao et al., 2015).
Again, data from the levothyroxine exposure indicated no change from control correlating with the assessment that the effects of exogenous thyroid hormone exposure are time and dose specific, and can be at least partially alleviated by compensatory growth (Howie et al., 2016; Durham et al., 2017b). Interestingly, the effects of the nicotine exposure on the specific CD44+, Sca1+, CD34−, CD45− stem phenotype cells were most dramatic in the coronal suture derived populations. This may be due to the patency of this suture as compared to the fusing posterior interfrontal suture that may contain relatively fewer cells with a stem phenotype. The shift in the intensity of staining, particularly for Sca1, indicates a reduction in the presence of that specific stem cell marker further indicating a change in the phenotype of the heterogeneous cell population resident to the sutures. Citalopram exposure reduced the stem population of cells from the posterior interfrontal suture in correlation with the dramatic phenotype noted with this exposure. The variability observed in this assessment is the result of inter-individual variability. Importantly, each pharmacologically treated population of cells was compared to a matching untreated population of cells isolated from the same individual. Taken together, these data indicate that the population of cells resident to calvarial sutures is heterogeneous and the balance of cell types within this undifferentiated space may be specific to each individual precipitating the spectrum of suture fusion observed (Durham et al., 2017a). Environmental exposures can affect cells within the calvarial sutures in a cell type specific manner, including by depleting resident suture stem cells.
5. Conclusions
Together, these data indicate that environmental exposures, such as these pharmacological interventions can precipitate abnormal craniofacial growth. This corroborates the CDC data which indicates that maternal thyroid disorder, nicotine use, and SSRI use are correlated with increased risk for craniofacial abnormalities including premature calvarial suture fusion (craniosynostosis) (Browne et al., 2011; Carmichael et al., 2008; Grewal et al., 2008). An apparent limitation to this investigation is the singular incidence of fusion in the normally patent murine coronal suture in the control group. As in humans, murine sutures exist across a spectrum between patency and fusion allowing for proper growth and development (Grova et al., 2012; Lenton et al., 2005). Some of this data resulting from different experimental modalities seems to be contradictory. For example, the down-regulation of vimentin in both sutures from individuals exposed to levothyroxine is not corroborated by the suture phenotype of those individuals. This disparity potentially highlights differences in cell type and signaling between these two sutures. Additionally, the loss of Gli1 stem cells in the coronal sutures in the citalopram exposure model validates the premature fusion phenotype identified but does not extend to the other suture investigated. The increased incidence of fusion in both sutures of individuals exposed to citalopram without corroboratory protein or gene expression changes may be indicative of multiple mechanisms for suture fusion.
Our characterization of the suture stem cell population further verifies calvarial sutures as a stem cell niche and defines the resident stem population using a multi-factorial phenotype CD44+, Sca1+, CD34−, CD45− (Zhao et al., 2015; Maruyama et al., 2016; Wilk et al., 2017). As there were differential effects of these pharmacological agents, it seems unlikely that suture stem cell depletion can be identified as a unifying mechanism of craniosynostosis, however, these data do indicate that maintenance of a balance of the heterogeneous cell phenotypes within the calvarial sutures is necessary for proper craniofacial growth and development. Further, homeostasis of cell functions including differentiation, apoptosis and proliferation is vital for proper maintenance of the calvarial sutures. Most importantly, though these medications may be required during pregnancy to manage thyroid disorders and depression, there is evidence that these exposures may negatively affect fetal development signaling a need for additional investigation.
Acknowledgements
This study was supported by research grants from the National Institutes of Health (NIH) National Institute of Dental and Craniofacial Research [F31DE026684 to ELD; R03DE023350A and R03DE02619-01A to JC; 5T32DE017551 to MUSC], the Cleft Palate Foundation Cleft/Craniofacial Anomalies Grant Award to JC, the Plastic Surgery Education Foundation Pilot Award to JC, the NIH National Institute on Aging (NIA) 1P01AG036675 (Augusta University), the NIH National Institute of General Medicine [P30GM103331 to MUSC] and the South Carolina Clinical and Translational Research Institute (NIH/NCATS NIH/NCATS UL1TR000062). This study utilized the expertise, resources and facilities of the Center for Oral Health Research (COHR) and the Hollings Cancer Center Flow Cytometry and Cell Sorting Unit. The authors would like to thank Dr. Michael Kern, Dr. Jeremy Barth, and Dr. Robin Muise-Helmericks for their early input on this project.
Abbreviations
- SSRI
Selective Serotonin Re-uptake Inhibitor
- CDC
Center for Disease Control
- μCT
Micro-Computed Tomography
- EDTA
Ethylenediaminetetraacetic acid
- HRP
Horseradish Peroxidase
- IHC
Immunohistochemistry
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
Fetal Bovine Serum
- PCR
Polymerase Chain Reaction
- PIF
Posterior Interfrontal Suture
Footnotes
Declaration of Competing Interest
The authors declare no competing interests.
References
- Agresti M, Gosain AK, 2005. Detection of apoptosis in fusing versus nonfusing mouse cranial sutures. J Craniofac Surg 16, 572–578. [DOI] [PubMed] [Google Scholar]
- Alkam T, Kim HC, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, Yamada K, Nabeshima T, 2013. Evaluation of emotional behaviors in young offspring of C57BL/6J mice after gestational and/or perinatal exposure to nicotine in six different time-windows. Behav. Brain Res. 239, 80–89. [DOI] [PubMed] [Google Scholar]
- Arnaud E, Renier D, Marchac D, 1995. Prognosis for mental function in scaphocephaly. J. Neurosurg 83, 476–479. [DOI] [PubMed] [Google Scholar]
- Berrettini WH, Lerman CE, 2005. Pharmacotherapy and pharmacogenetics of nicotine dependence. Am. J. Psychiatry 162, 1441–1451. [DOI] [PubMed] [Google Scholar]
- Boyadjiev SA, 2007. Genetic analysis of non-syndromic craniosynostosis. Orthod. Craniofacial Res. 10, 129–137. [DOI] [PubMed] [Google Scholar]
- Bradley JP, Levine JP, Roth DA, McCarthy JG, Longaker MT, 1996. Studies in cranial suture biology: IV. Temporal sequence of posterior frontal cranial suture fusion in the mouse. Plast. Reconstr. Surg 98, 1039–1045. [DOI] [PubMed] [Google Scholar]
- Browne ML, Hoyt AT, Feldkamp ML, Rasmussen SA, Marshall EG, Druschel CM, Romitti PA, 2011. Maternal caffeine intake and risk of selected birth defects in the National Birth Defects Prevention Study, birth defects research. Part A, Clin. and Mol. Teratol. 91, 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JW, Albright AL, Losken HW, Biglan AW, 1995. Intracranial hypertension after cranial vault decompression for craniosynostosis. Pediatr. Neurosurg 22, 270–273. [DOI] [PubMed] [Google Scholar]
- Capelo LP, Beber EH, Huang SA, Zorn TM, Bianco AC, Gouveia CH, 2008. Deiodinase-mediated thyroid hormone inactivation minimizes thyroid hormone signaling in the early development of fetal skeleton. Bone 43, 921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuco AV, Kahl S, Jack LJ, Bishop JO, Wallace H, 1999. Prolactin and growth hormone stimulation of lactation in mice requires thyroid hormones. Proc. Soc. Exp. Biol. Med 221, 345–351. [DOI] [PubMed] [Google Scholar]
- Cardinale A, Nastrucci C, Cesario A, Russo P, 2012. Nicotine: specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol 42, 68–89. [DOI] [PubMed] [Google Scholar]
- Carmichael SL, Ma C, Rasmussen SA, Honein MA, Lammer EJ, Shaw GM, 2008. National Birth Defects Prevention, Craniosynostosis and maternal smoking, Birth defects research. Part A, Clin. and Mol. Teratol. 82, 78–85. [DOI] [PubMed] [Google Scholar]
- Chadduck WM, Chadduck JB, Boop FA, 1992. The subarachnoid spaces in craniosynostosis. Neurosurgery 30, 867–871. [DOI] [PubMed] [Google Scholar]
- Chatterjee JS, Mahmoud M, Karthikeyan S, Duncan C, Dover MS, Nishikawa H, 2009. Referral pattern and surgical outcome of sagittal synostosis. J. Plast. Reconstr. Aesthet. Surg 62, 211–215. [DOI] [PubMed] [Google Scholar]
- Chistyakov V, Patkina N, Tammimaki A, Talka R, Salminen O, Belozertseva I, Galankin T, Tuominen R, Zvartau E, 2010. Nicotine exposure throughout early development promotes nicotine self-administration in adolescent mice and induces long-lasting behavioural changes. Eur. J. Pharmacol 640, 87–93. [DOI] [PubMed] [Google Scholar]
- Cray JJ Jr., Khaksarfard K, Weinberg SM, Elsalanty M, Yu JC, 2013. Effects of thyroxine exposure on osteogenesis in mouse calvarial pre-osteoblasts. PLoS One 8, e69067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cray JJ, Weinberg SM, Parsons TE, Howie RN, Elsalanty M, Yu JC, 2014. Selective serotonin reuptake inhibitor exposure alters osteoblast gene expression and craniofacial development in mice, birth defects research. Part A, Clin. and Mol. Teratol 100, 912–923. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Morse D, Klasson-Wehler E, Brouwer A, 1996. Binding of a 3,3′, 4,4′-tetrachlorobiphenyl (CB-77) metabolite to fetal transthyretin and effects on fetal thyroid hormone levels in mice. Toxicology 106, 105–114. [DOI] [PubMed] [Google Scholar]
- Dodmane PR, Arnold LL, Pennington KL, Cohen SM, 2014. Orally administered nicotine induces urothelial hyperplasia in rats and mice. Toxicology 315, 49–54. [DOI] [PubMed] [Google Scholar]
- Durham E, Jen S, Wang L, Nasworthy J, Elsalanty M, Weinberg S, Yu J, Cray J, 2015. Effects of citalopram on sutural and calvarial cell processes. PLoS One 10, e0139719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham EL, Howie RN, Black L, Bennfors G, Parsons TE, Elsalanty M, Yu JC, Weinberg SM, Cray JJ, 2016. Effects of thyroxine exposure on the Twist 1 +/− phenotype: a test of gene-environment interaction modeling for craniosynostosis. Birth Defects Res. Part A: Clin. and Mol. Teratol 106, 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham EL, Howie RN, Cray JJ, 2017a. Gene/environment interactions in craniosynostosis: a brief review. Orthod. Craniofacial Res. 20 (Suppl. 1), 8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham E, Howie RN, Parsons T, Bennfors G, Black L, Weinberg SM, Elsalanty M, Yu JC, Cray JJ Jr., 2017b. Thyroxine exposure effects on the cranial base. Calcif. Tissue Int. 101, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham E, Howie RN, Warren G, LaRue A, Cray J, 2019. Direct effects of nicotine exposure on murine calvaria and calvarial cells. Sci. Rep 9, 3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H, Jaddoe VW, Hudziak JJ, Roza SJ, Steegers EA, Hofman A, Verhulst FC, White TJ, Stricker BH, Tiemeier H, 2012. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch. Gen. Psychiatry 69, 706–714. [DOI] [PubMed] [Google Scholar]
- Esparza J, Hinojosa J, 2008. Complications in the surgical treatment of craniosynostosis and craniofacial syndromes: apropos of 306 transcranial procedures. Childs Nerv. Syst 24, 1421–1430. [DOI] [PubMed] [Google Scholar]
- Fong KD, Song HM, Nacamuli RP, Franc BL, Mari C, Fang TD, Warren SM, Contag CH, Blankenberg FG, Longaker MT, 2004. Apoptosis in a rodent model of cranial suture fusion: in situ imaging and gene expression analysis. Plast. Reconstr. Surg 113, 2037–2047. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, Zatonski W, Hsia J, Morton J, Palipudi KM, Asma S, Group GC, 2012. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet 380, 668–679. [DOI] [PubMed] [Google Scholar]
- Grewal J, Carmichael SL, Ma C, Lammer EJ, Shaw GM, 2008. Maternal periconceptional smoking and alcohol consumption and risk for select congenital anomalies, birth defects research. Part A, Clin. and Mol. Teratol 82, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grova M, Lo DD, Montoro D, Hyun JS, Chung MT, Wan DC, Longaker MT, 2012. Models of cranial suture biology. The J. of Craniofac. Surg 23, 1954–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GL, 2014. Smoking during pregnancy, vitamin C supplementation, and infant respiratory health. JAMA: The J. of the Am. Med. Assoc 311, 2070–2071. [DOI] [PubMed] [Google Scholar]
- Holmes G, van Bakel H, Zhou X, Losic B, Jabs EW, 2015. BCL11B expression in intramembranous osteogenesis during murine craniofacial suture development. Gene Expr. Patterns 17, 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie RN, Durham EL, Black L, Bennfors G, Parsons TE, Elsalanty ME, Yu JC, Weinberg SM, Cray JJ Jr., 2016. Effects of in utero thyroxine exposure on murine cranial suture growth. PLoS One 11, e0167805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie RN, Herberg S, Durham E, Grey Z, Bennfors G, Elsalanty M, LaRue AC, Hill WD, Cray JJ, 2018. Selective serotonin re-uptake inhibitor sertraline inhibits bone healing in a calvarial defect model. Int. J. of Oral Sci 10 (3), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabs EW, 2002. Genetic Etiologies of Craniosynostosis. In: Mooney MP, Siegel MI (Eds.), Understanding Craniofacial Anomalies: The Etiopathogenesis of Craniosynostoses and Facial Clefting. John W. Wiley and Sons, New York, pp. 125–146. [Google Scholar]
- Jiao J, Nitzke AM, Doukas DG, Seiglie MP, Dulawa SC, 2011. Antidepressant response to chronic citalopram treatment in eight inbred mouse strains. Psychopharmacology 213, 509–520. [DOI] [PubMed] [Google Scholar]
- Johnson D, Wilkie AO, 2011. Craniosynostosis. Eur. J. of Human Genet.: EJHG 19, 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG, 2010. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein LC, Stine MM, Pfaff DW, Vandenbergh DJ, 2003. Laternal nicotine exposure increases nicotine preference in periadolescent male but not female C57B1/6J mice. Nicotine Tob. Res 5, 117–124. [DOI] [PubMed] [Google Scholar]
- Krause K, Weiner J, Hones S, Kloting N, Rijntjes E, Heiker JT, Gebhardt C, Kohrle J, Fuhrer D, Steinhoff K, Hesse S, Moeller LC, Tonjes A, 2015. The effects of thyroid hormones on gene expression of acyl-coenzyme a Thioesterases in adipose tissue and liver of mice. Eur. Thyroid J. 4, 59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JCT, Harris MW, McKinney JD, Birnbaum LS, 1986. Effects of thyroid hormones on the induction of cleft palate by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6N mice. Toxicol. Appl. Pharmacol 84, 115–124. [DOI] [PubMed] [Google Scholar]
- Lamberg BA, Helenius T, Liewendahl K, 1986. Assessment of thyroxine suppression in thyroid carcinoma patients with a sensitive immunoradiometric TSH assay. Clin. Endocrinol. 25, 259–263. [DOI] [PubMed] [Google Scholar]
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT, 2005. Cranial suture biology. In: Current Topics in Developmental Biology. Academic Press, pp. 287–328. [DOI] [PubMed] [Google Scholar]
- Maguire CT, Demarest BL, Hill JT, Palmer JD, Brothman AR, Yost HJ, Condic ML, 2013. Genome-wide analysis reveals the unique stem cell identity of human amniocytes. PLoS One 8, e53372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm H, Artama M, Gissler M, Ritvanen A, 2011. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstet. Gynecol. 118, 111–120. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Jeong J, Sheu TJ, Hsu W, 2016. Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat. Commun 7, 10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxson R, Ishii M, 2008. The Bmp pathway in skull vault development. Front Oral. Biol. 12, 197–208. [DOI] [PubMed] [Google Scholar]
- Miller MT, 2000. Ocular findings in craniosynostosis. In: Cohen MM Jr., MacLean RE (Eds.), Craniosynostosis: Diagnosis, Evaluation, and Management. Oxford University Press, New York, pp. 184–196. [Google Scholar]
- Morrison SJ, Spradling AC, 2008. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AOM, 2005. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J. Anat 207, 637–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier JD, Blom T, Arentsen T, Homberg JR, 2011. The age-dependent effects of selective serotonin reuptake inhibitors in humans and rodents: a review. Prog. NeuroPsychopharmacol. Biol. Psychiatry 35, 1400–1408. [DOI] [PubMed] [Google Scholar]
- Opperman LA, 2000. Cranial sutures as intramembranous bone growth sites. Dev. Dyn.: An Off. Publ. of the Am. Assoc. of Anatomists 219, 472–485. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Rawlins JT, 2005. The extracellular matrix environment in suture morphogenesis and growth. Cells Tissues Organs 181, 127–135. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Adab K, Gakunga PT, 2000. Transforming growth factor-beta 2 and TGF-beta 3 regulate fetal rat cranial suture morphogenesis by regulating rates of cell proliferation and apoptosis. Dev. Dyn.: An Off. Publ. of the Am. Assoc. of Anatomists 219, 237–247. [DOI] [PubMed] [Google Scholar]
- Parsons TE, Weinberg SM, Khaksarfard K, Howie RN, Elsalanty M, Yu JC, Cray JJ Jr., 2014. Craniofacial shape variation in Twist1+/− mutant mice. Anat. Rec 297, 826–833. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno MR, Serti Eacute AE, Jehee FS, Fanganiello R, Yeh E, 2008. Genetics of craniosynostosis: genes, syndromes, mutations and genotype-phenotype correlations. Front. of Oral Biol 12, 107–143. [DOI] [PubMed] [Google Scholar]
- Rantamaki T, Hendolin P, Kankaanpaa A, Mijatovic J, Piepponen P, Domenici E, Chao MV, Mannisto PT, Castren E, 2007. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacol.: Off. Publ. of the Am. Coll. of Neuropsychopharmacol 32, 2152–2162. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Yazdy MM, Frias JL, Honein MA, 2008. Priorities for public health research on craniosynostosis: summary and recommendations from a Centers for Disease Control and Prevention-sponsored meeting. Am. J. Med. Genet. A 146A, 149–158. [DOI] [PubMed] [Google Scholar]
- Rawlins JT, Opperman LA, 2008. Tgf-beta regulation of suture morphogenesis and growth. Front. of Oral Biol. 12, 178–196. [DOI] [PubMed] [Google Scholar]
- Renda A, Nashmi R, 2014. Chronic nicotine pretreatment is sufficient to upregulate alpha4* nicotinic receptors and increase oral nicotine self-administration in mice. BMC Nenrosci 15 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin NH, 1999. Molecular genetic advances in understanding craniosynostosis. Plast. Reconstr. Surg 103, 1060–1070. [PubMed] [Google Scholar]
- Schaal C, Chellappan SP, 2014. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol. Cancer Res.: MCR 12, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D, 2006. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav. Pharmacol 17, 271–287. [DOI] [PubMed] [Google Scholar]
- Thordarson G, Fielder P, Lee C, Hom YK, Robleto D, Ogren L, Talamantes F, 1992. Mammary gland differentiation in hypophysectomized, pregnant mice treated with corticosterone and thyroxine. Biol. Reprod 47, 676–682. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM, 2013. 50-year trends in smoking-related mortality in the United States. N. Engl. J. Med 368, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese F, Bukhari AB, Malhotra R, De A, 2014. IHC profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One 9, e96801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk K, Yeh S-CA, Mortensen LJ, Ghaffarigarakani S, Lombardo CM, Bassir SH, Aldawood ZA, Lin CP, Intini G, 2017. Postnatal calvarial skeletal stem cells expressing PRX1 reside exclusively in the Calvarial sutures and are required for bone regeneration. Stem Cell Rep. 8, 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota M, Kobayashi Y, Morita J, Suzuki H, Hashimoto Y, Sasaki Y, Akiyoshi K, Moriyama K, 2014. Therapeutic effect of nanogel-based delivery of soluble FGFR2 with S252W mutation on Craniosynostosis. PLoS One 9, e101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN Jr., 2006. Statistical analysis of real-time PCR data. BMC Bioinformav 7, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jiao J, 2015. Molecular biomarkers for embryonic and adult neural stem cell and neurogenesis. Biomed. Res. Int 2015, 727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Feng J, Ho T-V, Grimes W, Urata M, Chai Y, 2015. The suture provides a niche for mesenchymal stem cells of craniofacial bones. Nat. Cell Biol 17, 386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]