Abstract
Background:
3,4-methylenedioxypyrovalerone (MDPV) toxicity includes intense neurological and cardiovascular events. We examined MDPV-induced cardiovascular, temperature, and locomotor effects following escalating and repeated MDPV administration in adult male and female Sprague-Dawley rats and compared these effects to cocaine in male rats.
Methods:
Telemetry devices were surgically implanted to allow continuous measurement of cardiovascular, temperature, and locomotor activity over a 22 h period after dosing. Rats were administered increasing intraperitoneal (IP) MDPV doses (1–5.6 mg/kg) every other day, followed two days later by a binge regimen of four injections of 3 mg/kg MDPV at 2 h intervals. MDPV serum concentrations were measured by LC-MS/MS. Cocaine (3–30 mg/kg) and four injections of 30 mg/kg IP were administered to male rats for comparison with male MDPV data.
Results:
The duration of MDPV cardiovascular effects was significantly greater (p<0.05) in male rats than female rats at 3–5.6 mg/kg. The ED50 for MDPV-induced locomotor was significantly lower in males (2.4 ± 0.3) than females (3.4 ± 0.2). Males showed significantly greater variability in MDPV serum concentrations than females after binge dosing. MDPV produced five-fold more potent cardiovascular effects than cocaine in male rats. MDPV did not alter thermoregulation in either sex, but cocaine binge administration decreased temperature.
Conclusion:
Effects of MDPV on temperature were not significantly different between sexes. MDPV-induced cardiovascular and locomotor effects in males lasted significantly longer and were more potent than in females. These differences appeared to be related to pharmacokinetic factors leading to greater variance in MDPV serum concentrations in males.
Keywords: cardiovascular, MDPV, telemetry, rat sex
1. Introduction
MDPV (3,4-methylenedioxypyrovalerone) is an abused stimulant in the synthetic cathinone class. MDPV is a potent monoamine reuptake inhibitor highly selective for dopamine and norepinephrine transporters with little affinity for the serotonin transporter (Baumann et al., 2013; Harvey and Baker, 2016; Wakabayashi et al., 2015). While the drug is likely taken with a recreational intent, MDPV intoxication can lead to unintended adverse physiological effects including tachycardia, hypertension, arrhythmias, agitation, sympathomimetic syndrome, hallucinations, psychosis, and suicidal actions (Sivagnanam et al., 2013; Wyman et al., 2013).
Schindler et al. (2016) report the cardiovascular (CV) effects of MDPV following subcutaneous (SC) administration in male Sprague-Dawley (SD) rats. They find that MDPV elicits increases in heart rate (HR) and blood pressure (BP). Of importance, they show that the ganglionic blocker, chlorisondamine, and adrenoceptor antagonists, prazosin, propranolol, and atenolol, block MDPV-induced cardiovascular effects. These findings suggest the CV effects of MDPV are centrally-mediated which has been shown with other psychostimulants such as cocaine and MDMA (Hysek et al., 2010; Ramoska and Sacchetti, 1985; Schindler et al., 1992). They also report that neither of the two hydroxylated Phase I MDPV metabolites alter CV effects. There are no studies that report the sex-dependent CV effects of MDPV in rats.
Several studies report the pharmacokinetic properties of MDPV in rats after different routes of administration. In male rats, the elimination half-life of MDPV is reported at 1–2 h among several studies using either SC, intraperitoneal (IP), or intravenous (IV) administration (Anizan et al., 2016; Baumann et al., 2016; Hambuchen et al., 2017a; Horsley et al., 2018). A study of the pharmacokinetics of MDPV after IP dosing of males and female rats (Hambuchen et al., 2017a). shows the most substantive sex differences are in the volume of distribution and IP bioavailability. In particular, there is a significantly greater bioavailability of MDPV in male rats, along with a greater variability in male MDPV serum concentrations. The increased magnitude and variance in IP bioavailability values suggests that the IP route of administration could potentially lead to sex-dependent differences in pharmacological effects of MDPV in rats.
In the present study we determined cardiovascular, temperature, and locomotor effects of MDPV following escalating single and binge IP administration of MDPV in male and female SD rats and compared these effects to cocaine in males. We hypothesized that there would be sex-related differences in these MDPV-induced effects and that these differences would be greater following binge administration.
2. Methods
2.1. Drugs and Chemicals
Racemic 3,4-methylenedioxypyrovalerone HCl was obtained from the National Institute on Drug Abuse (Bethesda, MD, USA). Cocaine was purchased from Sigma Chemical Company (St. Louis, MO, USA). All drug concentrations were expressed as free base. Other chemicals were purchased from Sigma Chemical Company or Thermo Fisher Scientific Inc. (Waltham, MA, USA) unless otherwise noted. MDPV and cocaine doses were prepared in pH 7.3 administration buffer containing 15 mM phosphate and 150 mM sodium chloride. All MDPV and cocaine doses were administered by the IP route (1 mL/kg).
2.2. Animals
Adult male (300–325 g) and female (250–275 g) SD rats (9–15 weeks) were purchased from Charles Rivers Laboratories International Inc. (Wilmington, MA, USA). One week after arrival, HD-S10 telemetry devices (Data Science International, St. Paul, MN) were surgically implanted into the aorta. Rats were anesthetized using 5% isoflurane for induction and 2% isoflurane for maintenance of anesthesia. The abdomen was shaved and disinfected prior to making a 5 cm incision down the midline of the abdomen. The abdominal skin, abdominal muscle, and intestines were retracted and the aorta isolated between renal and iliolumbar arteries. Blood supply to the aorta was briefly cutoff and the catheter of the telemetry device was implanted. The intestines were repositioned; the telemetry device was secured with a suture; and the abdominal cavity was closed. The skin was closed using staples that were removed 10 days later. Meloxicam (2 mg/kg) was administered prior to surgery and for two days following surgery to reduce pain and inflammation. Rats were given 10–14 days to recover before experimentation.
Rats were housed in individual cages with a 10-h light/14-h dark cycle, 22°C environment with free access to water and fed 20 g of pellets daily to maintain constant weights. All studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted and promulgated by the National Institutes of Health. All experiments were approved by the Animal Care and Use Committee of the University of Arkansas for Medical Sciences.
2.3. Telemetry Measurements and Drug Administration
Telemetry device data was collected using hardware and software (Dataquest ART 4.33 Gold Software) from Data Science International. On experiment days, rats were weighed before implants were turned on and before each repeated dose. Heart rate (HR), diastolic and systolic blood pressure (BP), core body temperature, and behavioral activity were reported every minute from 90 min before until 22 h after saline, MDPV, or cocaine administration. Injections occurred in the morning at ~10:00 am for single doses and ~8:00 am for binge doses. Injection sites for IP doses were alternated to minimize discomfort from multiple injections with the first injection on the left side. The plunger was pulled back before dosing to assure the needle was not in, for instance, a blood vessel, the bladder, or gastrointestinal tract.
Drugs were administered (IP) in ascending order (1, 3, and 5.6 mg/kg MDPV or 3, 10, and 30 mg/kg cocaine) every other day to SD rats (n=7/sex MDPV) (n=6/male cocaine). Two days later, a binge-like regimen of 3 mg/kg MDPV or 30 mg/kg cocaine every 2 h for 6 h (a total of 4 doses) was administered. Experimentation was conducted in home cages.
Cocaine was chosen for comparison with MDPV because the two drugs have a similar mechanism of action as norepinephrine-dopamine reuptake inhibitors. This comparison was only conducted in males.
2.4. Blood Samples
Blood samples were collected via tail vein three days before the first MDPV injection, and 8 and 24 h after the first of four MDPV binge doses. Blood samples were kept at 4–8°C for 1 h to allow clotting, centrifuged at 20,817 rcf at 4°C for 7 min for serum collection, and stored at-20°C. Serum MDPV concentrations were determined by chiral liquid chromatography tandem mass spectrometry (LC-MS/MS) (Hambuchen et al., 2017b).
2.5. Data Analysis
Data were presented as mean ± standard deviation (SD). Time course-activity curves of HR and BP data within each sex were averaged into 30 min bins. Data were also reported as percent change from saline for each dose and sex. Duration of effects on HR and BP was defined as the post-injection time at which a drug-induced effect did not differ from or fell below saline-like effects for at least 20 min. Binge values for HR and BP data were analyzed by computing difference scores determined by subtracting the 8 hrs of saline-like effects from the 8 hours of drug-elicited effects within each sex or within cocaine and MDPV treatment (males only). Locomotor activity were analyzed as described above and locomotor activity counts were summed cumulatively every 2 h during the binge. Predicted MDPV serum concentration-time data during binge dosing was simulated using Phoenix WinNonlin software (V6.4, Certara USA, Princeton, NJ) and pharmacokinetic parameters derived from previous 3 mg/kg IP MDPV pharmacokinetics studies in male and female SD rats (Hambuchen et al., 2017a). These predicted concentration-time curves were then compared to the observed MDPV serum concentrations collected at 2 h after the last binge dose. All data were analyzed using analysis of variance (ANOVA) procedures, linear regression analyses (for slope and potency (ED50) estimations from dose-effect curves), area under the curve (AUC) analyses, and Welch’s t-tests. Full descriptions of statistical analyses can be found in the supplement file. Graphs and statistical analysis were performed using GraphPad Prism software (Version 7.0, La Jolla, CA, USA). Statistical significance was declared at p<.05.
3. Results
3.1.1. MDPV effects on HR and BP following escalating single and binge doses in male and female rats
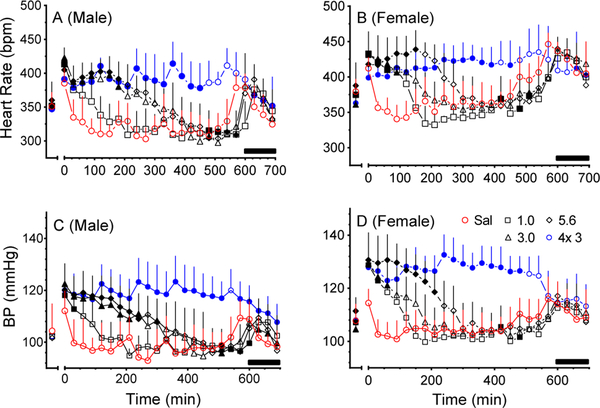
Injections of 3 and 5.6 mg/kg MDPV showed similar patterns of HR and BP effects over time in males (Fig. 1A, C), but females showed increasing MDPV-induced CV effects as dose increased (Fig. 1B, D). Although we monitored the animals for 22 h, HR and BP values appeared to blend into or synchronize with the dark cycle values beginning at about 550–600 min regardless of sex or MDPV dose (Fig. 1A–D).
Fig. 1:
Heart rate (A,B) and mean arterial BP (C,D) (+ SD) for male and female SD rats (n=7/sex) after IP injection of ascending, single doses (1–5.6 mg/kg MDPV) and binge dosing of MDPV (3 mg/kg MDPV x4 injections every 2 h). Black bars along the bottom right of the graph shows the dark cycle phase time (600 – 700 min). Data were collected 30 min prior to injection and for 22 h after dose administration, but only the first 700 min post-injection are shown. Statistical analysis was performed using a two-way ANOVA followed by Dunnett’s multiple comparison test to compare individual effects of drug treatment at each time point to saline control. Solid symbols indicate significant differences from saline (p<0.05).
Binge administration of MDPV potentiated elevated BP and HR in males and females for the duration of the light cycle (Fig. 1A–D). Peak effects following each injection during the binge were not statistically different for males or female (Fig. 1A–D). Termination of effects from binge administration could not be distinguished from the synchronization of HR and BP values to the dark cycle at ~600 m. Maximum effect on HR and BP during the binge did not appear to exceed the maximum effects achieved by any of the acute doses.
3.1.2. The duration of effect of MDPV-induced increases in HR and BP in male and female rats
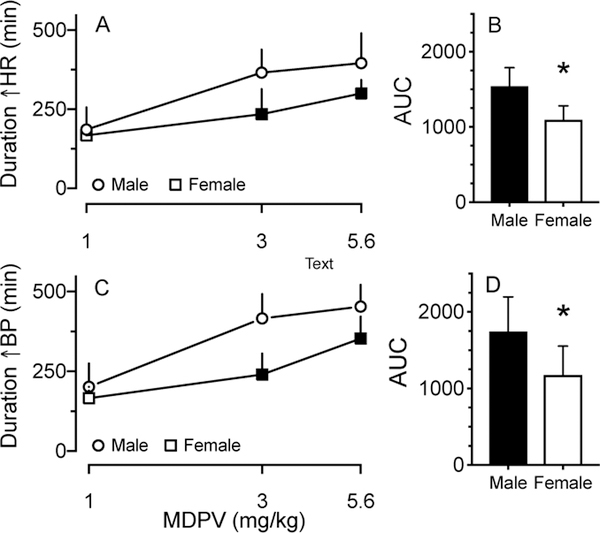
MDPV dose-dependently increased the duration of effect on HR and BP for males and females (Fig. 2A, C). There were significant main effects of dose and sex in MDPV-induced HR duration and an interaction between these two factors in MDPV-induced BP duration in the single dose acute injection experiments (Sup. Fig. S2). Males displayed significantly greater duration of effect of HR and BP than females after 3 and 5.6 mg/kg of MDPV (Fig. 2A, C). Duration of effect for HR and BP in males and females ranged from ~190 min for 1 mg/kg MDPV to greater than 660 min during binge administration. Males had significantly greater AUC values for duration of effect on HR and BP than females (Fig. 2C, D). During binge administration, increased HR and BP persisted throughout the light cycle, yet became confounded by the natural nocturnal acceleration (Fig. 1A–D). As such, the end of increased HR and BP from the binge administration could not be distinguished from the saline administration during the dark cycle.
Fig. 2:
Duration of MDPV-induced elevations in HR (A) and BP (C) in male and female SD rats (mean + SD, n=7/sex). Filled symbols indicate significant difference between sexes at corresponding doses determined by two-way ANOVA with Holm-Sidak’s multiple comparison test. The area under the MDPV-induced HR (B) and BP (D) versus MDPV dose curves (AUC) showed females had significantly lower (*p<0.05 Welch’s t-test) duration of MDPV-induced CV effects than males.
3.1.3. MDPV-induced 8-h HR and BP averages in male and female rats
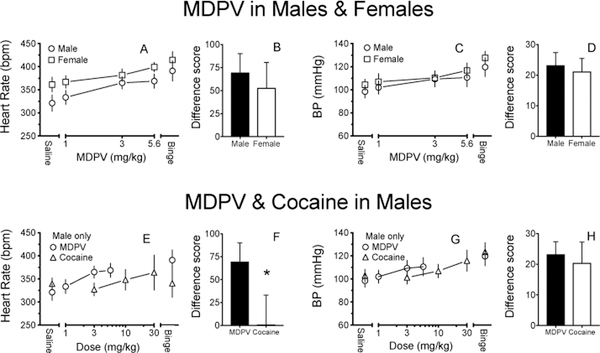
The average HR and BP effects of increasing doses of MDPV in male and female SD rats are depicted in Fig. 3A and C. There was no significant sex-dependent difference in ED50 values for the HR and BP verse MDPV dose (1–5.6 mg/kg) curves. There was no significant sex-dependent difference in the binge difference scores for HR and BP (Fig. 3B, D).
Fig. 3:
HR (A) and BP (C) following escalating doses of MDPV in male and female rats. HR (E) and BP (G) dose-effects following MDPV and cocaine administration in male rats. These data show average daytime MDPV-induced effects (8 h mean ± SD, n=6–7). The ED50 values (mg/kg; mean ± SD) for cardiovascular effects of MDPV in males (HR:2.4 ± 0.3; BP:2.6 ± 0.4) was not significantly different from females (HR: 3.3 ± 0.3; BP: 3.4 ± 0.2) (A, C), but was significantly different from cocaine (HR:12.7 ±1.5; BP: 14.9 ± 0.8). The * in plot 3F indicates cocaine treatment was significant different from MDPV treatment (p<0.05). The MDPV data for male rats in A and C are repeated in plots E and G for direct comparison with cocaine in male rats.
3.2. Cocaine-compared to MDPV-induced effects on HR and BP in male SD rats
The average HR and BP effects of increasing doses of MDPV and cocaine are depicted in Fig. 3E and G. The dose-response curves for acute MDPV-and cocaine-induced HR and BP revealed similar effectiveness, but cocaine was ~5-fold less potent (MDPV: ED50 = 2.4 ± 0.3 and 2.6 ± 0.4; Cocaine: ED50 = 12.7 ±1.5 and 14.9 ± 0.8, respectively) (Fig. 3C, D). The MDPV binge significantly increased HR and BP over saline; however, the cocaine binge did not produce a significantly different HR or BP from saline (Fig. 3C, D). The difference scores between the MDPV and cocaine binges were significantly different for HR, but not BP (Fig. 3F and H). Peak effects following each injection during the binge were not statistically different for MDPV or cocaine (Fig 1A and C; cocaine data not shown). No chronic changes in HR or BP were identified up to 12 days post binge MPDV or cocaine as compared to pre-treatment saline baseline (data not shown).
3.3. MDPV and cocaine effects on core body temperature
Core body temperature did not vary outside normal physiological ranges after a single or binge administration of MDPV in males and females or by single administrations of cocaine in male rats (Sup. Fig. S1A–D). During the light cycle, the maximum and minimum core body temperatures of SD rats were not significantly different between sexes after MDPV administration (Sup. Fig. S1A, B). There was a significant main effect of dose after MDPV administration for minimum and maximum temperatures and a significant interaction between sex and dose for minimum temperature; however, the minimum and maximum temperature were within normal physiological ranges for SD rats (Bishop et al., 2000). Maximum temperature during the binge was significantly greater for MDPV than cocaine, but occurred within normal, diurnal physiological temperature fluctuations. Cocaine produced a significant reduction in temperature of 1.9°C, which was below normal body temperature for SD rats, compared to MDPV during the binge dosing (Sup. Fig. S1D).
3.4. MDPV-induced locomotor effects in male and female rats
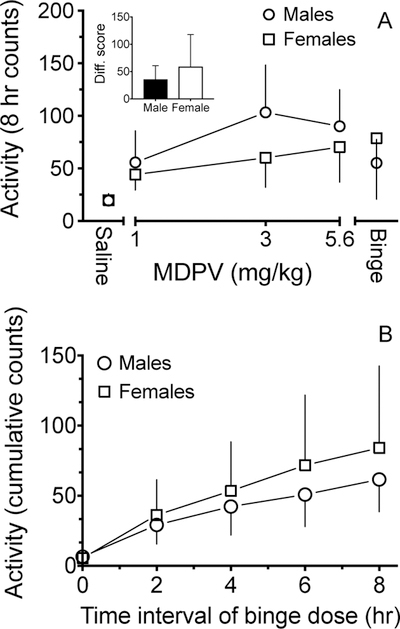
Activity during the diurnal cycle after IP MDPV administration is shown in Fig. 4. Females showed an apparent linear increase in activity from 1 to 5.6 mg/kg, whereas males showed an apparent inverted U-shaped dose-response function with the peak value at 3 mg/kg. The ED50 value for males (2.4 ± 0.3) was significantly lower than for females (3.4 ± 0.2). During the binge, each injection of 3 mg/kg MDPV resulted in an increase in cumulative activity for males and females (Fig. 4B). There was no significant sex-dependent difference in the binge difference scores for MDPV-induced locomotor effects (Fig. 4A inset).
Fig. 4:
MDPV-induced average locomotor activity (A) for 8 h ± SD and (B) cumulative locomotor activity between four binge doses (± SD) administered once every 2 h. The ED50 values (mg/kg; mean ± SD) for locomotor activity of MDPV in males (2.4 ± 0.3) was significantly different from females (3.4 ± 0.2) as determined by (A).
3.5. MDPV serum concentrations during binge dosing in male and female rats
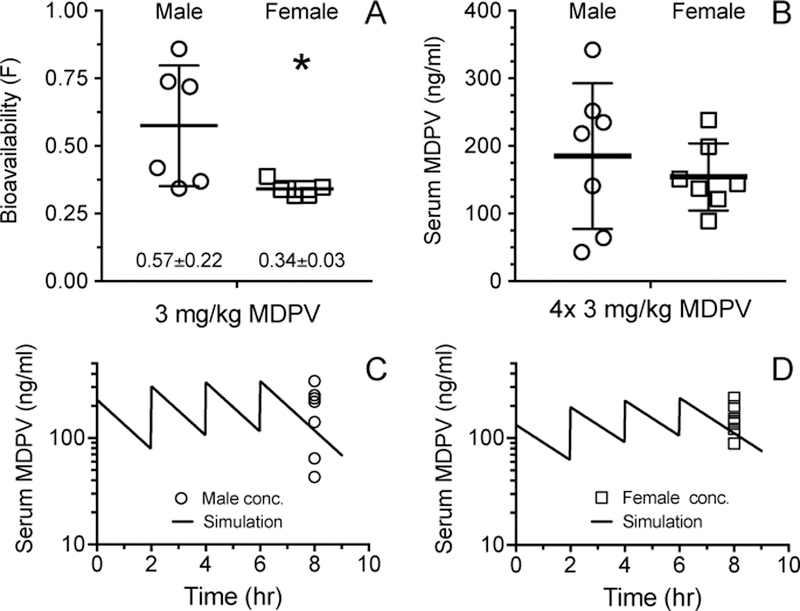
In a previous study by Hambuchen et al. (2017a), bioavailability of 3 mg/kg MDPV is shown to be significantly greater for males than females (Fig. 5A). Males have an apparent bimodal distribution in bioavailability after IP injection with half of the males having a similar bioavailability to females (0.34 ± 0.03); and half of the males showing a greater bioavailability of MDPV of ~0.8 to produce an average value of 0.57 ± 0.22 (Fig. 5A). In the current study, serum concentrations of MDPV 2 h after the last binge dose (480 min) were not significantly different between sexes (Fig. 5B); however, males displayed greater variability than females (Levene’s test; p=.04). There was no MPDV detected in serum collected before MDPV exposure or at 24 h after the last MDPV binge dose (data not shown). In the male rats, the average observed serum concentration was within 37% of the predicted value (Fig. 5C). In the female rats, this value was within 28% (Fig. 5D). In both sexes, the predicted value was within 1 SD of the observed values.
Fig. 5:
(A) Bioavailability of a 3 mg/kg IP MDPV dose in male and female SD rats, *p<0.05 statistically different from male rats (mean ± SD, n=5–6/sex, data from (Hambuchen et al., 2017a), (B) serum MDPV concentrations (mean ± SD, n=7/sex) 2 h after the final 4th binge dose in male and female SD rats, and (C) and (D) male and female (respectively) MDPV concentrations from Fig. 5B replotted on the simulated serum MDPV concentrations over a 9 h time period.
4. Discussion
The current study is the first to report sex-dependent MDPV-induced cardiovascular and temperature results for the full duration of effects following acute and binge administration of MDPV (IP). The MDPV-induced CV effects found in male SD rats were in agreement with previous research showing dose-dependent increases in HR and BP after acute SC injections (Schindler et al., 2016). In contrast to the studies by Schindler et al. (2016), we continuously monitored the CV parameters for the complete duration of MDPV-induced effects (Fig. 1A–D). Schindler reported CV effects for only three hours of MDPV-induced CV effects and did not completely characterize the effects of their highest dose tested (3 mg/kg MDPV, SC). In the current studies, the lowest dose of MDPV (1 mg/kg) produced about 3 h of CV effects; and the MDPV binge dose regimen produced CV effects for over 10 h in both sexes. Continuous monitoring of CV effects showed that, regardless of the MDPV dosing or rat sex, HR and BP synchronized to the increased values for HR and BP that occurred at the beginning of the nocturnal (dark) cycle at approximately 600 min after the initial injection of MDPV (Fig. 1). Thus, natural adaptive changes in the circadian rhythm limited our ability to fully characterize the duration of CV effects after binge dosing.
The maximum drug-induced CV effects were similar after each acute, repeated administration of drug. The HR and BP following injections during the binge of MDPV and cocaine were not significantly different. Acute tolerance to the CV effects of cocaine occurs in humans; however, in the present study the lack of change in peak HR and BP after each injection indicated that acute tolerance or sensitization to CV effects did not occur following binge dosing of MDPV or cocaine in SD rats (Fischman et al., 1985; Foltin and Haney, 2004; Mendelson et al., 1998). Different results may be found under alternative experimental conditions including the evaluation of different doses, dosing schedule, and in vivo model.
Other psychostimulants are reported to produce sex-dependent effects on the rat CV system (e.g., Rorabaugh et al., 2017; Xiao et al., 2009). Therefore, a primary goal of this study was to evaluate sex-dependent differences in MDPV-induced CV effects. In agreement with previous data in male rats (Schindler et al., 2016), there was a dose-dependent increase in HR and BP with MDPV administration in male and female rats. One exception was HR and BP changes after the 3.0 mg/kg MDPV in male rats which appeared identical to the results following 5.6 mg/kg MDPV (Fig. 1A and C). The termination and duration of MDPV-induced CV effects, however, was faster and shorter in females than in males, respectively (Fig. 1 and 2). Less robust drug-induced effects on BP in women than men has been reported in humans for cocaine (Lynch et al., 2008). Despite these differences, the average MDPV-induced HR and BP effect was not different between sexes during acute or binge (IP) administration. Adverse effects such as increases in HR and BP could be considered as deterrents to drug abuse, the current data suggest that females may have a higher abuse liability for MDPV than males due to less aversive CV effects. This supports findings of King et al. (2015) which found weaker MDPV-induced taste avoidance in females compared to males. A possible limitation of these studies is that experiments were not controlled for the estrous cycle which may attribute to MDPV-induced sex differences; however, IV self-administration of a similar, second generation cathinone, α-PVP, did not find differences across estrus phases (Javadi-Paydar et al., 2018).
Since cocaine is known to cause CV toxicity and has similar mechanisms of action as MDPV on norepinephrine and dopamine transporters, cocaine-induced BP and HR data were determined for comparisons to MDPV data in male rats. We chose to only study male rats for the cocaine analysis, since there were more published pharmacological data for the comparison. MDPV was five-fold more potent than cocaine in producing CV effects in males This finding supports other in vivo studies evaluating the relative potency of MDPV to cocaine in male rats based on behavioral endpoints including self-administration, locomotor activity, and discriminative stimulus effects. (Baumann et al., 2013; Gannon et al., 2017; Gatch et al., 2013).
Additionally, Simmons et al. (2016) reported MDPV is about 10 times more rewarding than cocaine based on ultrasonic vocalizations. During binge dosing, MPDV caused an increase in HR and BP, whereas the cocaine binge caused a decrease in HR with an increase in BP suggesting baroreceptor reflex activation. High doses of cocaine are known to produce bradycardia (Abrahams et al., 1996; Knuepfer and Branch, 1992); however, this bradycardic response did not occur with MDPV. It is important to note that while MDPV and cocaine are DAT and NET inhibitors, cocaine also has action at serotonin transporters (SERT), sodium channels, and muscarinic receptors which might influence CV parameters. While it is well documented that MDPV has weak effects at SERT, it is not known whether MDPV has action at sodium channels or muscarinic receptors (Marusich et al., 2014; Simmler et al., 2012)
Psychostimulants like 3,4-methylenedioxymethamphetamine (MDMA) and cocaine are known to affect core body temperature which is dependent on the drug, dose, and ambient temperature (Dafters, 1995; Dafters and Lynch, 1998; Gonzalez, 1993; Gordon et al., 1991; Malberg and Seiden, 1998). Elevated body temperatures are also associated with MDPV toxicity in humans (Froberg et al., 2015). Male Swiss mice (Fantegrossi et al., 2013) and male Long-Evans rats (Wakabayashi et al., 2015) show increases in brain and body temperature after single or binge doses of MDPV (dose range 0.1 to 1 mg/kg) at normal ambient temperature, which is exacerbated by increased environmental temperatures. Other studies in adult male rats, however, report that MDPV does not alter body temperature under normal ambient conditions (Aarde et al., 2015; 2013; Merluzzi et al., 2014). In the current studies, neither single or binge MDPV dosing increased or decreased core body temperature in SD rats beyond normal daily fluctuations at ambient temperatures (Sup. Fig. S1A–D). While single doses of cocaine did not produce changes in minimum and maximum core body temperature, binge dosing produced an approximate 2°C decrease in the minimum temperature (Sup. Fig. S1). Since serotonin influences thermoregulation, the decrease in body temperature after the cocaine binge, but not the MDPV binge, may be attributed to cocaine’s action at SERT.
Previous findings from our group show that there are sex-dependent differences in MPDV pharmacokinetics (Hambuchen et al., 2017a). Indeed, male SD rats have a greater bioavailability of MDPV after IP administration than female SD rats (Fig. 5A) which produces a higher average systemic dose in the males. In the current study, the average serum MDPV concentrations determined 2 h after the last binge dose were not significantly different between sexes; but, there was a significant difference in MDPV concentration variability between the sexes (Fig. 5B). This high variance in MDPV concentrations was consistent with the high variability in the bioavailability found in the Hambuchen pharmacokinetic studies (shown in Fig. 5A). Using pharmacokinetic parameters determined by Hambuchen et al. (2017a), MDPV concertation vs. time simulations were used to predict MDPV concentrations in male and females rats over a 9 hr period of binge dosing (Fig. 5C and D). In the simulated data, the predicted binge MDPV serum concentrations were within 1 SD of the observed value 2 h after the final MDPV dose. These data also predicted that male SD rats in the current study had higher concentrations of MDPV in the blood stream than females (Fig. 5C and D). Since we did not test alternative routes of administration, we cannot conclusively state that all differences can be accounted for by the IP route of administration. However, it is likely that the differences between sexes in MDPV-induced CV and locomotor effects in this study were due in part to the greater bioavailability of MDPV in males (Fig. 5A).
5. Conclusions
In these studies of male and female rats, differences were observed in the pattern of HR and BP response over a 10 h period following repeated IP administration of MDPV. Binge dosing with MDPV potentiated CV effects in male and female rats with no development of acute tolerance to repeated injections. Additionally, male rats showed significantly more variability in MDPV serum concentrations after binge dosing than females. MDPV elicited approximately 5 times more potent effects on HR and BP compared to cocaine in male SD rats. MDPV did not alter thermoregulation in male or female SD rats. Some of these sex differences with MDPV could be attributed to possible higher IP MDPV bioavailability in the male rats. Finally, since we did not find any overt toxicity in these studies, future research should consider evaluating higher MDPV doses with less variable routes of administration.
Supplementary Material
Acknowledgments
Role of funding source
Funding for this work was provided by the NIH National Institute on Drug Abuse (R01 DA039195, F31 DA046121 to SJM, T32 DA022981); the National Institute of General Medical Sciences (T32 GM106999); the National Center for Advancing Translational Sciences (Grant UL1TR000039) and the Arkansas Biosciences Institute (the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000). The funding sources had no role in the study design; collection, analysis, and interpretation of data; in the writing of this paper; or the decision to submit this paper.
Footnotes
Conflict of interest
No conflict declared.
References
- Aarde SM, Creehan KM, Vandewater SA, Dickerson TJ, Taffe MA, 2015. In vivo potency and efficacy of the novel cathinone α-pyrrolidinopentiophenone and 3,4-methylenedioxypyrovalerone: self-administration and locomotor stimulation in male rats. Psychopharmacology (Berl.) 232, 3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA, 2013. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: Self-administration and locomotor activity in rats. Neuropharmacology 71, 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams TP, Cuntapay M, Varner KJ, 1996. Sympathetic nerve responses elicited by cocaine in anesthetized and conscious rats. Physiol. Behav 59, 109–115. [DOI] [PubMed] [Google Scholar]
- Anizan S, Concheiro M, Lehner KR, Bukhari MO, Suzuki M, Rice KC, Baumann MH, Huestis MA, 2016. Linear pharmacokinetics of 3,4‐methylenedioxypyrovalerone (MDPV) and its metabolites in the rat: relationship to pharmacodynamic effects. Addict Biol. 21, 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Bukhari MO, Lehner KR, Anizan S, Rice KC, Concheiro M, Huestis MA, 2016. Neuropharmacology of 3,4-Methylenedioxypyrovalerone (MDPV), Its Metabolites, and Related Analogs, in: Neuropharmacology of New Psychoactive Substances (NPS), Springer, Cham, pp. 93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Tella SR, Cozzi NV, Schindler CW, 2013. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacol. 38, 552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop B, Silva G, Krasney J, Salloum A, Roberts A, Nakano H, Shucard D, Rifkin D, Farkas G, 2000. Circadian rhythms of body temperature and activity levels during 63 h of hypoxia in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol 279, R1378–R1385. [DOI] [PubMed] [Google Scholar]
- Dafters RI, 1995. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol. Behav 58, 877–882. [DOI] [PubMed] [Google Scholar]
- Dafters RI, Lynch E, 1998. Persistent loss of thermoregulation in the rat induced by 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) but not by fenfluramine. Psychopharmacology (Berl.) 138, 207–212. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC, 2013. In vivo effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacol. 38, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman MW, Schuster CR, Javaid J, Hatano Y, Davis J, 1985. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J. Pharmacol. Exp. Ther 235, 677–682. [PubMed] [Google Scholar]
- Foltin RW, Haney M, 2004. Intranasal cocaine in humans: acute tolerance, cardiovascular and subjective effects. Pharmacol. Biochem. Behav 78, 93–101. [DOI] [PubMed] [Google Scholar]
- Froberg BA, Levine M, Beuhler MC, Judge BS, Moore PW, Engebretsen KM, Mckeown NJ, Rosenbaum CD, Young AC, Rusyniak DE, ToxIC, O.B.O.T.A.T.I.C., 2015. Acute Methylenedioxypyrovalerone Toxicity. J. Med. Toxicol 11, 185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT, 2017. Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone under fixed and progressive Ratio schedules of reinforcement in rats. J. Pharmacol. Exp. Ther 361, 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ, 2013. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav. Pharmacol 24, 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez LP, 1993. Cocaine alters body temperature and behavioral thermoregulatory responses. NeuroReport 4, 106–108. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O’Callaghan JP, Miller DB, 1991. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol. Biochem. Behav 38, 339–344. [DOI] [PubMed] [Google Scholar]
- Hambuchen MD, Hendrickson HP, Gunnell MG, McClenahan SJ, Ewing LE, Gibson DM, Berquist MD, Owens SM, 2017a. The pharmacokinetics of racemic MDPV and its ( R ) and ( S ) enantiomers in female and male rats. Drug Alcohol Depend. 179, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambuchen MD, Hendrickson HP, Owens SM, 2017b. Chiral determination of 3,4-methylenedioxypyrovalerone enantiomers in rat serum. Anal. Methods 9, 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey EL, Baker LE, 2016. Differential effects of 3,4-methylenedioxypyrovalerone (MDPV) and 4-methylmethcathinone (mephedrone) in rats trained to discriminate MDMA or a d-amphetamine + MDMA mixture. Psychopharmacology (Berl.) 233, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsley RR, Lhotkova E, Hajkova K, Feriancikova B, Himl M, Kuchar M, Páleníč T, 2018. Behavioural, Pharmacokinetic, Metabolic, and Hyperthermic Profile of 3,4-Methylenedioxypyrovalerone (MDPV) in the Wistar Rat. Front. Psychiatry 9, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hysek CM, Vollenweider FX, Liechti ME, 2010. Effects of a β-blocker on the cardiovascular response to MDMA (Ecstasy). Emerg. Med. J 27, 586–589. [DOI] [PubMed] [Google Scholar]
- Javadi-Paydar M, Harvey EL, Grant Y, Vandewater SA, Creehan KM, Nguyen JD, Dickerson TJ, Taffe MA, 2018. Binge-like acquisition of α-pyrrolidinopentiophenone (α-PVP) self-administration in female rats. Psychopharmacology (Berl.) 235, 2447–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HE, Wakeford A, Taylor W, Wetzell B, Rice KC, Riley AL, 2015. Sex differences in 3,4-methylenedioxypyrovalerone (MDPV)-induced taste avoidance and place preferences. Pharmacol. Biochem. Behav 137, 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuepfer MM, Branch CA, 1992. Cardiovascular responses to cocaine are initially mediated by the central nervous system in rats. J. Pharmacol. Exp. Ther 263, 734–741. [PubMed] [Google Scholar]
- Lynch WJ, Kalayasiri R, Sughondhabirom A, Pittman B, Coric V, Morgan PT, Malison RT, 2008. Subjective responses and cardiovascular effects of self-administered cocaine in cocaine-abusing men and women. Addict. Biol 13, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Seiden LS, 1998. Small changes in ambient temperature cause large changes in 3,4-methylenedioxymethamphetamine (MDMA)-induced serotonin neurotoxicity and core body temperature in the rat. J. Neurosci 18, 5086–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Antonazzo KR, Wiley JL, Blough BE, Partilla JS, Baumann MH, 2014. Pharmacology of novel synthetic stimulants structurally related to the “bath salts” constituent 3,4-methylenedioxypyrovalerone (MDPV). Neuropharmacology 87, 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW, 1998. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacol. 18, 263–271. [DOI] [PubMed] [Google Scholar]
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, Riley AL, 2014. Age‐dependent MDPV‐induced taste aversions and thermoregulation in adolescent and adult rats. Dev. Psychobiol 56, 943–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramoska E, Sacchetti AD, 1985. Propranolol-induced hypertension in treatment of cocaine intoxication. Ann. Emerg. Med 14, 1112–1113. [DOI] [PubMed] [Google Scholar]
- Rorabaugh BR, Seeley SL, Stoops TS, D’Souza MS, 2017. Repeated exposure to methamphetamine induces sex-dependent hypersensitivity to ischemic injury in the adult rat heart. PLoS ONE 12, e0179129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler CW, Tella SR, Goldberg SR, 1992. Adrenoceptor mechanisms in the cardiovascular effects of cocaine in conscious squirrel monkeys. Life Sci. 51, 653–660. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Suzuki M, Rice KC, Baumann MH, 2016. Pharmacological mechanisms underlying the cardiovascular effects of the “bath salt” constituent 3,4-methylenedioxypyrovalerone (MDPV). Br. J. Pharmacol 173, 3492–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu L-H, Huwyler J, Chaboz S, Hoener MC, Liechti ME, 2012. Pharmacological characterization of designer cathinones in vitro. Br. J. Pharmacol 168, 458–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons SJ, Gregg RA, Tran FH, Mo L, Weltin E, Barker DJ, Gentile TA, Watterson LR, Rawls SM, Muschamp JW, 2016. Comparing rewarding and reinforcing properties between “bath salt” 3,4‐methylenedioxypyrovalerone (MDPV) and cocaine using ultrasonic vocalizations in rats. Addict. Biol 23, 102–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivagnanam K, Chaudari D, Lopez P, Sutherland ME, Ramu VK, 2013. “Bath salts” induced severe reversible cardiomyopathy. Am. J. Case Rep 14, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Ren SE, Kiyatkin EA, 2015. Methylenedioxypyrovalerone (MDPV) mimics cocaine in its physiological and behavioral effects but induces distinct changes in NAc glucose. Front. Neurosci 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman JF, Lavins ES, Engelhart D, Armstrong EJ, Snell KD, Boggs PD, Taylor SM, Norris RN, Miller FP, 2013. Postmortem tissue distribution of MDPV following lethal intoxication by “bath salts”. J. Anal. Toxicol 37, 182–185. [DOI] [PubMed] [Google Scholar]
- Xiao D, Yang S, Zhang L, 2009. Prenatal cocaine exposure causes sex-dependent impairment in the myogenic reactivity of coronary arteries in adult offspring. J. Hypertens 54, 1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.