Abstract
Fanconi anemia (FA)-associated severe aplastic anemia (SAA) requires allogeneic hematopoietic cell transplantation (HCT) for cure. With the evolution of conditioning regimens over time, outcomes of alternative donor HCT (AD-HCT) have improved dramatically. We compared outcomes of HLA-matched sibling donor HCT (MSD-HCT; n =17) and AD-HCT (n = 57) performed for FA-associated SAA at a single institution between 2001 and 2016. Overall survival at 5 years was 94% for MSD-HCT versus 86% for AD-HCT, neutrophil engraftment was 100% versus 95%, platelet recovery was 100% versus 89%, grade II-IV acute graft-versus-host disease (GVHD) was 6% versus 12%, grade III-IV acute GVHD was 6% versus 4%, and chronic GVHD was 0 versus 7%, with no statistically significant differences by type of transplant. The use of UCB was associated with decreased rates of neutrophil recovery in AD-HCT and platelet recovery in both MSD-HCT and AD-HCT. A trend toward a higher serious infection density before day +100 post-HCT was observed in AD-HCT compared with MSD-HCT (P= .02). These data demonstrate that AD-HCT should be considered at the same time as MSD-HCT for patients with FA-associated SAA.
Keywords: Fanconi anemia, Severe aplastic anemia, Hematopoietic cell, transplantation, Alternative donor, Pediatric
INTRODUCTION
Fanconi anemia (FA) is a rare disorder resulting from mutation in 1 of 22 genes [1,2] encoding critical proteins for DNA damage repair during replication. One major consequence of faulty DNA repair is apoptosis and progressive contraction of the hematopoietic stem cell (HSC) pool, ultimately manifesting as cytopenias and severe aplastic anemia (SAA). At present, the sole curative therapy for FA-associated SAA is allogeneic hematopoietic cell transplantation (HCT). Historically, HCT outcomes were far superior in patients with FA receiving bone marrow (BM) or umbilical cord blood (UCB) HSCs from an HLA-matched sibling donor (MSD) [3] compared with HBCs from an alternative donor (AD; HLA-matched or -mismatched nonsibling related donor or unrelated donor) [4–8]; however, recent efforts to optimize conditioning regimens and donor graft manipulation have greatly improved AD-HCT for FA-associated SAA [9].
HCT for FA is uniquely challenging given the intrinsic poor ability of FA-affected cells to tolerate DNA damage incurred by standard conditioning regimen chemotherapy [10] and radiation [11]. Recognizing these cellular sensitivities, in the mid-1980s, Gluckman et al. [12] and others [13,14] pioneered an effective reduced-toxicity HCT conditioning regimen combining low-dose alkylator cyclophosphamide with limited irradiation. For the past 20 years, in MSD recipients conditioned with low-dose cyclophosphamide, fludarabine, and antithymocyte globulin (ATG), the reported incidences of graft failure and severe grade III/IV acute graft-versus-host disease (GVHD) have been <10%, with a 5-year overall survival (OS) >90% [3]. These excellent outcomes have prompted some families lacking an MSD for their child affected by FA-associated SAA to pursue preimplantation genetic diagnosis and in vitro fertilization to select an FA-unaffected HLA-matched embryo to create an MSD while expanding their family [15,16]. Alternatively, AD-HCT has been delayed with the use of transfusions and androgen therapy, both of which have been identified as independent risk factors for poor survival after HCT [4,9,17,18].
Although conditioning regimens for MSD recipients have remained largely unchanged over the past 2 decades, HCT conditioning regimens for AD recipients have evolved to reduce the comparatively high morbidity and mortality. Lymphodepleting fludarabine was incorporated to support donor engraftment/reduce graft rejection, thymic shielding was added during radiation to improve neothymopoiesis and reduce post-HCT infectious complications, radiation dosing was optimized, and BM grafts were manipulated by CD34+ selection to control the donor T cell dose and reduce graft-versus-host disease (GVHD) [9,19,20]. The effects of these sequential changes on AD-HCT in the FA population have been reported recently [9]. Here we directly compare the results of MSD-HCT and AD-HCT for FA-associated SAA at a single center in the modern era.
MATERIALS AND METHODS
Study Design
Pediatric patients (age <18 years) with FA-associated SAA undergoing HCT at the University of Minnesota between June 2001 and June 2016 were identified from a prospectively recorded blood and marrow transplantation database of demographic, clinical, and laboratory outcome measures, with analysis completed as of April 2017. All parents/guardians provided signed Institutional Review Board-approved informed consent, in accordance with the Declaration of Helsinki.
Patients
Patients eligible for HCT had a diagnosis of FA confirmed by chromosomal breakage analysis and SAA, defined as persistent absolute neutrophil count <0.5 × 109/L, hemoglobin <8 g/dL, and/or platelet count <20 × 109/L. Patients with advanced myelodysplastic syndrome [21], leukemia, and/or BRCA2/FANCD1 mutations were exclded from this analysis, while patients with inadequate organ function (eg, left ventricular ejection fraction <45%, any liver function test >5 times the normal range, oxygen saturation <92% in room air), active uncontrolled infection, poor performance status (Lansky score <50%), or a personal history of squamous cell carcinoma within 2 years were ineligible for HCT. Thirty AD-HCT recipients reported here were included in a previous publication [9].
Transplantation Procedure
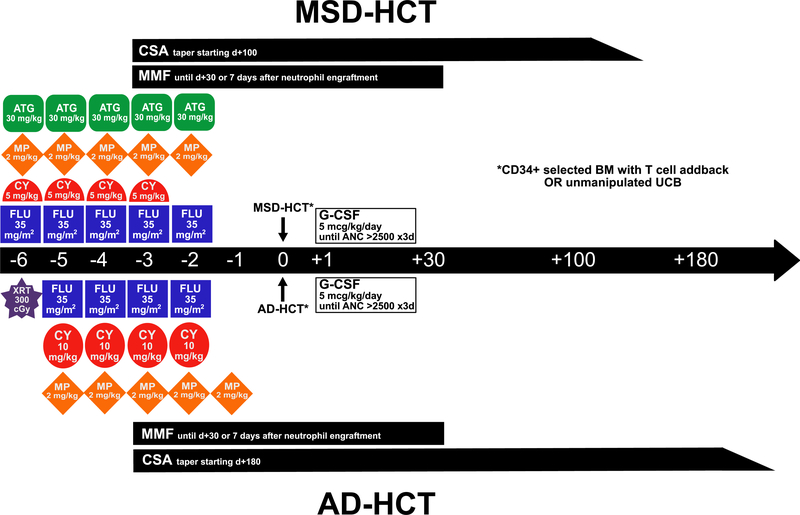
Patients with MSDs were conditioned as described previously [3] with fludarabine 175 mg/m2 (35 mg/m2 i.v. daily for 5 days, days −6 to −2), cyclophosphamide 20 mg/kg (5 mg/kg i.v. daily for 4 days, days −6 to −3), and equine ATG 150 mg/kg (30 mg/kg i.v. daily for 5 days, days −6 to −2, premedicated with methylprednisolone [MP] 2 mg/kg/day). Stem cell sources included HLA-identical BM and UCB.
Patients with ADs were conditioned with a single 300-cGy fraction of total body irradiation (TBI) on day −6, administered with thymic shielding as described previously [9]; fludarabine 140 mg/m2 (35 mg/m2 i.v. daily for 4 days, days −5 to −2); and cyclophosphamide 40 mg/kg (10 mg/kg i.v. daily for 4 days, days −5 to −2). AD recipients also received equine ATG 150 mg/kg (30 mg/kg i.v. daily for 5 days, days −5 to −1, premedicated with MP 2 mg/kg/day), MP alone, or neither during conditioning. AD stem cell sources included non-genotypically identical related donor or unrelated donor BM or UCB.
For both MSD and AD recipients, BM grafts underwent T cell depletion, using elutriation before 2002, Isolex 300 (Baxter, Deerfield, IL) from 2002 to 2010, and CliniMACs (Miltenyi Biotec, Auburn, CA) for CD34+ selection since 2010 [9]. An add-back of T cells to achieve a fixed graft dose of 1 × 105 CD3+ T cells/kg was provided. UCB stem cell sources were unmanipulated.
GVHD Prophylaxis
For all patients, GVHD prophylaxis began on day −3 before transplantation with a combination of cyclosporine (CSA) or sirolimus and either mycophenolate mofetil (MMF) or MP. CSA was dosed either orally or i.v., targeting a goal trough of 200 to 400 mg/L, sirolimus was dosed orally targeting a goal trough of 4 to 12 mg/L. A protocol-driven change from CSA to sirolimus was time-limited, affecting only 10 AD recipients, given the lack of an i.v. formulation and poor tolerance. MMF was dosed at 15 mg/kg/dose every 8 hours i.v. or orally with a maximum dose of 1 g, and was discontinued on day +30 or at 7 days after neutrophil engraftment, whichever occurred later. MP was dosed at 2 mg/kg/day until day +15, then tapered off by day +24. A 10- week CSA taper began for MSD recipients on day +100 and for AD recipients on day +180, in the absence of active GVHD or later, at least 1 month after control of acute GVHD was achieved.
Supportive Care
Before admission, patients were placed on yeast/mold antifungal prophylaxis for 1 month. On admission, patients were hospitalized in single-occupancy rooms with positive pressure high-efficiency particulate air filtration. Antibiotic prophylaxis was provided through neutrophil engraftment, and antifungal prophylaxis was provided until at least day +100. Patients seropositive for herpes simplex virus or cytomegalovirus (CMV), as well as those with a CMV-seropositive donor, received acyclovir antiviral prophylaxis until day +100. Prophylaxis against Pneumocystis jirovecii pneumonia was provided with trimethoprim-sulfamethoxazole (or an alternative in patients with medication allergy) after engraftment until 1 year post-HCT. Intravenous anti-infective coverage was broadened empirically for fever and subsequently adjusted per infection surveillance results. All patients received CMV-safe (CMV-seronegative or filtered) blood products. All patients received granulocyte-colony stimulating factor (G-CSF) 5 μg/kg i.v. daily from day +1 through neutrophil engraftment. Weekly blood CMV polymerase chain reaction (PCR) surveillance was prescribed until day +100 post-HCT, with preemptive ganciclovir or foscarnet therapy initiated on identification.
Endpoint Definitions
The time to neutrophil recovery was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥0.5× 109/L following ANC nadir. Inability to achieve an ANC of 0.5× 109/L by day +42 defined primary graft failure. Secondary graft failure was defined as a decline in ANC to <0.5× 109/L for 3 consecutive days or 0% donor DNA by molecular analysis, having previously achieved an ANC of ≥0.5× 109/L. Time to platelet recovery was the first of 3 consecutive days of a platelet count >20× 109/L without transfusion in the preceding 7 days. BM aspiration and biopsy were routinely performed at 21, 100, and 180 days and 1 and 2 years after HCT. Additional evaluations were prompted by concern for graft failure or malignant transformation. Donor chimerism was assessed by molecular analysis at these time points as well. GVHD was scored using standard criteria [22].
Infectious complication data were collected prospectively then audited retrospectively to ensure completeness and accuracy as described previously [23]. OS was defined as time from transplantation until death of any cause.
Statistical Analysis
Statistical comparisons by donor type (MSD versus AD) for categorical factors were completed with the chi-square test or Fisher’s exact test, in cases of limited expected counts, and for continuous factors by the general Wilcoxon rank-sum test for nonparametric data. Unadjusted estimates of OS were calculated by Kaplan-Meier curves [24]. Unadjusted estimates of neutrophil engraftment, platelet engraftment, and GVHD were analyzed using cumulate incidence, treating nonevent mortality as a competing risk [25]. Although primary comparisons in univariate analysis were outcomes by donor type, additional factors examined included patient sex (male versus female), patient-donor sex match (match versus mismatch), age (0–9 years versus 10–17 years), number of congenital malformations (0–2 versus 3+), pre-HCT transfusions (none versus any), pre-HCT renal function by glomerular filtrations rate (GFR; normal versus abnormal versus <40 mL/min/1.73 m2), diepoxybutane (DEB) chromosomal breakage mosaicism (≥90%−100% affected peripheral blood lymphocytes versus <90%), pre-HCT G-CSF use (no versus yes), and year of HCT (2001–2005 versus 2006–2010 versus 2011–2015). The independent effect of donor type on OS was assessed by Cox regression [26] and on engraftment and GVHD by Fine and Gray proportional hazards regression [27]. Visual plots and Martingdale residuals were used to test for violations of the proportional hazards assumption [28]. Rates of serious infections were reported from the time of transplantation to day +100 as infections per 1000 patient-days, allowing for multiple infections per patient, and compared across donor types using the Mantel-Haenszel chi-square test for incidence density. Given multiple comparisons, only P values <.01 are considered significant in analysis of serious infections. All reported P values are 2-sided. SAS version 9.3 (SAS Institute, Cary, NC) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria) were used for all statistical analyses.
RESULTS
Patient, Donor, and HCT Characteristics
Seventy-four patients with FA-associated SAA underwent HCT, including 17 with an MSD and 57 with an AD. There were no differences between MSD and AD recipients in terms of age at transplantation, sex, recipient-donor sex mismatch, BM versus UCB donor source, time from diagnosis to HCT, complementation group, DEB sensitivity (mean number of chromosome breaks/cell in vitro) or mosaicism (defined as present if >10% of cells revealed resistance to DEB), number of congenital malformations, incidence of androgen use, or number of blood product transfusions before HCT (Table 1). AD recipients did demonstrate a statistically significant greater use of G-CSF before HCT (53% vs 12%; P < .01). Evaluations immediately before HCT revealed no differences between MSD and AD recipients in terms of renal function, performance scale scoring, and current serious infection (the latter absent in all recipients).
Table 1.
Patient, Donor, and HCT Characteristics
| Characteristic | All Patients (n = 74) | MSD-HCT (n = 17) | AD-HCT (n = 57) | P Value |
|---|---|---|---|---|
| Pre-HCT characteristics | ||||
| Male patients, n (%) | 42 (57%) | 8 (47%) | 34 (60%) | .36 |
| Patient-donor sex mismatch | 34 (47%) | 9 (53%) | 25 (45%) | .59 |
| Age at HCT, yr, median (range) | 8 (2–15) | 8 (3–13) | 8 (2–15) | .74 |
| Time from diagnosis to HCT, mo, median (range) | 27 (2–143) | 23 (2–131) | 27 (3–143) | .29 |
| Complementation group, n (%) | NA | |||
| FANCA | 50 (68) | 12 (71) | 38 (67) | |
| FANCC | 10 (14) | 1 (6) | 9 (16) | |
| FANCD2 | 3 (4) | 3 (18) | 0 | |
| FANCF | 1 (1) | 0 | 1 (2) | |
| FANCG | 3 (4) | 0 | 3 (5) | |
| FANCJ | 2 (3) | 0 | 2 (4) | |
| FANCP | 1 (1) | 0 | 1 (2) | |
| Unknown | 4 (5) | 1 (6) | 3 (5) | |
| DEB sensitivity*, median (range) | 7.0 (.4–16.0) | 7.0 (1.2–13.5) | 6.9 (.4–16.0) | .89 |
| DEB mosaicism†, n (%) | 23 (31) | 6 (35) | 17 (30) | .73 |
| Congenital malformations, n (%) | .91 | |||
| 0–2 | 27 (36) | 6 (35) | 21 (37) | |
| 3+ | 47 (64) | 11 (65) | 36 (63) | |
| Previous therapies, n (%) | ||||
| Blood product transfusions ≥1 | 40 (54) | 6 (35) | 34 (60) | .08 |
| Androgen use | 3 (4) | 0 | 3 (5) | .33 |
| G-CSF use | 32 (43) | 2 (12) | 30 (53) | <.01 |
| Organ function and performance status, n (%) | ||||
| Serious infection | 0 | 0 | 0 | — |
| Patient CMV IgG-positive | 26 (35) | 7 (41) | 19 (33) | .55 |
| Glomerular filtration rate | >.99 | |||
| ≥40 mL/min/1.73 m2 | 69 (93) | 16 (94) | 53 (93) | |
| <40 mL/min/1.73 m2 | 5 (7) | 1 (6) | 4 (7) | |
| Lansky performance scale score <100% | 13 (18) | 4 (24) | 9 (16) | .48 |
| HCT characteristics | ||||
| Year of transplantation, n (%) | <.01 | |||
| 2001–2005 | 7 (9) | 7 (41) | 0 | |
| 2006–2010 | 37 (50) | 7 (41) | 30 (53) | |
| 2011–2015 | 30 (41) | 3 (18) | 27 (47) | |
| Stem cell source, n (%) | NE | |||
| 8/8 HLA MSD BM | 9 (12) | 9 (53) | ||
| 6/6 HLA MSD UCB | 8 (11) | 8 (47) | ||
| 8/8 HLA parental BM | 1 (1) | 1 (2) | ||
| 7/8 HLA mMSD BM | 1 (1) | 1 (2) | ||
| 7/8 HLA mm parental BM | 2 (3) | 2 (4) | ||
| 5/6 HLA mMSD sUCB + BM boost | 1 (1) | 1 (2) | ||
| 5–6/6 HLA (m)MUD BM | 32 (56) | |||
| 4–6/6 HLA (m)MUD sUCB | 52 (70) | 20 (35) | ||
| Stem cell dose, median (range) | ||||
| TNC (×107/kg) | 2.7 (.1–24.8) | 3.9 (.3–17.4) | 2.4 (.1–24.8) | .73 |
| BM | .7 (.1–24.8) | .5 (.3–17.4) | .7 (.1–24.8) | |
| UCB | 4.7 (2.2–12.0) | 4.6 (2.2–12.0) | 5.3 (2.2–10.2) | |
| CD34 (×106/kg), median (range) | 1.8 (.04–15.4) | 2.7 (.2–10.1) | 1.8 (.04–15.4) | .87 |
| BM | 3.1 (.04–15.4) | 4.1 (1.7–10.1) | 2.6 (.04–15.4) | |
| UCB | .7 (.1–2.7) | .5 (.2–2.7) | .7 (.1–1.8) | |
| GVHD prophylaxis, n (%) | NE | |||
| MP alone | 1 (1) | 1 (6) | ||
| CSA × MP | 41 (55) | 13 (76) | 28 (49) | |
| CSA + MMF | 22 (30) | 3 (18) | 19 (33) | |
| Sirolimus + MMF | 10 (14) | 10 (18) | ||
| HCT hospitalization, d, median (range) | 28 (11–211) | 24 (20–52) | 28 (11–211) | .02 |
| Follow-up, yr, median (IQR) | 7.0 (3.9–9.6) | 10.3 (8.0–14.0) | 6.2 (3.3–8.7) | <.01 |
Significant P values are in bold type.
NA indicates not applicable; NE, not evaluated; mMSD, mismatched sibling donor; mm, mismatched; sUCB, single umbilical cord blood; IQR, interquartile range.
DEB sensitivity: mean chromosome breaks/cell.
DEB mosaicism: presence of >10% DEB-resistant lymphocytes.
Conditioning regimens and GVHD prophylaxis were as described in the Methods as well as Figure 1. Stem cell sources for MSD recipients included BM in 9 (53%) and UCB in 8 (47%), with a median total nucleated cell (TNC) dose of 3.9 × 107 cells/kg of patient weight and .5 ×106 CD34+ cells/kg. For AD recipients, stem cell sources were heterogeneous in terms of donor and degree of HLA matching as shown in Table 1, with the majority of patients receiving BM (n = 36 [63%]; UCB, n = 20 [35%]; combination of BM and UCB, n = 1 [2%]). The median cell dose for AD-HCT recipients was 2.2 × 107 TNCs/kg and.7 × 106 CD34+ cells/kg. Cell doses were not statistically different between MSD-HCT and AD-HCT. The duration of hospitalization for HCT (including those patients who died before discharge) was a median of 4 days longer in AD-HCT recipients compared with MSD-HCT recipients (28 days [range, 11–211 days] versus 24 days [range, 20–52 days]; P = .02).
Figure 1.
MSD- and AD-HCT conditioning regimens and GVHD prophylaxis for FA-associated SAA. CY, cyclophosphamide; FLU, fludarabine; XRT, irradiation (with thymic shielding); SIRO, sirolimus.
Survival
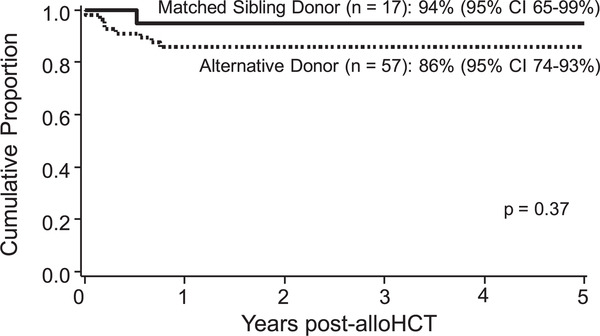
OS at 5 years post-HCT was not statistically significantly different between the 2 groups (94% [95% CI, 65%−99%] for MSD-HCT recipients and 86% [95% CI, 74%−93%] for AD-HCT recipients; P = .37) (Figure 2). The median duration of followup was 10.3 years for MSD-HCT recipients and 6.2 years for AD-HCT recipients is 10.3 and 6.2 years, with all deaths occurring within the first year after HCT. The risk of death increased with poor pre-HCT renal function, with only 60% OS for the 5 patients with a GFR <40 mL/min/1.73 m2, compared with 90% for the 69 patients with better renal function (univariate analysis, P = .02; multiple regression, HR, 5.4; P = .04). Pre-HCT androgen use also was associated with decreased 5-year OS, with a survival estimate of 33% for the 3 patients requiring androgen and 90% for the 71 patients without exposure (univariate analysis, P < .01; multiple regression, HR, 10.1; P < .01). However, for both of these factors, the low patient numbers reduce confidence in the reported significance.
Figure 2.
No difference in 5-year OS between MSD-HCT and AD-HCT.
Donor type had no association with OS in multiple regression (AD-HCT: HR, 1.9: 95% CI, .2–16.2; P = .54 compared with the MSD-HCT reference group). However, a statistically significant difference in OS by stem cell source was observed, with UCB associated with an increased risk of death compared with BM in multiple regression analysis (UCB: HR, 6.3; 95% CI, 1.3–31.0; P = .02). There were too few deaths to allow further analysis of the interaction between donor type (MSD versus AD) and stem cell source (BM versus UCB). The 9 patients who died following HCT included 1 MSD-HCT recipient who died of GVHD and 8 AD-HCT recipients who died from various attributable causes, including graft failure (n = 3), GVHD (n = 2), infection (n = 1), regimen-related toxicity (n = 1), and multiorgan failure (n = 1).
Engraftment
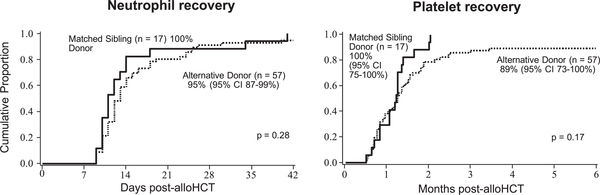
Neutrophil engraftment was achieved by day +42 after HCT in 96% of patients, with no statistically significant difference (P = .28) observed between MSD-HCT and AD-HCT recipients (Figure 3). The median day of neutrophil recovery was day +11 (range, days +9-+41) after HCT for MSD-HCT recipients and day +13 (range, days +9-+40) after HCT for AD-HCT recipients. In multiple regression, no factors were associated with neutrophil recovery, including donor type (AD-HCT: HR, .8; 95% CI, .5–1.4; P = .42 compared with MSD-HCT as the reference group). In a separate multiple regression model investigating the interaction between donor type (MSD versus AD) and stem cell source (BM versus UCB) with MSD BM as the reference group, both MSD UCB recipients (HR, .1; 95% CI, .04-.3; P < .01) and AD UCB recipients (HR, .1; 95% CI, .03-.2; P < .01) had an increased risk of failed neutrophil engraftment. However, AD UCB did not convey an increased risk of failed neutrophil engraftment compared with MSD UCB (HR, .9; 95% CI, .3–1.7; P = .49). There were too few graft failures to allow further evaluation of risk factors. In brief, the 3 affected patients received mismatched unrelated UCB before routine assessment of donor-specific anti-HLA antibodies. Cell doses were within the expected range for a high probability of engraftment (3.1–3.7 × 107 TNCs/kg and .3-.7 × 106 CD34+ cells/kg recipient weight).
Figure 3.
No difference in neutrophil or platelet count recovery between MSD-HCT and AD-HCT.
Platelet recovery was achieved by 6 months after HCT in 92% of patients, with no statistically significant differences between MSD-HCT and AD-HCT recipients (100% platelet engraftment; 95% CI, 75%−100% versus 89% platelet engraftment; 95% CI, 73%−100%; P = .17) (Figure 3). In multiple regression, only the need for blood product transfusions before HCT was associated with failed platelet recovery (HR, .5; 95% CI, .3-.9; P = .01). In a separate multiple regression model investigating the interaction between donor type (MSD versus AD) and stem cell source (BM versus UCB) with MSD BM as the reference group, the risk of failed platelet recovery was statistically significantly higher in all other groups (AD BM: HR, .4; 95% CI, .2-.8; P = .01; MSD UCB: HR, .4; 95% CI, .2-.8; P < .01; AD UCB: HR, .3; 95% CI, .1-.5; P < .01). However, as with neutrophil engraftment, no increased risk was seen for failed platelet recovery comparing AD UCB with MSD UCB (HR, .6; 95% CI, .3–1.2; P = .14).
GVHD
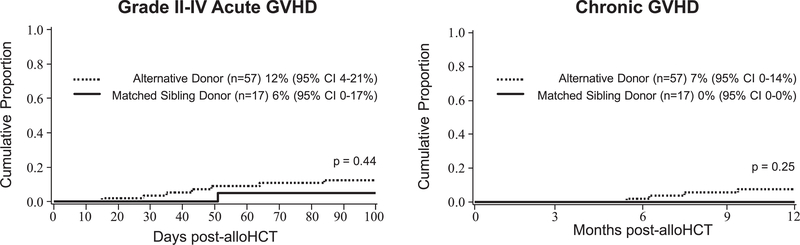
The incidence of grade II-IV acute GVHD by day +100 in all patients was 11% (95% CI, 4%−18%). Univariate analysis revealed no difference by donor type (6% [95% CI, 0–17%] for MSD-HCT versus 12% [95% CI, 4%−21%] for AD-HCT; P = .44) (Figure 4), but an increased risk with UCB as a stem cell source compared with BM (21% [95% CI, 6%−35%] for UCB versus 4% [95% CI, 0–10%] for BM; P = .02). In multiple regression, UCB was associated with grade II-IV acute GVHD (HR, 5.1; 95% CI, 1.1–24.3; P = .04). However, the incidence of acute GVHD was too rare to allow further evaluation of an interaction between donor type (MSD versus AD) and stem cell type (BM versus UCB) and this complication.
Figure 4.
No difference in incidence of acute or chronic GVHD between MSD-HCT and AD-HCT.
The overall incidence of grade III-IV acute GVHD by day +100 was 4% (95% CI, 0–9%) (Figure 2), occurring in 1 MSD recipient and in 2 AD recipients. No patient-related or transplantation-related factors were associated with severe acute GVHD. The incidence of chronic GVHD by 12 months post-HCT was 5% (95% CI, 0–11%), occurring only in 4 AD recipients, with no associated patient- or transplantation-related risk factors (Figure 4).
Infectious Complications
No significant differences in infection density were identified between MSD-HCT and AD-HCT recipients (Table 2). AD-HCT recipients trended toward more infections overall, with 10.9 serious infections per 1000 patient-days, compared with 4.71 for MSD-HCT recipients (P = .02). Specifically, AD-HCT recipients demonstrated higher rates of non-Clostridium difficile serious bacterial infections (infection density of 7.63 per 1000 patient-days in AD-HCT versus 2.35 per 1000 patient-days in MSD-HCT; P = .02), although this difference failed to reach statistical significance. There were no differences in Candida or Aspergillus fungal infections, CMV, Epstein-Barr virus, or other viral infections. Neither CD4+ T cell count nor IgG level at day +100 was associated with the incidence of bacterial, fungal, or viral infection (data not shown).
Table 2.
Infection Density: Number of Infections Per 1000 Patient-Days Occurring Before Day +100 Post-HCT
| Serious Infection | All Patients (n = 74) |
MSD-HCT (n = 17) |
AD-HCT (n = 57) |
P Value* |
|---|---|---|---|---|
| Fungal | ||||
| Aspergillus | .42 | 0 | .55 | .34 |
| Candida | .28 | .59 | .18 | .38 |
| Bacterial | ||||
| Non-Clostridium difficile | 6.39 | 2.35 | 7.63 | .02 |
| Viral | ||||
| CMV | 1.11 | 1.17 | 1.09 | .93 |
| Epstein-Barr virus | .42 | 0 | .55 | .34 |
| Other† | .83 | .59 | .91 | .69 |
| Total serious infections per 1000 patient-days | 9.44 | 4.71 | 10.90 | .02 |
Only P values < .01 should be considered significant due to multiple comparisons.
Other viral infections include adenoviremia, HHV-6 pneumonitis and viremia, HSV viremia, BK viremia with associated hemorrhagic cystitis requiring antiviral therapy, varicella, and influenza A.
DISCUSSION
Using modern strategies of conditioning and GVHD prophylaxis, we have demonstrated that children with FA undergoing HCT for SAA have excellent survival and neutrophil and platelet engraftment along with low rates of acute and chronic GVHD, with equivalent outcomes using MSD and AD stem cell sources. Current recommendations for monitoring of BM function in FA include complete blood count with differential every 3 months and annual BM biopsies with aspirate for cellularity, screening for myelodysplastic changes, cytogenetic evolution, and leukemia by flow cytometry [20]. HCT is indicated for SAA, defined as a persistent ANC <0.5 × 109/L, hemoglobin <8 g/dL, and/or platelet count <20 × 109/L. Optimal timing for HCT and donor selection is best accomplished at a blood and marrow transplantation center with FA expertise.
Historically, patients with FA-associated SAA lacking a MSD received androgen therapy and/or blood product transfusions to delay AD-HCT, given inferior outcomes. However, we now have evidence of comparable outcomes with MSD-HCT and AD-HCT, along with additional evidence that such delay strategies are deleterious. Here we report that pre-HCT androgen exposure is associated with decreased 5-year OS. Although our confidence in these findings is low given the small number of patients in these exposure groups (only 3 of 74 with androgen use), pre-HCT androgen use has been identified as an independent risk factor for poor survival in several studies of HCT for FA [4,17,29]. We also found platelet engraftment to be negatively correlated with pre-HCT blood product transfusion(s). Although it was not directly assessed, this association is suggestive of alloimmunization interfering with platelet recovery. With evidence of equivalent outcomes with MSD and AD-HCT for FA-associated SAA, patients lacking an MSD should proceed expeditiously to AD-HCT before exposure to androgens and/or blood product transfusions.
Among the 17 patients with FA undergoing MSD-HCT, 4 sibling donors (24%) were products of preimplantation genetic diagnosis/in vitro fertilization. This complex process involves genetic testing to ensure a selected embryo is both free of FA and an HLA match to the existing child with FA before pregnancy [30]. With dramatic improvements in the outcomes of AD-HCT for FA as demonstrated here, families may no longer feel obligated to undergo such costly and emotionally taxing measures to find a reliable donor source for their affected child.
UCB appears to be a less favorable stem cell source compared with BM, here associated with delayed neutrophil recovery, increased incidence of grade II-IV acute GVHD, and poorer survival, though we were unable to further associate this latter finding with donor type given the small number of deaths. HLA-mismatch of UCB is a known risk factor for transplantation-related mortality in patients with nonmalignant disease. Of the 5 AD-HCT deaths after UCBT, examination of high-resolution HLA-typing of HLA-A, -B, -C, and -DRB1 revealed donor-recipient pairs were mismatched at 2 of 8 alleles in 2 patients and in 3, 4, and 6 of 8 alleles in 1 patient each. MacMillan et al. [9] recently reported on a larger cohort of FA AD-HCT recipients from the University of Minnesota (n = 130, not limited to SAA as HCT indication) again showing delayed neutrophil recovery with UCB, but no statistically significant difference in OS between stem cell source (BM versus UCB). The association of increased grade II-IV acute GVHD and UCB likely reflects the heterogeneity within the AD UCB recipient group, encompassing HLAmatching ranging from 4 to 6/6, as well as the extremely low rates of GVHD realized with TCD of BM. In the literature on HCT for FA, T cell-depleted BM is the optimal stem cell source for this population, demonstrating low rates of GVHD (F03C10% for severe grade III-IV acute GVHD and <5% for chronic GVHD) [9,31]. Minimization of GVHD is critical in this patient population, in which this post-HCT complication contributes to development of head and neck cancers [7,32,33].
Recipients of AD grafts trend toward a higher incidence of infections and demonstrate statistically significantly longer duration of hospitalization after HCT compared with MSD recipients, highlighting future targets for improvement. Considerations for differences in infection rates between AD and MSD recipients include the role of serotherapy and/or UCB use in delayed T cell reconstitution, and the lack of virus-specific T cells available in the donor inoculum when using UCB stem cell sources. Higher rates of infection also may contribute to the slightly longer duration of hospitalization in AD-HCT recipients. The lack of association between day +100 CD4+ T cell count or IgG level with infection density is not surprising. Although lower cell counts and immunoglobulin levels might be expected to correlate with increased infection, increases in these values can be a reflection of an appropriate immune response to infection, making these measures poor biomarkers of infection risk.
Limitations of this study include the small number of subjects, particularly in the MSD-HCT group, and restriction to a single center. However, this latter limitation allowed for homogeneity in transplantation eligibility between the MSD-HCT and AD-HCT groups and consistent data collection and reporting.
In summary, patients with FA-associated SAA today have an equivalent chance of long-term survival with low risk of GVHD with either MSD or AD stem cell sources for HCT. Transplantation should not be delayed for lack of an MSD. To further improve on these AD-HCT outcomes, we are considering methods to reduce the incidence of post-HCT infections and subsequently improve hospital length of stay and OS. Such methods may include replacement of CD34+ selection of BM with TCR-αβ depletion, allowing for infusion of innate type γδ-T cells with strong antiviral effects [34] and prompt initiation of adoptive immunotherapy with virus-specific T cells to supplement current antiviral pharmacotherapies. T cell-depleted BM continues to show superior outcomes compared with UCB as a stem cell source in terms of OS, neutrophil recovery, and rate of GVHD, and is preferred when available. Methods of ex vivo stem cell expansion may be used to bolster stem cell numbers and improve hematopoietic recovery following UCBT [35].
ACKNOWLEDGMENTS
The authors thank their patients and families affected by Fanconi anemia.
Financial disclosure: This work was partially supported by the Fanconi Anemia Research Fund, Children’s Cancer Research Fund and Kidz1stFund.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Dong H, Nebert DW, Bruford EA, Thompson DC, Joenje H, Vasiliou V. Update of the human and mouse Fanconi anemia genes. Hum Genomics. 2015;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knies K, Inano S, Ramirez MJ, et al. Biallelic mutations in the ubiquitin ligase RFWD3 cause Fanconi anemia. J Clin Invest. 2017;127:3013–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan PL, Wagner JE, Auerbach AD, Defor TE, Slungaard A, Macmillan ML. Successful engraftment without radiation after fludarabine-based regimen in Fanconi anemia patients undergoing genotypically identical donor hematopoietic cell transplantation. Pediatr Blood Cancer. 2006;46:630–636. [DOI] [PubMed] [Google Scholar]
- 4.Guardiola P, Pasquini R, Dokal I, et al. Outcome of 69 allogeneic stem cell transplantations for Fanconi anemia using HLA-matched unrelated donors: a study on behalf of the European Group for Blood and Marrow Transplantation. Blood. 2000;95:422–429. [PubMed] [Google Scholar]
- 5.Gluckman E, Rocha V, Ionescu I, et al. Results of unrelated cord blood transplant in Fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant. 2007;13:10731082. [DOI] [PubMed] [Google Scholar]
- 6.Wagner JE, Eapen M, MacMillan ML, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007; 109:2256–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peffault de Latour R, Porcher R, Dalle JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:42794286. [DOI] [PubMed] [Google Scholar]
- 8.Zecca M, Strocchio L, Pagliara D, et al. HLA-haploidentical T cell-depleted allogeneic hematopoietic stem cell transplantation in children with Fanconi anemia. Biol Blood Marrow Transplant. 2014;20:571–576. [DOI] [PubMed] [Google Scholar]
- 9.MacMillan ML, DeFor TE, Young JA, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood. 2015;125:37983804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auerbach AD, Adler B, O’Reilly RJ, Kirkpatrick D, Chaganti RS. Effect of procarbazine and cyclophosphamide on chromosome breakage in Fanconi anemia cells: relevance to bone marrow transplantation. Cancer Genet Cytogenet. 1983;9:25–36. [DOI] [PubMed] [Google Scholar]
- 11.Gluckman E, Devergie A, Dutreix J. Radiosensitivity in Fanconi anaemia: application to the conditioning regimen for bone marrow transplantation. Br J Haematol. 1983;54:431–440. [DOI] [PubMed] [Google Scholar]
- 12.Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856–2862. [PubMed] [Google Scholar]
- 13.Dufour C, Rondelli R, Locatelli F, et al. Stem cell transplantation from HLA-matched related donor for Fanconi’s anaemia: a retrospective review of the multicentric Italian experience on behalf of Associazione Italiana di Ematologia ed Oncologia Pediatrica (AIEOP)–Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Br J Haematol. 2001;112:796805. [DOI] [PubMed] [Google Scholar]
- 14.Farzin A, Davies SM, Smith FO, et al. Matched sibling donor haematopoietic stem cell transplantation in Fanconi anaemia: an update of the Cincinnati Children’s experience. Br J Haematol. 2007;136:633–640. [DOI] [PubMed] [Google Scholar]
- 15.Verlinsky Y, Rechitsky S, Schoolcraft W, Strom C, Kuliev A. Preimplantation diagnosis for Fanconi anemia combined with HLA matching. JAMA. 2001;285:3130–3133. [DOI] [PubMed] [Google Scholar]
- 16.Grewal SS, Kahn JP, MacMillan ML, Ramsay NK, Wagner JE. Successful hematopoietic stem cell transplantation for Fanconi anemia from an unaffected HLA-genotype-identical sibling selected using preimplantation genetic diagnosis. Blood. 2004;103:1147–1151. [DOI] [PubMed] [Google Scholar]
- 17.Pasquini R, Carreras J, Pasquini MC, et al. HLA-matched sibling hematopoietic stem cell transplantation for Fanconi anemia: comparison of irradiation and nonirradiation containing conditioning regimens. Biol Blood Marrow Transplant. 2008;14:1141–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell R, Wagner JE, Hirsch B, DeFor TE, Zierhut H, MacMillan ML. Haematopoietic cell transplantation for acute leukaemia and advanced myelodysplastic syndrome in Fanconi anaemia. Br J Haematol. 2014;164:384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia—when and how? Br J Haematol. 2010;149:14–21. [DOI] [PubMed] [Google Scholar]
- 20.Ebens CL, MacMillan ML, Wagner JE. Hematopoietic cell transplantation in Fanconi anemia: current evidence, challenges and recommendations. Expert Rev Hematol 2017;10:81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cioc AM, Wagner JE, MacMillan ML, DeFor T, Hirsch B. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with Fanconi anemia: morphologic and cytogenetic characteristics. Am J Clin Pathol. 2010;133:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. [DOI] [PubMed] [Google Scholar]
- 23.Barker JN, Hough RE, van Burik JA, et al. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362–370. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. [DOI] [PubMed] [Google Scholar]
- 26.Cox DR. Regression models and life-tables. J Roy Stat Soc B. 1972;34:187220. [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28.Collett D Modelling Survival Data in Medical Research. 2nd ed Bristol, UK: Chapman & Hall/CRC Press; 2003. [Google Scholar]
- 29.Macmillan ML, Blazar BR, Defor T, et al. Alternate donor HCT for Fanconi anemia (FA): results of a total body irradiation (TBI) dose de-escalation study. Blood. 2008;112:2998.18840715 [Google Scholar]
- 30.Zierhut H, MacMillan ML, Wagner JE, Bartels DM. More than 10 years after the first “savior siblings”: parental experiences surrounding preimplantation genetic diagnosis. J Genet Couns. 2013;22:594–602. [DOI] [PubMed] [Google Scholar]
- 31.Aker M, Varadi G, Slavin S, Nagler A. Fludarabine-based protocol for human umbilical cord blood transplantation in children with Fanconi anemia. J Pediatr Hematol Oncol. 1999;21:237–239. [DOI] [PubMed] [Google Scholar]
- 32.Deeg HJ, Socié G, Schoch G, et al. Malignancies after marrow transplantation for aplastic anemia and Fanconi anemia: a joint Seattle and Paris analysis of results in 700 patients. Blood. 1996;87:386–392. [PubMed] [Google Scholar]
- 33.Guardiola P, Socié G, Li X, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103:73–77. [DOI] [PubMed] [Google Scholar]
- 34.Airoldi I, Bertaina A, Prigione I, et al. γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCRαβ+/CD19+ lymphocytes. Blood. 2015;125:2349–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner JE Jr, Brunstein CG, Boitano AE, et al. Phase I/II trial of StemRegenin-1 expanded umbilical cord blood hematopoietic stem cells supports testing as a stand-alone graft. Cell Stem Cell. 2016;18:144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]