Abstract
Background:
Children living near greenhouse agriculture may have an increased risk of pesticide exposure due to drift or direct contact with pesticide-treated areas. However, little is known about whether this increased potential for chronic exposure may impair their neurodevelopment.
Methods:
We examined 307 children aged 4-9 years, living in agricultural communities in Ecuador (ESPINA study). The two exposures calculated were residential distance from the nearest flower plantation perimeter and flower plantation surface area within 100m of homes. Five neurobehavioral domains were assessed: Attention/Inhibitory Control, Memory/Learning, Visuospatial processing and Sensorimotor (higher values reflect better performance). Low scores were defined according to the test’s cut-offs. Models were adjusted for demographic, socio-economic and growth variables.
Results:
The mean (SD) residential distance to the nearest flower plantation was 446m (344). Living 100m closer to crops was associated with increased odds (OR [95% CI]) of low scores in the domains of Memory/Learning (1.24 [1.05, 1.46]) and Language (1.09 [1.00, 1.19]). Associations were strongest among children living within 50m, having significantly lower scores in Language (−1.28 which is ~50% of a SD [−2.50, −0.06]), Attention/Inhibitory Control (−1.24 units, [−2.45, −0.04]), and Memory/Learning (−0.91, [−1.99, 0.17]), compared to children living farther than 500m. Analyses of areas of flower crops near homes concurred with these findings.
Conclusions:
Close residential proximity to greenhouse floricultural crops was associated with adverse neurobehavioral performance in Attention/Inhibitory Control, Language and Memory/Learning among children. This highlights the importance of reducing pesticide drift from plantations to nearby homes.
Keywords: Pesticides, Agriculture, Children, Residential proximity, Neurodevelopment, Drift
INTRODUCTION
Children of farmworkers or those residing in close proximity to crop production have an increased risk of exposure to pesticides (Coronado et al., 2011; Hyland and Laribi, 2017; Loewenherz et al., 1997; Lu et al., 2000). Exposures to neurotoxic pesticides in the prenatal period or early childhood may compromise healthy neurodevelopment (Bouchard et al., 2011; Burns et al., 2013; Eskenazi et al., 2007; Grandjean, 2006; Harari et al., 2010; Shelton et al., 2014; J. R. Suarez-Lopez et al., 2013). In a birth cohort in California, increasing levels of neurotoxic pesticides used within 1 km of maternal residence during pregnancy was associated with decreases in children’s cognitive function at 7-years (Gunier et al., 2017) and 10-years (Rowe et al., 2016) of age. For each standard deviation (SD) increase in agricultural use of total organophosphates (OPs) there was a 2-point decrease in Full-Scale IQ (Gunier et al., 2017). Three case-control studies of autism spectrum disorders (ASD) in California found that pregnant mothers had greater odds of having children later diagnosed with ASD if they lived near OP, pyrethroid organochlorine or other pesticide spray and application sites (Roberts et al., 2007; Carmichael et al., 2016; Ehrenstein et al., 2019).
The use of OPs and other classes of insecticides, fungicides and herbicides has been well documented throughout Pedro Moncayo County, Ecuador, a county with one of the highest concentrations of rose plantations on the continent and where flower production is a primary revenue driver (Grandjean et al., 2006; Handal et al., 2016; Harari, 2004; Suarez-Lopez et al., 2017a). Rose production is typically enclosed within greenhouses; however, pesticides may drift from the greenhouses through ventilation windows. Production of other flowers in open fields also exists, although in substantially smaller amounts. There is currently a need to understand not only the exposure patterns of children living near greenhouse agriculture, but also whether the neurobehavioral performance of such children may also be affected as a result of chronic pesticide drift from such crops. In the study of Secondary Exposures to Pesticides among Children and Adolescents (ESPINA: Estudio de la Exposición Secundaria a Plaguicidas en Niños y Adolescentes) based in Pedro Moncayo County, we observed that children living near flower crops had lower acetylcholinesterase (AChE) activity (reflecting greater exposure to cholinesterase inhibitor pesticides) and higher blood pressure compared to children living farther away (Suarez-Lopez et al., 2018). Because of these findings and our previously reported positive associations between AChE activity and neurobehavioral performance in boys (J.R. Suarez-Lopez et al., 2013) and transient alterations in neurobehavioral performance in relation to a peak pesticide spray season (Suarez-Lopez et al., 2017b), we hypothesized that children living near greenhouse floricultural production sites, assessed during the same time period with respect to harvest season, had worse neurobehavioral performance compared to children living farther away.
METHODS
Study Population
Seventy three percent of ESPINA participants were recruited using contact information from their participation in the 2004, county-wide, Survey of Access and Demand of Health Services in Pedro Moncayo County, a large representative survey of Pedro Moncayo County conducted by Fundacion Cimas del Ecuador in collaboration with the communities of Pedro Moncayo County. Using home addresses from this survey, 419 participants were contacted and invited to participate in the ESPINA study. Participant losses in this case were mostly due to missing or inaccurate information or changes in residential address. The remaining 27% of participants were recruited by word of mouth or community announcements. Children were selected after parents completed a pre-survey, which provided enough information to classify children who lived with a floricultural worker or without any agricultural workers. To be included, children who lived with a floricultural worker must have done so for at least one year. Children living without floricultural workers included children who had never cohabitated with any agricultural worker, had never inhabited a home where agricultural pesticides were stored and had no previous direct contact with agricultural pesticides. In 2008 a total of 313 children between the ages of 4 and 9 were examined in the months of July and August. Further assessment included parental surveys at the subjects’ homes and examinations of child participants in seven schools in Pedro Moncayo County. The present analyses include 304 participants who had information for all covariates of interest. Among the participants, informed consent was obtained from at least one parent in each case and verbal assent was obtained from all children older than seven years of age. Cases where parental consent or child assent were missing are not included in this study. Participant recruitment is described in more detail in Suarez-Lopez et al., 2012.
The ESPINA study was approved by the Institutional Review Boards of the University of Minnesota, The University of California San Diego, Universidad San Francisco de Quito and the Ministry of Public Health of Ecuador, and is endorsed by the Commonwealth of Pedro Moncayo County.
Proximity to Floriculture
Geographical coordinates of Pedro Moncayo County homes were obtained through portable global positioning system receivers in 2004, 2006 and 2010 by Fundacion Cimas del Ecuador, as part of the System of Local and Community Information (Sistema de Información Local y Comunitario [SILC]). Flower plantation edges (areal polygons) were created using satellite imagery from 2006 (Suarez-Lopez et al., 2018). Distance between the child’s home and the nearest 1m segment of the nearest flower plantation perimeter was calculated using ArcGIS 9.3. We also calculated the total area of flower crops within 100m of participants’ homes.
Neurobehavioral Performance
Neurodevelopmental assessments were conducted using the NEPSY-2, a standardized and validated neurodevelopment test battery for children of ages 3 to 16 years (Davis and Matthews 2010; Korkman et al., 2007). Children were tested in 11 age-appropriate subtests in five domains: Attention & Inhibitory Control (also known as Attention and Executive Functioning; subtests: auditory attention and response set, inhibition, statue), Language (comprehension of instructions, speeded naming), Memory & Learning (memory-for-faces immediate and delayed, narrative memory), Sensorimotor (Visuomotor precision) and Visuospatial Processing (design copying, geometric puzzles). Descriptions of each subtest have been described elsewhere (Korkman et al., 2007; J. R. Suarez-Lopez et al., 2013). Three subtests required translation into Spanish using terminology appropriate for the local population (auditory attention and response set, comprehension of instructions and narrative memory). Translation of the NEPSY-2 test has been found to be relatively unaffected by language and culture (Kofman et al., 2006; Mulenga et al., 2001). The neurodevelopmental assessments were conducted by trained examiners in a quiet and controlled setting, and the evaluations lasted for no more than two hours. Only one child and one examiner where allowed in a room. A parent was allowed in the examination room if the parents or children requested so. In such cases, the parent was required to be in complete silence and outside of the participant’s line of sight. Additional details of these assessments are described in previous publications (Suarez-Lopez et al., 2013; Suarez-Lopez et al., 2017b).
Neurobehavioral subtest scaled scores were calculated using the NEPSY-2 scoring assistant software (NCS Pearson Inc., San Antonio, TX). Most subtest scores consisted of primary scaled scores, which are age-adjusted values based on a national normative sample of US children (Korkman et al., 2007). Higher scores represent better performance. Neurobehavioral scaled scores were designed to range from 1 to 19 (mean: 10, SD: 3), with scores of 6 or 7 considered borderline and scores less than 6 (<9th percentile of the NEPSY-2 normative sample) considered below expected. For subtests that were composed of more than one primary scaled score (i.e. auditory attention and response set, inhibition and word list interference), we used the average of all available primary scaled scores per subtest for the domain score calculation. For subtests that included both correct and error components (i.e. auditory attention and response set) or time and error components (i.e. inhibition, speeded naming, visuomotor precision), we used the combined scaled scores (scores that combined both components) as primary scaled scores. The only subtest within the Sensorimotor domain was Visuomotor Precision; hence, the Sensorimotor domain score is equal to the Visuomotor Precision scaled score. We calculated a total neurobehavioral summary score as the average of primary scores of all eleven subtests. Domain scores and the neurobehavioral summary score were used as the measures of neurobehavioral performance, to reduce the number of associations tested thereby reducing the likelihood of type 1 errors.
Covariates
Children’s height was measured to the nearest 1 millimeter, using a height board and following recommended procedures (World Health Organization, 2008). Because height for age is a better indicator of under-nutrition than BMI (Mehta et al., 2013; “WHO Child Growth Standards,” 2006.), we calculated z-scores for height -for-age using the World Health Organization growth standards and used this variable as an indicator of malnutrition (World Health Organization Multicentre Growth Reference Study Group, 2006). Hemoglobin concentration was measured from a drop of blood from a finger-stick sample using the EQM Test-mate ChE Cholinesterase Test System 400 (EQM AChE Erythrocyte Cholinesterase Assay Kit 470; EQM Research, Inc, Cincinnati, OH). Other covariates, including self-reported maternal education, race, and cohabitation with a flower plantation worker, were obtained through interviews conducted at participants’ homes. Because previous ESPINA analyses have shown that the season during which a child was assessed may affect both exposure indicated by acetylcholine esterase activity as well as neurodevelopmental scores (Suarez-Lopez et al., 2017a, 2017b), time between Mother’s Day harvest and date of outcome assessment was also considered.
To include children with missing information in multivariable analyses, we created a “missing” race category, and imputed information of maternal education for 15 children with missing information by substituting the “household head’s” education reported in the SILC 2004.
Statistical Analysis
In primary analyses used linear regression analyses to test the associations between neurobehavior scores and residential distance to the nearest flower plantation. We also estimated odds ratios (ORs) of low neurodevelopmental scores associated with distance to the nearest flower plantation using multivariate logistic regression models. In both linear and logistic regression analyses, our main adjustment model included adjustment for child’s age, sex, race, maternal education, cohabitation with a flower plantation worker and 2 constructs of nutrition: height-for-age z-score and hemoglobin concentration. These variables are known to affect or correlate with neurobehavioral development in children. In sensitivity analysis, we assessed robustness of results to choice of covariates by additionally including income and household pesticide use. We also assessed effect modification by child sex using a multiplicative interaction term (distance + distance*sex) since we previously observed that the associations between AChE and neurobehavior were stronger among boys than girls in this study population (J. R. Suarez-Lopez et al., 2013).
In additional analyses, we assessed possible nonlinearities in the exposure-response relationship in a number of ways. We modeled categorical exposure as an alternative to analyzing exposure as a continuous measure (distance to nearest plantations). In all cases we used the same main adjustment models described above. We first analyzed exposure as quartiles of distance to the nearest plantation. Furthermore, considering that flower crops are enclosed within greenhouses, which reduce the amount of pesticide drift, we modeled residential proximity using finer categories within close proximity: 0-50m, 51-100m, 101-500m, and >500m. To estimate associations with binary outcomes, we combined the categories of 0-50m and 51-100 to improve the statistical power of analyses considering the small number of participants within each of the categories (17 and 19, respectively). We additionally applied general additive models (GAM), using smoothing spline functions to fit the relationship between continuous distances and subscale scores, adjusted for covariates.
In order to assess whether the amount of crop area near a residence is associated with the outcome we examined total flower crop areas within a 100m buffer around participants’ homes as a continuous variable and as a 3-category variable: no growing areas within 100m of the residence (reference group), crop area within 100m below the median and crop area within 100m above the median. In sensitivity analyses, we used the same approach with 150m and 200m buffers.
Analyses were conducted using proc glm and proc logistic in SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA) and GAM analyses were done in R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) using the mgcv package.
RESULTS
The mean age of children at the time of assessment was 6.6 years (SD = 1.6); 51% were male, 72% reported mestizo as their race, 21% indigenous, 2% other (white or black) and 5% had missing information (Table 1). of the 307 participants (50%) lived with a floricultural worker. The means of height-for-age z-score and hemoglobin concentration were −1.25 (SD: 0.96) and 12.7 mg/dL (SD: 1.2), respectively. On average, children in this sample lived 446 m from the edge of the nearest flower crop (SD: 344 m). In this sample of children, 6% lived within 50m, 11% lived within 100m, 27% lived within 200 m, and 61% lived within 500 m of a plantation’s edge. The average quantity of growing area within 100m of the residence was 292 m2 (SD: 1406 m2).
Table 1.
Characteristics of the study population, overall and by quartile of residential proximity to the edge of the nearest flower crop.
| Characteristic | Overall | Quartiles of residential proximity to nearest flower crop |
|||
|---|---|---|---|---|---|
| n=307 | 0-185m n=77 |
186-350m n=76 |
351-605m n=76 |
>605m n=78 |
|
| Child’s age, y | 6.6 (1.6) | 6.6 (1.6) | 6.5 (1.5) | 6.9 (1.6) | 6.5 (1.6) |
| Child sex, % | |||||
| Girls | 49 | 45 | 47 | 53 | 51 |
| Boys | 51 | 55 | 53 | 47 | 49 |
| Race, % | |||||
| Mestizo | 72 | 68 | 71 | 73 | 77 |
| Indigenous | 21 | 26 | 25 | 17 | 17 |
| Other | 2 | 0 | 3 | 5 | 0 |
| Missing | 5 | 6 | 1 | 5 | 6 |
| Height-for-age z score, SD | −1.25 (0.96) | −1.48 (0.97) | −1.15 (0.99) | −1.29 (0.92) | −1.05 (0.94) |
| Hemoglobin, mg/dL | 12.7 (1.2) | 12.6 (1.2) | 12.7 (1) | 12.9 (1.3) | 12.3 (1.1) |
| Acetylcholinesterase, U/mL | 3.14 (0.49) | 3.09 (0.52) | 3.09 (0.49) | 3.25 (0.49) | 3.12 (0.43) |
| Mother’s education, y | 7.3 (3.8) | 6.6 (3.1) | 8.8 (4.1) | 7.1 (3.9) | 6.5 (3.8) |
| Cohabitation with a flower plantation worker, % | 50 | 57 | 51 | 42 | 47 |
Values shown are mean (SD), unless otherwise noted.
The neurobehavioral subtest scores for subjects in our study sample were lower but with similar variability to those of the NEPSY-2 normative sample of U.S. children (data not shown)(J. R. Suarez-Lopez et al., 2013). The neurobehavioral domain scores of our participants ranged from 4 to 13, with mean (SD) scores of 6.6 (2.4) in Language, 8.8 (2.1) in Memory and Learning, 9.9 (3.3) in Sensorimotor, 9.6 (3.1) in Visuospatial and 8.5 (2.5) in the Attention and Inhibitory domain. The lowest domain scores were observed in the Language and Attention & Inhibitory Control domains. The percentages of children with scores of clinical concern for their age group were: 10% in the Memory domain, 12% in Visuospatial Processing, 15% in Attention & Inhibitory Control, 17% in Sensorimotor, and 33% in the Language domain (Korkman et al., 2007).
Residential distance to floricultural crops and neurobehavior
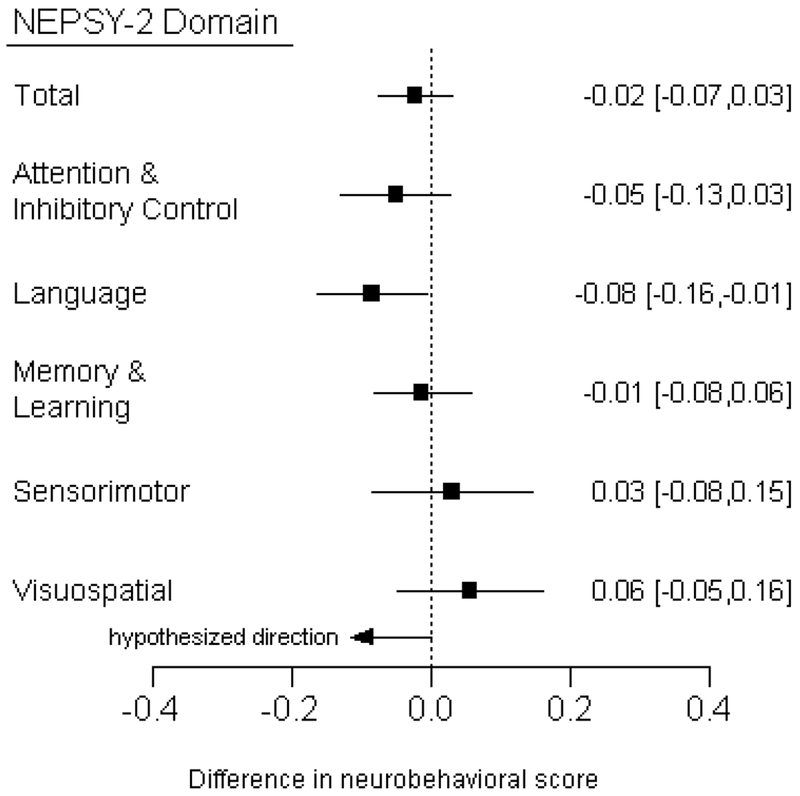
A decrement in the Language domain score was observed per 100m decrease in distance from the child’s residence to the edge of the nearest flower crop (β) of −0.08 units (95% CI: −0.01, −0.16, Figure 1). An association with the Attention & Inhibitory Control domain was also observed, although it was weaker (β: −0.05 units [95% CI: −0.13, 0.03] per 100m decrease in distance). No associations were observed with Memory & Learning, Sensorimotor or Visuospatial Processing in analyses of neurobehavior scores as continuous outcomes (higher values reflect better performance) and proximity to floricultural crops as a continuous exposure, adjusting for age, sex, race, height-for age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker. Additional adjustment for income or residential pesticide use did not meaningfully change the results (Table S4). We assessed evidence for effect modification by child sex and saw no statistically significant interaction (Table S5).
Figure 1.
Difference [95% confidence interval] in neurobehavioral scores associated with living 100m closer to the edge of the nearest floricultural crop. Linear regression models were adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker. We hypothesized that children residing closer to greenhouse agriculture had greater odds of having low neurobehavioral scores.
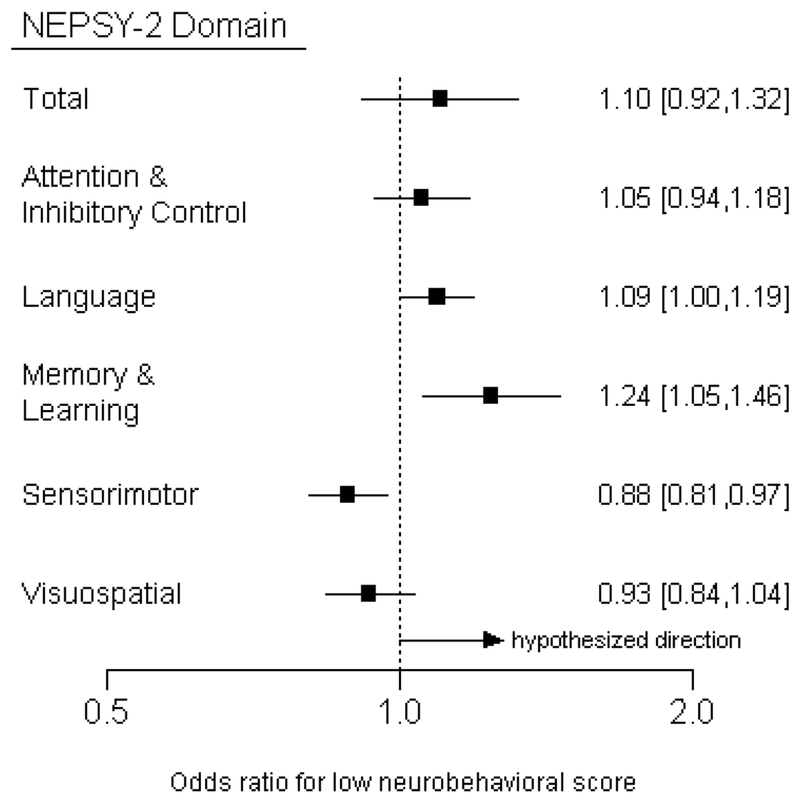
When using clinical thresholds to define a binary outcome we observed that for every 100m of closer residential distance to floricultural crops, the odds of low overall neurobehavioral score increased by 10% (OR: 1.10, 95% CI: 0.92, 1.32) (Figure 2). For every 100m closer in proximity to treated floricultural crops, the odds of low Language score increased by 9% (OR: 1.09, 95% CI: 1.00, 1.19), and the odds of low Memory & Learning score increased by 24% (OR: 1.24, 95% CI: 1.05, 1.46). In the Sensorimotor domain, we observed results in the direction opposite to that hypothesized: children living closer to crops had significantly lower odds of a low Sensorimotor score (OR 0.88 [95%CI: 0.81, 0.97] per 100 m decrease in distance).
Figure 2.
Odds ratios [95% confidence intervals] for low neurobehavioral scores associated with living 100m closer to the edge of the nearest floricultural crop. Logistic regression models were adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker. We hypothesized that children residing closer to greenhouse agriculture had greater odds of having low neurobehavioral scores.
Threshold effect
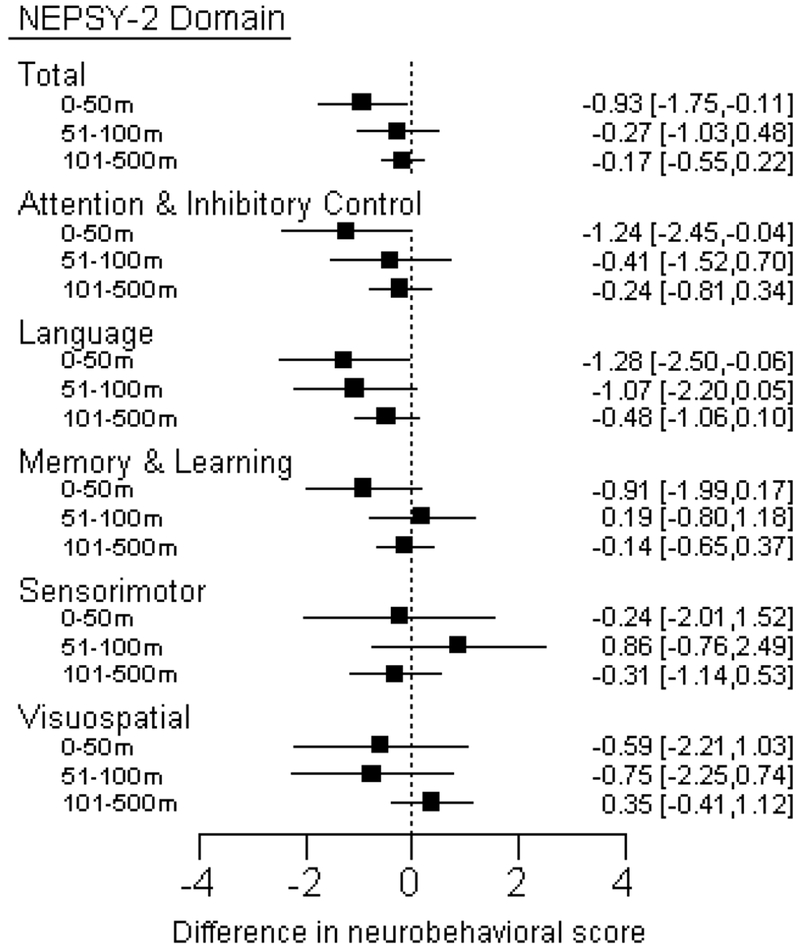
No clear threshold effect was observed using quartiles of exposure (Table S1). However, using categories to distinguish those living closest to floricultural crops, we observed that participants who lived within 50 m of a flower crop had the lowest neurobehavioral scores in three out of the five domains assessed (Figure 3). Compared to children living >500m from a flower crop, children living within 50m of a crop had statistically significant (or borderline significant) lower scores in the domains of Attention & Inhibitory Control, Language, and Memory & Learning. The scores in these domains were higher (improved) as the distance categories increased.
Figure 3.
Difference (95% confidence interval) in neurobehavioral scores associated with living within each category of residential distance to a floriculture crop (0-50m, 51-100m, or 101-500m) compared to living in the furthest category (>500m). There were 17 participants (5%) living within 0-50m, 19 (6%) between 51-100m, 152 (50%) between 101-500m, and 119 (39%) living more than 500m, from a flower plantation. Linear regression models were adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker. Children’s neurobehavior is altered for the language domain among those who lived within 50m and within 100m compared to those living more than 500m away, suggesting an adverse effect of living within close proximity to floriculture crops. Associations were also observed for living closest (0-50m) compared to furthest (>500m) to floriculture crops, in the Attention & Inhibitory Control domain and the Total score, consistent with the hypothesized adverse effect of residential proximity.
A similar trend was observed in logistic regression analyses of the dichotomous outcomes (Table 2). In these analyses, categories of 0-50m and 51-100m were combined to improve statistical power and model stability. Nonetheless, estimates still had wide confidence intervals for the overall score and the Memory & Learning domain. We observed substantially higher odds of low neurobehavioral scores (total, Attention & Inhibitory Control, Language, Memory & Learning), among children living within 100m of a plantation compared to children living at >500m; the odds ratios were weaker among participants living within 101m to 500m of a plantation. Finally, we inspected GAM plots for the relationship between continuous distance and neurobehavior scores for the three subdomains with the largest effect sizes (Figure S1). These are qualitatively similar to the results of analyses of distance by category (Table S1 and Figure 3), exhibiting a decrease in Attention & Inhibitory Control and Language scores at close proximity but do not provide strong evidence of nonlinear effects.
Table 2.
Odds ratios (95% CI)a for low neurobehavioral score when comparing living within 0-100m, or 101-500m, to living >500m from floriculture crops.
| Residential Proximity Category |
|||
|---|---|---|---|
| NEPSY-2 Domain | 0-100mb (n=36) |
101-500m (n=152) |
>500m (n=119) |
| Total | 5.07 (1.02, 25.20)c | 2.73 (0.63, 11.84) | ref |
| Attention & Inhibitory Control | 2.35 (0.85, 6.51) | 1.54 (0.69, 3.42) | ref |
| Language | 2.46 (1.06, 5.74)c | 1.41 (0.78, 2.54) | ref |
| Memory & Learning | 8.32 (2.46, 28.07)c | 2.70 (0.92, 7.99) | ref |
| Sensorimotor | 0.51 (0.17, 1.52) | 0.55 (0.27, 1.10) | ref |
| Visuospatial | 0.95 (0.28, 3.22) | 0.67 (0.29, 1.53) | ref |
Logistic regression models were adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker.
Categories of 0-50m and 51-100m were combined to improve statistical power and model stability.
p<0.05
Flower plantation areas near homes and neurobehavior
Only 12% of the study population lived within 100m of a plantation (n=42), which limited the power of our analyses of associations within very close distances to plantations. All results from linear regression analyses comparing growing area within 100m of participant residences (above or below the median of growing area) to those living further than 100m from floricultural crops were null (Table S2). In logistic regression analyses, children with the most growing area within 100m of their residence (defined as >546m2) had higher odds of low scores in the Language domain (OR=4.84, 95% CI: 1.59, 14.76), compared to children without any plantation land within 100m of their residence (Table S3).
DISCUSSION
We observed that close residential proximity to floricultural crops was associated with poorer neurobehavioral outcomes in the domains of Attention & Inhibitory Control, Language and Memory & Learning. Associations were strongest among children living within 50m of a flower crop and present to some extent among children living between 51 and 100m. These findings were partially corroborated by sensitivity analyses using areas of floricultural crops near homes as a related construct of pesticide drift from flower crops.
Unlike short-lived biomarkers of exposure, proximity of a child’s home to agricultural crops may approximate the child’s ongoing and historical low dose exposure to pesticides through off-target drift or direct access to pesticide-treated areas. In the ESPINA study, we previously described positive associations between AChE activity (lower values reflect greater exposure to cholinesterase inhibitor pesticides) and the domains of Attention & Inhibitory Control, Memory & Learning, and borderline associations with the Language domain affecting boys more than girls (J. R. Suarez-Lopez et al., 2013). Alterations in the same domains were observed in the present study, which is consistent with previous findings. Epidemiologic studies provide increasing evidence that pesticide exposure during key developmental periods may be a risk factor for a range of neurocognitive deficits later in life, including attention deficit and hyperactivity disorder, autism spectrum disorder, developmental delay, slowed reaction time, and slowed motor control, poor verbal comprehension (Bouchard et al., 2011; Burns et al., 2013; Eaton et al., 2008; Eskenazi et al., 2008, 2007; Fenske et al., 2000; Grandjean, 2006; Grandjean et al., 2006; Handal et al., 2008; Harari et al., 2010; Horton et al., 2012; Kofman et al., 2006; London et al., 2012; Rauh and Margolis, 2016; Shelton et al., 2014; J. R. Suarez-Lopez et al., 2013)
People living closest to agricultural crops are at increased risk of pesticide exposure. In our analyses, children living within 100m of a flower crop, and especially within 50m, had lower neurobehavioral scores compared to children living farther than 500m. These findings suggest that the amount of pesticide drift from crops onto nearby homes can especially affect the neurobehavioral performance of children living within 100m. However, alterations in neurobehavioral performance may also be present at greater distances but the limited statistical power of our study to detect smaller differences precluded us from assessing this further. In previous analyses of the ESPINA study, we observed positive associations between residential distances to flower crops and AChE activity, with the lowest AChE levels observed among children living within 232m of a greenhouse floricultural crop (Suarez-Lopez et al., 2018). This supports the construct of residential distance to flower plantations as a pathway of exposure to pesticides Furthermore, we previously observed that children living closer to flower crops had higher systolic blood pressures, which indicates that additional physiologic processes may be affected among children living near pesticide spray sites (Suarez-Lopez et al., 2018).
Multiple investigations have studied the association between proximity of homes to agricultural crops and pesticide exposure (Coronado et al., 2011; Deziel et al., 2015; Loewenherz et al., 1997; Lu et al., 2000; Simcox et al., 1995; Ward et al., 2006). While maximal exposure attributable to pesticide drift, among these studies, varied from 60 to 750 meters, this collection of studies rather consistently indicates that homes residing closer to pesticide treated fields tend to have higher pesticide levels and that children residing closer to pesticide treated fields tend to reflect higher pesticide exposure levels using biomonitoring studies. In this study, exposure was modeled as distance to the nearest plantation in the primary analyses, based on the assumption that increased distance reflects lower exposures. An alternative measure, area of plantations within varying buffer areas, which is likely a better proxy for exposure to pesticide drift, was also explored. As expected, results showed consistent associations between these two related but different constructs of pesticide exposure, which strengthens our findings.
Several studies have utilized residential proximity to agriculture as a metric of exposure to pesticides when studying its associations with neurodevelopment (Butler-Dawson et al., 2016; Coker et al., 2017; González-Alzaga et al., 2015; Gunier et al., 2017; Handal et al., 2007; Roberts et al., 2007; Rowe et al., 2016; Shelton et al., 2014; von Ehrenstein et al., 2019). A number of these studies used data from California State’s Pesticide Use Reporting System, finding positive correlations between proximity of prenatal residence to areas of agricultural pesticide applications and neurodevelopmental outcomes in early childhood, specifically ASD (Roberts et al., 2007; Shelton et al., 2014). In our analyses the observed effect size in the logistics models were small, but the magnitude may have been attenuated by the non-linear dose-response relationship shown in Figure 3. Furthermore, the linear regression models indicated that a difference of 100m in residential proximity to floricultural crops is associated with a greater likelihood of the child scoring in the subclinical ranges for the Language and the Memory and Learning domains by 9% and 24% respectively. In the context of measurable outcomes, this is clinically significant in that identifying children with delayed development warrants early intervention by clinicians as well as educators. The expected distance of pesticide drift from flower crops to nearby homes was smaller in our study population compared to those of other studies likely because rose production is enclosed within greenhouses. Greenhouses in Pedro Moncayo County have air circulation vents or windows, which could allow the escape of fumigated pesticides during and after spraying. However, these analyses suggest that pesticide drift, even in this setting, could still be problematic in the context of pesticide exposure affecting the neurodevelopment of children living nearby. This body of evidence coupled with the growing number of studies describing neurobehavioral alterations associated with pesticide exposures (Bouchard et al., 2011; Burns et al., 2013; Eskenazi et al., 2007; Grandjean, 2006; Harari et al., 2010; Shelton et al., 2014; J. R. Suarez-Lopez et al., 2013) suggests that extending buffer zones or protective areas that separate the industry from the neighboring communities, could be an effective way to protect developing children (including during prenatal development) from the adverse effects of pesticide exposure.
The present study was subject to several limitations. Residential distance from treated floricultural crops was used as a proxy for childhood exposure to floricultural pesticides. Though a crude exposure assessment, the use of this exposure metric is supported by the existing literature and validated within our study population (Suarez-Lopez et al., 2018). Prevailing winds were not accounted for in the present analyses. This provides potential for non-differential misclassification of the amount of pesticide drift from plantations to homes and may have biased our findings towards the null (Gordis, 2014). Also, while the vast majority of the floricultural production in Pedro Moncayo County includes roses, which are grown inside greenhouses, a small amount of production of other flowers also occurs in non-enclosed fields typically located near the greenhouses. For this reason, it is plausible that some of the pesticide drift from crops, and hence the associations observed in this study, may be a result of both greenhouse and open field floricultural production. Nonetheless, residential proximity to crops is a useful construct of exposure as it is an indicator of chronic pesticide exposure, and provides practical information about the distances in which populations may have an increased risk of pesticide exposure and/or adverse health. While it does not allow us to determine which specific chemicals are influencing this association, it indirectly accounts for a mixture of the various agrochemicals used in floriculture. The floricultural industry in Pedro Moncayo frequently uses various pesticides including insecticides (organophosphates, neonicotinoids and pyrethroids), many classes of fungicides and to lesser extent, herbicides (Grandjean et al., 2006; Handal et al., 2016; Harari, 2004; Suarez-Lopez et al., 2017a). Many of the studies assessing neurodevelopment and pesticide exposure, including the ESPINA Study, are limited in that they study biomonitoring of few pesticides, even though it is unusual for one pesticide to be used without multiple others. It is plausible that pesticides or other neurotoxicant agrochemical exposures explain the neurobehavioral deficits seen among participants living near the flower crops. Determining the quantity and types of agrochemicals used over time and by location would improve precision but would be very difficult to ascertain in this agricultural setting. Another limitation related to exposure assessment is that we were not able to account for all potential routes of exposure to pesticides, including dietary intake. We did not have information on time-activity patterns, which would have provided better insight into participants’ outdoor exposures (Ring et al., 2019). There is also some uncertainty associated with using home location only to estimate exposure to environmental pesticides. Some children in the cohort went to school during the day, while younger children attended daycare or stayed home with a relative. Modeling exposure experienced across all daily activities and locations is beyond the scope of this current study; we choose to focus on exposures at the children’s home locations.
Another limitation of this study is that neurobehavioral outcomes were assessed at only one point in time, and thus we are unable to assess if the neurodevelopmental effects are permanent. Additionally, the NEPSY-2 is based on a US normative sample. Although this does not affect the internal validity of our findings, it is unclear how accurately the cut-off values for “low performance” apply to this study population.
This study has several strengths and thus contributes to our understanding of the effects of pesticide use in floricultural communities. This study is unique in that there was a wide distribution of participants’ residential distance to crops, and we had a considerable number of children living in close proximity (<100m) to flower crops, which allowed us to estimate effect sizes at short distances. Additionally, all participants were examined during a period of relatively homogeneous flower production and pesticide use. Children in this study were examined during a period of lower flower production (July-August) and pesticide use compared to other times of the year. In theory, this would reduce the off-target pesticide drift potential from crops, with resulting lower exposures to children living nearby. Considering that pesticide spray seasons may also have short-term neurobehavioral alterations in children (Ismail et al., 2017; Rohlman et al., 2016, 2014; Suarez-Lopez et al., 2017b), it is plausible that these observed associations would be stronger during peak exposure periods. Lastly the existing studies have focused on residential distance to agricultural open fields. To our knowledge, the present study is the first to characterize the associations of neurobehavior in relation to greenhouse agricultural production, which is generally though to result in reduced pesticide drift from crops. Many types of crops involve the use of greenhouses such as flowers, tomatoes, cucumber, a variety of herbs, lettuce, bell peppers, and eggplants. The present study findings may be applicable to such agricultural production.
CONCLUSION
Children living in close proximity to floricultural crops had poorer neurobehavioral performance in the domains of Attention & Inhibitory Control, Language and Memory & Learning. These findings indicate that pesticide drift from agricultural plantations may affect the neurobehavioral development of children living nearby. This association between residential distances to agricultural industries and health outcomes could be studied further to facilitate the development of policies and practices that protect agricultural populations from adverse effects of pesticide drift.
Supplementary Material
Figure S1 (A-C). GAM plots for association between distance to nearest floriculture crop and neurobehavior measured as continuous scores. Models are adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker.
Table S1. Difference (95% confidence interval)a in neurobehavioral score associated within living in each quartile of residential proximity to the nearest floricultural plantation, compared to living furthest (>605m) from a plantation.
Table S2. Difference (95% confidence interval)a in neurobehavioral scores associated with total growing area within 100m of participants’ residences.
Table S3. Odds ratios (95% confidence intervals)a of low neurobehavioral score associated with total growing area within 100m participants’ residences.
Table S4. Difference (95% confidence interval)a in neurobehavioral scores associated with living 100m closer to the edge of the nearest floriculture crop in sensitivity analyses further adjusting for household income and household pesticide use.
Table S5. Effect modification of association between distance to the nearest floricultural crop and neurobehavior, by child sex.
Acknowledgments
Declaration of Interests / Funding: Funding for the ESPINA study: NIEHS (R01ES025792, R21ES026084-01), NIOSH (1R36OH009402). Dr. Friedman was supported by the NW PEHSU Fellowship program through EPA/ATSDR DW-75-9587701-4.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elizabeth Friedman, School of Medicine, and Department of Environmental & Occupational Health Sciences, University of Washington.
Marnie F. Hazlehurst, Department of Epidemiology, School of Public Health, University of Washington
Christine Loftus, Department of Epidemiology, School of Public Health, University of Washington.
Catherine Karr, Department of Pediatrics, University of Washington School of Medicine, and Departments of Environmental & Occupational Health Sciences and Epidemiology, School of Public Health, University of Washington.
Kelsey N. McDonald, Department of Geography, Macalester College
Jose Ricardo Suarez-Lopez, Department of Family Medicine and Public Health, University of California, San Diego.
References
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, Trujillo C, Johnson C, Bradman A, Barr DB, Eskenazi B, 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ. Health Perspect 119, 1189–95. 10.1289/ehp.1003185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CJ, McIntosh LJ, Mink PJ, Jurek AM, Li AA, 2013. Pesticide exposure and neurodevelopmental outcomes: Review of the epidemiologic and animal studies. J. Toxicol. Environ. Heal. - Part B Crit. Rev 10.1080/10937404.2013.783383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler-Dawson J, Galvin K, Thorne PS, Rohlman DS, 2016. Organophosphorus pesticide exposure and neurobehavioral performance in Latino children living in an orchard community. Neurotoxicology 53, 165–172. 10.1016/j.neuro.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael SL, Yang W, Ma C, Roberts E, Kegley S, English P, Lammer EJ, Witte JS, Shaw GM, 2016. Joint effects of genetic variants and residential proximity to pesticide applications on hypospadias risk. Birth Defects Res. A. Clin. Mol. Teratol 106, 653–658. 10.1002/bdra.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E, Gunier R, Bradman A, Harley K, Kogut K, Molitor J, Eskenazi B, 2017. Association between pesticide profiles used on agricultural fields near maternal residences during pregnancy and IQ at age 7 years. Int. J. Environ. Res. Public Health. 10.3390/ijerph14050506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronado GD, Holte S, Vigoren E, Griffith WC, Barr DB, Faustman E, Thompson B, 2011. Organophosphate pesticide exposure and residential proximity to nearby fields: Evidence for the drift pathway. J. Occup. Environ. Med 10.1097/JOM.0b013e318222f03a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel NC, Friesen MC, Hoppin JA, Hines CJ, Thomas K, Beane Freeman LE, 2015. A Review of Nonoccupational Pathways for Pesticide Exposure in Women Living in Agricultural Areas. Environ. Health Perspect 10.1289/ehp.1408273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS, 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol 38 Suppl 2, 1–125. 10.1080/10408440802272158 [DOI] [PubMed] [Google Scholar]
- Von Ehrenstein OS, Ling C, Cui X, Cockburn M, Park AS, Yu F, Wu J, Ritz B, 2019. Prenatal and infant exposure to ambient pesticides and autism spectrum disorder in children : population based case-control study 1–10. 10.1136/bmj.l962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP, 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ. Health Perspect 115, 792–8. 10.1289/ehp.9828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB, 2008. Pesticide toxicity and the developing brain, in: Basic and Clinical Pharmacology and Toxicology. 10.1111/j.1742-7843.2007.00171.x [DOI] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Simcox NJ, Loewenherz C, Touchstone J, Moate TF, Allen EH, Kissel JC, 2000. Strategies for assessing children’s organophosphorus pesticide exposures in agricultural communities. J. Expo. Anal. Environ. Epidemiol 10, 662–671. [DOI] [PubMed] [Google Scholar]
- González-Alzaga B, Hernández AF, Rodríguez-Barranco M, Gómez I, Aguilar-Garduño C, López-Flores I, Parrón T, Lacasaña M, 2015. Pre- and postnatal exposures to pesticides and neurodevelopmental effects in children living in agricultural communities from South-Eastern Spain. Environ. Int 10.1016/j.envint.2015.09.019 [DOI] [PubMed] [Google Scholar]
- Gordis L, 2014. Epidemiology, 5th ed ed. Saunders, Elsevier, Philadelphia: https://doi.org/9781455737338 [Google Scholar]
- Grandjean P, 2006. Pesticide Exposure and Stunting as Independent Predictors of Neurobehavioral Deficits in Ecuadorian School Children. Pediatrics 117, e546–e556. 10.1542/peds.2005-1781 [DOI] [PubMed] [Google Scholar]
- Grandjean P, Harari R, Barr DB, Debes F, 2006. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics 117, e546–56. 10.1542/peds.2005-1781 [DOI] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Harley KG, Eskenazi B, 2017. Will buffer zones around schools in agricultural areas be adequate to protect children from the potential adverse effects of pesticide exposure? PLoS Biol. 10.1371/journal.pbio.2004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handal AJ, Harlow SD, Breilh J, Lozoff B, 2008. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology 19, 851–9. 10.1097/EDE.0b013e318187cc5d [DOI] [PubMed] [Google Scholar]
- Handal AJ, Hund L, Páez M, Bear S, Greenberg C, Fenske RA, Barr DB, 2016. Characterization of Pesticide Exposure in a Sample of Pregnant Women in Ecuador. Arch. Environ. Contam. Toxicol 70, 627–639. 10.1007/s00244-015-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handal AJ, Lozoff B, Breilh J, Harlow SD, 2007. Neurobehavioral development in children with potential exposure to pesticides. Epidemiology. 10.1097/01.ede.0000259983.55716.bb [DOI] [PubMed] [Google Scholar]
- Harari R, 2004. Seguridad, salud y ambiente en la floricultura. IFA, PROMSA, Quito. [Google Scholar]
- Harari R, Julvez J, Murata K, Barr D, Bellinger DC, Debes F, Grandjean P, 2010. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ. Health Perspect 118, 890–6. 10.1289/ehp.0901582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Kahn LG, Perera F, Barr DB, Rauh V, 2012. Does the home environment and the sex of the child modify the adverse effects of prenatal exposure to chlorpyrifos on child working memory? Neurotoxicol. Teratol 34, 534–541. 10.1016/j.neuro.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland C, Laribi O, 2017. Review of take-home pesticide exposure pathway in children living in agricultural areas. Environ. Res 10.1016/j.envres.2017.04.017 [DOI] [PubMed] [Google Scholar]
- Ismail AA, Wang K, Olson JR, Bonner MR, Hendy O, Abdel Rasoul G, Rohlman DS, 2017. The impact of repeated organophosphorus pesticide exposure on biomarkers and neurobehavioral outcomes among adolescent pesticide applicators. J. Toxicol. Environ. Health. A 80, 542–555. 10.1080/15287394.2017.1362612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofman O, Berger A, Massarwa A, Friedman A, Jaffar AA, 2006. Motor inhibition and learning impairments in school-aged children following exposure to organophosphate pesticides in infancy. Pediatr. Res 60, 88–92. 10.1203/01.pdr.0000219467.47013.35 [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SL, 2007. NEPSY II: Clinical and Interpretive Manual, 2nd ed The Psychological Corporation, San Antonio, TX. [Google Scholar]
- Loewenherz C, Fenske RA, Simcox NJ, Bellamy G, Kalman D, 1997. Biological monitoring of organophosphorus pesticide exposure among children of agricultural workers in central Washington State. Environ. Health Perspect 105, 1344–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, Harari R, Kootbodien T, Kromhout H, Little F, Meijster T, Moretto A, Rohlman DS, Stallones L, 2012. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology. 10.1016/j.neuro.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fenske RA, Simcox NJ, Kalman D, 2000. Pesticide exposure of children in an agricultural community: Evidence of household proximity to farmland and take home exposure pathways, in: Environmental Research. 10.1006/enrs.2000.4076 [DOI] [PubMed] [Google Scholar]
- Mehta NM, Corkins MR, Lyman B, Malone A, Goday PS, Carney LN, Monczka JL, Plogsted SW, Schwenk WF, 2013. Defining Pediatric Malnutrition : A Paradigm Shift Toward Etiology-Related Definitions XX. 10.1177/0148607113479972 [DOI] [PubMed] [Google Scholar]
- Mulenga K, Ahonen T, Aro M, 2001. Performance of Zambian children on the NEPSY: a pilot study. Dev. Neuropsychol 20, 375–83. 10.1207/S15326942DN2001_4 [DOI] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE, 2016. Research Review: Environmental exposures, neurodevelopment, and child mental health – new paradigms for the study of brain and behavioral effects. J. Child Psychol. Psychiatry Allied Discip 10.1111/jcpp.12537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring CL, Arnot JA, Bennett DH, Egeghy PP, Fantke P, Huang L, Isaacs KK, Jolliet O, Phillips KA, Price PS, Shin H-M, Westgate JN, Setzer RW, Wambaugh JF, 2019. Consensus Modeling of Median Chemical Intake for the U.S. Population Based on Predictions of Exposure Pathways. Environ. Sci. Technol 53, 719–732. 10.1021/acs.est.8b04056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C, 2007. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ. Health Perspect 10.1289/ehp.10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Abdel-Rasoul G, Lasarev M, Hendy O, Olson JR, 2014. Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metab. Brain Dis 29, 845–855. 10.1007/s11011-014-9565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlman DS, Ismail AA, Rasoul GA, Bonner MR, Hendy O, Mara K, Wang K, Olson JR, 2016. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex. 74, 383–395. 10.1016/j.cortex.2015.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe C, Gunier R, Bradman A, Harley KG, Kogut K, Parra K, Eskenazi B, 2016. Residential proximity to organophosphate and carbamate pesticide use during pregnancy, poverty during childhood, and cognitive functioning in 10-year-old children. Environ. Res 10.1016/j.envres.2016.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, Hansen RL, Hertz-Picciotto I, 2014. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The charge study. Environ. Health Perspect 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA, 1995. Pesticides in household dust and soil: Exposure pathways for children of agricultural families. Environ. Health Perspect 103, 1126–1134. 10.1289/ehp.951031126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Butcher CR, Gahagan S, Checkoway H, Alexander BH, Al-Delaimy WK, 2017a. Acetylcholinesterase activity and time after a peak pesticide-use period among Ecuadorian children. Int. Arch. Occup. Environ. Health. 10.1007/s00420-017-1265-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Checkoway H, Jacobs DR, Al-Delaimy WK, Gahagan S, 2017b. Potential short-term neurobehavioral alterations in children associated with a peak pesticide spray season: The Mother’s Day flower harvest in Ecuador. Neurotoxicology. 10.1016/j.neuro.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Himes JH, Jacobs DR, Alexander BH, Gunnar MR, 2013. Acetylcholinesterase Activity and Neurodevelopment in Boys and Girls. Pediatrics 132, e1649–e1658. 10.1542/peds.2013-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Hong V, McDonald KN, Suarez-Torres J, Lopez D, De La Cruz F, 2018. Home proximity to flower plantations and higher systolic blood pressure among children. Int. J. Hyg. Environ. Health 221, 1077–1084. 10.1016/j.ijheh.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR, Himes JH, Alexander BH, Lazovich D, Gunnar M, 2012. Lower acetylcholinesterase activity among children living with flower plantation workers. Environ. Res 114, 53–59. https://doi.org/10.1016Zj.envres.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Lopez JR, Jacobs DR Jr., Himes JH, Alexander BH, 2013. Acetylcholinesterase activity, cohabitation with floricultural workers, and blood pressure in Ecuadorian children. Environ. Health Perspect 121 10.1289/ehp.1205431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward MH, Lubin J, Giglierano J, Colt JS, Wolter C, Bekiroglu N, Camann D, Hartge P, Nuckols JR, 2006. Proximity to crops and residential to agricultural herbicides in Iowa. Environ. Health Perspect 10.1289/ehp.8770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Child Growth Standards, n.d.
- World Health Organization, 2008. Training Course on Child Growth Assessment 7, 25–36. [Google Scholar]
- World Health Organization Multicentre Growth Reference Study Group, 2006. World Health Organization Child Growth Standards based on length/height, weight and age. Acta Paediatr. 450, 76–85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (A-C). GAM plots for association between distance to nearest floriculture crop and neurobehavior measured as continuous scores. Models are adjusted for age, sex, race, height-for-age z-score, hemoglobin, maternal education, and cohabitation with a flower plantation worker.
Table S1. Difference (95% confidence interval)a in neurobehavioral score associated within living in each quartile of residential proximity to the nearest floricultural plantation, compared to living furthest (>605m) from a plantation.
Table S2. Difference (95% confidence interval)a in neurobehavioral scores associated with total growing area within 100m of participants’ residences.
Table S3. Odds ratios (95% confidence intervals)a of low neurobehavioral score associated with total growing area within 100m participants’ residences.
Table S4. Difference (95% confidence interval)a in neurobehavioral scores associated with living 100m closer to the edge of the nearest floriculture crop in sensitivity analyses further adjusting for household income and household pesticide use.
Table S5. Effect modification of association between distance to the nearest floricultural crop and neurobehavior, by child sex.