Abstract
Background
Malaria extensively leads to mortality and morbidity in endemic regions, and the emergence of drug resistant parasites is alarming. Plant derived synthetic pharmaceutical compounds are found to be a foremost research to obtain diverse range of potent leads. Amongst them, the chalcone scaffold is a functional template for drug discovery. The present study involves synthesis of ten chalcones with various substitution pattern in rings A and B and assessment of their anti-malarial efficacy against chloroquine sensitive and chloroquine resistant strains as well as of their cytotoxicity and effect on haemozoin production.
Methods
The chalcones were synthesized by Claisen-Schmidt condensation between equimolar quantities of substituted acetophenones and aryl benzaldehydes (or indole-3-carboxaldehyde) and were screened for anti-malarial activity by WHO Mark III schizont maturation inhibition assay. The cytotoxicity profile of a HeLa cell line was evaluated through MTT viability assay and the selectivity index (SI) was calculated. Haemozoin inhibition assay was performed to illustrate mode of action on a Plasmodium falciparum strain.
Results
The IC50 values of all compounds were in the range 0.10–0.40 μg/mL for MRC-2 (a chloroquine sensitive strain) and 0.14–0.55 μg/mL for RKL-9 (a chloroquine resistant strain) of P. falciparum. All the chalcones showed low cellular toxicity with minimal haemolysis. The statistically significant reduction (p < 0.05) in the haemozoin production suggests a similar mechanism than that of chloroquine.
Conclusions
Out of ten chalcones, number 7 was found to be a lead compound with the highest potency (IC50 = 0.11 µg/mL), as compared to licochalcone (IC50 = 1.43 µg/mL) and with high selectivity index of 85.05.
Keywords: Malaria, Plasmodium falciparum, Chalcones, In vitro, Haemozoin
Background
Malaria control programmes are threatened due to a rapid expansion of resistance to distinct anti-malarial drugs. At present, 219 million cases are reported at a global scale, mostly in children under 5 years of age [1]. Out of the five species that cause human malaria, Plasmodium falciparum and Plasmodium vivax, are associated with life-threatening complications. There is confirmed resistance of both species against most of currently available anti-malarials. To combat drug resistant Plasmodium, artemisinin and its derivatives have been widely implicated all over in endemic regions, but appearance of artemisinin resistance, first in Cambodia in 2007 [2] and later its rapid spread to the south-east Asian region [3–7] has threatened all the previous success incurred by malaria control strategies.
Chalcones (1,3-diaryl-2-propen-1-ones) are basically plant secondary metabolites related to flavonoid family and are also crucial precursors of distinctive flavonoids and isoflavonoids [8]. They have been extensively studied due to their diverse pharmacological actions [9, 10], including anti-malarial activity (Fig. 1) [11, 12].
Fig. 1.
Diverse pharmacological activity of chalcones
Moreover, chalcones can be simply synthesized by the cost-efficient Claisen-Schmidt condensation between variously substituted benzaldehydes and acetophenones [13] thus, providing an array of distinctive potential analogues with potent pharmacological effects [14]. Anti-malarial activity of such chalcones is mostly attributed to the specificity of the substitution pattern, and hydrophobicity and size of ring B (Fig. 2) [15]. The anti-malarial property of chalcone was first reported after an in vitro evaluation of an oxygenated chalcone, “licochalcone A” exclusively obtained from Chinese licorice, as an anti-malarial agent against chloroquine sensitive and chloroquine resistant Plasmodium strains [16]. Further, many more potential analogues of licochalcone A with different substitution pattern have been reported for substantial anti-malarial activity [17]. The simple structure and unambiguous synthesis of chalcones have fascinated the consideration of many chemists to find and expand distinct analogues of this unusual scaffold for various infectious diseases including malaria. For more than a decade, a panel of alkoxylated, prenylated, hydroxylated, quinolinated, oxygenated chalcones derived from either syntheses or natural sources have been assessed for antiplasmodial activity with promising outcomes [17, 18]. Although several mechanisms have been postulated for various chalcones [15, 19–22], the exact mode of action still remains unclear. Besides, these chalcones are mostly supposed to show their anti-malarial activity through preventing host haemoglobin degradation by acting against malarial cysteine protease [23]. Molecular modelling research illustrated the linear and planar structure of chalcones, which enables them to fit appropriately within the active site of Plasmodium and Trypanosoma cysteine proteases suggesting a promising target for its action [23]. The present study describes synthesis of ten chalcones with different substitution pattern in rings A and B and assessment of their anti-malarial efficacy against chloroquine sensitive and chloroquine resistant strains as well as of their cytotoxicity and mode of action.
Fig. 2.
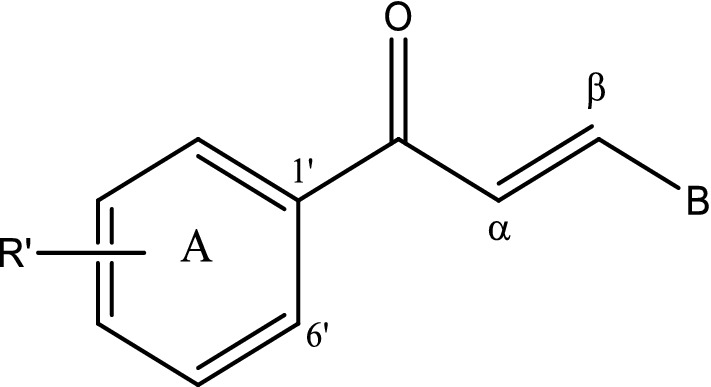
Basic structure of chalcones
Methods
Chemicals and reagents
The chalcones were synthesized at the Institute of Organic Chemistry with Centre of Phytochemistry, Bulgarian Academy of Sciences, Sofia, Bulgaria. Chloroquine phosphate, quinine hydrochloride, glutamine, sodium bicarbonate, and β-haematin were purchased from Sigma Aldrich while artemisinin was from IPCA. The study was approved by Institute Ethics Committee Project No. NK/1265/Ph.D/23991 at Post Graduate Institute of Medical Education and Research, Chandigarh, for maintenance of P. falciparum strains in human erythrocytes and AB+ve human serum.
Chemistry
The chalcones were synthesized by Claisen-Schmidt condensation between equimolar quantities of substituted acetophenones and aryl aldehydes (or indole-3-carboxaldehyde) [24, 25]. The progress of the reactions was monitored by thin-layer chromatography on silica gel plates. The condensation step was carried out over 6 h to 36 h. After purification by either column chromatography on silica gel or recrystallization from methanol, all corresponding chalcones were obtained in yields over 90%.
Stock solutions of chloroquine phosphate, quinine hydrochloride, artemisinin and each chalcone were prepared by dissolving each compound in DMSO to achieve concentration of 1.00 mg/mL. The DMSO amount in diluted concentrations (1%) had negligible effect on the parasite growth. DMSO was used as negative control.
In vitro anti-malarial activity
Parasites and culture
Two P. falciparum strains, MRC-2 (sensitive to chloroquine) and RKL-9 (resistant to chloroquine), obtained from National Institute of Malaria Research (NIMR), New Delhi, India, were used in this study. These strains were perpetuated in vitro in continuous culture according to the method of Trager and Jensen [26] with slight modifications. Briefly, both sensitive and resistant strains of P. falciparum were maintained in A+ erythrocytes in RPMI-1640 medium (having glutamine, but without any sodium bicarbonate) comprising 1.00 g of dextrose, 5.94 g of HEPES buffer, 40.00 mg of gentamycin. Additionally supplemented with 5% sodium bicarbonate and 10% (v/v) inactivated human AB+ serum then incubated in gas mixture of 5% CO2, 5% O2 and 90% N2 at 37 °C. Parasitized erythrocytes at initial 5% haematocrit were suspended in above mentioned culture medium and parasitaemia was regularly checked to maintain level between 2 and 4% with further sub-culturing for parasitaemia beyond 5%. Growth and multiplication of parasite was monitored by microscopy using Giemsa-stained slides.
Synchronization
To obtain ring stages of the parasite, the cultures were synchronized using d-sorbitol [27]. The cultures, with majority of ring stages, were treated with equal volume of aqueous 5% d-sorbitol for 5 min and then after centrifugation pellet were suspended in complete medium and fresh erythrocytes synchronized culture with 1% parasitaemia and 5% haematocrit were used for compound concentration response assay.
Compound concentration response assay
The concentration of each test compound needed to hinder multiplication of parasites by 50% (IC50) against P. falciparum strains were obtained through concentration response assay performed in 96-well sterile tissue culture plates. Synchronized parasite cultures were applied to different doses of each compound. Dilutions were performed in gentamycin-free culture medium, and incubated at 37 °C having gaseous mixture (5% CO2, 5% O2 and 90% N2) supply for 24 h. The results were expressed as IC50 values computed from HN-NonLin Regression analysis [28], as well as mean percentage inhibition ± standard error examined by thick smear Giemsa stained slides [29, 30].
Resistance index (RI)
The degree of resistance was determined by comparing the activity of chalcones on the chloroquine sensitive and chloroquine resistant strains of P. falciparum using the following formula [31]:
Cytotoxicity assay and evaluation of selective index
Cytotoxicity of the compounds on mammalian cells were accomplished employing HeLa cell line (NCCS, Pune) cultured in DMEM supplemented with 10% FBS by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) microenzymatic method with certain modifications [32]. Briefly, cells (104 cells/200 μL/well) were seeded into 96-well flat-bottom sterile tissue culture plates in complete medium. After 24 h of seeding, the test compounds at different dilutions were added and kept for another 24 h in a humidified chamber with 5% CO2 at 37 °C. Twenty microlitres of MTT (5.00 mg/mL in 1XPBS) stock solution were pipetted into each well, mixed and incubated for at least 3–4 h. After incubation, the plates were centrifuged at 1500 rpm for 5 min. The supernatant was disposed cautiously and 100 μL of DMSO were added to each well to lyse the cell and dissolve the insoluble purple formazan product into a coloured solution. Absorbance was taken at 570 nm to determine formazan formation as a measurement of cell viability. Experiments were performed in triplicate. The 50% cytotoxic concentration (CC50) was assesses by analysis of dose–response curves. Selectivity Index (SI) was calculated as [31]:
Haemolysis assay
Haemolytic effect of all chalcones and standard anti-malarial drugs, chloroquine, quinine and artemisinin, was examined by incubating normal erythrocytes with all above mentioned compounds in phosphate-buffered saline (PBS), respectively. Briefly, fresh erythrocytes were centrifuged for 5 min at 1600 rpm for at least thrice in PBS and then the remaining pellet was re-suspended in PBS at 2% hematocrit. One hundred microlitres of this suspended pellet was added to 96-well sterile culture plate having test compounds at different desired concentrations. PBS alone (for baseline values) and 0.4% Triton X-100 in PBS (for 100% haemolysis) were employed as controls. After keeping at 37 °C for 3 h, the test samples were centrifuged and the supernatant was used for determination of the haemolytic activity quantified in terms of haemoglobin release as monitored spectrophotometrically by taking absorbance at 415 nm [33]. The experiment was done in triplicate and the mean ± SD was calculated [33, 34].
Haemozoin inhibition assay
The haemozoin (β-haematin) inhibition by distinct drugs in P. falciparum cultures was assessed employing drug concentrations in the proximity of IC50 concentrations after completion of 48 h [35]. Briefly, the test cultures were centrifuged for 5–10 min at 1300 rpm to dispose of the culture medium. Infected erythrocyte pellet (mingled of β-haematin and erythrocyte membrane) were exposed to 0.01% saponin lysis for 10 min at 25 °C to lyse erythrocyte to release parasites. These released parasites were further washed three times with PBS, re-suspended in 2.5% sodium dodecyl sulfate buffer solution (SDS in PBS) and subjected to spin at 20,000 g for 1 h. The supernatant was disposed and the insoluble haemozoin pellet was washed in 2.5% SDS in PBS and then dissolved in 20 mM NaOH. The haemozoin content was quantified by taking the absorbance at 400 nm and using a standard curve prepared from β-haematin. The amount of haemozoin formed in relation to control was calculated. All assays were performed in triplicate.
Statistical analysis
Data were presented as mean ± SD. IBM SPSS Statistics version 21.0 was used for data analysis. p < 0.05 was taken as level of significance. Means were compared using one-way analysis of variance (ANOVA) followed by post hoc, Bonferroni multiple comparison test.
Results
Chemistry
The structures of the synthesized chalcones are represented in Table 1.
Table 1.
Structure of the synthesized chalcones 1–10
| Chalcone | R’ | B |
|---|---|---|
| 1 | 2′,4′,6′-Trimethoxy- | 3,4-Dimethoxyphenyl- |
| 2 | 2′,5′-Dimethoxy- | 4-Methoxyphenyl- |
| 3 | 2′,5′-Dimethoxy- | 3,4-Methylenedioxyphenyl- |
| 4 | 3′,4′,5′-Trimethoxy- | 4-Fluorophenyl- |
| 5 | 3′,4′,5′-Trimethoxy- | 4-Dimethylaminophenyl- |
| 6 | 3′,4′,5′-Trimethoxy- | 4-Methoxyphenyl- |
| 7 | 3′,4′,5′-Trimethoxy- | 3,4-Dimethoxyphenyl- |
| 8 | 3′,4′,5′-Trimethoxy- | 3,4-Methylenedioxyphenyl- |
| 9 | 4′-Chloro- | 1H-Indole-2-yl- |
| 10 | 4′-Iodo- | 1H-Indole-2-yl- |
Anti-malarial activity
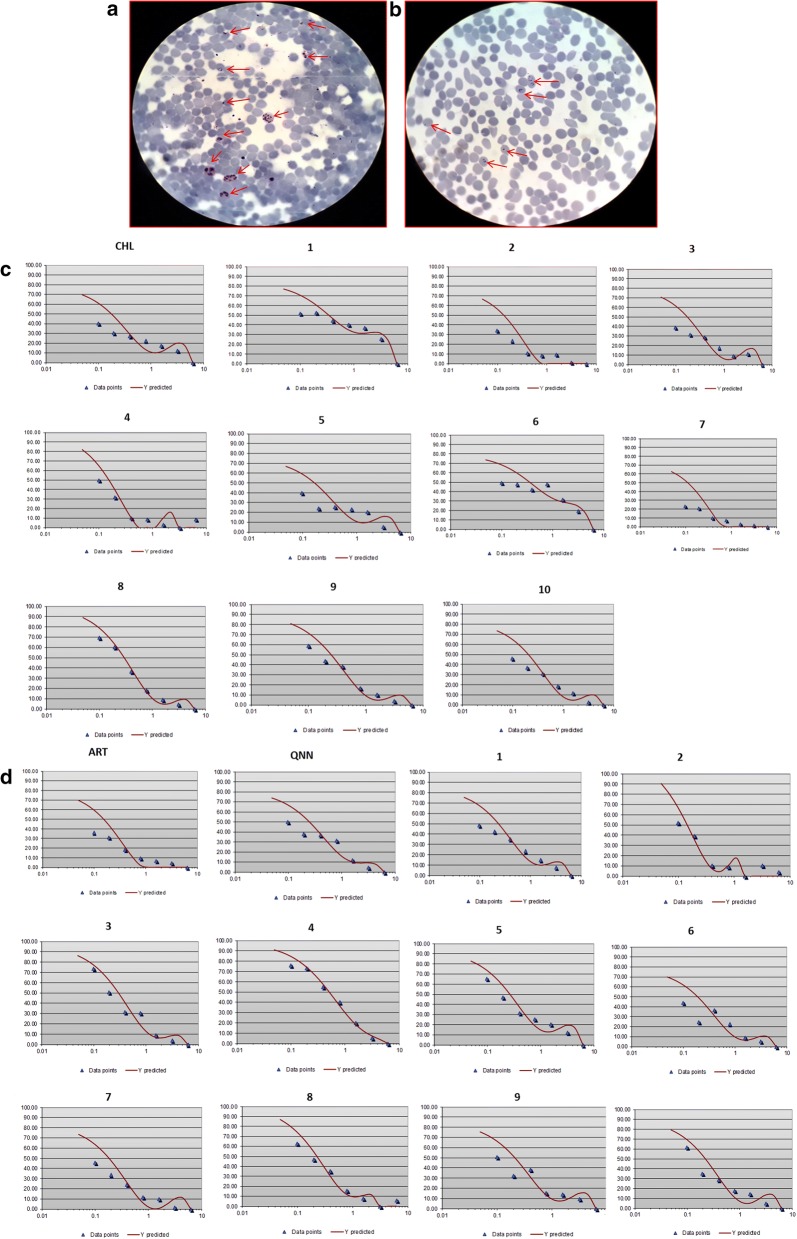
The chloroquine sensitive (MRC-2) and chloroquine resistant (RKL-9) strains of P. falciparum were cultured in vitro under sufficient gaseous mixture in RPMI1640 medium and the culture was synchronized by treating with 5% d-sorbitol to acquire mainly ring stage Plasmodium as depicted in Fig. 3a, b.
Fig. 3.
In vitro anti-malarial activity of chalcones on P. falciparum. a Unsynchronized culture of P. falciparum containing different stages of their life cycle; merozoites, early trophozoites (early ring stage), late trophozoites (late ring stage), schizonts, invading merozoites observed from Giemsa-stained slide under 1000× magnification. b Synchronized culture containing only ring stages of P. falciparum after treatment with 5% D-sorbitol observed from Giemsa-stained slide under 1000X magnification. c Dose–response curves (y-axis represents; % parasite matured into schizonts and x-axis represents; log10 concentration) of chloroquine sensitive P. falciparum strain (MRC-2) to different concentration of chalcones number 1,2, 3, 4, 5, 6, 7, 8, 9 and 10 and chloroquine (CHL). d Dose–response curves (y-axis represents; % parasite matured into schizonts and x-axis represents; log10 Concentration) of chloroquine resistant P. falciparum strain (RKL-9) to different concentration of chalcones number 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10, quinine hydrochloride (QNN) and artemisinin (ART)
Parasite at ring stage was used for compound concentration response assay with parasitaemia of 1% at 5% haematocrit. All chalcones, chloroquine, quinine and artemisinin were tested for anti-malarial activity on both strains by looking at percentage inhibition in schizont maturation following WHO Mark III protocol [36] in serially diluted range (6.25–0.09 μg/mL except artemisinin used in 6.25–0.09 ng/mL) of each drug concentration, Fig. 3c, d. The IC50 and IC90 values of all compounds were determined and the resistance index between the two sensitive and resistant strains was calculated (Table 2). The IC50 values acquired for all chalcones were in the range of 0.10–0.40 μg/mL for MRC-2 and 0.14–0.55 μg/mL for RKL-9. The chalcones 7 and 2 showed maximum potency with IC50 values of 0.11 and 0.13 μg/mL for MRC-2, and 0.18 and 0.14 μg/mL for RKL-9. The percentage inhibition in schizont maturation was also calculated after incubation of ring stage P. falciparum till 24 h at the same range of drug concentrations (Table 3).
Table 2.
In vitro anti-malarial activity of the chalcones on P. falciparum chloroquineS and P. falciparum chloroquineR strains, their HeLa cell cytotoxicity and resistance (RI) and selectivity indices (SI)
| Compounds/drugs code |
P. falciparum Chloroquines Strain (MRC-2) IC50 (µg/mL) |
P. falciparum Chloroquines Strain (MRC-2) IC90 (µg/mL) |
P. falciparum ChloroquineR Strain (RKL-9) IC50 (µg/mL) |
P. falciparum ChloroquineR Strain (RKL-9) IC90 (µg/mL) |
Resistance Index (RI) IC50 (RKL-9)/IC50 (MRC-2) |
HeLa Cell CC50 |
Selective index (SI) P.falciparum chloroquines (MRC-2) |
Selective index (SI) P.falciparum chloroquineR (RKL-9) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.34 | 5.54 | 0.23 | 1.42 | 0.68 | 4.36 | 12.82 | 18.96 |
| 2 | 0.13 | 0.51 | 0.14 | 0.36 | 1.08 | 1.06 | 8.15 | 7.57 |
| 3 | 0.17 | 0.75 | 0.29 | 1.15 | 1.71 | 7.79 | 45.82 | 26.86 |
| 4 | 0.15 | 0.40 | 0.51 | 2.26 | 3.40 | 0.84 | 5.60 | 1.65 |
| 5 | 0.16 | 1.08 | 0.23 | 0.92 | 1.43 | 8.45 | 52.81 | 36.74 |
| 6 | 0.35 | 5.23 | 0.19 | 0.98 | 0.54 | 1.66 | 4.74 | 8.74 |
| 7 | 0.11 | 0.46 | 0.18 | 0.67 | 1.64 | 15.31 | 139.18 | 85.05 |
| 8 | 0.29 | 1.01 | 0.26 | 5.31 | 0.90 | 2.20 | 7.59 | 8.46 |
| 9 | 0.25 | 0.96 | 0.21 | 0.95 | 0.84 | 1.65 | 6.60 | 7.85 |
| 10 | 0.20 | 0.89 | 0.19 | 0.81 | 0.95 | 1.88 | 9.40 | 9.90 |
| CHL | 0.17 | 1.14 | – | – | – | 31.04 | 182.58 | – |
| QNN | – | – | 0.25 | 1.59 | – | 30.31 | – | 121.24 |
| ART (ng/mL) | – | – | 0.15 | 0.15 | – | 49.11 | – | 327.4 |
ChloroquineS = Chloroquine Sensitive and ChloroquineR = Chloroquine Resistant
CHL chloroquine, QNN quinine hydrochloride, ART artemisinin
Table 3.
Schizont maturation inhibition (%) and haemolysis of normal erythrocytes (%) with effect to the chalcones
| Drugs/compound | % Schizont maturation inhibition ± SD (MRC-2) (Conc. = 6.25 μg/mL) | % Schizont maturation inhibition ± SD (RKL-9) (Conc. = 6.25 μg/mL) | % Hemolysis ± SD (Conc. = 12.5 μg/mL) |
|---|---|---|---|
| 1 | 82.43 ± 20.51 | 49.66 ± 25.46 | 1.44 ± 0.005 |
| 2 | 71.76 ± 10.61 | 58.02 ± 21.21 | 0.86 ± 0.002 |
| 3 | 59.26 ± 4.95 | 63.71 ± 2.83 | 1.01 ± 0.003 |
| 4 | 47.48 ± 17.68 | 57.69 ± 7.78 | 0.94 ± 0.006 |
| 5 | 58.65 ± 7.78 | 40 ± 20.51 | 0.65 ± 0.001 |
| 6 | 51.05 ± 42.42 | 43.85 ± 33.23 | 1.15 ± 0.005 |
| 7 | 94.24 ± 2.21 | 85.82 ± 6.36 | 1.08 ± 0.001 |
| 8 | 75.17 ± 12.02 | 77.78 ± 1.41 | 0.83 ± 0.003 |
| 9 | 50.32 ± 33.23 | 48.87 ± 25.46 | 1.30 ± 0.009 |
| 10 | 64.44 ± 9.90 | 49.62 ± 19.79 | 1.51 ± 0.013 |
| CHL | 88.39 ± 0.71 | – | 0.86 ± 0.001 |
| QNN | – | 71.87 ± 16.26 | 2.16 ± 0.035 |
| ART (ng/mL) | – | 87.88 ± 2.12 | 1.94 ± 0.026 |
CHL chloroquine, QNN quinine hydrochloride, ART artemisinin
Cytotoxicity assay and evaluation of selectivity index
Compound cytotoxicity performed on HeLa cell line showed 50% inhibitory cellular cytotoxicity at concentration range from 0.80 to 16.00 μg/mL. The results are summarized in Table 4. The calculated selectivity index shown in Table 2 was 139.18 for 7, 52.81 for 5 and 45.82 for 3 and others had < 15.00 on the chloroquine sensitive strain. Similarly, 7 had higher selectivity index (85.05) as compared to other derivatives on the chloroquine resistance strain.
Table 4.
Cell viability of chalcones and standard compounds on HeLa cell line (%)
| Compounds | % Cell viability ± SD (Conc. = 12.5 µg/mL) |
|---|---|
| 1 | 39.80 ± 0.06 |
| 2 | 47.57 ± 0.11 |
| 3 | 40.29 ± 0.07 |
| 4 | 33.98 ± 0.08 |
| 5 | 50.24 ± 0.08 |
| 6 | 41.74 ± 0.03 |
| 7 | 51.21 ± 0.04 |
| 8 | 42.71 ± 0.06 |
| 9 | 55.09 ± 0.09 |
| 10 | 47.08 ± 0.10 |
| CHL | 58.00 ± 0.06 |
| QNN | 62,62 ± 0.06 |
| ART (ng/mL) | 58.92 ± 0.06 |
CHL chloroquine, QNN quinine hydrochloride, ART artemisinin
The percentage viability of HeLa cells at different concentrations (12.5–0.09 μg/mL) of all compounds including standard anti-malarials is depicted in Additional file 1: Figure S1. At the highest concentration of 12.50 μg/mL (Table 4), the percentage cell viability of 9, 7, and 5 was more than 50%, which was found to be satisfactory compared to chloroquine (58.00 ± 0.06) and quinine (62.62 ± 0.06).
Effect on fresh erythrocytes (haemolysis)
Fresh erythrocytes treated with chalcones derivatives for 3 h at different concentrations in serial dilution (12.5–0.09 μg/mL) showed minimal percentages haemolysis below 5% (Table 3) when compared to the standard control triton X-100 (100% haemolysis).
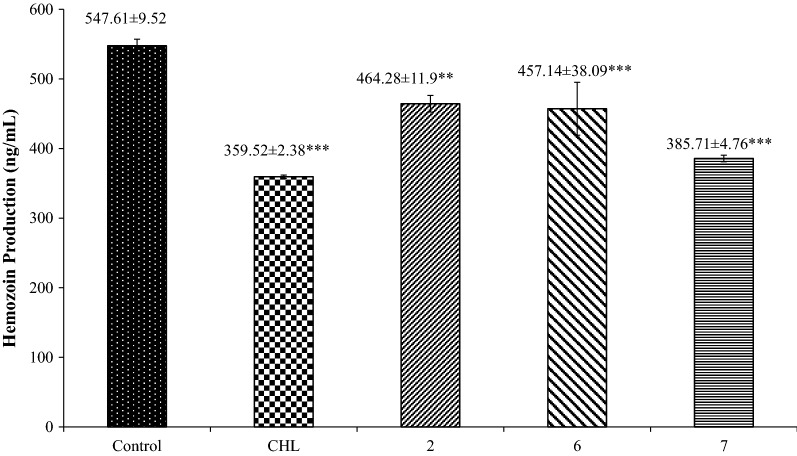
Effect on haemozoin production
The quantity of haemozoin formed is directly related to the level of haemoglobin digestion. Data for the haemozoin production by the Plasmodium in the effect of chloroquine and the three most potent screened chalcone 2, 6, and 7 derivatives are represented in Fig. 4. The haemozoin production in non-treated infected erythrocytes was used as the positive control. The level of haemozoin production of the chalcone 7 (385.71 ± 4.76) was slightly higher than that of chloroquine (359.52 ± 2.38). Other chalcones also had lower level as compared to that of the control (547.61 ± 9.52).
Fig. 4.
Amount of haemozoin production (ng/mL). n = The experiment was performed in triplicate. The data are represented as mean ± SD. Significant data are given as *p < 0.05; **p < 0.01;***p < 0.001
Discussion
Chloroquine and quinine retain anti-malarial efficacy for past several decades. Afterwhile, artemisinin-based combination therapy is the most recommended therapy to curb any malaria [37]. However, due to appearance of drug resistance and failure to achieve desired anti-malarial efficacy of existing drugs in several part of world [3] emphasizes the effort made by pharmaceutical companies and research organizations to search for new leads with high efficacy and minimal toxicity. Most anti-malarial drugs, such as chloroquine, quinine, mefloquine, halofantrine, pyrimethamine, sulfadoxine, sulfones, tetracyclines, act on the erythrocytic stage of parasite during the course of infection, which is the primary symptomatic phase of infection, thereby terminating the clinical attacks of malaria and addressing the constant threat of drug resistance [38]. Erythrocytic stages in culture of P. falciparum under in vitro conditions is practically feasible with easier manipulation step in the laboratory and found to be a major initial tool to screen schizontocidal compounds. Though this morphological microscopic method is cumbersome and labour intensive, it has been established because of its reproducibility and simplicity. It is also an inexpensive assay in comparison to the various other anti-malarial assays like [3H]-hypoxanthine incorporation assay, lactate dehydrogenase (pLDH) assay, Malaria SYBR Green I-based fluorescence (MSF) assay, double-site enzyme-linked lactate dehydrogenase enzyme immunodetection (DELI) assay, flow cytometric haemozoin detection assay, luciferase-based high-throughput screening (HTS) assay [39, 40], that can be set up in smaller laboratories. The evidence for the anti-malarial activity of chalcones from natural [16, 18, 41] and synthetic source is well documented [42–48].
In this study, 10 chalcones were analysed for anti-malarial activity and the results showed good activity against both chloroquine sensitive (MRC-2) strain (0.12–0.36 µg/mL) and chloroquine resistant (RKL-9) strain (0.15–0.52 µg/mL). The chalcones 7, 2 and 6 showed maximum anti-malarial potency as the most potent of them, 7, caused 94.24 ± 2.21% inhibition at concentration of 6.25 μg/mL (Table 3). In comparison, the chalcones with anti-malarial activity, described so far in the literature, have IC50 values between 1.1 and 12.3 µg/mL [11, 44, 47–49].
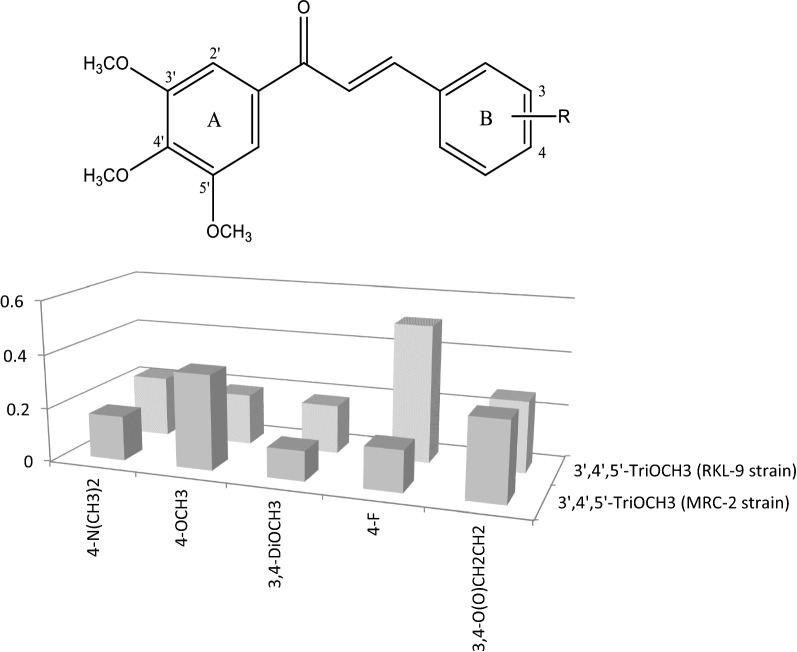
The anti-plasmodial activity of chalcones related to the position of methoxy groups on rings A and B. Concerning ring A, the most successful pattern was that of the 3′,4′,5′-trimethoxyphenyl motif (Fig. 5) shown by the chalcone 7, which is a pharmacophore with diverse range of biological actions including anticancer, anti-invasive, antioxidant, and anti-inflammatory activities. Its effectivity against the MRC-2 strain decreased depending on the substituents in ring B, as followed: 3,4-dimethoxyphenyl-(3,4-diOCH3)>4-fluorophenyl-(4-F)>4-dimethylaminophenyl-(4-N(CH3)2)>3,4-methylenedioxyphenyl-(3,4-O(O)CH2CH2)>4-methoxyphenyl-(4-OCH3), while against the resistant strain, RKL-9, this order changed to: 3,4-diOCH3>4-OCH3 > 4-N(CH3)2>3,4-O(O)CH2CH2>4-F. This result shows that the presence of methylated hydroxyl and amino groups in ring B is more relevant to activity of the 3′,4′,5′-trimethoxychalcones against the chloroquine resistant strain, which might be useful for a future design of more potent chalcones with anti-malarial activity. However, exact relation between such substitutions patterns on ring B and anti-malarial activity is not known.
Fig. 5.
Influence of the substitution of the 3′,4′,5′-trimethoxychalcones on their anti-malarial activity
Meanwhile, one of the most active chalcones (2) possesses methoxy groups at C-2′ and C-5′ positions in ring A and at C-3 and C-4 in ring B, meaning that exploring anti-malarial activity of a larger series of 2′,5′-dimethoxy chalcones with various substituents in ring B is also worthy.
Further, to investigate cytotoxic effect of all these derivatives, results demonstrate very low cytotoxic activity of all derivatives. The chalcones 2, 6 and 7 produced minimal cytotoxicity. The selectivity index is defined as relative effectiveness of investigational compound in inhibiting cell proliferation as compared to inducing cell death. Therefore, it is preferable to have higher selective index that means maximal activity with least cellular toxicity [50]. The comparison of the SI values obtained for 7 and the reference compounds (chloroquine, artemisinin, and quinine) demonstrates good therapeutic effect of 7 and activity close to that of the reference drugs. The chalcone 7 has comparatively higher CC50 values (> 15.30 μg/mL) and good selective index (> 136.60) that defines optimum selective anti-malarial. Previously, Lim et al. [49], showed the most active chalcone in their study also having 3,4-diOCH3 substituents in ring B, but 2′-OH and 4′-OCH3 groups in ring A had prominent cytotoxicity towards FM3A cells, a model of the host, that has comparatively low EC50 values (> 3.3 μg/mL), indicating that the compound has non-selective anti-malarial activity. This shows that finding out the specific anti-malarial target is crucial for the design of chalcones with anti-malarial activity.
To evaluate the effect of all chalcones on normal erythrocytes, percentage haemolysis was measured. All the derivatives irrespective of the concentration range used in the study illustrate minimal haemolytic effect and did not shows any adverse events on erythrocytes at drug concentrations at which they eliminate the parasite which suggests that the anti-malarial effect of these chalcones were primarily not due to erythrocyte lysis.
Next to locate the chalcones, anti-malarial target, the study used to appraise the feasible inhibitory activity of the potent chalcones in haemozoin inhibition assay. The chalcones are supposed to interface and prohibit the P. falciparum cysteine protease (falcipain) action, a vital enzyme believed to be intricate in the haemoglobin digestion present inside the acidic food vacuole of the intra-erythrocytic parasite. Hindrance in haemoglobin digestion process is catastrophic for the Plasmodium. It is anticipated that malarial aspartic proteases (plasmepsin) and cysteine proteases (falcipain) mediate the haemoglobin digestion for releasing amino acids that are needed for intra-erythrocytic parasite multiplication and growth [51]. Also, these proteases form an interesting anti-malarial drug target [51]. Structure based analysis anticipate anti-malarial chalcones restriction on trophozoite cysteine protease as the probable mode of action [23]. The results showed significant reduction in the production of haemozoin when infected erythrocytes were treated with chloroquine and three other potent derivatives (2, 6, and 7), compared to untreated infected erythrocytes. This also suggests the similar mechanism of anti-malarial action of chalcones as the chloroquine does. Similar results were shown in the previous studies where different chalcone derivatives showed hindrance of plasmodial haemozoin formation in culture suggesting that these chalcones act on haemozoin formation pathways [52–54]. However, few studies reported that some do not interfere with haemozoin formation [55, 56]. This variation is mostly due to substitution on the ring A or B of the chalcones.
Conclusion
Chalcones offer a very large repository of bioactive compounds with diverse molecular targets. Chalcones with even minor structural changes can result in targeting distinct cellular processes. The present in vitro study clearly indicates that finding the particular anti-malarial target is crucial for the design of potent chalcones. All chalcones here demonstrated potent anti-malarial activity in schizont maturation assay, with 7 having the highest potency (IC50 of 0.11 µg/mL) in contrast to licochalcone (1.43 µg/mL). Also, the inhibition in haemozoin production by these compounds suggests similar mechanism of action with chloroquine. However, extensive in vivo study is needed to confirm efficacy of these derivatives under influence of various physiological mechanism under-going inside animal models.
Supplementary information
Additional file 1: Figure S1. Cell viability (%) of chalcones and standard compound at different concentrations; CHL-Chloroquine; QNN-Quinine hydrochloride; ART-Artemisinin.
Acknowledgements
We are thankful to ICMR, New Delhi for providing financial support in form of junior research fellowship and senior research fellowship to Shweta Sinha, DST- Joint Indo Bulgarian Project for Funding Project Work (INT/BULGARIA/P-06/12). Special thanks are given to Neena Valecha (Professor, Director), C. R. Pillai (Emeritus Professor), NIMR, New Delhi, for providing the P. falciparum strains.
Abbreviations
- ANOVA
analysis of variance
- CC50
cytotoxicity concentration 50%
- DELI assay
double-site enzyme-linked lactate dehydrogenase enzyme immunodetection assay
- DMEM
Dulbecco Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- EC50
half maximal effective concentration
- HEPES buffer
N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) buffer
- HTS assay
high-throughput screening assay
- IC50
concentration for 50% inhibition
- MSF assay
malaria SYBR green I-based fluorescence assay
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NAOH
sodium hydroxide
- NCCS
National Centre for Cell Science
- NIMR
National Institute of Malaria Research
- PBS
phosphate-buffered saline
- pLDH assay
parasite lactate dehydrogenase assay
- RI
resistance index
- RPMI media
Roswell Park Memorial Institute media
- SD
standard deviation
- SDS
sodium dodecyl sulfate
- SI
selectivity index
- SPSS
Statistical Package for the Social Sciences
- WHO
World Health Organization
Authors’ contributions
RS, SS, DIB designed the study. BM, AB and BDR provided necessary input on the study design. SS and DIB conducted the experiments and data-analysis. SS wrote the initial draft of the manuscript. RS, DIB, BM and NM collaboratively revised the manuscript with SS. All authors contributed to reviewing the manuscript. All authors read and approved the final manuscript.
Funding
No funding support has been provided in the design of the studies, data collection, analysis, interpretation of data and in writing of the manuscript from any funding agencies (public, commercial, or not-for-profit sectors).
Availability of data and materials
All data generated or analysed during this study are included in this published article and its additional files.
Ethics approval and consent to participate
The ethics approval was given by Institute Ethics Committee, Project ref No. NK/1265/Ph.D/23991 at Post Graduate Institute of Medical Education and Research, Chandigarh for maintenance of P. falciparum strains in culture.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-019-3060-z.
References
- 1.WHO . World malaria report. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaratunga C, Sreng S, Suon S, Phelps ES, Stepniewska K, Lim P, et al. Artemisinin-resistant Plasmodium falciparum in Pursat province, western Cambodia: a parasite clearance rate study. Lancet Infect Dis. 2012;12:851–858. doi: 10.1016/S1473-3099(12)70181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2012;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phyo AP, Nkhoma S, Stepniewska K, Ashley EA, Nair S, McGready R, et al. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet. 2012;379:1960–1966. doi: 10.1016/S0140-6736(12)60484-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyaw MP, Nyunt MH, Chit K, Aye MM, Aye KH, Aye MM, et al. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS ONE. 2013;8:e57689. doi: 10.1371/journal.pone.0057689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion: a molecular epidemiology observational study. Lancet Infect Dis. 2017;17:491–497. doi: 10.1016/S1473-3099(17)30048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowakowska Z. A review of anti-infective and antiinflammatory chalcones. Eur J Med Chem. 2007;42:125–137. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Batovska DI, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 10.de Mello TF, Bitencourt HR, Pedroso RB, Aristides SM, Lonardoni MV, Silveira TG. Leishmanicidal activity of synthetic chalcones in Leishmania (Viannia) braziliensis. Exp Parasitol. 2014;136:27–34. doi: 10.1016/j.exppara.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Mishra N, Arora P, Kumar B, Mishra LC, Bhattacharya A, Awasthi SK, et al. Synthesis of novel substituted 1,3-diaryl propenone derivatives and their antimalarial activity in vitro. Eur J Med Chem. 2008;43:1530–1535. doi: 10.1016/j.ejmech.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Sinha S, Medhi B, Sehgal R. Chalcones as an emerging lead molecule for antimalarial therapy: a review. J Mod Med Chem. 2013;1:64–77. [Google Scholar]
- 13.Powers DG, Casebier DS, Fokas D, Ryan WJ, Troth JR, Coffen DL. Automated parallel synthesis of chalcone-based screening libraries. Tetrahedron. 1998;54:4085–4096. doi: 10.1016/S0040-4020(98)00137-9. [DOI] [Google Scholar]
- 14.Kumar R, Mohanakrishnan D, Sharma A, Kaushik NK, Kalia K, Sinha AK, et al. Reinvestigation of structure-activity relationship of methoxylated chalcones as antimalarials: synthesis and evaluation of 2,4,5-trimethoxy substituted patterns as lead candidates derived from abundantly available natural β-asarone. Eur J Med Chem. 2010;45:5292–5301. doi: 10.1016/j.ejmech.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Go ML, Liu M, Wilairat P, Rosenthal PJ, Saliba KJ, Kirk K. Antiplasmodial chalcones inhibit sorbitol-induced hemolysis of Plasmodium falciparum-infected erythrocytes. Antimicrob Agents Chemother. 2004;48:3241–3245. doi: 10.1128/AAC.48.9.3241-3245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, Theander TG, Christensen SB, Hviid L, Zhai L, Kharazmi A. Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob Agents Chemother. 1994;38:1470–1475. doi: 10.1128/AAC.38.7.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, Wilairat P, Go ML. Antimalarial alkoxylated and hydroxylated chalones: structure-activity relationship analysis. J Med Chem. 2001;44:4443–4452. doi: 10.1021/jm0101747. [DOI] [PubMed] [Google Scholar]
- 18.Narender T, Shweta, Tanvir K, Rao MS, Srivastava K, Puri SK. Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg Med Chem Lett. 2005;15:2453–2455. doi: 10.1016/j.bmcl.2005.03.081. [DOI] [PubMed] [Google Scholar]
- 19.Geyer JA, Prigge ST, Waters NC. Targeting malaria with specific CDK inhibitors. Biochim Biophys Acta. 2005;1754:160–170. doi: 10.1016/j.bbapap.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 20.Mi-Ichi F, Miyadera H, Kobayashi T, Takamiya S, Waki S, Iwata S, et al. Parasite mitochondria as a target of chemotherapy: inhibitory effect of licochalcone A on the Plasmodium falciparum respiratory chain. Ann N Y Acad Sci. 2005;1056:46–54. doi: 10.1196/annals.1352.037. [DOI] [PubMed] [Google Scholar]
- 21.Sriwilaijaroen N, Liu M, Go ML, Wilairat P. Plasmepsin II inhibitory activity of alkoxylated and hydroxylated chalcones. Southeast Asian J Trop Med Public Health. 2006;37:607–612. [PubMed] [Google Scholar]
- 22.Geyer JA, Keenan SM, Woodard CL, Thompson PA, Gerena L, Nichols DA, et al. Selectiveinhibition of Pfmrk, a Plasmodium falciparum CDK, by antimalarial 1, 3-diaryl-2-propenones. Bioorg Med Chem Lett. 2009;19:1982–1985. doi: 10.1016/j.bmcl.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Kenyon GL, Cohen FE, Chen X, Gong B, Dominguez JN, et al. In vitro antimalarial activity of chalcones and their derivatives. J Med Chem. 1995;38:5031–5037. doi: 10.1021/jm00026a010. [DOI] [PubMed] [Google Scholar]
- 24.Ivanova A, Batovska D, Engi H, Parushev S, Ocsovszki I, Kostova I, Molnar J. MDR-reversal activity of chalcones. In Vivo. 2008;22:379–384. [PubMed] [Google Scholar]
- 25.Mehandzhiyski A, Tsvetkova I, Najdenski C, Batovska D. Synthesis of chalcones and their heterocyclic analogues with potential antibacterial activity. Bulg J Chem. 2012;1:53–59. [Google Scholar]
- 26.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 27.Lambros E, Vanderberg JP. Synchronization of P. falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. doi: 10.2307/3280287. [DOI] [PubMed] [Google Scholar]
- 28.Noedl H. Non linear evaluation of malaria drug sensitivity data (HN-NonLin V1.1) Bangkok, Thailand: Armed Forces Research Institute for Medical Sciences; 2002. http://www.meduniwien.ac.at/user/harald.noedl/malaria/download.html.
- 29.WHO. In vitro micro-test (Mark III) for the assessment of P. falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimethamine and artemisinin. Geneva: World Health Organization; CTD/MAL/9720 Rev 2; 2001.
- 30.Mishra K, Dash AP, Swain BK, Dey N. Anti-malarial activities of Andrographis paniculata and Hedyotis corymbosa extracts and their combination with curcumin. Malar J. 2009;8:26. doi: 10.1186/1475-2875-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smit FJ, van Biljon RA, Birkholtz LM, N’Da DD. Synthesis and in vitro biological evaluation of dihydroartemisinyl-chalcone esters. Eur J Med Chem. 2015;90:33–44. doi: 10.1016/j.ejmech.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 33.Kaushik NK, Sharma J, Sahal D. Anti-plasmodial action of de novo-designed, cationic, lysine-branched, amphipathic, helical peptides. Malar J. 2012;11:256. doi: 10.1186/1475-2875-11-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, Sharma JD. In vitro hemolysis of human erythrocytes—by plant extracts with antiplasmodial activity. J Ethnopharmacol. 2001;74:239–243. doi: 10.1016/S0378-8741(00)00370-6. [DOI] [PubMed] [Google Scholar]
- 35.Akompong T, Ghori N, Haldar K. In vitro activity of riboflavin against the human malaria parasite Plasmodium falciparum. Antimicrob Agents Chemother. 2000;44:88–96. doi: 10.1128/AAC.44.1.88-96.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basco LK, Heseltine E. Field application of in vitro assays for the sensitivity of human malaria parasites to antimalarial drugs. Geneva: World Health Organization; 2007. http://www.who.int/iris/handle/10665/43610.
- 37.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 38.Fidock DA. Drug discovery: priming the antimalarial pipeline. Nature. 2010;465:297–298. doi: 10.1038/465297a. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya A, Mishra LC, Sharma M, Awasthi SK, Bhasin VK. Antimalarial pharmacodynamics of chalcone derivatives in combination with artemisinin against Plasmodium falciparum in vitro. Eur J Med Chem. 2009;44:3388–3393. doi: 10.1016/j.ejmech.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Sinha S, Sarma P, Sehgal R, Medhi B. Development in assay methods for in vitro antimalarial drug efficacy testing: a systematic review. Front Pharmacol. 2017;8:754. doi: 10.3389/fphar.2017.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yenesew A, Induli M, Derese S, Midiwo JO, Heydenreich M, Peter MG, et al. Anti-plasmodial flavonoids from the stem bark of Erythrina abyssinica. Phytochemistry. 2004;65:3029–3032. doi: 10.1016/j.phytochem.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 42.Chen M, Christensen S, Zhai L, Rasmussen MH, Theander TG, Frøkjaer S, et al. The novel oxygenated chalcone, 2,4-dimethoxy-4′-butoxychalcone, exhibits potent activity against human malaria parasite Plasmodium falciparum in vitro and rodent parasites Plasmodium berghei and Plasmodium yoelii in vivo. J Infect Dis. 1997;176:1327–1333. doi: 10.1086/514129. [DOI] [PubMed] [Google Scholar]
- 43.Domínguez JN, Charris JE, Lobo G, de Domínguez NG, Moreno MM, Riggione F, et al. Synthesis of quinolinylchalcones and evaluation of their antimalarial activity. Eur J Med Chem. 2001;36:555–560. doi: 10.1016/S0223-5234(01)01245-4. [DOI] [PubMed] [Google Scholar]
- 44.Awasthi SK, Mishra N, Kumar B, Sharma M, Bhattacharya A, Mishra LC, et al. Potent antimalarial activity of newly synthesized substituted chalcone analogs in vitro. Med Chem Res. 2009;18:407–420. doi: 10.1007/s00044-008-9137-9. [DOI] [Google Scholar]
- 45.Acharya BN, Saraswat D, Tiwari M, Shrivastava AK, Ghorpade R, Bapna S, et al. Synthesis and antimalarial evaluation of 1,3,5-trisubstituted pyrazolines. Eur J Med Chem. 2010;45:430–438. doi: 10.1016/j.ejmech.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 46.Domínguez JN, de Domínguez NG, Rodrigues J, Acosta ME, Caraballo N, León C. Synthesis and antimalarial activity of urenylBis-chalcone in vitro and in vivo. J Enzyme Inhib Med Chem. 2012;28:1267–1273. doi: 10.3109/14756366.2012.733383. [DOI] [PubMed] [Google Scholar]
- 47.Yadav N, Dixit SK, Bhattacharya A, Mishra LC, Sharma M, Awasthi SK, et al. Antimalarial activity of newly synthesized chalcone derivatives in vitro. Chem Biol Drug Des. 2012;80:340–347. doi: 10.1111/j.1747-0285.2012.01383.x. [DOI] [PubMed] [Google Scholar]
- 48.Tadigoppula N, Korthikunta V, Gupta S, Kancharla P, Khaliq T, Soni A, et al. Synthesis and insight into the structure activity relationships of chalcones as antimalarial agents. J Med Chem. 2013;56:31–45. doi: 10.1021/jm300588j. [DOI] [PubMed] [Google Scholar]
- 49.Lim SS, Kim HS, Lee DU. In vitro antimalarial activity of flavonoids and chalcones. Bull Korean Chem Soc. 2007;28:2495–2497. doi: 10.5012/bkcs.2007.28.12.2495. [DOI] [Google Scholar]
- 50.Guidance for Industry Antiviral Product Development—Conducting and Submitting Virology Studies to the Agency. (2006) http://www.fda.gov/cder/guidance/index.htmhttps://www.fda.gov/OHRMS/DOCKETS/98fr/05d-0183-gdl0002-01.pdf.
- 51.de Domínguez NDG, Rosenthal PJ. Cysteine proteinase inhibitors block early steps in hemoglobin degradation by cultured malaria parasites. Blood. 1996;87:4448–4454. doi: 10.1182/blood.V87.10.4448.bloodjournal87104448. [DOI] [PubMed] [Google Scholar]
- 52.Domínguez JN, León C, Rodrigues J, de Domínguez NG, Gut J, Rosenthal PJ. Synthesis and antimalarial activity of sulfonamide chalcone derivatives. Farmaco. 2005;60:307–311. doi: 10.1016/j.farmac.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Frölich S, Schubert C, Bienzle U, Jenett-Siems K. In vitro antiplasmodial activity of prenylated chalcone derivatives of hops (Humulus lupulus) and their interaction with haemin. J Antimicrob Chemother. 2005;55:883–887. doi: 10.1093/jac/dki099. [DOI] [PubMed] [Google Scholar]
- 54.Pandey AV, Singh N, Tekwani BL, Puri SK, Chauhan VS. Assay of β-hematin formation by malaria parasite. J Pharm Biomed Anal. 1999;20:203–207. doi: 10.1016/S0731-7085(99)00021-7. [DOI] [PubMed] [Google Scholar]
- 55.Mishra LC, Bhattacharya A, Bhasin VK. Phytochemical licochalcone A enhances antimalarial activity of artemisinin in vitro. Acta Trop. 2009;109:194–198. doi: 10.1016/j.actatropica.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 56.Sisodia BS, Negi AS, Darokar MP, Dwivedi UN, Khanuja SP. Antiplasmodial activity of steroidal chalcones: evaluation of their effect on hemozoin synthesis and the new permeation pathway of Plasmodium falciparum-infected erythrocyte membrane. Chem Biol Drug Des. 2012;9:610–615. doi: 10.1111/j.1747-0285.2012.01323.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Cell viability (%) of chalcones and standard compound at different concentrations; CHL-Chloroquine; QNN-Quinine hydrochloride; ART-Artemisinin.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its additional files.