Abstract
Volumetric muscle loss (VML) injuries exceed the considerable intrinsic regenerative capacity of skeletal muscle, resulting in permanent functional and cosmetic deficits. VML and VML-like injuries occur in military and civilian populations, due to trauma and surgery as well as due to a host of congenital and acquired diseases/syndromes. Current therapeutic options are limited, and new approaches are needed for a more complete functional regeneration of muscle. A potential solution is human hair-derived keratin (KN) biomaterials that may have significant potential for regenerative therapy. The goal of these studies was to evaluate the utility of keratin hydrogel formulations as a cell and/or growth factor delivery vehicle for functional muscle regeneration in a surgically created VML injury in the rat tibialis anterior (TA) muscle. VML injuries were treated with KN hydrogels in the absence and presence of skeletal muscle progenitor cells (MPCs), and/or insulin-like growth factor 1 (IGF-1), and/or basic fibroblast growth factor (bFGF). Controls included VML injuries with no repair (NR), and implantation of bladder acellular matrix (BAM, without cells). Initial studies conducted 8 weeks post-VML injury indicated that application of keratin hydrogels with growth factors (KN, KN+IGF-1, KN+bFGF, and KN+IGF-1+bFGF, n = 8 each) enabled a significantly greater functional recovery than NR (n = 7), BAM (n = 8), or the addition of MPCs to the keratin hydrogel (KN+MPC, KN+MPC+IGF-1, KN+MPC+bFGF, and KN+MPC+IGF-1+bFGF, n = 8 each) (p < 0.05). A second series of studies examined functional recovery for as many as 12 weeks post-VML injury after application of keratin hydrogels in the absence of cells. A significant time-dependent increase in functional recovery of the KN, KN+bFGF, and KN+IGF+bFGF groups was observed, relative to NR and BAM implantation, achieving as much as 90% of the maximum possible functional recovery. Histological findings from harvested tissue at 12 weeks post-VML injury documented significant increases in neo-muscle tissue formation in all keratin treatment groups as well as diminished fibrosis, in comparison to both BAM and NR. In conclusion, keratin hydrogel implantation promoted statistically significant and physiologically relevant improvements in functional outcomes post-VML injury to the rodent TA muscle.
Keywords: : FGF, functional recovery, IGF, keratin, myogenesis, volumetric muscle loss
Introduction
Recovery from muscle injury involves a series of interdependent and overlapping phases that include muscle degradation/inflammation, regeneration, and remodeling, respectively. These processes have been described in detail elsewhere.1 However, despite the well-documented regenerative capacity of skeletal muscle, there are numerous congenital and/or acquired diseases, surgical procedures (e.g., tumors or infection), and traumatic injuries suffered by soldiers and civilians2 that result in an irrecoverable loss of muscle tissue. These injuries are referred to as volumetric muscle loss (VML)3 and, by definition, they exceed the intrinsic regenerative capacity of skeletal muscle, resulting in permanent functional and cosmetic deficits.2
Present therapeutic options produce limited functional and cosmetic recovery, and further, they are associated with local morbidity at both the harvest and donor sites.4 As a result, recent attention has focused on the enormous potential of tissue engineering (TE) technologies to provide more effective treatment options for large muscle injuries. In fact, implantation of decellularized extracellular matrices (ECM), either without5–8 or with a cellular component,9–15 has been evaluated in several preclinical studies. In general, the inclusion of a cellular component provides for greater functional improvements.9,12,16 Nonetheless, recent clinical studies for treatment of VML injury via implantation of ECM alone17 have, indeed, provided some evidence of functional recovery, although with limited evidence for de novo muscle regeneration.
Given the uniqueness of each VML injury and the diversity of causes for VML and VML-like injuries, it seems logical that multiple TE technologies will be required to ensure more effective and widespread clinical applications. In fact, alternative TE approaches to implantation of cells and/or ECM are also being considered. For example, significant preclinical development efforts are also underway for the design and implantation of growth factor-releasing biological or synthetic scaffolds (including hydrogels).18 The ultimate goal of this strategy is to gain improved control of the spatiotemporal characteristics of growth factor release to ensure more complete functional restoration of muscle injuries in a fashion that is more analogous to morphogenesis.19–24
In this regard, keratin is an intermediate filament protein found in hair, wool, nails, and other epidermal appendageal structures.25 Keratin can be processed via oxidation, creating a noncovalently cross-linked form known as keratose (KSO), or via reduction, creating a disulfide cross-linked form known as kerateine (KTN). As mammalians do not naturally produce the enzyme that is responsible for keratin breakdown, the degradation rate of keratin based-biomaterials is easily manipulated through the combination of different ratios of the cross-linked and non-cross-linked forms.26 The easy manipulation of the degradation rate, in turn, enables the controlled release of physiologically relevant molecules (e.g., growth factors, antibiotics), potentially further increasing the clinical relevance of keratin-based biomaterials to skeletal muscle repair.27–30
With respect to the current studies, we were interested in evaluating the utility of keratin as a delivery vehicle for basic fibroblast growth factor (bFGF or FGF-2) and insulin-like growth factor 1 (IGF-1). bFGF is known to directly stimulate muscle regeneration by activating satellite cell proliferation, enhancing satellite cell recruitment to the injury site, and also, by virtue of its strong angiogenic effect.31–36 Likewise, IGF-1 also plays an important role in muscle maintenance and repair, influencing muscle metabolism, via myoblast differentiation and myotube formation,37–40 enhancing the role of bone marrow-derived cells to muscle regeneration, and contributing to the mitigation of fibrosis and the inflammatory response.41–45 The roles of IGF and bFGF in the skeletal muscle regeneration process are comprehensively discussed in Passipieri and Christ.18 Also of particular relevance to this article is the recent observation that macrophages can alter polarization in the presence of keratin proteins.46 In fact, the timing and nature of macrophage polarization is a key modulator of muscle repair/regeneration.47
Keratins biomaterials such as those used here are tissue compatible, biodegradable,48 nonantigenic, and nonimmunogenic.49 Medical and biotechnology applications of these biomaterials have been presented in several studies showing their in vitro compatibility with different cell types, including skeletal muscle.23,50–52 Their potential relevance to bone, cardiac muscle and peripheral nerve regeneration,53–57 as well as wound healing58,59 is now also becoming apparent. As such, the further development of keratin-based biomaterials may have major implications for improved TE approaches to muscle repair.
In this context, the goal of these studies was to investigate a novel skeletal muscle TE strategy, by which we build on our prior work with decellularized ECM to evaluate the utility of keratin hydrogels to support functional muscle regeneration/repair via provision of key skeletal muscle growth factors and/or muscle progenitor cells (MPCs). To this end, we used our established and biologically relevant rat tibialis anterior (TA) VML injury model to evaluate the ability of implantation/application of keratin hydrogels alone, or in combination with IGF-1 and/or bFGF and/or MPCs, to restore skeletal muscle tissue function and native tissue morphology.
Materials and Methods
Keratin extraction
Keratins were extracted by using a patented process in a quality system regulation/good manufacturing process, QSR/GMP, facility at KeraNetics, LLC (Winston-Salem, NC). Briefly, a cold solution of 0.5 M thioglycolic acid (TGA) in sodium hydroxide (reductive extraction for KTN) or a 2% peracetic acid (PAA) solution (oxidative extraction for KSO) was added to end-cut human hair. For reductive extraction, the reducing solution was added to hair in a mixing tank for 12 h at 37°C followed by two washes in 100 mM Tris base (Sigma) and water. The solution was then centrifuged, filtered, dialyzed against a 100 kDa molecular-weight cutoff cellulose membrane, and neutralized to pH 7.4. For oxidative extraction with PAA, the hair was handled in a similar manner. After acid extraction and Tris base/water washes, the extract was purified by centrifugation and dialyzed in water against a 50 kDa nominal low-molecular-weight cutoff filter. Both the oxidized and reduced solutions were lyophilized, ground, and sterilized by gamma irradiation.23
Bladder acellular matrix preparation
Bladder acellular matrix (BAM) scaffolds were prepared as previously described.16 Briefly, porcine-derived bladder was washed and trimmed to obtain the lamina propria, which was placed in 0.05% trypsin (Hyclone, Logan, UT) for 1 h at 37°C. The bladder was then transferred to Dulbecco's modified Eagle's medium (DMEM) solution that was supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic antimycotic (Hyclone), and it was kept overnight at 4°C. The preparation was then washed in a solution containing 1% Triton-X (Sigma) and 0.1% ammonium hydroxide (Fischer Scientific, Pittsburgh, PA) in de-ionized water for 4 days at 4°C. Finally, the bladder was then washed in deionized water for 3 days at 4°C. The decellularized scaffold was further dissected to obtain a scaffold of 0.2–0.4 mm thickness. The scaffolds were then cut and draped onto custom-made silicone molds with a 4.5 cm2 working area.
MPC isolation and culture
Rat MPCs were isolated from the soleus and TA muscles of 4–6 week-old male Lewis rats (Charles River Laboratories) as previously described.9,12,16 Briefly, after sterilization in iodine and consecutive washes in sterile phosphate-buffered saline (PBS), muscles were minced into small pieces by hand and incubated in 0.2% collagenase (Worthington Biochemicals) solution that was prepared in low-glucose DMEM (Hyclone) for 2 h at 37°C. Muscle tissue fragments were plated onto tissue culture dishes that were coated with Matrigel (1:50 dilution; BD Biosciences) in myogenic medium containing DMEM high glucose that was supplemented with 20% FBS, 10% horse serum, 1% chicken embryo extract, and 1% antibiotic/antimycotic (Hyclone). Cells were passaged at 70–80% confluence, cultured in low-glucose DMEM that was supplemented with 15% FBS and 1% antibiotic/antimycotic, and impregnated in the keratin gel at second passage.
KSO:KTN optimization and cell capacity
We have previously used enzyme-linked immunosorbant assays (ELISAs) to characterize the in vitro release profiles of both IGF-1 and bFGF from keratin hydrogels containing KSO, KTN, or a combination of the two.23,26 These previous in vitro studies demonstrated tunable and controlled release of both growth factors and also served as a basis for a previous in vivo subcutaneous model study.23 The gels were prepared with selected ratios of KSO to KTN in which varied concentrations (w/v) of keratin powder to sterile PBS were also examined. The results indicated that a 70:30 blend (weight:weight) of 15% KSO and 7% KTN resulted in the sustained release of both IGF-1 and bFGF over the course of a month.
Based on rheology data collected by Tomblyn et al.,23 the elastic modulus of hydrogel that allows the incorporation of cells and may still be extruded through a surgical syringe is ∼100 Pa. The maximum number of cells that could be incorporated into the optimized blend and still yield a hydrogel that was flowable in nature and capable of being extruded through a surgical syringe was 1.7 × 106 MPCs/mL in the KSO fraction. It may be assumed that the addition of this concentration of cells resulted in a hydrogel, which did not have an elastic modulus that was significantly greater than 100 Pa. As such, it is worth noting that the viscosity of the hydrogel limited the concentration of cells used in this study.
Hydrogel preparation
For the growth factor containing groups, IGF-1 and/or bFGF (Peprotech, Rocky Hill, NJ) were diluted in sterile water to yield a final concentration of 100 μg/mL of each growth factor. Growth factor solutions were added to KTN powders to make a 7% w/v hydrogel. KOS was added to either PBS or a serum-free media cell suspension of ∼1.65 × 106/mL passage 2 rat MPCs to form a 15% w/v hydrogel. The KTN and KSO hydrogels were then combined at a 70:30 KSO:KTN ratio (weight:weight) by passing the gels between coupled syringes.
Treatment groups
Treatment groups and group sizes are listed in Table 1 and are as follows: keratin (KN; n = 18), KN + I (n = 18), KN + b (n = 18), KN+I+b (n = 18), KN + M (n = 8), KN+M+I (n = 8), KN+M+b (n = 8), and KN+M+I+b (n = 8). Two negative control groups were included: no repair (NR) group (n = 17) and BAM group (n = 17).
Table 1.
Design of Experimental Groups Submitted to Tibialis Anterior Volumetric Muscle Loss Injury Model: List of Groups, Components, and Number of Animals (n) Are Described
| Components | ||||||
|---|---|---|---|---|---|---|
| Group | Group size (n) | Keratin | IGF-1 100 μg/mL | bFGF 100 μg/mL | MPC 1.65 × 106cells/mL | BAM |
| No repair | 17 | − | − | − | − | − |
| BAM | 17 | − | − | − | − | + |
| Keratin | 18 | + | − | − | − | − |
| KN + I | 18 | + | + | − | − | − |
| KN + b | 18 | + | − | + | − | − |
| KN+I+b | 18 | + | + | + | − | − |
| KN + M | 8 | + | − | − | + | − |
| KN+M+I | 8 | + | + | − | + | − |
| KN+M+b | 8 | + | − | + | + | − |
| KN+M+I+b | 8 | + | + | + | + | − |
b, bFGF, basic fibroblast growth factor; BAM, bladder acellular matrix; I, IGF-1, insulin-like growth factor 1; KN, keratin; M, MPC, muscle progenitor cell.
Animal care
This study was conducted in compliance with the Animal Welfare Act, the Implementing Animal Welfare Regulations, and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals. The Wake Forest University Health Sciences School of Medicine Animal Care and Use Committee approved all animal procedures. A total of 138 adult female Lewis rats (Charles River Laboratories) weighing 203.4 ± 0.8 g, at 11–13 weeks of age, were individually housed in a vivarium accredited by the American Association for the Accreditation of Laboratory Animal Care, and they were provided with food and water ad libitum.
Surgical procedures
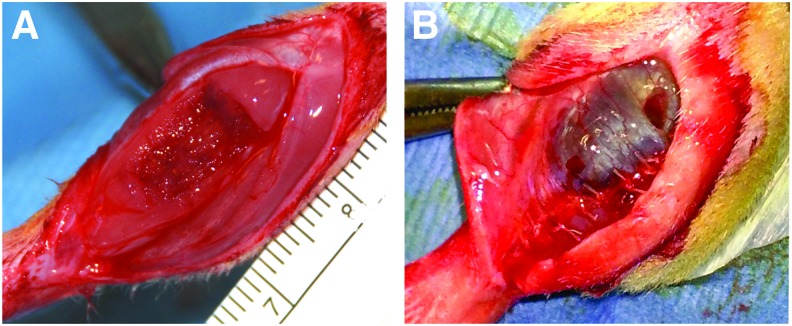
Surgical creation of VML injury was performed in the TA muscle as previously reported.16,60 A longitudinal incision was made on the lateral aspect of the left lower leg, followed by blunt separation of the skin from the underlying fascia. Similarly, the fascia covering the anterior crural muscles was separated by using blunt dissection. The proximal and distal tendons of the Extensor Hallicus Longus (EHL) and Extensor Digitorum Longus (EDL) muscles were then isolated and ablated. TA muscle corresponds to 0.17% of the total body weight, as previously determined.16,60 Excision of ∼20% of the TA muscle weight at the middle third of the muscle (Fig. 1A) characterized the VML injury model. The BAM scaffold was sutured directly over the defect as previously described.16 The fascia was closed with 6–0 vicryl sutures, and ∼200 μL of the hydrogel was injected by using a 19G needle over the muscle defect through the sutured fascia (Fig. 1B). The skin closure was performed with 5–0 prolene by using interrupted sutures, and skin glue was applied between the skin sutures to help prevent the incision from opening. Ketoprofen (0.03 mg/kg; subcutaneously) was administered every 24 h for 3 days.
FIG. 1.
Photograph of surgical site of the creation of the TA VML injury and subsequent treatment with a keratin-based hydrogel implant. (A) VML injury was created by excision of ∼20% of the TA muscle. Immediately after defect creation, the fascia layer was sutured and keratin-based hydrogel was injected through the incision, in the area between fascia and muscle (B). Skin was closed, and the animal was allowed to recover (not shown). TA, tibialis anterior; VML, volumetric muscle loss. Color images available online at www.liebertpub.com/tea
In vivo functional analysis
At 4, 8, and 12 weeks after surgery, rats were anesthetized (1.5–2.5% isoflurane) and the left hind limb was aseptically prepared. The rat was placed supine on a heated platform, and the left knee was bent to a 90° angle and was secured by using a stabilizing rod. The left foot was taped to a footplate that was attached to the shaft of an Aurora Scientific 305C-LR-FP servomotor, which was controlled by using a computer. Sterilized percutaneous needle electrodes were inserted through the skin for stimulation of the left common peroneal nerve. Electrical stimulus was applied by using an Aurora Scientific stimulator with a constant current SIU (Model 701C). Stimulation voltage and needle electrode placement were optimized with a series of twitch contractions at 1 Hz. Contractile function of the anterior crural muscles was assessed by measuring peak isometric tetanic torque derived from the maximal response to a range of stimulation frequencies (10–200 Hz). After functional testing, the animals were allowed to recover on the heated platform and returned to the vivarium. For terminal time points, animals were euthanized via CO2 inhalation and the TA muscle was harvested.
Gross morphology analysis of TA muscle
After animal euthanasia, skin on the hind limbs was removed and the fascia layer was gently detached and removed. Once exposed, the injured TA muscle and its contralateral uninjured control were imaged and harvested. The weights of the samples were measured, followed by preparation for histological analysis.
Histology and immunohistochemistry
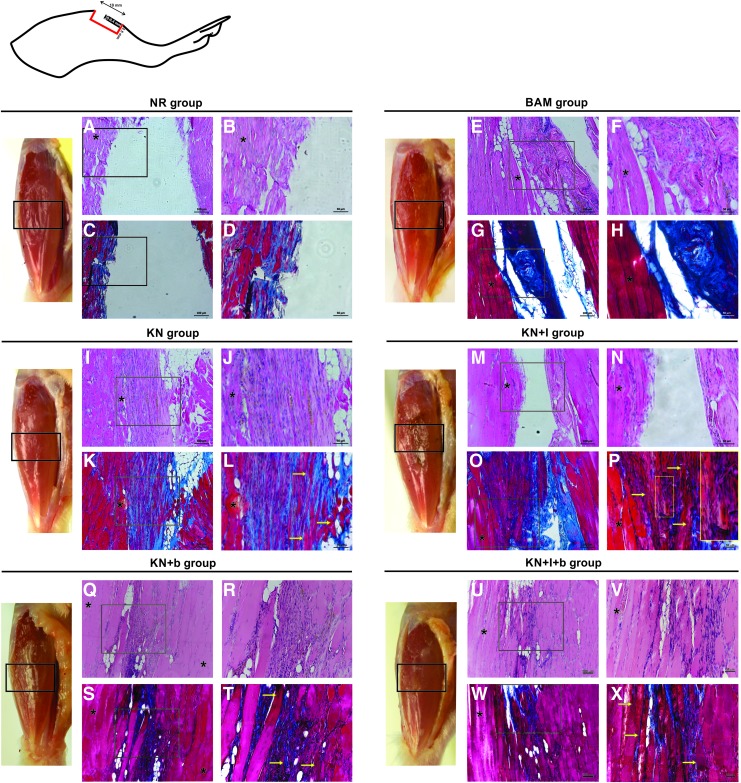
Muscles from all experimental groups were photographed before fixation in 4% paraformaldehyde overnight at 4°C and embedding in paraffin wax. At 12 weeks postinjury, longitudinal 5 μm-thick serial sections were retrieved up to 400 μm into the wound bed (n = 3 for each treatment group) (Figs. 5 and 6). Serial cross-sections were obtained from tissues that were retrieved at 12 weeks postinjury (n = 3 for each keratin treated group). Hematoxylin and eosin and Masson's trichrome stains were used to evaluate cellular morphology as well as for the presence of any inflammatory response in and around the implant. Cross-sectional areas of ≈150 muscle fibers adjacent to the injured area were manually measured by using ImageJ software (NIH). The number of centrally located nuclei was assessed by analyzing the nuclei location of 800–1000 fibers that were adjacent to the injured area.
FIG. 5.
Gross morphology and histological comparison of tissue retrieved from implant region of injured TA muscle 12 weeks after keratin-based hydrogel treatment. As shown by the representative macroscopic tissue images of the leg, there were quite obvious differences in the gross appearance of the injured TA region among treatment groups. For example, the injury site was quite apparent in the NR animals and to a much lesser extent in the KN-treated groups. In fact, the KN-alone (no growth factor) TA muscle is virtually indistinguishable from an uninjured TA muscle. Although BAM implantation did not promote robust functional recovery, significant volume reconstitution was apparent. Also shown are hematoxylin–eosin staining (A, B, E, F, I, J, M, N, Q, R, U, V), wherein nuclei are stained in blue-purple and cytoplasm and cellular proteins are stained in red-pink, as well as Mason's Trichrome (C, D, G, H, K, L, O, P S, T, W, X), in which tissue stains red, collagen stains blue, and nuclei stain black. In all cases, staining was performed on longitudinal sections of TA muscle obtained from the injured regions of the respective treatment groups. Schematic representation on the upper left corner shows the region of the tibialis anterior where histological analysis was performed. (A-–D) NR, (E–-H) BAM, (I-–L) KN, (M–-P) KN+I, (Q–-T) KN+b, and (U–-X) KN+I+b. Yellow arrows denote regions with new muscle fiber formation. *Denotes regions with native muscle. Yellow inset on (P) highlights new vessel formation. Color images available online at www.liebertpub.com/tea
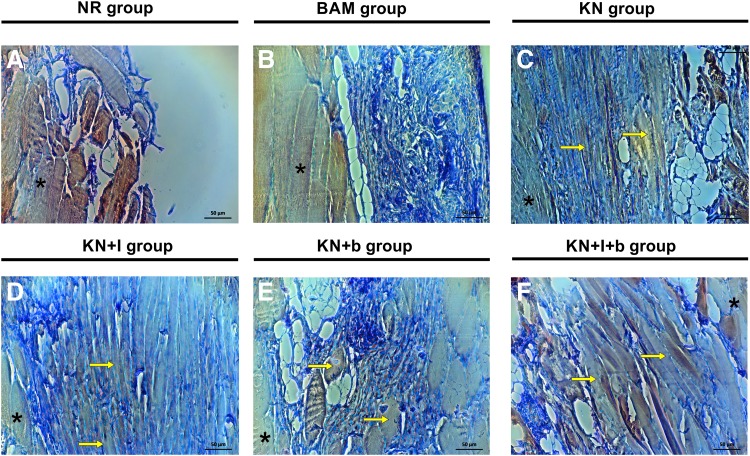
FIG. 6.
Representative images of myosin heavy chain expression at the injury site after treatment with keratin-based hydrogel. Longitudinal sections obtained at the interface between native tissue and injured area (see inset in Fig. 5) show the presence of newly formed myosin-positive fibers after treatment with KN (C), KN + I (D), KN + b (E), and KN+I+b (F). Samples from the NR group (A) and the BAM group (B) did not display the same level of myosin heavy chain expression, nor consistent evidence of significant regenerating fibers. Yellow arrows denote some of the regions with new muscle fiber formation; *denotes regions with native tissue. Color images available online at www.liebertpub.com/tea
Immunohistochemical staining was performed by using antibodies to detect myosin (MF-20, 1:10) acquired from Developmental Studies Hybridoma Bank (Iowa City, IA). Biotinylated anti-mouse IgG (MKB-2225, 1:250; Vector Laboratories, Inc.) was used as the secondary antibody. Sections were treated with Avidin Biotin Complex Reagent (PK-7100; Vector Laboratories, Inc.) and then visualized by using a NovaRED substrate kit (SK-4800; Vector Laboratories, Inc.). Finally, the sections were counterstained by using Gill's Hematoxylin (GHS280; Sigma-Aldrich). Tissue sections without primary antibody were used as negative controls. Images were captured and digitized (DM4000B Leica Upright Microscope) at varying magnifications.
Statistics
Numeric data are presented as mean ± standard error of the mean (SEM). Morphological and functional data were analyzed by using one- and two-way analyses of variance (ANOVAs), as indicated in the figure captions. On finding a significant ANOVA, post hoc comparison testing of parameters of interest was performed by using Fisher's least significant difference (LSD) test, with α set to 0.05. Statistical analysis was conducted by using GraphPad Prism 6.0 for Windows (La Jolla, CA).
Results
Creation of VML injury and in vivo functional analysis
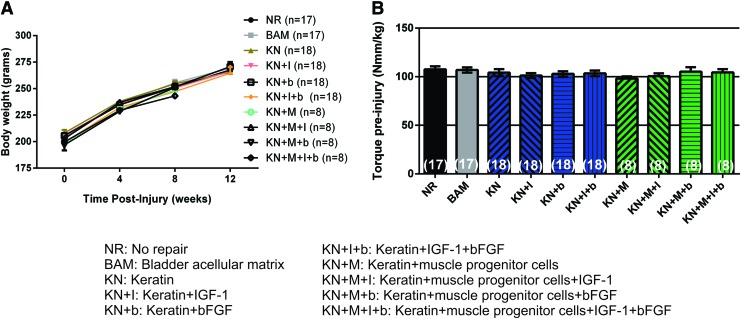
None of the animals died during the surgical procedure, and no postimplantation mortality was recorded. The weight of the TA muscle excised from the animals in each treatment group was similar, reflecting the creation of comparable VML injuries (NR: 73.3 ± 0.6 mg; BAM: 72.9 ± 0.7 mg; KN: 73.9 ± 0.6 mg; KN+I: 72.7 ± 1.1 mg; KN+b: 72.4 ± 0.9 mg; KN+I+b: 70.4 ± 1.0 mg; KN+M: 67.8 ± 0.5 mg; KN+ M+I: 73.9 ± 1.4 mg; KN+M+b: 68.1 ± 0.83 mg; KN+M+I+b: 72.4 ± 0.98 mg). Mean animal weights were similar among all treatment groups throughout the entire time course of the study (Fig. 2A), indicating that there was no adverse impact of any treatment. However, since animals in all the treatment groups gained weight over the course of the study, all statistical comparisons on functional measures were made on data normalized to body weight, to control for increases in in vivo torque production due to animal growth per se. Mean values for the baseline (preinjury) maximal isometric torque in response to peroneal nerve stimulation are shown in Figure 2B for all animals and treatment groups, and as illustrated, these responses were statistically indistinguishable.
FIG. 2.
Comparison of the body weight and functional baseline parameters among treatment groups. (A) Body weights of study animals reveal normal healthy weight gain in all treatment groups over the course of 8 weeks and/or 12 weeks. (B) Graphical comparison illustrating the equivalence of the mean baseline contraction force resulting from peroneal nerve stimulation and measured with a footplate force transducer in all treatment groups. In all cases, individual responses are normalized to their body weight. Data are presented as mean ± SEM. No statistical differences were observed by using a two-way and one-way ANOVA. Group sample sizes are listed in parentheses. ANOVA, analysis of variance; SEM, standard error of the mean. Color images available online at www.liebertpub.com/tea
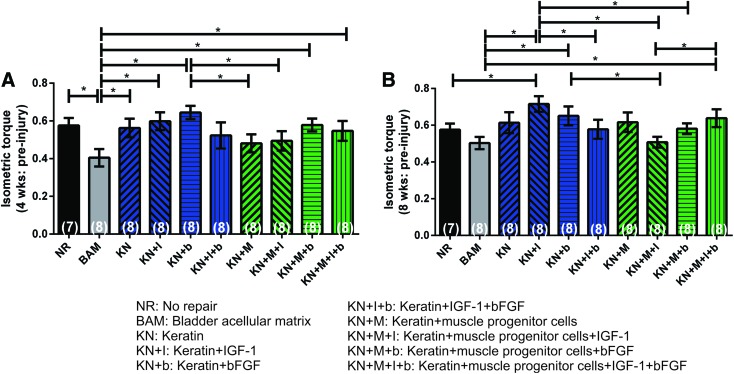
For the first phase of the study, 7–8 animals were randomized into 10 different treatment groups. The mean isometric torque values were normalized to preinjury baseline force and body weight at 4 and 8 weeks after surgery, respectively. Mean data for all parameters of interest, in all animals, are summarized in Table 2, and they are graphically illustrated in Figure 3A and B. A two-factor ANOVA was used to compare functional outcomes among the different treatment groups over time. Statistical analysis revealed a significant effect of treatment (p < 0.0354), a significant effect of time (p < 0.0001), but no interaction between time and treatment group (p > 0.1606). Post hoc analysis was then conducted at each time point, and it revealed that at 4 weeks most KN treatment groups showed improvement relative to implantation of BAM, but overall, there was no statistically significant improvement in functional recovery beyond the NR recovery level. However, at 8 weeks post-VML injury, the KN + I treatment group showed a degree of functional recovery and muscle wet weight greater than both NR and BAM, as well as several other treatment groups, both with and without inclusion of cells. Moreover, as illustrated, at 4 weeks (Fig. 3A) and 8 weeks post-VML injury (Fig. 3B and Table 2), in general, the groups treated with keratin alone, or keratin containing growth factor(s), actually showed significant improvement over the treatments groups with cells, but in no case, at either time point, was inclusion of cells found to produce a degree of functional recovery that was significantly greater than any of the KN treatment groups. Animals were euthanized at 8 weeks after surgery, and samples were subjected to histological analysis.
Table 2.
Summary of In Vivo Functional Data of Tibialis Anterior Muscle at 8 Weeks Postinjury
| NR | BAM | KN | KN + I | KN + b | KN+I+b | KN + M | KN+M+I | KN+M+b | KN+M+I+b | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (n) | 7 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Body wt (g) | 216 ± 0.9 | 206.6 ± 1.8 | 214.0 ± 4.3 | 206.1 ± 3.9 | 205.4 ± 2.6 | 194.8 ± 3.5 | 198.8 ± 2.3 | 202.9 ± 1.9 | 197.0 ± 5.3 | 199.8 ± 1.2 |
| TA muscle wet wt (mg) | 352.3 ± 16.6a | 385.0 ± 9.0b,c | 387.6 ± 16.8b,c | 370.3 ± 16.7a–d | 424.5 ± 14.4e | 364.8 ± 18.9a,c,d | 373.5 ± 13.9a–d,f | 355.7 ± 7.61a,b,d,f | 400.4 ± 13.2b,c,e | 392.5 ± 16.2b–e |
| Peak tetanic isometric torque | ||||||||||
| Nmm/kg body wt | 57.5 ± 3.8a | 50.0 ± 3.3a,b | 61.9 ± 5.8a,b | 66.5 ± 4.0c | 62.9 ± 5.0a,c,d | 56.1 ± 5.0a,b,d,e | 60.4 ± 5.3a–e | 51.1 ± 3.0a,b,e | 61.1 ± 3.1a,b,d,e | 66.6 ± 5.1a,c,d |
| Deficit (%)* | 28.0 ± 4.1a | 37.1 ± 4.1a,b | 23.3 ± 7.1a,b | 10.5 ± 5.3c | 18.6 ± 6.4a,c,d | 27.7 ± 6.4a,b,d,e | 22.9 ± 6.6a–e | 36.5 ± 3.6a,b,e | 27.3 ± 3.6a,b,d,e | 20.1 ± 6.0a,c,d |
| Nmm/g TA wet wt | 40.76 ± 1.5a | 33.4 ± 1.7a | 40.9 ± 3.6a | 44.1 ± 1.5a | 36.8 ± 3.4a | 37.6 ± 2.9a | 39.8 ± 2.8a | 36.1 ± 1.6a | 38.7 ± 2.6a | 41.0 ± 1.8a |
Values are mean ± SEM. Values denoted with the same letter are not significantly different (p > 0.05), whereas values without a similar letter denotation are significantly different (p < 0.05).
Deficit calculated from un-operated animals.
NR, no repair; SEM, standard error of the mean; TA, tibialis anterior; wt, weight.
FIG. 3.
Comparison of functional recovery observed after application of various keratin hydrogel formulations to TA VML injury at 4 and 8 weeks postimplantation. Peak isometric torque measured at (A) 4 weeks and (B) 8 weeks. There was no statistically significant functional benefit to the inclusion of cells in the various injected KN hydrogel treatments. In all cases, individual responses are presented as a ratio of the respective group mean of the initial maximum preinjury isometric torque response (presented in Fig. 2) as well as their body weight. Data are presented as mean ± SEM. *Significantly different at the p < 0.05 level using Fisher's PLSD post hoc test; after performing a two-way ANOVA for matched samples. Group sample sizes are listed in parentheses. KN, keratin. PLSD, protected least significant difference. Color images available online at www.liebertpub.com/tea
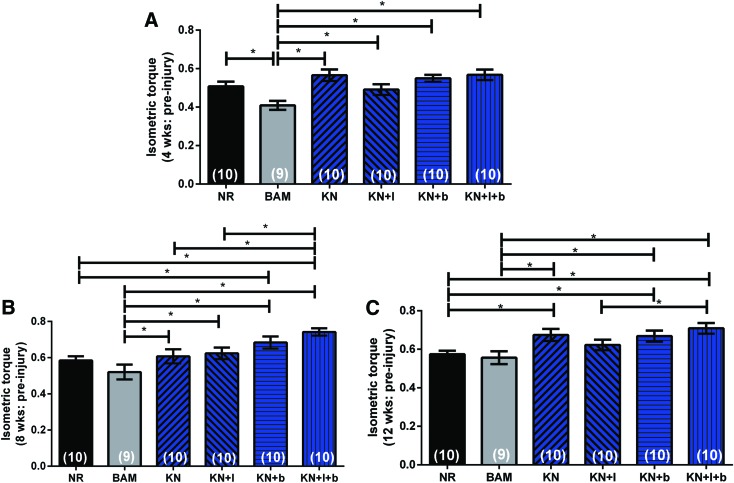
A prior study completed shortly after Phase I of this investigation demonstrated that for the TA VML rodent injury model, 12 weeks is required for a more complete functional restoration after treatment.16 In addition, the current data revealed that there was little impact of the inclusion of cells in KN hydrogels, as shown in Figure 3. As such, we conducted an independent second phase of the study in which 9–10 animals were randomly assigned to one of the 4 KN treatment groups and 2 negative control groups (NR and BAM) (i.e., the 4 groups containing cells were eliminated), and the functional evaluations were extended to a 12-week time point.
Once again, a two-factor ANOVA was used to compare functional outcomes among the different treatment groups over time. Statistical analysis revealed a significant effect of treatment (p < 0.0001), a significant effect of time (p < 0.0001), but no interaction between time and treatment group (p > 0.1157). Post hoc analysis revealed that in contrast to the single growth factor-loaded hydrogels (i.e., KN + I and KN+b), placement/implantation of the KN alone, as well as the KN+I+b hydrogels at the site of VML injury in the TA was associated with a significant and sustained functional recovery, relative to both NR and/or implantation of BAM alone, at all time points post-VML injury (Fig. 4). In particular, at 12 weeks post-VML injury, the peak isometric torque response to peroneal nerve stimulation in all keratin treatment groups (again, except for KN+I) was significantly greater than that observed for both the NR and BAM treatment groups (Fig. 4C and Table 3). Moreover, as shown in the companion paper to this work,61 the implantation of KN+I+b in a murine latissimus dorsi (LD) VML injury model had a similar functional outcome, among the same treatment groups (although again, not significantly different from KN alone). Analysis of TA muscle wet weight showed a significant reduction of muscle mass in all experimental groups relative to their contralateral control (contralateral control TA muscle wet weight: 467.9 ± 3.2 mg, p < 0.05). Interestingly, after 12 weeks, the TA wet weight of the samples in the KN + b and KN+I+b groups was similar, but it was significantly greater than all the other experimental groups (Table 3).
FIG. 4.
Evidence of improved functional recovery observed during 12 weeks postimplantation of keratin hydrogels in the TA VML injury model. Mean values for peak isometric torque production for each of the KN treatment groups are graphed at 4 weeks (A), 8 weeks (B), and 12 weeks (C) postinjury and implantation. As illustrated, the implantation of all keratin-based treatment groups, except KN+I, resulted in a significant and sustained functional recovery relative to BAM and/or NR. In all cases, individual responses are presented as a ratio of the respective group mean of the initial maximum preinjury isometric torque response (presented in Fig. 2) as well as their body weight. Data are presented as mean ± SEM. *Significantly different at the p < 0.05 level using Fisher's PLSD test; after performing a two-way ANOVA for matched samples. Group sample sizes are listed in parentheses. BAM, bladder acellular matrix; NR, no repair. Color images available online at www.liebertpub.com/tea
Table 3.
In vivo Functional Analysis of Tibialis Anterior Muscle at 12 Weeks Postinjury
| NR | BAM | KN | KN + I | KN + b | KN+I+b | |
|---|---|---|---|---|---|---|
| Sample size (n) | 10 | 9 | 10 | 10 | 10 | 10 |
| Body wt (g) | 267.7 ± 3.9 | 269.3 ± 4.9 | 265.5 ± 4.0 | 266.4 ± 3.7 | 270.8 ± 4.4 | 264.7 ± 5.5 |
| TA muscle wet wt (mg) | 375.8 ± 7.7a | 387.0 ± 16.6b | 400.4 ± 7.0b | 381.2 ± 9.6b | 427.7 ± 7.6c | 436.7 ± 7.9c |
| Peak tetanic isometric torque | ||||||
| Nmm/kg body wt | 65.0 ± 2.1a | 63.2.0 ± 3.8a | 71.9 ± 3.5b,c | 67.1 ± 2.9a,c | 72.1 ± 3.1b,c | 76.9 ± 2.9b |
| Deficit (%)* | 28.2 ± 2.3a | 30.5 ± 4.2a | 15.7 ± 3.9.b,c | 22.2 ± 3.4a,c | 16.5 ± 3.6b,c | 11.4 ± 3.4b |
| Nmm/g TA wet wt | 46.3 ± 1.3a | 42.4 ± 2.2a | 47.5 ± 1.6a | 46.8 ± 1.7a | 45.7 ± 2.1a | 46.4 ± 1.4a |
Values are mean ± SEM. Values denoted with the same letter are not significantly different (p > 0.05), whereas values without a similar letter denotation are significantly different (p < 0.05).
Deficit calculated from un-operated animals.
Gross morphology and histological analysis of TA muscle
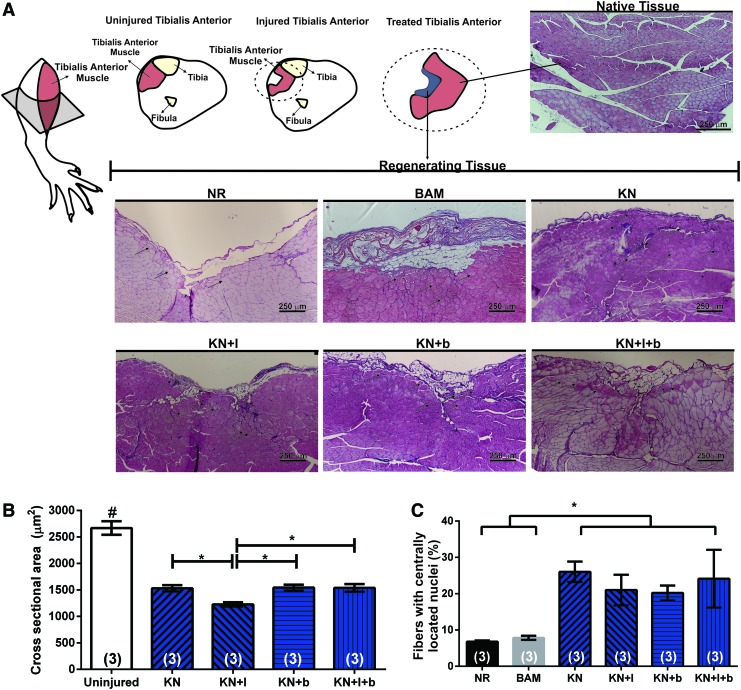
Macroscopically, the implants were well tolerated by the recipient animals, with no signs of infection, seroma, or rejection. Significant remodeling of the implanted region was evident 12 weeks after VML injury (Fig. 5). Representative histology from tissue sections in all six treatment groups is shown in Figures 5 and 6. These images were obtained in the first 400 μM from the surface of the TA. It is important to note that in the absence of new tissue formation (i.e., muscle regeneration), there should be no muscle tissue in this region, due to surgical excision during creation of the VML injury. In that regard, gross morphological analysis showed that the wound bed was similar in the KN, KN+b, and KN+I+b groups. More specifically, signs of muscle regeneration were evident by the presence of viable neo-tissue at the injury site close to the native muscle, as well as in regions of the implant that were much further removed from the native tissue interface. Although there was some evidence of residual fibrosis and inflammatory infiltrates at the 12-week time point, the groups that had a more robust functional recovery also exhibited more obvious de novo muscle regeneration (Fig. 6).
Of particular importance is the observation that new muscle tissue formation was observed at the 12-week time point in all KN-treated groups. As illustrated in Figures 5 and 6, this fact was reflected by the presence of striated myofibers in the keratin-implanted areas that were remote from the native tissue interface—where no tissue should be found, again, because the native muscle was harvested to create the VML injury. Furthermore, as seen in prior reports,10,12 significant vascularization was associated with myogenesis (e.g., see Fig. 5I, J, L, P). The BAM implant was also well integrated with the surrounding wound bed (Fig. 5) and resulted in significant volume reconstitution in the void created by the VML injury. However, from a histological perspective, there was little evidence of newly formed muscle tissue, and volume reconstitution occurred in the presence of significant fibrosis, as collagen is shown in blue with Masson's Trichrome stain (Fig. 5G, H). This latter observation is consistent with the observed diminution in functional recovery in BAM-treated animals relative to the KN-treated groups (Fig. 4). In stark contrast to all other treatment groups, there was a much more prominent “gap” present in the NR group in the VML injury region (Fig. 5A–D). There was little evidence of new muscle formation, and minimal fibrosis—in the initial 400 μm of sectioning—confirming the absence of significant endogenous muscle regeneration in this group.
Analysis of cross-sections of TA muscle retrieved at 12 weeks postinjury provided supporting evidence of new muscle formation after keratin-based treatments. As noted earlier, the defect area, represented schematically in Figure 7A as the absence of TA muscle in the VML injury region from the NR group, underwent significant volume reconstitution post-VML injury in the KN treatment groups. The cross-sectional areas of muscle fibers adjacent to the injury site were compared for three randomly selected animals in each of the keratin treatment groups (see the Materials and Methods section). Although there were numerous regenerating fibers, the cross-sectional area of newly formed fibers in all keratin-treated groups was significantly smaller than the age-matched control TA fiber (KN: 1531 ± 61.1 μm2; KN+I: 1226.9 ± 41.53 μm2; KN+b: 1544.33 ± 55.1 μm2; KN+I+b: 1538.8 ± 73.2 μm2; Uninjured control: 2669.56 ± 128.0 μm2, p < 0.05). Interestingly, the cross-sectional area of fibers in the KN + I group was also significantly smaller than that in the remaining keratin-treated groups (Fig. 7B). In addition, the keratin-treated group showed an increased number of fibers displaying centrally located nuclei when compared with the NR and BAM groups, although there was no difference among treatment groups (KN: 26.1% ± 2.8%; KN+I: 21.0% ± 4.5%; KN+b: 20.2% ± 2.0%; KN+I+b: 24.1% ± 7.9%; NR: 6.7% ± 0.3%; BAM: 7.8% ± 0.6%, p < 0.05) (Fig. 7C).
FIG. 7.
Cross-sectional analysis of keratin-treated TA muscles retrieved 12 weeks postinjury. (A) Schematic representation of anatomical location of TA injury is shown in the upper left corner and cross-sectional area of newly formed fiber in injured TA muscle. Black arrows denote representative centrally located nuclei in regenerating fibers. (B) Fiber cross-sectional area analysis of the keratin-treated muscles. (C) Quantification of percentage of fiber with centrally located nuclei among regenerated tissue in all treatment groups. *Significantly different at the p < 0.05 level using Fisher's PLSD test; after performing an one-way ANOVA for matched samples. Group sample sizes are listed in parentheses. Color images available online at www.liebertpub.com/tea
Discussion
Skeletal muscle has a tremendous capacity for regeneration after injury, in large part, as a result of the proliferation and differentiation of resident quiescent satellite cells.62,63 However, there are still no commercially available tissue-engineered products that have been able to leverage this regenerative ability to effectively treat the irreversible muscle damage/loss (i.e., VML) in patients that results from traumatic injuries, or acquired and/or congenital VML-like diseases/conditions. Despite encouraging results from recent clinical studies for treatment of VML injury via implantation of ECM, there is still plenty of room for therapeutic improvement.8,17,64–66 This fact has spurred a growing preclinical effort to develop tissue engineered/regenerative medicine technologies with greater efficacy and a wider range of clinical applications for VML and VML-like injuries.67–70
In pursuit of additional approaches to the treatment of the spectrum of VML injuries, this study evaluated the potential utility of keratin hydrogels. Keratin hydrogels are flowable biological scaffolds with material properties (e.g., degradation rate) that are amenable for muscle tissue implantation, and further, they can be easily tailored to promote the sustainable release of biological compounds.57,71 IGF-1 and bFGF are examples of locally produced molecules that play crucial roles in the complex signaling cascade that stimulates recruitment, proliferation, and differentiation of satellite cells during muscle repair and regeneration.71–73 Previous studies have shown that the incorporation of growth factors into keratin hydrogels permits controlled release over time.74,75 Several factors are involved in the controlled release, including diffusion from the hydrogel as well as binding between growth factors and keratin. It has previously been shown that growth factors released from keratin biomaterials maintain bioactivity.23,76 Thus, it is also likely that the keratin hydrogels protect growth factors from nonspecific enzymatic degradation and hydrolysis. One objective of this study was to determine whether the incorporation of growth factors into keratin hydrogels would improve functional regeneration in the context of VML injury.77 Of note, this article, as well as the accompanying manuscript on LD VML repair in a murine model61 represents the first application(s) of keratin hydrogels to treat VML injury.
To this end, we evaluated keratin hydrogels modified to include IGF-1 and/or bFGF and/or MPCs (see the Materials and Methods section, Table 1).71,78 The observed functional recovery of the TA muscle after VML injury for all treatment groups is graphically depicted in Figures 3 and 4. As illustrated, the mean maximal isometric torque in the NR and BAM groups is consistent with previously published data,16,60 indicating the reproducibility and applicability of the rodent TA VML injury model. If one uses statistically significant increases in isometric torque over NR and/or BAM as an indication of improved functional recovery, then it is reasonable to conclude that the implantation of KN hydrogel has a positive impact (Fig. 3). It also seems equally clear that the inclusion of cells, under these conditions (see section “Result - Creation of VML injury and in vivo functional analysis” for more details), imparted little additional benefits (Fig. 3; see section “Result - Creation of VML injury and in vivo functional analysis” for more details). In light of this latter observation, and in conjunction with the fact that another study completed shortly after Phase I of this study16 indicated that 12 weeks post-VML injury yields a more complete functional recovery in this VML injury model (see Results section), we conducted a second series of experiments that were focused on KN-treated groups only at a 12-week time point.
Although significant differences in isometric torque among KN treatment groups, relative to NR and BAM, were observed throughout the 12-week course of the study, only KN alone and KN+I+b were significantly different from both NR and BAM at the 12-week time point. This provides clear evidence that the implantation of KN hydrogels is associated with functional recovery from VML injury in the rat TA model. Moreover, as shown in Figures 5 and 6, the implantation of KN hydrogels resulted in both volume reconstitution and myogenesis. Consistent with these data, the TA wet weight at retrieval in both the KN alone and KN+I+b was significantly greater than that for all other treatment groups (Table 3). Interestingly, as shown in the companion paper to this work,61 the implantation of KN+I+b in a murine LD VML injury model had a similar functional outcome among the same treatment groups (again, indistinguishable from KN alone), at the end time point of that study (8 weeks in the mouse LD VML injury model versus 12 weeks in the rat TA VML injury model). These observations clearly highlight the efficacy of implantable keratin biomaterials as a potentially important platform for the treatment of VML injury.
To put these observations into perspective, it should be noted that in these studies, the synergists (EDL and EHL) are removed at the time of creation of the surgical VML injury, resulting in a permanent ≈20% functional deficit (see Corona et al.16 for details). This means that after removing approximately 20–30% of the TA muscle, the total functional defect is ≈50%, of which only 30% is recoverable. Thus, the maximal functional recovery possible is 0.8 of the preinjury baseline maximal isometric torque response. Both NR and BAM treatment groups exhibited mean maximal torque responses of ≤0.6, whereas the keratin hydrogel treatments (KN alone, KN+b, and KN+I+b) exhibited mean values of ≥0.7, ranging as high as 0.74. This is a very robust and reproducible average functional recovery of ≈0.7/0.8 or 90% on a substantial number of animals.
Qualitative and quantitative morphological and histological analyses also provide important insights regarding the potential mechanisms that are responsible for the observed functional recovery. For example, as illustrated by the representative examples provided in Figure 5, the TA muscles treated with KN hydrogels (in particular, Figure 5I–L (KN treatment group) and Figure 5U–X (KN+I+b treatment group)) showed an abundance of newly formed myofibers and blood vessels, along with diminished fibrosis (i.e., collagen formation) relative to the BAM-treated group. In addition, keratin hydrogel implantation was associated with significantly increased new tissue formation, including an abundance of new myofibers, presenting as smaller diameter fibers with centrally located nuclei identified in regions of surgical excision (i.e., the wound bed and empty space between the two sides of the TA muscle belly; see Fig. 7). These are established hallmarks for new muscle tissue formation,79–81 and they are consistent with an ongoing repair/regeneration/remodeling process that was particularly striking in the KN and KN+I+b treatment groups, but present, to some extent, in all KN-treated groups (Figs. 5–7). Again, these observations stand in stark contrast to observations on the untreated group (NR) as well as on the BAM-treated group (again, Figs. 5–7), which showed little or no evidence of myogenesis. As highlighted in Figure 7, these observations are consistent with the supposition that KN-based treatment promotes a microenvironment that is more favorable for muscle regeneration, resulting in an apparent active “zone of regeneration,” extending from the border of the remaining tissue to remote regions well within the VML excision area. Although a complete restoration of tissue volume, structure, and function was not achieved in this work, even with the most effective KN hydrogel formulations (i.e., KN alone and KN+I+b), these results still represent an important step forward.
The contributions of controlled time-released growth factors to muscle healing have been extensively discussed elsewhere (reviewed in Cezar and Mooney,70 Lee et al.,82 and Mao and Mooney83). Interestingly, keratin alone resulted in significant functional improvement in mean isometric torque at 12 weeks relative to both the BAM and NR groups (Fig. 4). As such, it appears that the main impact of growth factor addition in the present study is to increase the rate, but not the eventual magnitude, of the observed functional recovery. More specifically, as shown in Figures 3 and 4, only KN-treated groups with growth factors included displayed mean isometric torque responses that were significantly greater than NR before 12 weeks (i.e., at 8 weeks). Clearly, a further investigation of the benefits of inclusion of growth factors is warranted. Nevertheless, recovery from a VML injury requires replacement of lost myofibers, as well as restoration of the vascular and neurological network that will support muscle growth. In this scenario, growth factor-based tissue-engineered approaches have been shown to enhance the degree of functional recovery by playing a supporting role in all the stages of the muscle regeneration process. Recent advances in the field have been extensively described in Passipieri and Christ.18
Since keratin-only implantation promoted morphological and functional improvement even in the absence of growth factor, it begs the question of potential mechanisms of action. Possibly, this observation means that during muscle regeneration, implanted keratin biomaterial devices provide mechanical stability that modulates cell proliferation, cell adhesion, and gene expression in a similar manner as seen in nerve repair.53,78,84,85 Modulation of the immune response, as noted earlier for more favorable macrophage polarization (i.e., more M2 than M1), and the inherent binding affinities of the hydrogel may also be considered factors.74,75 However, determination of the precise mechanism(s) responsible for the improved functional outcomes observed after implantation of only a keratin hydrogel also remains the subject of future investigation.
The use of keratin hydrogel for growth factor delivery shows significant potential, whereas the inclusion of a cellular component, that is MPCs, did not further enhance functional muscle regeneration under the conditions reported here (Fig. 3). This stands in contrast to our prior observations with very distinct biological scaffolds, including our work with BAM, a decellularized sheet-like scaffold that was also subjected to biological preconditioning before implantation.9,12,13,16,86,87 Cell delivery through the use of hydrogels has been reported in a variety of injury models in vivo.88–98 Investigators have shown that the delivery of 1.5 × 107 myoblasts (a number of cells ∼37 × higher than what was used in this study) and 100 μg of bFGF via a gelatin hydrogel contributed to new muscle fiber formation in skeletal muscle injury in a rat gastrocnemius VML injury model. However, functional outcome was not evaluated.88
Of note, our studies did use donor male cells that were implanted into female recipients. The rationale for this approach was to minimize the requirements for donor animals (given the larger muscle sizes in males), and to ensure a more direct comparison with the functional recovery reported in our prior studies.9,12,16 In this regard, there are reports of the potential importance of gender differences in muscle stem cells.99,100 Although we cannot unequivocally rule out a potential role for gender differences in muscle-derived stem cells in our current observations, our previously published observations have reported robust functional regeneration in the same animal models of VML injury by using the same approach. As such, the reason for a lack of functional improvement after the inclusion of a cellular component with the keratin implants, as reported herein, is likely related to a combination of factors, including the much lower cell density achieved in the keratin hydrogel relative to BAM (26- to 50-fold less; Fig. 3), as well as the fact that the implants in these studies were not subjected to cyclic mechanical stretch before implantation. The lower cell density will likely alter key aspects of cell-to-cell interaction(s), whereas the absence of cyclic mechanical stretch clearly affects cell phenotypes.9,12,16 The absence of mechanical cues coupled with a reduced cell density may diminish the therapeutic effects that are typically attributed to cell-based therapies.9 In short, the current data suggest that a further optimization of keratin hydrogel properties is required before the impact of introducing cells on muscle repair and regeneration in the setting of VML injury can be rigorously evaluated.
In summary, we have shown for the first time that keratin hydrogels, which have been successfully used for bone and nerve regeneration,56,57,78,85 can also promote a significant functional recovery of severely damaged skeletal muscle in an established and a biologically relevant rodent model of TA VML injury. Finally, these studies provide further credence for the results shown in the companion paper to this work,61 in that implantation of KN+I+b in a rat TA VML injury model had exactly the same functional outcome (again, indistinguishable from KN alone), among the same treatment groups, as it does after implantation in a mouse model of VML injury. Nonetheless, keratin hydrogel implantation resulted in a significant functional recovery at the 12-week time point in the absence of both cells and growth factors. Although further studies are clearly required to better understand the mechanisms that are responsible for the active “zone of regeneration” after keratin implantation, taken together, these observations have important implications for improved therapeutics for VML injuries and VML-like conditions, as the KN hydrogel is a capable, cell-compatible, drug delivery and tunable biomaterials platform that could extend the range of clinical applications.
Acknowledgments
The authors would like to thank Mr. Daniel Lovell for technical assistance with histological procedures. This work was supported by the US Army Medical Research and Materiel Command under Contract No. W81XWH-15-C-0084. J.A.P. acknowledges support as a CNPq Postdoctoral Fellow (2013–2014).
Disclosure Statement
L.B. is an officer and a shareholder in KeraNetics, LLC, which contributed support for this project. All other authors have no competing financial interests.
References
- 1.Jarvinen T.A., Jarvinen T.L., Kaariainen M., Kalimo H., and Jarvinen M. Muscle injuries: biology and treatment. Am J Sports Med 33, 745, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Holcomb J.B., Stansbury L.G., Champion H.R., Wade C., and Bellamy R.F. Understanding combat casualty care statistics. J Trauma 60, 397, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Grogan B.F., and Hsu J.R. Volumetric muscle loss. J Am Acad Orthop Surg 19 Suppl 1, S35, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Lin C.H., Lin Y.T., Yeh J.T., and Chen C.T. Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast Reconstr Surg 119, 2118, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Merritt E.K., Hammers D.W., Tierney M., Suggs L.J., Walters T.J., and Farrar R.P. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A 16, 1395, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Perniconi B., Costa A., Aulino P., Teodori L., Adamo S., and Coletti D. The pro-myogenic environment provided by whole organ scale acellular scaffolds from skeletal muscle. Biomaterials 32, 7870, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Sicari B.M., Agrawal V., Siu B.F., Medberry C.J., Dearth C.L., Turner N.J., and Badylak S.F. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Part A 18, 1941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner N.J., Badylak J.S., Weber D.J., and Badylak S.F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J Surg Res 176, 490, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Corona B.T., Machingal M.A., Criswell T., Vadhavkar M., Dannahower A.C., Bergman C., Zhao W., and Christ G.J. Further development of a tissue engineered muscle repair construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss injury. Tissue Eng Part A 18, 1213, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corona B.T., Wu X., Ward C.L., McDaniel J.S., Rathbone C.R., and Walters T.J. The promotion of a functional fibrosis in skeletal muscle with volumetric muscle loss injury following the transplantation of muscle-ECM. Biomaterials 34, 3324, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Criswell T.L., Corona B.T., Wang Z., Zhou Y., Niu G., Xu Y., Christ G.J., and Soker S. The role of endothelial cells in myofiber differentiation and the vascularization and innervation of bioengineered muscle tissue in vivo. Biomaterials 34, 140, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Machingal M.A., Corona B.T., Walters T.J., Kesireddy V., Koval C.N., Dannahower A., Zhao W., Yoo J.J., and Christ G.J. A tissue-engineered muscle repair construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17, 2291, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merritt E.K., Cannon M.V., Hammers D.W., Le L.N., Gokhale R., Sarathy A., Song T.J., Tierney M.T., Suggs L.J., Walters T.J., and Farrar R.P. Repair of traumatic skeletal muscle injury with bone-marrow-derived mesenchymal stem cells seeded on extracellular matrix. Tissue Eng Part A 16, 2871, 2010 [DOI] [PubMed] [Google Scholar]
- 14.VanDusen K.W., Syverud B.C., Williams M.L., Lee J.D., and Larkin L.M. Engineered skeletal muscle units for repair of volumetric muscle loss in the tibialis anterior muscle of a rat. Tissue Eng Part A 20, 2920, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams M.L., Kostrominova T.Y., Arruda E.M., and Larkin L.M. Effect of implantation on engineered skeletal muscle constructs. J Tissue Eng Regen Med 7, 434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corona B.T., Ward C.L., Baker H.B., Walters T.J., and Christ G.J. Implantation of in vitro tissue engineered muscle repair constructs and bladder acellular matrices partially restore in vivo skeletal muscle function in a rat model of volumetric muscle loss injury. Tissue Eng Part A 20, 705, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sicari B.M., Rubin J.P., Dearth C.L., Wolf M.T., Ambrosio F., Boninger M., Turner N.J., Weber D.J., Simpson T.W., Wyse A., Brown E.H., Dziki J.L., Fisher L.E., Brown S., and Badylak S.F. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 6, 234ra58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passipieri J.A., and Christ G.J. The potential of combination therapeutics for more complete repair of volumetric muscle loss injuries: the role of exogenous growth factors and/or progenitor cells in implantable skeletal muscle tissue engineering technologies. Cells Tissues Organs 202, 202, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Grasman J.M., Do D.M., Page R.L., and Pins G.D. Rapid release of growth factors regenerates force output in volumetric muscle loss injuries. Biomaterials 72, 49, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammers D.W., Sarathy A., Pham C.B., Drinnan C.T., Farrar R.P., and Suggs L.J. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng 109, 1051, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho T.C., Chiang Y.P., Chuang C.K., Chen S.L., Hsieh J.W., Lan Y.W., and Tsao Y.P. PEDF-derived peptide promotes skeletal muscle regeneration through its mitogenic effect on muscle progenitor cells. Am J Physiol Cell Physiol 309, C159, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybalko V.Y., Pham C.B., Hsieh P.L., Hammers D.W., Merscham-Banda M., Suggs L.J., and Farrar R.P. Controlled delivery of SDF-1alpha and IGF-1: CXCR4(+) cell recruitment and functional skeletal muscle recovery. Biomater Sci 3, 1475, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomblyn S., Pettit Kneller E.L., Walker S.J., Ellenburg M.D., Kowalczewski C.J., Van Dyke M., Burnett L., and Saul J.M. Keratin hydrogel carrier system for simultaneous delivery of exogenous growth factors and muscle progenitor cells. J Biomed Mater Res B Appl Biomater 104, 864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M.H., Hong H.N., Hong J.P., Park C.J., Kwon S.W., Kim S.H., Kang G., and Kim M. The effect of VEGF on the myogenic differentiation of adipose tissue derived stem cells within thermosensitive hydrogel matrices. Biomaterials 31, 1213, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Crewther W.G., Fraser R.D.B., Lennox F.G., and Lindley H. The chemistry of keratins. In: Anfinsen C.B., Edsall J.T., and Richards F.M., eds. Advances in Protein Chemistry. New York: Academic Press, 1965, pp. 191. [DOI] [PubMed] [Google Scholar]
- 26.Ham T.R., Lee R.T., Han S., Haque S., Vodovotz Y., Gu J., Burnett L.R., Tomblyn S., and Saul J.M. Tunable keratin hydrogels for controlled erosion and growth factor delivery. Biomacromolecules 17, 225, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins C.A., and Partridge T.A. Self-renewal of the adult skeletal muscle satellite cell. Cell Cycle 4, 1338, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Hill M., Wernig A., and Goldspink G. Muscle satellite (stem) cell activation during local tissue injury and repair. J Anat 203, 89, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke M.S., Khakee R., and McNeil P.L. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci 106 (Pt 1), 121, 1993 [DOI] [PubMed] [Google Scholar]
- 30.DiMario J., Buffinger N., Yamada S., and Strohman R.C. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science 244, 688, 1989 [DOI] [PubMed] [Google Scholar]
- 31.Lafreniere J.F., Mills P., Tremblay J.P., and El Fahime E. Growth factors improve the in vivo migration of human skeletal myoblasts by modulating their endogenous proteolytic activity. Transplantation 77, 1741, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Efthimiadou A., Asimakopoulos B., Nikolettos N., Giatromanolaki A., Sivridis E., Lialiaris T.S., Papachristou D.N., and Kontoleon E. The angiogenetic effect of intramuscular administration of b-FGF and a-FGF on cardiac muscle: the influence of exercise on muscle angiogenesis. J Sports Sci 24, 849, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Han D., Zhao H., Parada C., Hacia J.G., Bringas P., Jr., and Chai Y. A TGFbeta-Smad4-Fgf6 signaling cascade controls myogenic differentiation and myoblast fusion during tongue development. Development 139, 1640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.House S.L., Castro A.M., Lupu T.S., Weinheimer C., Smith C., Kovacs A., and Ornitz D.M. Endothelial fibroblast growth factor receptor signaling is required for vascular remodeling following cardiac ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 310, H559, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nummenmaa E., Hamalainen M., Moilanen T., Vuolteenaho K., and Moilanen E. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand J Rheumatol 44, 321, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Oladipupo S.S., Smith C., Santeford A., Park C., Sene A., Wiley L.A., Osei-Owusu P., Hsu J., Zapata N., Liu F., Nakamura R., Lavine K.J., Blumer K.J., Choi K., Apte R.S., and Ornitz D.M. Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis. Proc Natl Acad Sci U S A 111, 13379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson E.M., Hsieh M.M., and Rotwein P. Autocrine growth factor signaling by insulin-like growth factor-II mediates MyoD-stimulated myocyte maturation. J Biol Chem 278, 41109, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Retamales A., Zuloaga R., Valenzuela C.A., Gallardo-Escarate C., Molina A., and Valdes J.A. Insulin-like growth factor-1 suppresses the Myostatin signaling pathway during myogenic differentiation. Biochem Biophys Res Commun 464, 596, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Gardner S., Gross S.M., David L.L., Klimek J.E., and Rotwein P. Separating myoblast differentiation from muscle cell fusion using IGF-I and the p38 MAP kinase inhibitor SB202190. Am J Physiol Cell Physiol 309, C491, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senesi P., Luzi L., Montesano A., Mazzocchi N., and Terruzzi I. Betaine supplement enhances skeletal muscle differentiation in murine myoblasts via IGF-1 signaling activation. J Transl Med 11, 174, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelosi L., Giacinti C., Nardis C., Borsellino G., Rizzuto E., Nicoletti C., Wannenes F., Battistini L., Rosenthal N., Molinaro M., and Musaro A. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J 21, 1393, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Caroni P., and Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol 110, 1307, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bikle D.D., Tahimic C., Chang W., Wang Y., Philippou A., and Barton E.R. Role of IGF-I signaling in muscle bone interactions. Bone 80, 79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philippou A., and Barton E.R. Optimizing IGF-I for skeletal muscle therapeutics. Growth Horm IGF Res 24, 157, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacco A., Doyonnas R., LaBarge M.A., Hammer M.M., Kraft P., and Blau H.M. IGF-I increases bone marrow contribution to adult skeletal muscle and enhances the fusion of myelomonocytic precursors. J Cell Biol 171, 483, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fearing B.V., and Van Dyke M.E. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater 10, 3136, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Brown B.N., Ratner B.D., Goodman S.B., Amar S., and Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Guzman R.C., Merrill M.R., Richter J.R., Hamzi R.I., Greengauz-Roberts O.K., and Van Dyke M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 32, 8205, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Kirfel J., Magin T.M., and Reichelt J. Keratins: a structural scaffold with emerging functions. Cell Mol Life Sci 60, 56, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tachibana A., Kaneko S., Tanabe T., and Yamauchi K. Rapid fabrication of keratin-hydroxyapatite hybrid sponges toward osteoblast cultivation and differentiation. Biomaterials 26, 297, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Yamauchi K., Maniwa M., and Mori T. Cultivation of fibroblast cells on keratin-coated substrata. J Biomater Sci Polym Ed 9, 259, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Reichl S. Films based on human hair keratin as substrates for cell culture and tissue engineering. Biomaterials 30, 6854, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Hill P.S., Apel P.J., Barnwell J., Smith T., Koman L.A., Atala A., and Van Dyke M. Repair of peripheral nerve defects in rabbits using keratin hydrogel scaffolds. Tissue Eng Part A 17, 1499, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Dias G.J., Peplow P.V., McLaughlin A., Teixeira F., and Kelly R.J. Biocompatibility and osseointegration of reconstituted keratin in an ovine model. J Biomed Mater Res Part A 92, 513, 2010 [DOI] [PubMed] [Google Scholar]
- 55.Shen D., Wang X., Zhang L., Zhao X., Li J., Cheng K., and Zhang J. The amelioration of cardiac dysfunction after myocardial infarction by the injection of keratin biomaterials derived from human hair. Biomaterials 32, 9290, 2011 [DOI] [PubMed] [Google Scholar]
- 56.Pace L.A., Plate J.F., Smith T.L., and Van Dyke M.E. The effect of human hair keratin hydrogel on early cellular response to sciatic nerve injury in a rat model. Biomaterials 34, 5907, 2013 [DOI] [PubMed] [Google Scholar]
- 57.de Guzman R.C., Saul J.M., Ellenburg M.D., Merrill M.R., Coan H.B., Smith T.L., and Van Dyke M.E. Bone regeneration with BMP-2 delivered from keratose scaffolds. Biomaterials 34, 1644, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Pechter P.M., Gil J., Valdes J., Tomic-Canic M., Pastar I., Stojadinovic O., Kirsner R.S., and Davis S.C. Keratin dressings speed epithelialization of deep partial-thickness wounds. Wound Repair Regen 20, 236, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Poranki D., Whitener W., Howse S., Mesen T., Howse E., Burnell J., Greengauz-Roberts O., Molnar J., and Van Dyke M. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J Biomater Appl 29, 26, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Wu X., Corona B.T., Chen X., and Walters T.J. A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies. Biores Open Access 1, 280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker H.B., Passipieri J.A., Siriwardane M., Ellenburg M.D., Vadhavkar M., Bergman C.R., Saul J.M., Tomblyn S., Burnett L., and Christ G.J. Cell and growth factor loaded keratin hydrogels for treatment of volumetric muscle loss (VML) in a mouse model. Tissue Eng 2017. DOI: 10.1089/ten.tea.2016.0457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., and Morgan J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Lepper C., Partridge T.A., and Fan C.M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138, 3639, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner N.J., Yates A.J., Jr., Weber D.J., Qureshi I.R., Stolz D.B., Gilbert T.W., and Badylak S.F. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A 16, 3309, 2010 [DOI] [PubMed] [Google Scholar]
- 65.Valentin J.E., Turner N.J., Gilbert T.W., and Badylak S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 31, 7475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F. Biologic scaffold composed of skeletal muscle extracellular matrix. Biomaterials 33, 2916, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Christ G.J., Siriwardane M.L., and de Coppi P. Engineering muscle tissue for the fetus: getting ready for a strong life. Front Pharmacol 6, 53, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christ G.J., Passipieri J.A., Treasure T.E., Freeman P.N., Wong M.E., Martin N.R.W., Player D., and Lewis M.P. Soft tissue reconstruction: skeletal muscle engineering. In: Vishwakarma A., Songtao Shi P.S., and Ramalingam M., eds. Stem Cell Biology and Tissue Engineering in Dental Sciences. Cambridge, MA: Academic Press, 2015, pp. 567 [Google Scholar]
- 69.Cittadella Vigodarzere G., and Mantero S. Skeletal muscle tissue engineering: strategies for volumetric constructs. Front Physiol 5, 362, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cezar C.A., and Mooney D.J. Biomaterial-based delivery for skeletal muscle repair. Adv Drug Deliv Rev 84, 188, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saul J.M., Ellenburg M.D., de Guzman R.C., and Van Dyke M. Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J Biomed Mater Res Part A 98, 544, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Musaro A., Giacinti C., Borsellino G., Dobrowolny G., Pelosi L., Cairns L., Ottolenghi S., Cossu G., Bernardi G., Battistini L., Molinaro M., and Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A 101, 1206, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yablonka-Reuveni Z., Seger R., and Rivera A.J. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47, 23, 1999 [DOI] [PubMed] [Google Scholar]
- 74.Han S., Ham T.R., Haque S., Sparks J.L., and Saul J.M. Alkylation of human hair keratin for tunable hydrogel erosion and drug delivery in tissue engineering applications. Acta Biomater 23, 201, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Guzman R.C., Tsuda S.M., Ton M.T., Zhang X., Esker A.R., and Van Dyke M.E. Binding interactions of keratin-based hair fiber extract to gold, keratin, and BMP-2. PloS One 10, e0137233, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kowalczewski C.J., Tombyln S., Wasnick D.C., Hughes M.R., Ellenburg M.D., Callahan M.F., Smith T.L., Van Dyke M.E., Burnett L.R., and Saul J.M. Reduction of ectopic bone growth in critically-sized rat mandible defects by delivery of rhBMP-2 from kerateine biomaterials. Biomaterials 35, 3220, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andreopoulos F.M., and Persaud I. Delivery of basic fibroblast growth factor (bFGF) from photoresponsive hydrogel scaffolds. Biomaterials 27, 2468, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Sierpinski P., Garrett J., Ma J., Apel P., Klorig D., Smith T., Koman L.A., Atala A., and Van Dyke M. The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29, 118, 2008 [DOI] [PubMed] [Google Scholar]
- 79.Aurora A., Garg K., Corona B.T., and Walters T.J. Physical rehabilitation improves muscle function following volumetric muscle loss injury. BMC Sports Sci Med Rehabil 6, 41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corona B.T., Garg K., Ward C.L., McDaniel J.S., Walters T.J., and Rathbone C.R. Autologous minced muscle grafts: a tissue engineering therapy for the volumetric loss of skeletal muscle. Am J Physiol Cell Physiol 305, C761, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Hawke T.J., and Garry D.J. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91, 534, 2001 [DOI] [PubMed] [Google Scholar]
- 82.Lee K., Silva E.A., and Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8, 153, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mao A.S., and Mooney D.J. Regenerative medicine: current therapies and future directions. Proc Natl Acad Sci U S A 112, 14452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Apel P.J., Garrett J.P., Sierpinski P., Ma J., Atala A., Smith T.L., Koman L.A., and Van Dyke M.E. Peripheral nerve regeneration using a keratin-based scaffold: long-term functional and histological outcomes in a mouse model. J Hand Surg 33, 1541, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Pace L.A., Plate J.F., Mannava S., Barnwell J.C., Koman L.A., Li Z., Smith T.L., and Van Dyke M. A human hair keratin hydrogel scaffold enhances median nerve regeneration in nonhuman primates: an electrophysiological and histological study. Tissue Eng Part A 20, 507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ayele T., Zuki A.B., Noorjahan B.M., and Noordin M.M. Tissue engineering approach to repair abdominal wall defects using cell-seeded bovine tunica vaginalis in a rabbit model. J Mater Sci Mater Med 21, 1721, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Moon du G., Christ G., Stitzel J.D., Atala A., and Yoo J.J. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A 14, 473, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Hagiwara K., Chen G., Kawazoe N., Tabata Y., and Komuro H. Promotion of muscle regeneration by myoblast transplantation combined with the controlled and sustained release of bFGFcpr. J Tissue Eng Regen Med 10, 325, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Dosier C.R., Uhrig B.A., Willett N.J., Krishnan L., Li M.T., Stevens H.Y., Schwartz Z., Boyan B.D., and Guldberg R.E. Effect of cell origin and timing of delivery for stem cell-based bone tissue engineering using biologically functionalized hydrogels. Tissue Eng Part A 21, 156, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen C.H., Chang M.Y., Wang S.S., and Hsieh P.C. Injection of autologous bone marrow cells in hyaluronan hydrogel improves cardiac performance after infarction in pigs. Am J Physiol Heart Circ Physiol 306, H1078, 2014 [DOI] [PubMed] [Google Scholar]
- 91.Rossi C.A., Flaibani M., Blaauw B., Pozzobon M., Figallo E., Reggiani C., Vitiello L., Elvassore N., and De Coppi P. In vivo tissue engineering of functional skeletal muscle by freshly isolated satellite cells embedded in a photopolymerizable hydrogel. FASEB J 25, 2296, 2011 [DOI] [PubMed] [Google Scholar]
- 92.Seebach E., Freischmidt H., Holschbach J., Fellenberg J., and Richter W. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater 10, 4730, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Lee S.H., Kim I.G., Jung A.R., Shrestha K.R., Lee J.H., Park K.D., Chung B.H., Kim S.W., Kim K.H., and Lee J.Y. Combined effects of brain-derived neurotrophic factor immobilized poly-lactic-co-glycolic acid membrane with human adipose-derived stem cells and basic fibroblast growth factor hydrogel on recovery of erectile dysfunction. Tissue Eng Part A 20, 2446, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soler-Botija C., Bago J.R., Llucia-Valldeperas A., Valles-Lluch A., Castells-Sala C., Martinez-Ramos C., Fernandez-Muinos T., Chachques J.C., Pradas M.M., Semino C.E., and Bayes-Genis A. Engineered 3D bioimplants using elastomeric scaffold, self-assembling peptide hydrogel, and adipose tissue-derived progenitor cells for cardiac regeneration. Am J Transl Res 6, 291, 2014 [PMC free article] [PubMed] [Google Scholar]
- 95.Huang J., Wang S., Wei C., Xu Y., Wang Y., Jin J., and Teng G. In vivo differentiation of adipose-derived stem cells in an injectable poloxamer-octapeptide hybrid hydrogel. Tissue Cell 43, 344, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Mulyasasmita W., Cai L., Dewi R.E., Jha A., Ullmann S.D., Luong R.H., Huang N.F., and Heilshorn S.C. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Controlled Release 191, 71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathieu E., Lamirault G., Toquet C., Lhommet P., Rederstorff E., Sourice S., Biteau K., Hulin P., Forest V., Weiss P., Guicheux J., and Lemarchand P. Intramyocardial delivery of mesenchymal stem cell-seeded hydrogel preserves cardiac function and attenuates ventricular remodeling after myocardial infarction. PloS One 7, e51991, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fuoco C., Sangalli E., Vono R., Testa S., Sacchetti B., Latronico M.V., Bernardini S., Madeddu P., Cesareni G., Seliktar D., Rizzi R., Bearzi C., Cannata S.M., Spinetti G., and Gargioli C. 3D hydrogel environment rejuvenates aged pericytes for skeletal muscle tissue engineering. Front Physiol 5, 203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deasy B.M., Schugar R.C., and Huard J. Sex differences in muscle-derived stem cells and skeletal muscle. Crit Rev Eukaryot Gene Expr 18, 173, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Deasy B.M., Lu A., Tebbets J.C., Feduska J.M., Schugar R.C., Pollett J.B., Sun B., Urish K.L., Gharaibeh B.M., Cao B., Rubin R.T., and Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol 177, 73, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]