Abstract
Background: Levothyroxine suppression of thyrotropin (TSH) is broadly applied to patients with thyroid cancer despite lack of consensus on the optimal TSH concentration necessary to reduce cancer recurrence while minimizing toxicity from subclinical hyperthyroidism. The objectives of this study were to examine the beneficial effects and the cardiac and skeletal toxicity of TSH suppression in well-differentiated thyroid carcinoma (DTC).
Methods: A total of 771 patients (569 women) at ATA low or intermediate risk of recurrence, with a mean age of 48±14 years, and undergoing total thyroidectomy at a tertiary care center between 2000 and 2006 were followed for a median of six and a half years. They were divided into a suppressed TSH group (median TSH ≤0.4 mIU/L) and a nonsuppressed group (median TSH >0.4 mIU/L). Structural recurrence of thyroid cancer, postoperative atrial fibrillation (AF), and osteoporosis were examined in the two groups. Osteoporosis was only examined in women.
Results: A total of 43/771 (5.6%) patients recurred, 29/739 (3.9%) patients were diagnosed with postoperative osteoporosis, and 17/756 (2.3 %) were diagnosed with postoperative AF. Despite similar rates of recurrence (HR 1.02, p=0.956 [CI 0.54–1.91]), patients treated to a median TSH ≤0.4 mIU/L were at increased postoperative risk of a composite outcome of AF and osteoporosis (HR 2.1, p=0.05 [CI 1.001–4.3]) compared to those not suppressed. A differential risk of AF alone (HR 0.78, p=0.63 [CI 0.3–2.1]) was not detected, but postoperative osteoporosis was increased among women with a suppressed TSH compared to those not suppressed (HR 3.5, p=0.023 [CI 1.2–10.2]). The increased risk of postoperative osteoporosis disappeared when the patient's median TSH was maintained around 1 mIU/L.
Conclusion: TSH suppression significantly increases the risk of postoperative osteoporosis without changing tumor recurrence in ATA low- and intermediate-risk patients with DTC. Future interventions should focus on avoiding harm in indolent disease.
Introduction
Total thyroidectomy with or without 131I ablation followed by long-term levothyroxine suppression of thyrotropin (TSH) is the traditional treatment for well-differentiated thyroid carcinoma (DTC) (1–4). Currently, most patients with thyroid cancer are given a dose of levothyroxine that suppresses TSH levels below the normal range, inducing a state of subclinical hyperthyroidism. The rationale for this approach stems from experimental and clinical data showing that TSH stimulates thyroid cell proliferation, radioiodine uptake, and thyroglobulin (Tg) production (5–8). Removing this stimulus, at least theoretically, will inhibit growth of residual neoplastic tissue (5,6,9).
In patients affected by DTC, TSH suppression with levothyroxine is associated with a decreased risk of tumor recurrence (5,10–14), and endogenous or exogenous increases in TSH may occasionally induce clinical progression of thyroid cancer (15,16). Doses of levothyroxine that reduce circulating TSH to 0.4 mIU/L reportedly induce maximum suppression of serum Tg (17), suggesting that increasing the degree of TSH suppression beyond this threshold may not further decrease tumor function (18). Others have found that serum Tg continues to decrease in thyroid cancer patients when TSH is further suppressed to undetectable levels (<0.1 mIU/L) (19).
Despite clinical practice guidelines addressing the need for TSH suppression in patients with DTC (1–4), there is currently no evidence-based consensus on the optimal TSH concentration that would reduce tumor recurrence while ensuring minimal adverse effects from subclinical hyperthyroidism. Also, no recommendations currently take into account the patient's age, underlying comorbidities, tumor stage, or response to therapy to balance the benefits of levothyroxine suppressive treatment with the cardiovascular and skeletal risks of iatrogenic thyrotoxicosis. So, prolonged TSH suppression in relatively low-risk patients could easily lead to more harm than good.
The aims of this study are thus twofold; first, to examine the impact of TSH suppression on recurrence in a well-characterized cohort of patients with DTC at low and intermediate risk of recurrence as defined by the American Thyroid Association (ATA) (20); and second, to examine the harmful effects of TSH suppression as measured by the diagnosis of postoperative osteoporosis and atrial fibrillation (AF) in the same cohort.
Materials and Methods
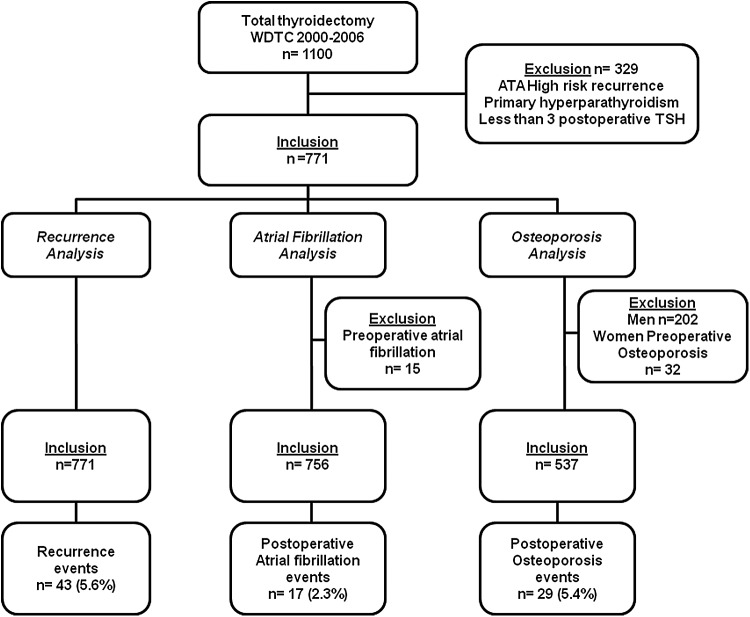
Following Institutional Review Board approval, the charts of 1100 consecutive patients who had undergone total thyroidectomy at our institution for DTC between January 1, 2000, and December 31, 2006, were reviewed. Patients were excluded if they were at high risk of tumor recurrence as defined by the ATA (macroscopic tumor invasion, gross residual disease, distant metastases) (20), as it is believed that there is evidence to support the beneficial effect of TSH suppression in a significant subset of these patients (21,22). Patients with a preexisting diagnosis of hyperparathyroidism were also excluded, as this is an independent risk factor for the development of osteoporosis; men, since they are not routinely screened for osteoporosis; and patients who had fewer than three postoperative TSH laboratory measurements, to ensure adequate follow-up to evaluate this variable. No patients had a diagnosis of permanent hypoparathyroidism. A total of 771 patients were considered for analysis. Patients with known preoperative AF and osteoporosis were excluded from the respective event-specific analyses (Fig. 1).
FIG. 1.
Inclusion criteria.
The cohort was divided into TSH-suppressed and TSH-nonsuppressed groups based on a median TSH level of 0.4 mIU/L. Postoperative TSH values were analyzed up to the date of the event or the last follow-up. TSH values within seven days of radioactive iodine (RAI) scan or therapy were excluded from the analysis, as our institution commonly administers recombinant human TSH, and blood determinations of TSH are often confounded by exogenously administered TSH. The TSH-suppressed group had a mean±standard deviation (SD) of 12±6 TSH determinations, and the TSH-nonsuppressed group had a mean±SD of 9.7±6 TSH determinations. Patient demographic and clinicopathological characteristics were collected as outlined in Table 1. Preoperative risk categories for osteoporosis and AF were adapted from Biondi and Cooper (23) and are described in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy). Low-, intermediate-, and high-risk categories were given values of one, two, and three, respectively, and all calculations of postoperative AF and osteoporosis events were adjusted by this preoperative risk. Locoregional recurrence required tissue confirmation; distant recurrence was diagnosed by appropriate imaging criteria with or without tissue confirmation. Patients with biochemical recurrences defined by elevated Tg levels without a structural correlate on imaging were purposefully excluded, as it is believed that structural recurrences are a more robust endpoint to measure this outcome. Postoperative AF was defined by EKG evidence of persistent arrhythmia or new documentation of disease in the notes of a physician; transient episodes of AF attributed to acute illness or operative procedures were excluded. Postoperative osteoporosis was defined by a bone mineral density (BMD) T-score <−2.5 standard deviations below that of a young white adult at the anteroposterior lumbar spine, femoral neck, or total hip. New osteoporosis was also considered if the patient had been started on bisphosphonate therapy in the absence of a known indication such as metastases or Paget's disease, or if specified in the notes of the treating physician. Postoperative AF and osteoporosis were adjudicated as events whether they had been diagnosed within or outside the institution, and whether they had been detected by the treating endocrinologist or by the general practitioner. Two reviewers scrutinized the medical records of patients to adjudicate the events of recurrence, AF, and osteoporosis, and two additional reviewers reexamined these medical records in instances of disagreement.
Table 1.
Comparison of Suppressed and Nonsuppressed Groups
| Characteristics | Suppressed TSH ≤0.4 mIU/L (n=465) | Nonsuppressed TSH >0.4 mIU/L (n=306) | p-Value |
|---|---|---|---|
| Age, years (mean±SD) | 46.6±13.9 | 49.8±14.9 | <0.01 |
| Sex, females, n (%) | 353 (76%) | 215 (70%) | 0.08 |
| Histology | 0.245 | ||
| Classical type | 153 (33%) | 83 (27%) | |
| Follicular variant | 135 (29%) | 79 (26%) | |
| Tall cell variant | 73 (16%) | 43 (14%) | |
| Other | 51 (11%) | 45 (15%) | |
| Microcarcinomas | 53 (11%) | 56 (18%) | <0.01 |
| Extrathyroidal extension | 165 (36%) | 89 (29%) | 0.16 |
| Vascular invasion | 11 (2.4%) | 15 (4.9%) | 0.30 |
| N stage | 0.05 | ||
| N0 | 179 (38%) | 124 (41%) | |
| N1a | 102 (22%) | 53 (17%) | |
| N1b | 78 (17%) | 38 (12%) | |
| Nx | 106 (23%) | 91 (30%) | |
| RAI therapy | 348 (75%) | 184 (60%) | <0.01 |
| ATA risk | <0.01 | ||
| Low | 187 (40%) | 154 (50%) | |
| Intermediate | 278 (60%) | 152 (50%) |
TSH, thyrotropin; SD, standard deviation; RAI, radioactive iodine; ATA, American Thyroid Association.
Statistical analysis was carried out using Stata Statistical Software v12 (StataCorp., College Station, TX). Student's t-test was used to compare continuous variables in the TSH treatment arms and Pearson's chi-square test to examine categorical variables. Recurrence and harm were analyzed using survival analysis. Kaplan–Meier curves were built and the log-rank test was used to assess for significance of the surviving function. Cox proportional hazards models were built to allow for multivariate adjustment by variables that proved to be statistically different in the TSH treatment arms. Additionally, to account for indication biases of levothyroxine administration and balance differences in prescription practices in this retrospective study, propensity score analysis was used. A p-value of <0.05 was considered statistically significant.
Results
The clinicopathological characteristics of patients with median TSH levels ≤0.4 mIU/L and >0.4 mIU/L are outlined in Table 1. No significant differences were found in terms of sex, histological subtype of thyroid cancer, vascular invasion, extrathyroidal extension, or nodal stage between the suppressed and nonsuppressed groups. However, clinicians were more likely to suppress younger patients (p<0.01), patients at higher risk of tumor recurrence evidenced by a tumor size >1 cm (p<0.01), and ATA intermediate risk group (p<0.01; Table 1). Suppressed patients were also more likely to have received 131I therapy (p<0.01).
Tumor recurrence
A total of 43/771 (5.6%) patients developed a structural tumor recurrence during a median follow-up of 6.5 years (Fig. 2). Fifteen patients out of 306 (4.9%) treated to a TSH level >0.4 mIU/L developed tumor recurrence compared with 28/465 (6.0%) in the suppressed group. There was no statistically significant difference in the disease-free survival (DFS) rate of TSH-suppressed compared to TSH-nonsuppressed patients (HR 1.02 [CI 0.54–1.91], p=0.956). Given the retrospective and nonrandomized nature of this study, it was found that physicians tended to suppress younger patients and patients at higher risk of recurrence (Table 1). To account for these differences in therapeutic practices, multivariate analyses were conducted as well as adjustment by propensity scores. TSH suppression did not significantly decrease the risk of recurrence in low- and intermediate-risk patients when adjusting for age, sex, RAI administration, and ATA risk category (HR 0.88 [CI 0.46–1.66], p=0.692; Table 2). Male sex and ATA “intermediate risk” were independent predictors of tumor recurrence (p=0.038 and p=0.001, respectively). Interestingly, when RAI was incorporated into the multivariate model, it did not independently predict for increased risk of recurrence (p=0.437). In addition to conducting multivariate analyses, propensity scores analyses were performed to account for indication biases at the time of prescribing levothyroxine suppressive therapy. TSH suppression ≤0.4 mIU/L was not associated with a decreased risk of recurrence when stratified on propensity score (HR 1.08 [CI 0.45–2.63], p=0.856).
FIG. 2.
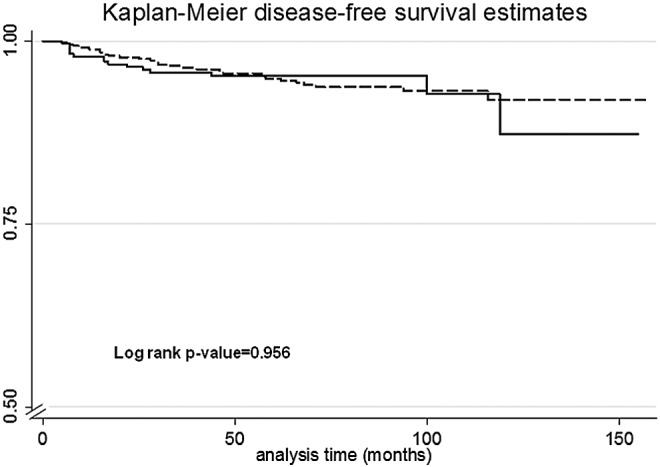
Recurrence-free survival in patients treated to a median thyrotropin (TSH) of ≤0.4 mIU/L (dashed) or >0.4 mIU/L (solid).
Table 2.
Multivariate Analysis for Tumor Recurrence
| Multivariate analysis | HR | CI | p-Value |
|---|---|---|---|
| TSH suppression | 0.88 | [0.46–1.66] | 0.692 |
| Age | 0.99 | [0.97–1.02] | 0.862 |
| Sex | 0.53 | [0.29–0.96] | 0.038 |
| RAI therapy | 1.5 | [0.55–3.94] | 0.437 |
| ATA risk | 6.5 | [2.2–19.3] | 0.001 |
HR, hazard ratio; CI, confidence interval.
Composite event of skeletal and cardiovascular toxicity
Despite similar rates of recurrence between the suppressed and nonsuppressed groups, subjects treated to a median TSH ≤0.4 mIU/L developed an adverse effect to levothyroxine suppression, defined as the first event of AF or osteoporosis, at 2.1 times the rate of their nonsuppressed counterparts (HR 2.1 [CI 1.001–4.3], p=0.05; Fig. 3A).
FIG. 3.
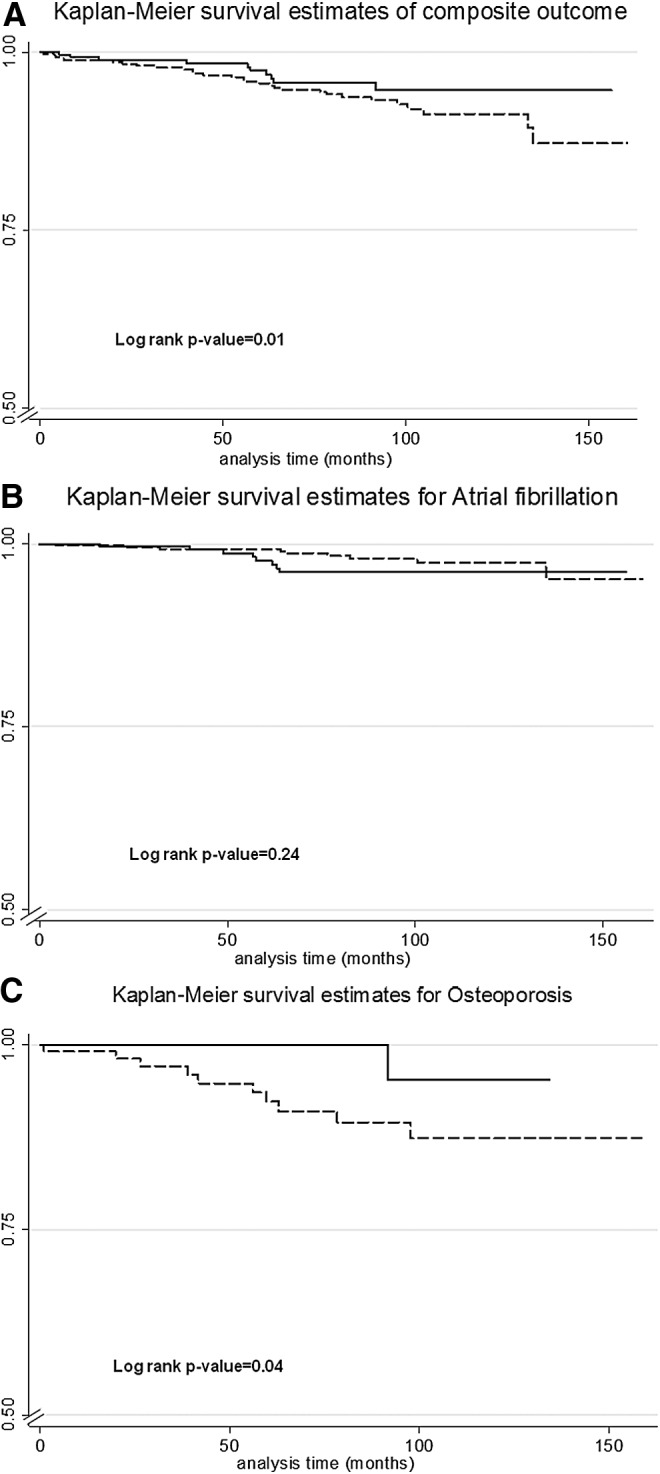
(A) Composite of harm (first event of atrial fibrillation or osteoporosis), (B) atrial fibrillation, and (C) osteoporosis in patients treated to a median TSH of ≤0.4 mIU/L (dashed) or >0.4 mIU/L (solid).
AF risk
Fifteen patients had a preoperative diagnosis of AF in this study and were thus excluded from the postoperative AF analysis. Of the remaining 756 patients, 17 (2.3%) developed AF during the course of their follow-up (Fig. 3B). Given the small number of events, no differential risk of postoperative AF was detected among patients suppressed versus those not suppressed (HR 0.78 [CI 0.3–2.1], p=0.63). No difference in the development of AF was detected, even after adjusting for preoperative risk of AF (Supplementary Table S1).
Osteoporosis risk
The osteoporosis analysis was limited to female patients without a preoperative diagnosis of osteoporosis. Men were excluded from the analysis, as they are not routinely screened for osteoporosis. All calculations were adjusted by preoperative risk of developing osteoporosis (Supplementary Table S1). Among 537 women, 29 (5.4%) were diagnosed with postoperative osteoporosis. The risk of postoperative osteoporosis among women was 3.5 times greater (HR 3.5 [CI 1.2–10.2], p=0.023) when they were suppressed (TSH ≤0.4 mIU/L) compared to those who were not suppressed (Fig. 3C). Given that age is a known risk factor for the development of osteoporosis, multivariate analyses were conducted adjusting for this variable. Women had a 4.3 times higher risk of developing osteoporosis when they were suppressed compared to the nonsuppressed group when age was taken into account (HR 4.3 [CI 1.45–12.85], p=0.009). The higher HR of 4.3 in the multivariate model demonstrates a synergistic effect between increasing age and TSH suppression, suggesting that TSH suppression in elderly women may cause even greater bone toxicity.
Optimal TSH level
The ideal TSH level for patients with low and intermediate DTC would be one that does not increase the risk of adverse cardiovascular and skeletal events while maintaining beneficial effects on tumor recurrence. Figure 4 demonstrates the risk of osteoporosis and tumor recurrence at incremental TSH levels between 0.4 and 1.0 mIU/L. Each bar represents a comparison of patients below a certain TSH median value and the rest of the cohort. As median TSH levels approach 1.0 mI/L, the hazard ratio of post-operative osteoporosis becomes nonsignificant while the risk of recurrence remains unchanged. Interestingly, the data suggest that at the lower limit of normal TSH (between 0.5 and 0.7 mIU/L), there is also increased risk of osteoporosis. It would appear that a TSH level around 0.9 or 1 mIU/L is optimal for maintenance treatment of ATA low- to intermediate-risk patients, as the risk of osteoporosis disappears yet the risk of recurrence remains unchanged.
FIG. 4.
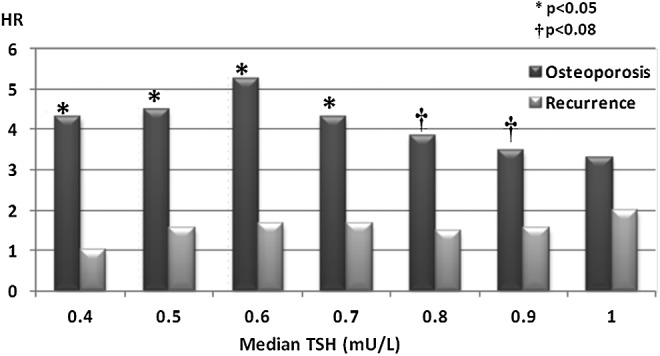
Risk of osteoporosis and tumor recurrence as a function of median TSH level.
Discussion
In this study, the beneficial effects of TSH suppression on thyroid cancer recurrence and the cardiovascular and skeletal toxicity that results from long-term iatrogenic thyrotoxicosis in patients at ATA low and intermediate risk of recurrence were examined. It was found that TSH suppression increased the postoperative likelihood of being diagnosed with osteoporosis, and it did not improve recurrence rates in this population. This finding was not altered after a multivariate analysis or a propensity score analysis, suggesting that TSH suppression is an independent predictor of skeletal toxicity that may not improve recurrence rates of patients with low- and intermediate-risk DTC.
The lack of a beneficial effect of TSH suppression on recurrence of low- and intermediate-risk patients with DTC in this study is supported by other groups. Cooper et al. found that TSH suppression to very low levels reduced recurrence in Stage 3 and 4 patients, but not in low-risk patients. When RAI was included in the model, the effect of TSH suppression in high-risk patients disappeared (24). Similarly, Jonklaas et al. were not able to show a beneficial impact of TSH suppression in Stage 1 patients (25). A comprehensive review of the literature by Biondi and Cooper concluded that aggressive TSH suppression is likely to be important in high-risk patients and less critical in low-risk patients (23). The only prospective randomized controlled trial published to date in all risk thyroid cancer patients showed that DFS, especially in low-risk patients without TSH suppression, was not inferior to that of patients with TSH suppression (26). The findings in this study support this conclusion and suggest that TSH suppression may not improve DFS in low- and intermediate-risk patients.
The cardiovascular morbidity and mortality associated with subclinical hyperthyroidism has been confirmed in multiple studies (27,28), and there is increasing awareness of this toxicity, as more severe effects have been documented with advanced age (29,30). Due to the small number of events in this study, there was not enough power to detect the effect of TSH suppression on the risk of postoperative AF. Also, the hazard ratio of <1 in the AF results suggests that clinicians were averse to suppressing patients with preexisting cardiac conditions.
In terms of the skeletal events, a significantly increased risk of osteoporosis was detected in the women who had a TSH suppressed ≤0.4 mIU/L compared to those not suppressed. Menopausal status was not specifically collected, but older women were at a higher risk of osteoporosis than younger women were. Two recent reviews on the effects of TSH suppression in DTC concluded that postmenopausal women were at increased risk of bone loss when TSH was suppressed, but the effect of hyperthyroidism on premenopausal women and men was conflicting (31,32). Furthermore, TSH levels <0.1 mIU/L and even 0.1–0.5 mIU/L were associated with increased risk of hip and vertebral fractures (27,33). In 2011, Sugitani et al. published the results of a randomized controlled trial of TSH suppression and its impact on bone mineral density. TSH suppression to <0.1 mIU/L conferred a significant reduction in BMD in older patients within one year of suppression comparable to the reduction in BMD seen after five years in nonsuppressed patients (34). The reduction in BMD in the nonsuppressed group was attributed to the natural bone density decline in postmenopausal women. It was found that the effect of TSH and age was synergistic in terms of bone loss; the combined effect of TSH suppression and older age was more toxic to the bone than each of these were individually.
A biological explanation for the observed skeletal toxicity may be related to the well-documented effects of triiodothyronine on osteoblasts to stimulate osteoclasts and in turn bone resorption (35), or to the reported direct effects of TSH on bone. TSH has been shown to bypass the thyroid to exert direct protective effects on the skeleton. Through a fast-forward short loop involving Wnt5a production, TSH may enhance osteoblast differentiation and stimulates osteoprotegerin to attenuate bone resorption by osteoclasts (36,37). Lowering TSH levels in DTC may result in bone loss from a direct effect of thyroid hormones or from failure to maintain this TSH protective effect.
Total thyroidectomy with or without remnant ablation with 131I followed by long-term levothyroxine suppression of TSH is the traditional treatment for DTC (38). TSH suppression is generally recommended in well-meaning efforts to prevent or decrease the likelihood of tumor recurrence (5,10–14), and increases in TSH are thought to lead to clinical progression of thyroid cancer (15,16). Thus, TSH suppression has been associated with increased survival or delayed progression to recurrence, especially in high-risk patients (21,22). This viewpoint is reflected in current ATA and ETA guidelines where TSH suppression <0.1 mIU/L is recommended for high-risk thyroid cancer patients, and suppression between 0.1 and 1–2 mIU/L is suggested for low-risk patients (1,2). In addition to this, there is clear indication that driver mutations in thyroid cancer have distinct effects on thyroid-differentiated properties, including TSH responsiveness. For instance, RAS mutant tumors, such as follicular variant papillary thyroid cancers, retain expression of the TSH receptor and may remain dependent on TSH signaling (39). Other cancers lose expression of the TSH receptor (40,41), particularly those with BRAF mutations, which represent approximately 50% of the low- and intermediate-risk ATA categories. Hence, some tumors progress independent of the effects of TSH (42), others are cured by the initial intervention (43), and yet TSH suppression as a goal of therapy is applied indiscriminately to most patients with the disease, in many cases as a life-long treatment.
While the evidence for the recommendations of the major thyroid societies is clearer for patients at high risk of recurrence, the optimal TSH maintenance level for patients at low and intermediate risk of recurrence or patients with tumors that may not respond to TSH suppression remains elusive. These results suggest that a TSH cutoff of 0.9–1.0 mIU/L is optimal for low- and intermediate-risk patients to balance the risk of osteoporosis development whilst not increasing the risk of tumor recurrence. Furthermore, no further benefit from TSH suppression may be obtained after formal documentation of absent residual or recurrent disease (44). A large, prospective, randomized, controlled study is required, however, to clarify definitively the optimal TSH level that would minimize the adverse effects of iatrogenic hyperthyroidism and maximize the beneficial effects of TSH suppression on recurrence in this growing population of ATA low- and intermediate-risk patients.
Due to its retrospective nature, this study has several limitations. The TSH treatment groups were not randomized and therefore suffered from inherent indication biases; ATA intermediate-risk patients were treated more aggressively than low-risk patients in that they were more likely to be suppressed and also more likely to have received RAI therapy. This may have prevented a beneficial effect of TSH suppression on recurrence from being detected. An attempt was made, however, to account for this limitation by performing multivariate analyses as well as propensity score analyses, but even after these adjustments, a statically significant effect of TSH suppression on tumor recurrence in ATA low- or intermediate-risk patients could not be detected. Not every patient had a pre and postoperative bone density test. Hence, it is possible that treating clinicians may have been more likely to investigate and thus diagnose osteoporosis and AF in patients on TSH suppression. However, the majority of these adverse events were found by nonendocrinologists within or outside the institution so the real impact of the above limitation is likely to be minimal. Information was not collected on estrogen replacement therapy, calcium and vitamin D supplementation, but the majority of patients were prescribed calcium and vitamin D after thyroidectomy. Clinicians may have been less likely to suppress patients at greater risk of AF or osteoporosis. This would have resulted in an underestimation of harm. Even so, more than a fourfold increase in the rate of age-adjusted osteoporosis was detected in patients with a median TSH ≤0.4 mIU/L compared to those with a nonsuppressed TSH. Initiation of bisphosphonate therapy was considered for indications other than bone metastasis or Paget's disease as one of the criteria to adjudicate osteoporosis. It is possible that some physicians may have begun bisphosphonate therapy for osteopenia or for prevention of osteoporosis. Given that information was not collected on menopausal status, a T-score of <−2.5 was used as a diagnosis of osteoporosis, although a Z-score is often used in premenopausal women. In addition, a low T-score may not always reflect osteoporosis, and may sometimes reflect osteomalacia or other conditions associated with a low BMD. Finally, the osteoporosis analysis was only performed in women, as men are generally not screened for osteoporosis at our institution. It is therefore not possible to comment directly on the skeletal effects of TSH suppression in men.
Conclusion
TSH suppression ≤0.4 mIU/L increases the risk of osteoporosis without changing tumor recurrence in thyroid cancer patients at ATA low and intermediate risk of recurrence. The findings of this study suggest that further research is required to delineate the role of TSH suppression in low- and intermediate-risk patients with thyroid carcinoma to avoid causing more harm than good. In particular, strategies currently employed to prevent recurrence need to be redefined to avoid long-term cardiovascular and skeletal toxicity in this population. The observations in this study extend to a median follow-up of 6.5 years where most recurrences would have been found. Longer TSH suppression may result in an even worse risk–benefit ratio. Definitive prospective, randomized, controlled studies that take into account individual patients' risks of osteoporosis and AF would ultimately be required to confirm these results. In the meantime, counseling on calcium and vitamin D supplementation, exercise, and screening of vitamin D levels may be considered where applicable. The paradigm outlined in this study could be extended to examine other types of thyroid cancer where the biology of the disease may drive the progression of the tumor independent of the effects of TSH suppression.
Supplementary Material
Author Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. 2009. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–1214 [DOI] [PubMed] [Google Scholar]
- 2.Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W, European Thyroid Cancer Taskforce 2006. European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. Eur J Endocrinol 154:787–803 [DOI] [PubMed] [Google Scholar]
- 3.Sundram F, Robinson BG, Kung A, Lim-Abrahan MA, Bay NQ, Chuan LK, Chung JH, Huang SM, Hsu LC, Kamaruddin N, Cheah WK, Kim WB, Koong SS, Lin HD, Mangklabruks A, Paz-Pacheco E, Rauff A, Ladenson PW. 2006. Well-differentiated epithelial thyroid cancer management in the Asia Pacific region: a report and clinical practice guideline. Thyroid 16:461–469 [DOI] [PubMed] [Google Scholar]
- 4.Pitoia F, Ward L, Wohllk N, Friguglietti C, Tomimori E, Gauna A, Camargo R, Vaisman M, Harach R, Munizaga F, Corigliano S, Pretell E, Niepomniszcze H. 2009. Recommendations of the Latin American Thyroid Society on diagnosis and management of differentiated thyroid cancer. Arq Bras Endocrinol Metabol 53:884–887 [DOI] [PubMed] [Google Scholar]
- 5.Balme HW. 1954. Metastatic carcinoma of the thyroid successfully treated with thyroxine. Lancet 266:812–813 [DOI] [PubMed] [Google Scholar]
- 6.Carayon P, Thomas-Morvan C, Castanas E, Tubiana M. 1980. Human thyroid cancer: membrane thyrotropin binding and adenylate cyclase activity. J Clin Endocrinol Metab 51:915–920 [DOI] [PubMed] [Google Scholar]
- 7.Spencer CA, LoPresti JS, Fatemi S, Nicoloff JT. 1999. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid 9:435–441 [DOI] [PubMed] [Google Scholar]
- 8.Heemstra KA, Liu YY, Stokkel M, Kievit J, Corssmit E, Pereira AM, Romijn JA, Smit JW. 2007. Serum thyroglobulin concentrations predict disease-free remission and death in differentiated thyroid carcinoma. Clin Endocrinol 66:58–64 [DOI] [PubMed] [Google Scholar]
- 9.Brabant G. 2008. Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? J Clin Endocrinol Metab 93:1167–1169 [DOI] [PubMed] [Google Scholar]
- 10.Crile G., Jr 1966. Endocrine dependency of papillary carcinomas of the thyroid. JAMA 195:721–724 [PubMed] [Google Scholar]
- 11.Mazzaferri EL, Jhiang SM. 1994. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med 97:418–428 [DOI] [PubMed] [Google Scholar]
- 12.Wang PW, Wang ST, Liu RT, Chien WY, Tung SC, Lu YC, Chen HY, Lee CH. 1999. Levothyroxine suppression of thyroglobulin in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 84:4549–4553 [DOI] [PubMed] [Google Scholar]
- 13.Cady B, Cohn K, Rossi RL, Sedgwick CE, Meissner WA, Werber J, Gelman RS. 1983. The effect of thyroid hormone administration upon survival in patients with differentiated thyroid carcinoma. Surgery 94:978–983 [PubMed] [Google Scholar]
- 14.Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J, Jaffiol C. 1996. Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab 81:4318–4323 [DOI] [PubMed] [Google Scholar]
- 15.Goldberg LD, Ditchek NT. 1981. Thyroid carcinoma with spinal cord compression. JAMA 245:953–954 [PubMed] [Google Scholar]
- 16.Sfakianakis GN, Skillman TG, George JM. 1975. Thyroxine withdrawal in thyroid cancer. Ohio State Med J 71:79–82 [PubMed] [Google Scholar]
- 17.Burmeister LA, Goumaz MO, Mariash CN, Oppenheimer JH. 1992. Levothyroxine dose requirements for thyrotropin suppression in the treatment of differentiated thyroid cancer. J Clin Endocrinol Metab 75:344–350 [DOI] [PubMed] [Google Scholar]
- 18.Kamel N, Gullu S, Dagci Ilgin S, Corapcioglu D, Tonyukuk Cesur V, Uysal AR, Baskal N, Erdogan G. 1999. Degree of thyrotropin suppression in differentiated thyroid cancer without recurrence or metastases. Thyroid 9:1245–1248 [DOI] [PubMed] [Google Scholar]
- 19.Spencer CA, Lai-Rosenfeld AO, Guttler RB, LoPresti J, Marcus AO, Nimalasuriya A, Eigen A, Doss RC, Green BJ, Nicoloff JT. 1986. Thyrotropin secretion in thyrotoxic and thyroxine-treated patients: assessment by a sensitive immunoenzymometric assay. J Clin Endocrinol Metab 63:349–355 [DOI] [PubMed] [Google Scholar]
- 20.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. 2010. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA, Smit JW. 2007. Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab 92:2610–2615 [DOI] [PubMed] [Google Scholar]
- 22.Diessl S, Holzberger B, Mader U, Grelle I, Smit JW, Buck AK, Reiners C, Verburg FA. 2012. Impact of moderate vs stringent TSH suppression on survival in advanced differentiated thyroid carcinoma. Clin Endocrinol 76:586–592 [DOI] [PubMed] [Google Scholar]
- 23.Biondi B, Cooper DS. 2010. Benefits of thyrotropin suppression versus the risks of adverse effects in differentiated thyroid cancer. Thyroid 20:135–146 [DOI] [PubMed] [Google Scholar]
- 24.Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, Ain KB, Bigos ST, Brierley JD, Haugen BR, Klein I, Robbins J, Sherman SI, Taylor T, Maxon HR., 3rd 1998. Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 8:737–744 [DOI] [PubMed] [Google Scholar]
- 25.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI. 2006. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 26.Sugitani I, Fujimoto Y. 2010. Does postoperative thyrotropin suppression therapy truly decrease recurrence in papillary thyroid carcinoma? A randomized controlled trial. J Clin Endocrinol Metab 95:4576–4583 [DOI] [PubMed] [Google Scholar]
- 27.Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, Leese GP. 2010. Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 95:186–193 [DOI] [PubMed] [Google Scholar]
- 28.Klein Hesselink EN, Klein Hesselink MS, de Bock GH, Gansevoort RT, Bakker SJ, Vredeveld EJ, van der Horst-Schrivers AN, van der Horst IC, Kamphuisen PW, Plukker JT, Links TP, Lefrandt JD. 2013. Long-term cardiovascular mortality in patients with differentiated thyroid carcinoma: an observational study. J Clin Oncol 31:4046–4053 [DOI] [PubMed] [Google Scholar]
- 29.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 30.Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quan ML, Pasieka JL, Rorstad O. 2002. Bone mineral density in well-differentiated thyroid cancer patients treated with suppressive thyroxine: a systematic overview of the literature. J Surg Oncol 79:62–69; discussion 69–70 [DOI] [PubMed] [Google Scholar]
- 32.Heemstra KA, Hamdy NA, Romijn JA, Smit JW. 2006. The effects of thyrotropin-suppressive therapy on bone metabolism in patients with well-differentiated thyroid carcinoma. Thyroid 16:583–591 [DOI] [PubMed] [Google Scholar]
- 33.Bauer DC, Ettinger B, Nevitt MC, Stone KL, Study of Osteoporotic Fractures Research Group 2001. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 134:561–568 [DOI] [PubMed] [Google Scholar]
- 34.Sugitani I, Fujimoto Y. 2011. Effect of postoperative thyrotropin suppressive therapy on bone mineral density in patients with papillary thyroid carcinoma: a prospective controlled study. Surgery 150:1250–1257 [DOI] [PubMed] [Google Scholar]
- 35.Williams GR. 2013. Thyroid hormone actions in cartilage and bone. Eur Thyroid J 2:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baliram R, Latif R, Berkowitz J, Frid S, Colaianni G, Sun L, Zaidi M, Davies TF. 2011. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc Natl Acad Sci U S A 108:16277–16282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baliram R, Sun L, Cao J, Li J, Latif R, Huber AK, Yuen T, Blair HC, Zaidi M, Davies TF. 2012. Hyperthyroid-associated osteoporosis is exacerbated by the loss of TSH signaling. J Clin Invest 122:3737–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzaferri E, Young R, Oertel J, Kemmerer W, Page C. 1977. Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore) 56:171–196 [PubMed] [Google Scholar]
- 39.Cancer Genome Atlas Research Network 2014. Integrated genomic characterization of papillary thyroid carcinoma. Cell 159:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berlingieri MT, Akamizu T, Fusco A, Grieco M, Colletta G, Cirafici AM, Ikuyama S, Kohn LD, Vecchio G. 1990. Thyrotropin receptor gene expression in oncogene-transfected rat thyroid cells: correlation between transformation, loss of thyrotropin-dependent growth, and loss of thyrotropin receptor gene expression. Biochem Biophys Res Comm 173:172–178 [DOI] [PubMed] [Google Scholar]
- 41.Brabant G, Maenhaut C, Kohrle J, Scheumann G, Dralle H, Hoang-Vu C, Hesch RD, von zur Muhlen A, Vassart G, Dumont JE. 1991. Human thyrotropin receptor gene: expression in thyroid tumors and correlation to markers of thyroid differentiation and dedifferentiation. Mol Cell Endocrinol 82:R7–12 [DOI] [PubMed] [Google Scholar]
- 42.Franco AT, Malaguarnera R, Refetoff S, Liao XH, Lundsmith E, Kimura S, Pritchard C, Marais R, Davies TF, Weinstein LS, Chen M, Rosen N, Ghossein R, Knauf JA, Fagin JA. 2011. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci U S A 108:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahimpasic T, Nixon IJ, Palmer FL, Whitcher MM, Tuttle RM, Shaha A, Patel SG, Shah JP, Ganly I. 2012. Undetectable thyroglobulin after total thyroidectomy in patients with low- and intermediate-risk papillary thyroid cancer—is there a need for radioactive iodine therapy? Surgery 152:1096–1105 [DOI] [PubMed] [Google Scholar]
- 44.Durante C, Montesano T, Torlontano M, Attard M, Monzani F, Tumino S, Costante G, Meringolo D, Bruno R, Trulli F, Massa M, Maniglia A, D'Apollo R, Giacomelli L, Ronga G, Filetti S, Group PTCS 2013. Papillary thyroid cancer: time course of recurrences during postsurgery surveillance. J Clin Endocrinol Metab 98:636–642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.