Abstract
Background: Iodine deficiency has long been recognized as an important public health problem. Global approaches such as salt iodization that aim to overcome iodine deficiency have been successful. Meanwhile, they have led to excessive iodine consumption in some populations, thereby increasing the risks of iodine-induced thyroid dysfunction, as well as the comorbidities and mortality associated with hypothyroidism and hyperthyroidism. This study aimed to elucidate whether iodine intake is associated with mortality among U.S. adults.
Methods: This was an observational study to estimate mortality risks according to urinary iodine concentration (UIC) utilizing a nationally representative sample of 12,264 adults aged 20–80 years enrolled in the National Health and Nutrition Examination Survey (NHANES) III. Crude and multivariable Cox proportional hazards regression models were employed to investigate the association between UIC (<50, 50–99, 100–299, 300–399, and ≥400 μg/L) and mortalities (all-cause, cardiovascular, and cancer). In sensitivity analyses, the study adjusted for total sodium intake and fat/calorie ratio in addition to other potential confounders. Stratum-specific analyses were also conducted to estimate the effects of UIC on mortality according to age, sex, race/ethnicity, and estimated glomerular filtration rate category.
Results: Over a median follow-up of 19.2 years, there were 3159 deaths from all causes. Participants with excess iodine exposure (UIC ≥400 μg/L) were at higher risk for all-cause mortality compared to those with adequate iodine nutrition (hazard ratio = 1.19 [confidence interval 1.04–1.37]). Elevated hazard ratios of cardiovascular and cancer mortality were also found, but the confidence interval of the effect estimates included the null value for both outcomes. Low UIC was not associated with increased mortality. Restricted cubic spline models showed similar results for all outcomes. The results did not change substantially after adjusting for total sodium intake and fat/calorie ratio. None of the potential interactions were statistically significant on a multiplicative scale.
Conclusion: Higher all-cause mortality among those with excess iodine intake compared to individuals with adequate iodine intake highlights the importance of monitoring population iodine status. Further studies with longitudinal measures of iodine status are needed to validate these results and to assess the potential risks excess iodine intake may have on long-term health outcomes.
Keywords: : urinary iodine, iodine deficiency, iodine excess, hypothyroidism, mortality, NHANES
Introduction
Iodine is an essential micronutrient necessary for the synthesis of thyroid hormones that are important for various metabolic processes (1,2). Iodine deficiency has been associated with the development of goiter, hypothyroidism, hyperthyroidism, and thyroid autoimmunity (1,3). Iodine deficiency disorders (IDDs) have been recognized as important public health problem, and IDD elimination programs, mainly salt iodization, have been adopted by most countries (3,4). A daily intake of 150 μg of iodine has been recommended for adults who are not pregnant or lactating by the Institute of Medicine, the World Health Organization (WHO), the United Nations Children's Fund (UNICEF), and the Iodine Global Network (5,6). Iodized salt is fortified at around 45 mg of iodide/kg in the United States (2). Currently, approximately 70% of households worldwide and almost 90% of households in North and South America use iodized salt, with other common sources such as seaweed, seafood, and iodine-containing supplement (5), and the concerted global approach to eliminate IDDs has been successful at substantially decreasing the number of countries with iodine deficiency (7,8).
In contrast, the number of countries in which individuals consume more than adequate or excessive amounts of iodine has increased (9). Through failure to adapt to the acute Wolff–Chaikoff effect and the Jod–Basedow phenomenon (9), iodine excess can induce hypo- and hyperthyroidism, respectively, and thus represents an emerging public health challenge following the decrease of IDDs (9). Given the increase in risk of cardiovascular disease, cancer, and overall mortality in patients with thyroid dysfunction (10–12), excessive iodine intake might also contribute to mortality from these chronic diseases as mediated through thyroid dysfunction. However, longitudinal data regarding iodine status and mortality are scarce. This study investigated whether iodine status is associated with all-cause and/or cause-specific mortalities among adults in the general U.S. population.
Methods
Data sources and study population
The U.S. National Health and Nutrition Examination Survey (NHANES) III (1988–1994) is a stratified, multistage probability sample of individuals in the general population selected at random through a complex statistical process. Subjects completed structured interviews and a physical examination, and some provided urine and/or blood samples (13). The NHANES III study protocols were approved by the National Center for Health Statistics (NCHS) Institutional Review Board (14), with all participants providing informed written consent. Among adults enrolled in the NHANES III, the unweighted response rates for the household interview and the physical examination were 86% and 78%, respectively (15). Participants have been prospectively followed for mortality through December 2011.
There were 15,081 NHANES III participants aged ≥20 years and ≤80 years at enrollment for whom urinary iodine concentration (UIC) values were available. Participants lacking data regarding education (n = 94), body mass index (BMI; n = 26), or missing death records (n = 11) were excluded. The study cohort was further restricted by excluding subjects who were pregnant or lactating (n = 265), reported use of iodine-rich medications or those which alter thyroid function (amiodarone, n = 20; thyroid hormone replacement and/or antithyroidal drugs, n = 290), had abnormal serum thyrotropin concentrations (reference range 0.39–4.60 mIU/L; n = 1964) (16). Subjects with severe renal dysfunction (estimated glomerular filtration rate [eGFR] mL/min/1.73 m2) <30 (n = 147) were also excluded. The final analytical cohort contained 12,264 participants (6064 males).
UIC and other measurements
In the NHANES III, spot urine specimens were collected from participants, and frozen (≤–20°C) aliquots of these specimens were stored until shipped. Frozen urine samples were sent in batches in Styrofoam-insulated shipping containers with dry ice to the laboratory. Specimen stability was demonstrated at 5°C and ≤–20°C (17). UIC was determined by the Iodine Research Laboratory, University of Massachusetts Medical Center (Worcester, MA), using the Sandell–Koltoff reaction as modified by Benotti et al. (18,19). In reference to WHO guidelines for UIC as a marker of population iodine status (3), NHANES III participants were categorized into the following five groups according to UIC (μg/L): very low UIC, 0–49; low UIC, 50–99; normal UIC, 100–299; high UIC, 300–399; and very high UIC, ≥400 μg/L. Measured weights and heights were used to calculate BMI. Serum creatinine (Scr) measurements were performed according to the laboratory procedure manual for NHANES III (20,21), from which an eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (GFR = 141 × min [Scr/κ, 1]α × max [Scr/κ, 1]−1.209 × 0.993Age × 1.018 [if women] × 1.159 [if black]; κ = 0.7 for women and 0.9 for men, α = −0.329 for women and −0.411 for men, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1) (22). Dietary sodium (mg/day), calorie (kcal/day), and fat (kcal/day) intakes were obtained from 24-hour dietary recall collected by trained interviewers using a personal computer-based interactive platform and coded by the University of Minnesota's Nutrition Coordinating Center (20,21).
Outcome ascertainment
The primary outcome of the present study was all-cause mortality, and secondary outcomes were two cause-specific mortalities: cardiovascular and cancer deaths. Mortality data were ascertained by the NCHS from death certificate information provided by the National Death Index (NDI) (23) after record matching by social security number, name, date of birth, race/ethnicity, sex, state of birth, and state of residence. The cause of death was determined according to the International Classification of Diseases, 10th version (ICD–10). Cardiovascular disease was classified using ICD–10 codes I00–09, I11, I13, I20–51, and I60–69. Cancer was classified using ICD–10 codes C00–C97.
Statistical analyses
Patient characteristics and generated descriptive statistics were explored using chi-square tests for categorical variables and analysis of variance for continuous variables. Crude and multivariable Cox proportional hazards regression models were employed, adjusting for potential confounders. First, the study adjusted for age (per 10 years), sex (men or women), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican American or others), education status (less than high school, high school, or general education degree, or more than high school), and active smoking (self-reported; Model 1). Further, the study adjusted for diabetes (self-reported), hypertension (self-reported), hypercholesterolemia (self-reported), previous cardiovascular disease (self-reported), previous cancer (self-reported), BMI (<18.5, 18.5 to <25.0, 25.0 to <30.0, or ≥30.0 kg/m2), and eGFR (30 to <60, or ≥60 mL/min/1.73 m2) in addition to covariates in Model 1 (Model 2). In sensitivity analyses, the effects of UIC on all-cause, cardiovascular-specific, and cancer-specific mortalities were estimated after further adjustment for total sodium intake (categorized in quintiles) and fat/calorie ratio (categorized in quintiles), as iodine intake can be affected by dietary habits. To assess effect measure modification, stratum-specific analyses were conducted to estimate the effects of UIC on mortality according to age (20–39, 40–59, and ≥60 years), sex, race/ethnicity, and eGFR category by insertion of a multiplicative term into each model. Those with extremely high values of iodine were also excluded to assess the impact of these potentially mismeasured outliers on the results (99th percentile; UIC >1100 μg/L; n = 120). Finally, continuous associations were investigated between UIC and mortality by fitting restricted cubic spline models with four knots at 5th, 35th, 65th, and 95th percentile of UIC (24).
All statistical analyses were conducted using Stata v12.1 (StataCorp LP). Appropriate sample weights were selected to account for unequal probabilities of selecting NHANES participants, as well as nonresponse of those eligible and approached. The spline curve was constructed using the “mkspline” command in Stata.
Results
The mean age ± standard deviation (SD) of participants was 45.0 ± 17.0 years, and 49.5% were male (Table 1). Urinary iodine levels in participants were generally lower in females, white non-Hispanics, and those with hypercholesterolemia, but higher among those with less than a high-school education, who were obese, and those with higher sodium intake.
Table 1.
Baseline Characteristicsa of NHANES III Participants in the Study Sample, According to UICb (N = 12,264)
| Total | Very low UIC | Low UIC | Normal UIC | High UIC | Very high UIC | |
|---|---|---|---|---|---|---|
| Total, n (%) | 12,264 | 1398 (11.4) | 2597 (21.2) | 6500 (53.0) | 804 (6.6) | 965 (7.9) |
| UIC, μg/L | 0–49 | 50–99 | 100–299 | 300–399 | 400– | |
| Age, years | 45.0 ± 17.0 | 46.8 ± 15.3 | 46.1 ± 16.8 | 44.1 ± 17.1 | 45.0 ± 17.5 | 45.4 ± 18.0 |
| Sex (men), n (%) | 6064 (49.5) | 462 (33.1) | 1173 (45.2) | 3394 (52.2) | 460 (57.2) | 575 (60.0) |
| Race/ethnicity, n (%) | ||||||
| Non-Hispanic white | 4761 (38.8) | 699 (50.0) | 1053 (40.6) | 2385 (36.7) | 287 (35.7) | 337 (34.9) |
| Non-Hispanic black | 3490 (28.5) | 352 (25.2) | 847 (32.6) | 1835 (28.2) | 204 (25.4) | 252 (26.1) |
| Mexican American | 3498 (28.5) | 283 (20.2) | 574 (22.1) | 2023 (31.1) | 283 (35.2) | 335 (34.7) |
| Others | 515 (4.2) | 64 (4.6) | 123 (4.7) | 257 (4.0) | 30 (3.7) | 41 (4.3) |
| Education status, n (%) | ||||||
| Less than high school | 24688 (21.9) | 244 (17.5) | 496 (19.1) | 1442 (22.2) | 223 (27.7) | 283 (29.3) |
| High school or GED | 2012 (16.4) | 215 (15.4) | 418 (16.1) | 1057 (16.3) | 150 (19.0) | 172 (17.8) |
| Higher than high school | 7564 (61.7) | 939 (67.2) | 1683 (64.8) | 4001 (61.6) | 431 (53.6) | 510 (52.9) |
| Active smoking, n (%) | 3426 (27.9) | 410 (29.3) | 720 (27.7) | 1793 (27.6) | 206 (25.6) | 297 (30.8) |
| Diabetes, n (%) | 900 (7.3) | 80 (5.7) | 180 (6.9) | 506 (7.8) | 61 (7.6) | 73 (7.6) |
| Hypertension, n (%) | 3113 (25.4) | 358 (25.6) | 695 (26.8) | 1607 (24.7) | 205 (25.5) | 248 (25.7) |
| Hypercholesterolemia, n (%) | 2108 (17.2) | 277 (19.8) | 480 (18.5) | 1088 (16.7) | 115 (14.3) | 148 (15.3) |
| Previous cardiovascular disease, n (%) | 759 (6.2) | 74 (5.3) | 149 (5.7) | 439 (6.8) | 44 (5.5) | 53 (5.5) |
| Previous cancer, n (%) | 697 (5.7) | 91 (6.5) | 148 (5.7) | 351 (5.4) | 41 (5.1) | 66 (6.8) |
| BMI (kg/m2), n (%) | ||||||
| <18.5 | 233 (1.9) | 38 (2.7) | 31 (1.2) | 135 (2.1) | 12 (1.5) | 17 (1.8) |
| 18.5 to <25.0 | 4490 (36.6) | 568 (40.6) | 944 (36.4) | 2332 (35.9) | 281 (35.0) | 365 (37.8) |
| 25.0 to <30.0 | 4308 (35.1) | 493 (35.3) | 960 (37.0) | 2232 (34.3) | 288 (35.8) | 335 (34.7) |
| ≥30.0 | 3233 (26.4) | 299 (21.4) | 662 (25.5) | 1801 (27.7) | 223 (27.7) | 248 (25.7) |
| eGFR (30 to <60 mL/min/1.73 m2), n (%) | 1580 (12.8) | 178 (12.7) | 348 (13.4) | 804 (12.4) | 109 (13.6) | 141 (14.6) |
| Total sodium intake ( × 102 mg/day) | 34.2 ± 20.3 | 32.5 ± 18.6 | 33.3 ± 20.7 | 34.6 ± 20.2 | 34.4 ± 19.5 | 35.5 ± 22.7 |
| Fat/calorie ratio, % | 33.5 ± 9.4 | 33.6 ± 9.7 | 33.1 ± 9.4 | 33.6 ± 9.3 | 33.5 ± 9.1 | 33.4 ± 9.2 |
Data are presented as count (percentage) or mean ± SD.
UIC status was categorized in reference to WHO guidelines: very low UIC (0–49 μg/L), low UIC (50–99 μg/L), normal UIC (100–299 μg/L), high UIC (300–399 μg/L), and very high UIC (400– μg/L).
NHANES, National Health and Nutrition Examination Survey; UIC, urinary iodine concentration; GED, General Educational Development; HR, hazard ratio; CI, confidence interval; BMI, body mass index; eGFR, estimated glomerular filtration rate.
UIC groups and mortality
The median duration of follow-up for mortality ascertainment was 19.2 (interquartile range 17.4–21.0) years, from which 3159 all-cause deaths were identified (717 cardiovascular, 799 cancer, and 1643 deaths from other causes). The adjusted all-cause mortality hazard ratios (HR) were higher for the very high UIC group compared to the normal UIC group (HR = 1.19 [confidence interval (CI) 1.04–1.37]), but no differences were observed for other UIC groups (Table 2). For this very high UIC group, effect estimates >1 were also estimated for both cardiovascular and cancer mortality. However, the CIs included the null value (cancer, HR = 1.39 [CI 0.93–2.07]; cardiovascular, HR = 1.16 [CI 0.90–1.34]; Table 2). Similar results were found for cancer mortality in the very low UIC group (HR = 1.34 [CI 0.95–1.90]). However, effect estimates of all-cause and cardiovascular mortality in this group were <1 (all-cause, HR = 0.93 [CI 0.77–1.14]; cardiovascular, HR = 0.87 [CI 0.66–1.15]; Table 2). Adjustment for potential intermediate variables (diabetes, hypertension, hypercholesterolemia, previous cardiovascular disease, and previous cancer) did not alter these results. Results were also similar after exclusion of participants with extremely high UIC (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
Table 2.
Associations Between UIC groups and All-Cause, Cardiovascular, and Cancer Mortality in NHANES IIIa
| Adjusted | ||||
|---|---|---|---|---|
| Event N/total N | Unadjusted HR [CI] | Model 1bHR [CI] | Model 2cHR [CI] | |
| All-cause mortality | ||||
| Very low UIC | 327/1398 | 0.91 [0.75–1.12] | 0.88 [0.73–1.06] | 0.93 [0.77–1.14] |
| Low UIC | 679/2597 | 1.00 [0.87–1.16] | 0.91 [0.80–1.0]) | 0.96 [0.84–1.09] |
| Normal UIC (reference) | 1664/6500 | 1 | 1 | 1 |
| High UIC | 206/804 | 1.09 [0.83–1.44] | 0.91 [0.70–1.18] | 0.88 [0.66–1.17] |
| Very high UIC | 283/965 | 1.32 [1.12–1.58] | 1.20 [1.04–1.39] | 1.19 [1.04–1.37] |
| Cardiovascular mortality | ||||
| Very low UIC | 71/1398 | 0.77 [0.58–1.03] | 0.79 [0.60–1.06] | 0.87 [0.66–1.15] |
| Low UIC | 146/2597 | 0.87 [0.70–1.08] | 0.79 [0.64–0.98] | 0.87 [0.70–1.08] |
| Normal UIC (reference) | 390/6500 | 1 | 1 | 1 |
| High UIC | 46/804 | 0.92 [0.55–1.52] | 0.72 [0.42–1.23] | 0.68 [0.38–1.22] |
| Very high UIC | 64/965 | 1.34 [1.01–1.76] | 1.18 [0.94–1.48] | 1.16 [0.90–1.34] |
| Cancer mortality | ||||
| Very low UIC | 99/1398 | 1.45 [1.01–2.08] | 1.26 [0.89–1.76] | 1.34 [0.95–1.90] |
| Low UIC | 177/2597 | 1.24 [0.92–1.65] | 1.09 [0.82–1.44] | 1.14 [0.87–1.49] |
| Normal UIC (reference) | 395/6500 | 1 | 1 | 1 |
| High UIC | 51/804 | 1.26 [0.76–2.09] | 1.09 [0.69–1.71] | 1.09 [0.69–1.74] |
| Very high UIC | 77/965 | 1.53 [1.03–2.28] | 1.39 [0.92–2.10] | 1.39 [0.93–2.07] |
The reference is normal UIC.
HR adjusted for age, sex, race/ethnicity, education status. and active smoking.
HR adjusted for diabetes, hypertension, hypercholesterolemia, previous cardiovascular disease, previous cancer, BMI, and eGFR in addition to covariates in Model 1.
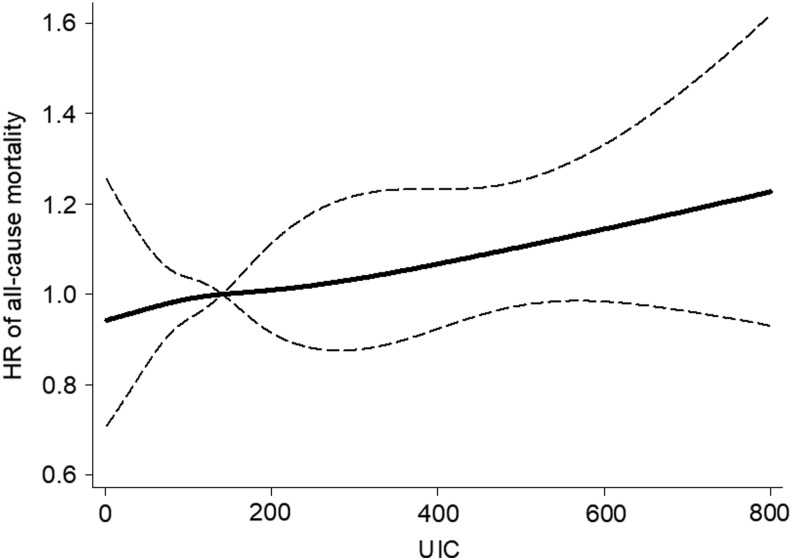
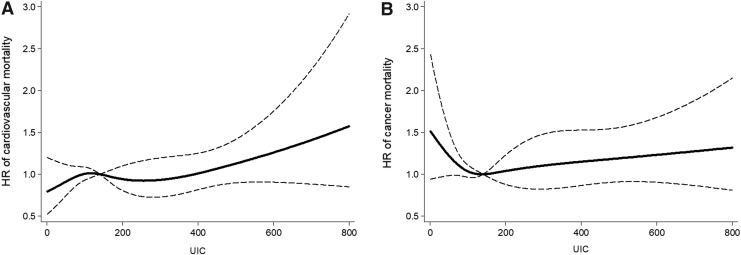
Models of UICs utilizing restricted cubic splines showed similar results for all outcomes (Figs. 1 and 2). In sensitivity analyses, the study also adjusted for total sodium intake and fat/calorie ratio in addition to all other covariates, and results for all-cause, cardiovascular-specific, and cancer-specific mortalities did not change substantially (Supplementary Table S2).
FIG. 1.
Association between urinary iodine concentration (UIC) and all-cause mortality using a restricted cubic spline regression model with four knots at 5th, 35th, 65th, and 95th percentile of UIC (30, 102, 182, and 450 μg/L). The dashed lines represent the confidence intervals (Cis) for the spline model (reference is the median, i.e., 140 μg/L). The range of UICs was restricted to 0–800 μg/L because predictions >800 μg/L are based on too few data points.
FIG. 2.
Association between UIC and cardiovascular (A) and cancer (B) mortality using a restricted cubic spline regression model with four knots at the 5th, 35th, 65th, and 95th percentile of UIC (30, 102, 182, and 450 μg/L). The dashed lines represent the CIs for the spline model (reference is the median, i.e., 140 μg/L). The range of UICs was restricted to 0–800 μg/L because predictions >800 μg/L are based on too few data points.
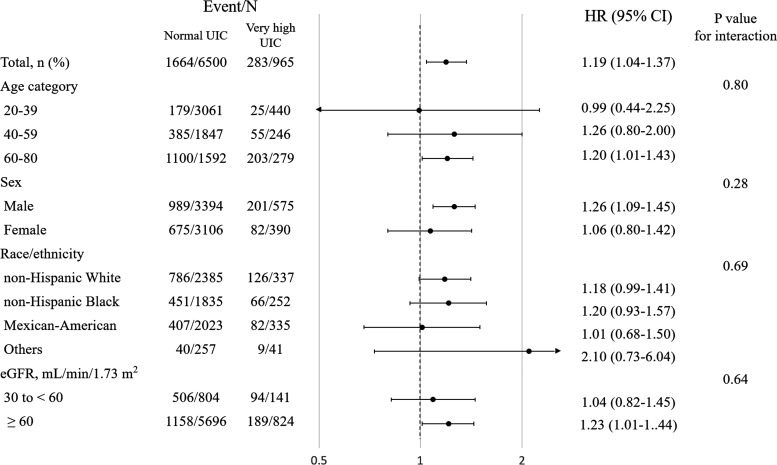
In stratified fully adjusted analyses comparing the very high UIC group to those having normal UICs, all-cause mortality risk was greater in participants >40 years of age, in men, and in every race/ethnicity except Mexican-Americans. Finally, for participants with eGFR ≥60 mL/min/1.73 m2, a larger HR was estimated for all-cause mortality than for those with eGFR of 30 to <60 mL/min/1.73 m2. Overall, however, none of these potential interactions was statistically significant on a multiplicative scale (Fig. 3).
FIG. 3.
Stratum-specific analysis (hazard ratios [HRs] for very high UIC compared to normal UIC). HRs were calculated in Cox proportional hazards regression models for all-cause mortality adjusting for age, sex, race/ethnicity, education status, active smoking, diabetes, hypertension, hypercholesterolemia, previous cardiovascular disease, previous cancer, body mass index, and estimated glomerular filtration rate (Model 2). p-Values test for homogeneity among subgroups.
Discussion
To the best of the authors' knowledge, this is the first epidemiologic study assessing the association between iodine status and mortality among a representative sample of the U.S. adult population. The results suggest that very high UIC (≥400 μg/L), consistent with excessive iodine intake, was associated with an increased risk of all-cause mortality. However, associations of all-cause mortality were not observed for any of the other UIC groups when compared to those in the normal UIC group.
Some epidemiologic studies have reported a high incidence of hypothyroidism and autoimmune thyroiditis in populations with more than adequate iodine intake (25–28). Other studies also reported an increased incidence of hyperthyroidism following periods of mandatory salt iodization (29,30). Thyroid dysfunction is known to induce endothelial and cardiac dysfunction, and thus may increase the risk of cardiovascular disease (12,31–34). There are also limited studies showing an association between thyroid dysfunction and increased cancer risks, although the underlying mechanism has not been established (12,32,33). Moreover, iodine excess can induce goiter, a risk factor for the development of thyroid cancer (25), an association that has been reported in some populations (35–37). The present study excluded participants with existing thyroid dysfunction. Since the likelihood of insufficient or excessive iodine intake might be even higher in this group, the present findings may also underestimate the impact of iodine status on mortality among those with very low UICs. In stratum-specific analyses, some differences between very high UIC and normal UIC were suggested with age, sex, race/ethnicity, and eGFR, but the models did not provide evidence for interaction on a multiplicative scale. Larger studies are needed to clarify whether these factors modify the effect of excessive iodine intake on mortality.
Some studies have reported increased odds of hypothyroidism among low UIC populations (25,38), suggesting that iodine deficiency may also be adversely related to cardiovascular outcomes (39). This study sample did not find that low UIC was associated with all-cause mortality. The trend observed between increased iodine intake and decreased risk for cardiovascular mortality that was observed may in part help explain these findings. In addition, owing to the successful implementation of IDD eradication (4,8), some subjects in the low UIC category at the time of the NHANES assessment might have subsequently increased their iodine intake during the follow-up period. Such a potential shift to an increased intake would cause misclassification of exposure and potentially bias the mortality estimates toward the null. One possible explanation for the findings showing a lower risk of cardiovascular-specific mortality among those with low UICs compared to those with normal UICs is that among those with low UICs, both iodized salt and sodium intake may be lower, thus leading to decreased cardiovascular mortality in this group. Additionally, adjusting for total sodium intake and a fat/calorie ratio did not change the results, but information regarding iodized salt intake was not available in the NHANES III data set to investigate this hypothesis further. Increased cancer mortality was found in the low UIC group, although the confidence intervals did not exclude the null due to insufficient sample size. Thyroid dysfunction may mediate both the effects of insufficient and excessive iodine intake to increase cancer mortality risk (12,31,32).
The present study has several limitations. First, iodine status in this study sample was estimated only from a single spot urine measurement. UIC is an easy and cost-effective tool recommended by the WHO to assess population iodine status (5). However, urinary iodine excretion (UIE) obtained from a 24-hour urine collection has also been used to estimate population iodine intake (40), and further prospective studies using UIE are needed to validate the present results. The urinary iodine/creatinine ratio was not used, as its utility is still controversial (5,41). There was also no information in this data set regarding trends in UIC over the follow-up period, and this might cause misclassification of exposure to affect mortality risk. Second, the susceptibility of individuals to excessive or insufficient iodine intake is dependent on the geographic area of residence in chronically iodine sufficient or insufficient regions, as well as the presence of thyroid dysfunction due to other causes (1). Participants with hypothyroidism, hyperthyroidism, and use of iodine-containing medications were excluded, but information on area of residence was not available. Third, the possibility cannot be excluded that participants had received iodinated contrast media (ICM) through computed tomography (CT) or angiography prior to the collection of urine for UIC measurements, as this information was not available in the data set. It is known that UICs acutely increase as a result of the iodine load present in ICM, but these normalize within one to two months (42). Participants in the NHANES are non-institutionalized civilians, and few would be expected to have diseases requiring contrast CT or angiography. Also, the study adjusted for cardiovascular disease and cancer, which are diseases that might have required these procedures. Fourth, residual confounding and competing risks for cause-specific mortalities cannot be completely excluded. Further studies with longitudinal measures of iodine status and serum thyroid function are needed to overcome these limitations, possibly establish causality, and reassess the UIC cutoff values that may be related to negative health effects. Lastly, relying on NDI death certificate records could have introduced potential outcome misclassification, but this would be non-differential (independent of UIC) and most likely generate a bias toward the null.
In conclusion, populations with ≥400 μg/L UIC are at increased risk of all-cause mortality compared to those with normal-range UIC. Iodine intake parameters, including UIC, have been monitored in countries with iodine deficiency (5), but have usually not been monitored in iodine-sufficient regions. Although global public health interventions addressing IDDs have been hugely successful (7,8) and should not be abandoned, population iodine intake monitoring may be required in order to assess the potential risks of excess iodine on long-term health outcomes (3,9).
Supplementary Material
Acknowledgments
We would like to thank Kimberly C. Paul, PhD (Department of Epidemiology, UCLA Fielding School of Public Health) for her help with the statistical analyses. Dr. Ritz received funding from NIH with the following grants: R01ES023451, R56ES026600.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Zimmermann MB, Boelaert K. 2015. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol 3:286–295 [DOI] [PubMed] [Google Scholar]
- 2.Leung AM, Braverman LE, Pearce EN. 2012. History of U.S. iodine fortification and supplementation. Nutrients 4:1740–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Eastman CJ. 2012. The changing epidemiology of iodine deficiency. Nat Rev Endocrinol 8:434–440 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization 1994. Iodine and health: eliminating iodine deficiency disorders safely through salt iodisation: a statement by the World Health Organization. Available at: http://apps.who.int/iris/bitstream/10665/58693/1/WHO_NUT_94.4.pdf?ua=1 (accessed January10, 2018)
- 5.WHO, UNICEF, and ICCIDD 2007. Assessment of the iodine deficiency disorders and monitoring their elimination. Available at: http://apps.who.int/iris/bitstream/10665/43781/1/9789241595827_eng.pdf (accessed January10, 2018)
- 6.Food and Nutrition Board of the U.S. Institute of Medicine 2006. Dietary Reference Intakes. National Academy Press, Washington, DC, pp 320–327 [Google Scholar]
- 7.Andersson M, Karumbunathan V, Zimmermann MB. 2012. Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–750 [DOI] [PubMed] [Google Scholar]
- 8.Pretell EA, Pearce EN, Moreno SA, Dary O, Kupka R, Gizak M, Gorstein J, Grajeda R, Zimmermann MB. 2017. Elimination of iodine deficiency disorders from the Americas: a public health triumph. Lancet Diabetes Endocrinol 5:412–414 [DOI] [PubMed] [Google Scholar]
- 9.Leung AM, Braverman LE. 2014. Consequences of excess iodine. Nat Rev Endocrinol 10:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Journy NM, Bernier MO, Doody MM, Alexander BH, Linet M, Kitahara CM. 2017. Hyperthyroidism, hypothyroidism and cause-specific mortality in a large cohort of women. Thyroid 27:1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodondi N, Den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J; Thyroid Studies Collaboration 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K, Tsujimoto T, Saito J, Sugiyama T. 2016. Association between serum thyrotropin levels and mortality among euthyroid adults in the United States. Thyroid 26:1457–1465 [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics, Centers for Disease Control and Prevention 2011. National Health and Nutrition Examination Survey. Available at: www.cdc.gov/nchs/nhanes.htm (accessed January10, 2018)
- 14.National Center for Health Statistics, Centers for Disease Control and Prevention 2010. NCHS research ethics review board (ERB) approval. Available at: www.cdc.gov/nchs/nhanes/irba98.htm (accessed January10, 2018)
- 15.National Center for Health Statistics, Centers for Disease Control and Prevention. NHANES response rates and CPS totals. Available at: https://wwwn.cdc.gov/nchs/data/nhanes3/ResponseRates/nh3_rr.pdf (accessed January10, 2018)
- 16.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. 2002. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87:489–499 [DOI] [PubMed] [Google Scholar]
- 17.National Center for Health Statistics, Centers for Disease Control and Prevention 1996. Laboratory procedures used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Available at: https://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf (accessed January10, 2018)
- 18.Benotti J, Benotti N, Pino S, Gardyna H. 1965. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem 11:932–936 [PubMed] [Google Scholar]
- 19.Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL, DeLozier DM, Jackson RJ. 1998. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 83:3401–3408 [DOI] [PubMed] [Google Scholar]
- 20.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. 2013. National Health and Nutrition Examination Survey: plan and operations, 1999–2010. Vital Health Stat 1 56:1–37 [PubMed] [Google Scholar]
- 21.1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1 32:1–407 [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fillenbaum GG, Burchett BM, Blazer DG. 2009. Identifying a national death index match. Am J Epidemiol 170:515–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenland S. 1995. Avoiding power loss associated with categorization and ordinal scores in dose–response and trend analysis. Epidemiology 6:450–454 [DOI] [PubMed] [Google Scholar]
- 25.Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, Jin Y, Yu X, Fan C, Chong W, Yang F, Dai H, Yu Y, Li J, Chen Y, Zhao D, Shi X, Hu F, Mao J, Gu X, Yang R, Tong Y, Wang W, Gao T, Li C. 2006. Effect of iodine intake on thyroid diseases in China. New Engl J Med 354:2783–2793 [DOI] [PubMed] [Google Scholar]
- 26.Konno N, Makita H, Yuri K, Iizuka N, Kawasaki K. 1994. Association between dietary iodine intake and prevalence of subclinical hypothyroidism in the coastal regions of Japan. J Clin Endocrinol Metab 78:393–397 [DOI] [PubMed] [Google Scholar]
- 27.Laurberg P, Jorgensen T, Perrild H, Ovesen L, Knudsen N, Pedersen IB, Rasmussen LB, Carlé A, Vejbjerg P. 2006. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol 155:219–228 [DOI] [PubMed] [Google Scholar]
- 28.Sang Z, Wang PP, Yao Z, Shen J, Halfyard B, Tan L, Zhao N, Wu Y, Gao S, Tan J, Liu J, Chen Z, Zhang W. 2012. Exploration of the safe upper level of iodine intake in euthyroid Chinese adults: a randomized double-blind trial. Am J Clin Nutr 95:367–373 [DOI] [PubMed] [Google Scholar]
- 29.Galofre JC, Fernandez-Calvet L, Rios M, Garcia-Mayor RV. 1994. Increased incidence of thyrotoxicosis after iodine supplementation in an iodine sufficient area. J Endocrinol Invest 17:23–27 [DOI] [PubMed] [Google Scholar]
- 30.Todd CH, Allain T, Gomo ZA, Hasler JA, Ndiweni M, Oken E. 1995. Increase in thyrotoxicosis associated with iodine supplements in Zimbabwe. Lancet 346:1563–1564 [DOI] [PubMed] [Google Scholar]
- 31.Galli E, Pingitore A, Iervasi G. 2010. The role of thyroid hormone in the pathophysiology of heart failure: clinical evidence. Heart Fail Rev 15:155–169 [DOI] [PubMed] [Google Scholar]
- 32.Gencer B, Collet T-H, Virgini V, Bauer DC, Gussekloo J, Cappola AR, Nanchen D, den Elzen WP, Balmer P, Luben RN, Iacoviello M, Triggiani V, Cornuz J, Newman AB, Khaw KT, Jukema JW, Westendorp RG, Vittinghoff E, Aujesky D, Rodondi N; Thyroid Studies Collaboration 2012. Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from six prospective cohorts. Circulation 126:1040–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuijpens JL, Nyklictek I, Louwman MW, Weetman TA, Pop VJ, Coebergh JW. 2005. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid 15:1253–1259 [DOI] [PubMed] [Google Scholar]
- 34.Guarino V, Castellone MD, Avilla E, Melillo RM. 2010. Thyroid cancer and inflammation. Mol Cell Endocrinol 321:94–102 [DOI] [PubMed] [Google Scholar]
- 35.Blomberg M, Feldt-Rasmussen U, Andersen KK, Kjaer SK. 2012. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int J Cancer 131:2360–2366 [DOI] [PubMed] [Google Scholar]
- 36.Dong W, Zhang H, Zhang P, Li X, He L, Wang Z, Liu Y. 2013. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med Sci Monit 19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michikawa T, Inoue M, Shimazu T, Sawada N, Iwasaki M, Sasazuki S, Yamaji T, Tsugane S; Japan Public Health Center-based Prospective Study Group 2012. Seaweed consumption and the risk of thyroid cancer in women: the Japan Public Health Center-based Prospective Study. Eur J Cancer Prev 21:254–260 [DOI] [PubMed] [Google Scholar]
- 38.Lee KW, Shin D, Song WO. 2016. Low urinary iodine concentrations associated with dyslipidemia in US adults. Nutrients 8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venturi S, Donati FM, Venturi A, Venturi M. 2000. Environmental iodine deficiency: a challenge to the evolution of terrestrial life? Thyroid 10:727–729 [DOI] [PubMed] [Google Scholar]
- 40.Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, Ovesen L, Jørgensen T. 2009. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 19:1281–1286 [DOI] [PubMed] [Google Scholar]
- 41.Perrine CG, Cogswell ME, Swanson CA, Sullivan KM, Chen TC, Carriquiry AL, Dodd KW, Caldwell KL, Wang CY. 2014. Comparison of population iodine estimates from 24-hour urine and timed-spot urine samples. Thyroid 24:748–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee SY, Chang DL, He X, Pearce EN, Braverman LE, Leung AM. 2015. Urinary iodine excretion and serum thyroid function in adults after iodinated contrast administration. Thyroid 25:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.