Abstract
Background
Schistosomiasis is one of the most important infectious parasitic diseases in the world. The most important was to control schistosomiasis is through a combination of medical therapy and immunization. The membrane antigens Tsp2 and 29 from Schistosoma are promising anti-schistosomiasis vaccine candidates.
Material/Methods
In this study, the pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, and pcDNA3.1 (+)-SjTsp2-29 eukaryotic expression vectors were successfully constructed as DNA vaccines, and the protective abilities of these vaccines were evaluated in mice.
Results
The results showed that vaccination with SjTsp2, Sj29, and SjTsp2-29 reduced parasite burden and hepatic pathology compared to the control group, and the protective effect of the bivalent SjTsp2-29 DNA vaccine was better than that of the univalent SjTsp2 or Sj29 DNA vaccines. We also found high levels of IgG, IgG1, and IgG2a against SjTsp2, Sj29, and SjTsp2-29 DNA vaccines, with high expression of IFN-γ and no IL-4 in the mice.
Conclusions
The double-membrane antigen DNA vaccine SjTsp2-29 elicited protection against Schistosoma infection and might serve as a vaccine candidate.
MeSH Keywords: DNA, Schistosoma japonicum, Vaccine Potency
Background
Schistosomiasis, one of the most important infectious diseases in humans, is caused by infection with 3 important Schistosome species: Schistosoma haematobium, Schistosoma mansoni, and Schistosoma japonicum [1,2]. There are estimated to be about 77 000 schistosomiasis patients in China and about 30 800 advanced schistosomiasis cases were diagnosed in 2015 [3]. Although praziquantel is a widely-used, high-efficiency, broad-spectrum oral antiparasitic drug, chemotherapy does not prevent reinfection or transmission of schistosomiasis in highly endemic areas [4].
Additionally, the repeated and large-scale use of praziquantel in epidemic areas has led to drug resistance and a reduced cure rate [4–6]. The best long-term strategies are intermediate host management, environmental and water sanitation, good personal hygiene maintenance, and vaccines [7–9]. Although the development of vaccines against schistosomiasis has had more setbacks than successes, some inspiring results have emerged recently based on the use of membrane protein antigens of Schistosoma [10–14].
The Schistosome tegument is involved in nutrition, immune evasion and modulation, excretion, osmoregulation, sensory reception, and signal transduction, and membrane proteins of parasite are vulnerable to attack by the host immune system [15–17]. Thus, the tegument is crucial to the parasite and is a target for vaccines and drugs. The membrane antigens of S. mansoni, the extracellular loop 2 of a tetraspanin (TSP-2) and 29, are promising anti-schistosomiasis vaccine candidates that have approximately 50% protection rate in mice infected with S. mansoni cercariae [11,12]. The membrane antigen of S. japonicum, Sj-TSP-2, has been confirmed as an effective anti-schistosomiasis vaccine in mice [18,19]. The Sj29 protein also has been identified and confirmed to be a membrane protein by our group [20]. The use of multivalent vaccines is a promising method that induces greater protection than with the use of a single antigen [21,22].
Thus, we developed a high-performance bivalent DNA vaccine including the membrane proteins SjTsp2 and Sj29 of S. japonicum. Based on the constructions of the pcDNA3.1(+)-SjTsp2 and pcDNA3.1(+)-Sj29 groups, the SjTsp2 and Sj29 fusion gene connected with (Gly4Ser)3 was amplified by SOEing (Splicing by Overlap Extension) PCR and cloned into the eukaryotic expression vector pcDNA3.1(+). After endotoxin-free plasmids were prepared, we evaluated the protective abilities of the bivalent pcDNA3.1(+)-SjTsp2-29 DNA vaccine in mice.
Material and Methods
Parasites and animals
Oncomelania hupensis snails infected with a mainland Chinese strain of S. japonicum were provided from the Jiangsu Provincial Institute of Parasitic Diseases. Female Kunming mice (6 weeks old) were purchased from the Animal Centre of Anhui Medical University and used for the vaccine trials.
Ethics approval
The mice received care that complied with the Guidelines for the Care and Use of Laboratory Animals. All of the work presented here was approved by the Anhui Experimental Animal Training Base (reference number LLSC20140061).
Preparation of DNA vaccines
The pET28a-SjTsp2, pET28a-Sj29, and pET28a-SjTsp2–29 recombinant plasmids were constructed. The pcDNA3.1(+)-SjTsp2 DNA vaccine construct was modified from pET28a-SjTsp2, which contains a larger extracellular loop of TSP-2 from S. japonicum. Larger extracellular loop of Sj-TSP-2 gene products were digested with pET28a-SjTsp2 with BamHI/XhoI, and then subcloned into the pcDNA3.1(+) vector. Extracellular region of Sj29 gene was digested pET28a-SjTsp2 with BamHI/XhoI, and then subcloned into the pcDNA3.1(+) vector to construct pcDNA3.1(+)-29 DNA vaccine. Based on the constructions of the pcDNA3.1(+)-SjTsp2 and pcDNA3.1(+)-Sj29 groups, the SjTsp2 and Sj29 fusion gene connected with (Gly4Ser)3 was amplified by SOEing PCR and cloned into the pcDNA3.1(+) with BamHI/Xho I. The fusion SjTsp2 and Sj29 genes connected by (Gly4Ser)3 were digested pET28a-SjTsp2–29 with BamHI/Xho I, and then subcloned into the pcDNA3.1(+) vector to construct the pcDNA3.1(+)-SjTsp2–29 DNA vaccine. The 3 constructs were confirmed by restriction enzyme analyses and sequencing, and a large number of endotoxin-free plasmids were then extracted in accordance with the instructions of the plasmid extraction kit (CW Biotech, Beijing, China).
Administration of the DNA vaccine
Fifty 6-week-old female Kunming mice were randomly divided into 5 groups of 10 mice each: a normal saline (NS) group, a pcDNA3.1(+) plasmid group, a pcDNA3.1(+)-SjTsp2 group, a pcDNA3.1(+)-Sj29 group, and a pcDNA3.1(+)-SjTsp2–29 group. To enhance the uptake of foreign DNA vaccine, all mice were injected with 30 μl bupivacaine hydrochloride (7.5 mg/ml) (Harvest Pharmaceutical Co., Shanghai, China) in the right tibialis anterior muscle 24 h before immunization. Each group was immunized with 3 intramuscular injections of 75 μg DNA vaccine 3 times biweekly, and the control group was injected with 100 μl normal saline.
Challenge infection and worm and egg burden recovery
Two weeks after the last immunization, the 30 infected Oncomelania were exposed to sunlight for 4 h at 25–28°C and cercariae were shed in a beaker. Mice were infected with 24±2 S. japonicum cercariae by percutaneous infection. At 6 weeks after infection, the worm reduction rates and the egg reduction rates in the livers were calculated. The worms were collected and counted by perfusion from the hepatic portal system, and the eggs were also collected. The weight of liver tissues was measured before being homogenized in 50 ml PBS. We mixed 5 ml homogenate with 1 ml 10% NaOH and incubated it at 56°C for 1 h. The average of 3 counts per 100 μl mixture was considered as the number of eggs in each sample tested, and this was converted to EPG (eggs per gram). The rate of reduction in the worm and egg counts was calculated as follows: percentage reduction in worm burden=(mean worm burden of control group–mean worm burden of vaccinated group)/mean worm burden of control group×100%; percentage reduction in liver egg count=(mean EPG from control group–mean EPG from vaccinated group)/mean EPG from control group×100%.
Detection of antibodies and cytokines levels by enzyme-linked immunosorbent assay (ELISA)
Before the mice were infected with S. japonicum, the sera were collected. The anti-AWA IgG, IgG1, and IgG2 antibody levels in the serum samples were detected by ELISA. Each well of a plate was coated with 1 μg AWA (adult worm antigen). SjAWA was prepared as described previously by Abán [23]. Plates were blocked by 200 μl/well of 2.5% BSA (bovine serum albumin) in 0.02 M PBS with 0.05% Tween 20 (PBST) (pH 7.2). After the serum samples were diluted 100 times with PBST, 100 μl of the diluted sera was added to each well and incubated for 1 h. Finally, the plates were incubated with peroxidase-conjugated goat anti-mouse IgG, IgG1, and IgG2a (Sigma) diluted to 1: 10 000, 1: 5000, and 1: 5000, respectively, for 1 h at room temperature (RT). The reaction was visualized by addition of 200 μl/well of 3,3′, 5,5′-Tetramethylbenzidine substrate, and then was stopped by adding 50 μl 2 M H2SO4. The optical density (OD) was measured at 450 nm.
To evaluate the cytokine profile generated by the immunization of mice with DNA vaccine, the sera were collected before the mice were infected with S. japonicum. We detected the levels of a panel of cytokines (IFN-γ and IL-4) in the sera, and mouse IFN-γ and IL-4 ELISA kits (eBioscience, USA) were used according to the manufacturer’s instructions.
Histology and immunohistochemistry analysis
To detect the DNA vaccine expression in vivo, 3 days after the third immunization, 1 mouse was taken from each group, then the injected muscle tissues were removed, fixed in 10% formalin, embedded in paraffin, and sectioned for histology. Another mouse from the pcDNA3.1(+) group was chosen randomly as a control. The muscle tissue sections were incubated for 30 min with DNA vaccine immunized serum (dilution 1: 1000) for immunohistochemistry. The immunohistochemistry tests were conducted according to the instructions of the Power Vision Two-Step detection system (Zhongshan Biotechnology Co., Beijing, China), which was used to view the muscle tissues at 20× image magnification. At 6 weeks after infection, all mice were sacrificed, livers were excised and immediately fixed in 10% formalin in PBS, and embedded in paraffin. The liver tissues sections (4 μm) were stained with hematoxylin and eosin (HE) and examined for quantitative and qualitative changes. The granuloma areas were measured with computer-assisted morphometric software (Image-Pro Plus) to calculate the granuloma areas as percentages of the total areas for each side. At least 3 discontinuous slides were measured for each specimen, and the mean values of 8 mice were taken for statistical analysis [24].
Statistical analyses
The results were analyzed with one-way ANOVA with post hoc testing using the least significant difference test. All data are shown as the mean±the standard error of the mean (SEM). P values <0.05 were considered statistically significant.
Results
Construction of the pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, and pcDNA3.1(+)-SjTsp2-29 DNA vaccine
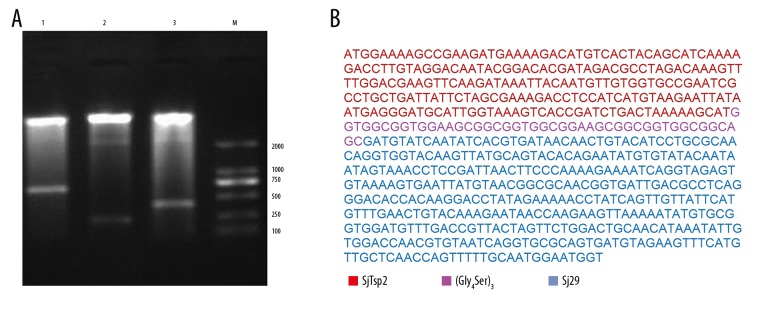
pcDNA3.1(+)-SjTsp2–29, pcDNA3.1(+)-SjTsp2, and pcDNA3.1(+)-Sj29 were digested with BamHI and XhoI. The sizes of digestive products were consistent with those expected: 660 bp, 225 bp, and 390 bp, respectively. According to the agarose gel results (Figure 1A) and sequencing results (Figure 1B), SjTsp2, Sj29, and SjTsp2–29 were successfully incorporated into the backbone vector pcDNA3.1(+). Therefore, the pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, and pcDNA3.1(+)-SjTsp2–29 DNA vaccines were successfully constructed.
Figure 1.
Identification of recombinant plasmids pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, and pcDNA3.1(+)-SjTsp2–29. (A) Identification with restriction enzyme digestion. pcDNA3.1(+)-SjTsp2–29, pcDNA3.1(+)-SjTsp2, and pcDNA3.1(+)-Sj29 were digested with BamHI and XhoI, and the sizes of digestive products were consistent with those expected (i.e., 660, 225, and 390bp, respectively). Lane M: DNA marker; lane 1: pcDNA3.1(+)-SjTsp2–29; lane 2: pcDNA3.1(+)-SjTsp2; lane 3: pcDNA3.1(+)-Sj29. (B) SjTsp2–29 nucleotide sequence alignment with Sj-TSP-2 and Sj29. a: -SjTsp2; b: (Gly4Ser)3 linker; c: Sj29
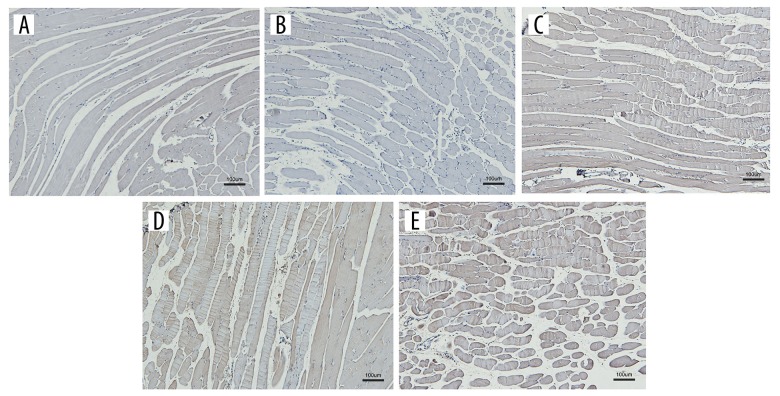
Immunohistochemistry analyses of the injected muscle tissues
Immunohistochemical analyses of the injected muscles were performed to identify the expression of the DNA vaccines in muscle following injection. The results revealed that immunostaining of the injected muscle for the DNA vaccine antibody was positive, whereas immunostaining of the pcDNA3.1(+) and NS-injected muscles was negative (Figure 2).
Figure 2.
Gene expressions in the muscle tissues of mice injected with the DNA vaccines. (A) NS, (B) pcDNA3.1(+), (C) pcDNA3.1(+)-SjTsp2, (D) pcDNA3.1(+)-Sj29, (E) pcDNA3.1(+)-SjTsp2–29.
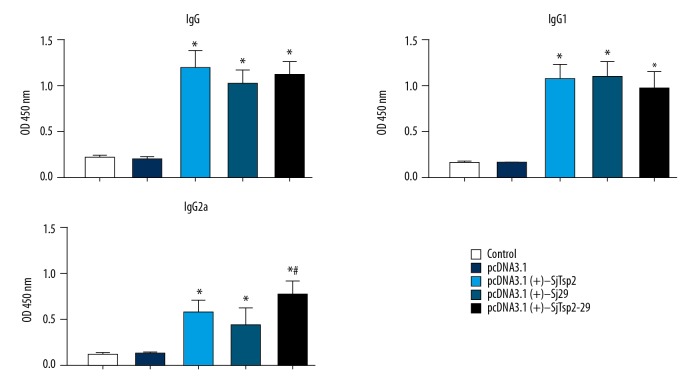
Antibody response to DNA vaccination
To assess antigen-specific anti-AWA antibodies production, the sera were collected before the infection of mice by S. japonicum. ELISA was performed to detect the levels of IgG and IgG subtypes in immunized mouse sera using AWA as antigen. High total IgG titers were recorded for all vaccinated groups relative to the infected control groups that were vaccinated with pcDNA3.1(+) or NS (P<0.05). pcDNA3.1(+)-SjTsp2–29 induced higher levels of IgG2a compared to pcDNA3.1(+)-Sj-TSP-2 or pcDNA3.1(+)-Sj29 alone (Figure 3).
Figure 3.
Detection of anti-AWA IgG, IgG1, and IgG2a in sera of different groups by ELISA. All data are expressed as the means±the SEMs (n=10 for each group). * P<0.05 versus the corresponding pcDNA3.1 or NS group.
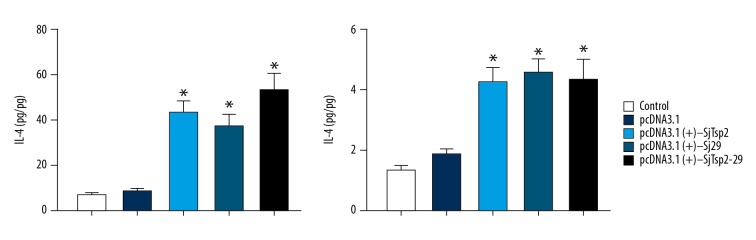
Cytokine profile
To evaluate the cytokine profile generated by the immunization of mice with DNA vaccine, we measured the levels of cytokines (IFN-γ and IL-4) in mouse sera. Significantly higher levels of IFN-γ were detected in sera of DNA-vaccinated mice compared to the control group (Figure 4). However, lower levels of IL-4, a signature of Th2-immune response, were detected.
Figure 4.
Detection of IFN-γ and IL-4 of different groups by ELISA. All data are expressed as the means±the SEMs (n=10 for each group). * P<0.05 versus the corresponding pcDNA3.1 or NS group, # P<0.05 versus the corresponding pcDNA3.1(+)-Sj29 group.
DNA vaccine induces immune protection against S. japonicum
To determine the protective efficacies of the pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, and pcDNA3.1(+)-SjTsp2–29 DNA vaccines, the infected mice were sacrificed 42 days after infection to measure the adult worm and egg reduction rates in the liver. As shown in Table 1, the mice that were injected with pcDNA3.1(+)-SjTsp2 or pcDNA3.1(+)-Sj29 exhibited significantly greater reduction in worm burden and liver eggs compared to the control group (P<0.05). Most interestingly, the pcDNA3.1(+)-SjTsp2–29 group exhibited significantly greater reduction in the worm burden and liver eggs compared to the pcDNA3.1(+)-SjTsp2 and pcDNA3.1(+)-Sj29 groups (P<0.05). The pcDNA3.1(+)-SjTsp2–29 group exhibited a worm reduction rate of 53.2% and an egg reduction rate of 51.4%.
Table 1.
Parasitology data of mice vaccinated with pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29 and pcDNA3.1(+)-SjTsp2–29.
| Group | Adult worms mean±SD | Worm reduction rate (%) | Liver eggs mean±SD | Liver eggs reduction rate (%) |
|---|---|---|---|---|
| NS | 15.8±3.9 | 30942±4123 | ||
| pcDNA3.1(+) | 14.7±4.1 | 7.0 | 28131±3056 | 9.1 |
| pcDNA3.1(+)-SjTsp2 | 10.2±3.1 | 35.4* | 19305±1978 | 37.6* |
| pcDNA3.1(+)-Sj29 | 11±3.9 | 30.4* | 21234±1927 | 31.4* |
| pcDNA3.1(+)-SjTsp2–29 | 7.4±2.1 | 53.2*# | 15038±1801 | 51.4*# |
All data are expressed as the means±the SEMs (n=10 for each group).
P<0.05 versus the corresponding NS or pcDNA3.1(+) group,
P<0.05 versus the corresponding pcDNA3.1(+)-SjTsp2 or pcDNA3.1(+)-Sj29 group.
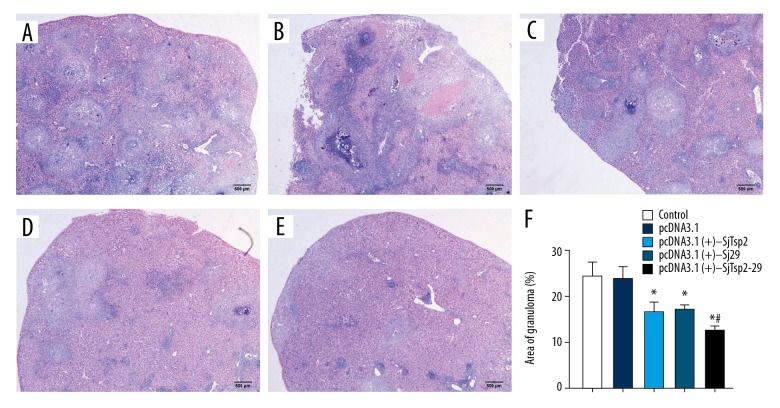
DNA vaccination affects the formation of S. japonicum egg-induced liver granulomas
To investigate the effect of the DNA vaccines on S. japonicum egg-induced liver granulomas, liver tissues were fixed and stained with HE. Strikingly, compared to the control mice, the liver granulomas were attenuated in the groups that were injected with the pcDNA3.1(+)-SjTsp2, pcDNA3.1(+)-Sj29, or pcDNA3.1(+)-SjTsp2–29 DNA vaccines. pcDNA3.1(+)-SjTsp2–29 caused a significantly greater granuloma reduction in the liver compared to pcDNA3.1(+)-SjTsp2 and pcDNA3.1(+)-Sj29 (P<0.05) (Figure 5).
Figure 5.
Effects of the DNA vaccines on the sizes of the hepatic granulomas in the livers of the Schistosoma japonicum-infected mice. (A) NS, (B) pcDNA3.1(+), (C) pcDNA3.1(+)-SjTsp2, (D) pcDNA3.1(+)-Sj29, (E) pcDNA3.1(+)-SjTsp2–29. (F) The hepatic egg granuloma sizes were measured with computer-assisted histomorphometric analyses. All data are expressed as the means±the SEMs (n=10 for each group). * P<0.05 versus the corresponding NS or pcDNA3.1(+) group, # P<0.05 versus the corresponding pcDNA3.1(+)-SjTsp2 or pcDNA3.1(+)-Sj29 group.
Discussion
Some schistosomiasis vaccines (e.g., Sh28GST, SmTSP2, and Sm14) are already in early human clinical trials [7,25,26], but these trials are not yet finished. The failure to develop effective vaccines may be due to a variety of factors, including the fact that the current understanding of schistosomiasis immunity and immune mechanisms is largely dependent on studies in mice, but vaccines conducted in studies only in mouse models may have undesirable effects in human clinical trials. A vigorous humoral response (IgG, IgM, and IgE) is found in patients with acute and chronic schistosomiasis, which is different from that in mice [7,27,28]. Therefore, new Schistosome vaccine molecules and vaccination are still needed for study.
Tetraspanins of Schistosoma mansoni have 4 transmembrane regions, which are connected by 2 extracellular loops, and the loops are speculated to interact with ligands [6]. The larger extracellular loop of Sm-TSP-2 has been confirmed to be valuable anti-schistosomiasis vaccine, which induces approximately 50% reduction in the worm burden [11]. Sm-TSP-2, which fuses to a thioredoxin partner, has been confirmed to be a more effective anti-schistosomiasis vaccine than Sm-TSP-2 alone [29]. Sj-TSP-2 is highly polymorphic [30,31] and therefore has limited utility as a vaccine. The Sj-TSP-2e protein is recognized by the sera of some patients who are infected with S. japonicum, but this protein does not protect mice against a S. japonicum challenge infection [30]. However, Yuan [19] used a subclass of the Sj-TSP-2 sequence to express recombinant Sj-TSP-2 protein as a vaccine, and observed significant efficacy in mice. Similarly, we found 3 subclasses of Sj-TSP and confirmed that the Sj-Tsp2-A DNA vaccine induced partial protective immunity against S. japonicum infection [18].
The Sm29 protein is strongly recognized by IgG1 and IgG3 antibodies of naturally resistant individuals, as well as by patients who are resistant to reinfection and live in areas that are endemic for schistosomiasis [12,32]. Sm29 is a promising candidate as a vaccine against schistosomiasis, and is confirmed to have about a 50% protection level against experimental S. mansoni infection [12]. Pinheiro et al. [33] confirmed that vaccination using Sm-TSP-2 linked with the terminus of Sm29 induced reductions in parasite burden and hepatic pathology relative to control; the resultant protection levels ranged from 27.84% to 34.83%. The combination of Sm29 and Sm14 induces significant protective immunity in mice infected with S. mansoni [34]. A vigorous humoral immune response can be induced by a vaccine formulation containing rSm29 adsorbed to alum, which successfully protects against S. mansoni reinfection in mice, indicating a potentially effective vaccine formulation that could be used in humans [35]. Sj29 is homologous to Sm29 of S. mansoni, which was characterized by our group as a membrane-bound antigen in S. japonicum [20].
S. japonicum is a multi-cellular parasite with complex life cycle stages and can evade the immune recognition system via mechanisms of antigenic variation. Furthermore, not all individuals have immunogenic responses to the identical antigens that occur in natural infections. The bivalent vaccine SjTsp2–29, which is composed of the Sj-TSP-2 and Sj29 schistosomal genes, can induce more broad-range protection against Schistosoma than can the univalent Sj-TSP-2 and Sj29 DNA vaccine. The method of using 2 promising vaccine molecules in combination merits further investigations for improving protection efficacy. In the present study, we used a flexible peptide (Gly4Ser)3 as a linker to connect the Sj-TSP-2 and Sj29 schistosomal genes to construct the DNA vaccine SjTsp2–29. Immunohistochemistry analyses revealed the expressions of specific antigens in the quadriceps muscles of the Sj-TSP-2, Sj29, and SjTsp2–29 groups. The present study shows that it is possible to induce protection in a murine schistosomiasis model by DNA vaccines Sj-TSP-2, Sj29, and SjTsp2–29. Kunming mice immunized with DNA vaccines Sj-TSP-2, Sj29, and SjTsp2–29 revealed high levels of IgG, IgG1, and IgG2a after the last immunization. However, the highest levels of IgG2a were induced after immunization with SjTsp2–29 compared to Sj-TSP-2 or Sj29 alone. The Th1-type of immune response was confirmed to be induced by DNA vaccines Sj-TSP-2, Sj29, and SjTsp2–29, which was shown by the production of IFN-γ and no significant IL-4 by cytokine analysis. Some researchers [31,36–38] have found that the Th1 type of immune response plays a more important role in providing protection against Schistosoma infection than does Th2. Moreover, these results were involved in increases in the expression of IFN-γ. The protective response mechanism of IFN-γ is macrophage activation to impede schistosomula migration and kill parasites in a nitric oxide-dependent approach [39,40]. Sm-TSP-2 and Sm29 are promising anti-schistosomiasis vaccine candidates that have been shown to elicit approximately 50% protection in mice [11,12]. However, S. japonicum differs from S. mansoni, and we found that the Sj-TSP-2 and Sj29 DNA vaccines achieved more than 30% protection and reduced the pathological inflammation in the mouse livers. The reduction rate of the granuloma areas elicited in the Sj-TSP-2 and Sj29 groups was approximately 30%. We found that the SjTsp2–29 DNA vaccine achieved greater than 50% protection and also reduced the hepatic pathological inflammation in mice. The reduction rate of the granuloma area in the SjTsp2–29 group was 50%. Thus, the protective effect of the bivalent DNA vaccine was better than that of the univalent DNA vaccines. We found that the bivalent DNA vaccine not only reduced worm and egg burden, but also reduced the pathological lesions caused by Schistosoma infection.
Conclusions
The findings of the present study suggest that the bivalent membrane SjTsp2–29 DNA vaccine might be a promising strategy for immunoprophylaxis against S. japonicum and might also provide a basis for the development of an effective vaccine against Schistosoma in the future.
Footnotes
Source of support: This work was supported by the National Natural Science Foundation of China (#30901251), Grants for Scientific Research of BSKY from Anhui Medical University (XJ201321), and the Natural Science Foundation of Anhui Province of China (KJ2012A174 and KJ2017A691)
Availability of data and materials
The datasets used and analyzed are available from the corresponding author on reasonable request. The results of analysis of the datasets are presented as tables, figures, and supplementary information files.
References
- 1.Steinmann P, Keiser J, Bos RM, et al. Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux DH, Malecela MN. Neglected tropical diseases and the millennium development goals: why the “other diseases” matter: Reality versus rhetoric. Parasit Vectors. 2011;4:234. doi: 10.1186/1756-3305-4-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang LJ, Xu ZM, Dang H, et al. [Endemic status of schistosomiasis in People’s Republic of China in 2012]. Chinese Journal of Schistosomiasis Control. 2016;28:611–17. doi: 10.16250/j.32.1374.2020263. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 4.Oliveira SC, Fonseca CT, Cardoso FC, et al. Recent advances in vaccine research against schistosomiasis in Brazil. Acta Tropica. 2008;108:256–62. doi: 10.1016/j.actatropica.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 5.Pica-Mattoccia L, Doenhoff MJ, Valle C, et al. Genetic analysis of decreased praziquantel sensitivity in a laboratory strain of Schistosoma mansoni. Acta Tropica. 2009;11:82–85. doi: 10.1016/j.actatropica.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: Mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infect Dis. 2008;21:659–67. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- 7.Merrifield M, Hotez PJ, Beaumier CM, et al. Advancing a vaccine to prevent human schistosomiasis. Vaccine. 2016;34:2988–91. doi: 10.1016/j.vaccine.2016.03.079. [DOI] [PubMed] [Google Scholar]
- 8.Gray DJ, Li YS, Williams GM, et al. A multi-component integrated approach for the elimination of schistosomiasis in the People’s Republic of China: Design and baseline results of a 4-year cluster-randomised intervention trial. Int J Parasitol. 2014;44:659–68. doi: 10.1016/j.ijpara.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Mo AX, Agosti JM, Walson JL, et al. Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg. 2014;90:54–60. doi: 10.4269/ajtmh.13-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganley-Leal LM, Guarner J, Todd CW, et al. Comparison of Schistosoma mansoni irradiated cercariae and Sm23 DNA vaccines. Parasite Immunol. 2005;27:341–49. doi: 10.1111/j.1365-3024.2005.00785.x. [DOI] [PubMed] [Google Scholar]
- 11.Tran MH, Pearson MS, Bethony JM, et al. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–40. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 12.Cardoso FC, Macedo GC, Gava E, et al. Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection. PLoS Negl Trop Dis. 2008;2:e308. doi: 10.1371/journal.pntd.0000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carvalho GB, Pacífico LG, Pimenta DL, et al. Evaluation of the use of C-terminal part of the Schistosoma mansoni 200kDa tegumental protein in schistosomiasis diagnosis and vaccine formulation. Exp Parasitol. 2014;139:24–32. doi: 10.1016/j.exppara.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Martins VP, Morais SB, Pinheiro CS, et al. Sm10.3, a member of the micro-exon gene 4 (MEG-4) family, induces erythrocyte agglutination in vitro and partially protects vaccinated mice against Schistosoma mansoni infection. PLoS Negl Trop Dis. 2014;8:e2750. doi: 10.1371/journal.pntd.0002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–63. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Mulvenna J, Moertel L, Jones MK, et al. Exposed proteins of the Schistosoma japonicum tegument. Int J Parasitol. 2010;40:543–54. doi: 10.1016/j.ijpara.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME. New vaccines for neglected parasitic diseases and dengue. Transl Res. 2013;162:144–55. doi: 10.1016/j.trsl.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Zhang WN, Ren CP, et al. [Construction of DNA vaccine pcDNA3.1(+)/tetraspanin 2-A against Schistosoma japonicum and its immune-protective effect in mice]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009;27(6):534–36. [in Chinese] [PubMed] [Google Scholar]
- 19.Yuan C, Fu YJ, Li J, et al. Schistosoma japonicum: Efficient and rapid purification of the tetraspanin extracellular loop 2, a potential protective antigen against schistosomiasis in mammalian. Exp Parasitol. 2010;126:456–61. doi: 10.1016/j.exppara.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Ren CP, Liu M, Zhao ZR, et al. [Cloning, expression and preliminary identification of the coding genetic sequences of the 29 000 extra membranous protein from Schistosoma japonicum]. Chinese Journal of Laboratory Medicine. 2008;831:685–86. [in Chinese] [Google Scholar]
- 21.Li C, Yu L, Liu Z, et al. Schistosoma japonicum: The design and experimental evaluation of a multivalent DNA vaccine. Cell Mol Biol Lett. 2006;11:449–60. doi: 10.2478/s11658-006-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Liu HF, Lu MB, et al. Construction, purification, and evaluation of multivalent DNA vaccine against Schistosoma japonicum. Parasitol Res. 2011;108:115–21. doi: 10.1007/s00436-010-2040-6. [DOI] [PubMed] [Google Scholar]
- 23.Abán JL, Ramajo V, Arellano JL, et al. A fatty acid binding protein from Fasciola hepatica induced protection in C57/BL mice from challenge infection with Schistosoma bovis. Vet Parasitol. 1999;83(2):107–21. doi: 10.1016/s0304-4017(99)00053-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu M, Chen P, Büchele B, et al. A boswellic acid-containing extract attenuates hepatic granuloma in C57BL/6 mice infected with Schistosoma japonicum. Parasitol Res. 2013;112:1105–11. doi: 10.1007/s00436-012-3237-7. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca CT, Oliveira SC, Alves CC. Eliminating schistosomes through vaccination: what are the best immune weapons? Front Immunol. 2015;6:95. doi: 10.3389/fimmu.2015.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riveau G, Schacht AM, Dompnier JP, et al. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: A phase 3 randomized, controlled trial in Senegalese children. PLoS Negl Trop Dis. 2018;12:e0006968. doi: 10.1371/journal.pntd.0006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caldas IR, Campi-Azevedo AC, Oliveira LF, et al. Human schistosomiasis mansoni: Immune responses during acute and chronic phases of the infection. Acta Trop. 2008;108:109–17. doi: 10.1016/j.actatropica.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Egesa M, Lubyayi, Tukahebwa EM, et al. Schistosoma mansoni schistosomula antigens induce Th1/Pro-inflammatory cytokine responses. Parasite Immunol. 2018;40:e12592. doi: 10.1111/pim.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering DA, McSorley HJ, Bethony JM, et al. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis. 2012;6:e1564. doi: 10.1371/journal.pntd.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Li J, Duke M, et al. protective efficacy and marked polymorphism limits the value of Schistosoma japonicum tetraspanin-2 as a vaccine target. PLoS Negl Trop Dis. 2011;5:e1166. doi: 10.1371/journal.pntd.0001166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, Xu X, Qing X, Pan W. Identification and characterization of six novel tetraspanins from Schistosoma japonicum. Parasit Vectors. 2011;4:190. doi: 10.1186/1756-3305-4-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardoso FC, Pacífico RN, Mortara RA, Oliveira SC. Human antibody responses of patients living in endemic areas for schistosomiasis to the tegumental protein Sm29 identified through genomic studies. Clin Exp Immunol. 2006;144:382–91. doi: 10.1111/j.1365-2249.2006.03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinheiro CS, Ribeiro AP, Cardoso FC, et al. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP-2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014;36:303–12. doi: 10.1111/pim.12118. [DOI] [PubMed] [Google Scholar]
- 34.Ewaisha RE, Bahey-El-Din M, Mossallam SF, et al. Combination of the two schistosomal antigens Sm14 and Sm29 elicits significant protection against experimental Schistosoma mansoni infection. Exp Parasitol. 2014;145:51–60. doi: 10.1016/j.exppara.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Alves CC, Araujo N, Bernardes WPOS, et al. A Strong humoral immune response induced by a vaccine formulation containing rSm29 adsorbed to alum is associated with protection against Schistosoma mansoni reinfection in mice. Front Immunol. 2018;9:2488. doi: 10.3389/fimmu.2018.02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacifico LG, Fonseca CT, Chiari L, Oliveira SC. Immunization with Schistosoma mansoni 22.6 kDa antigen induces partial protection against experimental infection in a recombinant protein form but not as DNA vaccine. Immunobiology. 2006;211:97–104. doi: 10.1016/j.imbio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Garcia TC, Fonseca CT, Pacifico LG. Peptides containing T cell epitopes, derived from Sm14, but not from paramyosin, induce a Th1 type of immune response, reduction in liver pathology and partial protection against Schistosoma mansoni infection in mice. Acta Trop. 2008;106:162–67. doi: 10.1016/j.actatropica.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Pinho JM, Cardoso FC, Lopes DO. Immunization with SmIg, a novel tegument protein from Schistosoma mansoni, fails to induce protection in mice but reduces liver pathology. Parasitology. 2010;137:1079–88. doi: 10.1017/S0031182009991387. [DOI] [PubMed] [Google Scholar]
- 39.Jankovic D, Wynn TA, Kullberg MC, et al. Optimal vaccination against Schistosoma mansoni requires the induction of both B cell- and IFN-gamma-dependent effector mechanisms. J Immunol. 1999;162:345–51. [PubMed] [Google Scholar]
- 40.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]