Abstract
Objectives
To determine associations between farm- and flock-level antimicrobial usage (AMU), farm biosecurity status and the abundance of faecal antimicrobial resistance genes (ARGs) on broiler farms.
Methods
In the cross-sectional pan-European EFFORT study, conventional broiler farms were visited and faeces, AMU information and biosecurity records were collected. The resistomes of pooled faecal samples were determined by metagenomic analysis for 176 farms. A meta-analysis approach was used to relate total and class-specific ARGs (expressed as fragments per kb reference per million bacterial fragments, FPKM) to AMU (treatment incidence per DDD, TIDDDvet) per country and subsequently across all countries. In a similar way, the association between biosecurity status (Biocheck.UGent) and the resistome was explored.
Results
Sixty-six (38%) flocks did not report group treatments but showed a similar resistome composition and roughly similar ARG levels to antimicrobial-treated flocks. Nevertheless, we found significant positive associations between β-lactam, tetracycline, macrolide and lincosamide, trimethoprim and aminoglycoside antimicrobial flock treatments and ARG clusters conferring resistance to the same class. Similar associations were found with purchased products. In gene-level analysis for β-lactams and macrolides, lincosamides and streptogramins, a significant positive association was found with the most abundant gene clusters blaTEM and erm(B). Little evidence was found for associations with biosecurity.
Conclusions
The faecal microbiome in European broilers contains a high diversity of ARGs, even in the absence of current antimicrobial selection pressure. Despite this, the relative abundance of genes and the composition of the resistome is positively related to AMU in European broiler farms for several antimicrobial classes.
Introduction
Antimicrobial usage (AMU) is considered an important driver for the selection of antimicrobial resistance (AMR) in human, animal and environmental bacteria.1 AMR in pathogenic bacteria hampers treatment and results in increased healthcare costs.2 Next to human healthcare, one of the main users of antimicrobials is the intensive livestock industry. Resistance development in livestock is a great concern for the animal population and could be a source of bacteria transferring AMR to the human population.3
Broiler production is a major industry within livestock farming.4 Broilers are produced in a highly optimized way, characterized by a pyramidal structure consisting of a small number of pedigree and great-grandparent stock farms at the top of the pyramid and a large number of broiler farms at the bottom.5 Broilers are raised for consumption within 6–7 weeks on average, which results in over 10 million tons of chicken meat produced in 2014 in the EU.6 In these conditions, antimicrobials are regularly administered to the whole flock to prevent or control infectious diseases.7,8
In Europe, AMU in broiler production is mostly reported at country level and based on national sales-data monitoring systems. When national sales data are related to national AMR data, mostly based on MIC determinations for the bacterial indicator Escherichia coli, positive associations have been observed for several antimicrobial classes.1,9,10 Evidence beyond these ‘ecological’ associations is limited due to the absence of more detailed epidemiological data within countries and species at the farm level. Research at farm or even flock level enables analysis of such relationships in the same epidemiological unit and allows adjustment for potential confounding variables, which are generally not available for country-level analyses and might potentially lead to ecological fallacy.11,12 For broilers, only a few association studies have been performed at farm level. These studies provide evidence for a positive association between flock- or farm-level AMU and AMR in specific commensal or pathogenic bacteria.13,14
One possibly related (risk) factor or confounder that can be addressed with farm- or flock-level data is farm biosecurity. Farm biosecurity has been defined as the total of all measures taken to prevent both introduction and spread of infectious agents15 and thus represents a collection of many potential factors that might influence introduction and further spread of AMR.13,14,16
In this study, metagenomic shotgun sequencing is applied for the analysis of the resistome, in DNA from the total community of faecal bacteria. Metagenomic sequencing enables a broad, culture-independent and semi-quantitative reflection of resistance present in a broiler flock.17 The aim of this study was to determine the relationship between the broiler faecal resistome and farm- and flock-level usage of antimicrobials and farm biosecurity status in nine European countries.
Materials and methods
Study design
This cross-sectional study relates potential risk factors to the resistome of a pooled faecal sample from one flock of each broiler farm. In total, 181 flocks from 181 farms from nine European countries (Belgium, Bulgaria, Denmark, France, Germany, Italy, the Netherlands, Poland and Spain) were sampled. In this paper, samples from 176 of the 181 farms were included. Five samples were excluded due to errors made during processing or incomplete data. All farms have been anonymized to ensure that results cannot be traced back to individual farms. Country was anonymized as this was required by the farming organization in one participating country.
Selection of farms and sampling
In the nine collaborating countries, 20 non-mixed conventional broiler farms per country were visited between May 2014 and June 2016. Eligible farms needed to have, among other criteria, all-in all-out production (thinning from day 30 onwards allowed), no intended slaughter age higher than 50 days and no production of animals other than broilers. Further farm characteristics and (country-specific) deviations from the selection protocol can be found in the supplement of Munk et al.18 Per farm, 25 fresh faecal droppings from the floor from one flock (one batch) were collected, transported at 4°C and stored at −80°C within 24 hours. In this study a pooled sample of the 25 individual samples was used, resulting in one faecal pool per flock and per farm.
Laboratory analysis and bioinformatics analysis
After DNA extraction and metagenomic shotgun sequencing (Illumina HiSeq 3000, 50 million paired-end reads per sample), the cleaned reads were mapped to the ResFinder antimicrobial resistance gene (ARG) database (accessed 17 November 2016) of the Centre for Genomic Epidemiology.19 The output was clustered at an ARG sequence identity level of 90%. The unit of outcome is a normalized read count FPKM (fragments per kb reference per million bacterial fragments). FPKM was calculated by dividing the mapped resistance fragments by the length of the respective resistance gene and the total number of bacterial fragments per sample and multiplying by 109. In the analyses, the following outcomes per flock were used: (i) the sum of FPKM of all resistance gene clusters; (ii) the sum of FPKM per antimicrobial class; and (iii) the FPKM per 90% identity-level gene cluster for two antimicrobial classes.
More details on the laboratory analysis and metagenomic shotgun sequencing can be found in the Supplementary Methods (available as Supplementary data at JAC Online) and in Munk et al.18
Quantification of AMU and farm biosecurity
Information on AMU, biosecurity status and several other characteristics of the farm and flock was collected through a questionnaire by interviewing the farmer on the day of the visit. The quantification of AMU is described in detail by Joosten et al.7 Two measures of AMU have been derived: (i) antimicrobials administered via group treatment to the flock from which the samples were taken (during its lifespan until sampling close to the age of slaughter); and (ii) antimicrobials purchased for the whole farm (which may contain more flocks than the sampled flock) in the year before sampling. The treatment incidence (TI) of DDDs (TIDDDvet) was calculated by dividing the amount of antimicrobials administered or purchased by the dose multiplied by days at risk multiplied by kg of animal. TIDDDvet can be read as the percentage of the life of a broiler for which it is treated. In the analyses, the following explanatory variables were used: (i) the total sum of TIDDDvet per flock (group treatment data) or farm (purchase data); and (ii) the sum per antimicrobial class.
The questionnaire also contained items relevant for the calculation of the biosecurity score with the Biocheck.Ugent method.16 More details are provided in the Supplementary Methods. The biosecurity score is expressed as a value between 0 (no biosecurity measures are in place) and 100% (all biosecurity measures are in place and used). In the analyses, the external and internal biosecurity scores were tested as explanatory variables.
Data analysis
A country effect appears in both AMR and AMU data and has been described before.7,18 For example, the country of origin of the samples is significantly associated with the resistome and explains roughly 25% of the variation observed. To address this effect, considering the total number of farms per country included in the analysis (18 or 20), we used country-specific models as input for a random-effects meta-analysis (R package Metafor, DerSimonian-Laird heterogeneity estimator).20 Meta-analysis allows results to be obtained and visualized in a transparent way.21 Outcome and AMU data were log10 transformed because of skewness (1 was added to keep zeros), and the outcome was standardized (mean 0, SD 1). Thus, associations were first calculated with linear regression per country and subsequently a meta- or overall association was calculated across countries. Concurrent usage and observations of the corresponding resistance did not occur at all farms and occasionally not in each country, resulting in specific meta-analyses with data from fewer than nine countries. The analysis was performed stepwise: first the association between AMU and ARGs was calculated for each corresponding antimicrobial class (e.g. tetracycline resistance versus tetracycline use), followed by non-corresponding classes. Confounding by biosecurity status of the farm and sampling age of the broilers was tested. A sensitivity analysis was performed by calculating the association between corresponding ARGs and AMU as a binary variable, with 0 meaning no AMU reported at flock or farm level and 1 meaning (any) AMU reported at flock or farm level.
The same meta-analysis approach was used to test the association between ARGs and internal and external biosecurity status, with and without adjusting for AMU. For two antimicrobial groups that showed a robust association with corresponding ARGs, an additional analysis was performed to test which gene clusters drive the association with the respective antimicrobial class. Again, random-effects meta-analysis was used with individual gene clusters as the outcome and corresponding AMU as the explanatory variable.
To control for multiple testing we applied a false discovery rate (FDR) of 0.1, using the Benjamini–Hochberg procedure.22 This was done separately for the six analyses described above. All descriptive and statistical analyses were done in R (version 3.3.1).23 An explanation of the interpretability of our results can be found in the Supplementary Methods.
Data availability
The DNA sequences (reads) from 363 metagenomic samples from 359 herds are deposited in the European Nucleotide Archive under the project accession number PRJEB22062.
Results
Farms
The average flock size at setup over all nine countries was 27971 one-day-old chicks (Table 1). On average, the smallest flocks were sampled in country I (16413 one-day-old chicks) and the largest flocks in country C (35035 one-day-old chicks). Age of sampled flocks was 34 days on average, with the youngest broilers sampled in country C (26 days) and the oldest in country D (42 days). The average weight at slaughter of the broilers from the sampled farms was 2372 g (range: 1744 g in country E to 2693 g in country H).
Table 1.
General characteristics of the sampled farms and flocks by country and overall countries
| Country | Number of farms included in the analyses | Average number of broilers present at the farm |
Average number of rounds per year |
Number of broilers set up in the sampled barn |
Age of broilers during sampling (days) |
Average weight of broilers during sampling (g) |
Average weight of broilers at slaughter (g) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean |
median |
mean |
median |
mean |
median |
mean |
median |
mean |
median |
mean |
median |
||
| (range) | (range) | (range) | (range) | (range) | (range) | ||||||||
| A | 20 | 77322 | 80000 | 7 | 7 | 29952 | 28300 | 34 | 35 | 2042 | 2125 | 2529 | 2500 |
| (24530–180000) | (3–8) | (16500–46700) | (27–39) | (1300–2490) | (2385–2750) | ||||||||
| B | 18 | 106059 | 76600 | 8 | 8 | 29827 | 32750 | 31 | 33 | 1861 | 1900 | 2320 | 2400 |
| (17200–240000) | (7.3–8.5) | (17200–41400) | (19–40) | (1350–2700) | (1550–2700) | ||||||||
| C | 20 | 46255 | 35150 | 8 | 8 | 35035 | 34450 | 26 | 27 | 1361 | 1322 | 2236 | 2193 |
| (25000–144000) | (7–8.5) | (25000–53300) | (16–32) | (530–2000) | (2000–2800) | ||||||||
| D | 20 | 96390 | 60000 | 5 | 5 | 23398 | 20950 | 42 | 43 | 2440 | 2450 | 2645 | 2600 |
| (24000–400000) | (4–5.5) | (11340–50000) | (34–51) | (1600–3650) | (2100–3700) | ||||||||
| E | 20 | 54810 | 47500 | 6 | 6 | 30605 | 29580 | 31 | 29 | 1479 | 1214 | 1744 | 1625 |
| (21400–216000) | (3–9) | (21420–42886) | (21–48) | (575–3000) | (1300–2700) | ||||||||
| F | 20 | 108258 | 110000 | 7 | 7 | 30849 | 31200 | 36 | 36 | 1939 | 1940 | 2375 | 2388 |
| (32000–200000) | (6–8.5) | (17550–49700) | (29–42) | (1500–2500) | (2065–2721) | ||||||||
| G | 20 | 56135 | 41500 | 6 | 6 | 31473 | 33565 | 36 | 36 | 2009 | 2070 | 2528 | 2500 |
| (19000–150000) | (5–6) | (18500–41800) | (30–42) | (1300–2500) | (2050–2900) | ||||||||
| H | 20 | 41680 | 30210 | 5 | 6 | 23219 | 22440 | 36 | 37 | 1907 | 1900 | 2693 | 2750 |
| (20000–114141) | (2–6.5) | (14000–33864) | (22–44) | (859–2500) | (1750–3000) | ||||||||
| I | 18 | 54873 | 20000 | 6 | 6 | 16413 | 16725 | 31 | 29 | 1529 | 1645 | 2264 | 2200 |
| (8000–250000) | (4–7) | (8000–27000) | (19–54) | (730–2600) | (1950–2700) | ||||||||
| Overall | 176 | 71101 | 50000 | 6.35 | 6.15 | 27971 | 26500 | 33.8 | 34 | 1844 | 1850 | 2372 | 2450 |
| (8000–400000) | (2–9) | (8000–53300) | (19–54) | (575–3650) | (1300–3700) | ||||||||
Associations between usage and resistance
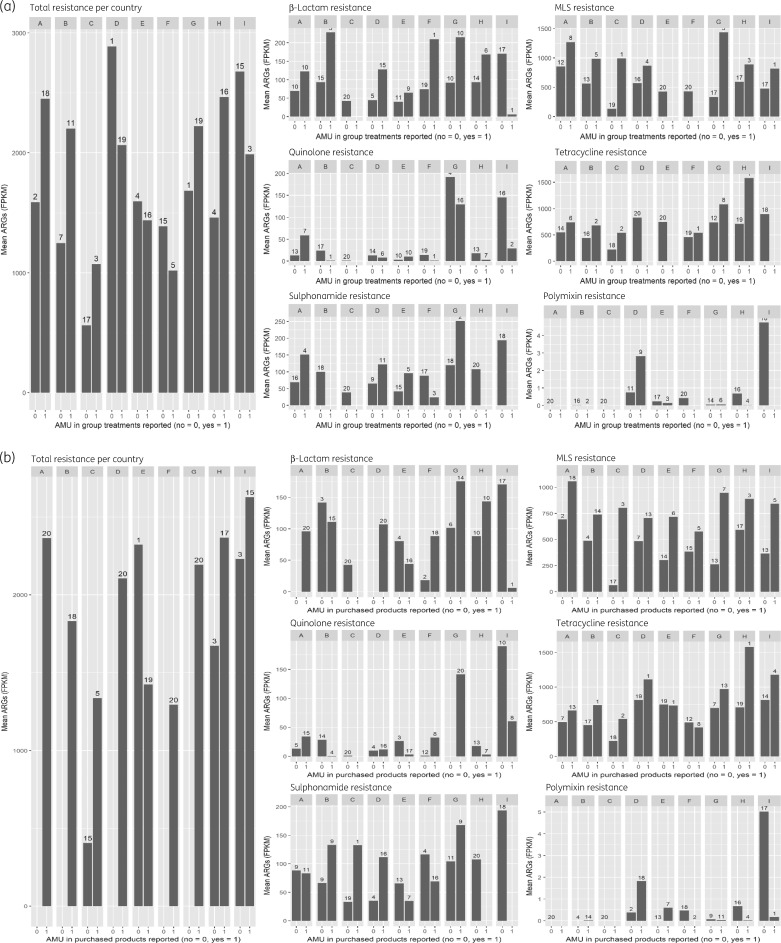
Of the 176 analysed flocks, 66 (38%) did not report any use of group treatments in the sampled flock up to the day of sampling. In total, 22 farms (13%) reported not to have purchased any antimicrobials in the year before sampling. Most of the non-users (47 of 66) were present in three countries (C, F and I) However, these untreated flocks showed similar diversity and only slightly reduced ARG clusters (overall mean of 1677 FPKM) compared with treated flocks (overall mean of 1880 FPKM) (Figure 1a and b).
Figure 1.
(a) Mean sum of ARGs in FPKM of farms that did or did not report AMU in group treatments for the sampled flock, grouped by country. Left: total ARGs versus total AMU per flock with number of farms shown above the bars. Right: ARGs of several (handpicked) antimicrobial classes/groups versus corresponding AMU per flock with number of farms shown above the bars. (b) Mean sum of ARGs in FPKM of farms that did or did not report AMU in purchased products by the whole farm in the year before sampling, grouped by country. Left: total ARGs versus total AMU per farm with number of farms shown above the bars. Right: ARGs of several (handpicked) antimicrobial classes/groups versus corresponding AMU per farm with number of farms shown above the bars.
Both possible confounders (farm biosecurity and sampling age of the broilers) were not significantly associated with the outcome and, when added to the models, estimates of the associations did not change more than 10%. Therefore, these variables were not included in the final and presented models. For discussion of our results we applied an FDR of 0.1; the FDR per comparison is given in all tables.
Although high levels of resistance were present in flocks or farms without AMU in the sampled rearing period, we did find associations between AMU and the corresponding ARGs (Table 2). Significant positive associations were found between flock group treatments and ARGs for MLS antibiotics (macrolides, lincosamides and streptogramins), tetracyclines, aminoglycosides, β-lactams (Figure 2a) and trimethoprim and their respective resistance. MLS antibiotics used included macrolide and lincosamide treatments. The MLS resistance group included macrolide, lincosamide and streptogramin gene clusters.
Table 2.
Results of meta-analysis between ARGs in FPKM and AMU as TIDDDvet of corresponding antimicrobial classes/groups
| Class/group of ARGs | Class/group of AMUa | Estimate | P value | FDR | 95% CI | Country and number of farms with reported AMU |
|---|---|---|---|---|---|---|
| MLS (macrolide, lincosamide, streptogramin) | MLS (macrolide, lincosamide) (group) | 1.18 | <0.001 | <0.001 | 0.74–1.62 | A-8, B-5, C-1, D-4, G-3, H-3, I-1 |
| Tetracycline | tetracycline (group) | 0.98 | <0.001 | <0.001 | 0.57–1.39 | A-6, B-2, C-2, F-1, G-8, H-1 |
| Trimethoprim | trimethoprim/sulphonamide (purchased) | 1.19 | 0.004 | 0.026 | 0.37–2.01 | A-11, B-9, C-1, D-16, E-7, F-16, G-9 |
| Tetracycline | tetracycline (purchased) | 0.83 | 0.005 | 0.026 | 0.25–1.41 | A-13, B-1, C-2, D-1, E-1, F-8, G-13, H-1, I-4 |
| Total | total (purchased) | 0.58 | 0.008 | 0.028 | 0.15–1.00 | A-20, B-18, C-5, D-20, E-19, F-20, G-20, H-17, I-15 |
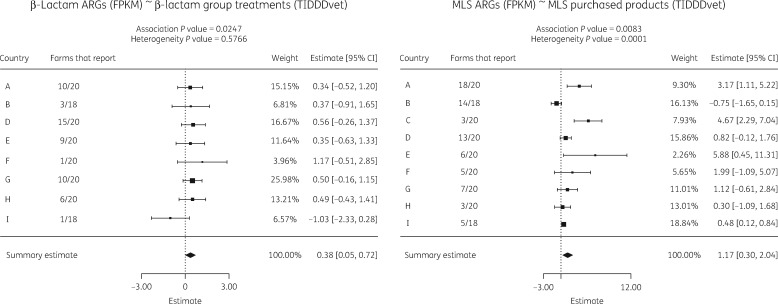
| MLS (macrolide, lincosamide, streptogramin) | MLS (macrolide, lincosamide) (purchased) | 1.17 | 0.008 | 0.028 | 0.30–2.04 | A-18, B-14, C-3, D-13, E-6, F-5, G-7, H-3, I-5 |
| Amphenicol | amphenicol (purchased) | 7.76 | 0.016 | 0.045 | 1.47–14.04 | D-2, G-2, I-1 |
| β-Lactam | β-lactam (group) | 0.38 | 0.025 | 0.058 | 0.05–0.72 | A-10, B-3, D-15, E-9, F-1, G-10, H-6, I-1 |
| Trimethoprim | trimethoprim/sulphonamide (group) | 0.86 | 0.026 | 0.058 | 0.10–1.61 | A-4, D-11, E-5, F-3, G-2 |
| Aminoglycoside | aminoglycoside (group) | 1.23 | 0.031 | 0.063 | 0.11–2.35 | G-2, H-7 |
| Aminoglycoside | aminoglycoside (purchased) | 1.04 | 0.047 | 0.086 | 0.01–2.07 | G-4, H-7 |
| Sulphonamide | trimethoprim/sulphonamide (purchased) | 0.72 | 0.106 | 0.177 | −0.15–1.59 | A-11, B-9, C-1, D-16, E-7, F-16, G-9 |
| Total | total (group) | 0.33 | 0.207 | 0.319 | −0.18–0.85 | A-18, B-11, C-3, D-19, E-16, F-5, G-19, H-16, I-3 |
| Sulphonamide | trimethoprim/sulphonamide (group) | 0.47 | 0.238 | 0.340 | −0.31–1.26 | A-4, D-11, E-5, F-3, G-2 |
| Amphenicol | amphenicol (group) | 1.10 | 0.266 | 0.354 | −0.84–3.04 | G-1 |
| β-Lactam | β-lactam (purchased) | 0.30 | 0.298 | 0.373 | −0.26–0.86 | A-20, B-15, D-20, E-16, F-18, G-14, H-10, I-1 |
| Polymyxin | polymyxin (purchased) | −0.07 | 0.668 | 0.786 | −0.38–0.24 | B-14, D-18, E-7, F-2, G-11, H-4, I-1 |
| Quinolone | quinolone (purchased) | 0.20 | 0.738 | 0.820 | −0.96–1.36 | A-15, B-4, D-16, E-17, F-8, G-20, H-7, I-8 |
| Quinolone | quinolone (group) | −0.02 | 0.915 | 0.964 | −0.41–0.36 | A-7, B-1, D-6, E-10, F-1, G-16, H-7, I-2 |
| Polymyxin | polymyxin (group) | 0.00 | 0.987 | 0.987 | −0.35–0.35 | B-2, D-9, E-3, G-6, H-4 |
Associations in bold have a false discovery rate <0.1.
(group) indicates AMU in group treatments of the sampled flock; (purchased) indicates AMU in purchased products by the farm in the year before sampling.
Figure 2.
Two example forest plots of the country-specific associations and meta-analysis results. Left: β-lactam ARGs in FPKM and β-lactam group treatments as TIDDDvet. Right: MLS ARGs (FPKM) and purchased MLS products (TIDDDvet). The number of farms that report AMU, the weight of the individual association in the summary estimate and the 95% CI per country are also shown. The summary estimates with confidence intervals for the overall association are shown at the bottom.
For AMU defined as products purchased by the whole farm, significant associations were found for total, MLS antibiotics (Figure 2b), tetracycline, amphenicol and trimethoprim products and their respective resistance. The analysis between corresponding resistance and AMU as a binary variable gave the same results as the analysis with AMU as a continuous variable except for the association with total purchased products (Table S1).
To investigate co- or cross-resistance, associations between total ARGs or ARGs per antimicrobial class and total and non-corresponding usage or purchased products were tested. After controlling for the FDR, none of these associations remained significant (Table S2).
The resistance reported per antimicrobial class is the sum of FPKM of many different resistance gene clusters. For most classes the contribution of single resistance gene clusters to the overall class-level ARG is highly skewed (with a few genes largely determining the sum of ARG per class) (Table 3). A detailed analysis was performed of the association between β-lactam group treatments and individual β-lactam resistance gene clusters and between purchased MLS products and MLS resistance gene clusters (Tables S3 and S4). Within both of these antimicrobial classes/groups we observed a significant positive association with the most abundant gene cluster [blaTEM and erm(B), respectively]. For β-lactam group treatments the only other significant positive association was with the blaACT cluster. For purchased MLS products we also saw significant positive associations with several different erm gene clusters and lsaA and mefB.
Table 3.
Ten most abundant gene clusters per antimicrobial class/group (which gave an overall significant association with AMU) and their contribution to the total sum of ARGs in percentages
| Rank | β-Lactam | % | MLS | % | Aminoglycoside | % | Tetracycline | % | Amphenicol | % | Trimethoprim | % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | bla TEM | 84.65 | erm(B) | 41.11 | aadA cluster 1 | 23.06 | tet(W) | 57.35 | cmx | 29.90 | dfrA1 | 47.50 |
| 2 | bla CMY–blaBIL–blaLAT | 2.43 | lnu(A) | 21.65 | spc | 12.73 | tet(A) | 8.67 | catpC194 | 23.49 | dfrD | 15.34 |
| 3 | bla SHV | 1.70 | lnu(C) | 17.97 | strB | 10.50 | tet(L) | 6.71 | cml | 16.25 | dfrK | 7.90 |
| 4 | bla OXA-61 | 1.67 | erm(C) | 3.80 | aadE 1 KF864551 | 9.78 | tet(M) | 6.06 | floR | 12.92 | dfrA12 | 7.14 |
| 5 | bla ACT-9 | 1.65 | erm(X) | 2.40 | strA | 9.27 | tet(Q) | 3.55 | catA1 | 5.26 | dfrA14 | 5.17 |
| 6 | bla ACT | 1.43 | vat(E) | 1.86 | aph3 III | 8.15 | tet(Z) | 2.94 | cat3 | 2.71 | dfrG | 4.35 |
| 7 | cfxA | 1.35 | erm(F) | 1.59 | ant6 Ia | 3.24 | tet(O) | 2.66 | cat2 | 1.64 | dfrA16 | 3.39 |
| 8 | bla CTX-M cluster 1 | 1.26 | lnu(B) | 1.49 | aac3 Iva | 3.08 | tet(4) | 1.92 | cat | 1.51 | dfrA7–dfrA17 | 3.25 |
| 9 | cepA | 0.59 | erm(G) | 0.95 | aph4 Ia | 2.98 | tet(B) | 1.75 | fexA | 1.17 | dfrA15 | 2.96 |
| 10 | mecA cluster 1 | 0.53 | lnu(F) | 0.91 | aadE 1 KF421157 | 2.98 | tet(33) | 1.51 | catpC221 | 1.06 | dfrA5–dfrA30 | 1.90 |
Biosecurity
We found two statistically significant associations between ARGs (analysed in total and per class) and internal or external biosecurity (Table S5). We observed a significant, positive association between oxazolidinone resistance and internal biosecurity (i.e. higher internal biosecurity is associated with more oxazolidinone resistance genes) and a significant, negative association between tetracycline resistance and internal biosecurity. After adjustment for AMU, only the former association remained.
Discussion
In this study we quantified resistance using the resistome of pooled faecal flock samples obtained by metagenomic analysis and related this to AMU data of the broiler flocks and farms from different countries. Our results confirm the hypothesis that higher antimicrobial exposure at flock or farm level is associated with more AMR.
Positive associations between AMU and ARGs
Our AMR and AMU data showed country-specific differences7,18 and therefore random-effects meta-analysis was used to test the relationship between ARGs and AMU. Almost all associations between ARGs and AMU of corresponding antimicrobial classes were positive. The abundance of genes coding for tetracycline, MLS, trimethoprim and aminoglycoside resistance was significantly positively related to the corresponding flock treatments and corresponding products purchased by the farm. Our data thus showed that current use in a flock is associated with a higher abundance of resistance genes in the same flock, although antimicrobial products from these classes have been used in broilers for a long time now. An increase in several tet genes and the use of chlortetracycline has also been shown by others.24 In the frequent—and expected—case that not all classes of antibiotics were used in a specific flock in its lifespan, when this occurred countrywide this country was not included in the meta-analysis. The results per antibiotic class were therefore often based on fewer than nine countries. β-Lactams, quinolones and polymyxins were the classes used most in this study.7 For quinolones and polymyxins, no significant relation was found with their corresponding ARGs. For polymyxins, this is probably due to relatively low gene abundances in the samples (Figure 1). For quinolones, usage is reported in almost all countries and an association with resistance has been described before (though not with metagenomic analysis).13,25 One likely reason for this lack of association is that quinolone resistance is partly due to point mutations that could not be detected sufficiently with the resistance gene database used here, and is difficult to detect in metagenomic studies altogether.26 The association between β-lactam resistance and use in the sampled flock was significant in our study. Within the class of β-lactam resistance, blaTEM turned out to be the gene cluster with the highest FPKM, in agreement with the significant positive association between blaTEM and β-lactam use in the flock. The blaTEM cluster is large: it includes >150 TEM-type β-lactamases,18 of which a large part have an ESBL- or inhibitor-resistant phenotype. Genes of special interest, such as blaCTX-M and blaCMY, were observed in these flocks. Probably due to the fact that these genes were restricted to relatively rare species and that usage of the respective antibiotics was low, we did not find significant associations. Within the MLS gene cluster, there were a number of genes that were significantly positively associated with MLS products purchased by the farm in the year before sampling, including not only the expected highly abundant gene clusters erm(B) and erm(C), but also less prevalent genes. All in all, we conclude that higher reported AMU is associated with higher relative gene abundance, while the resulting veterinary and public health implications are yet difficult to conclude upon.
Flock- versus farm-level AMU
Our results show a similar, but not identical, picture of associations between ARGs and usage at flock level (use in the sampled flock specifically) as for usage at farm level (purchased products over one year). Flock-level data are considered to be superior to data on purchased products if associations between AMU and AMR are thought to occur by selection in the actual flock. The overall correlation between TIDDDvet of group treatments and purchased products over one year is moderate (0.547) and data on these purchased products might resemble general and/or historic use by the farm and thereby give an additional perspective on the association between usage and resistance, which might also occur through recirculation of resistant bacteria within a farm from flock to flock. Moreover, the presence of residual amounts of antimicrobials might be sufficient to maintain the presence of resistant bacteria.
ARGs without current antimicrobial pressure
Overall, the observed positive associations between ARG and AMU were relatively weak, and the presence of many of the measured resistance genes seems not to be explained by current use. This can also be concluded from the roughly similar abundance of resistance genes in the flocks and farms that do not report any AMU. Several reasons for resistance genes being present without current antimicrobial pressure have been suggested in the literature. Roughly since the 1950s, increasing amounts and types of antimicrobials have been used in the livestock industry exerting selective pressures on the development and spread of AMR.27 Also, usage in other (higher) sections of the broiler pyramid might influence AMR in lower sections through vertical transmission.5,28
Once resistance genes are present at a farm, recirculation of resistance genes via the (farm) environment is possible.29,30 Furthermore, resistance gene carriage does not necessarily compromise microbial fitness, which makes presence of resistance genes in the absence of AMU pressure more likely.31,32 Taken together, the drivers for resistance genes to be present in poultry samples are diverse, complicating quantification of the associations between AMU and AMR. From these results, it can also be questioned to what extent resistance can be reduced only through reducing the use in specific flocks.
Biosecurity
External and internal biosecurity include all possible measures to minimize the introduction and spread of disease at the farm. Possibly, the introduction and spread of ARGs could also be influenced by these measures. No data exist on the association between internal and external biosecurity scores and AMR yet, but associations with a few individual measures have been reported.14,33 Within the EFFORT study, the same associations have been explored within European pig farming. This resulted in a positive association between internal biosecurity and macrolide gene clusters.21 Our analysis, after adjusting for AMU, resulted in one association: higher internal biosecurity was associated with a higher relative abundance of oxazolidinone ARGs. Oxazolidinone antibiotics are not used in broiler production though and we do not have an explanatory hypothesis for this specific association. Due to the limited degree of association overall, we conclude that our data are not sufficient to support the hypothesis that introduction and spread of ARGs is influenced by biosecurity measures.
Co- or cross-resistance
The analysis of the relationship between non-corresponding antimicrobial classes of resistance and use did not result in significant associations. The analysis was based on short metagenomics reads, which implies that the actual origin and genomic context is unknown, hindering focused searches for co- or cross-resistance within one species or genomic context. However, within the data generated in this study, the role of co- or cross-resistance is minor compared with usage of the corresponding class.
Strengths and limitations of the study
With 176 broiler flocks included in these analyses this is, to our knowledge, the largest metagenomic cross-country study that has been performed in poultry, which enabled us to look at the whole faecal resistome instead of specific resistance in specific bacteria. Despite this large number of samples, insufficient power might still be a reason for not detecting certain associations in our study. Although sampling was performed in nine countries, all information concerning AMU and biosecurity was collected in a harmonized way with the use of protocols and close collaboration between the researchers. Despite this, bias might be introduced by misclassification of biosecurity and underreporting of AMU. Bias might also be introduced by the DNA extraction procedure and the library preparation. It often favours certain bacteria, thereby biasing retrieved gene frequencies; however, this bias should be consistent across all samples and countries. The current selection of farms in each country is based on preset inclusion criteria and in agreement with local farming organizations, and partially also based on convenience (e.g. distances to farms). As a result, the sample of farms in each country cannot be considered representative for the livestock sector in that country.
With respect to the methodology, another limitation of the focus on similarity of short reads to known resistance genes is that the function of the assumed resistance genes can only be assigned with a certain probability and it is unknown whether their presence implies functional/expressed resistance. However, it has been shown that tetracycline resistance measurements in the same sample in cfu counting of aerobic bacteria and metagenomics do correlate significantly.34 Another limitation is the fact that due to the large but still limited sequencing depth relatively rare genes might be underrepresented in the results. Also, resistance genes from unculturable bacteria are probably underrepresented in the ResFinder database and therefore in our analysis.
Conclusions
This study applied metagenomics to establish associations between AMU and the resistome on European broiler farms. Clearly positive associations between corresponding AMU and resistance genes were observed. Significant results were shown for both flock-level and farm-level usage, highlighting that both actual and historic use can contribute to AMR presence. Our data did not support associations with ARGs and non-corresponding AMU or biosecurity status of the farm. We do, however, show that the faecal microbiome harbours many resistance genes in the absence of current AMU.
Supplementary Material
Acknowledgements
Parts of the results have been presented orally at the 5th International One Health Congress in Saskatoon, Canada, June 2018 (abstract number 4 AMR 02).
We thank all broiler-herd owners and their veterinarians who participated in this study, and all researchers of the EFFORT consortium for close collaboration, especially those involved in visiting and sampling the participating farms and laboratory work: M. Schlepers (Belgium); T. Ivanova, N. Cholakov, E. Gurova-Mehmedova and K. Penchev (Bulgaria); F. Nienhaus (Germany); C. L. Nielsen, P. Ryt-Hansen, B. Hølstad, B. Rasmussen and K. Nielsen (Denmark); C. Jenna, D. Virginie, E. Florent, E. Eric, S. Le Bouquin, L. Denis and T. Rodolphe (France); M. Gherpelli, M. Pegoraro and V. Carfora (Italy); D. de Vries (the Netherlands); and B. Gawlik, D. Krasucka and A. Hoszowski (Poland).
Members of the EFFORT consortium
Haitske Graveland (UUVM), Alieda van Essen (WBVR), Bruno Gonzalez-Zorn (UCM), Gabriel Moyano (UCM), Pascal Sanders (ANSES), Claire Chauvin (ANSES), Julie David (ANSES), Antonio Battisti (IZSLT), Andrea Caprioli (IZSLT), Thomas Blaha (TIHO), Katharina Wadepohl (TIHO), Maximiliane Brandt (TIHO), Tine Hald (DTU), Ana Sofia Ribeiro Duarte (DTU), Dariusz Wasyl (NVRI), Magdalena Skarzyńska (NVRI), Magdalena Zajac (NVRI), Hristo Daskalov (NDRVI), Helmut W. Saatkamp (BEC) and Katharina D.C. Stärk (SAFOSO).
Funding
This work was supported by the European Community’s Seventh Framework Program (FP7/2007–2013) under grant agreement number: 613754. (http://www.effort-against-amr.eu/).
Transparency declarations
None to declare.
Contributor Information
EFFORT consortium:
Haitske Graveland, Alieda vanEssen, Bruno Gonzalez-Zorn, Gabriel Moyano, Pascal Sanders, Claire Chauvin, Julie David, Antonio Battisti, Andrea Caprioli, Thomas Blaha, Katharina Wadepohl, Maximiliane Brandt, Tine Hald, Ana Sofia Ribeiro Duarte, Dariusz Wasyl, Magdalena Skarzyńska, Magdalena Zajac, Hristo Daskalov, Helmut W Saatkamp, and Katharina D C Stärk
References
- 1. Chantziaras I, Boyen F, Callens B. et al. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: a report on seven countries. J Antimicrob Chemother 2014; 69: 827–34. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Antimicrobial Resistance: Global Report on Surveillance 2014 2014. https://www.who.int/drugresistance/documents/surveillancereport/en/.
- 3. Tang KL, Caffrey NP, Nóbrega DB. et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. Lancet Planet Health 2017; 1: e316–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Medicines Agency ESoVAC. Sales of Veterinary Antimicrobial Agents in 30 European Countries in 2016 2018. https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-30-european-countries-2016-trends-2010-2016-eighth-esvac_en.pdf.
- 5. Dierikx CM, van der Goot JA, Smith HE. et al. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: a descriptive study. PLoS One 2013; 8: e79005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eurostat. Meat Production Statistics: Poultry Meat.2014. https://ec.europa.eu/eurostat/statistics-explained/index.php? title=Archive:Meat_production_statistics#Poultry_meat.
- 7. Joosten P, Sarrazin S, Van Gompel L. et al. Quantitative and qualitative analysis of antimicrobial usage at farm and flock level on 181 broiler farms in nine European countries. J Antimicrob Chemother 2019; 74: 798–806. [DOI] [PubMed] [Google Scholar]
- 8. Landoni MF, Albarellos G.. The use of antimicrobial agents in broiler chickens. Vet J 2015; 205: 21–7. [DOI] [PubMed] [Google Scholar]
- 9. Dorado-Garcia A, Mevius DJ, Jacobs JJ. et al. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in the Netherlands. J Antimicrob Chemother 2016; 71: 3607–19. [DOI] [PubMed] [Google Scholar]
- 10.ECDC, EFSA, EMA. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals. EFSA J 2017; 15: 4872.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sjolund M, Postma M, Collineau L. et al. Quantitative and qualitative antimicrobial usage patterns in farrow-to-finish pig herds in Belgium, France, Germany and Sweden. Prev Vet Med 2016; 130: 41–50. [DOI] [PubMed] [Google Scholar]
- 12. Magouras I, Carmo LP, Stärk KDC. et al. Antimicrobial usage and -resistance in livestock: where should we focus? Front Vet Sci 2017; 4: 148.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor NM, Wales AD, Ridley AM. et al. Farm level risk factors for fluoroquinolone resistance in E. coli and thermophilic Campylobacter spp. on poultry farms. Avian Pathol 2016; 45: 559–68. [DOI] [PubMed] [Google Scholar]
- 14. Persoons D, Haesebrouck F, Smet A. et al. Risk factors for ceftiofur resistance in Escherichia coli from Belgian broilers. Epidemiol Infect 2011; 139: 765–71. [DOI] [PubMed] [Google Scholar]
- 15. Barceló J, Marco E.. On farm biosecurity. In: Proceedings of the 15th International Pig Veterinary Society Congress, Birmingham, England, 1998. pp. 129–33. IPVS, Birmingham, UK. [Google Scholar]
- 16. Gelaude P, Schlepers M, Verlinden M. et al. Biocheck.UGent: a quantitative tool to measure biosecurity at broiler farms and the relationship with technical performances and antimicrobial use. Poult Sci 2014; 93: 2740–51. [DOI] [PubMed] [Google Scholar]
- 17. Bengtsson-Palme J, Larsson DGJ, Kristiansson E.. Using metagenomics to investigate human and environmental resistomes. J Antimicrob Chemother 2017; 72: 2690–703. [DOI] [PubMed] [Google Scholar]
- 18. Munk P, Knudsen BE, Lukjancenko O. et al. Abundance and diversity of the faecal resistome in slaughter pigs and broilers in nine European countries. Nat Microbiol 2018; 3: 898–908. [DOI] [PubMed] [Google Scholar]
- 19. Zankari E, Hasman H, Cosentino S. et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Viechtbauer W. Conducting meta-analyses in R with the Metafor package. J Stat Softw 2010; 36: 1–48. [Google Scholar]
- 21. Van Gompel L, Luiken REC, Sarrazin S. et al. The antimicrobial resistome in relation to antimicrobial use and biosecurity in pig farming, a metagenome-wide association study in nine European countries. J Antimicrob Chemother 2019; 74: 865–76. [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995; 57: 289–300. [Google Scholar]
- 23.R-Core-Team. R: A Language and Environment for Statistical Computing Vienna, Austria: R Foundation for Statistical Computing, 2017.
- 24. Xiong W, Wang Y, Sun Y. et al. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome 2018; 6: 34.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chantziaras I, Smet A, Haesebrouck F. et al. Studying the effect of administration route and treatment dose on the selection of enrofloxacin resistance in commensal Escherichia coli in broilers. J Antimicrob Chemother 2017; 72: 1991–2001. [DOI] [PubMed] [Google Scholar]
- 26. Ellington MJ, Ekelund O, Aarestrup FM. et al. The role of whole genome sequencing in antimicrobial susceptibility testing of bacteria: report from the EUCAST Subcommittee. Clin Microbiol Infect 2017; 23: 2–22. [DOI] [PubMed] [Google Scholar]
- 27. Davies J, Davies D.. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010; 74: 417–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Borjesson S, Guillard T, Landen A. et al. Introduction of quinolone resistant Escherichia coli to Swedish broiler population by imported breeding animals. Vet Microbiol 2016; 194: 74–8. [DOI] [PubMed] [Google Scholar]
- 29. Nilsson O, Borjesson S, Landen A. et al. Vertical transmission of Escherichia coli carrying plasmid-mediated AmpC (pAmpC) through the broiler production pyramid. J Antimicrob Chemother 2014; 69: 1497–500. [DOI] [PubMed] [Google Scholar]
- 30. Huijbers PM, Graat EA, van Hoek AH. et al. Transmission dynamics of extended-spectrum β-lactamase and AmpC β-lactamase-producing Escherichia coli in a broiler flock without antibiotic use. Prev Vet Med 2016; 131: 12–9. [DOI] [PubMed] [Google Scholar]
- 31. Holmes AH, Moore LSP, Sundsfjord A. et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016; 387: 176–87. [DOI] [PubMed] [Google Scholar]
- 32. Fischer EA, Dierikx CM, van Essen-Zandbergen A. et al. The IncI1 plasmid carrying the blaCTX-M-1 gene persists in in vitro culture of a Escherichia coli strain from broilers. BMC Microbiol 2014; 14: 77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mo SS, Kristoffersen AB, Sunde M. et al. Risk factors for occurrence of cephalosporin-resistant Escherichia coli in Norwegian broiler flocks. Prev Vet Med 2016; 130: 112–8. [DOI] [PubMed] [Google Scholar]
- 34. Munk P, Andersen VD, de Knegt L. et al. A sampling and metagenomic sequencing-based methodology for monitoring antimicrobial resistance in swine herds. J Antimicrob Chemother 2016; 72: 385–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DNA sequences (reads) from 363 metagenomic samples from 359 herds are deposited in the European Nucleotide Archive under the project accession number PRJEB22062.