Abstract
Background
Cataract is the leading cause of blindness in the world, and clinically significant astigmatism may affect up to approximately 20% of people undergoing cataract surgery. Pre‐existing astigmatism in people undergoing cataract surgery may be treated, among other techniques, by placing corneal incisions near the limbus (limbal relaxing incisions or LRIs) or by toric intraocular lens (IOLs) specially designed to reduce or treat the effect of corneal astigmatism on unaided visual acuity.
Objectives
To assess the effects of toric IOLs compared with LRIs in the management of astigmatism during phacoemulsification cataract surgery.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register; 2019, Issue 9); Ovid MEDLINE; Ovid Embase and four other databases. The date of the search was 27 September 2019.
Selection criteria
We included randomised controlled trials (RCTs) comparing toric IOLs with LRIs during phacoemulsification cataract surgery.
Data collection and analysis
We used standard methods expected by Cochrane. We graded the certainty of the evidence using GRADE. Our primary outcome was the proportion of participants with postoperative residual refractive astigmatism of less than 0.50 dioptres (D) six months or more after surgery. We also collected data on mean residual refractive astigmatism. Secondary outcomes included: uncorrected distance visual acuity, vision‐related quality of life, spectacle independence and adverse effects including postoperative lens rotation requiring re‐alignment. To supplement the main systematic review assessing the effects of toric IOLs compared with LRIs in the management of astigmatism during phacoemulsification cataract surgery, we sought to identify economic evaluations on the subject.
Main results
We identified 10 relevant studies including 517 people (626 eyes). These studies took place in China (three studies), UK (three), Brazil (one), India (one), Italy (one) and Spain (one). The median age of participants was 71 years. The level of corneal astigmatism specified in the inclusion criteria of these studies ranged from 0.75 D to 3 D. A variety of toric IOLs were used in these studies, in all but one study, these were monofocal. Studies used three different nomograms to determine the size and placement of the LRI. Two studies did not specify this. None of the studies were at low risk of bias in all domains, but two studies were at low risk of bias in all domains except selective outcome reporting, which was unclear. The remaining studies were at a mixture of low, unclear or high risk of bias.
People receiving toric IOLs were probably more likely to achieve a postoperative residual refractive astigmatism of less than 0.5 D six months or more after surgery (risk ratio (RR) 1.40, 95% confidence interval (CI) 1.10 to 1.78; 5 RCTs, 262 eyes). We judged this to be moderate‐certainty evidence, downgrading for risk of bias. In the included studies, approximately 500 eyes per 1000 achieved postoperative astigmatism less than 0.5 D in the LRI group compared with 700 per 1000 in the toric IOLs group. There was a small difference in residual astigmatism between the two groups, favouring toric IOLs (mean difference (MD) –0.32 D, 95% CI –0.48 to –0.15 D; 10 RCTs, 620 eyes). Although all studies favoured toric IOLs, the results of individual studies were inconsistent (range of effects –0.02 D to –0.71 D; I² = 89%). We considered this to be low‐certainty evidence, downgrading for risk of bias and inconsistency. People receiving a toric IOL probably have a small improvement in visual acuity at six months or more after surgery compared to people receiving LRI, but the difference is small and probably clinically insignificant (MD –0.04 logMAR, 95% CI –0.07 to –0.02; 8 RCTs, 474 eyes; moderate‐certainty evidence). Low‐certainty evidence from one study of 40 people suggested little difference in vision‐related quality of life measured using the Visual Function Index (VF‐14) (MD –3.01, 95% CI –8.56 to 2.54). Two studies reported spectacle independence and suggested that people receiving toric IOLs may be more likely to be spectacle independent (RR 1.56, 95% CI 1.14 to 2.15; 100 people; low‐certainty evidence). There were no cases of lens rotation requiring surgery (very low‐certainty evidence). Five studies (320 eyes) commented on a range of other adverse effects including corneal oedema, endophthalmitis and corneal ectasia. All these studies reported that there were no adverse events with the exception of one study (40 eyes) where one participant in the LRI group had a central de‐epithelisation which recovered over 10 days.
We found no economic studies that compared toric IOLs with LRIs.
Authors' conclusions
Toric IOLs probably provide a higher chance of achieving astigmatism within 0.5 D after cataract surgery compared with LRIs. There may be a small mean difference in postoperative astigmatism, favouring toric IOLs, but this difference is likely to be clinically unimportant. There was no evidence of an important difference in postoperative visual acuity or quality of life between the techniques. Evidence on adverse effects was uncertain. The apparent shortage of relevant economic evaluations indicates that economic evidence regarding the costs and consequence of these two procedures is currently lacking.
Plain language summary
Toric intraocular lenses versus limbal relaxing incisions for astigmatism in cataract surgery
What is the aim of this review? The aim of this Cochrane Review was to find out how toric intraocular lenses (IOLs) compare with limbal relaxing incisions (LRIs) for correcting astigmatism during cataract surgery. Cochrane researchers collected and analysed all relevant studies to answer this question and found 10 studies.
Key messages The review shows that toric IOLs probably provide a higher chance of a good outcome with respect to astigmatism after cataract surgery compared with LRIs. The difference in average astigmatism may be small and there may be little or no difference in vision or quality of life. There was a lack of evidence on which of these techniques represents best value for money.
What was studied in the review? As people get older, the lens within the eye can become cloudy: this is known as a cataract. Eye doctors can perform an operation to remove the cataract and replace it with a clear artificial IOL. The clear window at the front of the eye (the cornea) focuses light onto the ‘film’ at the back of the eye (the retina). The normal cornea is not perfectly dome‐shaped; it is commonly described as being shaped like a rugby ball. Because of this shape, the eye focuses light imperfectly onto the retina and this is known as astigmatism. It is measured in units called dioptres. In most eyes, astigmatism is slight and does not cause any symptoms. In some people, astigmatism is large enough to cause significant visual blurring. Usually this astigmatism is corrected by spectacles. However, during cataract surgery there are two possible ways of correcting the astigmatism, either by putting in a special "toric" lens, or by performing special incisions known as limbal relaxing incisions. Cataract surgery is a common operation and astigmatism is also a common condition. In order to achieve best possible vision after surgery for people with astigmatism it is important to understand the best way to correct it.
What are the main results of the review? Cochrane researchers found 10 relevant studies. These studies took place in China (three studies), UK (three), Brazil (one), India (one), Italy (one) and Spain (one). The studies compared toric IOLs with LRIs for people with astigmatism who were having cataract surgery.
Cochrane researchers assessed how certain the evidence is for each review finding. They looked for factors that can make the evidence less certain, such as problems with the way the studies were done, very small studies, and inconsistent findings across studies. They graded each finding as very low‐, low‐, moderate‐ or high‐certainty.
The review shows that:
⇒ People receiving toric IOLs were probably more likely to achieve a good outcome with respect to astigmatism (that means astigmatism of less than 0.5 dioptres) six months or more after surgery compared to people receiving LRIs (moderate‐certainty evidence). ⇒ On average, there may be a small difference in astigmatism between the two groups after surgery, favouring toric IOLs (low‐certainty evidence).
⇒ People receiving a toric IOL probably have a small improvement in visual acuity at six months or more after surgery compared to people receiving LRIs, but the difference is small and may be clinically unimportant (moderate‐certainty evidence).
⇒ There may be little difference in vision‐related quality of life (low‐certainty evidence).
⇒ People receiving toric IOLs may be more likely not to require spectacles to achieve their best distance vision compared with people receiving LRIs (low‐certainty evidence).
⇒ There was only very low‐certainty evidence on adverse effects.
⇒ Cochrane researchers found no economic studies that compared toric IOLs with LRIs.
How up‐to‐date is this review? Cochrane researchers searched for studies that had been published up to September 2019.
Summary of findings
Summary of findings for the main comparison. Toric intraocular lens (IOL) compared to limbal relaxing incisions (LRIs) for corneal astigmatism after cataract surgery (phacoemulsification).
| Toric IOL compared to LRIs for corneal astigmatism after cataract surgery (phacoemulsification) | ||||||
| Patient or population: people with astigmatism who are having cataract surgery (phacoemulsification) Setting: eye hospital Intervention: toric IOL Comparison: LRIs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Risk with LRIs | Risk with toric IOL | |||||
|
Postoperative residual refractive astigmatism of less than 0.50 D Follow‐up: ≥ 6 months |
500 per 1000 | 700 per 1000 (550 to 890) | RR 1.40 (1.10 to 1.78) | 262 eyes (5 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
|
Postoperative residual refractive astigmatism in dioptres Follow‐up: ≥ 6 months |
The mean postoperative residual refractive astigmatism ranged from 0.23 D to 1.23 D | MD 0.32 D less astigmatism (0.48 D less to 0.15 D less) | — | 620 eyes (10 RCTs) | ⊕⊕⊝⊝ Lowb | — |
|
Uncorrected postoperative distance visual acuity (logMAR) LogMAR score ranges from –1.3 to +1.3 with lower scores representing better vision (logMAR of 0 = 6/6 visual acuity) Follow‐up: ≥ 6 months |
The mean uncorrected postoperative distance visual acuity (logMAR) ranged from 0.09 to 0.336 logMAR | MD 0.04 logMAR lower (0.07 lower to 0.02 lower) | — | 474 eyes (8 RCTs) | ⊕⊕⊕⊝ Moderatea | — |
|
Spectacle independence for distance as reported by the participant Follow‐up: ≥ 6 months |
500 per 1000 | 780 per 1000 (570 to 1000) | RR 1.56 (1.14 to 2.15) | 100 people (2 RCTs) | ⊕⊕⊝⊝ Lowc | — |
|
Vision‐related quality of life measured using the VF‐14 index Scale 0–100: 0 = unable to do all applicable activities because of vision and 100 = able to do all applicable activities because of vision) Follow‐up: ≥ 6 months |
The mean vision‐related quality of life score was 93.7 | MD 3.01 lower (8.56 lower to 2.54 higher) | — | 40 people (1 RCT) | ⊕⊕⊝⊝ Lowd | — |
|
Adverse effects: postoperative lens rotation requiring second procedure to re‐align toric IOL Follow‐up: any time point |
There were no events. | 318 (5 RCTs) | ⊕⊝⊝⊝ Very lowe | — | ||
| Adverse effects: other | 6 studies commented on adverse effects including corneal oedema, endophthalmitis and corneal ectasia. All reported no events with the exception of 1 study (40 eyes) where 1 participant in the LRI group had a central de‐epithelialisation which recovered over 10 days and 1 study (70 eyes) where there was 1 case of dry eye in the LRI group which resolved after 3 months and 1 case of cystoid macular oedema in the toric IOL group which resolved after 3 months with medical treatment, and 1 case of posterior capsular opacification, which underwent Nd:YAG capsulotomy. | 410 (6 RCTs) | ⊕⊝⊝⊝ Very lowf | — | ||
| *The basis for the assumed risk is the pooled risk in the LRI groups in the included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IOL: intraocular lens; LRI: limbal relaxing incision; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for risk of bias (studies were poorly reported and some studies were at high risk of performance and detection bias). bDowngraded one level for risk of bias (studies were poorly reported and some studies were at high risk of performance and detection bias) and one level for serious inconsistency (study effect sizes ranged from –0.71 D to –0.02 D and I² = 87%). cDowngraded one level for risk of bias (both studies were poorly reported and at high risk of bias in one or more domain) and one level for publication bias (this outcome was only reported by 2/10 studies and unclear if it was collected by the other studies). dDowngraded one level for risk of bias (study was poorly reported) and one level for serious imprecision (confidence intervals included 0 and could not exclude important differences). eDowngraded one level for risk of bias (studies were poorly reported and some studies were at high risk of performance and detection bias) and two levels for very serious imprecision (studies were underpowered to address this rare event and there were no events). fDowngraded one level for risk of bias (studies were poorly reported and some studies were at high risk of performance and detection bias) and two levels for very serious imprecision (studies were underpowered to address adverse effects).
Background
Description of the condition
Cataract is the leading cause of treatable blindness and visual impairment (Congdon 2003; Pascolini 2012). The provision of cataract surgical service delivery is up part of the World Health Organization (WHO) vision 2020 strategy in the management of preventable blindness (WHO 2013). It has been estimated that successful management of cataracts would potentially avert over 3.5 million disability‐adjusted life years per year globally (Baltussen 2004). In modern cataract surgery, the cataractous natural lens can be removed through a microscopic incision, a technique known as phacoemulsification (Kelman 1967). An intraocular lens (IOL) is then placed within the natural lens capsule to restore the optics of the eye, simultaneously addressing pre‐existing refractive errors such as myopia (short sight) and hyperopia (long sight) (Hirnschall 2014; Visser 2013). Foldable IOLs made from silicone or acrylic material can be inserted through incisions as small as 1.8 mm (Kohnen 2009). These advances have led to increasing expectations of visual outcomes following cataract surgery, with many people wanting to be able to see at distance without spectacles. Consequently, there are now divergent approaches in the surgical management of a third refractive error: astigmatism (described below). Corneal astigmatism can be treated either by placing incisions in the cornea (limbal relaxing incisions (LRIs)) or by inserting specially designed IOLs (toric IOLs) that can cancel out pre‐existing astigmatism. Astigmatism is relatively common in people attending for cataract surgery. In one study of over 100,000 people undergoing cataract surgery in the UK, 78% had astigmatism of 0.5 dioptres (D) or more, 42% of 1 D or more and 21% of 1.5 D or more (Day 2019). Other studies have similar estimates (Ferrer‐Blasco 2009; Khan 2011; Lyall 2014).
Corneal astigmatism
The eye is formed anteriorly by a transparent dome called the cornea. The natural lens is an encapsulated structure suspended by ligaments posterior to the pupil. The eye can be likened to a photographic camera, with the cornea and lens functioning as a camera lens. The pupil can be compared to the diaphragm aperture. The inner layer of the eye, the retina, is analogous to the film. Refractive errors exist when the eye is not able to focus effectively on distant objects. The cornea is not normally perfectly spherical, being steepest in one meridian and flattest in the perpendicular meridian. A sufficiently large difference in the refractive power of each meridian can result in a blurry image, a condition known as astigmatism. Regular astigmatic errors can be neutralised by toric lenses, which work by optically cancelling out the refractive power of both the steep and flat meridians. Irregular astigmatism occurs when the steep and flat meridians are not perpendicular, and cannot be fully neutralised by a toric lens (de Freitas 2007; Nissman 2006; Saunders 1995; Weale 1983).
Refractive power is measured in dioptres. Corneal astigmatism can be assessed using different devices. Optical keratometry is a method used to evaluate corneal curvature at points on the front surface of the central cornea, while in corneal topography, illuminated rings (Placido rings) are projected onto the cornea to measure its curvature up to the periphery. Corneal tomography is a technique that can be used to assess the curvature of both the front and back surfaces of the cornea (Ferrer‐Blasco 2009; Hoffmann 2010; O'Brart 1994; Talamo 1991; Visser 2013). These investigations are important for planning surgical correction of astigmatism (de Freitas 2007; Hoffmann 2010).
Description of the intervention
Incisions across the steep meridian of the cornea have the effect of inducing flattening, thereby reducing corneal astigmatism. The size of this effect depends on the depth, length and position of the incision. LRIs are circumferential incisions placed at the edge of the cornea (limbus) at each end of the steep meridian, using a guarded blade with a preset depth to avoid inadvertent perforation (Arraes 2006; Hirnschall 2014; Kaufmann 2005). The length of the incisions are determined by a nomogram, depending on the extent of astigmatism the surgeon wishes to treat (Hirnschall 2014). It has become possible to perform these incisions with extreme precision using a femtosecond laser. Alternative surgical methods of modifying astigmatism include operating on the steep corneal axis, opposite clear corneal incisions and femtosecond intrastromal incisions. The treatment effects of these procedures are not the subject of this review.
Toric corrections can be incorporated into IOLs, allowing the treatment of corneal astigmatism in increments of 0.50 D or 0.75 D (Braga‐Mele 2014). One key aspect of toric IOL placement is alignment of the lens axis with the steep axis of the cornea. The first models of toric IOL had high incidences of postoperative rotation, but advances in IOL material and design have resulted in improved rotational stability (Visser 2013). Following insertion of the IOL into the capsule, it must be rotated to align with the steep corneal axis, which is identified from preoperative markings on the cornea (Sheppard 2013; Visser 2013). It has become possible to use image‐guided technology to indicate the steep axis to the surgeon without prior marking of the cornea (Schultz 2016).
How the intervention might work
LRIs produce a flattening effect that results in a concomitant steepening of the flatter meridian to produce a more spherical corneal shape, this effect has been termed coupling of the meridians. Toric IOLs incorporate different refractive powers along perpendicular meridians to optically neutralise the corresponding corneal axes (Visser 2013). It is commonly accepted that LRIs are more suitable for lower levels of astigmatism (less than 2 D), whereas toric IOLs may be more suitable for higher levels (more than 2 D).
Why it is important to do this review
Widespread advances in cataract surgery have raised expectations of visual results, with both surgeons and patients aiming for spectacle independence. Since astigmatism may affect quality of vision after cataract surgery, its treatment should be predictable and stable. LRIs and toric IOLs are two very different strategies. A systematic review of these techniques may help to produce evidence‐based guidelines for managing astigmatism. A review of the costs of the postsurgical corneal astigmatism found that costs were largely driven by spectacle costs (Anderson 2018). The cost ranged from USD 1786 to USD 4629 in 2017, so there are significant resource implications associated with the refractive outcomes of surgery.
Objectives
To assess the effects of toric IOLs compared with LRIs in the management of astigmatism during phacoemulsification cataract surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
We included trials in which participants had cataract associated with corneal astigmatism above 0.50 D. We excluded studies that included other associated ocular diseases such as keratoconus, retinal diseases, irregular astigmatisms, or a combination of these.
Types of interventions
We included trials that compared toric IOLs to LRIs. We only included studies where cataracts were extracted using phacoemulsification.
Types of outcome measures
We did not select studies on the basis of reporting of outcomes. Our prespecified time point was six months or more postoperative.
Primary outcomes
Proportion of participants with postoperative residual refractive astigmatism of less than 0.50 D.
Mean postoperative residual refractive astigmatism in dioptres.
Secondary outcomes
Mean postoperative uncorrected distance visual acuity (logMAR).
Spectacle independence for distance as reported by the participant.
Mean vision‐related quality of life.
Adverse effects
We reported any adverse effects present in the studies, for example, postoperative lens rotation requiring second procedure to re‐align the toric IOL.
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following electronic databases for RCTs and controlled clinical trials. An additional search was carried out on MEDLINE and Embase using economic search filters to specifically identify economic studies. There were no restrictions to language or year of publication. The date of the search was 27 September 2019.
Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 9, which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 27 September 2019; Appendix 1).
MEDLINE Ovid (1946 to 27 September 2019; Appendix 2).
MEDLINE Ovid – economic search (1946 to 27 September 2019; Appendix 3).
Embase Ovid (1980 to 27 September 2019; Appendix 4).
Embase Ovid – economic search (1980 to 27 September 2019; Appendix 5).
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 27 September 2019; Appendix 6).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 27 September 2019; Appendix 7).
WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 27 September 2019; Appendix 8).
Searching other resources
We searched the reference lists of the studies included in the review.
Data collection and analysis
Selection of studies
Two review authors (JCL, JE) independently screened the titles and abstracts resulting from the searches using web‐based software (Covidence). We resolved disagreements by discussion. Citations considered not relevant at this stage were not documented further in the review, but the number of these were recorded in a flow chart. We obtained full‐text copies of potentially relevant trials.
Two review authors (JCL, JE) independently assessed the full‐text copies for inclusion according to the Criteria for considering studies for this review. We resolved disagreements by discussion. We planned to correspond with investigators to clarify study eligibility, as appropriate. We were not masked to the names of the authors, institutions or journal publication when we selected studies.
We listed all studies excluded after full‐text review and provided a brief justification for exclusion in the Characteristics of excluded studies table.
For potentially eligible studies identified on trials' registers, we did the following.
If the study had a completion date more than two years previously, we looked for publications of this trial and contacted the investigators if necessary to obtain published or unpublished data from the trial. If eligible, the study was included in the review irrespective of whether we could identify a publication or not.
If the study had a completion date within two years, or in the future, we documented the study in the Characteristics of ongoing studies section.
One review author (AK) screened the economic search results.
Data extraction and management
Two review authors (JCL, JE) independently extracted data using an online form developed by Cochrane Eyes and Vision in Covidence, which we piloted before data extraction. We resolved discrepancies by discussion. We contacted trial investigators for missing data as required. One review author (JE) imported all data directly into Review Manager 5 (Review Manager 2014); a second review author (JCL) checked the accuracy of the data.
Study characteristics
We collected the following information on study characteristics.
Study design: parallel‐group RCT/within‐person RCT/one or both eyes reported.
Participants: country, total number of participants, age, sex, inclusion criteria and exclusion criteria.
Intervention and comparator details: including number of people (eyes) randomised to each group.
Primary and secondary outcomes as measured and reported in the trials; adverse events.
Length of follow‐up.
Date study conducted.
Funding and conflicts of interest.
Included on trials registry 'yes/no' including registration number if available.
Outcome data
We extracted the following data from each included study for intervention and comparator groups separately.
For continuous variables, such as residual refractive astigmatism, uncorrected distance visual acuity and quality of life: mean, standard deviation and number of participants on which outcome measured.
For dichotomous variables, such as postoperative residual refractive astigmatism of less than 0.50 D, spectacle independence and adverse events: number of people with the event and number of people on which outcome data collected.
For multi‐arm studies, we used data relevant to our intervention and comparator groups. If two groups contained relevant data, we combined the groups using the calculator within Review Manager 5 (Review Manager 2014). We also used Review Manager 5 to combine data when outcome data were only reported on stratified groups.
Data on refractive astigmatism were reported inconsistently with respect to whether it was defined as a negative or positive value. We extracted the absolute value only (i.e. we ignored the minus/plus sign). We checked that the direction of effect in our forest plot was consistent with the published paper for each study.
Assessment of risk of bias in included studies
Using Cochrane's 'Risk of bias' tool, two review authors (JCL, JE) independently assessed the risk of bias in each included study as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved disagreements by discussion.
We considered and reported on the following sources of bias.
Selection bias (random sequence generation, allocation concealment): was the sequence of allocation generated using a random procedure and was the allocation concealed to people recruiting/enrolling participants and to participants?
Performance bias (masking of participants and researchers): were the recipients of care unaware of their assigned intervention? Were people providing care unaware of the assigned intervention?
Detection bias (masking of outcome assessors). Were people evaluating outcomes unaware of the assigned intervention?
Attrition bias: were the rates of follow‐up and compliance similar in the groups? Was the analysis by intention‐to‐treat (ITT) and were there any post randomisation exclusions?
Selective outcome reporting bias: is there any evidence that the outcomes that were measured were not reported?
We graded each domain as low risk of bias, high risk of bias or unclear risk of bias (lack of information or uncertainty of potential for bias). We contacted trial investigators for clarification of parameters graded as 'unclear'.
Measures of treatment effect
We calculated the mean difference (MD) with 95% confidence intervals (CI) for the following continuous outcomes: residual refractive astigmatism in dioptres, uncorrected distance visual acuity and quality of life. Where possible, we checked for the skewness of continuous data (Altman 1996). We calculated the risk ratio (RR) with 95% CI for the following dichotomous outcomes: proportion of participants with postoperative residual refractive astigmatism of less than 0.50 D and spectacle independence for distance as reported by the participant.
Unit of analysis issues
Eyes and people
Trials may randomise one or both eyes to the intervention or comparator. If people are randomly allocated to treatment but only one eye per person is included in the trial then there will be no unit of analysis issue. In these cases, we documented how the eye was selected. If people were randomly allocated to treatment but both eyes were included and reported, we planned to analyse as 'clustered data' (i.e. adjust for within‐person correlation). If the study was a within‐person study (i.e. one eye was randomly allocated to intervention and the other eye received the comparator), then we planned to analyse as paired data. We contacted investigators for further clarification as needed.
Dealing with missing data
We planned to conduct an ITT analysis where possible, using imputed data if computed by the trial investigators using an appropriate method. We did not impute missing data ourselves. As ITT data were not available, we did an 'available case' analysis. This assumes that data are missing at random. We assessed whether this assumption was reasonable by collecting data from each included trial on the number of participants excluded or lost to follow‐up and reasons for loss to follow‐up by treatment group, if reported.
Assessment of heterogeneity
We examined the overall characteristics of the studies, in particular the type of participants and types of interventions, to assess the extent to which the studies were similar enough to make pooling study results sensible. We examined the forest plots of study results to see how consistent the results of the studies were, in particular the size and direction of effects.
We calculated the I² statistic, which is the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) (Higgins 2002). We considered I² values over 50% to indicate substantial inconsistency but also considered Chi² P values. As the Chi² test may have low power when the number of studies are few, we considered P less than 0.1 to indicate statistical significance of the Chi² test.
Assessment of reporting biases
We used the Cochrane 'Risk of bias' tool to assess selective or incomplete reporting (see Assessment of risk of bias in included studies). When there were 10 trials or more included in a meta‐analysis, we constructed funnel plots and considered tests for asymmetry for assessment of publication bias, according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
Data synthesis
We decided in advance that we would pool data using a random‐effects model. However, in cases where the data were sparse (e.g. fewer than three studies contributing to a model), we judged that a random‐effects model would not provide a robust estimate and we used a fixed‐effect model (Deeks 2019).
If there was inconsistency between individual study results such that a pooled result was probably not a good summary of the individual trial results (e.g. the effects were in different directions or the I² statistic was greater than 50% and P was less than 0.1), we did not pool the data but described the pattern of the individual study results.
If there was statistical heterogeneity but all the effect estimates were in the same direction such that a pooled estimate would seem to provide a good summary of the individual trial results, we provided a summary estimate.
Subgroup analysis and investigation of heterogeneity
We did not do any subgroup analyses. See Differences between protocol and review for analyses planned in the protocol.
Sensitivity analysis
We compared fixed‐effect and random‐effects models for our primary outcomes, as planned in our protocol (Lake 2017). We did not do any of the other planned sensitivity analyses (see Differences between protocol and review).
We examined the effect of excluding studies with unit of analysis errors. This analysis was not planned in our protocol (Lake 2017; see Differences between protocol and review).
Brief economic commentary
To report the current economic evidence base, one review author (AK) prepared a brief economic commentary based on current methods guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Shemilt 2019). We planned to summarise the availability and principal findings of full economic evaluations (cost‐effectiveness analyses, cost‐utility analyses, cost‐benefit analyses) that compare toric IOLs and LRIs for the management of astigmatism. This commentary was also planned to focus on the extent to which principal findings of eligible economic evaluations indicate that an intervention might be judged favourably (or unfavourably) from an economic perspective, when implemented in different settings.
Summary of findings and assessment of the certainty of the evidence
We prepared a 'Summary of findings' table presenting relative and absolute risks. Two review authors (JE, JCL working together) graded the overall certainty of the evidence for each outcome using the GRADE classification (GRADEpro 2015).
Results
Description of studies
Results of the search
The electronic searches yielded 1043 records (Figure 1). After removal of 336 duplicates, we screened the remaining 707 records. We obtained the full‐text reports of 29 records for further assessment. We included 12 reports of 10 studies: see Characteristics of included studies for details. We excluded 16 reports of 15 studies: see Characteristics of excluded studies table. We identified one ongoing study that met the inclusion criteria and this will be assessed for inclusion in the review when data become available (NCT03633851).
1.
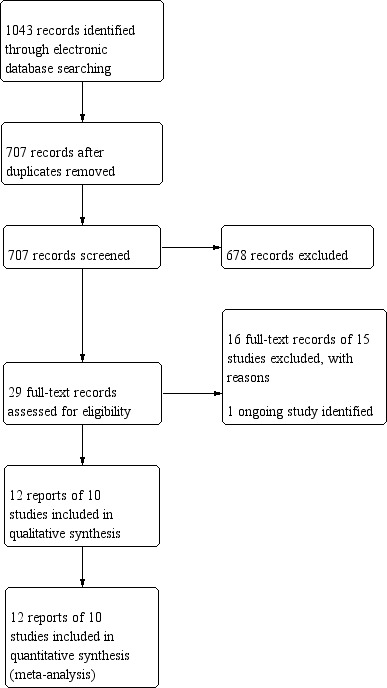
Study flow diagram.
Brief economic commentary searches
To supplement the main systematic review assessing the effects of toric IOLs compared with LRIs in the management of astigmatism during phacoemulsification cataract surgery, we sought to identify economic evaluations on the subject. Searching for economic studies on MEDLINE, Embase and DARE, NHS EED and HTA on the CRD database identified 43 records. After removal of 19 duplicates, one review author (AK) screened the remaining 24 records. No economic studies were identified that compared toric IOLs with LRIs.
Included studies
We identified 10 relevant studies. These studies took place in China (three studies), UK (three), Brazil (one), India (one), Italy (one) and Spain (one).
Types of studies
Eight studies randomised people to treatment; two studies were within‐person studies and randomly allocated eyes to treatment (Gangwani 2014; Hirnschall 2014). Neither of these two within‐person studies reported an appropriate paired analysis.
Of the eight studies that randomly allocated people to treatment, six studies included one eye per person in the study (Lam 2016; Leon 2015; Liu 2014; Mingo‐Botin 2010; Nanavaty 2017; Titiyal 2014). In five of these studies, it was not clear how the study eye was selected. In one study, when both eyes fulfilled the inclusion criteria, the right eye was selected for analysis (Liu 2014).
Of the eight studies that randomly allocated people to treatment, two studies included one or both eyes per person in the analysis (Dong 2015; Freitas 2014). Neither of these studies reported an appropriate analysis (i.e. adjusted for within‐person correlation).
Types of participants
See Table 2.
1. Characteristics of participants.
| Study | Country | Number of people (eyes) | Mean age in years (range) | % Men | Level of corneal astigmatism in inclusion criteria in dioptres | Mean preoperative corneal astigmatism in dioptres | Mean preoperative astigmatism in dioptres |
| Dong 2015 | China | 66 (84) | 72 (60–79) | 55% | 1.5–4.0 | NR | NR |
| Freitas 2014 | Brazil | 31 (62) | 69 (51–84) | 39% | 0.75–2.50 | 1.36 (range 0.75–2.5) | NR |
| Gangwani 2014 | UK | 30 (60)a | 75 (NR) | 48% | 0.75–2.50 | 1.75 (max 2.58) | NR |
| Hirnschall 2014 | UK | 30 (60)a | 71 (NR) | 43% | 1.0–2.5 | 1.57 (SD 0.44) | NR |
| Lam 2016 | China (Hong Kong) | 60 (60) | 66 (NR) | 42% | ≤ 3.0 | –1.31 | 1.66 |
| Leon 2015 | Italy | 102 (102) | 70 (53–88) | 47% | 1.0 to 2.0 | 1.75 | 1.75 |
| Liu 2014 | China | 54 (54) | 70 (NR) | 50% | 0.75–2.5 | NR | 1.58 |
| Mingo‐Botin 2010 | Spain | 40 (40) | 74 (44–90) | NR | 1.00–3.00 | 1.78 | 2.03 |
| Nanavaty 2017 | UK | 70 (70) | 74 (50–89) | 43% | > 0.75 to < 2.5 | 1.26 | NR |
| Titiyal 2014 | India | 34 (34) | 61 (NR) | NR | 1.25–3.0 | 2.10 | 1.95 |
| Total | — | 517 (626) | Median 71 | Median 45% | — | — | — |
aWithin‐person study. max: maximum; NR: not reported; SD: standard deviation.
The studies included 517 people (626 eyes). The median age of participants was 71 years. The level of corneal astigmatism specified in the inclusion criteria of these studies ranged from 0.75 D to 3 D.
Types of interventions and comparators
See Table 3.
2. Characteristics of interventions and comparators.
| Study | Type of toric IOL | Type of non‐toric IOL | Details of LRI | Nomogram |
| Dong 2015 | Tecnis Toric (Abbott Medical Optics) | Spherical IOL, unspecified | Conventional temporal clarity cornea incision phacoemulsification and spherical IOL implantation combined with LRIs on steep axial position. | Not specified |
| Freitas 2014 | AcrySof Toric (Alcon) | AcrySof Natural (Alcon) | LRIs were placed inside the limbus using a calibrated diamond knife with a preset blade depth of 600 μm. | Donnenfeld |
| Gangwani 2014 | M‐Flex multifocal toric (Rayner Intraocular Lenses) | M‐Flex multifocal non‐toric (Rayner Intraocular Lenses) | Both surgeons used an identical 600 μm guided steel blade (Micro Feather ophthalmic scalpel with aluminium handle, No: 7360G, Feather Safety Razor Co., Ltd.). The depth of the PCRIs was 600 μm. | Donnenfeld |
| Hirnschall 2014 | T‐Flex toric (Rayner Intraocular Lenses) | C‐Flex or Superflex non‐toric (Rayner Intraocular Lenses) | A CCI on the steep meridian was preferred and combined with a single opposite PCRI. In cases in which a CCI on the steep meridian was awkward, such as in cases of superonasal incisions in deep‐set eyes, a temporal incision combined with 2 PCRIs was created. In all cases, PCRIs were made at the end of surgery using a 600 μm guided steel blade (Micro Feather ophthalmic scalpel with aluminium handle No: 7360G, Feather Safety Razor Co., Ltd.) | Donnenfeld |
| Lam 2016 | Tecnis toric ZCT150, ZCT225 and ZCT300 (Abbott Medical Optics) | Tecnis monofocal ZCB00 (Abbott Medical Optics) | The LRI incision was made before the commencement of phacoemulsification using a 600 μm Accutome guarded diamond knife. | Nichamin Age and Pachymetry Adjusted nomogram |
| Leon 2015 | AcrySof IQ toric (Alcon) | AcrySof IQ Aspheric monofocal (Alcon) | Based on the procedure described by Langerman, a vertical limbal relaxing wound was created with a guarded micrometer diamond blade by making a groove concentric to the limbus. The incision depth was set at 600 μm equal to approximately 85% of the peripheral corneal thickness at the axis to be cut and the incisions were approximately a length of 3 mm. After the paired incision was made, the penetrating CCI was made along the steepest axis in the upper area for the cataract surgery, along the same axis as the LRI. | Nichamin Age and Pachymetry Adjusted nomogram |
| Liu 2014 | AcrySof toric (Alcon) | AcrySof (Alcon) | The incisions were as deep as 80–90% of the corneal thickness and were located in the peripheral cornea of the steep axis. | Gills and Gayon's |
| Mingo‐Botin 2010 | AcrySof Toric (Alcon) | AcrySof Natural (Alcon) | PCRIs were created inside the limbus using a calibrated diamond knife with the blade depth set at 600 μm. In eyes with against‐the‐rule astigmatism, the main incision for phacoemulsification was created to match the location of the LRI. At the end of surgery, the PCRIs were performed including the previous incision, and a paired incision was made on the opposite side. In eyes with with‐the‐rule astigmatism, both PCRIs were created on the steep axis before the globe was entered at the beginning of the procedure. | Nichamin Age and Pachymetry Adjusted nomogram |
| Nanavaty 2017 | T‐Flex (Rayner Intraocular Lenses) | Rayner C‐flex (Rayner Intraocular Lenses) | A single or double PCRI was placed on the limbus after draping and before starting cataract surgery using the recommendation from www.LRIcalculator.com and using 0‐ and 180‐degree ink marks as a reference and using the surgically induced astigmatism of 0.5 D at 120 degrees. After the participant had been draped, a standard 600 μm disposable PCRI blade (Feather, Osaka, Japan) was used to perform the PCRIs in all cases before any incisions for cataract surgery. | Donnenfeld |
| Titiyal 2014 | AcrySof IQ Toric IOL (Alcon) | AcrySof IQ Aspheric IOL (Alcon) | A 12‐blade radial keratotomy marker was placed on the 7.0 mm optical zone mark. Paired arcuate keratotomy incisions were made in the 7.0 mm optical zone. The 30‐degree paired arcuate keratotomy cuts were made on the steeper meridian using a micrometer‐guided diamond knife (Meyco) that was set at 100% of the thinnest paracentral pachymetry. | Nomogram not specified. |
CCI: clear corneal incision; IOL: intraocular lens; LRI: limbal relaxing incision; PCRI: peripheral corneal relaxing incision.
The studies used a variety of different toric IOLs.
Tecnis Toric IOL (Abbott Medical Optics) (Dong 2015; Lam 2016).
AcrySof Toric (Alcon) (Freitas 2014; Leon 2015; Liu 2014; Mingo‐Botin 2010; Titiyal 2014).
M‐Flex or T‐flex (Rayner Intraocular lenses) (Gangwani 2014; Hirnschall 2014; Nanavaty 2017).
In the LRI group, studies used non‐toric lenses from the same manufacturers as the toric IOLs.
Tecnis monofocal non‐toric IOL (Abbott Medical Optics) (Dong 2015; Lam 2016).
AcrySof Natural or aspheric IQ (Alcon) (Freitas 2014; Leon 2015; Liu 2014; Mingo‐Botin 2010; Titiyal 2014).
C‐Flex or Superflex (Rayner Intraocular lenses) (Gangwani 2014; Hirnschall 2014; Nanavaty 2017).
Types of outcomes
All studies measured and reported distance visual acuity, both uncorrected and best‐corrected, or spectacle‐corrected, and also provided some measures of either corneal or refractive astigmatism. Other outcomes were included but these were more variably reported:
contrast sensitivity (Gangwani 2014; Lam 2016; Mingo‐Botin 2010);
near vision (Gangwani 2014; Nanavaty 2017);
quality of life or participant satisfaction, or both (Gangwani 2014; Mingo‐Botin 2010; Nanavaty 2017);
need for spectacles (Lam 2016);
endothelial cell count or density (Lam 2016; Titiyal 2014);
intraocular pressure (Liu 2014).
The most commonly reported adverse event was IOL rotation. One study reported glare and halos, but not by treatment group (Gangwani 2014). Otherwise adverse effects were inconsistently reported, but some studies mentioned lack of adverse events such as:
intraoperative or postoperative complications, or both (Liu 2014; Mingo‐Botin 2010);
cystoid macular oedema (Nanavaty 2017);
posterior capsule opacification (Nanavaty 2017);
dry eye (Nanavaty 2017);
corneal ectasia (Titiyal 2014);
hyperopic shift (Titiyal 2014);
unspecified (Lam 2016);
persistent corneal oedema (Freitas 2014);
pupillary block (Freitas 2014);
retinal detachment (Freitas 2014);
endophthalmitis (Freitas 2014).
Follow‐up ranged from one month to 12 months. Only one study reported data at 12 months (Nanavaty 2017).
Excluded studies
We excluded 16 studies (Characteristics of excluded studies table). In all cases it was because the study did not consider either toric IOLs or LRIs. In one study, although both intervention and comparator were evaluated, they were not randomly allocated (Solomon 2019).
Risk of bias in included studies
Risk of bias is summarised in Figure 2 and Figure 3.
2.
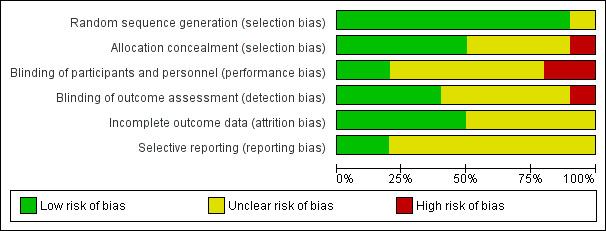
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
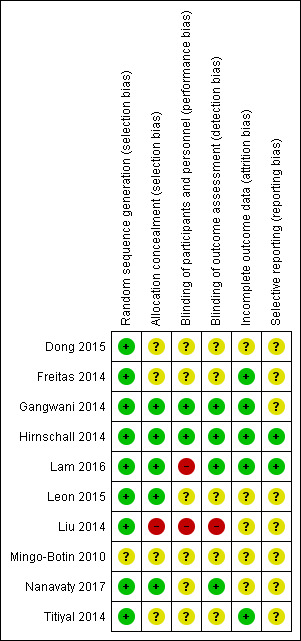
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged that the allocation sequence had been generated using an adequate method in nine out of the 10 included studies. In Mingo‐Botin 2010, the published report did not provide enough information to judge this adequately. We contacted study authors for clarification but received no reply.
Five studies specific allocation concealment (Gangwani 2014; Hirnschall 2014; Lam 2016; Leon 2015; Nanavaty 2017). In one study, Liu 2014, we judged the allocation was probably unconcealed because concealment was not described and the random number table involved use of odd/even numbers which suggested it was not concealed. For the other four studies, we judged the risk of bias as unclear and sought clarification from the investigators but received no replies.
Blinding
Two studies described adequate methods to mask participants, personnel and outcome assessors (Gangwani 2014; Hirnschall 2014). Two studies were not masked and so we initially judged both studies at high risk of performance and detection bias (Lam 2016; Liu 2014). Lam 2016 subsequently clarified by correspondence that the outcome assessors were masked. Nanavaty 2017 described masking outcome assessors but not participants and personnel. We sought clarification from the other investigators but received no replies.
Incomplete outcome data
We assessed five studies at low risk of attrition bias (Freitas 2014; Gangwani 2014; Hirnschall 2014; Lam 2016; Titiyal 2014). In the other five studies, there was insufficient information in the report and we sought more information from investigators, but received no replies.
Selective reporting
We did not have access to the registered protocols and trials registry entries. We asked investigators for clarification. Lam 2016 and Hirnschall 2014 responded confirming that all outcomes were reported.
Effects of interventions
See: Table 1
Primary outcomes
Proportion of participants with postoperative residual refractive astigmatism of less than 0.5 D
People receiving a toric IOL were more likely to achieve residual refractive astigmatism of less than 0.5 D at six months or more after surgery (RR 1.40, 95% CI 1.10 to 1.78; 5 studies, 262 eyes; Analysis 1.1; Figure 4). Two of the included studies had a unit of analysis error (Freitas 2014; Hirnschall 2014). Excluding these studies in sensitivity analysis did not appreciably change the estimate of effect but did reduce its precision (RR 1.44, 95% CI 0.89 to 2.34; 144 eyes; data not shown). We judged this to be moderate‐certainty evidence, downgrading one level for risk of bias (the studies were poorly reported and some studies were at high risk of performance and detection bias). The results suggest that after cataract surgery with LRIs, approximately 500 per 1000 people will achieve residual refractive astigmatism of less than 0.5 D at six months or more after surgery compared with 700 per 1000 people receiving toric IOLs (95% CI 550 per 1000 to 890 per 1000). Results from a sensitivity analysis using a fixed‐effect model provided similar results (RR 1.36, 95% CI 1.14 to 1.62).
1.1. Analysis.
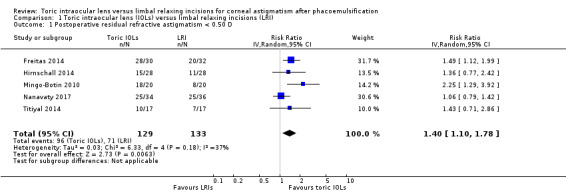
Comparison 1 Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI), Outcome 1 Postoperative residual refractive astigmatism < 0.50 D.
4.
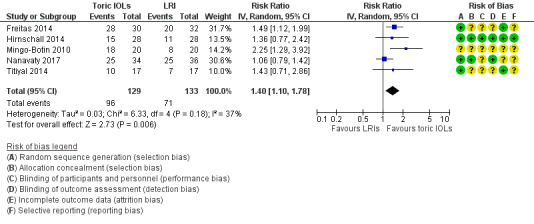
Only half of the included studies reported our primary outcome in the way that we had prespecified it. All the studies reported a measure of residual refractive astigmatism as a continuous variable. We made the post‐hoc decision to collect data in this format as well.
Mean postoperative residual refractive astigmatism
Analysis 1.2 and Figure 5 shows the postoperative residual refractive astigmatism at six months or more after surgery. People receiving a toric IOL on average had a small reduced astigmatism (MD –0.32 D, 95% CI –0.48 to –0.15; 10 studies, 620 eyes). However, there was high unexplained heterogeneity: study results ranged from –0.71 D (Mingo‐Botin 2010) to –0.70 D (Leon 2015) to –0.02 D (Nanavaty 2017) (I² = 87%; P < 0.00001). We excluded four studies with unit of analysis errors (Dong 2015; Freitas 2014; Gangwani 2014; Hirnschall 2014), which did not change materially the estimate of effect (MD –0.37, 95% CI –0.62 to –0.12; I² = 89%; 360 eyes; data not shown). We judged this low‐certainty evidence downgrading one level for risk of bias and one level for serious inconsistency.
1.2. Analysis.
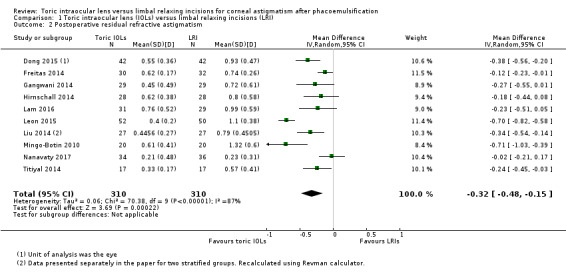
Comparison 1 Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI), Outcome 2 Postoperative residual refractive astigmatism.
5.
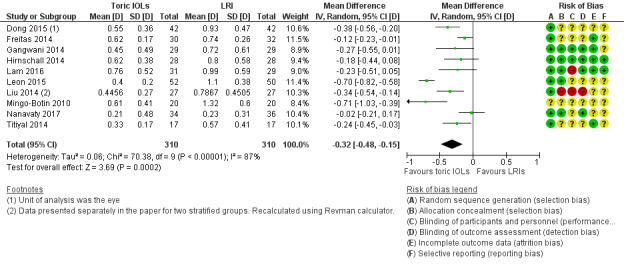
It was difficult to assess reasons for heterogeneity. In this case, Mingo‐Botin 2010 used an AcrySof toric IOL and in the LRI group, an AcrySof Natural (non‐toric) IOL, and Nichamin age and pachymetry adjusted nomogram. Leon 2015 also used AcrySof lenses and a Nichamin nomogram. Nanavaty 2017 used Rayner T‐Flex toric and C‐Flex non‐toric lenses; LRIs were assisted with a Donnenfeld nomogram.
A funnel plot to investigate publication bias did not suggest any obvious asymmetry (i.e. small‐study effects; Figure 6). Results from a sensitivity analysis using a fixed‐effect model provided similar results but with narrower CIs (MD –0.33 D, 95% CI –0.38 to –0.27).
6.
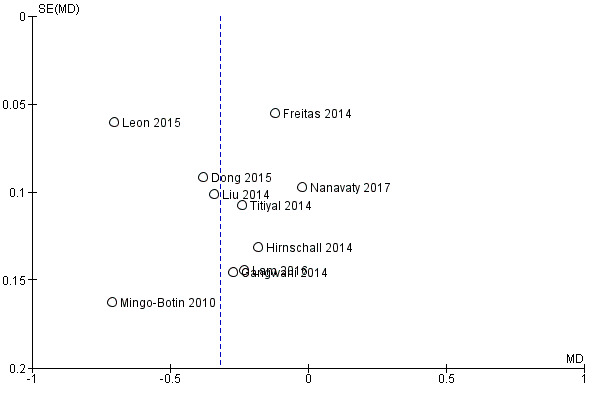
Secondary outcomes
Mean postoperative uncorrected distance visual acuity
People receiving a toric IOL had a small improvement in visual acuity at six months or more after surgery compared to people receiving LRI (MD –0.04 logMAR, 95% CI –0.07 to –0.02; 8 studies, 474 eyes; Analysis 1.3), but this small difference is probably clinically unimportant. We excluded two studies with unit of analysis errors (Gangwani 2014; Hirnschall 2014), which did not change materially the estimate of effect (MD –0.05, 95% CI –0.08 to –0.02; 360 eyes; data not shown). We judged this moderate‐certainty evidence downgrading one level for risk of bias.
1.3. Analysis.
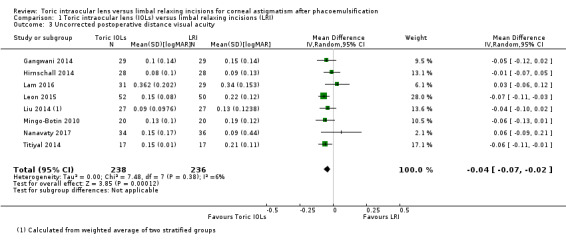
Comparison 1 Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI), Outcome 3 Uncorrected postoperative distance visual acuity.
Spectacle independence for distance
Two studies including 100 people reported spectacle independence. People receiving toric IOLs were more likely to report spectacle independence (RR 1.56, 95% CI 1.14 to 2.15; Analysis 1.4). We judged this low‐certainty evidence downgrading one level for risk of bias and one level for publication bias: this outcome was only reported by 2/10 studies and it was unclear if it was collected and not reported by the other studies.
1.4. Analysis.
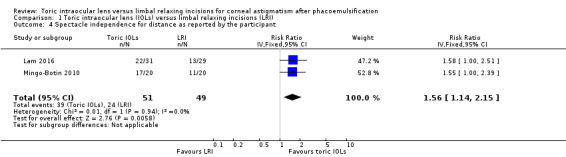
Comparison 1 Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI), Outcome 4 Spectacle independence for distance as reported by the participant.
One of the within‐person studies also reported spectacle use but it was not possible to assess between‐group differences in this study (Gangwani 2014); quote: "On the questionnaire, all patients reported that they were not wearing spectacles most of the time, although 9 patients (52.9%) needed spectacles when reading for a long time."
Mean vision‐related quality of life
One study of 40 people reported vision‐related quality of life measured using the Visual Function Index (VF‐14) (Mingo‐Botin 2010). The mean quality of life score in the LRI group was 93.7. The mean difference with toric IOLs compared with LRI was 3.01 lower (95% CI 8.56 lower to 2.54 higher). We judged this low‐certainty evidence downgrading one level for risk of bias and one level for imprecision; CIs included 0 and we could not exclude important differences.
Nanavaty 2017 collected quality of life data, but did not fully report them. Quote: "At 1 month, tIOL [toric IOL] patients were happier than PCRI [LRI] group patients. At 12 months, more tIOL patients reported no trouble in being able to use off‐the‐shelf (nonprescription) sunglasses. There was no significant difference in any other parameter assessed on the questionnaire between the groups for preoperative, 1 month postoperatively, and 12 months postoperatively."
Adverse effects
Postoperative lens rotation requiring second procedure to re‐align toric intraocular lens
None of the five studies (318 eyes) that reported this outcome reported any events. We judged this very low‐certainty evidence. We downgraded one level for risk of bias (studies were poorly reported and some studies were at high risk of performance and detection bias) and two levels for very serious imprecision (studies were underpowered to address this rare event and there were no events).
Other adverse effects
Six studies (410 eyes) reported adverse effects.
Freitas 2014 (62 eyes): quote: "No potentially sight‐threatening complications such as persistent corneal edema, pupillary block, retinal detachment, or endophthalmitis were observed."
Lam 2016 (60 eyes): quote: "There was no other complication seen in any study eye for both groups."
Liu 2014 (54 eyes): quote: "All patients underwent successful operations without any intra or post operation complications."
Mingo‐Botin 2010 (40 eyes): quote: "Other than the previously described IOL rotation, there were no postoperative complications in the toric IOL group. One patient in the relaxing incisions group had a central deepithelialization; recovery, aided by a bandage contact lens, was slow but complete over 10 days."
Nanavaty 2017 (70 eyes): quote: "In the PCRI arm there was 1 case of postoperative dryeye, which resolved after 3 months. In the tIOL [toric IOL] arm there was 1 case of cystoid macular edema, which resolved after 3 months with medical treatment, and 1 case of posterior capsular opacification, which underwent Nd:YAG capsulotomies."
Titiyal 2014 (34 eyes): quote: "Potential complications of AK [astigmatic keratotomy], such as corneal ectasia or a hyperopic shift, were not seen."
Only one study reported on glare/haloes but did not disaggregate by study group: quote: "Seven patients (41%) reported glare and 6 (35%) reported halos; no patient reported being bothered by these phenomena. One patient identified the eye with a toric IOL as the better eye and 1 patient the eye with the PCRI" (Gangwani 2014).
Discussion
Summary of main results
We identified 10 studies including 517 people (626 eyes). These studies took place in China (three studies), UK (three), Brazil (one), India (one), Italy (one) and Spain (one). The median age of participants was 71 years. The level of corneal astigmatism specified in the inclusion criteria of these studies ranged from 0.75 D to 3 D. The studies used a variety of toric IOLs, in all but one study these were monofocal. They used three different nomograms to determine the size and placement of the LRI. None of the studies were at low risk of bias in all domains, but two studies were at low risk of bias in all domains except selective outcome reporting. The remaining studies were a mixture of low, unclear or high risk of bias.
People receiving toric IOLs were more likely to achieve a postoperative residual refractive astigmatism of less than 0.5 D six months or more after surgery (RR 1.40, 95% CI 1.10 to 1.78; 5 RCTs, 262 eyes). We judged this moderate‐certainty evidence, downgrading one level for risk of bias. In the included studies, approximately 500 eyes per 1000 achieved postoperative astigmatism less than 0.5 D in the LRI group compared with 700 per 1000 in the toric IOLs group. There was a small difference in residual astigmatism between the two groups, favouring toric IOLs (mean difference (MD) –0.32 D, 95% CI –0.48 to –0.15; 10 RCTs, 620 eyes). Although all studies favoured toric IOLs, the results of individual studies were inconsistent (range of effects –0.02 D to –0.71 D; I² = 89%). We considered this low‐certainty evidence, downgrading for both risk of bias and inconsistency. Uncorrected visual acuity was similar between the two groups (MD –0.04 logMAR, 95% CI –0.07 to –0.02; 8 RCTs, 474 eyes; moderate‐certainty evidence). Low‐certainty evidence from one study of 40 people suggested little difference in vision‐related quality of life measured using VF‐14 (MD –3.01, 95% CI –8.56 to 2.54). Two studies reported spectacle independence and suggested that people receiving toric IOLs may be more likely to be spectacle independent (RR 1.56, 95% CI 1.14 to 2.15; 100 people; low‐certainty evidence). There were no cases of lens rotation requiring surgery (very low‐certainty evidence). Five studies (320 eyes) commented on a range of other adverse effects including corneal oedema, endophthalmitis and corneal ectasis. All these studies reported that there were no adverse events with the exception of one study (40 eyes) where one participant in the LRI group had a central de‐epithelialisation which recovered over 10 days.
Overall completeness and applicability of evidence
The studies took place in a range of locations and evaluated IOLs that are in common use currently. We consider the evidence to be largely applicable to current practice. Since studies evaluated IOLs of different models from the same manufacturer, costs of IOL should have been included. Toric IOLs usually present double the cost of standard IOLs in global markets. A cost‐effective analysis would have been useful to assess economic impacts of one technique over the other, especially when considering that toric IOLs presented statistical differences that clinically might not be so relevant.
The treatment effect of LRIs is limited by their physical dimensions, whereas that of toric IOLs is determined by the much more generous parameters afforded by modern lens manufacture. It is likely that toric IOLs may be more effective than LRIs at higher degrees of astigmatism. However, we could not address this question in this review, since the upper limit of astigmatism in the included studies was 3 D.
Quality of the evidence
We downgraded all outcomes for risk of bias as the studies were poorly reported and some studies were at high risk of performance and detection bias. There was considerable unexplained heterogeneity in the refractive outcome with estimates of effect ranging from –0.71 D to –0.02 D. There were some differences in these studies (e.g. in the techniques used for LRIs), but with relatively small numbers of studies it was difficult to attribute the difference in to any one factor. Relatively few studies reported quality of life and spectacle independence and we downgraded for imprecision and, in the case of spectacle independence, possible selective reporting as we were surprised so few studies considered this as an outcome. The studies were generally underpowered to consider rare but important adverse effects and so we considered this very low‐certainty evidence.
Brief economic commentary
To supplement the main systematic review assessing the effects of toric IOLs compared with LRIs in the management of astigmatism during phacoemulsification cataract surgery, we sought to identify economic evaluations on the subject. We found no economic studies that compared toric IOLs with LRIs. The apparent shortage of relevant economic evaluations indicates that economic evidence regarding the costs and consequence of these two procedures is currently lacking.
Potential biases in the review process
Our prespecified primary outcomes (proportion of people achieving postoperative residual refractive astigmatism of less than 0.5 D) was not uniformly reported, so we also collected data on refractive astigmatism as a continuous variable which was reported in some form by all studies. We do not think that this will bias the results of the study: an analysis of refractive astigmatism stratified by whether or not studies reported our dichotomous primary outcome did not suggest any difference in astigmatism between these studies (data not shown).
Agreements and disagreements with other studies or reviews
Kessel 2016 published a systematic review and meta‐analysis of toric IOLS versus non‐toric IOLs for treatment of astigmatism. This study included a broader range of studies, with comparators of non‐toric IOLs, corneal and LRIs. Overall, the results of Kessel 2016 are of the same order of magnitude as the current review but the authors concluded with an overall more positive judgement on toric IOLs than the current review. There are several differences worth noting.
The two reviews consider different comparator groups. Kessel 2016 evaluated the effect of astigmatism correction of toric IOLs with or without other techniques for astigmatism correction. This might have increased the amount of eyes with uncorrected astigmatism in the non‐toric IOL group, thus increasing favourable results for toric IOLs.
There were some differences in the studies included. Kessel 2016 included two studies that we excluded (Maedel 2014; Mendicute 2009). These RCTs evaluated opposite clear corneal incisions for correction of astigmatism. These are full penetration incisions performed along with the standard surgical phacoemulsification incision. This technique presents a different approach and, in our view, could not be considered an LRI. The current review includes three studies published after searches were done for Kessel 2016 (Dong 2015; Leon 2015; Nanavaty 2017).
Kessel 2016 did not downgrade for risk of bias in the included studies, although grading of risk of bias of the individual studies was similar to the current review. The difference appears to be in the weight given to unclear risk of bias. In our view lack of information, particularly for performance and detection bias, is probably an indicator that masking was not done and therefore the study was likely to be at risk of bias. We contacted all study authors regarding these unclear risk of bias judgements but to date have only received clarification from one study team.
Kessel 2016 did not consider whether or not the effects were clinically important. For example, the difference in uncorrected visual acuity for the comparison between toric IOLs and relaxing incisions was –0.06 logMAR units (95% CI –0.10 to –0.02), which is equivalent to approximately 3 letters of difference. In the current review, it was –0.04 logMAR units, which is about 2 letters. Kessel 2016 reported that more people with toric IOLs achieved 20/25 vision, which was not an outcome considered in the current view; the size of the effect was similar irrespective of whether LRIs were done in the control group.
Kessel 2016 also concluded there was moderate‐certainty evidence of no difference in complications but we considered the evidence to be uncertain.
Authors' conclusions
Implications for practice.
Treatment of astigmatism to a certain degree was obtained by both toric intraocular lens (IOL) and limbal relaxing incisions. The high heterogeneity and low certainty detected in these studies may be due to inherent difficulties in assessing and reporting astigmatism within a group of people. Decisions regarding the choice of one technique over the other should take into account economic factors and population‐based treatment.
Individual practice settings in which patients present coverage of higher‐cost materials in cataract surgery may chose toric IOLs for their slight advantage regarding residual refractive astigmatic error; however, expectations of these patients should be managed regarding possibilities of residual astigmatism.
When dealing with population‐based treatment of cataract surgery, in which surgical costs are an issue, this review supports the use of LRIs for treatment of astigmatism since toric IOLs present higher costs than standard spherical IOLs from the same manufacturer. The clinical importance of differences in the order of 0.32 D and 0.04 logMAR units could also be considered: 0.02 logMAR units represent 1 letter on a logMAR Chart, a difference of 0.04 units, or 2 letters in a logMAR chart, might not justify the higher investment related to toric IOLs. However, we note that we were unable to identify relevant economic evidence regarding the costs and consequence of these two procedures.
Implications for research.
Statistical differences should be noted according to their clinical impact when comparing refractive correction. Future trials should include data on costs of the procedure and cost‐effectiveness. Reports on visual acuity or refractive errors might benefit from standardised evaluation of visual function and spectacle independence. Longer time‐point evaluations (over 12 months) might be useful in comparing long‐term effects of both techniques. A core outcome set including recommendations for essential outcomes in this field is needed. At the minimum, this should include specification of measures and metrics for astigmatism, visual acuity, quality of life and spectacle independence.
We identified a proportion of studies that had unit of analysis errors. Either they included two eyes per person in the study but did not take within‐person correlation into account, or they compared intervention in one eye with comparator in the contralateral eye (i.e. a within‐person study), without performing a correct paired analysis. Although we could identify little impact of these unit of analysis errors in the current review, it would seem prudent to ensure that future studies are designed and analysed carefully, preferably with appropriate statistical support.
Acknowledgements
The methods section of this review was based on a standard template prepared by Cochrane Eyes and Vision (CEV) (eyes.cochrane.org).
CEV created and executed the electronic search strategies. We thank Alex Shortt, Richard Wormald, Mohammed Ziaei and Catey Bunce for their comments on the protocol, review or both and Anupa Shah for her assistance throughout the review process.
We thank Marcony Santhiago for his contributions to the published protocol.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Cataract Extraction] explode all trees #2 MeSH descriptor: [Pseudophakia] this term only #3 pseudophakia #4 pha?oemulsif* #5 phaco* or phako* #6 (extract* or aspirat* or operat* or remov* or surg* or excis*) near/3 cataract* #7 capsulorhexis #8 MeSH descriptor: [Lenses, Intraocular] explode all trees #9 MeSH descriptor: [Lens Implantation, Intraocular] explode all trees #10 (intraocular or intra ocular) near/3 lens* #11 IOL or IOLs #12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 #13 (limbal or cornea*) near/3 relax* #14 LRI or LRIs or CRI or CRIs #15 toric or tIOL* #16 SN6AT3 or SN6AT4 or SN6AT5 or SN6AT6 or SN6AT7 or SN6AT8 or SN6AT9 #17 SND1T2 or SND1T3 or SND1T4 or SND1T5 or SND1T6 #18 TFNT20 or TFNT30 or TFNT40 or TFNT50 or TFNT60 #19 ZCT150 or ZCT225 or ZCT300 or ZCT400 #20 Symfony or ZXT150 or ZXT225 or ZXT300 or ZXT375 #21 ZKB00 or ZMB00 or ZLB00 #22 AT TORBI or AT LISA or M‐flex or T‐flex #23 FineVision Toric or HOYA iSert or AA4203 or AA4203 or Trulign toric or BL1UT or enVista toric or MX60T #24 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 #25 #12 and #24
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp cataract extraction/ 14. exp pseudophakia/ 15. pseudophakia.tw. 16. pha?oemulsif$.tw. 17. (phaco or phako).tw. 18. ((extract$ or aspirat$ or operat$ or remov$ or surg$ or excis$) adj3 cataract$).tw. 19. capsulorhexis.tw. 20. exp lens implantation intraocular/ 21. exp lenses intraocular/ 22. ((intraocular or intra ocular) adj3 lens$).tw. 23. (IOL or IOLs).tw. 24. or/13‐23 25. ((limbal or cornea$) adj3 relax$).tw. 26. (LRI or LRIs or CRI or CRIs).tw. 27. toric.tw. 28. tIOL$.tw. 29. (SN6AT3 or SN6AT4 or SN6AT5 or SN6AT6 or SN6AT7 or SN6AT8 or SN6AT9).tw. 30. (SND1T2 or SND1T3 or SND1T4 or SND1T5 or SND1T6).tw. 31. (TFNT20 or TFNT30 or TFNT40 or TFNT50 or TFNT60).tw. 32. (ZCT150 or ZCT225 or ZCT300 or ZCT400).tw. 33. (Symfony or ZXT150 or ZXT225 or ZXT300 or ZXT375).tw. 34. (ZKB00 or ZMB00 or ZLB00).tw. 35. (AT TORBI or AT LISA or M‐flex or T‐flex).tw. 36. (FineVision Toric or HOYA iSert or AA4203 or AA4203 or Trulign toric or BL1UT or enVista toric or MX60T).tw.
37. or/25‐36 38. 24 and 37 39. 12 and 38
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. MEDLINE Ovid economics search strategy
1. exp cataract extraction/ 2. exp pseudophakia/ 3. pseudophakia.tw. 4. pha?oemulsif$.tw. 5. (phaco or phako).tw. 6. ((extract$ or aspirat$ or operat$ or remov$ or surg$ or excis$) adj3 cataract$).tw. 7. capsulorhexis.tw. 8. exp lens implantation intraocular/ 9. exp lenses intraocular/ 10. ((intraocular or intra ocular) adj3 lens$).tw. 11. (IOL or IOLs).tw. 12. or/1‐11 13. ((limbal or cornea$) adj3 relax$).tw. 14. (LRI or LRIs or CRI or CRIs).tw. 15. toric.tw. 16. tIOL$.tw. 17. (SN6AT3 or SN6AT4 or SN6AT5 or SN6AT6 or SN6AT7 or SN6AT8 or SN6AT9).tw. 18. (SND1T2 or SND1T3 or SND1T4 or SND1T5 or SND1T6).tw. 19. (TFNT20 or TFNT30 or TFNT40 or TFNT50 or TFNT60).tw. 20. (ZCT150 or ZCT225 or ZCT300 or ZCT400).tw. 21. (Symfony or ZXT150 or ZXT225 or ZXT300 or ZXT375).tw. 22. (ZKB00 or ZMB00 or ZLB00).tw. 23. (AT TORBI or AT LISA or M‐flex or T‐flex).tw. 24. (FineVision Toric or HOYA iSert or AA4203 or AA4203 or Trulign toric or BL1UT or enVista toric or MX60T).tw. 25. or/13‐24 26. 12 and 25 27. Economics/ 28. exp "costs and cost analysis"/ 29. Economics, Dental/ 30. exp economics, hospital/ 31. Economics, Medical/ 32. Economics, Nursing/ 33. Economics, Pharmaceutical/ 34. (economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 35. (expenditure$ not energy).ti,ab. 36. value for money.ti,ab. 37. budget$.ti,ab. 38. or/27‐37 39. ((energy or oxygen) adj cost).ti,ab. 40. (metabolic adj cost).ti,ab. 41. ((energy or oxygen) adj expenditure).ti,ab. 42. or/39‐41 43. 38 not 42 44. letter.pt. 45. editorial.pt. 46. historical article.pt. 47. or/44‐46 48. 43 not 47 49. exp animals/ not humans/ 50. 48 not 49 51. bmj.jn. 52. "cochrane database of systematic reviews".jn. 53. health technology assessment winchester england.jn. 54. or/51‐53 55. 50 not 54 56. 26 and 55
Appendix 4. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp cataract extraction/ 34. exp pseudophakia/ 35. pseudophakia.tw. 36. pha?oemulsif$.tw. 37. (phaco or phako).tw. 38. ((extract$ or aspirat$ or operat$ or remov$ or surg$ or excis$) adj3 cataract$).tw. 39. capsulorhexis.tw. 40. exp lens implant/ 41. exp lens implantation/ 42. ((intraocular or intra ocular) adj3 lens$).tw. 43. (IOL or IOLs).tw. 44. or/33‐43 45. ((limbal or cornea$) adj3 relax$).tw. 46. (LRI or LRIs or CRI or CRIs).tw. 47. toric.tw. 48. tIOL$.tw. 49. (SN6AT3 or SN6AT4 or SN6AT5 or SN6AT6 or SN6AT7 or SN6AT8 or SN6AT9).tw. 50. (SND1T2 or SND1T3 or SND1T4 or SND1T5 or SND1T6).tw. 51. (TFNT20 or TFNT30 or TFNT40 or TFNT50 or TFNT60).tw. 52. (ZCT150 or ZCT225 or ZCT300 or ZCT400).tw. 53. (Symfony or ZXT150 or ZXT225 or ZXT300 or ZXT375).tw. 54. (ZKB00 or ZMB00 or ZLB00).tw. 55. (AT TORBI or AT LISA or M‐flex or T‐flex).tw. 56. (FineVision Toric or HOYA iSert or AA4203 or AA4203 or Trulign toric or BL1UT or enVista toric or MX60T).tw. 57. or/45‐56 58. 44 and 57 59. 32 and 58
Appendix 5. Embase Ovid economics search strategy
1. exp cataract extraction/ 2. exp pseudophakia/ 3. pseudophakia.tw. 4. pha?oemulsif$.tw. 5. (phaco or phako).tw. 6. ((extract$ or aspirat$ or operat$ or remov$ or surg$ or excis$) adj3 cataract$).tw. 7. capsulorhexis.tw. 8. exp lens implant/ 9. exp lens implantation/ 10. ((intraocular or intra ocular) adj3 lens$).tw. 11. (IOL or IOLs).tw. 12. or/1‐11 13. ((limbal or cornea$) adj3 relax$).tw. 14. (LRI or LRIs or CRI or CRIs).tw. 15. toric.tw. 16. tIOL$.tw. 17. (SN6AT3 or SN6AT4 or SN6AT5 or SN6AT6 or SN6AT7 or SN6AT8 or SN6AT9).tw. 18. (SND1T2 or SND1T3 or SND1T4 or SND1T5 or SND1T6).tw. 19. (TFNT20 or TFNT30 or TFNT40 or TFNT50 or TFNT60).tw. 20. (ZCT150 or ZCT225 or ZCT300 or ZCT400).tw. 21. (Symfony or ZXT150 or ZXT225 or ZXT300 or ZXT375).tw. 22. (ZKB00 or ZMB00 or ZLB00).tw. 23. (AT TORBI or AT LISA or M‐flex or T‐flex).tw. 24. (FineVision Toric or HOYA iSert or AA4203 or AA4203 or Trulign toric or BL1UT or enVista toric or MX60T).tw. 25. or/13‐24 26. 12 and 25 27. Health Economics/ 28. exp Economic Evaluation/ 29. exp Health Care Cost/ 30. pharmacoeconomics/ 31. or/27‐30 32. (econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. 33. (expenditure$ not energy).ti,ab. 34. (value adj2 money).ti,ab. 35. budget$.ti,ab. 36. or/32‐35 37. 31 or 36 38. letter.pt. 39. editorial.pt. 40. note.pt. 41. or/38‐40 42. 37 not 41 43. (metabolic adj cost).ti,ab. 44. ((energy or oxygen) adj cost).ti,ab. 45. ((energy or oxygen) adj expenditure).ti,ab. 46. or/43‐45 47. 42 not 46 48. animal/ 49. exp animal experiment/ 50. nonhuman/ 51. (rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. 52. or/48‐51 53. exp human/ 54. human experiment/ 55. or/53‐54 56. 52 not (52 and 55) 57. 47 not 56 58. 0959‐8146.is. 59. (1469‐493X or 1366‐5278).is. 60. 1756‐1833.en. 61. or/58‐60 62. 57 not 61 63. Conference abstract.pt. 64. 62 not 63 65. 26 and 64
Appendix 6. ISRCTN search strategy
(limbal relax OR corneal relax OR toric) AND (cataract OR phaco OR IOL)
Appendix 7. ClinicalTrials.gov search strategy
(limbal relax OR corneal relax OR toric) AND (cataract OR phaco OR IOL) AND astigmatism
Appendix 8. WHO ICTRP search strategy
toric AND non toric AND astigmatism
Appendix 9. DARE, NHS EED and HTA on CRD Database
(limbal relax OR corneal relax OR toric) AND (cataract OR phaco OR IOL)
Data and analyses
Comparison 1. Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative residual refractive astigmatism < 0.50 D | 5 | 262 | Risk Ratio (IV, Random, 95% CI) | 1.40 [1.10, 1.78] |
| 2 Postoperative residual refractive astigmatism | 10 | 620 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.48, ‐0.15] |
| 3 Uncorrected postoperative distance visual acuity | 8 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.02] |
| 4 Spectacle independence for distance as reported by the participant | 2 | 100 | Risk Ratio (IV, Fixed, 95% CI) | 1.56 [1.14, 2.15] |
| 5 Vision‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.5. Analysis.
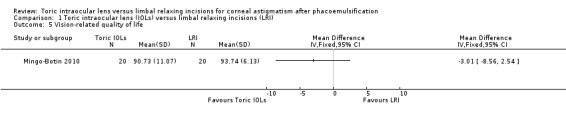
Comparison 1 Toric intraocular lens (IOLs) versus limbal relaxing incisions (LRI), Outcome 5 Vision‐related quality of life.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dong 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 or 2 eyes per person, unclear how the eyes were selected and whether analysed adjusted for within‐person correlation. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: aged ≥ 60 years; diagnosed senile cataract before surgery; pupils of normal round shape and diameter > 3 mm; hardness of lens nuclear categorised according LOCS – from II to IV level; regular corneal astigmatism within range 1.5–4.0 D. Exclusion criteria: people with retinal disease, glaucoma, keratitis, irregular corneal astigmatism, recent acute eye infections, pterygium; history of ocular trauma and surgical procedure. Pretreatment differences: none reported |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Visual acuity, corneal astigmatism and refraction Adverse effects: not reported Follow‐up: 3 months |
|
| Identification |
Sponsorship source: not reported Country: China Authors name: Yong‐Xiao Dong Email: 187299703@qq.com Conflict of interest: not reported Trial registration ID: not reported Date study conducted: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: random allocation done using random number table. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: information not provided. We contacted study authors for clarification but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: information not provided. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: information not provided. We contacted study authors for clarification but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: no description of participants who withdrew or were lost to follow‐up. We contacted study authors for clarification but received no response. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trial registration entry. We contacted study authors for clarification but received no response. |
Freitas 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: people randomly allocated to treatment, both eyes included in study. Analysis probably not adjusted for within‐person correlation. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: aged > 40 years; visually significant cataract, defined as spectacle distance corrected visual acuity (SDCVA) worse than Snellen 20/40 (LogMAR scale of 0.3); regular corneal astigmatism ranging 0.75–2.50 D; pharmacological mydriasis ≥ 6.0 mm (measured at the slit lamp) to facilitate proper intraoperative visualisation of axis marks on surface of the toric IOL. Exclusion criteria: history of previous surgery, pterygium, ocular disease that would lead to poor postoperative corrected visual acuity (corneal scarring, uveitis, advanced glaucoma, neuro‐ophthalmic disease, and significant macular disease or other retinopathy), or zonule or pupil abnormalities. Pretreatment differences: LRI group older (mean age: 66 years in IOL group vs 72 years in LRI group). |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Manifest refraction, uncorrected distance visual acuity and spectacle distance corrected visual acuity, spherical equivalent refraction
Adverse effects: persistent corneal oedema, pupillary block, retinal detachment, endophthalmitis Follow‐up: 1, 3 and 6 months after surgery |
|
| Identification |
Sponsorship source: financial contributors: private: Alcon Labs. Brazil Sao Paulo (SP), provided all IOLs at no cost for scientific purposes. Public: Municipal Health Authority of Uberlândia funded surgical procedures as part of a regular governmental assistance policy. Country: Brazil Authors name: Giuliano Oliveira Freitas Email: gofreitas@ufmg.br Conflict of interest: none Trial registration ID: not reported Date study conducted: May 2010 to June 2012 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned using the Microsoft Excel TM “=RANDBETWEEN(1;2)”" |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: information not provided. We contacted study authors for clarification but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: no statement of any blinding of participant or surgeon in the text. Information not provided. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: no statement of blinding of the examiner. We contacted study authors for clarification but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients completed the follow‐up period of 6 months." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no obvious selective outcome reporting from methods but difficult to judge because no access to protocol or trial registration record (or both). We contacted study authors for clarification but received no response. |
Gangwani 2014.
| Methods |
Study design: randomised controlled trial Study grouping: within‐person study. The second eye was operated on within 4 weeks of the first surgery and received the alternate treatment. Group allocation of the first eye was random. Eyes/people: eyes randomly allocated to treatment but not analysed as a paired study |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: aged ≥ 40 years; bilateral corneal astigmatism 1.0–2.5 D on automated keratometry performed with partial coherence interferometry (IOLMaster, software version 5.x, Carl Zeiss Meditec AG) and visually significant cataract in both eyes. Exclusion criteria: irregular astigmatism or forme fruste keratoconus on Scheimpflug corneal tomography (Pentacam HR, Oculus Optikger€ate GmbH), corneal scars, phacodonesis, pseudoexfoliation syndrome, traumatic cataract, and other ophthalmic copathology that could have an impact on capsular bag stability or postoperative visual function. Pretreatment differences: not reported but was a within‐person study. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Corneal astigmatism, IOL rotation, monocular uncorrected distance visual acuity (ETDRS chart), corrected distance visual acuity, autorefraction (Topcon RM‐8800), subjective refraction (Jackson cross‐cylinder method and trial frames), near vision (near‐vision ETDRS chart at 40 cm), glare testing (straylight meter, C‐Quant), contrast sensitivity (Pelli‐Robson charts, Precision Vision, at 1 m), spectacle use, participant satisfaction (questionnaire), self‐reported glare or halos and symptoms. Adverse effects: not reported Follow‐up: 1 and 3 months |
|
| Identification |
Sponsorship source: Rayner Surgical, UK. Supported by the Department of Health through an award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. Country: UK Authors name: Vincenzo Maurino Email: vincenzo.maurino@moorfields.nhs.uk Conflict of interest: no author had financial or proprietary interest in any material or method mentioned. Date study conducted: December 2009 to December 2010 Trial registration ID: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a computer‐based system by a person otherwise not involved in the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "The patient and examiners were masked to the group allocation, and the surgeon was masked to allocation until the time of IOL implantation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patient‐masked and examiner‐ masked study included patients who were scheduled for cataract surgery in both eyes and who desired spectacle independence." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To avoid observer bias due to knowledge of the IOL orientation on the previous follow‐up photograph of a patient, all images were imported in random to ensure that the images of each patient were not analyzed consecutively." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Three patients were lost to follow‐up, 1 due to general health problems and 2 due to noncompliance, and were excluded from analysis." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: outcomes not clearly specified in the methods section and no access to protocol or trial registration record. We contacted study authors for clarification but received no response. |
Hirnschall 2014.
| Methods |
Study design: randomised controlled trial Study grouping: within‐person study. Surgery comprising the alternate treatment was performed in the second eye within 4 weeks after the first surgery. Group allocation of the first eye was randomly chosen. Eyes/people: eyes randomly allocated to treatment but probably not analysed as a paired study. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: aged ≥ 40 years, corneal astigmatism 1.0–2.5 D on partial coherence interferometry automated keratometry (IOLMaster, software version 5.x, Carl Zeiss Meditec AG) and cataract in both eyes. Exclusion criteria: irregular astigmatism or forme fruste keratoconus on corneal tomography (Pentacam HR, Oculus Optikgerate GmbH), corneal scars, phacodonesis, pseudoexfoliation syndrome, traumatic cataract and other ophthalmic pathology that could have an impact on capsular bag stability and on postoperative visual function. Pretreatment differences: not relevant for age and gender. Preoperative corneal astigmatism similar. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Monocular uncorrected distance visual acuity (ETDRS Chart), corrected distance visual acuity, autorefraction (RM‐8800), subjective refraction (Jackson cross‐cylinder method and trial frames), IOL toric axis (retroillumination photographs), rotational stability analysis (Keynote software), astigmatism vector analysis, corneal astigmatism, residual refractive astigmatism Adverse effects: not reported Follow‐up: 1 and 6 months |
|
| Identification |
Sponsorship source: partial financial support provided by the Department of Health through an award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and University College London Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology. The views expressed in the publication were those of the authors and not necessarily those of the Department of Health. Supported by an unrestricted grant from Rayner Surgical, London, UK. Country: UK Authors name: Oliver Findl Email: oliver@findl.at Conflict of interest: none Date study conducted: January 2009 to July 2009 Trial registration ID: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group allocation of the first eye was randomly chosen. Randomization was performed using a computer‐based system by a person otherwise not involved in the trial." |
| Allocation concealment (selection bias) | Low risk | Quote: "The patient and examiners were masked to the group allocation, and the surgeon was masked to allocation until the time of IOL implantation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patient and examiners were masked to the group allocation, and the surgeon was masked to allocation until the time of IOL implantation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "avoid observer bias resulting from knowledge of the IOL orientation of the previous follow‐up photograph of a patient, all images were imported in random to ensure that the images of each patient were not analyzed consecutively." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study enrolled 60 eyes of 30 patients. Two patients were lost to follow‐up, 1 as a result of general health problems and the other for noncompliance; both were excluded from analysis." Judgement comment: number of participants was not specified in the tables; however, the number was stated in the text. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: outcomes were not clearly specified in the methods section and we did not have access to protocol or trial registration record. We contacted study authors for clarification and they confirmed that all outcomes specified in the protocol were reported. |
Lam 2016.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 eye per person included in the study (study eye); unclear how this study eye was selected. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: preoperative BCVA < 0.2 logarithm of the minimum angle of resolution, calculated spherical IOL power 12.0–25.0 D, pupil dilation ≥ 6 mm and astigmatism ≤ 3.0 D (with‐the‐rule, oblique or against‐the‐rule regular corneal astigmatism) as measured by IOL Master 500 (Carl Zeiss, Meditec AG, Jena, Germany). Exclusion criteria: pregnancy, lactation, corneal abnormalities, previous corneal surgery, amblyopia, uncontrolled glaucoma, clinically significant macular changes, history of macular oedema, proliferative diabetic retinopathy, iris neovascularisation, history of retinal detachment, history of uveitis or iritis, optic atrophy, microphthalmos, recurrent intraocular inflammation of unknown aetiology, blind or absent fellow eye; intraoperative events such as requirement of additional surgery (e.g. glaucoma), vitreous loss, significant anterior chamber hyphaema, uncontrollable positive intraocular pressure, zonular damage, capsulorhexis tear, capsular rupture or inability to place the optic and both haptics of the IOL into the capsular bag. Pretreatment differences: LRI group slightly older (68 years versus 65 years on average) and slightly less refractive and corneal astigmatism preoperatively. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Vector analysis of astigmatism, uncorrected visual acuity, contrast sensitivity, refractive astigmatism, corneal astigmatism, need for spectacles Adverse effects: lens misalignment, other Follow‐up: 3 months |
|
| Identification |
Sponsorship source: Abbott Medical Optics, Abbott Park, IL, USA sponsored all the TECNIS 1‐Piece IOL and TECNIS Toric IOL (correspondence with study author). Country: Hong Kong, China Authors name: Vishal Jhanji Email: vishaljhanji@cuhk.edu.hk Conflict of interest: none declared Date study conducted: March 2012 to April 2014 (correspondence with study author) Trial registration ID: REC/IRB# KC/KE‐12‐0115/FR1 (correspondence with study author) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent optometrist generated the randomisation chart. A dynamic allocation scheme was used to create an even number of blocks. Simple randomisation was then used to allocate eyes of each patient to receive aspheric monofocal IOL or aspheric toric IOL." |
| Allocation concealment (selection bias) | Low risk | Quote: "The subjects were not informed of their draws until the completion of the surgery." Judgement comment: "An independent optometrist…" implies allocation was kept concealed. This was confirmed by correspondence with study author. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: participants were informed at the end of surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (correspondence with study author): "The assessors were not informed of the allocation (therefore masked) as they were not allowed to use the microscopes to check for LRI or dilating the pupil to examine the IOLs. Hence, post‐op VA [visual acuity] and spherical and cylindrical aberrations were taken without knowing the allocation. Only the surgeons were not masked as they would perform post‐op follow‐ups." Judgement comment: outcome assessors were masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Overall, 60 patients (29 for LRI group and 31 for toric IOL group) completed the 3‐month follow‐up postoperatively. One patient suffered a stroke 2 months postoperatively and was not able to attend the clinic, and another two patients were lost to follow‐up." |
| Selective reporting (reporting bias) | Low risk | Judgement comment: study author confirmed all planned outcomes were reported. |
Leon 2015.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 eye per person (study eye); unclear how this study eye was selected. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: significant cataract (II–IV group LOCS III), regular corneal astigmatism (1.0–2.0 D), with‐the‐rule astigmatism, mean axial length 23–24 mm, regular and symmetric astigmatism shape at the corneal topographic map, regular and with‐the‐rule astigmatism of the posterior corneal surface, pharmacologic mydriasis > 6.00 mm diameter to allow intraoperative and postoperative visualisation of axis marks on the toric IOLs. Exclusion criteria: previous surgery in the eye under study, irregular astigmatisms of the anterior or the posterior corneal surfaces, against‐the‐rule astigmatism, ocular diseases (pupil or zonular abnormalities, corneal scaring, uveitis, glaucoma, neuro‐ophthalmic diseases, significant macular disease or other retinopathy). Pretreatment differences: none |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Uncorrected distance visual acuity, best‐corrected distance visual acuity, topographic and keratometric changes in cornea, refractive evaluation and residual astigmatism, lens misalignment, endothelial cell count Adverse effects: not reported Follow‐up: 1, 3 and 6 months |
|
| Identification |
Sponsorship source: not reported Country: Italy Authors name: Pia Leon Email: pialeon@libero.it Conflict of interest: none Date study conducted: January 2013 to June 2013 Trial registration ID: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the two treatments computer. A randomized number was assigned to each patient when the inclusion and exclusion criteria were satisfied." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly assigned to one of the two treatments computer. A randomized number was assigned to each patient when the inclusion and exclusion criteria were satisfied." Judgement comment: as the random allocation done after inclusion, implication is that the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
Liu 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: when both eyes of the same participant fulfilled the inclusion criteria, only the right eye was included for analysis. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: LogMAR BCVA > 0.5, spherical equivalent < ± 6 D, corneal astigmatism 0.75–2.5 D. Exclusion criteria: marginal degeneration, corneal scar, pterygium, fundus lesions, optic neuropathy, age‐related macular degeneration, retinal artery/vein occlusion, lens exfoliation, mental problems. Pretreatment differences: stratified by degree of corneal astigmatism. 0.75–1.5 D and 1.75–2.5 D. No obvious group differences. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Uncorrected visual acuity, BCVA, subjective refraction, corneal curvature, intraocular pressure Adverse effects: intra‐ and postoperative complications (not specified) Follow‐up: 1 month |
|
| Identification |
Sponsorship source: not reported Country: China Authors name: Zhiping Liu Email: liuzhiping0318@163.com Conflict of interest: not reported Trial registration ID: not reported Date study conducted: March 2011 to March 2012 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Everyone in each group received a random number from the random number table to establish the consulting order. The patients with even numbers received PCRIs, while those with odd numbers received toric‐IOL." |
| Allocation concealment (selection bias) | High risk | Judgement comment: this was not discussed and the odd/even number procedure for the random number table appeared unconcealed. We contacted study authors for clarification but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: study was not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: study was not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
Mingo‐Botin 2010.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 eye per person included in the study (study eye); unclear how this study eye was selected. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: visually significant cataract, regular corneal astigmatism 1.00–3.00 D, and pharmacological mydriasis ≥ 6.0 mm to allow intraoperative and postoperative visualisation of axis marks on the toric IOL. Exclusion criteria: previous surgery in the eye under study, ocular disease that would lead to poor postoperative corrected visual acuity (corneal scarring, uveitis, advanced glaucoma, neuro‐ophthalmic disease, significant macular disease or other retinopathy), zonule or pupil abnormalities, and irregular astigmatism or astigmatism outside the defined range. Pretreatment differences: LRI group slightly older. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Uncorrected distance visual acuity, corrected distance visual acuity, intraocular pressure, refraction, keratometry, corneal topography, contrast sensitivity with/without glare, participant satisfaction, quality of life (VF‐14) Adverse effects: intraoperative complications Follow‐up: 1 day, 1 and 3 months |
|
| Identification |
Sponsorship source: not reported Country: Spain Authors name: David Mingo‐Botin Email: davimnbot@gmail.com Conflict of interest: none Date study conducted: May 2008 to June 2009 Trial registration ID: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients were randomly assigned to receive a toric IOL (AcrySof Toric, Alcon, Inc.) or a spherical IOL (AcrySof Natural, Alcon, Inc.) associated with peripheral corneal relaxing incisions." Judgement comment: insufficient information to judge. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
Nanavaty 2017.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 eye per person. Although the first eye of the participant was enlisted into study, the second eye underwent the same intervention (toric IOL or PCRI) and the surgery was performed according to normal National Health Service protocols. Data were collected only from the first study eye. Unclear how the first study eye was selected. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: symptomatic cataract, postoperative visual potential of 0.2 logMAR or better, and corneal astigmatism between > 0.75 D and < 2.5 D on topography (Pentacam HR; Oculus, Wetzlar, Germany). Exclusion criteria: aged < 18 years; any ocular comorbidity with cornea, uvea, retina or optic nerve that may be detrimental to visual outcomes; diabetes; people with glaucoma, abnormal corneal topography or any other coexisting retinal or cornea conditions; people with astigmatism outside the study range; people concurrently using ocular medications, including lubricants; and people unable to consent and unable to attend follow‐up visits. Pretreatment differences: not fully reported. More women in the LRI group. |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Uncorrected distance visual acuity, best‐corrected distance visual acuity (EDTRS charts at 4 m), uncorrected near visual acuity, manifest refractive sphere, cylinder and spherical equivalent, keratometric astigmatism and mean keratometry, aberration, alignment of toric IOL, quality of life (Quality‐of‐Life Impact of Refractive Correction questionnaire). Adverse effects: cystoid macular oedema, posterior capsule opacification, dry eye Follow‐up: 1, 3, 6 and 12 months |
|
| Identification |
Sponsorship source: funded by an unrestricted research grant from Rayner, Hove, UK. Country: UK Authors name: Mayank A Nanavaty Email: mayank.nanavaty@bsuh.nhs.uk Conflict of interest: quote: "Mayank A. Nanavaty received research grants from Rayner and Ziemer and is a consultant to Rayner, Alcon, and Ziemer. The following authors have no financial disclosures: Kaveeta K. Bedi, Shahnaz Ali, Mathew Holmes, and Saul Rajak." Date study conducted: March 2014 to May 2015 Trial registration ID: NCT02067429 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomly allocated to either intervention arm (tIOL [toric IOL] and PCRI) using an online random number generator that was created by a member of the research and development team who was not involved in participant assessment or surgery." |
| Allocation concealment (selection bias) | Low risk | Quote: "The research team was informed about the required intervention (tIOL [toric IOL] or PCRI) just before the patient went into the operating room for the surgery. They prepared a blocked randomization sequence (blocks of random size; n ¼ 2, 4, and 6) with 2 strata. Two sets of envelopes were generated. One contained 80 randomization allocation codes for participants with 0.75 to 1.5 D and the other contained 80 randomization allocation codes for participants with 1.5 to 2.5 D to ensure even distribution of patients over the entire corneal astigmatism range of 0.75 to 2.5 D." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "and refraction measurement without the information on the type of intervention during the patient’s follow‐up visit, to reduce the unrecognized biases." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: 80 people enrolled, 10 withdrawn, apparently before randomisation. 2 groups: 37 in toric IOL group vs 33 in LRI group. Some additional losses to follow‐up but unclear how many people included in the final analysis. We contacted study authors for clarification but received no response. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: difficult to judge as only a limited number of outcomes on the trials registry record (NCT02067429). We contacted study authors for clarification but received no response. |
Titiyal 2014.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group Eyes/people: 1 eye per person included in the study (study eye); unclear how this study eye was selected. |
|
| Participants |
Baseline characteristics Toric IOLs
LRI
Overall
Inclusion criteria: aged 45–65 years, presented to the outpatient department or anterior segment services of the centre with visually significant immature senile cataract, regular bow‐tie moderate corneal astigmatism (1.25–3.0 D) and no ocular or systemic contraindications to surgery. Exclusion criteria: complicated cataract, posterior segment pathology, astigmatism < 1.25 D or > 3.00 D, systemic condition likely to result in an unpredictable response to surgery (e.g. collagen vascular disease, diabetes mellitus), inability to attend follow‐up visits or who were not willing to provide written consent. Pretreatment differences: no obvious group differences |
|
| Interventions |
Intervention characteristics Toric IOLs
LRI
|
|
| Outcomes | Uncorrected distance visual acuity, corrected distance visual acuity, corneal topography, endothelial cell density, subjective refraction and manual keratometry, residual astigmatism (vector analysis) Adverse effects: IOL alignment, corneal ectasia, hyperopic shift Follow‐up: 1 day, 1 week, 1 and 3 months |
|
| Identification |
Sponsorship source: not reported Country: India Authors name: Namrata Sharma Email: namrata.sharma@gmail.com Conflict of interest: none Date study conducted: not reported Trial registration ID: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients who met the inclusion criteria were recruited and randomized into 2 groups with an equal number of eyes. Randomization was performed using a table of random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Judgement comment: not reported. We contacted study authors for clarification but received no response. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The study enrolled 34 eyes of 34 patients; each of the 2 groups comprised 17 eyes. There were no dropouts in either group, and all patients were followed regularly for a minimum of 3 months." |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: insufficient information to judge. We contacted study authors for clarification but received no response. |
BCVA: best corrected visual acuity; CCI: clear corneal incision; ETDRS: Early Treatment Diabetic Retinopathy Study; IOL: intraocular lens; LOCS: Lens Opacities Classification System; LRI: limbal relaxing incision; PCRI: peripheral corneal relaxing incision; SD: standard deviation; SIA: surgically induced astigmatism; VF‐14: Visual Function Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carvalho 2007 | No toric IOLs |
| Coloma‐González 2007 | No toric IOLs |
| Eliwa 2016 | No toric IOLs |
| Holland 2010 | No LRIs |
| Kaufmann 2005 | No toric IOLs |
| Maedel 2014 | No LRIs |
| Mendicute 2009 | No LRIs |
| Miyata 2011 | No toric IOLs |
| Muller‐Jensen 1999 | No toric IOLs |
| Nagpal 2015 | No LRIs |
| Ouchi 2010 | No toric IOLs |
| Roberts 2018 | No toric IOLs |
| Shen 2004 | No toric IOLs |
| Solomon 2019 | Although this study considered both toric IOLs and LRIs, they were not randomly allocated to treatment. |
| Wang 2013 | No toric IOLs |
IOL: intraocular lens; LRI: limbal relaxing incisions.
Characteristics of ongoing studies [ordered by study ID]
NCT03633851.
| Trial name or title | Toric IOL vs non‐toric IOL with LRI for corneal astigmatism |
| Methods | Within‐person study |
| Participants | 30 |
| Interventions | Toric intraocular MX60T lens Standard MX60 plus corneal incisions |
| Outcomes | From clinical trials registry entry: Primary outcomes: Astigmatism reduction evaluated with optical biometry, corneal topography Astigmatism reduction evaluated with manifest refraction, autorefraction
Secondary outcomes: Measurement of uncorrected visual acuity, monocular and binocular, using a back‐lit EDTRS chart placed at 4 m. Although these assessments appear to have different units of measurements, but all the measurements will be in the same units of measure (timeframe: 6 and 12 months). Measurement of best‐spectacle corrected visual acuity, monocular and binocular, using a back‐lit EDTRS chart placed at 4 m (timeframe: 6 and 12 months). Quality of vision evaluated with the Quality of Vision questionnaire score, a validated, Rasch‐adjusted questionnaire in which people are asked to rate 10 dysphotopsia items illustrated by standard photographs, scoring each item (0, 1, 2, 3) in relation to how frequent, severe and bothersome their symptoms are (30 items in total) (timeframe: 6 and 12 months). Visual disability evaluated with the Catquest 9‐SF cataract visual disability questionnaire, a Rasch‐adjusted cataract visual disability questionnaire that asks people to rate difficulty with a range of vision‐related daily activities (timeframe: 6 and 12 months). Overall satisfaction evaluated with vision rating questionnaire. It will be obtained by asking people to rate whether they were very satisfied, satisfied, neither satisfied nor unsatisfied, unsatisfied or very unsatisfied (timeframe: 6 and 12 months). Dysphotopsia evaluated with the Dysphotopsia questionnaire. 4 questions regarding dysphotopsia symptoms (halo, glare or dazzle, unwanted images, or shadows), scores as no symptoms at all ("none") or they were "barely noticeable," "annoying" or "debilitating" (timeframe: 6 and 12 months). |
| Starting date | October 2017. Estimated study completion date: August 2019 |
| Contact information | Isaac John, PhD; Isaac.John@nhs.net Freda Gomes, MSc; Freda.Gomes@nhs.net |
| Notes | clinicaltrials.gov/ct2/show/nct03633851 |
EDTRS: Early Treatment Diabetic Retinopathy Study; IOL: intraocular lens.
Differences between protocol and review
We considered postoperative residual astigmatism as a dichotomous variable in the protocol. However, all studies reported this outcome as a continuous variable, so we made the post‐hoc decision to also collect these data.
We added a new co‐author to the team, JE.
We added a brief economic commentary into the review with the addition of an additional co‐author, health economist, AK.
We planned the following subgroup analyses but did not do them because of:
techniques of limbal relaxing incisions: it was difficult to assign studies to clearly different subgroups with respect to the types of incision;
differential effects of treatment of less than 2 D and more than 2 D of astigmatism (addressing the hypothesis that LRIs are better for lesser degrees and toric lenses for higher degrees of astigmatism). There was not much heterogeneity in inclusion criteria in this respect with only one study restricted to higher levels of astigmatism. In general, studies did not report data disaggregated by levels of astigmatism;
age groups: 65 years or more versus less than 65 years. Studies had a similar mean age and did not report data disaggregated by age;
materials and models of toric IOLs. While nearly half of studies used AcrySof Toric, there was heterogeneity in the other lenses used and we judged subgroup analysis would not be helpful.
We planned to perform the following sensitivity analyses on the primary outcome considering but there were not enough studies to make this a useful exercise:
excluding studies at high risk of bias in one or more domains;
excluding industry‐funded studies
We did one additional sensitivity analysis not planned in our protocol. We examined the effect of excluding studies with a unit of analysis error. This was in response to a comment by a peer referee with a specialisation in statistics.
Contributions of authors
JCL: writing of the protocol, screening search results, data extraction, writing the review. GV: revision and proofing. GC: editing protocol and review. GJMP: final revision and editing. AK: preparation of the brief economic commentary. JE: screening search results, data extraction, data analysis, writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base and also through the NIHR Cochrane Incentive Scheme..
The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
JCL: none. GV: none. GC: none. GJMP: none. AK: none. JE: none.
Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) signed off the review for publication. Peter Tugwell, Senior Editor and Nuala Livingstone, Associate Editor for the Cochrane Musculoskeletal, Oral, Skin and Sensory (MOSS) Network reviewed a draft prior to publication. This was to avoid a potential conflict of interest as one of the authors (JE) is the joint Co‐ordinating Editor for CEV.
New
References
References to studies included in this review
Dong 2015 {published data only}
- Dong YX, Huang L, Guan XR, Ma Y, Han WT, Zhao J, et al. Efficacy of phacoemulsification combined with intraocular lens implantation for senile cataract with corneal astigmatism. International Eye Science 2015;15(11):1923‐6. [Google Scholar]
Freitas 2014 {published data only}
- Freitas GO, Boteon JE, Carvalho MJ, Pinto RM. Treatment of astigmatism during phacoemulsification. Arquivos Brasileiros de Oftalmologia 2014;77(1):40‐6. [DOI] [PubMed] [Google Scholar]
Gangwani 2014 {published data only}
- Gangwani V, Hirnschall N, Findl O, Maurino V. Multifocal toric intraocular lenses versus multifocal intraocular lenses combined with peripheral corneal relaxing incisions to correct moderate astigmatism. Journal of Cataract and Refractive Surgery 2014;40(10):1625‐32. [DOI] [PubMed] [Google Scholar]
Hirnschall 2014 {published data only}
- Hirnschall N, Gangwani V, Crnej A, Koshy J, Maurino V, Findl O. Correction of moderate corneal astigmatism during cataract surgery: toric intraocular lens versus peripheral corneal relaxing incisions. Journal of Cataract and Refractive Surgery 2014;40(3):354‐61. [DOI] [PubMed] [Google Scholar]
Lam 2016 {published data only}
- Lam DK, Chow VW, Ye C, Ng PK, Wang Z, Jhanji V. Comparative evaluation of aspheric toric intraocular lens implantation and limbal relaxing incisions in eyes with cataracts and ≤3 dioptres of astigmatism. British Journal of Ophthalmology 2016;100(2):258‐62. [DOI] [PubMed] [Google Scholar]
Leon 2015 {published data only}
- Leon P, Pastore MR, Zanei A, Umari I, Messai M, Negro C, et al. Correction of low corneal astigmatism in cataract surgery. International Journal of Ophthalmology 2015;8(4):719‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu Z, Sha X, Liang X, Wang Z, Liu J, Huang D. Toric intraocular lens vs. peripheral corneal relaxing incisions to correct astigmatism in eyes undergoing cataract surgery. Eye Science 2014;29(4):198‐203. [PubMed] [Google Scholar]
- Tang WM, Chen QD, Liang XW, Wang ZH. Toric intraocular lens versus peripheral corneal relaxing incisions to correct astigmatism in eyes having cataract surgery. International Eye Science 2013;13(3):552‐6. [Google Scholar]
Mingo‐Botin 2010 {published data only}
- Mingo‐Botín D, Muñoz‐Negrete FJ, Won Kim HR, Morcillo‐Laiz R, Rebolleda G, Oblanca N. Comparison of toric intraocular lenses and peripheral corneal relaxing incisions to treat astigmatism during cataract surgery. Journal of Cataract and Refractive Surgery 2010;36(10):1700‐8. [DOI] [PubMed] [Google Scholar]
Nanavaty 2017 {published data only}
- Nanavaty MA, Bedi KK, Ali S, Holmes M, Rajak S. Toric intraocular lenses versus peripheral corneal relaxing incisions for astigmatism between 0.75 and 2.5 diopters during cataract surgery. American Journal of Ophthalmology 2017;180:165‐77. [DOI] [PubMed] [Google Scholar]
- Zukaite I, Bedi KK, Ali S, Nanavaty MA. Influence of peripheral corneal relaxing incisions during cataract surgery for corneal astigmatism up to 2.5 dioptres on corneal densitometry. Eye 2019;33(4):804‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Titiyal 2014 {published data only}
- Titiyal JS, Khatik M, Sharma N, Sehra SV, Maharana PK, Ghatak U, et al. Toric intraocular lens implantation versus astigmatic keratotomy to correct astigmatism during phacoemulsification. Journal of Cataract and Refractive Surgery 2014;40(5):741‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carvalho 2007 {published data only}
- Carvalho MJ, Suzuki SH, Freitas LL, Branco BC, Schor P, Lima AL. Limbal relaxing incisions to correct corneal astigmatism during phacoemulsification. Journal of Refractive Surgery 2007;23(5):499‐504. [DOI] [PubMed] [Google Scholar]
Coloma‐González 2007 {published data only}
- Coloma‐González I, González‐Herrera M, Mengual‐Verdú E, Hueso‐Abancens JR. Limbal relaxing incisions and cataract surgery: our experience. Archivos de la Sociedad Espanola de Oftalmologia 2007;82(9):551‐4. [DOI] [PubMed] [Google Scholar]
Eliwa 2016 {published data only}
- Eliwa TF, Abdellatif MK, Hamza II. Effect of limbal relaxing incisions on corneal aberrations. Journal of Refractive Surgery 2016;32(3):156‐62. [DOI] [PubMed] [Google Scholar]
Holland 2010 {published data only}
- Holland E, Lane S, Horn JD, Ernest P, Arleo R, Miller KM. The AcrySof Toric intraocular lens in subjects with cataracts and corneal astigmatism: a randomized, subject‐masked, parallel‐group, 1‐year study. Ophthalmology 2010;117(11):2104‐11. [DOI] [PubMed] [Google Scholar]
Kaufmann 2005 {published data only}
- Kaufmann C, Peter J, Ooi K, Phipps S, Cooper P, Goggin M, et al. Limbal relaxing incisions versus on‐axis incisions to reduce corneal astigmatism at the time of cataract surgery. Journal of Cataract and Refractive Surgery 2005;31(12):2261‐5. [DOI] [PubMed] [Google Scholar]
Maedel 2014 {published data only}
- Maedel S, Hirnschall N, Chen YA, Findl O. Rotational performance and corneal astigmatism correction during cataract surgery: aspheric toric intraocular lens versus aspheric nontoric intraocular lens with opposite clear corneal incision. Journal of Cataract and Refractive Surgery 2014;40(8):1355‐62. [DOI] [PubMed] [Google Scholar]
Mendicute 2009 {published data only}
- Mendicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer‐Blasco T, Montés‐Micó R. Toric intraocular lens versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. Journal of Cataract and Refractive Surgery 2009;35(3):451‐8. [DOI] [PubMed] [Google Scholar]
Miyata 2011 {published data only}
- Miyata K, Miyai T, Minami K, Bissen‐Miyajima H, Maeda N, Amano S. Limbal relaxing incisions using a reference point and corneal topography for intraoperative identification of the steepest meridian. Journal of Refractive Surgery 2011;27(5):339‐44. [DOI] [PubMed] [Google Scholar]
Muller‐Jensen 1999 {published data only}
- Muller‐Jensen K, Fischer P, Siepe U. Limbal relaxing incisions (LRI) for correcting astigmatism in clear corneal cataract surgery. Ophthalmologe 1999;96(7):432‐6. [DOI] [PubMed] [Google Scholar]
- Muller‐Jensen K, Fischer P, Siepe U. Limbal relaxing incisions to correct astigmatism in clear corneal cataract surgery. Journal of Refractive Surgery 1999;15(5):586‐9. [DOI] [PubMed] [Google Scholar]
Nagpal 2015 {published data only}
- Nagpal R, Sharma N, Vasavada V, Maharana PK, Titiyal JS, Sinha R, et al. Toric intraocular lens versus monofocal intraocular lens implantation and photorefractive keratectomy: a randomized controlled trial. American Journal of Ophthalmology 2015;160(3):479‐86. [DOI] [PubMed] [Google Scholar]
Ouchi 2010 {published data only}
- Ouchi M, Kinoshita S. Prospective randomized trial of limbal relaxing incisions combined with microincision cataract surgery. Journal of Refractive Surgery 2010;26(8):594‐9. [DOI] [PubMed] [Google Scholar]
Roberts 2018 {published data only}
- Roberts HW, Wagh VK, Sullivan DL, Archer TJ, O'Brart DP. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. Journal of Cataract and Refractive Surgery 2018;44(8):955‐63. [DOI] [PubMed] [Google Scholar]
Shen 2004 {published data only}
- Shen Y, Tong JP, Li YM. Corneal relaxing incision combined with phacoemulsification and IOL implantation. Journal of Zhejiang University. Science 2004;5(8):985‐8. [DOI] [PubMed] [Google Scholar]
Solomon 2019 {published data only}
- Solomon KD, Sandoval HP, Potvin R. Correcting astigmatism at the time of cataract surgery: toric IOLs and corneal relaxing incisions planned with an image‐guidance system and intraoperative aberrometer versus manual planning and surgery. Journal of Cataract and Refractive Surgery 2019;45(5):569‐75. [DOI] [PubMed] [Google Scholar]
Wang 2013 {published data only}
- Wang XL, Zhang R. Estimation of the optical quality after implanting Acrysof ReSTOR multifocal intraocular lenses with corneal incision to correct astigmatism in cataract patients. International Eye Science 2013;13(11):2319‐21. [Google Scholar]
References to ongoing studies
NCT03633851 {published data only}
- NCT03633851. Toric IOL vs non‐toric IOL with LRI for corneal astigmatism. clinicaltrials.gov/ct2/show/NCT03633851 (first received 16 August 2018).
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2018
- Anderson DF, Dhariwal M, Bouchet C, Keith MS. Global prevalence and economic and humanistic burden of astigmatism in cataract patients: a systematic literature review. Clinical Ophthalmology 2018;12:439‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arraes 2006
- Arraes JC, Cunha F, Arraes TA, Cavalvanti R, Ventura M. Limbal relaxing incisions during cataract surgery: one‐year follow‐up. Arquivos Brasileiros de Oftalmologia 2006;69(3):361‐4. [DOI] [PubMed] [Google Scholar]
Baltussen 2004
- Baltussen R, Sylla M, Mariotti SP. Cost‐effectiveness analysis of cataract surgery: a global and regional analysis. Bulletin of the World Health Organisation 2004;82(5):338‐45. [PMC free article] [PubMed] [Google Scholar]
Braga‐Mele 2014
- Braga‐Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. Journal of Cataract and Refractive Surgery 2014;40(2):313‐22. [DOI] [PubMed] [Google Scholar]
Congdon 2003
- Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA 2003;290(15):2057‐60. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 16 May 2019. Melbourne: Veritas Health Innovation.
Day 2019
- Day AC, Dhariwal M, Keith MS, Ender F, Perez Vives C, Miglio C, et al. Distribution of preoperative and postoperative astigmatism in a large population of patients undergoing cataract surgery in the UK. British Journal of Ophthalmology 2019;103(7):993‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Freitas 2007
- Freitas W, Melo LA Jr, Schor P, Campos M. Comparative analyses between clinical refraction and automatic refraction obtained through a wave front sensor. Arquivos Brasileiros de Oftalmologia 2007;70(4):677‐82. [DOI] [PubMed] [Google Scholar]
Deeks 2019
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta‐analyses. Draft version (29 January 2019). In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. editor(s). Cochrane Handbook of Systematic Reviews. London: Cochrane, 2019. [Google Scholar]
Ferrer‐Blasco 2009
- Ferrer‐Blasco T, Montés‐Micó R, Peixoto‐de‐Matos SC, González‐Méijome JM, Cerviño A. Prevalence of corneal astigmatism before cataract surgery. Journal of Cataract and Refractive Surgery 2009;35(1):70‐5. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association : JMLA 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 16 May 2019. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Hoffmann 2010
- Hoffmann PC, Hutz WW. Analysis of biometry and prevalence data for corneal astigmatism in 23,239 eyes. Journal of Cataract and Refractive Surgery 2010;36(9):1479‐85. [DOI] [PubMed] [Google Scholar]
Kelman 1967
- Kelman CD. Phaco‐emulsification and aspiration. A new technique of cataract removal. A preliminary report. American Journal of Ophthalmology 1967;64(1):23‐35. [PubMed] [Google Scholar]
Kessel 2016
- Kessel L, Andresen J, Tendal B, Erngaard D, Flesner P, Hjortdal J. Toric intraocular lenses in the correction of astigmatism during cataract surgery: a systematic review and meta‐analysis. Ophthalmology 2016;123(2):275‐86. [DOI] [PubMed] [Google Scholar]
Khan 2011
- Khan MI, Muhtaseb M. Prevalence of corneal astigmatism in patients having routine cataract surgery at a teaching hospital in the United Kingdom. Journal of Cataract and Refractive Surgery 2011;37(10):1751‐5. [DOI] [PubMed] [Google Scholar]
Kohnen 2009
- Kohnen T. How far we have come: from Ridley's first intraocular lens to modern IOL technology. Journal of Cataract and Refractive Surgery 2009;35(12):2039. [DOI] [PubMed] [Google Scholar]
Lyall 2014
- Lyall DA, Srinivasan S, Ng J, Kerr E. Changes in corneal astigmatism among patients with visually significant cataract. Canadian Journal of Ophthalmology. Journal Canadien d'Ophtalmologie 2014;49(3):297‐303. [DOI] [PubMed] [Google Scholar]
Nissman 2006
- Nissman SA, Tractenberg RE, Saba CM, Douglas JC, Lustbader JM. Accuracy, repeatability, and clinical application of spherocylindrical automated refraction using time‐based wavefront aberrometry measurements. Ophthalmology 2006;113(4):577e1‐2. [DOI] [PubMed] [Google Scholar]
O'Brart 1994
- O'Brart DP, Corbett MC, Saunders DC, Rosen ES. The topography of corneal astigmatism. European Journal of Implant and Refractive Surgery 1994;6(6):361‐9. [Google Scholar]
Pascolini 2012
- Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. British Journal of Ophthalmology 2012;96(5):614‐8. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saunders 1995
- Saunders KJ. Early refractive development in humans. Survey of Ophthalmology 1995;40(3):207‐16. [DOI] [PubMed] [Google Scholar]
Schultz 2016
- Schultz M, Oberheide U, Kermani O. Comparability of an image‐guided system with other instruments in measuring corneal keratometry and astigmatism. Journal of Cataract and Refractive Surgery 2016;42(6):904‐12. [DOI] [PubMed] [Google Scholar]
Shemilt 2019
- Shemilt I, Aluko P, Graybill E, Craig D, Henderson C, Drummond M, et al. on behalf of the Campbell and Cochrane Economics Methods Group. Chapter 20: Economic evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sheppard 2013
- Sheppard AL, Wolffsohn JS, Bhatt U, Hoffmann PC, Scheider A, Hutz WW, et al. Clinical outcomes after implantation of a new hydrophobic acrylic toric IOL during routine cataract surgery. Journal of Cataract and Refractive Surgery 2013;39(1):41‐7. [DOI] [PubMed] [Google Scholar]
Talamo 1991
- Talamo JH, Stark WJ, Gottsch JD, Goodman DF, Pratzer K, Cravy TV, et al. Natural history of corneal astigmatism after cataract surgery. Journal of Cataract and Refractive Surgery 1991;17(3):313‐8. [DOI] [PubMed] [Google Scholar]
Visser 2013
- Visser N, Bauer NJ, Nuijts RM. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. Journal of Cataract and Refractive Surgery 2013;39(4):624‐37. [DOI] [PubMed] [Google Scholar]
Weale 1983
- Weale RA. Ocular anatomy and refraction. Documenta Ophthalmologica 1983;55(4):361‐74. [DOI] [PubMed] [Google Scholar]
WHO 2013
- WHO. Universal eye health: a global action plan 2014–2019. World Health Organization. Geneva (Switzerland): World Health Organization, 2013:1‐22. [Google Scholar]
References to other published versions of this review
Lake 2017
- Lake JC, Victor G, Santhiago MR, Clare G, Porfírio GJM. Limbal relaxing incisions versus toric intraocular lens for corneal astigmatism after phacoemulsification. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD012801] [DOI] [PMC free article] [PubMed] [Google Scholar]


