Abstract
Active coral restoration typically involves two interventions: crossing gametes to facilitate sexual larval propagation; and fragmenting, growing, and outplanting adult colonies to enhance asexual propagation. From an evolutionary perspective, the goal of these efforts is to establish self‐sustaining, sexually reproducing coral populations that have sufficient genetic and phenotypic variation to adapt to changing environments. Here, we provide concrete guidelines to help restoration practitioners meet this goal for most Caribbean species of interest. To enable the persistence of coral populations exposed to severe selection pressure from many stressors, a mixed provenance strategy is suggested: genetically unique colonies (genets) should be sourced both locally as well as from more distant, environmentally distinct sites. Sourcing three to four genets per reef along environmental gradients should be sufficient to capture a majority of intraspecies genetic diversity. It is best for practitioners to propagate genets with one or more phenotypic traits that are predicted to be valuable in the future, such as low partial mortality, high wound healing rate, high skeletal growth rate, bleaching resilience, infectious disease resilience, and high sexual reproductive output. Some effort should also be reserved for underperforming genets because colonies that grow poorly in nurseries sometimes thrive once returned to the reef and may harbor genetic variants with as yet unrecognized value. Outplants should be clustered in groups of four to six genets to enable successful fertilization upon maturation. Current evidence indicates that translocating genets among distant reefs is unlikely to be problematic from a population genetic perspective but will likely provide substantial adaptive benefits. Similarly, inbreeding depression is not a concern given that current practices only raise first‐generation offspring. Thus, proceeding with the proposed management strategies even in the absence of a detailed population genetic analysis of the focal species at sites targeted for restoration is the best course of action. These basic guidelines should help maximize the adaptive potential of reef‐building corals facing a rapidly changing environment.
Keywords: adaptive potential, assisted gene flow, biomarkers, coral restoration, genetic diversity, inbreeding, outbreeding, phenotypic resilience, population enhancement, species selection, unintended selection
Introduction
Coral reef ecosystems face unparalleled destruction due to anthropogenic climate change (McClenachan et al. 2017, Hughes et al. 2018), and protective coping mechanisms observed in extant coral populations may be overwhelmed by the rate of ocean temperature change (Ainsworth et al. 2016). Thus, the recovery of reef ecosystems hinges on immediate and decisive actions to reduce CO2 emissions globally. Meanwhile, large investments in coral reef restoration and the population enhancement of critical reef‐building species have been made in an effort to revive coral communities and maintain some ecosystem function and services (Ladd et al. 2018). Technologies such as artificial selection or genetic engineering are being contemplated to “design” coral populations capable of withstanding current and future environmental challenges. However, field applications of these technologies are not yet ready (van Oppen et al. 2001, Torda et al. 2014, Cleves et al. 2018). Challenges include our rudimentary understanding of the genetic basis of traits that will be essential in the future and limited knowledge of their interactions (i.e., trade‐offs; Muller et al. 2018), as well as logistical difficulties of propagating and outplanting engineered corals at ecologically significant scales. Protocols for assessing the risks associated with such interventions are being developed but have yet to be widely adopted (Baums 2008, IUCN/SSC 2013).
We contend that currently the best approach to improve reef resilience via direct, restoration‐focused intervention is to harness and foster the adaptive genetic diversity that is already present in coral populations while efforts to reduce greenhouse gas emissions continue (Kleypas et al. 2016, Bay et al. 2017, Matz et al. 2018). Every coral species exists across a variety of environmental gradients, some of which occur over small spatial scales (e.g., fore, back, and patch reefs) while others occur over very large ones (e.g., across ocean‐basins). There is mounting evidence that corals genetically adapt to their local conditions (Polato et al. 2010, Barshis et al. 2013, Kenkel et al. 2013, Dixon et al. 2015), so each species is in fact a system of sub‐populations adapted to diverse environments and connected by larval migration. In linked sets of metapopulations, global change could be rapidly matched by the recombination of pre‐existing adaptive genetic variants, i.e., adaptation based on standing genetic variation (Hermisson and Pennings 2005, Baums 2008, Whiteley et al. 2011). Increasingly severe and frequent mortality events have already led to widespread and continued loss of coral cover (Eakin et al. 2010, Smith et al. 2013, Precht et al. 2016, Walton et al. 2002) and exerted strong selection pressure on these metapopulations. Therefore, adaptation is likely to be rapid: survivors of high mortality events are particularly robust and may be expected to eventually spawn a new generation of better‐adapted corals (Libro and Vollmer 2016, Muller et al. 2018). However, reduced connectivity between these metapopulations, lower fertilization rates because colonies are spread further apart (i.e., allee effects; Knowlton 2001), and universally declining environmental conditions across most reef sites reduce the realization of this potential. If local genetic variation can be maintained, environmental conditions can be stabilized, and genetic exchange among populations can continue unimpeded, adaptation may allow corals to keep pace with climate change for the next 100 years or longer (Matz et al. 2018). In this context, many interventions are available now that can help maintain high local genetic variation and continued exchange among populations.
From this evolution‐centric perspective, the goal of restoration is to establish self‐sustaining, sexually reproducing coral populations thereby promoting continuous genetic adaptation of the species, both locally and throughout its range. This approach is congruent with endangered species recovery goals and aligned with but distinct from some current restoration guidelines focused on ecological goals such as coral cover or habitat provision. Measuring success using these metrics by themselves may be insufficient to ensure the long‐term future of restored corals, as ecological success may only be temporary in the absence of self‐sustaining coral populations. Instead, our focus is on providing practical guidelines to maintain the genetic diversity and phenotypic resilience required for corals to survive and produce genetically diverse and viable offspring that could serve as raw material for natural selection. Layering both evolutionary and ecological components will maximize the resilience of restored coral populations.
The need for concrete guidelines is particularly pressing in the Caribbean, where investments in active restoration of coral populations have occurred over the last two decades and continue to increase at a rate that exceeds similar efforts in other regions (Young et al. 2017, Chamberland et al. 2015, Lirman and Schopmeyer 2016). Caribbean reefs also tend to be highly degraded and suffer from long‐term recruitment failure of the two major reef‐building species, Acropora palmata (Williams et al. 2015) and Orbicella faveolata (Hughes and Tanner 2000), Although some reefs still feature substantial stands of these species, without sexual recruitment they may already be effectively extinct (Honnay and Bossuyt 2005). Most alarmingly, since there is no ecological redundancy in the Caribbean to replace these species in their reef‐accretion function, many Caribbean reefs have shifted from net accretional to net erosional states (Kuffner and Toth 2016, Yates et al. 2018). Shifts to net erosional states are now a global phenomenon (Perry et al. 2018), with concomitant increases in the vulnerability of coastal communities to inundation and shoreline erosion throughout the tropical world (Beck et al. 2018, Storlazzi et al. 2018). Although outplanting corals using nursery‐propagated stock can help restore some ecological functions (Montoya Maya et al. 2016) and may buy time to prevent regional extirpation, without sexual recombination and the shuffling of alleles to promote adaptation, the long‐term future of these corals appears bleak.
The guidelines presented here aim to re‐establish populations that are capable of sexual recruitment and genetic exchange (Fig. 1). These include recommendations on how different reef habitats should be strategically sampled to capture much of the adaptive genetic variants existing in coral populations, and how sampled genets should be outplanted and monitored. We discuss possible enhancements to restoration practices such as routine genotyping of propagated stock, trait‐based assessment of genet performance, jump‐starting genetic admixture by producing first‐generation offspring for outplanting, and promoting long‐range genetic exchange (“assisted gene flow,” AGF). In addition, because corals host photosynthetic micro‐algae [family Symbiodiniaceae sensu LaJeunesse et al. (2018)], we also consider possibilities for the management of endosymbiont diversity in restored populations. Finally, we discuss the balance of risks and benefits associated with implementing the recommended strategies. The review is the product of the Coral Restoration Genetics Working group, one of the five original working groups of the Coral Restoration Consortium (Appendix S1: Section 1).
Figure 1.
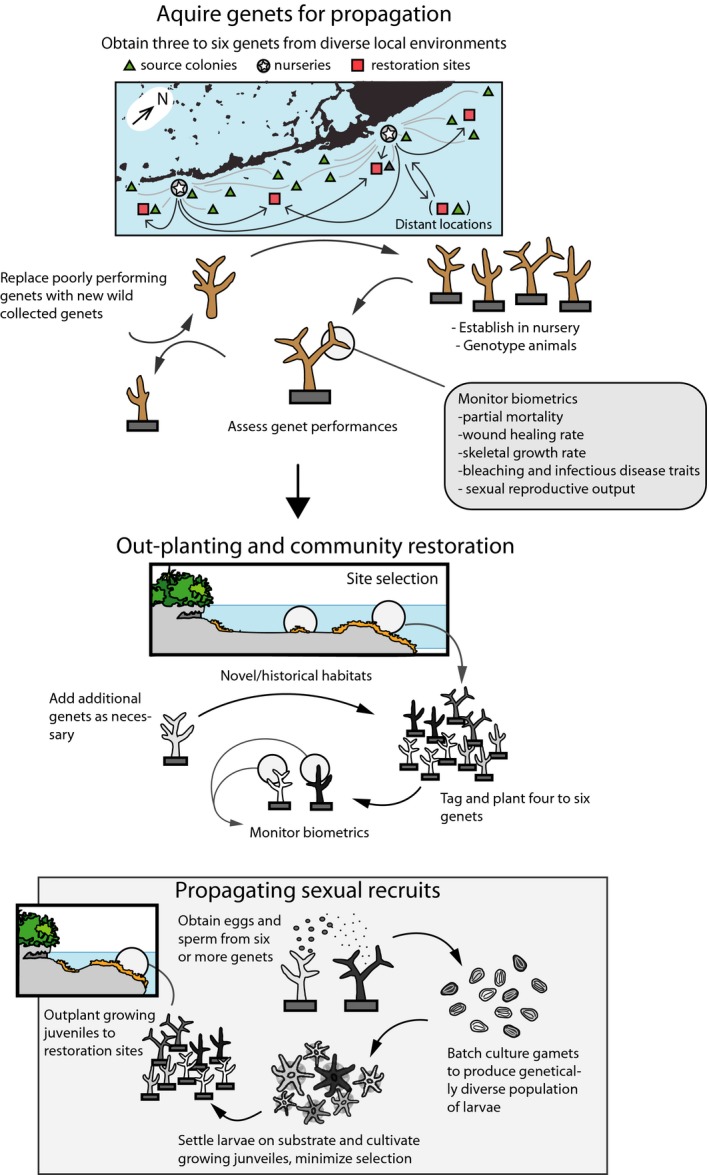
Overview of basic restoration guidelines to maximize the adaptive potential of reef‐building corals facing a rapidly changing environment. A genet is defined as a genetically unique colony or collection of colonies (ramets) that can trace their ancestry back to the same sexual reproductive event (i.e., they stem from the same settler and, hence, share the same genome).
Prioritizing Coral Species for Restoration Efforts
Coral species selection for restoration will depend upon the goals of the project, which could include shoreline protection, fisheries habitat provisioning, species conservation, tourism, or a combination thereof. Given that funding, nursery space, and time are limited, we propose that ecosystem‐based restoration efforts for Caribbean corals should prioritize species that are (1) foundation reef builders; (2) experiencing severe declines in cover (Appendix S2: Fig. S1); and (3) consistently failing to sexually recruit (Fig. 2). In the Caribbean and western north Atlantic, only a limited number of coral species build reef framework, the overall species diversity is lower, and functional redundancy is limited compared to the Indo‐Pacific (Bellwood et al. 2004), making species selection comparatively straightforward. There is a striking lack of sexual recruitment for many of the framework‐building species throughout the basin (Hughes and Tanner 2000, Edmunds and Elahi 2007, Williams et al. 2015, Davies et al. 2013a) and recruitment has shifted from long‐lived broadcast‐spawning species to more weedy brooding species (Rogers et al. 1984, Hughes and Tanner 2000, Green et al. 2008, but see Vermeij et al. 2014).
Figure 2.
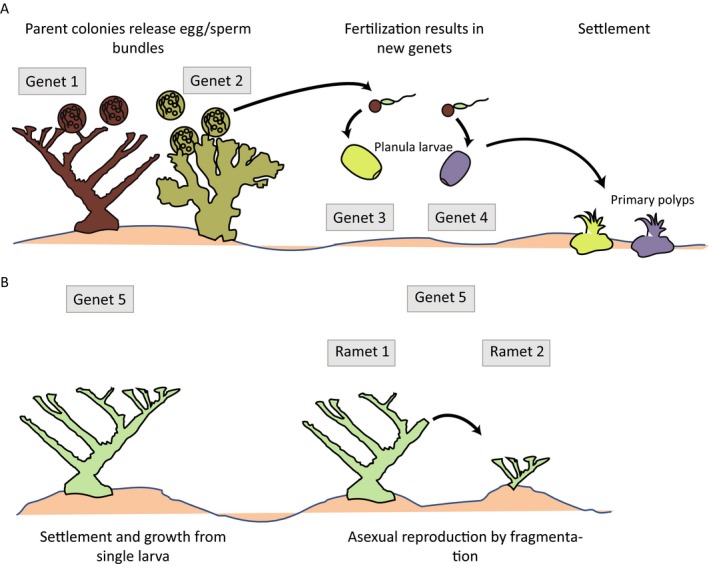
Coral reproduction. (A) Most reef‐building species in the Caribbean are self‐incompatible hermaphroditic broadcast spawners. Adult colonies release egg–sperm bundles that float to the surface where they break apart and mix with gametes from other colonies of the same species. After fertilization and development, larvae settle onto the reef, metamorphose into primary polyps, and grow into new genets. (B) Over time, genets may fragment. These fragments can reattach to form new ramets of the same genet. A genet is defined as a genetically unique colony or collection of colonies (ramets) that can trace their ancestry back to the same sexual reproductive event (i.e., they stem from the same settler and, hence, share the same genome). Adopted from Devlin‐Durante et al. (2016).
Current restoration efforts target Acropora cervicornis, A. palmata, Orbicella faveolata, and O. annularis. All are broadcast‐spawning species and are among those experiencing widespread population declines and sexual recruitment failure (van Woesik et al. 1993). A. cervicornis takes up the most space in current nurseries (Lirman and Schopmeyer 2016). On some reefs in Belize and the Dominican Republic, A. cervicornis has been restored to high densities (Lirman and Schopmeyer 2016) and outplants are spawning (Carne and Baums 2016). In Florida, a shift to A. palmata and multi‐species restoration with an emphasis on reef‐building is now underway. Additional efforts have been extended locally to species where extirpation might be imminent (i.e., Dendrogyra cylindrus in the Florida Keys). We suggest that efforts be expanded to target Pseudodiploria spp., Siderastrea siderea, Stephanocoenia intersepta, Montastrea cavernosa, Colpophyllia natans, and Orbicella franksi, as these species each meet two prioritization criteria (Appendix S2: Fig. S1), are already managed in some locations, and are good candidate species for rebuilding reef structure long‐term, in part because these species still successfully produce sexual recruits.
For coral taxa that are rare or not major reef builders (e.g., Dendrogyra, Agaricia, Porites, Dichocoenia, Favia), we propose strategic genetic banking as a complementary activity to active restoration (Hagedorn et al. 2012). This compromise will free up resources for major reef builders of immediate concern while safeguarding genetic material for future action on these rare members that increase community diversity and may play important, if unknown, roles in ecosystem processes (Bellwood et al. 2006). Thus, efficient cryo‐banking techniques should be developed for species whose gametes can be collected, and ex situ gene banking (i.e., keeping live animals in aquaria) might be considered for others. We also recommend that restoration feasibility studies be conducted for rare species experiencing massive population declines, multi‐year sexual recruitment failure, and those that exhibit unique life histories such as Dendrogyra cylindrus (Neely et al. 2018, Chan et al. 2019), to help identify and address challenges associated with restoring these species. Lastly, we propose that species prioritizations should follow an adaptive management strategy under which demographic monitoring of natural and outplanted populations is evaluated every 5–10 years to determine changes in population growth rates (λ) or sexual recruitment rates (which serve as a reasonable proxy when λ cannot be estimated). Sexual recruitment rates can be monitored via both recruitment tiles (Humanes and Bastidas 2015) and genotyping (Box 1; Tables 1, 2; Appendix S3: Section S1).
Table 1.
An overview of different genotyping methods employed for Caribbean coral species and their symbionts
| Species | Allozymes | AFLP | Msats | SNPs | References |
|---|---|---|---|---|---|
| Coral | |||||
| Acropora cervicornis | ✓ (12) | ✓ | Baums et al. (2005a, 2009), Drury et al. (2016) | ||
| Acropora palmata | ✓ | ✓ (13) | ✓ | Baums et al. (2005a, 2009), Devlin‐Durante and Baums (2017) | |
| Dendrogyra cylindrus | ✓ (11) | Chan et al. (2019) | |||
| Favia fragum | ✓ (15) | Carlon and LippE (2008) | |||
| Orbicella annularis | ✓ | ✓ | ✓ (14) | ✓ | Van Veghel and Bak (2011), Lopez et al. (1999), Fukami et al. (2004), Severance et al. (2004), Davies et al. (2013b), Prada et al. (2016) |
| Orbicella faveolata | ✓ | ✓ (9) | ✓ | Fukami et al. (2004), Lopez et al. (1999), Davies et al. (2013b), Prada et al. (2016) | |
| Montastrea cavernosa | ✓ | ✓ (14) | Shearer and Coffroth (2004), Brazeau et al. (2013), Serrano et al. (2014), Jarett et al. (2017) | ||
| Porites astreoides | ✓ | ✓ | ✓ (15) | Weil (2018), Brazeau et al. (1998), Shearer and Coffroth (2004), Kenkel et al. (2013), Serrano et al. (2016) | |
| Symbiont | |||||
| Symbiodinium microadriaticum (ITS2 type A1) | ✓ | Schoenberg and Trench (1980) | |||
| S. “fitti” (ITS2 type A3) | ✓ (13) | Baillie et al. (2000a, b), Pinzón et al. (2011), Baums et al. (2014a) | |||
| Breviolum “dendrogyrum” (ITS2 type B1) | ✓ (7) | Santos and Coffroth (2003), Pettay and LaJeunesse (2007), Chan et al. (2019) | |||
| B. endomadracis (ITS2 type B7) | ✓ (3) | Santos and Coffroth (2003), Pettay and LaJeunesse (2007) | |||
| B. minutum (ITS2 type B1) | ✓ (3) | Santos and Coffroth (2003), Pettay and LaJeunesse (2007) | |||
| B. psygmophilum (ITS2 type B2) | ✓ (6) | Pettay and LaJeunesse (2007), Grupstra et al. (2017) | |||
| Durusdinium trenchii (ITS2 type D1a) | ✓ (17) | Pettay and LaJeunesse (2009), Wham et al. (2001) | |||
AFLP, amplification fragment length polymorphism; Msats, microsatellites; SNPs, single nucleotide polymorphisms. A ✓ indicates that the method was used for that species. Numbers in parenthesis give the number of microsatellite loci available.
Table 2.
Comparison of the microsatellites and SNP genotyping methods
| Measure | Msats | SNP‐based methods | ||||
|---|---|---|---|---|---|---|
| Targeted‐enrichment capture | Microarray | Microfluidics | Traditional GBS/RAD‐tag | RAD capture | ||
| No. markers | 101 | 102 | 103–105 | 102 | 103–105 | 102–103 |
| Minimum no. samples | 1 | 6 | 96 | 96 | 1 | 1 |
| Sample preparationa | moderate | moderate | low‐moderate | low | low‐moderate | low‐ moderate |
| Technical expertiseb | moderate | high | moderate | moderate‐high | high | moderate |
| Computational resourcesc | low | high | moderate | moderate | high | moderate |
| Reproducible between labsd | moderate | high | high | high | high | high |
| Estimated price per sample | US$50 | US$450 | US$50 | US$10 | US$75 | US$50–70 |
Low, library preparation and sequencing can be completed at a core‐facility provider; moderate, multiple‐day process but limited hands‐on time.
Based on training requirements. Low, minimal training; moderate, some advanced training; high, highly advanced training (Grover and Sharma 2016).
Based on the computational resource demands. Low, analyzed on a standard computer with minimal storage; moderate, analyzed on standard computer with large data storage requirements; high, analyzed on high‐performance computer with large data storage.
Sensitivity of the method to differences between laboratories or sequencing facilities. Moderate, analysis can be impacted by laboratory conditions (e.g., different PCR buffer or PCR machine can result in loci running at different sizes) and experience of user; High, analysis can be impacted by high sequencing error rates, but genotype calls are not influenced by user.
Box 1: Determining genetic and genotypic diversity of coral hosts and symbionts.
Coral host genotypic diversity: In this review, we discuss the importance of both genotypic and genetic diversity on restoration decisions. A “genet” is defined as a genetically unique colony or collection of colonies (“ramets”) that can trace their ancestry back to the same sexual reproductive event (i.e., they stem from the same settler and, hence, share the same genome). Genotypic diversity is the total number of genetically distinct individuals (genets) within a population, whereas genetic diversity is the amount of variation between genotypes on the level of individual genes (Fig. 3). To track the genotypic diversity of corals and their symbionts, it is necessary to determine their unique multilocus genotypes (MLGs) via genetic analysis (Appendix S3: Section 1). While various genotyping approaches have been developed for many of the Caribbean coral species, including allozymes, amplification fragment length polymorphisms (AFLP), microsatellites, and single nucleotide polymorphisms (SNPs; Table 1), the latter two are more routinely used in conservation genetics today (Puckett 2017). Furthermore, microsatellite and SNP loci provide higher allelic variation that can be used to discriminate colonies that share a MLG because they were generated via asexual fragmentation versus those colonies that share an MLG because they are closely related (such as siblings).
Tracking symbiont community diversity is also recommended for fragments and reasonably sized juveniles when possible (see Role of Symbionts). The resolution of Symbiodiniaceae genotypic diversity is constrained by the genetic tools available. The standard internal transcribed spacer 2 (ITS2; LaJeunesse 2001) and chloroplast 23S (cp23S; Santos et al. 2002) rDNA markers are usually sufficient to resolve symbionts to approximately the species level. High‐throughput methods for ITS2 have been developed (Arif et al. 2014, Quigley et al. 2014, Thomas et al. 2018, Smith et al. 2017), although they inevitably underestimate diversity because ITS2 does not resolve all Symbiodiniaceae species (Parkinson et al. 2015b). Methods thus need to be adjusted depending on whether a particular colony is expected to host only one numerically dominant symbiont species (in which case direct sequencing is appropriate) or if it hosts multiple co‐dominant symbiont species (in which case denaturing gradient gel electrophoresis or high‐throughput amplicon sequencing are required; Pochon et al. 2018). Finer‐scale resolution of symbiont diversity at the sub‐species level could be provided by the psbA minicircle non‐coding region (Moore et al. 2003, Barbrook et al. 2006, LaJeunesse and Thornhill 2011), microsatellites (Table 1), or SNP markers (currently under development).
Genotyping technology: With the increasing scale of restoration activities, genotyping efforts need to be simplified at minimal costs (Appendix S3: Section 1). Technological advancements in high‐throughput SNP‐based methods such as genotype‐by‐sequencing (GBS) and reduced representation sequencing methods (collectively called RAD‐seq) have made it possible to assess genetic diversity at a large number of single nucleotide variant (SNV) loci for a reasonable cost (Altshuler et al. 2000). However, there is no guarantee that the same set of SNV loci is recovered from each sample in a run or between runs, making clone identification more challenging. Other SNP‐based methods using standardized markers, such as target‐enrichment capture, RAD capture (Hoffberg et al. 2016), microarrays, and microfluidics, also provide information on the order of 102–105 of variants reproducibly (Table 2; Dixon et al. 2016). Regardless of which new approach is chosen, all these methods present additional hidden challenges, requiring advanced bioinformatic training, computational infrastructure, and increased data storage (Table 2). Because resource allocation is an important consideration for practitioners when selecting a genotyping method, we consider two options in depth for obtaining coral MLGs based on the availability of common laboratory equipment, computational resources, and overall budget (Appendix S3: Section S1).
Figure 3.
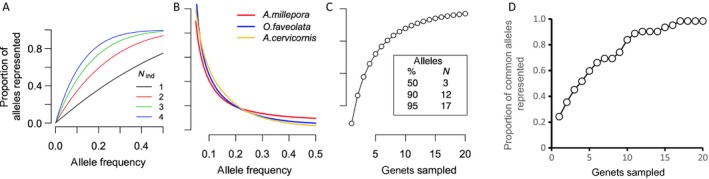
Capturing allelic diversity for coral conservation. Sampling of relatively few genets from a population is sufficient to capture most common alleles. (A) Proportion of alleles observed when sampling a certain number of genets (N ind) from a population, depending on the allele frequency. For an allele of frequency P, the probability of not observing it among N diploid genets is (1 − P)2N. Hence, the probability of observing such an allele, which is the same as the proportion of such alleles across the whole genome that are observed, is 1 − (1 − P)2N. (B) The proportion of common alleles (allele frequency P > 0.05) depends on their allele frequency in three species of reef‐building corals and can be calculated based on single nucleotide polymorphism (SNP) data. The three species (Acropora millepora, Orbicella faveolata, and Acropora cervicornus) have broadly similar distributions of their common alleles, despite substantial phylogenetic and geographic separation. (C) Proportion of common SNP alleles represented within a certain number of sampled genets. A sample of just three genets captures >50% of all SNP alleles found in more than 5% of all genomes in the population, and 12 genets capture 90% of them. (D) Proportion of common alleles represented calculated based on microsatellite loci for Acropora palmata. Note that due to higher mutation rate of microsatellites they require four rather than three genets to represent >50% of alleles.
Choosing Coral Colonies for Restoration: Who and From Where?
Active restoration of the major Caribbean reef builders, specifically the acroporids and the orbicellids, is well underway, and restoration practitioners have already chosen many genets for propagation and outplanting. The classical precept of primum non nocere (“first, do no harm”) has traditionally been translated into precautionary concerns about genetic risks, especially preventing genetic swamping or outbreeding depression from affecting the integrity of local populations (Edmands 2007). This premise underlies why the sourcing of restoration material from local areas has been preferred traditionally. This “local is best” (LIB) provenance strategy is sometimes operationally regulated (Tringali et al. 2018), placing strict limits on the geographic range of sourcing corals. However, the poor performance of most local coral populations in recent years suggests that whatever local adaptation is present in these populations may not be adequate to assure persistence as environments continue to change (Williams et al. 2015, b , Chan et al. 2019). Hence, we advise considering more flexible provenancing strategies.
Plant restoration planners have similarly articulated provenance strategies that may improve the prospects for climate adaptation in restored populations (Sgrò et al. 2011, Williams et al. 2008 a, Prober et al. 2015, Espeland et al. 2017). The simple approach of selecting warmer adapted genets assumes that predictions of environmental conditions are known and that there are no ecological trade‐offs in phenotypes (e.g., poor reproductive performance or poor disease resistance in heat‐tolerant genets). Hence, a so‐called “climate‐adjusted provenance” strategy (sensu Prober et al. 2015) is favored, which incorporates representation of both local genets and genets from across an environmental gradient skewed toward those coming from populations already experiencing predicted future environmental conditions (Fig. 1).
Assisted gene flow
A climate‐adjusted provenance strategy is recommended when designing assisted gene flow (AGF) interventions. Such AGF interventions recognize that local adaptation is a characteristic of local populations that can be used to improve the fitness of distant populations, especially in scenarios of rapid environmental change. Deliberate translocation of organisms, propagules, or genes among populations can be an effective means to facilitate the spread of adaptive alleles, particularly in areas where populations have declined to the point where colony density or genetic diversity may be insufficient to support successful sexual production of offspring (e.g., where only one known genet persists, (Baums et al. 2006, 2014b). Foundation species with wide ranges and large population sizes can be particularly good candidates for AGF to yield ecosystem‐scale benefits (Aitken and Whitlock 2013). Many coral species fit this profile, and AGF was suggested as a viable management strategy for corals as long as a decade ago (Hoegh‐Guldberg et al. 2008, Riegl et al. 2011).
Embracing AGF interventions has been suggested by several studies (Hoegh‐Guldberg et al. 2008, Dixon et al. 2015, Matz et al. 2018) because corals adapt to local temperature conditions at the genetic level (Polato et al. 2010, Bay and Palumbi 2014, Palumbi et al. 2014, Dixon et al. 2015) and therefore genetic variants conferring heat tolerance should already be present at high frequencies in warm‐adapted populations. In some cases, natural migration appears to be sufficient to exchange these adaptive genetic variants among thermal environments (Matz et al. 2018). Nevertheless, assisted translocation could help ensure such exchange, especially because warming ocean temperatures are expected to change dispersal patterns by shortening planktonic periods and altering current speeds and direction (Heyward and Negri 2010, Baums et al. 2013, Figueiredo et al. 2014, Wood et al. 2006). Models based on population genetic data from Acropora millepora, a common coral on the Great Barrier Reef, show that even when immigrants account for as much as 1–3% of the total population there is still no risk of “migrational meltdown” (a reduction of fitness of local population because of an influx of maladaptive alleles) (Matz et al. 2018). This suggests that human‐assisted migration would not pose risks for coral populations still numbering in the tens of thousands, although this may become an issue for populations that have experienced severe declines, such as those in the Florida Keys. Another consideration is the possible limiting effects of cold tolerance when out planting corals sourced from warner environments to colder ones (Howells et al. 2013).
Nursery and breeding stock selection
In situ nurseries have become an important source for coral stock used in outplanting and breeding efforts. One goal of a nursery should be to ensure good representation of adaptive alleles that are beneficial in various present‐day reef environments, encompassing a sizeable portion of adaptive genetic diversity existing within the species. At this time, we rely on only a handful of traits to identify resilient coral colonies that may carry potentially adaptive alleles, we do not know the identity of the potentially adaptive alleles, and we have very few reliable biomarkers (Box 2). Therefore, we propose the following strategies to maximize the chance that nursery collections will capture alleles that help coral species adapt to various environments (Fig. 3).
Box 2: Biomarkers.
Biomarkers have emerged as useful tools in many fields, from plant breeding to human disease prediction. Recently, they have been suggested as attractive candidates for use in coral restoration. In theory, restoration practitioners would use a simple bioassay to uncover information about coral colony performance. This information could then be applied to select colonies for parental stocks in larval propagation, rearing in nurseries, or matching outplants to particular sites in anticipation of future environmental challenges. However, biomarkers are often context‐ and species‐specific (Parkinson et al. 2018a), and therefore multiple markers may be needed depending on the types of information desired. In addition, there are many steps between identifying a potential biomarker and deploying it in the field, and the intermediate steps involved in biomarker development are often overlooked. The process involves four major phases: discovery, validation, field trials, and implementation. At present, the majority of basic scientific research has not progressed past the discovery phase (Parkinson et al. 2018a). Consequently, the costs associated with developing practical biomarkers as predictive tools for selective restoration may be high. While the potential savings in terms of time and effort may justify the initial investment, a cost‐benefit analysis must be considered when prioritizing funding for further research in this area at this time.
Identify environmentally diverse source reef patches. Within the largest practical area, identify reef sites that differ in their environmental conditions. Ideally, as part of a climate‐adjusted provenance strategy, some of these sites would feature projected future conditions, such as elevated temperature, more variable temperature regimes, and/or lower aragonite saturation state. Our current understanding is that local adaptation in corals happens most prominently with respect to depth (Bongaerts et al. 2011, Prada and Hellberg 2013, Cohen and Dubinsky 2015). But other factors can play a role in adaptation such as temperature (Howells et al. 2013), especially its daily range (Palumbi et al. 2014, Kenkel et al. 2015a). Other environmental parameters such as pH (Comeau et al. 2014, Schoepf et al. 2017), turbidity (Anthony 2000, Anthony and Fabricius 2000), or levels of inorganic nutrients may also play a role in local adaptation, though more research into these specific factors will be required. Even in the absence of detailed environmental data, distinct reef habitats can be identified as sites hosting noticeably different communities of reef organisms. For the success of the sourcing strategy it is important to sample as widely as possible along environmental gradients, in the same depth range as the target restoration sites. The latter is because corals from deep reef habitats (>15–20 m) may be partially or completely reproductively isolated from shallow populations of the same species (Prada and Hellberg 2013, Serrano et al. 2014, 2016, Bongaerts et al. 2017) and may be unsuitable for propagation in shallow nurseries or for restoration of shallow sites.
Select colonies for propagation. Collect three to six coral genets per patch, selecting healthy colonies that show some difference in morphology and/or size. They should be growing some distance apart (>5 m; Baums et al. 2006, Foster et al. 2007) to maximize the chance of sampling distinct genets rather than clonemates (Box 1). Collecting just a few colonies per patch might seem insufficient but just three or four diploid colonies would contain about one‐half of all common alleles (frequency >5%) present in a population (Fig. 3). Thus, sampling of three to four colonies from a patch would capture many, if not most, alleles that are locally adaptive since such alleles are expected to be common within the patch due to natural selection.
Monitor, replace, and repeat. Propagate selected colonies in the nursery and monitor their performance (see Phenotypic traits of propagated corals). If some corals show poor fitness in the nursery, replace them with other genets from the same patch or habitat type. Excluding poorly performing genets is unlikely to affect representation of the adaptive genetic diversity in the nursery provided representation of all habitat types is maintained. However, it is possible that local adaptation to some reef environments would prove incompatible with the nursery, which is itself a distinct habitat. Although genetic variants in these corals are still valuable as part of the adaptive genetic diversity of the species, we suggest triaging such cases (i.e., omitting genets that perform poorly in nurseries) or omitting the nursery stage by rearing fragments at the native reef (protocols for this are under development; Fragments of Hope, personal communication). If survival and growth is merely suboptimal in nurseries rather than absent, then propagating poorly performing genets in the nursery at a minimal level may be warranted if they are performing particularly well during outplanting and have other desirable traits (O'Donnell et al. 2018).
The resulting minimum number of coral genets for each species propagated in a nursery should be on the order of 20–25 and include representatives from reef sites spanning the full range of environmental variation occupied by the species within the restoration jurisdiction. This number of genets would contain >95% of the common alleles present locally within a species (Fig. 3). This approach still omits most of the genetic diversity existing within a species simply because the clear majority of natural genetic variants are rare. In fact, by far the most common type of genetic variant is a singleton, an allele present in a single genet of the species. However, to capture these would require sampling essentially all genets in a species, an approach that is not practical. Rare alleles are by no means useless; while they might not be adaptive at present (given their low abundance) they might become adaptive in the future, when conditions change. Indeed, long‐term adaptation will eventually require replacement of present‐day common adaptive alleles with new ones that are currently rare or even non‐existent (requiring a new mutation to arise). Despite this, comprehensive sampling of alleles that are currently common serves a very important purpose: it gives the restored population a better chance of surviving in the immediate future. With climate‐adjusted provenancing, survival trajectories might be extended by several decades. The longer‐term survival of a restored population will only be ensured if gene flow occurs with adjacent populations (introducing immigrants from other populations that bear novel adaptive alleles), or if adaptive mutations occur within the restored population.
Sexual propagation and selection of donor colonies
The short‐term adaptive response based on common alleles outlined in the previous section can only happen if outplanted genets reproduce sexually to generate novel allele combinations. Without offspring of such crosses recruiting back to the restored reef, the scope for natural selection cannot extend beyond the originally outplanted genets, and no further adaptation would be possible (barring somatic mutations and genotypic mosaicism; Van Oppen et al. 2015). In this regard, outplanting hundreds or thousands of lab‐reared, sexually produced offspring instead of (or in addition to) adult coral fragments is promising because it would accelerate the process of genetic restoration by one generation. Outplanting sexually produced offspring would be especially valuable for species experiencing long‐term failure of natural recruitment, such as the Caribbean acroporids and orbicellids. Currently in the western Atlantic, gametes for larval production are harvested from wild populations, as well as from fragments in nurseries, via colony netting and subsequent ex situ fertilization in culture vessels. Larval offspring are eventually returned to the field at various stages of development. Sexual propagation and selection of donor colonies should be based on the following strategies.
Maximize fertilization success
Most major reef‐building coral species are broadcast spawners that are highly outbred and genetically diverse (Baums 2008, Baird et al. 2009). Therefore, it is usually not necessary to determine their relatedness to avoid inbreeding because the likelihood of picking two parents in natural populations that are closely related as a result of sexual reproduction (i.e., are siblings) is vanishingly low (Fig. 2). It must be noted, however, that some Caribbean reef builders (Acropora spp., Orbicella spp., D. cylindrus) can be highly clonal (Fig. 2), where neighboring colonies are genetically identical due to asexual fragmentation (Baums et al. 2006, Foster et al. 2007, Miller et al. 2017). These species are hermaphroditic, but are practically self‐incompatible, and consequently successful sexual reproduction requires gametes to be available from (at least) two different genets (Fogarty et al. 2012, Baums et al. 2013). To minimize the chance of harvesting gametes from the same genet, it can be helpful to sample colonies that are relatively far apart (>5 m) (Baums et al. 2006, Foster et al. 2013). The only definitive solution to the clonality problem is to genotype sampled corals (Box 1), which is highly recommended if the budget allows (Appendix S1–S3). Because broadcast‐spawning species typically acquire their algal symbionts from the environment horizontally each generation, effects of breeding design on the population structure and diversity of these symbionts are not a major concern (however, the situation may be different for species that transmit their Symbiodiniaceae from parent to offspring vertically each generation; see Role of Symbionts). In some regions, sexual compatibility can be surprisingly low among conspecific Caribbean acroporids and orbicellids (Fogarty et al. 2012, Baums et al. 2013, Miller et al. 2017), for unknown reasons. For example, there are no data supporting the notion that corals from contrasting environments have lower cross‐fertilization rates. Dixon et al. (2015) crossed colonies from very different thermal regimes and did not observe any differences in fertilization rates (it was nearly 100% for both between‐ and within‐population crosses). In addition, the typical lack of genetic structure across environmental gradients for broadcast‐spawning corals speaks of the absence of strong reproductive barriers imposed by environmental differences (Ayre and Hughes 2000, Baums et al. 2005b, Davies et al. 2015b), except across large depth gradients (Prada and Hellberg 2013). Whether sexual compatibility is determined entirely by genetics or is also context‐dependent remains an open question. Nevertheless, because even a modest percentage of unfertilized eggs in larval cultures can cause significant overall mortality (Pollock et al. 2017), the fertilization success of a coral genet should be monitored whenever possible to improve breeding outcomes. Fertilization success between two‐parent crosses is easily measured by counting the proportion of cleaving eggs two hours post‐fertilization, whereas measuring fertilization success of single parental genets in batch cultures requires genotyping of offspring (Baums et al. 2013, Davies et al. 2015a). Fertilization success is highly dependent on gamete concentration (Oliver and Babcock 1992, Levitan et al. 2004) with optimum in the range of 106 cells/mL. Because, fertilization also declines with gamete age (Oliver and Babcock 1992, Fogarty et al. 2012), the recommended strategy is to use fresh gametes whenever possible. Maximizing parental diversity of batch cultures (more than two parental donors) is the next most important goal. Detailed instructions on how to increase larval survival rates are available from The Nature Conservancy (2018).
Use larval crosses to increase local genetic diversity or sexual recruit numbers
During spawning, gamete output is often disproportionate, where one genet provides more spawn than other parents. When designing batch cultures, considerations about the combination of parents and the relative contribution of each parent to the batch will depend on the goal of the breeding program. If the goal is to maximize the genetic diversity of offspring, it is important to ensure equal contribution of parents to the batch culture. However, if the goal is to create the largest number of larvae regardless of their genetic diversity, then all gametes should be added to the culture. We propose that batch cultures for restoration should include at least six parents (Iwao et al. 2014), and more when possible.
Maximize settler survival
Survival of outplanted recruits is currently low and presents a bottleneck to the success of sexual recruitment strategies. However, examples of sexual recruits that have survived to sexual maturity and now spawn predictably each year exist (Guest et al. 2014, Chamberland et al. 2016, dela Cruz and Harrison 2017). Settlers survive better when competition with benthic macro‐ and turf algae is low and predation pressure from coral predators is reduced (e.g., Hermodice carunculata, Coralliophila abbreviata), thus careful site selection for outplanting is paramount (see Outplanting strategies).
Enhance adaptation potential via assisted gene flow
Crossing corals and outplanting offspring may be a particularly effective means of AGF. Donor colonies sourced from widely separated and environmentally divergent reefs might be expected to show low survivorship if directly transplanted from one site to another. In contrast, their first‐generation offspring would be expected to show greater phenotypic variation, allowing at least some of them to thrive in either parental habitat. In corals, non‐heritable maternal effects can also have an important impact on the physiology and stress tolerance of early life cycle stages (Dixon et al. 2015, Kenkel et al. 2015b). This can potentially be harnessed to further improve the chance of survival of first‐generation offspring upon transplantation: whenever possible, mothers (i.e., egg donor colonies) should come from the habitat into which the first‐generation offspring are going to be outplanted. While the occurrence of outbreeding depression has never been directly tested in corals, observations in other animals as well as plant species indicate that the risk of outbreeding depression is likely low and should not prevent coral restoration action, especially in local populations that are rapidly declining (Ralls et al. 2018).
Interspecies hybridization
Scleractinian corals have a long history of interspecies hybridization (Veron 2011). General evolutionary principles dictate that the long‐term fitness of hybrids must be lower than the fitness of the “purebred” species, otherwise the species would have merged and would not exist as separate units. However, if hybrids can successfully back‐cross (reproduce with the purebreds), they could provide a way to exchange adaptive genetic variation among constituent species. Hybrid corals occur naturally in the Indo‐Pacific as well as the Caribbean (Willis et al. 2014b). In Caribbean acroporids, hybrids between A. palmata and A. cervicornis exhibit a wide range of morphological variation, and are often found in very shallow, high light environments (Vollmer and Palumbi 1995, Fogarty 2012). As a source for novel morphological diversity, hybridization is an attractive restoration target to enhance reef habitat structure (though clearly not for species recovery). There is little concern about genetic swamping of parental species with hybrid alleles: although these hybrids produce viable gametes (Fogarty 2012), later generation genets are still rare in the population and both parental species have very low rates of successful natural sexual reproduction. In the Orbicella species complex, interspecies hybrids are also observed but occur at different frequencies across the Caribbean and perhaps over depth gradients (Fukami et al. 2004). In both hybrid complexes, the hybrid phenotypes may be useful for unique restoration applications such as restoring reef structure in deep, very shallow, or sheltered habitats. Additionally, preliminary lab studies have verified that hybrid Acropora offspring can show improved performance in exposure to elevated temperature and CO2 conditions. The limited space in nurseries may dictate that practitioners prioritize rearing purebred parents, but at least for sexual larval production, gametes of opportunity may be used to generate interspecies crosses. The outplanting strategy for these hybrid settlers should follow the same recommendations as for the purebred parents (see Outplanting Strategies).
Phenotypic traits of propagated corals
Given the lack of predictive tools for selecting genets a priori (Box 2), genets will likely perform differently during the propagation phase than they do on the native reef. Performance depends on interactions between genets and their local environment that are difficult to predict (Lirman et al. 2014b, Drury et al. 2017, O'Donnell et al. 2018) and trade‐offs between desirable traits, such as growth and thermal tolerance, may occur (Ladd et al. 2017). Thus, maintaining phenotypic diversity in outplant populations is essential. To that end, tracking key traits in nursery populations will not only help restoration practitioners optimize nursery stocks but can ensure that a diverse suite of potentially important traits is included in outplanting designs. However, traits are not all equally relevant to success and measuring some traits can be challenging, time consuming, and cost prohibitive, diverting resources from essential tasks such as outplanting and nursery maintenance. Therefore, establishing consistent practical guidelines for collecting the most informative data will be important to optimally manage nurseries.
While most genets can survive and propagate in nursery conditions, there are often more genets available within a nursery's area of interest than can be maintained. When this is the case, traits that can help guide which genets to propagate are (1) partial mortality, (2) rate of wound healing, (3) skeletal growth rate, (4) bleaching and infectious disease resistance or resilience, and (5) sexual reproductive output (Table 3).
Table 3.
Phenotypic traits that can help in selecting coral genets for propagation and restoration
| Trait | Measurement |
|---|---|
| Partial mortality | amount of tissue loss |
| Wound healing rate | days to heal from fragmentation |
| Skeletal growth rate | buoyant weight or “crown area” |
| Bleaching and infectious disease resistance/resilience | no bleaching/infectious disease or recovers quickly |
| Sexual reproduction output | spawning and sperm motility |
Partial mortality
Partial mortality is common in coral colonies due to a variety of impacts, including corallivory, competition, fragmentation, and temperature stress (Lirman et al. 2014a). Partial mortality can lead to increased susceptibility to infectious diseases. Thus, percent recent mortality is a reliable indicator of recent stress across a range of coral species (Cooper et al. 2009, Lirman et al. 2014a) and can be visually estimated directly or from photographs.
Wound healing rate
Corals that heal rapidly after sustaining lesions or fragmentation have a lower probability of secondary infection (Palmer et al. 2011) and therefore higher probability of survival. Furthermore, healing rate may be indicative of improved physiological condition (Fisher et al. 2007) and/or greater energy reserves (Denis et al. 2011), which can help corals survive bleaching events (Rodrigues and Grottoli 2007, Levas et al. 2013, 2018, Grottoli et al. 2014, Camp et al. 2018).
Skeletal growth rate
Corals that grow rapidly in nurseries (i.e., high calcification rate) can be fragmented more frequently to produce more stock. Corals that grow rapidly in the field generate more structural complexity, which is often a primary goal of reef restoration. Unfortunately, linear extension rate (a frequently applied measure of coral growth) is not always correlated in the nursery and the field (O'Donnell et al. 2018) and growth rates are often not correlated with survival (Ladd et al. 2017). However, there is evidence that calcification rate may be genetically influenced and therefore represents a good measure of genet performance, whereas linear extension rate and skeletal density vary with environmental conditions (Kuffner et al. 2017). Although quantifying calcification rate by measuring buoyant weight is time‐consuming (Jokiel et al. 1978, Herler and Dirnwöber 2011, Morrison et al. 2013), investing the time and resources initially to quantify calcification in this way when selecting genets could have long‐term payoffs in nursery and outplanting efficacy. However, for tracking genet growth in the field, we suggest a metric commonly used in silviculture termed the “crown area” (CA) of a tree (Uzoh and Ritchie 2007). Crown area defines the two‐dimensional footprint under the tree canopy and, thus, would work for branching and massive coral morphologies alike, and represents the coral's capacity for harvesting direct, downwelling solar irradiance. The measurements needed to calculate crown area are the long width of the crown (CWL) and the short width of the crown (CWS, perpendicular to the long width), both of which can be easily estimated using a tailor's tape, and the area of the footprint calculated using the formula
In addition to yielding important information on how much reef‐substratum area each coral colony claims per unit of time, it is a simple and straightforward measurement of planar coral‐surface area with which to normalize calcification rates (Kuffner et al. 2013) or other physiological measurements.
Bleaching and disease resistance and/or resilience
Threats to coral reefs are numerous but disease and bleaching are two of the main drivers of coral decline in the wild (Halpern et al. 2008, Harborne et al. 2017) and in restoration projects (Drury et al. 2017, Ladd et al. 2017). Importantly, infectious disease prevalence and the frequency and severity of bleaching events are expected to increase over the coming century (Miller et al. 2014, Maynard et al. 2015, van Hooidonk et al. 1996) and bleached corals are often less resistant to infectious diseases (Muller et al. 2018). Therefore, to maximize the chances of successful restoration outcomes, nurseries should expend efforts to identify and maintain genets that are resistant or resilient to thermal stress and disease (i.e., do not bleach and do not contract infectious disease, or rapidly recover from both). Although multiple traits can impart resilience to one or both of these stressors, such as the identity and diversity of algal symbionts, heterotrophic capacity, energy reserves, and environmental history (Baker et al. 2004, Berkelmans and van Oppen 2006, Anthony et al. 2009, Hughes and Grottoli 2013), many of these traits are currently time‐consuming or difficult to measure, and require specialized training and equipment. Instead, restoration practitioners should focus on simple, qualitative surveys to track the prevalence of mortality, bleaching, and infectious diseases among genets. Survey data should be collected at least twice a year to coincide with the timing of bleaching and/or infectious disease outbreaks in that region (although more frequent surveys are desirable). Surveys should involve recording the number and proportion of ramets from each genet experiencing bleaching or disease and whether the recorded stressor resulted in partial or complete ramet mortality, or if the ramet survived (Muller et al. 2018). Genets that show high mortality due to infectious disease or bleaching should be replaced within the nursery, preferably from the same source site and from parent colonies that appear to also have recovered from the stress or were less susceptible to the stress.
Sexual reproduction
Fecundity is a necessary, but insufficient, trait to assure reproductive success given the requirements for spawning synchrony and fertilization compatibility (see Sexual propagation and selection of donor colonies). The cultivation of large adult fragments as “spawning stocks” within nurseries can provide opportunities to monitor the presence/absence of spawning and synchrony in spawn time among genets as well as to collect and rear sexual offspring. Reproductive size will vary considerably among species (Soong and Lang 1992) so maintaining multiple colonies of all genets above spawning size in nurseries may be more feasible for some species than others. Genets with low reproductive output that are low performing in the other phenotypic trait categories should be reevaluated as candidates for propagation stocks. A low‐cost method to assess gamete quality is to observe sperm motility (Hagedorn et al. 2006), which is highly predictive of fertilization success. Sperm motility can be scored by observing live sperm through a low‐end phase contrast microscope and estimating the proportion of sperm that are moving (M. Hagedorn, personal communication). This is cheaper and faster than assessing egg quality (e.g., by analyzing their lipid content). Sperm motility is likely influenced by genetic factors as well as environmental factors (such as heat stress (Hagedorn et al. 2016)) and is consequently a good measure of genet performance in a given environment.
Outplanting Strategies
The selection of sites to establish restored populations is a strong factor in the ultimate success of outplants. Many previous outplanting efforts show high variation in survivorship among sites that are not easily attributable to specific ecological drivers (Bowden‐Kerby 2008, Bowden‐Kerby and Carne 2012, Lirman et al. 2014b, Drury et al. 2017). Potential contributing factors include protection status, visitation, intact trophic structure of the reef (including herbivory and corallivory levels), water quality, historical occurrence of the species, and alleviation of the stressors that caused the original coral decline (The Nature Conservancy checklist; available online).11 Individual species need to be outplanted to sites within their general habitat niche (e.g., A. palmata prefers relatively shallow, high energy habitats) but more specific guidelines can be difficult to establish due to the lack of baseline data, species‐specific differences in niches (Goreau 1959, Dollar 1982), and uncertainty as to how those niches may change in the future. Historical and fossil records may be used as guides to where species flourished recently and during the warmer mid‐Holocene, providing insight for restoration siting (Toth et al. 2017).
Site selection for fragments
Within species, there is an expectation that genets should survive better in a habitat similar to their native habitat. Direct reciprocal transplant experiments with various species support this expectation (Howells et al. 2013, Kenkel et al. 2013). However, studies examining habitat‐specific performance of nursery‐reared Acropora spp. do not show patterns of preferential performance (e.g., in linear growth or mortality) in native sites or habitat types (Drury et al. 2017, O'Donnell et al. 2018, Pausch et al. 2018). There are various reasons this expectation may not play out, such as a lack of local adaptation in the first place, or acclimation during the nursery propagation period that decouple this affinity. Stochastic disturbance as well as rapid change in environmental conditions within individual sites may also exacerbate the mismatch of genets with native habitats. In the absence of better predictive capacity in matching genet performance (see Box 2), our overall recommendation is to adopt a similar climate‐adjusted provenance strategy in outplanting genets among individual sites (Fig. 1), with proportional representation of all genets from available stocks outplanted among sites.
Increasing potential for sexual reproduction
To maximize the potential for sexual reproduction in the restored population of broadcast‐spawning species, more than one genet is required. Two genets may provide only marginal fertilization potential for outcrossing hermaphrodites, while mixtures of four to six parents showed high fertilization success in Acropora spp. (Baums et al. 2013, Iwao et al. 2014, Miller et al. 2017). Higher numbers of parental genets (~10) are likely needed for gonochoristic species because sex ratios are often skewed, or for Acropora spp. due to lack of synchrony during spawning and variability in fertility among genets (Fogarty et al. 2012, Miller et al. 2016). Hence, we recommend mixtures of 5–10 genets for batch culturing of all species. Optimal distance between outplants is unknown but 2–3 m may work well for fast growing acroporids and orbicellids (not too far for successful fertilization, not too close that colonies compete for resources). If more genets are available in nursery stocks, they can be allocated among sites in different combinations or, if phenotypic information on stress‐resistant genets is available, they may be stratified among sites to ensure that some resistant phenotypes are outplanted to each site. In special cases where certain genets display multiple positive traits (e.g., low partial mortality and high skeletal growth rate), these “winners” may be included at as many sites as feasible, keeping in mind that these nursery “winners” may not necessarily exhibit superior performance in the wild.
Site selection for sexual recruits
In the case of restoration based on sexual reproduction, each juvenile represents a unique genet. Thus, the number of genets outplanted to a site will be dramatically higher than can be achieved when outplanting nursery‐reared coral fragments. We propose that similar strategies in site selection be employed for the outplanting of sexual recruits as for fragments. The goal is to mix and match as many genets as possible to maximize genetic diversity, which is the basis of adaptive resilience (Hermisson and Pennings 2005, Baums 2008, Whiteley et al. 2011).
Monitoring
It is too time consuming and challenging to monitor very small sexual propagules, but as recruit sizes increases, they are relatively easy to track. Subsequent monitoring of the success of genets within each outplant site can provide important insights into their performance. Where tracking the phenotypic performance of genets across sites is feasible, resulting data should be contributed to genet/phenotype databases (see Future research needs ). In the simpler case where the fate of genets is not tracked, colonies that expire should be replaced with fragments from genets that were not originally included at that site.
Role of Symbionts
Role of symbiont in an asexual restoration program
Reef corals are mutualistic symbioses between diverse and taxonomically divergent taxa that collectively comprise the coral “holobiont” (Rohwer et al. 2001), and the identity of these partners can have important effects on the phenotypic characteristics of coral colonies (Baker 2003). In particular, diversity in the algal symbiont community (family Symbiodiniaceae) has been linked to variation in holobiont thermotolerance (Glynn et al. 2001, Baker et al. 2004, Berkelmans and van Oppen 2006, LaJeunesse et al. 2010), growth rate (Jones et al. 2008, Cunning et al. 2015, Pettay et al. 2015), irradiance optima (Rowan and Knowlton 1995, Rowan et al. 1997, Iglesias‐Prieto et al. 2004), and possibly disease resistance (Correa et al. 2009), all of which are relevant to restoration objectives. Thus, algal symbionts may affect restoration outcomes, though evidence for such impacts in nurseries or outplant sites is currently lacking in the few cases where they were investigated (Lirman et al. 2014b, Parkinson and Baums 2014, O'Donnell et al. 2018, Parkinson et al. 2018b).
Because many coral species commonly used for restoration are capable of hosting several species and strains of Symbiodiniaceae, at least when sampled across their biogeographic range, algal symbiont diversity may create phenotypic and genetic differences among restoration units that are not attributable to the coral host (Parkinson and Baums 2014). However, unlike host genetic differences, symbiont genetic differences may not be fixed over a coral genet's lifespan, as the symbiont community can potentially change over time in response to disturbances and/or prevailing environmental conditions. However, barring direct collaboration with scientists to identify and monitor changes in symbiont communities using genetic methods, it may not be practical for most restoration practitioners to take explicit account of Symbiodiniaceae diversity for the time being. Although an active area of research, prescriptive methods of manipulating symbionts as part of routine nursery propagation and outplanting have yet to be identified, and the longevity of these manipulations has yet to be established in the field (Peixoto et al. 2017). At present, it should be sufficient for practitioners to realize that exposure of nursery‐grown corals to specific environmental conditions might lead to changes in symbiont communities, leading to variation in outplanting success. Climate‐adjusted provenancing might represent a pragmatic approach to dealing with this uncertainty. Warmer local environments are expected to have a higher abundance of both thermotolerant coral host genets and thermotolerant symbionts, so sourcing holobionts from such locations may help outplanted corals survive long‐term under climate change conditions.
Role of symbionts in a sexual restoration program
An important question in sexual propagation is when (i.e., at what age) to outplant sexual offspring. Evidence suggests that increasing recruit size increases outplant survivorship (Raymundo and Maypa 2004) such that an extended grow‐out period in the nursery may be worthwhile provided costs of ex situ rearing can be minimized. For species with larvae that lack symbionts (i.e., those that acquire symbionts via horizontal transmission), the process of symbiont colonization during early life deserves attention. The onset of symbiosis can be as early as 2–6 days after fertilization (Schwarz et al. 1999, Edmunds et al. 2005, Harii et al. 2009). In rare cases, the association is highly specific from the outset, where the host harbors one algal species from juvenile to adult stages (e.g., Fungia scutaria; Weis et al. 1992). More commonly, during early onset the association is plastic (with the formation of partnerships sometimes involving multiple symbiont species), but ultimately shifts to a stable dominant species of Symbiodiniaceae during the juvenile stage (Coffroth et al. 2001, van Oppen 2016, Abrego et al. 2009, Cumbo et al. 2013, Yamashita et al. 2013). While colonization itself does not seem to be a limiting step, symbiont community composition is another major determinant (along with host genet) of physiological performance of the coral (Little et al. 2004, Parkinson et al. 2015a, Grottoli et al. 2018) and is expected to vary with outplant environment.
Provisioning of sexual recruits
In the natural environment, typical Symbiodiniaceae sources for coral recruits include the water column, sediments, and adult corals (Coffroth et al. 2006, Adams et al. 2009, Nitschke et al. 2016, Cunning et al. 2017, Ali et al. 2018, Quigley et al. 2018). It is straightforward to provision coral juveniles with mixtures of cultivated micro‐algae when they are kept in small aquaria ex situ, but only a subset of the Symbiodiniaceae diversity is currently cultured. Further, relatively little is known regarding how to provision particular symbionts, in situ, in such a way as to maximize their uptake by coral larvae or juveniles. Environmental conditions affect larval symbiont acquisition, with increasing temperatures appearing to select for thermotolerant symbionts, at least in Acropora from the Great Barrier Reef (Abrego et al. 2012). One strategy to facilitate the acquisition of appropriate symbionts is thus to outplant settlers prior to symbiont colonization. That way, Symbiodiniaceae are acquired from the outplant environment, likely promoting symbionts that are specialized for that environment (LaJeunesse et al. 2004, 2010, Abrego et al. 2012, Howells et al. 2012). However, maturation of the symbiont community can take 1.5–3.5 years (Abrego et al. 2009, Yamashita et al. 2016), and does not always match that of the adult community at the outplant site (Little et al. 2004). There is relatively less information on the acquisition of different symbionts by Caribbean scleractinian corals, but laboratory studies suggest that an uptake window of up to 4 months exists (McIlroy and Coffroth 2017), and that it can take up to 4 years before the community stabilizes (Coffroth et al. 2010, Poland and Coffroth 2017). A hybrid approach has been taken by managers and researchers in Mexico who transfer outplants to the reef for two weeks to take up local symbionts and then return them to the nursery to achieve additional growth before final outplanting (Claudia Padilla and Ania Banaszak, personal communication). Additional research on the optimal timing of outplanting sexual recruits is needed.
Rare species
Rare coral species that also associate with rare symbionts such as the threatened Caribbean pillar coral, Dendrogyra cylindrus and its symbiont Breviolum dendrogyrum (Lewis et al. 2018, Chan et al. 2019) face additional challenges. Because adult D. cylindrus are the only known reservoirs for this symbiont, and these populations are in rapid decline, it cannot be assumed that natural sexual recruits will readily be able to acquire appropriate symbionts. Consequently, larval recruits might have to be exposed to adult conspecifics, or special efforts made to culture these Symbiodiniaceae specifically for restoration.
Future Research Needs
The recommendations in this review are based on the best available science, but knowledge gaps exist that should be the focus of future research. In general, it has to be recognized that nursery rearing of both larvae and adult fragments imposes unintended selection for nursery‐adapted genets (Christie et al. 2012, 2016, Morvezen et al. 2016, Horreo et al. 2018, O'Donnell et al. 2018), although the strength of the selection is not yet known but could be estimated. For example, larval culturing selects gametes and larvae that can survive netting, high‐density rearing, handling during water changes, settling on artificial substrates, and so on. Data from other systems demonstrate that after removal of initial culture selection, species can adapt to wild conditions rapidly, especially if time spent in captivity is short (Espeland et al. 2017, but see Horreo et al. 2018). Our review already includes recommendations to reduce unintended selection such as diversified provenancing (over different habitats and times), intentionally promoting gene flow, and matching the nursery and reef environments as closely as possible (Frankham 2008, Espeland et al. 2017). Both outbreeding and inbreeding depression have yet to be tested experimentally in corals and should be a focus of future research. Outbreeding depression could occur despite our expectations, especially if the number of outplanted genets approaches the number of genets surviving in the wild. Consequences include (1) wasting resources by transplanting maladapted genets and (2) preventing rapid local adaptation when foreign genotypes reproduce with local genets. We are not aware of definitive evidence for outbreeding depression in corals, but the topic is receiving renewed attention in marine species (Pritchard et al. 2013, Pereira et al. 2014, Ellingson and Krug 2016, Phillips et al. 2017). Inbreeding depression could occur after repeated rounds of sexual reproduction among closely related genets in a nursery setting but that seems a far‐off risk, given the challenges of long‐term coral culture and breeding combined with relatively long generation times.
Unintended introduction of invasive pests when moving corals over large distances is another concern. This risk is particularly relevant for corals given the dire toll infectious disease have had on coral populations (Walton et al. 2002) and lack of screening tools for pathogens. Translocating larvae or gametes (i.e., cryo‐preserved sperm), when undertaking AGF, rather than adult fragments, is a good option to minimize this risk (Hagedorn et al. 2012) and further refinement of these methods is a research priority. Basic biosecurity practices such as visual inspections and quarantine can also reduce risk of unintended pest introduction (IUCN/SSC 2013).
To improve the prospects for leveraging specific genets and traits in future restoration, it is crucial to link coral genets with phenotypic performance and fine‐grain environmental data. We call for an open‐access database to catalog all managed genets in nurseries and outplants. This would also address a specific aim (Action 4a) in the Endangered Species Act recovery plan for A. palmata and A. cervicornis (NMFS 2015). Achieving this goal will require a coordinated effort by practitioners and researchers to report standardized metadata for each genet in the database allowing integration with other ongoing monitoring efforts (e.g., Atlantic and Gulf Rapid Reef Assessment database; available online).12 At a minimum, the database should contain information about the collection site (Geographical Positioning System coordinates), collection date, sample depth, and collector information. Additional information on the phenotypic traits and environmental variables discussed should be collected when possible. Finally, if the budget permits genotyping (Box 1), a clonal identification number should be assigned to genets based on the comparison of their multilocus genotype (MLG) pattern against a background of archived genotypes (for example, previously detected acroporid genomic variants; Ktichen et al. 2019).
A way forward is the integration of the recommended actions into a decision‐making framework to decide on how and when interventions should be made, and the development of such a framework is another recommended research priority (Anthony et al. 2015).
Conclusions
Reef restoration has made significant progress via the development of innovative ways to grow and outplant corals. While the task of restoring reefs in the face of rapid climate change remains daunting and will prove futile if current carbon emissions continue unabated, coral species still harbor a significant amount of genetic variation that can be leveraged to enable rapid adaptation if the restoration community adopts an evolution‐centric strategy. The heterogeneity in response to stress is what provides the hope that reef‐restoration work is worth the time and effort. We posit that this natural heterogeneity can be capitalized upon, using the recommendations herein, in a way that may speed up natural selection and provide a means for certain species and coral ecosystems to persist.
Supporting information
Acknowledgments
The Coral Restoration Consortium (CRC) formed in the fall of 2016 and established five initial working groups to provide best management practices for coral restoration with collaborative participation of practitioners, reef managers, and scientists. The synthesis provided here is the first product of the coral genetics and science working group. Thanks to S. R. Palumbi for advising the working group. Special thanks to M. L. Loewe for her logistical support of the working group. We are grateful to the reviewers and editor for helping us improve the manuscript. All authors contributed to the writing and editing of the manuscript. I. B. Baums organized the workshop and led the writing. The CRC, the National Oceanographic and Atmospheric Administration, the Pennsylvania State University's Institute for Sustainability, Institute for Energy and the Environment and the Center for Marine Science and Technology are acknowledged for travel and logistics support. I. B. Baums was supported by NSF grants OCE 1537959 and IOS 1810959. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Baums, I. B. , Baker A. C., Davies S. W., Grottoli A. G., Kenkel C. D., Kitchen S. A., Kuffner I. B., LaJeunesse T. C., Matz M. V., Miller M. W., Parkinson J. E., and Shantz A. A.. 2019. Considerations for maximizing the adaptive potential of restored coral populations in the western Atlantic. Ecological Applications 29(8):e01978 10.1002/eap.1978
Corresponding Editor: Éva Elizabeth Plaganyi.
Notes
Literature Cited
- Abrego, D. , van Oppen M. J. H., and Willis B. L.. 2009. Onset of algal endosymbiont specificity varies among closely related species of Acropora corals during early ontogeny. Molecular Ecology 18:3532–3543. [DOI] [PubMed] [Google Scholar]
- Abrego, D. , Willis B. L. and van Oppen M. J. H.. 2012. Impact of Light and Temperature on the Uptake of Algal Symbionts by Coral Juveniles. PLoS ONE 7:e50311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, L. M. , Cumbo V. R., and Takabayashi M.. 2009. Exposure to sediment enhances primary acquisition of Symbiodinium by asymbiotic coral larvae. Marine Ecology Progress Series 377:149–156. [Google Scholar]
- Ainsworth, T. D. , Heron S. F., Ortiz J. C., Mumby P. J., Grech A., Ogawa D., Eakin C. M., and Leggat W.. 2016. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352:338–342. [DOI] [PubMed] [Google Scholar]
- Aitken, S. N. , and Whitlock M. C.. 2013. Assisted Gene Flow to Facilitate Local Adaptation to Climate Change. Annual Review of Ecology, Evolution, and Systematics 44:367–388. [Google Scholar]
- Ali, A. , Kriefall N. G., Emery L. E., Kenkel C. D., Matz M. V. and Davies S. W.. 2018. Recruit symbiosis establishment and Symbiodiniceae composition influenced by adult corals and reef sediment. bioRxiv 10.1101/421339. [DOI] [Google Scholar]
- Altshuler, D. , Pollara V. J., Cowles C. R., Van Etten W. J., Baldwin J., Linton L., and Lander E. S.. 2000. An SNP map of the human genome generated by reduced representation shotgun sequencing. Nature 407:513–516. [DOI] [PubMed] [Google Scholar]
- Anthony, K. R. N. 2000. Enhanced particle‐feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19:59–67. [Google Scholar]
- Anthony, K. R. N. , and Fabricius K. E.. 2000. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. Journal of Experimental Marine Biology and Ecology 252:221–253. [DOI] [PubMed] [Google Scholar]
- Anthony, K. R. N. , Hoogenboom M. O., Maynard J. A., Grottoli A. G., and Middlebrook R.. 2009. Energetics approach to predicting mortality risk from environmental stress: a case study of coral bleaching. Functional Ecology 23:539–550. [Google Scholar]
- Anthony, K. R. N. , et al. 2015. Operationalizing resilience for adaptive coral reef management under global environmental change. Global Change Biology 21:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif, C. , Daniels C., Bayer T., Banguera‐Hinestroza E., Barbrook A., Howe C. J., LaJeunesse T. C., and Voolstra C. R.. 2014. Assessing Symbiodinium diversity in scleractinian corals via next‐generation sequencing‐based genotyping of the ITS2 rDNA region. Molecular Ecology 23:4418–4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayre, D. J. , and Hughes T. P.. 2000. Genotypic diversity and gene flow in brooding and spawning corals along the Great Barrier Reef, Australia. Evolution 54:1590–1605. [DOI] [PubMed] [Google Scholar]
- Baillie, B. K. , Belda‐Baillie C. A., and Maruyama T.. 2000a. Conspecificity and Indo‐Pacific distribution of Symbiodinium genotypes (Dinophyceae) from giant clams. Journal of Phycology 36:1153–1161. [Google Scholar]
- Baillie, B. K. , Belda‐Baillie C. A., Silvestre V., Sison M., Gomez A. V., Gomez E. D., and Monje V.. 2000b. Genetic variation in Symbiodinium isolates from giant clams based on random‐amplified‐polymorphic DNA (RAPD) patterns. Marine Biology 136:829–836. [Google Scholar]
- Baird, A. H. , Guest J. R., and Willis B. L.. 2009. Systematic and Biogeographical Patterns in the Reproductive Biology of Scleractinian Corals. Annual Review of Ecology Evolution and Systematics 40:551–571. [Google Scholar]
- Baker, A. C. 2003. Flexibility and specificity in coral‐algal symbiosis: Diversity, ecology, and biogeography of Symbiodinium . Annual Review of Ecology Evolution and Systematics 34:661–689. [Google Scholar]
- Baker, A. C. , Starger C. J., McClanahan T. R., and Glynn P. W.. 2004. Coral reefs: corals’ adaptive response to climate change. Nature 430:741. [DOI] [PubMed] [Google Scholar]
- Barbrook, A. C. , Visram S., Douglas A. E., and Howe C. J.. 2006. Molecular diversity of dinoflagellate symbionts of Cnidaria: the psbA minicircle of Symbiodinium . Protist 157:159–171. [DOI] [PubMed] [Google Scholar]
- Barshis, D. J. , Ladner J. T., Oliver T. A., Seneca F. O., Traylor‐Knowles N., and Palumbi S. R.. 2013. Genomic basis for coral resilience to climate change. Proceedings of the National Academy of Sciences USA 110:1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baums, I. B. 2008. A restoration genetics guide for coral reef conservation. Molecular Ecology 17:2796–2811. [DOI] [PubMed] [Google Scholar]
- Baums, I. B. , Hughes C. R., and Hellberg M. H.. 2005a. Mendelian microsatellite loci for the Caribbean coral Acropora palmata . Marine Ecology Progress Series 288:115–127. [Google Scholar]
- Baums, I. B. , Miller M. W., and Hellberg M. E.. 2005b. Regionally isolated populations of an imperiled Caribbean coral, Acropora palmata . Molecular Ecology 14:1377–1390. [DOI] [PubMed] [Google Scholar]
- Baums, I. B. , Miller M. W., and Hellberg M. E.. 2006. Geographic variation in clonal structure in a reef building Caribbean coral, Acropora palmata . Ecological Monographs 76:503–519. [Google Scholar]
- Baums, I. B. , Devlin‐Durante M. K., Brown L., and Pinzón J. H.. 2009. Nine novel, polymorphic microsatellite markers for the study of threatened Caribbean acroporid corals. Molecular Ecology Resources 9:1155–1158. [DOI] [PubMed] [Google Scholar]
- Baums, I. B. , Devlin‐Durante M. K., Polato N. R., Xu D., Giri S., Altman N. S., Ruiz D., Parkinson J. E., and Boulay J. N.. 2013. Genotypic variation influences reproductive success and thermal stress tolerance in the reef building coral, Acropora palmata . Coral Reefs 32:703–717. [Google Scholar]
- Baums, I. B. , Devlin‐Durante M. K., and LaJeunesse T. C.. 2014a. New insights into the dynamics between reef corals and their associated dinoflagellate endosymbionts from population genetic studies. Molecular Ecology 23:4203–4215. [DOI] [PubMed] [Google Scholar]
- Baums, I. B. , Durante M. D., Laing A. A., Feingold J., Smith T., Bruckner A., and Monteiro J.. 2014b. Marginal coral populations: the densest known aggregation of Pocillopora in the Galápagos Archipelago is of asexual origin. Frontiers in Marine Science 1: 10.3389/fmars.2014.00059. [DOI] [Google Scholar]
- Bay, R. A. , and Palumbi S. R.. 2014. Multilocus adaptation associated with heat resistance in reef‐building corals. Current Biology 24:2952–2956. [DOI] [PubMed] [Google Scholar]
- Bay, R. A. , Rose N. H., Logan C. A., and Palumbi S. R.. 2017. Genomic models predict successful coral adaptation if future ocean warming rates are reduced. Science Advances 3:e1701413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, M. W. , Losada I. J., Menéndez P., Reguero B. G., Díaz‐Simal P., and Fernández F.. 2018. The global flood protection savings provided by coral reefs. Nature Communications 9:2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellwood, D. R. , Hughes T. P., Folke C., and Nystrom M.. 2004. Confronting the coral reef crisis. Nature 429:827–833. [DOI] [PubMed] [Google Scholar]
- Bellwood, D. R. , Hughes T. P., and Hoey A. S.. 2006. Sleeping functional group drives coral‐reef recovery. Current Biology 16:2434–2439. [DOI] [PubMed] [Google Scholar]
- Berkelmans, R. , and van Oppen M. J. H.. 2006. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society B 273:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts, P. , Riginos C., Hay K. B., van Oppen M. J., Hoegh‐Guldberg O., and Dove S.. 2011. Adaptive divergence in a scleractinian coral: physiological adaptation of Seriatopora hystrix to shallow and deep reef habitats. BMC Evolutionary Biology 11:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts, P. , Riginos C., Brunner R., Englebert N., Smith S. R., and Hoegh‐Guldberg O.. 2017. Deep reefs are not universal refuges: Reseeding potential varies among coral species. Science. Advances 3:2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden‐Kerby, A. 2008. Restoration of threatened Acropora cervicornis corals: intraspecific variation as a factor in mortality, growth, and self‐attachment. Pages 1200–1204 in 11th International Coral Reef Symposium, Fort Lauderdale, Florida, USA.
- Bowden‐Kerby, A. , and Carne L.. 2012. Thermal tolerance as a factor in Caribbean Acropora restoration. in 12th International Coral Reef Symposium, Cairns, Australia.
- Brazeau, D. A. , Gleason D. F., and Morgan M. E.. 1998. Self‐fertilization in brooding hermaphroditic Caribbean corals: Evidence from molecular markers. Journal of Experimental Marine Biology and Ecology 231:225–238. [Google Scholar]
- Brazeau, D. A. , Lesser M. P., and Slattery M.. 2013. Genetic structure in the coral, Montastraea cavernosa: assessing genetic differentiation among and within mesophotic reefs. PLoS ONE 8:e65845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp, E. F. , Schoepf V., and Suggett D. J.. 2018. How can “Super Corals” facilitate global coral reef survival under rapid environmental and climatic change? Global Change Biology 24:2755–2757. [DOI] [PubMed] [Google Scholar]
- Carlon, D. B. , and LippE C.. 2008. Fifteen new microsatellite markers for the reef coral Favia fragum and a new Symbiodinium microsatellite. Molecular Ecology Resources 8:870–873. [DOI] [PubMed] [Google Scholar]
- Carne, L. , and Baums I. B.. 2016. Demonstrating effective Caribbean acroporid population enhancement: all three nursery‐grown, out‐planted taxa spawn August 2015 & 2016 in Belize. Reef Encounter 31:42–43. [Google Scholar]
- Chamberland, V. F. , Vermeij M. J. A., Brittsan M., Carl M., Schick M., Snowden S., Schrier A., and Petersen D.. 2015. Restoration of critically endangered elkhorn coral (Acropora palmata) populations using larvae reared from wild‐caught gametes. Global Ecology and Conservation 4:526–537. [Google Scholar]
- Chamberland, V. F. , Petersen D., Latijnhouwers K. R. W., Snowden S., Mueller B., and Vermeij M. J. A.. 2016. Four‐year‐old Caribbean Acropora colonies reared from field‐collected gametes are sexually mature. Bulletin of Marine Science 92:263–264. [Google Scholar]
- Chan, A. N. , Lewis C. L., Neely K. L. and Baums I. B.. 2019. Fallen pillars: The past, present, and future population dynamics of a rare, specialist coral–algal symbiosis. Frontiers in Marine Science 6: 10.3389/fmars.2019.00218. [DOI] [Google Scholar]
- Christie, M. R. , Marine M. L., French R. A., and Blouin M. S.. 2012. Genetic adaptation to captivity can occur in a single generation. Proceedings of the National Academy of Sciences USA 109:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie, M. R. , Marine M. L., Fox S. E., French R. A., and Blouin M. S.. 2016. A single generation of domestication heritably alters the expression of hundreds of genes. Nature Communications 7:10676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves, P. A. , Strader M. E., Bay L. K., Pringle J. R., and Matz M. V.. 2018. CRISPR/Cas9‐mediated genome editing in a reef‐building coral. Proceedings of the National Academy of Sciences USA 115:5235–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffroth, M. A. , Santos S. R., and Goulet T. L.. 2001. Early ontogenetic expression of specificity in a cnidarian‐algal symbiosis. Marine Ecology Progress Series 222:85–96. [Google Scholar]
- Coffroth, M. A. , Lewis C. F., Santos S. R., and Weaver J. L.. 2006. Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians. Current Biology 16:R985–R987. [DOI] [PubMed] [Google Scholar]
- Coffroth, M. A. , Poland D. M., Petrou E. L., Brazeau D. A. and Holmberg J. C.. 2010. Environmental symbiont acquisition may not be the solution to warming seas for reef‐building corals. PLoS ONE 5:e13258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, I. and Dubinsky Z.. 2015. Long term photoacclimation responses of the coral Stylophora pistillata to reciprocal deep to shallow transplantation: photosynthesis and calcification. Frontiers in Marine Science 2: 10.3389/fmars.2015.00045. [DOI] [Google Scholar]
- Comeau, S. , Edmunds P. J., Spindel N. B., and Carpenter R. C.. 2014. Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Marine Ecology Progress Series 501:99–111. [Google Scholar]
- Cooper, T. F. , Gilmour J. P., and Fabricius K. E.. 2009. Bioindicators of changes in water quality on coral reefs: review and recommendations for monitoring programmes. Coral Reefs 28:589–606. [Google Scholar]
- Correa, A. M. S. , Brandt M. E., Smith T. B., Thornhill D. J., and Baker A. C.. 2009. Symbiodinium associations with diseased and healthy scleractinian corals. Coral Reefs 28:437–448. [Google Scholar]
- Cumbo, V. , Baird A., and van Oppen M.. 2013. The promiscuous larvae: flexibility in the establishment of symbiosis in corals. Coral Reefs 32:111–120. [Google Scholar]
- Cunning, R. , Gillette P., Capo T., Galvez K., and Baker A. C. J. C. R.. 2015. Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs 34:155–160. [Google Scholar]
- Cunning, R. , Gates R. D., and Edmunds P. J.. 2017. Using high‐throughput sequencing of ITS2 to describe Symbiodinium metacommunities in St. John, US Virgin Islands. PeerJ 5:e3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S. W. , Matz M. V., and Vize P. D.. 2013a. Ecological complexity of coral recruitment processes: effects of invertebrate herbivores on coral recruitment and growth depends upon substratum properties and coral species. PLoS ONE 8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S. W. , Rahman M., Meyer E., Green E. A., Buschiazzo E., Medina M., and Matz M. V.. 2013b. Novel polymorphic microsatellite markers for population genetics of the endangered Caribbean star coral, Montastraea faveolata . Marine Biodiversity 43:167–172. [Google Scholar]
- Davies, S. W. , Scarpino S. V., Pongwarin T., Scott J., and Matz M. V.. 2015a. Estimating trait heritability in highly fecund species. G3‐Genes Genomes Genetics 5:2639–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies, S. W. , Treml E. A., Kenkel C. D., and Matz M. V.. 2015b. Exploring the role of Micronesian islands in the maintenance of coral genetic diversity in the Pacific Ocean. Molecular Ecology 24:70–82. [DOI] [PubMed] [Google Scholar]
- dela Cruz, D. W. and Harrison P. L.. 2017. Enhanced larval supply and recruitment can replenish reef corals on degraded reefs. Scientific Reports 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis, V. , Debreuil J., De Palmas S., Richard J., Guillaume M. M. M., and Bruggemann J. H.. 2011. Lesion regeneration capacities in populations of the massive coral Porites lutea at Reunion Island: environmental correlates. Marine Ecology Progress Series 428:105–117. [Google Scholar]
- Devlin‐Durante, M. K. , and Baums I. B.. 2017. Genome‐wide survey of single‐nucleotide polymorphisms reveals fine‐scale population structure and signs of selection in the threatened Caribbean elkhorn coral, Acropora palmata . PeerJ 5:e4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin‐Durante, M. K. , Miller M. W., Precht W. F. and Baums I. B.. 2016. How old are you? Genet age estimates in a clonal animal Molecular Ecology 25:5628–5646. [DOI] [PubMed] [Google Scholar]
- Dixon, G. B. , Davies S. W., Aglyamova G. V., Meyer E., Bay L. K., and Matz M. V.. 2015. Genomic determinants of coral heat tolerance across latitudes. Science 348:1460–1462. [DOI] [PubMed] [Google Scholar]
- Dixon, G. , Bay L. K., and Matz M. V.. 2016. Genic DNA methylation drives codon bias in stony corals. Molecular Biology and Evolution 33:2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollar, S. J. 1982. Wave stress and coral community structure in Hawaii. Coral Reefs 1:71–81. [Google Scholar]
- Drury, C. , Dale K. E., Panlilio J. M., Miller S. V., Lirman D., Larson E. A., Bartels E., Crawford D. L., and Oleksiak M. F.. 2016. Genomic variation among populations of threatened coral: Acropora cervicornis . BMC Genomics 17:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury, C. , Manzello D., and Lirman D.. 2017. Genotype and local environment dynamically influence growth, disturbance response and survivorship in the threatened coral, Acropora cervicornis . PLoS ONE 12:e0174000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eakin, C. M. , et al. 2010. Caribbean corals in crisis: record thermal stress, bleaching, and mortality in 2005. PLoS ONE 5:e13969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmands, S. 2007. Between a rock and a hard place: evaluating the relative risks of inbreeding and outbreeding for conservation and management. Molecular Ecology 16:463–475. [DOI] [PubMed] [Google Scholar]
- Edmunds, R. J. , and Elahi R.. 2007. The demographics of a 15‐year decline in cover of the Caribbean reef coral Montastraea annularis . Ecological Monographs 77:3–18. [Google Scholar]
- Edmunds, P. J. , Gates R. D., Leggat W., Hoegh‐Guldberg O., and Allen‐Requa L.. 2005. The effect of temperature on the size and population density of dinoflagellates in larvae of the reef coral Porites astreoides . Invertebrate Biology 124:185–193. [Google Scholar]
- Ellingson, R. A. , and Krug P. J.. 2016. Reduced genetic diversity and increased reproductive isolation follow population‐level loss of larval dispersal in a marine gastropod. Evolution 70:18–37. [DOI] [PubMed] [Google Scholar]
- Espeland, E. K. , Emery N. C., Mercer K. L., Woolbright S. A., Kettenring K. M., Gepts P., and Etterson J. R.. 2017. Evolution of plant materials for ecological restoration: insights from the applied and basic literature. Journal of Applied Ecology 54:102–115. [Google Scholar]
- Figueiredo, J. , Baird A., Harii S., and Connolly S. R.. 2014. Increased local retention of reef coral larvae as a result of ocean warming. Nature Climate Change 4:498–502. [Google Scholar]
- Fisher, E. M. , Fauth J. E., Hallock P., and Woodley C. M.. 2007. Lesion regeneration rates in reef‐building corals Montastraea spp. as indicators of colony condition. Marine Ecology Progress Series 339:61–71. [Google Scholar]
- Fogarty, N. D. 2012. Caribbean acroporid coral hybrids are viable across life history stages. Marine Ecology Progress Series 446:145–159. [Google Scholar]
- Fogarty, N. D. , Vollmer S. V., and Levitan D. R.. 2012. Weak prezygotic isolating mechanisms in threatened Caribbean Acropora corals. PLoS ONE 7:e30486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, N. L. , Baums I. B., and Mumby P. J.. 2007. Sexual vs. asexual reproduction in an ecosystem engineer: the massive coral Montastraea annularis . Journal of Animal Ecology 76:384–391. [DOI] [PubMed] [Google Scholar]
- Foster, N. L. , Baums I. B., Sanchez J. A., Paris C. B., Chollett I., Agudelo C. L., Vermeij M. J. A., and Mumby P. J.. 2013. Hurricane‐driven patterns of clonality in an ecosystem engineer: The Caribbean coral Montastraea annularis . PLoS ONE 8:e53283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankham, R. 2008. Genetic adaptation to captivity in species conservation programs. Molecular Ecology 17:325–333. [DOI] [PubMed] [Google Scholar]
- Fukami, H. , Budd A. F., Levitan D. R., Jara J., Kersanach R., and Knowlton N.. 2004. Geographic differences in species boundaries among members of the Montastraea annularis complex based on molecular and morphological markers. Evolution 58:324–337. [PubMed] [Google Scholar]
- Glynn, P. W. , Mate J. L., Baker A. C., and Calderon M. O.. 2001. Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Nino‐Southern oscillation event: Spatial/temporal patterns and comparisons with the 1982–1983 event. Bulletin of Marine Science 69:79–109. [Google Scholar]
- Goreau, T. F. 1959. The ecology of Jamaican coral reefs I: species composition and zonation. Ecology 40:67–90. [Google Scholar]
- Green, D. H. , Edmunds P. J., and Carpenter R. C.. 2008. Increasing relative abundance of Porites astreoides on Caribbean reefs mediated by an overall decline in coral cover. Marine Ecology Progress Series 359:1–10. [Google Scholar]
- Grottoli, A. G. , Warner M. E., Levas S. J., Aschaffenburg M. D., Schoepf V., McGinley M., Baumann J., and Matsui Y.. 2014. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Global Change Biology 20:3823–3833. [DOI] [PubMed] [Google Scholar]
- Grottoli, A. G. , et al. 2018. Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS ONE 13:e0191156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover, A. , and Sharma P. C.. 2016. Development and use of molecular markers: past and present. Critical Reviews in Biotechnology 36:290–302. [DOI] [PubMed] [Google Scholar]
- Grupstra, C. G. B. , Coma R., Ribes M., Leydet K. P., Parkinson J. E., McDonald K., Catllà M., Voolstra C. R., Hellberg M. E., and Coffroth M. A.. 2017. Evidence for coral range expansion accompanied by reduced diversity of Symbiodinium genotypes. Coral Reefs 39:981–985. [Google Scholar]
- Guest, J. R. , Baria M. V., Gomez E. D., Heyward A. J., and Edwards A. J.. 2014. Closing the circle: is it feasible to rehabilitate reefs with sexually propagated corals? Coral Reefs 33:45–55. [Google Scholar]
- Hagedorn, M. , Carter V. L., Steyn R. A., Krupp D., Leong J. C., Lang R. P., and Tiersch T. R.. 2006. Preliminary studies of sperm cryopreservation in the mushroom coral, Fungia scutaria . Cryobiology 52:454–458. [DOI] [PubMed] [Google Scholar]
- Hagedorn, M. , et al. 2012. Preserving and using germplasm and dissociated embryonic cells for conserving Caribbean and Pacific coral. PLoS ONE 7:e33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn, M. , Carter V. L., Lager C., Camperio Ciani J. F., Dygert A. N., Schleiger R. D., and Henley E. M.. 2016. Potential bleaching effects on coral reproduction. Reproduction Fertility and Development 28:1061–1071. [Google Scholar]
- Halpern, B. S. , et al. 2008. A global map of human impact on marine ecosystems. Science 319:948–952. [DOI] [PubMed] [Google Scholar]
- Harborne, A. R. , Rogers A., Bozec Y. M., Mumby P. J. and Annual R.. 2017. Multiple stressors and the functioning of coral reefs. Pages 445–468 in Annual Review of Marine Science, Volume 9. Annual Reviews, Palo Alto, California, USA. [DOI] [PubMed]
- Harii, S. , Yasuda N., Rodriguez‐Lanetty M., Irie T., and Hidaka M.. 2009. Onset of symbiosis and distribution patterns of symbiotic dinoflagellates in the larvae of scleractinian corals. Marine Biology 156:1203–1212. [Google Scholar]
- Herler, J. , and Dirnwöber M.. 2011. A simple technique for measuring buoyant weight increment of entire, transplanted coral colonies in the field. Journal of Experimental Marine Biology and Ecology 407:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermisson, J. , and Pennings P. S.. 2005. Soft sweeps: molecular population genetics of adaptation from standing genetic variation. Genetics 169:2335–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyward, A. J. , and Negri A. P.. 2010. Plasticity of larval pre‐competency in response to temperature: observations on multiple broadcast spawning coral species. Coral Reefs 29:631–636. [Google Scholar]
- Hoegh‐Guldberg, O. , Hughes L., McIntyre S., Lindenmayer D. B., Parmesan C., Possingham H. P., and Thomas C. D.. 2008. Assisted colonization and rapid climate change. Science 321:345–346. [DOI] [PubMed] [Google Scholar]
- Hoffberg, S. L. , Kieran T. J., Catchen J. M., Devault A., Faircloth B. C., Mauricio R., and Glenn T. C.. 2016. RADcap: sequence capture of dual‐digest RADseq libraries with identifiable duplicates and reduced missing data. Molecular Ecology Resources 16:1264–1278. [DOI] [PubMed] [Google Scholar]
- Honnay, O. , and Bossuyt B.. 2005. Prolonged clonal growth: escape route or route to extinction? Oikos 108:427–432. [Google Scholar]
- Horreo, J. L. , Valiente A. G., Ardura A., Blanco A., Garcia‐Gonzalez C., and Garcia‐Vazquez E.. 2018. Nature versus nurture? Consequences of short captivity in early stages. Ecology and Evolution 8:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells, E. J. , Beltran V. H., Larsen N. W., Bay L. K., Willis B. L., and van Oppen M. J. H.. 2012. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nature Climate Change 2:116–120. [Google Scholar]
- Howells, E. J. , Berkelmans R., van Oppen M. J. H., Willis B. L., and Bay L. K.. 2013. Historical thermal regimes define limits to coral acclimatization. Ecology 94:1078–1088. [DOI] [PubMed] [Google Scholar]
- Hughes, A. D. , and Grottoli A. G.. 2013. Heterotrophic compensation: A possible mechanism for resilience of coral reefs to global warming or a sign of prolonged stress? PLoS ONE 8:e81172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, T. P. , and Tanner J. E.. 2000. Recruitment failure, life histories, and long‐term decline of Caribbean corals. Ecology 81:2250–2263. [Google Scholar]
- Hughes, T. P. , et al. 2018. Global warming transforms coral reef assemblages. Nature 556:492–496. [DOI] [PubMed] [Google Scholar]
- Humanes, A. , and Bastidas C.. 2015. In situ settlement rates and early survivorship of hard corals: a good year for a Caribbean reef. Marine Ecology Progress Series 539:139–151. [Google Scholar]
- Iglesias‐Prieto, R. , Beltran V. H., LaJeunesse T. C., Reyes‐Bonilla H., and Thome P. E.. 2004. Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proceedings of the Royal Society B 271:1757–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUCN/SSC . 2013. Guidelines for Reintroductions and Other Conservation Translocations. Version 1.0. IUCN Species Survival Commission, Gland, Switzerland.
- Iwao, K. , Wada N., Ohdera A., and Omori M.. 2014. How many donor colonies should be cross‐fertilized for nursery farming of sexually propagated corals? Journal of Natural Resources 5:521–526. [Google Scholar]
- Jarett, J. K. , MacManes M. D., Morrow K. M., Pankey M. S., and Lesser M. P.. 2017. Comparative genomics of color morphs in the coral Montastraea cavernosa . Scientific Reports 7:16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokiel, P. , Maragos J. E., and Franzisket L.. 1978. Coral growth: buoyant weight technique Pages 529–541 in Stoddart D. and Johannes R., editors. Coral reefs: research methods. UNESCO, Paris, France. [Google Scholar]
- Jones, A. M. , Berkelmans R., van Oppen M. J. H., Mieog J. C., and Sinclair W.. 2008. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proceedings of the Royal Society B 275:1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel, C. D. , Goodbody‐Gringley G., Caillaud D., Davies S. W., Bartels E., and Matz M. V.. 2013. Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Molecular Ecology 22:4335–4348. [DOI] [PubMed] [Google Scholar]
- Kenkel, C. D. , Almanza A. T., and Matz M. V.. 2015a. Fine‐scale environmental specialization of reef‐building corals might be limiting reef recovery in the Florida Keys. Ecology 96:3197–3212. [DOI] [PubMed] [Google Scholar]
- Kenkel, C. D. , Setta S. P., and Matz M. V.. 2015b. Heritable differences in fitness‐related traits among populations of the mustard hill coral, Porites astreoides . Heredity 115:509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleypas, J. A. , Thompson D. M., Castruccio F. S., Curchitser E. N., Pinsky M., and Watson J. R.. 2016. Larval connectivity across temperature gradients and its potential effect on heat tolerance in coral populations. Global Change Biology 22:3539–3549. [DOI] [PubMed] [Google Scholar]
- Knowlton, N. 2001. The future of coral reefs. Proceedings of the National Academy of Sciences USA 98:5419–5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ktichen, S. A. , Ratan A., Bedoya‐Reina O. C., Burhans R., Fogarty N. D., Miller W. and Baums I. B.. 2019. Genomic Variants Among Threatened Acropora Corals. G3: Genes¦Genomes¦Genetics 9:1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffner, I. B. , and Toth L. T.. 2016. A geological perspective on the degradation and conservation of western Atlantic coral reefs. Conservation Biology 30:706–715. [DOI] [PubMed] [Google Scholar]
- Kuffner, I. B. , Hickey T. D., and Morrison J. M.. 2013. Calcification rates of the massive coral Siderastrea siderea and crustose coralline algae along the Florida Keys (USA) outer‐reef tract. Coral Reefs 32:987–997. [Google Scholar]
- Kuffner, I. B. , Bartels E., Stathakopoulos A., Enochs I. C., Kolodziej G., Toth L. T., and Manzello D. P.. 2017. Plasticity in skeletal characteristics of nursery‐raised staghorn coral, Acropora cervicornis . Coral Reefs 36:679–684. [Google Scholar]
- Ladd, M. C. , Shantz A. A., Bartels E., and Burkepile D. E.. 2017. Thermal stress reveals a genotype‐specific tradeoff between growth and tissue loss in restored Acropora cervicornis . Marine Ecology Progress Series 572:129–139. [Google Scholar]
- Ladd, M. C. , Miller M. W., Hunt J. H., Sharp W. C., and Burkepile D. E.. 2018. Harnessing ecological processes to facilitate coral restoration. Frontiers in Ecology and the Environment 16:239–247. [Google Scholar]
- LaJeunesse, T. C. 2001. Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the its region: In search of a “species” level marker. Journal of Phycology 37:866–880. [Google Scholar]
- LaJeunesse, T. C. , and Thornhill D. J.. 2011. Improved resolution of reef‐coral endosymbiont (Symbiodinium) species diversity, ecology, and evolution through psbA non‐coding region genotyping. PLoS ONE 6:2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse, T. C. , Bhagooli R., Hidaka M., DeVantier L., Done T., Schmidt G. W., Fitt W. K., and Hoegh‐Guldberg O.. 2004. Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Marine Ecology Progress Series 284:147–161. [Google Scholar]
- LaJeunesse, T. C. , et al. 2010. Host‐symbiont recombination versus natural selection in the response of coral‐dinoflagellate symbioses to environmental disturbance. Proceedings of the Royal Society B 277:2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse, T. C. , Parkinson J. E., Gabrielson P. W., Jeong H. J., Reimer J. D., Voolstra C. R., and Santos S. R.. 2018. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Current Biology 28:2570–2580.e2576. [DOI] [PubMed] [Google Scholar]
- Levas, S. J. , Grottoli A. G., Hughes A., Osburn C. L. and Matsui Y.. 2013. Physiological and biogeochemical traits of bleaching and recovery in the mounding species of coral Porites lobata: Implications for resilience in mounding corals. PLoS ONE 8:e63267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levas, S. , Schoepf V., Warner M. E., Aschaffenburg M., Baumann J., and Grottoli A. G.. 2018. Long‐term recovery of Caribbean corals from bleaching. Journal of Experimental Marine Biology and Ecology 506:124–134. [Google Scholar]
- Levitan, D. R. , Fukami H., Jara J., Kline D., McGovern T. M., McGhee K. E., Swanson C. A., and Knowlton N.. 2004. Mechanisms of reproductive isolation among sympatric broadcast‐spawning corals of the Montastraea annularis species complex. Evolution 58:308–323. [PubMed] [Google Scholar]
- Lewis, A. M. , Chan A. N., and LaJeunesse T. C.. 2018. New species of closely related endosymbiotic dinoflagellates in the greater Caribbean have niches corresponding to host coral phylogeny. Journal of Eukaryotic Microbiology 66:469–482. [DOI] [PubMed] [Google Scholar]
- Libro, S. , and Vollmer S. V.. 2016. Genetic signature of resistance to White Band Disease in the Caribbean staghorn coral Acropora cervicornis . PLoS ONE 11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirman, D. , and Schopmeyer S.. 2016. Ecological solutions to reef degradation: optimizing coral reef restoration in the Caribbean and Western Atlantic. PeerJ 4:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirman, D. , Formel N., Schopmeyer S., Ault J., Smith S., Gilliam D., and Riegl B.. 2014a. Percent recent mortality (PRM) of stony corals as an ecological indicator of coral reef condition. Ecological Indicators 44:120–127. [Google Scholar]
- Lirman, D. , Schopmeyer S., Galvan V., Drury C., Baker A. C., and Baums I. B.. 2014b. Growth dynamics of the threatened Caribbean staghorn coral Acropora cervicornis: influence of host genotype, symbiont identity, colony size, and environmental setting. PLoS ONE 9:e107253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little, A. F. , van Oppen M. J. H., and Willis B. L.. 2004. Flexibility in algal endosymbioses shapes growth in reef corals. Science 304:1492–1494. [DOI] [PubMed] [Google Scholar]
- Lopez, J. V. , Kersanach R., Rehner S. A., and Knowlton N.. 1999. Molecular determination of species boundaries in corals: genetic analysis of the Montastraea annularis complex using amplified fragment length polymorphisms and a microsatellite marker. Biological Bulletin 196:80–93. [DOI] [PubMed] [Google Scholar]
- Matz, M. V. , Treml E. A., Aglyamova G. V., and Bay L. K.. 2018. Potential and limits for rapid genetic adaptation to warming in a Great Barrier Reef coral. PLos Genetics 14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard, J. , et al. 2015. Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nature Climate Change 5:688. [Google Scholar]
- McClenachan, L. , O'Connor G., Neal B. P., Pandolfi J. M., and Jackson J. B. C.. 2017. Ghost reefs: Nautical charts document large spatial scale of coral reef loss over 240 years. Science Advances 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy, S. E. , and Coffroth M. A.. 2017. Coral ontogeny affects early symbiont acquisition in laboratory reared recruits. Coral Reefs 36:927–932. [Google Scholar]
- Miller, M. W. , Lohr K. E., Cameron C. M., Williams D. E., and Peters E. C.. 2014. Disease dynamics and potential mitigation among restored and wild staghorn coral, Acropora cervicornis . PeerJ 2:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W. , Williams D. E. and Fisch J.. 2016. Genet‐specific spawning patterns in Acropora palmata . Coral Reefs 35:1393–1398. [Google Scholar]
- Miller, M. W. , Baums I. B., Pausch R. E., Bright A. J., Cameron C. M., Williams D. E., Moffitt Z. J., and Woodley C. M.. 2017. Clonal structure and variable fertilization success in Florida Keys broadcast‐spawning corals. Coral Reefs 37:239–249. [Google Scholar]
- Montoya Maya, P. H. , Smit K. P., Burt A. J. and Frias‐Torres S.. 2016. Large‐scale coral reef restoration could assist natural recovery in Seychelles, Indian Ocean. Nature Conservation 35:1393–1398. [Google Scholar]
- Moore, R. B. , Ferguson K. M., Loh W. K. W., Hoegh‐Guldberg C., and Carter D. A.. 2003. Highly organized structure in the non‐coding region of the psbA minicircle from clade C Symbiodinium . International Journal of Systematic and Evolutionary Microbiology 53:1725–1734. [DOI] [PubMed] [Google Scholar]
- Morrison, J. M. , Kuffner I. B. and Hickey T. D.. 2013. Methods for monitoring corals and crustose coralline algae to quantify in‐situ calcification rates. U.S. Geological Survey Open‐File Report 2013‐1159, 11p. http://pubs.usgs.gov/of/2013/1159/.
- Morvezen, R. , Boudry P., Laroche J., and Charrier G.. 2016. Stock enhancement or sea ranching? Insights from monitoring the genetic diversity, relatedness and effective population size in a seeded great scallop population (Pecten maximus). Heredity 117:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, E. M. , Bartles E., and Baums I. B.. 2018. Bleaching causes loss of disease resistance within the threatened coral species, Acropora cervicornis . eLife 7:e35066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely, K. L. , Lewis C., Chan A. N., and Baums I. B.. 2018. Hermaphroditic spawning by the gonochoric pillar coral Dendrogyra cylindrus . Coral Reefs 37:1087–1092. [Google Scholar]
- Nitschke, M. R. , Davy S. K., and Ward S.. 2016. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs 35:335–344. [Google Scholar]
- NMFS . 2015. Recovery plan for Elkhorn (Acropora palmata) and Staghorn (A. cervciornis) Corals. Prepared by the Acropora Recovery Team for the National Marine Fisheries Service. National Marine Fisheries Service, Silver Spring, Maryland, USA. [Google Scholar]
- O'Donnell, K. E. , Lohr K. E., Bartels E., Baums I. B., and Patterson J. T.. 2018. Acropora cervicornis genet performance and symbiont identity throughout the restoration process. Coral Reefs 37:1109–1118. [Google Scholar]
- Oliver, J. , and Babcock R.. 1992. Aspects of the fertilization ecology of broadcast spawning corals ‐ sperm dilution effects and insitu measurements of fertilization. Biological Bulletin 183:409–417. [DOI] [PubMed] [Google Scholar]
- Palmer, C. V. , Traylor‐Knowles N. G., Willis B. L., and Bythell J. C.. 2011. Corals use similar immune cells and wound‐healing processes as those of higher organisms. PLoS ONE 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbi, S. R. , Barshis D. J., Traylor‐Knowles N., and Bay R. A.. 2014. Mechanisms of reef coral resistance to future climate change. Science 344:895–898. [DOI] [PubMed] [Google Scholar]
- Parkinson, J. E. and Baums I. B.. 2014. The extended phenotypes of marine symbioses: ecological and evolutionary consequences of intraspecific genetic diversity in coral‐algal associations. Frontiers in Microbiology 5:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, J. E. , Banaszak A. T., Altman N. S., LaJeunesse T. C., and Baums I. B.. 2015a. Intraspecific diversity among partners drives functional variation in coral symbioses. Scientific Reports 5:15667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson, J. E. , Coffroth M. A., and LaJeunesse T. C.. 2015b. New species of Clade B Symbiodinium (Dinophyceae) from the greater Caribbean belong to different functional guilds: S. aenigmaticum sp. nov., S. antillogorgium sp. nov., S. endomadracis sp. nov., and S. pseudominutum sp. nov. Journal of Phycology 51:850–858. [DOI] [PubMed] [Google Scholar]
- Parkinson, J. , et al. 2018a. Molecular tools for coral reef restoration: beyond biomarker discovery. OSF Preprints.
- Parkinson, J. E. , Bartels E., Devlin‐Durante M. K., Lustic C., Nedimyer K., Schopmeyer S., Lirman D., LaJeunesse T. C., and Baums I. B.. 2018b. Extensive transcriptional variation poses a challenge to thermal stress biomarker development for endangered corals. Molecular Ecology 27:1103–1119. [DOI] [PubMed] [Google Scholar]
- Pausch, R. E. , Williams D. E., and Miller M. W.. 2018. Impacts of fragment genotype, habitat, and size on outplanted elkhorn coral success under thermal stress. Marine Ecology Progress Series 592:109–117. [Google Scholar]
- Peixoto, R. S. , Rosado P. M., Leite D. C., Rosado A. S., and Bourne D. G.. 2017. Beneficial microorganisms for corals (BMC): Proposed mechanisms for coral health and resilience. Frontiers in Microbiology 8:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, R. J. , Barreto F. S., and Burton R. S.. 2014. Ecological novelty by hybridization: experimental evidence for increased thermal tolerance by transgressive segregation in Tigriopus californicus . Evolution 68:204–215. [DOI] [PubMed] [Google Scholar]
- Perry, C. T. , et al. 2018. Loss of coral reef growth capacity to track future increases in sea level. Nature 558:396–400. [DOI] [PubMed] [Google Scholar]
- Pettay, D. T. , and LaJeunesse T. C.. 2007. Microsatellites from clade B Symbiodinium spp. specialized for Caribbean corals in the genus Madracis . Molecular Ecology Notes 7:1271–1274. [Google Scholar]
- Pettay, D. T. , and LaJeunesse T. C.. 2009. Microsatellite loci for assessing genetic diversity, dispersal and clonality of coral symbionts in ‘stress‐tolerant’ clade D Symbiodinium. Molecular Ecology Resources 9:1022–1025. [DOI] [PubMed] [Google Scholar]
- Pettay, D. T. , Wham D. C., Smith R. T., Iglesias‐Prieto R., and LaJeunesse T. C.. 2015. Microbial invasion of the Caribbean by an Indo‐Pacific coral zooxanthella. Proceedings of the National Academy of Sciences USA 112:7513–7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, K. P. , Jorgensen T. H., Jolliffe K. G., and Richardson D. S.. 2017. Evidence of opposing fitness effects of parental heterozygosity and relatedness in a critically endangered marine turtle? Journal of Evolutionary Biology 30:1953–1965. [DOI] [PubMed] [Google Scholar]
- Pinzón, J. , Devlin‐Durante M., Weber M., Baums I., and LaJeunesse T.. 2011. Microsatellite loci for Symbiodinium A3; (S. fitti) a common algal symbiont among Caribbean Acropora; (stony corals) and Indo‐Pacific giant clams (Tridacna). Conservation Genetics Resources 3:45–47. [Google Scholar]
- Pochon, X. , Wecker P., Stat M., Berteaux‐Lecellier V., and Lecellier G.. 2018. Towards an in‐depth characterization of Symbiodiniaceae in tropical giant clams via multiplex metabarcoding. PeerJ Preprints 6:e27313v27311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland, D. M. , and Coffroth M. A.. 2017. Trans‐generational specificity within a cnidarian–algal symbiosis. Coral Reefs 36:119–129. [Google Scholar]
- Polato, N. R. , Voolstra C. R., Schnetzer J., DeSalvo M. K., Randall C. J., Szmant A. M., Medina M., and Baums I. B.. 2010. Location‐specific responses to thermal stress in larvae of the reef‐building coral Montastraea faveolata . PLoS ONE 5:e11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock, F. J. , et al. 2017. Coral larvae for restoration and research: a large‐scale method for rearing Acropora millepora larvae, inducing settlement, and establishing symbiosis. PeerJ 5:e3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada, C. , and Hellberg M. E.. 2013. Long prereproductive selection and divergence by depth in a Caribbean candelabrum coral. Proceedings of the National Academy of Sciences USA 110:3961–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada, C. , Hanna B., Budd A. F., Woodley C. M., Schmutz J., Grimwood J., Iglesias‐Prieto R., Pandolfi J. M., Levitan D., and Johnson K. G.. 2016. Empty niches after extinctions increase population sizes of modern corals. Current Biology 26:3190–3194. [DOI] [PubMed] [Google Scholar]
- Precht, W. F. , Gintert B. E., Robbart M. L., Fura R., and van Woesik R.. 2016. Unprecedented disease‐related coral mortality in southeastern Florida. Scientific Reports 6:31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard, V. L. , Knutson V. L., Lee M., Zieba J., and Edmands S.. 2013. Fitness and morphological outcomes of many generations of hybridization in the copepod Tigriopus californicus . Journal of Evolutionary Biology 26:416–433. [DOI] [PubMed] [Google Scholar]
- Prober, S. , Byrne M., McLean E., Steane D., Potts B., Vaillancourt R. and Stock W.. 2015. Climate‐adjusted provenancing: a strategy for climate‐resilient ecological restoration. Frontiers in Ecology and Evolution 3 10.3389/fevo.2015.00065. [DOI] [Google Scholar]
- Puckett, E. E. 2017. Variability in total project and per sample genotyping costs under varying study designs including with microsatellites or SNPs to answer conservation genetic questions. Conservation Genetics Resources 9:289–304. [Google Scholar]
- Quigley, K. M. , Davies S. W., Kenkel C. D., Willis B. L., Matz M. V., and Bay L. K.. 2014. Deep‐sequencing method for quantifying background abundances of Symbiodinium types: exploring the rare Symbiodinium biosphere in reef‐building corals. PLoS ONE 9:e94297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley, K. M. , Bay L. K., and Willis B. L.. 2018. Leveraging new knowledge of Symbiodinium community regulation in corals for conservation and reef restoration. Marine Ecology Progress Series 600:245–253. [Google Scholar]
- Ralls, K. , Ballou J. D., Dudash M. R., Eldridge M. D. B., Fenster C. B., Lacy R. C., Sunnucks P., and Frankham R.. 2018. Call for a paradigm shift in the genetic management of fragmented populations. Conservation Letters 11:e12412. [Google Scholar]
- Raymundo, L. J. , and Maypa A. P.. 2004. Getting bigger faster: Mediation of size‐specific mortality via fusion in juvenile coral transplants. Ecological Applications 14:281–295. [Google Scholar]
- Riegl, B. M. , Purkis S. J., Al‐Cibahy A. S., Abdel‐Moati M. A., and Hoegh‐Guldberg O.. 2011. Present limits to heat‐adaptability in corals and population‐level responses to climate extremes. PLoS ONE 6:e24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, L. J. , and Grottoli A. G.. 2007. Energy reserves and metabolism as indicators of coral recovery from bleaching. Limnology and Oceanography 52:1874–1882. [Google Scholar]
- Rogers, C. S. , Fitz H. C., Gilnack M., Beets J., and Hardin J.. 1984. Scleractinian coral recruitment patterns at Salt River Submarine Canyon, St‐Croix, United States Virgin Islands. Coral Reefs 3:69–76. [Google Scholar]
- Rohwer, F. , Breitbart M., Jara J., Azam F., and Knowlton N.. 2001. Diversity of bacteria associated with the Caribbean coral Montastraea franksi . Coral Reefs 20:85–91. [Google Scholar]
- Rowan, R. , and Knowlton N.. 1995. Intraspecific diversity and ecological zonation in coral‐algal symbiosis. Proceedings of the National Academy of Sciences USA 92:2850–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan, R. , Knowlton N., Baker A., and Jara J.. 1997. Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269. [DOI] [PubMed] [Google Scholar]
- Santos, S. R. , and Coffroth M. A.. 2003. Molecular genetic evidence that dinoflagellates belonging to the genus Symbiodinium freudenthal are haploid. Biological Bulletin 204:10–20. [DOI] [PubMed] [Google Scholar]
- Santos, S. R. , Taylor D. J., Kinzie R. A. III, Hidaka M., Sakai K., and Coffroth M. A.. 2002. Molecular phylogeny of symbiotic dinoflagellates inferred from partial chloroplast large subunit (23S)‐rDNA sequences. Molecular Phylogenetics and Evolution 23:97–111. [DOI] [PubMed] [Google Scholar]
- Schoenberg, D. , and Trench R.. 1980. Genetic variation in Symbiodinium (= Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. I. Isoenzyme and soluble protein patterns of axenic cultures of Symbiodinium microadriaticum . Proceedings of the Royal Society B 207:405–427. [Google Scholar]
- Schoepf, V. , Jury C. P., Toonen R. J. and McCulloch M. T.. 2017. Coral calcification mechanisms facilitate adaptive responses to ocean acidification. Proceedings of the Royal Society B 284 10.1098/rspb.2017.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, J. A. , Krupp D. A., and Weis V. M.. 1999. Late larval development and onset of symbiosis in the scleractinian coral fungia scutaria. Biological Bulletin 196:70–79. [DOI] [PubMed] [Google Scholar]
- Serrano, X. , Baums I. B., O'Reilly K., Smith T. B., Jones R. J., Shearer T. L., Nunes F. L., and Baker A. C.. 2014. Geographic differences in vertical connectivity in the Caribbean coral Montastraea cavernosa despite high levels of horizontal connectivity at shallow depths. Molecular Ecology 23:4226–4240. [DOI] [PubMed] [Google Scholar]
- Serrano, X. M. , Baums I. B., Smith T. B., Jones R. J., Shearer T. L., and Baker A. C.. 2016. Long distance dispersal and vertical gene flow in the Caribbean brooding coral Porites astreoides . Scientific Reports 6:21619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance, E. G. , Szmant A. M., and Karl S. A.. 2004. Microsatellite loci isolated from the Caribbean coral, Montastraea annularis . Molecular Ecology Notes 4:74–76. [Google Scholar]
- Sgrò, C. M. , Lowe A. J., and Hoffmann A. A.. 2011. Building evolutionary resilience for conserving biodiversity under climate change. Evolutionary Applications 4:326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer, T. , and Coffroth M.. 2004. Isolation of microsatellite loci from the scleractinian corals, Montastraea cavernosa and Porites astreoides . Molecular Ecology Resources 4:435–437. [Google Scholar]
- Smith, T. B. , Brandt M. E., Calnan J. M., Nemeth R. S., Blondeau J., Kadison E., Taylor M., and Rothenberger P.. 2013. Convergent mortality responses of Caribbean coral species to seawater warming. Ecosphere 4:art87. [Google Scholar]
- Smith, E. G. , Ketchum R. N., and Burt J. A.. 2017. Host specificity of Symbiodinium variants revealed by an ITS2 metahaplotype approach. ISME Journal 11:1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong, K. , and Lang J. C.. 1992. Reproductive integration in reef corals. Biological Bulletin 183:418–431. [DOI] [PubMed] [Google Scholar]
- Storlazzi, C. D. , et al. 2018. Most atolls will be uninhabitable by the mid‐21st century because of sea‐level rise exacerbating wave‐driven flooding. Science Advances 4:eaap9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nature Conservancy . 2018. Larval propagation. vhttp://www.reefresilience.org/restoration/coral‐populations/larval‐propagation/
- Thomas, L. , Kendrick G. A., Kennington W. J., Richards Z. T., and Stat M.. 2014. Exploring Symbiodinium diversity and host specificity in Acropora corals from geographical extremes of Western Australia with 454 amplicon pyrosequencing. Molecular Ecology 23:3113–3126. [DOI] [PubMed] [Google Scholar]
- Torda, G. , et al. 2017. Rapid adaptive responses to climate change in corals. Nature Climate Change 7:627–636. [Google Scholar]
- Toth, L. T. , Kuffner I. B., Stathakopoulos A., and Shinn E. A.. 2018. A 3,000‐year lag between the geological and ecological shutdown of Florida's coral reefs. Global Change Biology 24:5471–5483. [DOI] [PubMed] [Google Scholar]
- Tringali, M. D. , et al. 2007. Genetic Policy for the Release of Finfishes in Florida. Publication No. IHR‐2007‐001. Florida Fish and Wildlife Research Institute, Talahassee, Florida, USA.
- Uzoh, F. C. C. and Ritchie M. W.. 1996. Crown area equations for 13 species of trees and shrubs in Northern California and Southwestern Oregon. USDA Forest Service Pacific Southwest Research Station Research Paper, Albany, CA. [Google Scholar]
- van Hooidonk, R. , Maynard J., Tamelander J., Gove J., Ahmadia G., Raymundo L., Williams G., Heron S. F., and Planes S.. 2016. Local‐scale projections of coral reef futures and implications of the Paris Agreement. Scientific Reports 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oppen, M. 2001. In vitro establishment of symbiosis in Acropora millepora planulae. Coral Reefs 20:200–200. [Google Scholar]
- van Oppen, M. J. H. , Oliver J. K., Putnam H. M., and Gates R. D.. 2015. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences USA 112:2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oppen, M. J. H. , Souter P., Howells E. J., Heyward A., and Berkelmans R.. 2011. Novel genetic diversity through somatic mutations: fuel for adaptation of reef corals? Diversity 3:405–423. [Google Scholar]
- Van Veghel, M. L. and Bak R. P.. 1993. Intraspecific variation of a dominant Caribbean reef building coral, Montastrea annularis: genetic, behavioral and morphometric aspects. Marine Ecology Progress Series 92:255–265. [Google Scholar]
- van Woesik, R. , Scott W. J., and Aronson R. B.. 2014. Lost opportunities: Coral recruitment does not translate to reef recovery in the Florida Keys. Marine Pollution Bulletin 88:110–117. [DOI] [PubMed] [Google Scholar]
- Vermeij, M. J. A. , Bakker J., Hal N. V. d. and Bak R. P. M.. 2011. Juvenile coral abundance has decreased by more than 50% in only three decades on a small Caribbean island. Diversity 3:296–307. [Google Scholar]
- Veron, J. E. N. 1995. Corals in space and time. The biogeography and evolution of the Scleractinia. Cornell University Press, Ithaca, New Jeresey, USA. [Google Scholar]
- Vollmer, S. V. , and Palumbi S. R.. 2002. Hybridization and the evolution of reef coral diversity. Science 296:2023–2025. [DOI] [PubMed] [Google Scholar]
- Walton, C. J. , Hayes N. K. and Gilliam D. S.. 2018. Impacts of a regional, multi‐year, multi‐species coral disease outbreak in Southeast Florida. Frontiers in Marine Science 5 10.3389/fmars.2018.00323. [DOI] [Google Scholar]
- Weil, E. 1992. Genetic and morphological variation in Caribbean and eastern Pacific Porites (Anthozoa, Scleractinia). Preliminary results Pages 643–655 in Proceedings of the Seventh International Coral Reef Symposium, Guam, Volume 2 [Google Scholar]
- Weis, V. M. , Reynolds W. S., deBoer M. D., and Krupp D. A.. 2001. Host‐symbiont specificity during onset of symbiosis between the dinoflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria . Coral Reefs 20:301–308. [Google Scholar]
- Wham, D. C. , Pettay D. T., and LaJeunesse T. C.. 2011. Microsatellite loci for the host‐generalist “zooxanthella” Symbiodinium trenchi and other Clade D Symbiodinium . Conservation Genetics Resources 3:541–544. [Google Scholar]
- Whiteley, A. R. , Fitzpatrick S. W., Funk W. C., and Tallmon D. A.. 2015. Genetic rescue to the rescue. Trends in Ecology & Evolution 30:42–49. [DOI] [PubMed] [Google Scholar]
- Williams, D. E. , Miller M. W., and Kramer K. L.. 2008. Recruitment failure in Florida Keys Acropora palmata, a threatened Caribbean coral. Coral Reefs 27:697–705. [Google Scholar]
- Williams, A. V. , Nevill P. G., and Krauss S. L.. 2014a. Next generation restoration genetics: applications and opportunities. Trends in Plant Science 19:529–537. [DOI] [PubMed] [Google Scholar]
- Williams, D. E. , Miller M. W., and Baums I. B.. 2014b. Cryptic changes in the genetic structure of a highly clonal coral population and the relationship with ecological performance. Coral Reefs 33:595–606. [Google Scholar]
- Willis, B. L. , van Oppen M. J. H., Miller D. J., Vollmer S. V., and Ayre D. J.. 2006. The role of hybridization in the evolution of reef corals. Annual Review of Ecology Evolution and Systematics 37:489–517. [Google Scholar]
- Wood, S. , Baums I. B., Paris C. B., Ridgwell A., Kessler W. S., and Hendy E. J.. 2016. El Niño and coral larval dispersal across the eastern Pacific marine barrier. Nature Communications 7:12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, H. , Suzuki G., Hayashibara T., and Koike K.. 2013. Acropora recruits harbor “rare” Symbiodinium in the environmental pool. Coral Reefs 32:355–366. [Google Scholar]
- Yamashita, H. , Suzuki G., Shinzato C., Jimbo M., and Koike K.. 2018. Symbiosis process between Acropora larvae and Symbiodinium differs even among closely related Symbiodinium types. Marine Ecology Progress Series 592:119–128. [Google Scholar]
- Yates, K. K. , Zawada D. G., Smiley N. A., and Tiling‐Range G.. 2017. Divergence of seafloor elevation and sea level rise in coral reef ecosystems. Biogeosciences 14:1739–1772. [Google Scholar]
- Young, C. N. , Schopmeyer S. A., and Lirman D.. 2012. A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and Western Atlantic. Bulletin of Marine Science 88:1075–1098. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


