Abstract
Summary:
Objective
The aim of this study was to determine if insular damage is associated with markers of autonomic dysfunction.
Methods
We studied patients who underwent temporal lobe and/or insular resections for epilepsy surgery between April 2010 and June 2015 at UHCMC. Pre-surgical T1-weighted MPRAGE, standard T1, T2 and FLAIR sequences were compared with post-surgical MRI by a neuroradiologist and classified as type 0 (no involvement of insula), type 1 (minimal involvement of insular margin), type 2 (insular involvement <25%) and type 3 (insular involvement ≥25%).
Analysis of heart rate variability (HRV) was carried out in pre-and post-operative video electroencephalography (vEEG) recording. Time-domain parameters were calculated: (MNN [mean of RR intervals], RMSSD [root mean square difference of successive RR intervals], SDNN [standard deviation of RR intervals] and CV [coefficient of variation]). In addition, frequency-domain parameters were calculated: (low frequency [LF], high frequency [HF] and LF/HF).
Results
Twenty-one patients (14 females) with mean age of 36.2 ± 14.4 (30; 22–75) were studied. Insular involvement was classified as type 0 (4 patients [19%]), type 1 (9 [43%]), type 2 (7 [33%]) and type 3 (1 [5%]). Significant decrease in RMSSD (p=0.025) and CV (p=0.008) was seen in insular damage types 2 and 3 compared with no or minimal insular involvement (types 0 and 1). Right-sided resections were associated with increase in LF power (p=0.010) and the LF/HF ratio (p=0.017).
Conclusions
This study indicates that insular resection may lead to autonomic function changes.
Keywords: SUDEP, Insula, Heart rate variability, Epilepsy Surgery, Autonomic function
Introduction
The insula, by virtue of its role in autonomic and cardiovascular control, has been linked to stroke-related mortality [1, 2] and more recently to sudden unexpected death in epilepsy (SUDEP) [3–5]. Right or left lesional insular epilepsy may result in ictal bradycardia and asystole [6, 7] and may also be related to post-ictal cardiac dysrhythmia [5]. It is therefore possible that the SUDEP may result from a predisposition toward fatal cardiac rhythms due to an insular epileptogenic zone [4, 5] or damage to insular structures [3] that alters autonomic tone. Insular damage may arise from several different mechanisms in epilepsy including direct insular resections during epilepsy surgery to generalized convulsive seizures that induce damage to the insula [8, 9]. Cortical thinning [8] and increased insular connectivity [9] are known to occur in high-risk patients, although their association with peri-ictal cardiovascular dysfunction remains to be proven [10]. Heart rate variability (a measure of the autonomic function) is known to be significantly reduced in epilepsy, and decreased HRV is associated with increased risk of sudden cardiac death [10]. We set out to determine the presence and extension of insular involvement in different types of temporal lobe and/or insular epilepsy and the effect of such resection on changes in baseline heart rate variability (HRV) measures before and after surgical resection.
Methods
We retrospectively analyzed the epilepsy surgery database at University Hospitals Cleveland Medical Center (UHCMC) for all patients who underwent temporal lobe and/or insula resections between April 2010 and June 2015. Exclusion criteria for analysis were patients with insular damage prior to epilepsy surgery, unavailable post-operative brain MRI and/ or post-operative vEEG recordings for HRV analysis.
Neuroimaging and surgical details
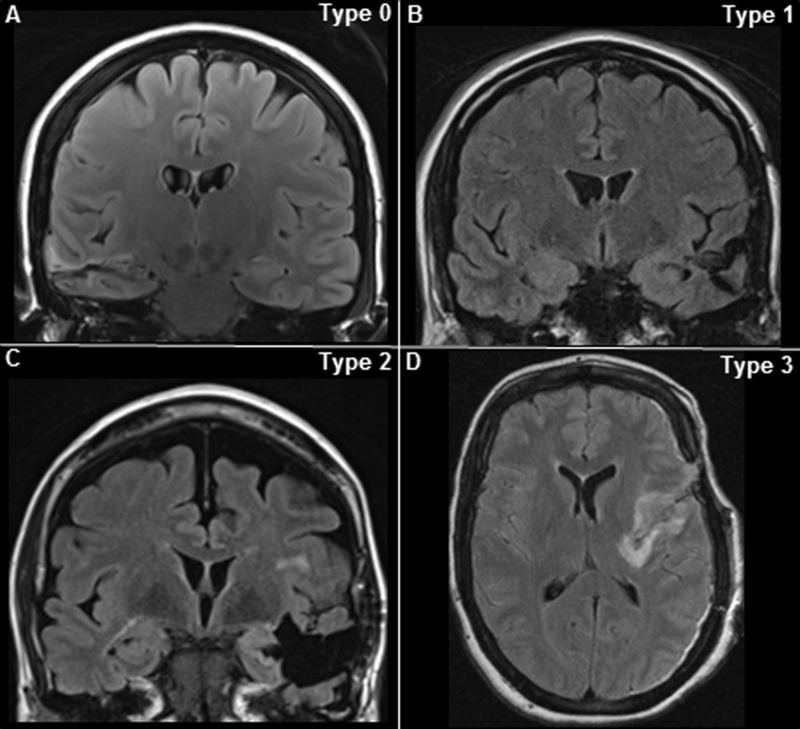
Pre-surgical thin-cut (1 mm) T1-weighted MPRAGE, standard T1, T2, T2-weighted FLAIR pre-contrast and/or post-contrast sequences were compared to post-surgical MRI by two neuroradiologists and classified according to presence and severity of insular involvement or resection as type 0 (no involvement of insula), type 1 (minimal involvement of insular margin), type 2 (insular involvement <25%) and type 3 (insular involvement ≥25%) (Figure 1).
Figure 1. Types of insular radiological involvement.
FLAIR MRI brain sequences showing: A) no involvement of insula (type 0) after right-sided selective surgery, B) minimal involvement of posterior insular border (type 1) after left-selective extensive surgery, C) <25% of insular involvement (type 2) after left-sided extensive surgery, and D) ≥25% (type 3) after left-sided extensive surgery.
Autonomic function analysis
Analysis of heart rate variability (HRV) metrics was carried out in a subset of patients who underwent post-operative long-term epilepsy monitoring, usually as a result of continuing seizures, in whom pre-and post-operative HRV comparisons were possible. Video electroencephalography (vEEG) records were analyzed to exclude movement, activity and electroclinical seizures, so as to ensure similar pre-and post-operative interictal records using a MATLAB program.
In this study, we used linear analytical methods to study differences in HRV patterns between patients with and without radiological insular resection (type 2–3 vs type 0–1). Several time-domain parameters were calculated including MNN (mean of the RR intervals), RMSSD (root mean square difference of successive RR intervals), SDNN (standard deviation of the RR intervals) and CV (coefficient of variation). In addition, frequency-domain parameters were calculated including normalized low frequency (LF) power (0.04–0.15 Hz), normalized HF power (0.15–0.4 Hz) and LF/HF power ratio. These were calculated over 5-minute periods during 30 minutes of identical inter-ictal awake states in two successive vEEG evaluations in each patient.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) IBM, corp. version 24. Summary statistics were reported as mean +/− standard deviation (median, range). Chi-square test was used to assess the association between the dichotomous variable radiological insular resection (yes/no) with nominal variables. Paired sample t-test between 5-minute periods averaged in the pre-operative vEEG with 5-minute periods averaged in the post-operative vEEG were carried out with each patient. Kruskal-Wallis one-way analysis of variance was used to assess the relationship between insular resection extent with HRV changes and Mann-Whitney U test to compare HRV in between both hemispheres. Spearman’s correlation coefficient was used to assess the relationship between epilepsy surgery outcome and HRV changes. Significance was set at p<0.05 using two-sided tests. The study was reviewed and approved by the local Institutional Review Board.
Results
Seventy-two patients underwent temporal lobe and/or insular resection during the period April 2010-June 2015. Two SUDEP cases previously reported were not included in this study [3]. Forty-nine patients were excluded due to presence of insular damage prior to epilepsy surgery, unavailable post-operative brain MRI or post-operative vEEG recordings for HRV analysis or because of absence of artifact-free EKG recordings.
Thus, 21 patients were analyzed (14 females) with a mean age of 36.2 years ± 14.4 (30; 22–75) were included in the study (Table 1). The mean time between pre-and post-surgical video-EEG recordings was 40.7± 31 months (35; 9–168). Mean time between surgery and pre-surgical video EEG recoding was 14.04± 13.6 months (7; 1–46), surgery to post-surgical video EEG recording was 20.5±17.04 months (20; 1–70).
Table 1.
Epilepsy surgery characteristics, radiological insular involvement, outcome.
| Case | Age | Operation | Side | Pre-operative Brain MRI |
Insular Involvement on post-operative MRI |
Outcome (ILAE) |
|---|---|---|---|---|---|---|
| 1 | 25 | Selective temporal lobectomy + hippocampal transections | L | Negative | 2 | 2 |
| 2 | 30 | Selective temporal lobectomy | R | Negative | 2 | 1 |
| 3 | 60 | Hippocampal transections | L | Negative | 0 | 3 |
| 4 | 75 | Selective temporal lobectomy + hippocampal transections | L | Negative | 1 | 1 |
| 5 | 30 | Selective temporal lobectomy + hippocampal transections | L | Negative | 1 | 4 |
| 6 | 44 | Selective temporal lobectomy + hippocampal transections | L | Negative | 1 | 3 |
| 7 | 22 | Selective temporal lobectomy + hippocampectomy | L | Negative | 2 | 1 |
| 8 | 23 | Selective temporal lobectomy + hippocampal transections | L | Negative | 2 | 1 |
| 9 | 48 | Selective temporal lobectomy | R | Negative | 0 | 4 |
| 10 | 39 | Temporo-occipital lesionectomy | R | Positive | 0 | 4 |
| 11 | 43 | Anterior temporal lobectomy | L | Negative | 1 | 1 |
| 12 | 22 | Temporo-insular lobectomy | L | Negative | 3 | 5 |
| 13 | 23 | Anterior temporal lobectomy | L | Positive | 1 | 3 |
| 14 | 24 | Hippocampal transections | L | Negative | 1 | 1 |
| 15 | 26 | Hippocampectomy | R | Negative | 1 | 5 |
| 16 | 24 | Selective temporal lobectomy + hippocampal transections | R | Negative | 1 | 1 |
| 17 | 40 | Selective temporal lobectomy + hippocampal transections | L | Negative | 2 | 1 |
| 18 | 26 | Selective temporal lobectomy + hippocampal transections | L | Negative | 0 | 1 |
| 19 | 44 | Selective temporal lobectomy + hippocampal transections | R | Positive | 2 | 4 |
| 20 | 34 | Anterior temporal lobectomy | R | Negative | 1 | 1 |
| 21 | 58 | Anterior temporal lobectomy | L | Negative | 2 | 1 |
Selective temporal lobectomy= resection of amygdala, temporal pole and/or hippocampus; anterior temporal lobectomy= resection of temporal neocortex and mesial temporal structures, R: right, L: left, RMSSD: root mean square difference of successive RR intervals.
Statistically significant results (p<0.05)
Insular radiological involvement was classified as type 0 (4 patients [19%]), type 1 (9 [43%]), type 2 (7 [33%]) and type 3 (1 [5%]) [Table 1].
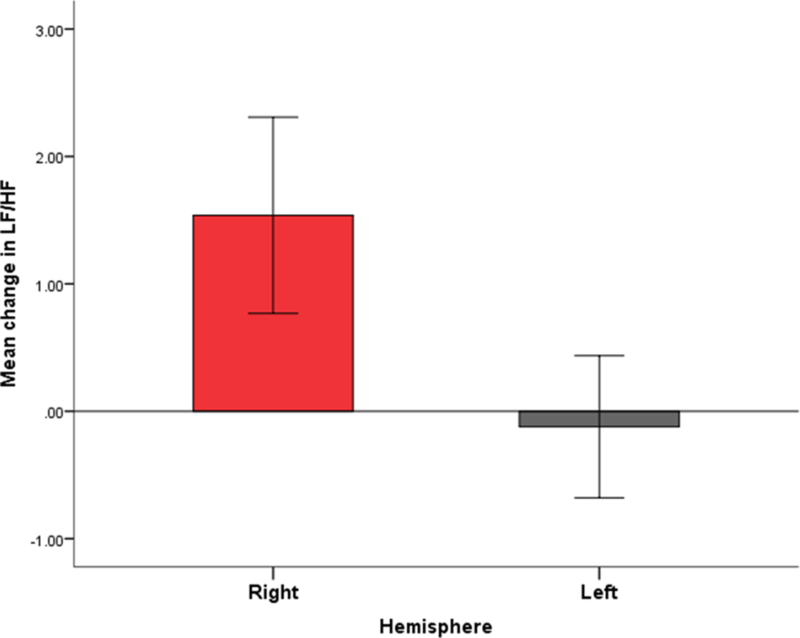
We analyzed autonomic function changes (pre and post-surgery) comparing extent of radiological insular involvement (type 2–3 vs type 0–1). Significant differences were found on RMSSD (p=0.025), and CV (p=0.008) between patients with no or minimal insular involvement (types 0 and 1) and patient with more extent insular involvement (types 2 and 3), such that the latter had comparatively decreased RMSSD and CV indices (Table 2). No significant differences were found on frequency-domain parameters of HF, LF and LF/HF ratio and extent of insular resection (Table 2). Right-sided resections were significantly correlated with an increase in LF power (p=0.010) and the LF/HF ratio (p=0.017) [Figure 2]. There was no association between epilepsy surgery outcome and pre and post-operative HRV changes (p>0.05).
Table 2.
Autonomic function changes (Pre and Post-surgery) comparing extent of radiological insular involvement.
| Pre vs Post Q (p-value) |
Estimated Mean | Pre vs Post with Severity interaction |
Estimated Mean | |||
|---|---|---|---|---|---|---|
| Pre-Q | Post-Q | Type 0–1 | Type 2–3 | |||
| MNN (ms) | 0.973 | 0.810 | 0.811 | 0.583 | 0.806 | 0.814 |
| SDNN (ms) | 0.649 | 0.056 | 0.054 | 0.086 | 0.056 | 0.055 |
| RMSSD (ms) | 0.654 | 0.044 | 0.044 | 0.025* | 0.044 | 0.042 |
| CV (%) | 0.210 | 0.070 | 0.065 | 0.008* | 0.069 | 0.066 |
| LFP (%) | 0.102 | 5.469 | 6.386 | 0.203 | 6.471 | 5.384 |
| HFP (ms2) | 0.639 | 1.926 | 2.038 | 0.459 | 2.023 | 1.941 |
| LF/HF (ms2) | 0.531 | 5.115 | 5.613 | 0.486 | 5.183 | 5.545 |
MNN (mean of the RR intervals), SDNN (standard deviation of the RR intervals), RMSSD (root mean square difference of successive RR intervals), CV (coefficient of variation), LF (low frequency), HF (high frequency), ms (milliseconds).
Statistically significant.
Figure 2. Autonomic function changes (pre and post-surgery) comparing laterality (right and left hemisphere) of radiological insular involvement.
The y axis represents mean change in LF/HF ratio comparing pre to post-surgery, and the x axis denotes the hemisphere of epilepsy surgery. Right-sided surgery was associated with increased LF/HF ratio (p=0.017).
HF: high frequency, LF: low frequency.
Discussion
We found autonomic changes, characterized by marked differences in HRV patterns, in patients with radiological evidence of insular involvement after epilepsy surgery. We previously reported two such patients, who subsequently succumbed to SUDEP [3]. Two important questions arose from their analysis and led to the current study: first, the incidence and extent of insular involvement in temporal lobe epilepsy surgery, and second, the presence and extent of autonomic dysfunction in insular damage. Analysis of HRV metrics in post-surgical insular involvement suggests significant HRV changes based on the extent of resection and the hemisphere. This may reflect tendency to specific types of autonomic dysfunction [11] in these patients.
HRV noninvasively reflects sympathetic and parasympathetic balance as a measure of cardiac autonomic tone using EKG recordings [11]. It reflects oscillations in the interval between consecutive heartbeats defined as adjacent QRS complexes of a continuous artifact-free EKG and includes measures based on time and frequency domain analysis. A close relationship is known to exist between increased sympathetic activity and/or decreased parasympathetic activity with a consequent tendency toward development of fatal arrhythmia [11]. HRV is known to be significantly reduced in epilepsy, and decreased HRV is associated with increased risk of sudden cardiac death [10]. We found that patients with minimal or no insular involvement had increased HRV, suggesting predominance of vagal tone, whereas those with more marked insular damage had significantly larger decreases in HRV, implying decreased parasympathetic tone or overall increased sympathetic tone. The implications of such changes in patients with intractable generalized convulsive seizures in particular may include tendency to cardiac rhythm changes seen in near-SUDEP and SUDEP phenomena. A progressive, serial increase in interictal and pre-ictal HRV was reported in one case of SUDEP over a seven-month period, followed by a monitored death characterized by shortened QTc interval (335–358 milliseconds) and post-ictal tachycardia followed by bradycardia/asystole, atrial fibrillation, and ventricular fibrillation [12]. Based on these observations, increased parasympathetic tone was posited as a potential causative mechanism. The converse scenario of decreased HRV and autonomic instability is also thought to contribute to SUDEP [13]. RMSSD (root mean square differences of successive R-R intervals), a measure of HRV, is inversely correlated with the SUDEP-7 risk inventory since epilepsy subjects with low RMSSD have higher inventory scores and greater risk [13]. RMSSD has been suggested as a biomarker for SUDEP risk. Such decreased HRV metrics have been associated with ventricular tachyarrhythmias and increased risk of mortality [11–14].
The bidirectional change in HRV observed in cardiac risk is consistent with the variable pattern of cardiac autonomic and respiratory dysfunction observed in the phenomenological heterogeneity of monitored SUDEP cases [5, 15]. The role of autonomic cortical control structures in SUDEP is an intriguing unknown since several such structures (insula, amygdala, hippocampus, orbitofrontal cortex) are either primary epileptogenic zones or part of putative seizure networks. The insula has been directly or indirectly implicated in both cardiac autonomic control and mortality in stroke patients. Prolonged direct electrical stimulation of the rat insular cortex produces lethal cardiac arrhythmia and sudden death [16]. Electrical stimulation of the human insula produced cardiac chronotropic and blood pressure responses in five patients with insular epilepsy [17]. Left-sided insular dominance for parasympathetic cardiovascular effects was noted [17]. Insular damage in patients with cerebral infarction is associated with increased sympathetic activity, cardiac arrhythmias, conduction blocks and risk of mortality including due to sudden death [14, 18].
The low frequency (LF) HRV domain is thought to represent both sympathetic and parasympathetic activity whereas the high frequency (HF) domain predominantly reflects parasympathetic activity. Consistent with autonomic changes and mortality noted in earlier studies of stroke patients, we observed that right sided insular resection is associated with increased LF domain and LF/HF ratio and therefore a significant increase in overall sympathetic tone [11]. Although the number of cases was small in each group and more severe damage cases were predominant in the left-sided group, our results may indicate that even minor insular involvement in the right-sided group was enough to produce autonomic changes. SUDEP mortality may not be exclusive to right sided insular resection; both right and left insular epilepsy have been implicated in SUDEP [6, 7].
Radiological evidence of insular damage as detected by MRI may be a more prominent manifestation of the subtle changes reported by imaging studies of SUDEP and high-risk patients. Prominent cortical thinning may be seen in several structures related to autonomic and breathing control such as the orbitofrontal, anterior cingulate, and insular cortices [8]. Autonomic indices and HRV metrics have yet to be reported in these imaging cohorts. However, in the population with post-surgical insular involvement, findings of HRV change appear to be robust. Whether insular involvement in epilepsy surgery translates to increased mortality is uncertain. Post-operative seizure freedom entails no greater mortality risk than the seizure free population who have not had epilepsy surgery [19]. Failed epilepsy surgery patients understandably continue to be at high risk (10.4/1000 vs 5.2/1000 person years) [20]. However, SUDEP accounts only partly for mortality; a comprehensive epilepsy surgery mortality study of 89 deaths reported only 15 definite or probable SUDEP cases [20]. Much larger cohort studies are required to address whether resection of insular tissue contributes to mortality in general and SUDEP in particular.
Successful temporal lobe surgery undoubtedly mitigates mortality, including risk of SUDEP, but avoidable insular resection may lead to increased risk in those who are not rendered seizure-free by surgery. As a result, minimally invasive techniques such as laser interstitial thermotherapy and radiofrequency thermocoagulation may be advantageous, particularly for mesial temporal epilepsy with a localized epileptogenic zone. For insular epileptogenic zones, the extent of surgical resection should be carefully planned to balance risk of continued seizures against avoidable insular resection, both of which may contribute to increased risk of SUDEP. Confirmation of these findings in a larger study is needed, with special emphasis on mortality outcomes in relation to operative approaches and insular involvement.
The sample size was small and the severity of insular involvement between right and left hemisphere were not equally distributed, with more severe insular damage cases in the left-sided group. In addition, patient records were retrospectively analyzed, with resulting potential attendant biases. Post-surgical imaging studies in the post-operative period were not carried out at a fixed time interval and thus scan acquisition was not standardized. We did not assess the impact of medications on HRV since our study was not sufficiently powered to do so. Patients have not been longitudinally followed up for mortality outcomes and hence the influence of HRV change on actual SUDEP incidence is unknown, but deserving of further study.
Acknowledgments
Samden Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407.
Footnotes
Financial Disclosure Statement:
Nuria Lacuey, Vasant Garg, Barbara Bangert, Johnson D. Hampson and Jonathan Miller report no disclosures.
Bibliography
- [1].Colivicchi F, Bassi A, Santini M, Caltagirone C, Prognostic implications of right-sided insular damage, cardiac autonomic derangement, and arrhythmias after acute ischemic stroke, Stroke, 36 (2005) 1710–1715. [DOI] [PubMed] [Google Scholar]
- [2].Laowattana S, Zeger SL, Lima JA, Goodman SN, Wittstein IS, Oppenheimer SM, Left insular stroke is associated with adverse cardiac outcome, Neurology, 66 (2006) 477–483; discussion 463. [DOI] [PubMed] [Google Scholar]
- [3].Lacuey N, Zonjy B, Theerannaew W, Loparo KA, Tatsuoka C, Sahadevan J, Lhatoo SD, Left-insular damage, autonomic instability, and sudden unexpected death in epilepsy, Epilepsy Behav, 55 (2016) 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ryvlin P, Avoid falling into the depths of the insular trap, Epileptic Disord, 8 Suppl 2 (2006) S37–56. [PubMed] [Google Scholar]
- [5].Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T, Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study, Lancet Neurol, 12 (2013) 966–977. [DOI] [PubMed] [Google Scholar]
- [6].Tayah T, Savard M, Desbiens R, Nguyen DK, Ictal bradycardia and asystole in an adult with a focal left insular lesion, Clin Neurol Neurosurg, 115 (2013) 1885–1887. [DOI] [PubMed] [Google Scholar]
- [7].Seeck M, Zaim S, Chaves-Vischer V, Blanke O, Maeder-Ingvar M, Weissert M, Roulet E, Ictal bradycardia in a young child with focal cortical dysplasia in the right insular cortex, Eur J Paediatr Neurol, 7 (2003) 177–181. [DOI] [PubMed] [Google Scholar]
- [8].Ogren JA, Tripathi R, Macey PM, Kumar R, Stern JM, Eliashiv DS, Allen LA, Diehl B, Engel JJ, Rani S, Lhatoo SD, Harper RM, Regional Cortical Thickness Changes Accompanying Generalized Tonic-Clonic Seizures, Neuroimage: Clinical, 20 (2018) 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allen LA, Harper RM, Kumar R, Guye M, Ogren JA, Lhatoo SD, Lemieux L, Scott CA, Vos SB, Rani S, Diehl B, Dysfunctional Brain Networking among Autonomic Regulatory Structures in Temporal Lobe Epilepsy Patients at High Risk of Sudden Unexpected Death in Epilepsy, Front Neurol, 8 (2017) 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stein PK, Fauchier L, Babuty D, Sudden death, arrhythmic events and measurements of heart rate variability, J Am Coll Cardiol, 34 (1999) 2148–2149. [DOI] [PubMed] [Google Scholar]
- [11].Heart rate variability. Standards of measurement, physiological interpretation, and clinical use., Eur Heart J, 17 (Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology; 1996) 354–381. [PubMed] [Google Scholar]
- [12].Jeppesen J, Fuglsang-Frederiksen A, Brugada R, Pedersen B, Rubboli G, Johansen P, Beniczky S, Heart rate variability analysis indicates preictal parasympathetic overdrive preceding seizure-induced cardiac dysrhythmias leading to sudden unexpected death in a patient with epilepsy, Epilepsia, 55 (2014) e67–71. [DOI] [PubMed] [Google Scholar]
- [13].DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, Gornbein J, Harper RM, RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory, Epilepsy Behav, 19 (2010) 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Abboud H, Berroir S, Labreuche J, Orjuela K, Amarenco P, Insular involvement in brain infarction increases risk for cardiac arrhythmia and death, Ann Neurol, 59 (2006) 691–699. [DOI] [PubMed] [Google Scholar]
- [15].Lhatoo SD, Nei M, Raghavan M, Sperling M, Zonjy B, Lacuey N, Devinsky O, Nonseizure SUDEP: Sudden unexpected death in epilepsy without preceding epileptic seizures, Epilepsia, 57 (2016) 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Oppenheimer SM, Cechetto DF, Hachinski VC, Cerebrogenic cardiac arrhythmias. Cerebral electrocardiographic influences and their role in sudden death, Arch Neurol, 47 (1990) 513–519. [DOI] [PubMed] [Google Scholar]
- [17].Oppenheimer S, Hachinski V, Complications of acute stroke, Lancet, 339 (1992) 721–724. [DOI] [PubMed] [Google Scholar]
- [18].Christensen H, Boysen G, Christensen AF, Johannesen HH, Insular lesions ECG abnormalities, and outcome in acute stroke, J Neurol Neurosurg Psychiatry, 76 (2005) 269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sperling MR, Feldman H, Kinman J, Liporace JD, O’Connor MJ, Seizure control and mortality in epilepsy, Ann Neurol, 46 (1999) 45–50. [DOI] [PubMed] [Google Scholar]
- [20].Sperling MR, Barshow S, Nei M, Asadi-Pooya AA, A reappraisal of mortality after epilepsy surgery, Neurology, 86 (2016) 1938–1944. [DOI] [PubMed] [Google Scholar]