Abstract
Radiation effects on colorectal cancer rates, adjusted for smoking, alcohol intake and frequency of meat consumption and body mass index (BMI) by anatomical subsite (proximal colon, distal colon and rectum) were examined in a cohort of 105,444 atomic bomb survivors. Poisson regression methods were used to describe radiation‐associated excess relative risks (ERR) and excess absolute rates (EAR) for the 1958–2009 period. There were 2,960 first primary colorectal cancers including 894 proximal, 871 distal and 1,046 rectal cancers. Smoking, alcohol intake and BMI were associated with subsite‐specific cancer background rates. Significant linear dose–responses were found for total colon (sex‐averaged ERR/Gy for 70 years old exposed at age 30 = 0.63, 95% confidence interval [CI]: 0.34; 0.98), proximal [ERR = 0.80, 95% CI: 0.32; 1.44] and distal colon cancers [ERR = 0.50, 95% CI: 0.04; 0.97], but not for rectal cancer [ERR = 0.023, 95% CI: −0.081; 0.13]. The ERRs for proximal and distal colon cancers were not significantly different (p = 0.41). The ERR decreased with attained age for total colon, but not for proximal colon cancer, and with calendar year for distal colon cancer. The ERRs and EARs did not vary by age at exposure, except for decreasing trend in EAR for proximal colon cancer. In conclusion, ionizing radiation is associated with increased risk of proximal and distal colon cancers. The ERR for proximal cancer persists over time, but that for distal colon cancer decreases. There continues to be no indication of radiation effects on rectal cancer incidence in this population.
Keywords: ionizing radiation, colorectal cancer, radiation risk, proximal colon, distal colon
Short abstract
What's new?
Increasing radiation dose is associated with elevated colon cancer incidence among atomic bomb survivors. Questions remain, however, about differences in radiation‐related increases in risk by anatomical subsite, particularly the proximal and distal colon and the rectum. In this study, analyses of radiation and colorectal cancer risk for Japanese atomic bomb survivors in the Life Span Study cohort show that ionizing radiation is associated specifically with elevated risk of proximal and distal colon cancers. Adjustment for body mass index and lifestyle factors had little effect on radiation risk estimates. No association was found between radiation exposure and rectal cancer.
Abbreviations
- AHS
Adult Health Study
- AIC
Akaike's information criteria
- BMI
body mass index
- DS02R1
Dosimetry System 2002 Revision 1
- EAR
excess absolute rate
- ERR
excess relative risk
- ICD‐O‐3
International Classification of Disease for Oncology Third Edition
- LSS
Life Span Study
- NIC
not in either city
- PYs
person‐years
Introduction
Strong evidence has been found for a relationship between ionizing radiation exposure and colon cancer risk, although evidence for rectal cancer has been inconsistent.1, 2 Among atomic bomb survivors, the colon cancer rates increased with radiation dose in studies of incidence and mortality, while there was no evidence of radiation effects on rectal cancer rates.3, 4, 5, 6 Nakatsuka et al. reported that all colon subsites (cecum and ascending, transverse and descending and sigmoid colon) were equally sensitive to radiation among atomic bomb survivors followed through 1980, whereas rectum was not.3
Lifestyle factors such as smoking, alcohol drinking, meat consumption and body mass index (BMI) have also been linked with increased risk of colorectal cancer in Western and several Japanese cohort studies.7, 8, 9, 10, 11 Recently, evidence has emerged that time trends of incidence,12 associations with lifestyle and environmental risk factors, occurrence of heredity disease, carcinogenic pathways13, 14 and prognosis15 of colon cancer differ by anatomical subsite (i.e., proximal vs. distal location). Genomic studies also support different pathogenesis of sporadic proximal and distal colon cancers with proximal colon cancer likely being related to microsatellite instability (MSI) and distal colon cancer to chromosomal instability (CIN).14 After 1992 when the colorectal cancer screening of fecal occult blood test allowing for removal of colon polyps started in Japan, the increasing trend of proximal colon cancer incidence attenuated, the incidence trend of distal colon leveled off, and trend of rectal cancer started decreasing.12 Several epidemiological studies found a shift with calendar time in the topographical distribution of colon cancer incidence proximally and to the right.12, 16, 17
Earlier studies of the atomic bomb survivors noted that BMI and radiation exposure have effects on the incidence of colon cancer.18 However, other lifestyle factors have not been considered to adjust for background rate or effect modification of radiation risk of colorectal cancer incidence in previous analyses in the survivors. The subsite‐specific analyses of colorectal cancer risk in atomic bomb survivors have not been updated and there remain questions concerning the difference in radiation risks for proximal colon, distal colon and rectum after controlling for lifestyle factors.
The aim of our study was to evaluate radiation risk of colorectal cancer in the Life Span Study (LSS) cohort of atomic bomb survivors by adding 11 years of follow up, adjusting for smoking, alcohol intake, frequency of meat consumption and BMI, then to compare radiation risks by anatomical subsite of colorectal cancer.
Subjects and Methods
Subjects
The LSS cohort comprises 120,321 Japanese atomic bomb survivors who were identified from the 1950 National Census. The LSS includes 26,580 persons (22% of the cohort) who were residents in Hiroshima or Nagasaki in 1950, 1951, or 1952, but who were not in either city (NIC) at the time of the bombings. Detailed sampling methods of LSS were described elsewhere.19 Vital status was ascertained by the Japanese family registry system (koseki) which is virtually complete, while cancer incidence was ascertained mainly through Hiroshima or Nagasaki cancer registries, depending on where the LSS subjects were exposed to atomic bombing or where NIC subjects resided when they were selected. The incident cancer analyses are based on 105,444 cohort members including 80,205 survivors with estimated radiation doses (see below) and 25,239 NIC subjects, who were alive and not known to have had cancer as of January 1, 1958—the year in which the cancer registries in Hiroshima and Nagasaki became operational.
Radiation doses
Dosimetry System 2002 Revision 1 (DS02R1) was used to estimate individual organ doses received by those exposed to radiation from the bombings.20 Estimated doses were adjusted to account for implausibly large estimates (shielded kerma more than 4 Gy) and random errors in dose assignments. Weighted absorbed organ dose was calculated as the sum of gamma dose plus 10 times the neutron dose and used for cancer risk analysis at the organ. Because rectum dose was not estimated, weighted absorbed bladder dose was used as its surrogate.
Case ascertainment
The main study outcome was the first primary colorectal cancer diagnosed from 1958 to 2009. To be consistent with other incident cancer definitions, we did not include mucosal cancers. Cases diagnosed only at autopsy were treated as noncases in order to avoid a potential bias related to high autopsy rates among people exposed to high doses of radiation in the early years of follow‐up.19 Colorectal cancers were further divided into three anatomic subsites: proximal colon, distal colon and rectum. Proximal colon included the cecum (International Classification of Disease for Oncology, Third Edition [ICD‐O‐3] topography code: C18.0), appendix (C18.1), ascending colon (C18.2), hepatic flexure (C18.3), transverse colon (C18.4) and splenic flexure (C18.5). Distal colon included the descending colon (C18.6) and sigmoid colon (C18.7). Rectum included the rectosigmoid junction (C19.9), rectum not otherwise specified (C20.9) and anus, anal canal and anorectum (C21.0‐C21.8). Cancers of overlapping colon subsites (C18.8) and colon cancers, not otherwise specified (C18.9) were included in the total colon cancer group but not in the subsite‐specific groups.
Lifestyle data
Information on lifestyle and anthropometric factors were obtained from four LSS mailed questionnaire surveys and three Adult Health Study (AHS) clinic‐based questionnaires administered between 1963 and 1991.21, 22, 23, 24, 25 In the current analyses, we evaluated information on smoking history, alcohol intake, meat consumption and BMI. The availability of LSS survey data varied from 47% for meat consumption to 67% for alcohol intake. See Supporting Information Materials for more details.
We summarized smoking history in terms of total pack‐years smoked calculated from the reported intensity and duration of smoking, as described in Furukawa et al.26 Alcohol intake was characterized as the number of alcohol‐containing drinks consumed per day (assuming 10 g ethanol per drink) based on the largest amount reported on any questionnaire. BMI was calculated as weight divided by squared height and expressed in kg/m2 using data from the earliest completed questionnaire. BMI was evaluated as a categorical variable with the cut‐offs corresponding to underweight (<18.5), normal weight (18.5–24.9) and overweight or obese (≥25.0) according to the WHO definition.27 The frequency of meat consumption was summarized as the number of days consuming a meat‐containing meal per week based on the highest value reported on any questionnaire. In the analyses, the frequency of meat consumption was evaluated using three categories: ≤1 day/week, 2–4 days/week and 5–7 days/week. Distributions of lifestyle factors and BMI among individuals with known information are presented in Supporting Information Tables S1–S3.
To avoid a bias arising from “immortal person time”,28 lifestyle information (e.g., smoking history) for all LSS subjects was considered unknown until they reported it for the first time and known thereafter. Individuals who did not respond to any questionnaire retained unknown status throughout the entire follow‐up. Because most Japanese females as same generation of LSS subjects are thought not to smoke or consume alcohol, while most males do, we allowed the effect of unknown smoking history and alcohol consumption to vary by sex.
Statistical analysis
The analytical data file consisted of grouped person‐years (PYs) of observation and counts of colorectal cases. The stratification variables and their respective categories are described in the Supporting Information Materials. PYs were computed from January 1, 1958, until the earliest of the date of diagnosis of any cancer, date of death, date of 110th birthday or December 31, 2009. Since only cancers that were diagnosed inside the Hiroshima or Nagasaki cancer registry catchment areas were included in the analysis, PYs were adjusted for migration in and out of the study area based on the updated AHS migration rates. See more details elsewhere.19
Radiation effects were described using excess relative risks (ERR) and excess absolute rates (EAR) estimated from the person‐years table using the Poisson regression. The general background incidence rate, that is, the rate in the absence of radiation exposure from the atomic bombs (radiation dose = 0 Gy), was modeled parametrically as explained below.
The ERR model had the form:
λ0(c, s, cy, a, n, sm, smuk, al, aluk, b, m) × [1 + ρ(d)ε(s, a, e, cy)].
The EAR model had the form:
λ0(c, s, cy, a, n, sm, smuk, al, aluk, b, m) + ρ(d)ε(s, a, e, cy).
In the models, λ0(.) represents the background rate of colorectal cancer as a logarithmic function of conventional determinant variables, including city (c), sex (s), calendar year (cy), attained age (a) and an indicator of whether the subjects were not in the city at the time of bombings (n). In addition, we modeled the background rates as a function of smoking intensity described in pack‐years (sm) and alcohol drinks consumed per day (al) allowing for unknown status by sex, BMI (b) and frequency of meat consumption (m). Smoking intensity was treated as time‐dependent variable, while alcohol intake, frequency of meat consumption and BMI were treated as time‐independent variables.
The functions ρ(.) and ε(.) in ERR and EAR models describe the dose–response function (see below) and effect modification, respectively; ε(.) is modeled as a log‐linear function. Potential effect modifiers included sex (s), attained age (a), age at exposure (e) and calendar year (cy). Throughout the article we present the ERR and EAR averaged over sex for a 70‐year‐old individual exposed at age 30 unless otherwise specified. The parametric form of ERR model is shown in the supplement.
We tested two forms of risk function.
ρ(d) = β1d Linear
ρ(d) = β1d + β2d2 Linear‐quadratic
The best‐fitting dose–response model was selected using the Akaike's information criteria (AIC) from linear and linear‐quadratic models while simultaneously modeling the background and effect modifications.
The difference in ERRs for proximal and distal colon cancer was tested using a joint endpoint analysis analogous to the analysis of competing risks.29 This approach allowed us to fit separate background parameters for each endpoint (as in subsite‐specific analyses) while simultaneously testing for a difference in ERRs or effect modifiers across the endpoints.
Parameter estimates, likelihood‐ratio tests (LRT) and 95% confidence intervals (95% CI) based on profile likelihood or Wald‐type were computed using Poisson regression models as implemented in the AMFIT program of the Epicure version 2.002 and the gnm package of R version 3.5.2. All statistical tests were two‐sided with type‐I error of 0.05.
Ethical considerations
Our study was approved by the Institutional Review Board of the Radiation Effects Research Foundation via approval of RPs 1–75 (Research Protocol for Life Span Study of A‐bomb survivors, Hiroshima and Nagasaki) and 18–61 (Tumor registry study in Hiroshima and Nagasaki). The Hiroshima Prefecture, Hiroshima City and Nagasaki Prefectures approved the linkages between LSS cohort members and data from the Cancer Registries.
Results
Colorectal cancer cases and crude incidence rates
We ascertained 2,960 cases of colorectal cancer diagnosed between 1958 and 2009, including 1,914 colon and 1,046 rectal cancers. This represented a 26% increase since 1998, the end of the last reporting period,5 and made colorectal cancer the second most common cancer in the LSS. About 88% of colon cancer cases were histologically confirmed, while 6% had diagnosis based on death certificate only. The corresponding numbers for rectal cancer cases were 91 and 5%, respectively. Overall, adenocarcinoma was the most frequent histological type of colorectal cancer (88%). By anatomical location within the colon, there were 894 proximal (47%), 871 distal (45%) colon cancers and 149 (8%) colon cancers with overlapping locations or not otherwise specified location.
Characteristics of colorectal cancer cases in the LSS are shown in Table 1. Crude incidence rates (per 10,000 PYs) were 6.2 for total colon (2.9 for proximal, 2.8 for distal colon) and 3.4 for rectal cancer. The crude incidence rates for all subsites were comparable in Hiroshima and Nagasaki survivors. Males had higher rates of distal colon and rectal cancers than females, while the rates of proximal colon cancer in males and females were comparable. For rectal cancer, the rates increased with increasing age at exposure/older birth cohorts (these are perfectly correlated with the LSS). The rates increased with attained age and dose for colon cancer (total, proximal and distal). Rates did not increase with dose for rectal cancer.
Table 1.
Characteristics of subjects and colorectal cancer cases among LSS subjects, 1958–2009
| Subjects | Person‐years | Cancer cases and crude rate | Subjects | Person‐years | Cancer cases and crude rate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total colon | Proximal | Distal | Rectum | |||||||||
| n | n | Rate | n | Rate | n | Rate | n | n | Rate | |||
| City | ||||||||||||
| Hiroshima | 73,401 | 2,193,360 | 1,346 | 6.1 | 631 | 2.9 | 610 | 2.8 | 731 | 3.3 | ||
| Nagasaki | 32,043 | 886,209 | 568 | 6.4 | 263 | 3.0 | 261 | 2.9 | 315 | 3.6 | ||
| Sex | ||||||||||||
| Men | 42,910 | 1,142,190 | 782 | 6.8 | 305 | 2.7 | 419 | 3.7 | 518 | 4.5 | ||
| Women | 62,534 | 1,937,390 | 1,132 | 5.8 | 589 | 3.0 | 452 | 2.3 | 528 | 2.7 | ||
| Age at exposure (year)1 | ||||||||||||
| 0–19 | 45,787 | 1,629,030 | 810 | 5.0 | 370 | 2.3 | 413 | 2.5 | 444 | 2.7 | ||
| 20–39 | 30,089 | 988,542 | 802 | 8.1 | 408 | 4.1 | 325 | 3.3 | 374 | 3.8 | ||
| 40– | 29,568 | 461,993 | 302 | 6.5 | 116 | 2.5 | 133 | 2.9 | 228 | 4.9 | ||
| Attained age (year) | ||||||||||||
| <40 | 56,657 | 646,198 | 15 | 0.2 | 9 | 0.1 | 5 | 0.1 | 13 | 0.2 | ||
| 40– | 15,260 | 486,300 | 43 | 0.9 | 18 | 0.4 | 22 | 0.5 | 41 | 0.8 | ||
| 50– | 16,637 | 614,645 | 209 | 3.4 | 87 | 1.4 | 115 | 1.9 | 145 | 2.4 | ||
| 60– | 11,258 | 651,188 | 559 | 8.6 | 254 | 3.9 | 280 | 4.3 | 341 | 5.2 | ||
| 70– | 4,649 | 457,182 | 621 | 13.6 | 282 | 6.2 | 300 | 6.6 | 340 | 7.4 | ||
| 80– | 983 | 224,056 | 467 | 20.8 | 244 | 10.9 | 149 | 6.7 | 166 | 7.4 | ||
| DS02R1 colon dose (Gy) | DS02R1 bladder dose (Gy) | |||||||||||
| NIC2 | 25,239 | 761,539 | 475 | 6.2 | 214 | 2.8 | 227 | 3.0 | 25,239 | 761,539 | 239 | 3.1 |
| <0.005 | 35,978 | 1,032,560 | 615 | 6.0 | 284 | 2.8 | 281 | 2.7 | 35,935 | 1,031,360 | 356 | 3.5 |
| −0.1 | 27,511 | 807,891 | 467 | 5.8 | 213 | 2.6 | 215 | 2.7 | 27,543 | 808,629 | 285 | 3.5 |
| −0.2 | 5,594 | 164,117 | 112 | 6.8 | 61 | 3.7 | 43 | 2.6 | 5,595 | 163,959 | 49 | 3.0 |
| −0.5 | 5,926 | 169,182 | 115 | 6.8 | 54 | 3.2 | 51 | 3.0 | 5,850 | 166,935 | 58 | 3.4 |
| −1 | 3,136 | 88,997 | 71 | 8.0 | 43 | 4.8 | 25 | 2.8 | 3,184 | 90,623 | 38 | 4.3 |
| −2 | 1,565 | 42,240 | 41 | 9.7 | 19 | 4.5 | 19 | 4.5 | 1,604 | 43,527 | 17 | 4.0 |
| 2+ | 495 | 12,956 | 18 | 13.9 | 6 | 4.6 | 10 | 7.7 | 494 | 12,802 | 4 | 3.1 |
| Total | 105,444 | 3,079,570 | 1,914 | 6.2 | 894 | 2.9 | 871 | 2.8 | 105,444 | 3,079,380 | 1,046 | 3.4 |
Age at exposure is equivalent to the duration that time of bombing (1945) minus year of birth, so that age at exposure are perfectly correlated with year of birth.
NIC subjects were residents when they were selected from 1950 National Census.
Background rates
As in previous analyses, we described the background incidence rates in terms of sex, attained age, city and location at the time of bombings (NIC vs. In‐City). Based on preliminary analyses, we found that calendar year described the temporal pattern of background rates better than birth year (AIC difference = 52.5). Therefore, unlike before, we modeled the background rates in terms of calendar year rather than birth year (Supporting Information Table S4).
The overall background rates of total colon cancer increased since 1958, peaked around 1990 and decreased. The background rates of distal colon and rectal cancers followed a similar pattern, while the rates of proximal colon cancer stabilized after 1990. For the same calendar time period, the background rates of proximal colon cancer were comparable in males and females, increasing roughly in proportion to the fourth–fifth power of attained age. The rates of distal colon and rectal cancer were higher in males than females and increased in proportion to the third power of age. The fitted background rates by age and sex for five calendar periods are presented in Figure 1 for total, proximal and distal colon cancer and rectal cancers.
Figure 1.
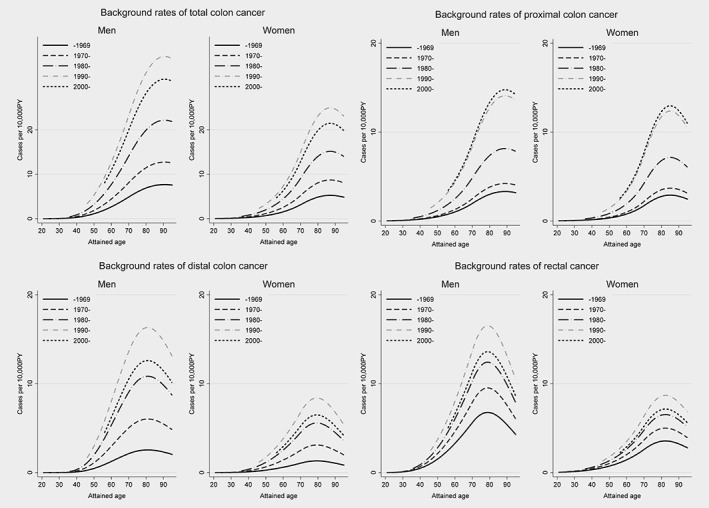
Background rates for total, proximal and distal colon cancers and rectal cancer.
We also analyzed the background rates in terms of selected lifestyle factors and BMI. Smoking intensity, alcohol consumption (amount) and BMI were associated with a modest but significant increase in risk of total colon cancer (Table 2). These associations were largely attributed to the association of smoking intensity and alcohol consumption with distal colon cancer and to the association of BMI with proximal colon cancer. Only alcohol consumption was significantly associated with increased risk of rectal cancer. The frequency of meat consumption was not associated with risk of colorectal cancer for any subsites.
Table 2.
Association of potential risk factors with background rate of colorectal cancer by anatomical site
| Colon | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total colon | Proximal | Distal | Rectum | |||||
| Lifestyle and BMI | Relative risk | (95% CI) | Relative risk | (95% CI) | Relative risk | (95% CI) | Relative risk | (95% CI) |
| Smoking | ||||||||
| Pack‐years at age 70 | 1.28 | (1.10; 1.48) | 1.11 | (0.89; 1.39) | 1.33 | (1.08; 1.64) | 1.04 | (0.85; 1.29) |
| Men, unknown smoking status | 0.70 | (0.53; 0.93) | 0.45 | (0.29; 0.69) | 1.09 | (0.74; 1.60) | 1.02 | (0.73; 1.42) |
| Alcohol drinking | ||||||||
| Amount, drink/day | 1.06 | (1.02; 1.10) | 1.04 | (0.98; 1.10) | 1.08 | (1.03; 1.14) | 1.08 | (1.03; 1.14) |
| Men, unknown drinking status | 1.17 | (0.90; 1.54) | 0.99 | (0.65; 1.50) | 1.27 | (0.87; 1.86) | 0.82 | (0.59; 1.12) |
| Women, unknown drinking status | 0.67 | (0.55; 0.81) | 0.64 | (0.48; 0.84) | 0.79 | (0.59; 1.06) | 0.73 | (0.56; 0.94) |
| BMI | ||||||||
| 0–18.5 | 0.83 | (0.69; 0.99) | 0.73 | (0.55; 0.96) | 0.97 | (0.75; 1.25) | 1.00 | (0.79; 1.26) |
| 18.5–25 | Reference | Reference | Reference | Reference | ||||
| 25+ | 1.21 | (1.05; 1.39) | 1.28 | (1.05; 1.56) | 1.20 | (0.97; 1.48) | 1.08 | (0.88; 1.3) |
| Unknown | 1.51 | (1.28; 1.79) | 1.58 | (1.23; 2.01) | 1.46 | (1.14; 1.88) | 1.38 | (1.11; 1.71) |
| Meat | ||||||||
| None or less than 1 day/week | 1.04 | (0.90; 1.19) | 0.98 | (0.80; 1.20) | 1.08 | (0.87; 1.34) | 0.97 | (0.79; 1.20) |
| 2–4 days/week | Reference | Reference | Reference | Reference | ||||
| Almost every day | 0.98 | (0.83; 1.16) | 0.93 | (0.73; 1.17) | 1.06 | (0.83; 1.35) | 1.05 | (0.83; 1.32) |
| Unknown | 1.14 | (0.98; 1.31) | 1.09 | (0.88; 1.34) | 1.05 | (0.84; 1.32) | 1.38 | (1.13; 1.68) |
Total colon cancer includes proximal, distal and other colon cancers. BMI was calculated as weight (kg)/height (m2).
Excess relative risk
We found a significant dose–response for total colon (excluding rectum) cancer based on a linear ERR model with the basic background adjustment (i.e., sex, attained age, calendar year, city and NIC) and effect modification by sex, age at exposure, attained age or calendar year (Table 3). Adjustment for lifestyle factors had little impact on radiation risk estimates or female: male (F:M) ratio of ERRs, although, AIC indicated that a richer background model adjusted for lifestyle factors and BMI described data substantially better (Table 3). Based on the richer model, the sex‐averaged ERR per Gy at age 70 after exposure at age 30 was 0.63 (95% CI: 0.34; 0.98) for total colon cancer and 0.025 (95% CI: −0.087; 0.14) for rectal cancer. The point estimate for the sex‐averaged ERR per Gy for proximal colon cancer (0.80; 95% CI: 0.32; 1.44) was higher than that for distal colon cancer (0.50; 95% CI: 0.04; 0.97), but the difference of ERRs between proximal and distal colon cancer was not statistically significant (0.30; 95%CI: −0.67; 1.10, p = 0.41).
Table 3.
ERRs of radiation and effect modifications based on the linear model with background model adjusted by conventional variables and with background model adjusted by conventional variables, smoking, alcohol intake, frequency of meat consumption and BMI
| Cancer sites | ERR/Gy (95%CI) | Effect modifiers (95%CI) | Calendar year1 | AIC difference2 | ||||
|---|---|---|---|---|---|---|---|---|
| Men | Women | Sex‐averaged | F:M ratio | Age at exposure3 | Attained age (power) | |||
| With background model adjusted by conventional variables4 | ||||||||
| Total colon5 | 0.66 (0.29; 1.16)w | 0.47 (0.18; 0.86)w | 0.57 (0.29; 0.89) | 0.71 (0.26; 1.69)w | 19% (−21%; 78%) | −3.53 (−6.13; −0.96) | ||
| Proximal colon5 | 0.88 (0.10; 1.66)w | 0.55 (0.05; 1.06)w | 0.72 (0.19; 1.24)w | 0.63 (0.15; 1.76)w | −11% (−49%; 39%) | −1.97 (−5.24; 2.95) | ||
| Distal colon6 | 0.69 (0.04; 1.34) | 0.27 (−0.18; 0.72) | 0.48 (0.03; 0.88) | 0.40 (0.08; 2.02) | −29% (−61.8%; 22.4%) | −64% (−82.8%; −33.0%) | ||
| Rectum5 | 0.0077 (−0.036; 0.051)w | 0.026 (−0.103; 0.155)w | 0.017 (−0.068; 0.101)w | 3.36 (0.25; 45.35)w | −84.2% (−98%; 15.0%)w | 4.4 (−5.5; 14.3)w | ||
| With background model adjusted by conventional variables4, smoking, alcohol intake, frequency of meat consumption and BMI | ||||||||
| Total colon5 | 0.77 (0.36; 1.30) | 0.50 (0.20; 0.90) | 0.63 (0.34; 0.98) | 0.65 (0.24; 1.48) | 24% (−16%; 82%) | −3.63 (−6.17; −1.14) | −52.4 | |
| Proximal colon5 | 0.97 (0.15; 1.80) | 0.62 (0.09; 1.16) | 0.80 (0.32; 1.44) | 0.64 (0.17; 1.77) | −6.0% (−44%; 47%) | −2.10 (−5.27; 2.54) | −26.9 | |
| Distal colon6 | 0.73 (0.06; 1.41)w | 0.27 (−0.17; 0.71)w | 0.50 (0.04; 0.97)w | 0.37 (0.08; 1.78)w | −32% (−62%; 14%) | −65% (−83%; −35%) | −11.5 | |
| Rectum5 | 0.011 (−0.045; 0.068)w | 0.041 (−0.14; 0.22)w | 0.025 (−0.087; 0.14)w | 3.63 (0.28; 47.71)w | −81% (−98%; 57%)w | 4.1 (−6.76; 14.99)w | −22.4 | |
Total colon cancer includes proximal, distal and other colon cancer. DS02R1 weighted absorbed colon and bladder dose were used to estimate colon and rectum cancer ERRs, respectively. Confidence intervals (CI) were likelihood bound or Wald‐type (w).
Coefficient of calendar year means the percentage change per decade increase in calendar year (common to males and females).
AIC difference means the difference from the Akaike information criteria of models with background model adjusted by conventional variables for each subsite.
Coefficient of age at exposure means the percentage change per decade increase in age at exposure (common to males and females).
Conventional variables used for adjustment by background model were age, sex, calendar year and indicator of NIC.
ERR/Gy shows at age 70 years old after exposure at age 30 years old.
ERR/Gy shows in 1985 after exposure at age 30 years old.
The fitted linear dose–response for total colon cancer is presented in Figure 2. The quadratic term was not statistically significant over the full dose range or under 2 Gy (p > 0.5 and p = 0.41, respectively). Similar nonsignificant dose–responses for the quadratic term were observed for all colon‐related subsites and dose ranges in both sex‐averaged and sex‐specific tests.
Figure 2.
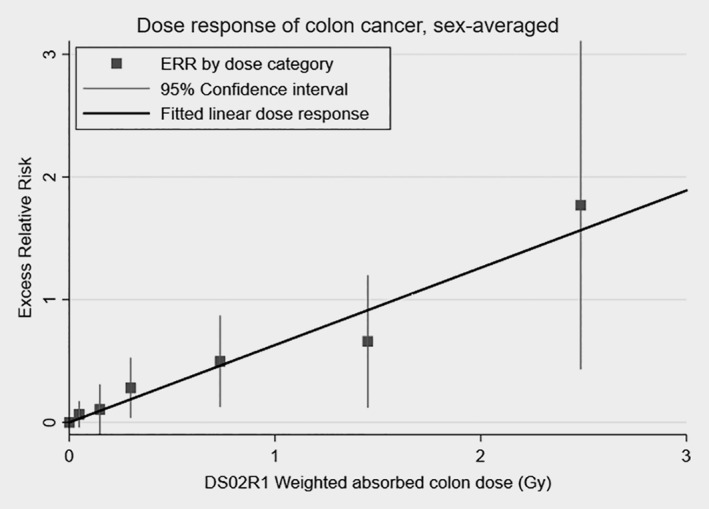
Dose–response function for total colon cancer among the LSS subjects. Solid line shows the fitted linear sex‐averaged ERR dose–response. Points show ERR estimates with 95% confidence intervals by dose category. The ERRs are given for subjects at attained age of 70 years after exposure at age 30 years.
We found a significant modification of the ERR at 1 Gy for total colon cancer by attained age, but not by age at exposure (Table 3). The ERR decreased per each year increase in attained age proportional to the −3.63 power of attained age. The ERR for distal colon cancer also decreased with attained age (−5.78, 95% CI: −9.58; −2.23) and increased with age at exposure (86%, 95% CI: 8.0%; 229%), significantly (not shown in Table 3). However, since a model allowing for modification of the ERR by calendar year fit the data marginally better (AIC difference 1.4) than a model with simultaneous modification of the ERR by attained age and age at exposure, we chose the model with calendar year effect modification as the preferred model. Based on this model, the ERR for distal colon decreased by 65% (95% CI: 83%; 35%) per decade of calendar year increase becoming not significant after 1995 (ERR in 1995 after exposure at age 30 = 0.175, 95%CI: <−0.004; 0.52). The effects of attained age and age at exposure on the ERR for proximal and rectal cancers were not significant. Sex did not modify the ERR for any anatomical subsite. Adjustment for lifestyle factors in the background had no appreciable effect on magnitude or significance of tested effect modifiers. We also evaluated effect modification of ERR by lifestyle factors and BMI, but the results were null (data not shown).
Excess absolute rate
The EARs for colon cancer overall and by anatomical subsite were estimated based on the linear EAR model with the richer background and effect modification by sex, age at exposure and attained age (Table 4). As the ERR per Gy for rectal cancer was near zero, we did not estimate the respective EAR or fraction of cases attributable to radiation exposure (see below). While the EAR was higher in males than females for each colon subsite, this difference was not significant. For each subsite, the EAR increased with attained age in proportion to inverse attained age to the third–fourth power. All EARs tended to decrease with age at exposure, but the effect was significant only for proximal colon cancer. For this subsite, the EAR decreased by 32% (95% CI: 52%; 2.5%) per decade increase in age at exposure. For an individual aged 70 who was exposed at age 30, the sex‐averaged EAR was 6.01 per 10,000 PY‐Gy (95% CI: 3.29; 8.72) for total colon cancer, 3.29 (95% CI: 1.39; 5.18) for proximal colon cancer and 2.26 (95% CI: 0.42; 4.11) for distal colon cancer. In contrast to the ERR, the EAR for distal colon cancer did not vary by calendar year (57%, 95% CI: −8.5%; 141%).
Table 4.
Excess absolute rates for colon cancer and effect modifiers by subsite among the LSS subjects
| Cancer sites | EAR per 10,000 person‐years per Gy | Effect modifiers (95% CI) | ||||
|---|---|---|---|---|---|---|
| Men | Women | Sex‐averaged | F:M ratio | Age at exposure | Attained age (power) | |
| Colon | 7.88 (3.45; 12.3) | 4.13 (1.62; 6.64) | 6.01 (3.29; 8.72) | 0.52 (0.21; 1.06) | −25% (−43%; 0.7%) | 3.83 (2.17; 5.48) |
| Proximal colon | 4.06 (1.19; 6.94) | 2.51 (0.67; 4.36) | 3.29 (1.39; 5.18) | 0.62 (0.21; 1.44) | −32% (−52%; −2.5%) | 3.77 (1.79; 5.75) |
| Distal colon | 3.68 (0.46; 6.90) | 0.85 (−0.48; 2.18) | 2.26 (0.42; 4.11) | 0.23 (−0.06; 0.79) | −6.3% (−42.3%; 52.2%) | 2.95 (1.79; 5.75) |
Total colon cancer includes proximal, distal and other colon cancer. EAR is the excess cases per 10,000 person‐years per Gy at age 70 years after exposure at age 30 years.
DS02R1 weighted absorbed colon dose were used to estimate EAR with background model with conventional variables (age, sex, calendar year and indicator of NIC), smoking, alcohol intake, frequency of meat consumption and BMI. Confidence intervals (CI) are Wald‐type.
Excess cancer cases
The number of background cases, radiation excess cases and attributable fraction due to radiation exposure were estimated for each category of radiation dose based on the preferred ERR model (Supporting Information Table S5). The number of total colon cancer cases due to radiation exposure was 89 (50 in males and 39 in females) corresponding to an attributable fraction of 10.7% (14.2% in males and 8.2% in females) among cohort members with doses more than 5 mGy. The subsite‐specific number of radiation excess cases (attributable fraction) was 53 (13.3%) for proximal colon cancer and 34 (9.3%) for distal colon cancer.
Discussion
We found that the radiation risk of total colon cancer remains elevated more than six decades after exposure, although the radiation risk of distal colon attenuated with calendar time. Rectal cancer continues to lack an association with radiation exposure. The dose–response for colon cancer is consistent with linearity. The ERRs per Gy are significantly elevated for both proximal and distal colon cancer and do not differ significantly from each other. Adjustment for smoking, frequency of alcohol and meat consumption, and BMI has little or no effect on estimated ERRs.
The overall pattern of background rate trends for colon cancer in the LSS was consistent with that of the general cancer incidence trend in Japan.12 Colorectal cancer screening started in 1992 in Japan and has been thought to have attenuated the increase of incidence rate. The background rates of distal colon and rectal cancers followed a similar pattern of Japanese general cancer incidence, while the rates of proximal colon cancer leveled off after 1990.
Our main results are consistent with previous results from this cohort, which showed a significant radiation‐related risk for colon but not for rectal cancer.3, 5, 6 Sex‐averaged ERRs/Gy for colon cancer in our study and in the latest study were 0.54 (95% CI: 0.30; 0.81) and 0.63 (90% CI: 0.34; 0.98), respectively, while ERR/Gy for rectum cancer were 0.22 (95% CI: −0.081; 0.13) and 0.19 (90% CI: −0.04; 0.47), respectively.5 Our findings of significant radiation risk of colon cancer are also consistent with the radiation risk reported in studies of medically irradiated populations.1, 2 The positive association between BMI and overall colon cancer risk observed in our study is compatible with the results of a systematic review11 and meta‐analysis of prospective studies.30 It is also in agreement with an earlier LSS study by Semmens et al.18 As before,18 radiation and BMI risk estimates changed little after mutual adjustment and radiation risk did not vary according to BMI levels.
For total colon cancer, a linear dose–response model fit better, based on AIC, than a linear quadratic model both in males and females. The linear ERR and EAR estimated from models with effect modification and adjustments for lifestyle factors were not significantly different between males and females, although point estimates were higher in males in each case. The decreasing trend in ERR with increasing attained age, which was suggested in the previous analysis,5 has become statistically significant in the present study. Although patterns for effect modification of ERR and EAR for colon by attained age appeared, no significant variation in ERR or EAR by age at exposure was found for overall colon cancer. In fact, the decrease in EAR with increasing age at exposure was smaller in the current study compared to the previous study (−25% vs. −56%) and no longer significant.5 These changing patterns of effect modification by age at exposure over time underscore the importance of long, possibly life‐long follow‐up to uncover the true patterns of radiation risk.
Although both the ERR and EAR were higher for proximal than for distal colon cancer, neither risk estimates differed significantly between the two anatomical sites. We also found that the ERR for distal colon cancer decreased with calendar year, while the ERR for proximal colon cancer did not vary by age or time. In contrast, the EAR for distal cancer increased with attained age, but not with calendar time, while the EAR for proximal cancer increased with attained age and decreased with age at exposure. Thus, our findings are mixed and do not exclude the possibility of different tumorigenic pathways in proximal and distal colon after radiation exposure.
The proximal and distal colons have different characteristics based on embryologic origin and risk factors. Epidemiological studies of populations unexposed to radiation suggest that associations with lifestyle factors vary by anatomical subsite of colon.9 For example, association with alcohol consumption appears to be stronger for distal than proximal colon cancer,31 whereas the pattern of risk with smoking is opposite.32 Comparable data for populations exposed to radiation are sparse. We found that BMI was associated with proximal colon cancer, whereas smoking and alcohol consumption were associated with distal colon cancer.
Sporadic proximal colon cancer is likely to be related to MSI and distal colon cancer to CIN.14 Hains et al. found a higher level of MSI in mice irradiated by neutrons.33 In our results, radiation risk for proximal colon cancer tended to be higher than distal colon cancer, although the difference was not significant. Our results seemed to be consistent with the above findings. However, mechanisms of radiation carcinogenesis in proximal and distal colon remain poorly understood in human. Molecular studies of radiation‐related cancers are needed.
Although rectal cancer continued to indicate no association with radiation exposure in the LSS, increased risk of rectal cancer was reported among prostate and cervical cancer patients exposed to high‐dose radiation by radiotherapy.34, 35, 36, 37 At low‐dose levels, an increased risk of rectal cancer incidence was observed in the study of the National Registry for Radiation Workers (NRRW‐3) in UK38 and an increased risk of mortality was observed in the INWORKS.39 Conversely, neither study showed increased risk of colon cancer. Reasons for the lack of association between radiation exposure and rectal cancer in the LSS remain unclear.
The strengths of our study include a well‐designed cohort, more than 50 years of follow‐up, nearly complete ascertainment of vital status and cancer cases from high‐quality population‐based cancer registries, and individual dose estimates. In the analysis, we adjusted radiation risk estimates for smoking, alcohol intake, frequency of meat consumption and BMI and estimated risks by anatomical subsite of colon. The study's limitations include incomplete lifestyle data, ranging from 47% to 67% complete, depending on the specific factor. This mainly reflects the proportion of subjects deceased prior to the initiation of surveys in the 1960s. To minimize the potential for associated biases, we allowed background rates in individuals with unknown and known status for lifestyle factors and BMI to differ. In addition to evaluating variations in radiation risk estimates by lifestyle data, for example, smoking intensity and duration, we also tested for variations in risk estimates according to the availability of such data (data not shown), but the risk estimates were quite stable. Therefore, we do not think that radiation risk estimates are seriously biased. Also, we cannot exclude the possibility that some individuals changed their lifestyle because the last mail survey was conducted in 1991. However, about 76% of all PYs were accumulated before 1990 and it usually requires several years for changes in exposure levels to translate into changes in risk.
In conclusion, the Life Span Study data continue to show a radiation effect on colon but not for rectal cancer. The excess radiation risk of proximal colon cancer in the LSS cohort has persisted for more than 60 years after exposure and is likely to throughout the life of the survivors. The radiation risks for both proximal and distal colon cancers are elevated, but are not significantly different from each other, although conventional risk factors and possible carcinogenesis pathways are thought to be different. However, it is notable that the excess radiation risk of distal colon seems to have attenuated with time. Further follow‐up will shed light on the temporal patterns of colon cancer, especially possible divergent patterns for proximal vs. distal cancer. Molecular studies related to carcinogenesis pathways are needed to improve our understanding of radiation risks for different anatomical subsites.
Supporting information
Table S1. Number of subjects by drinking status and mean drink amount (drinks/day) among the LSS subjects
Table S2. LSS questionnaire meat consumption by sex and radiation dose
Table S3. LSS questionnaire earliest self‐reported BMI by sex and radiation dose
Table S4. Association of conventional risk factors with background rate of colorectal cancer by anatomical site.
Table S5. Observed and excess cases by dose category and sex based on the linear model among the LSS subjects.
Acknowledgements
The authors appreciate all LSS members who have contributed to our study. We would like to thank Hiroshima Prefecture, Hiroshima City and Nagasaki Prefecture Cancer Registries, for approval to use the data and also thank all staff of the RERF Tumor and Tissue Registry Office and the Masterfile section for their efforts. The assistance of Ms. Hiroko Moriwaki, Ms. Sachiyo Funamoto is gratefully acknowledged. The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan is a public interest incorporated foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE). The research was also funded in part through DOE award DE‐HS0000031 to the National Academy of Sciences and contract HHSN261201400009C through the U.S. National Cancer Institute (NCI), with additional support from the Division of Cancer Epidemiology and Genetics in the NCI Intramural Research Program. This publication was supported by RERF Research Protocol 1–75 and 18–61. The views of the authors do not necessarily reflect those of the two governments.
References
- 1. Silver K, Greene T, Latowsky G. Cancer and workers exposed to ionizing radiation, a review of the research literature, vol. 34 Boston, MA: Center for Environmental Health Studies, 2003. 37. [Google Scholar]
- 2. Kamran SC, Berrington De Gonzalez A, Ng A, et al. Therapeutic radiation and the potential risk of second malignancies. Cancer 2016;122:1809–21. [DOI] [PubMed] [Google Scholar]
- 3. Nakatsuka H, Shimizu Y, Yamamoto T, et al. Colorectal cancer incidence among atomic bomb survivors, 1950‐80. J Radiat Res 1992;33:342–61. [DOI] [PubMed] [Google Scholar]
- 4. Thompson DE, Mabuchi K, Ron E, et al. Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958‐1987. Radiat Res 1994;137:S17–67. [PubMed] [Google Scholar]
- 5. Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958‐1998. Radiat Res 2007;168:1–64. [DOI] [PubMed] [Google Scholar]
- 6. Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950‐2003: an overview of cancer and noncancer diseases. Radiat Res 2012;177:229–43. [DOI] [PubMed] [Google Scholar]
- 7. Mizoue T, Inoue M, Tanaka K, et al. Tobacco smoking and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2006;36:25–39. [DOI] [PubMed] [Google Scholar]
- 8. Akhter M, Kuriyama S, Nakaya N, et al. Alcohol consumption is associated with an increased risk of distal colon and rectal cancer in Japanese men: the Miyagi cohort study. Eur J Cancer 2007;43:383–90. [DOI] [PubMed] [Google Scholar]
- 9. Matsuo K, Mizoue T, Tanaka K, et al. Association between body mass index and the colorectal cancer risk in Japan: pooled analysis of population‐based cohort studies in Japan. Ann Oncol 2012;23:479–90. [DOI] [PubMed] [Google Scholar]
- 10. Kimura Y, Kono S, Toyomura K, et al. Meat, fish and fat intake in relation to subsite‐specific risk of colorectal cancer: the Fukuoka colorectal cancer study. Cancer Sci 2007;98:590–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pham NM, Mizoue T, Tanaka K, et al. Meat consumption and colorectal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol 2014;44:641–50. [DOI] [PubMed] [Google Scholar]
- 12. Nakagawa H, Ito H, Hosono S, et al. Changes in trends in colorectal cancer incidence rate by anatomic site between 1978 and 2004 in Japan. Eur J Cancer Prev 2017;26:269–76. [DOI] [PubMed] [Google Scholar]
- 13. Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Y, Oki E, Ando K, et al. The impact of a high‐frequency microsatellite instability phenotype on the tumor location‐related genetic differences in colorectal cancer. Cancer Genet Cytogenet 2010;196:133–9. [DOI] [PubMed] [Google Scholar]
- 15. Yahagi M, Okabayashi K, Hasegawa H, et al. The worse prognosis of right‐sided compared with left‐sided colon cancers: a systematic review and meta‐analysis. J Gastrointest Surg 2016;20:648–55. [DOI] [PubMed] [Google Scholar]
- 16. Cheng L, Eng C, Nieman LZ, et al. Trends in colorectal cancer incidence by anatomic site and disease stage in the United States from 1976 to 2005. Am J Clin Oncol 2011;34:573–80. [DOI] [PubMed] [Google Scholar]
- 17. Chauvenet M, Cottet V, Lepage C, et al. Trends in colorectal cancer incidence: a period and birth‐cohort analysis in a well‐defined French population. BMC Cancer 2011;11:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Semmens EO, Kopecky KJ, Grant E, et al. Relationship between anthropometric factors, radiation exposure, and colon cancer incidence in the life span study cohort of atomic bomb survivors. Cancer Causes Control 2013;24:27–37. [DOI] [PubMed] [Google Scholar]
- 19. Grant EJ, Brenner A, Sugiyama H, et al. Solid cancer incidence among the life span study of atomic bomb survivors: 1958‐2009. Radiat Res 2017;187:513–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cullings HM, Grant EJ, Egbert SD, et al. DS02R1: improvements to atomic bomb survivors’ input data and implementation of dosimetry system 2002 (DS02) and resulting changes in estimated doses. Health Phys 2017;112:56–97. [DOI] [PubMed] [Google Scholar]
- 21. Sagan L, Ishimaru T, Onishi S. Epidemiologic survey, Adult Health Study sample, Hiroshima and Nagasaki Study of cardiovascular disease Hiroshima and Nagasaki: Mortality related to family history and habits, Research Protocol No. 26–63. Hiroshima: Radiation Effects Research Foundation, 1963. [Google Scholar]
- 22. Kato H, Yano K, Johnson KG. Study of cardiovascular disease, Hiroshima and Nagasaki: Mortality related to family history and habits, Research Protocol No. 9–65. Hiroshima: Radiation Effects Research Foundation, 1965. [Google Scholar]
- 23. Wakabayashi T. Mail questionnaire survey for epidemiologic data on females in the JNIH‐ABCC Life Span Study sample, Research Protocol No. 11–69. Hiroshima: Radiation Effects Research Foundation, 1969. [Google Scholar]
- 24. Chief of Department of Epidemiology and Statistics . Mail questionnaire survey for epidemiologic data on the life span study extended sample, Research Protocol No. 14–78. Hiroshima: Radiation Effects Research Foundation, 1978. [Google Scholar]
- 25. Akiba S, Shibata Y, Kasagi F, et al. Mail survey on epidemiologic factors in the extended life span study sample, Research Protocol No. 4–91. Hiroshima: Radiation Effects Research Foundation, 1991. [Google Scholar]
- 26. Furukawa K, Preston DL, Lönn S, et al. Radiation and smoking effects on lung cancer incidence among atomic bomb survivors. Radiat Res 2010;174:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet (London, England) 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- 28. Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol 2008;167:492–9. [DOI] [PubMed] [Google Scholar]
- 29. Pierce DA, Preston DL. Joint analysis of site‐specific cancer risks for the atomic bomb survivors. Radiat Res 1993;134:134–42. [PubMed] [Google Scholar]
- 30. Renehan AG, Tyson M, Egger M, et al. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- 31. Hjartaker A, Aagnes B, Robsahm TE, et al. Subsite‐specific dietary risk factors for colorectal cancer: a review of cohort studies. J Oncol 2013;2013:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wei EK, Colditz GA, Giovannucci EL, et al. A comprehensive model of colorectal cancer by risk factor status and subsite using data from the nurses’ health study. Am J Epidemiol 2017;185:224–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haines J, Bacher J, Coster M, et al. Microsatellite instability in radiation‐induced murine tumours; influence of tumour type and radiation quality. Int J Radiat Biol 2010;86:555–68. [DOI] [PubMed] [Google Scholar]
- 34. Desautels D, Czaykowski P, Nugent Z, et al. Risk of colorectal cancer after the diagnosis of prostate cancer: a population‐based study. Cancer 2016;122:1254–60. [DOI] [PubMed] [Google Scholar]
- 35. Wallis CJD, Mahar AL, Choo R, et al. Second malignancies after radiotherapy for prostate cancer: systematic review and meta‐analysis. BMJ 2016;352:i851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rombouts AJM, Hugen N, van Beek JJP, et al. Does pelvic radiation increase rectal cancer incidence?—a systematic review and meta‐analysis. Cancer Treat Rev 2018;68:136–44. [DOI] [PubMed] [Google Scholar]
- 37. Rodriguez AM, Kuo Y‐F, Goodwin JS. Risk of colorectal cancer among long‐term cervical cancer survivors. Med Oncol 2014;31:943. [DOI] [PubMed] [Google Scholar]
- 38. Haylock RGE, Gillies M, Hunter N, et al. Cancer mortality and incidence following external occupational radiation exposure: an update of the 3rd analysis of the UK national registry for radiation workers. Br J Cancer 2018;119:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richardson DB, Cardis E, Daniels RD, et al. Site‐specific solid cancer mortality after exposure to ionizing radiation. Epidemiology 2018;29:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of subjects by drinking status and mean drink amount (drinks/day) among the LSS subjects
Table S2. LSS questionnaire meat consumption by sex and radiation dose
Table S3. LSS questionnaire earliest self‐reported BMI by sex and radiation dose
Table S4. Association of conventional risk factors with background rate of colorectal cancer by anatomical site.
Table S5. Observed and excess cases by dose category and sex based on the linear model among the LSS subjects.


