Abstract
When dealing with smart polymers, in particular with shape memory polymers, the polymer type and composition specify the overall material properties and in particular the extent of the shape memory effect. Polybenzoxazines as a polymer with high potential for structural applications represent a promising component for materials with both shape memory effect and structurally interesting material properties. This minireview gives insight into how the shape memory effect, in particular the shape recovery event, is influenced by internal factors such as polymer structure, morphology and external factors such as filler addition.
Keywords: polybenzoxazines, shape memory polymer composites, shape memory polymers, shape recovery
Polybenzoxazines as a polymer with high potential for structural applications represent a promising component for shape memory polymers. This minireview gives insight into how the shape memory effect, in particular the shape recovery event, is influenced by internal factors such as polymer structure, morphology and external factors such as filler addition.

1. Introduction
Shape memory polymers (SMPs) are stimuli‐responsive materials that can regain their original permanent shape after being deformed to a temporary shape in response to an external stimulus. Various kinds of external stimuli are used for actuation,1, 2, 3, 4, 5 such as change in temperature,6 light induction,7 electrical current,8 solvents,9 or ion strength.9 One of the most common stimulus to induce a change in shape is temperature10, 11 as it allows convenient programming of SMP materials.12, 13 SMPs can be comprised of thermoplastics with physically induced crosslinks (TP‐SMP) and thermosetting polymers with covalent crosslinks (TS‐SMP) as long as the molecular network structure contains at least two separate phases. Their T trans are categorized into two types by their network structure that is, melting temperature (T m) for thermoplastic‐shape memory polymers (TP‐SMPs) or glass transition temperature (T g) for thermoset‐shape memory polymers (TS‐SMPs).14 There are three main stages in the classic process of the thermally induced SMPs as shown in Figure 1.15 The first stage is the deformation of SMPs from their original shape (permanent shape received after conventional processing) to temporary shape after heating beyond transition temperature (T trans) of the polymer.14 The fixation of the polymer in its temporary shape by cooling below T trans represents the second stage, which is called programming. The ability of a polymer to fix the mechanical deformation is described/quantified by the shape fixity rate. The recovery is the final stage when SMP autonomously regain its original shape upon heating up to T trans. The shape recovery ratio and strain recovery rate describe the SMPs ability and the required force to restore the deformation. Upon cooling down the SMP below T trans the material solidifies and no recovery of the temporary shape can be observed. In this so called one‐way shape memory effect the programming step is required to rebuild temporary shape. A triple‐shape or multi‐shape polymer is able to memorize more than one temporary shape. Multiple SMPs require an appropriate polymer structure and morphology featuring a wide range of thermal transition temperatures, which could be T m or T g. Different T gs or a wide range of T g enable deformation to multiple temporary shapes. Multiple SMP can be also accomplished by heating a programmed SMP first above the T g and then above the T m of the switching segment.
Figure 1.

Main stages of thermally induced SMPs comparing thermosetting (TS) and thermplastic (TP) based SMPs with T g as T trans of TS‐SMPs and T m as T trans for TP‐SMPs.
Over the last few decades, shape memory research has significantly increased prompting developments of novel SMPs for advanced applications in, for example, structural parts,16 automotive,17 aerospace and life sciences.18 The focus is mainly on the improvement of the shape memory performance including shape fixity, shape recovery and recovery stress. One of the most common strategies is to alloy with polymers and reinforcing with fillers or fibers that affect the shape changing parameters or SMPs mechanical performance. Various attempts have been described with respect to the studied polymers, for example, polyurethanes, epoxies,5 and composites, for example, particles (silica or POSS), layered materials (graphite), fibrous materials (nanofibers19). The SMP systems of thermosets blended with polybenzoxazines have drawn great attention due to many interesting properties. Polybenzoxazines have been continuously investigated as a thermosetting polymer acting as a stable network segment in TS‐SMPs. They feature various beneficial properties such as high thermal stability, excellent mechanical properties, low water absorption and near zero shrinkage after processing. Moreover, it was already shown that benzxoazine chemistry allows alloying with various types of polymers and additives, which provides SMPs with outstanding shape memory performance.20, 21, 22, 23 Therefore, this minireview focuses on TS‐SMPs based on polybenzoxazine alloys, and composites describing recent advances in their SMP behavior.
2. Thermoset‐shape memory polymers (TS‐SMPs) based on polybenzoxazine alloys
The ring opening polymerization of benzoxazine monomers proceeds at elevated temperature without the need of catalysts or curing agents, and without releasing any by‐products.24, 25, 26 Upon polymerization phenolic moieties evolve that present reactive sites for further modifications and reactions with other resins or polymers.20, 21, 27, 28 Furthermore, benzoxazine monomers exhibit a very low melt viscosity facilitating compounding with various fillers or fibers. Thus, the combination of polybenzoxazines structure and morphology with its convenient chemistry enables specific adjustment of SMPs attributes in particular the strain recovery rate.
Polybenzoxazine/poly(ϵ‐caprolactone) based blends, copolymers and alloys
Arnebold et al. and Lützen et al. have shown that by introducing partially crystalline thermoplastic domains into an epoxy based polymer network could result shape memory polymers depending on the thermoplastic content.29, 30 The epoxy network is responsible for the permanent shape, whereas the incorporated partially‐crystalline oligomers (poly(ϵ‐caprolactone) PCL, poly(ω‐pentadecalactone) PPDL) enable shape changes into a temporary shape. Basically, the melting temperature of the covalently incorporated thermoplastic domains is used for switching between the permanent and temporary shape.15 The cationic ring opening polymerization of a cycloaliphatic epoxy is responsible for the covalent incorporation of the hydroxyl‐terminated oligomers via the activated monomer mechanism. That approach, however, does not work for benzoxazines. The hydroxyl‐terminated oligomers do not take part in the ring opening polymerization of benzoxazines due to their low nucleophilicity. Moreover, H. Ishida et al. have described phase separation events for PCL and benzoxazine mixtures at concentrations higher than 10 wt % PCL during the polymerization process.31 Thus, attempts to alloy hydroxyl‐terminated PCL with bisphenol A based benzoxazine resulted immiscible blends due to strong phase separation. That could be overcome by end group tosylation of PCLs hydroxyl end groups, leading to a covalent incorporation of the polyester by a nucleophilic attack of the benzoxazine onto the tosylated polyester.32, 33, 34 Gel content determinations proved that the neat hydroxyl‐terminated PCL oligomers form phase separated blend like samples, whereas the tosyl bearing PCL resulted copolymer like materials as depicted in Figure 2.
Figure 2.

Proposed structure of PCL/PBA‐a copolymer and blend (reprinted with permission from source 34).
DMA measurements showed that the cross‐link density of the copolymer like samples decreased, due to the covalent incorporation of the PCL chains.34 However, covalent integration of the PCL oligomers into benzoxazines network is not complete as proven by decreasing gel contents related to the increasing PCL content (Figure 3 a). Thus, around 40 wt % free, unbound PCL exists in PCL/benzoxazine alloys with PCL contents ≥60 wt % featuring a mixed bonding mode (Figure 3 b). That influences the overall material properties and shape memory behavior. Schäfer et al. showed that exclusively copolymer‐like samples with mixed bonding mode showed shape memory behavior.
Figure 3.

a) Sample composition of PCL/PBA‐a polymers with mixed bonding mode (reprinted with permission from source 33), b) possible bonding modes depending on PCLs end group modification (reprinted with permission from source 32).
With the aim to elucidate the role of the free PCL, soxhlet extractions were performed with PCL containing PBA‐a blends and copolymers, resulting extracted blends and copolymers, respectively (Figure 4).
Figure 4.

Possible bonding modes depending on PCLs end group modification after extraction.
Morphological and mechanical studies were performed comparing the samples properties before and after extraction. SEM images of fractured surfaces before and after extraction are presented in Figure 5. As described by the groups of H. Ishida, S. Zheng and K. Koschek PCL/PBA‐a blends (represented here by the sample Bz‐OH‐70) show a strong phase separation evidenced by a rough fractured surface.31, 33, 35 Extraction of free PCL leaves solely an agglomerated PBA‐a particle structure. In contrast, the copolymer like alloys exhibited a smooth surface pointing to homogenous distribution and incorporation of PCL before and after extraction. Activating the hydroxyl end groups by tosylation allows the PCL chains to get incorporated into benzoxazines network preventing a macroscopic phase separation.32
Figure 5.

Pictures of fracture surfaces of (un)modified PCL/PBA‐a samples and pure PBA‐a done by SEM (reprinted with permission from 32).
The strong phase separation in case of the PCL/PBA‐a blends featured higher degrees of crystallinity compared to the (homogeneous) copolymer‐like samples due to PCLs local fixation in the PBA‐a network. After PCL extraction, crystallinity vanished for the extracted blends, whereas the copolymer like samples showed X‐ray diffraction pattern proving that crystallinity could evolve despite covalent fixation of PCL in a PBA‐a network. However, thermoanalytical studies showed that the bonding state seems to affect the crystallization process. From their studies the authors concluded that the crystallization process of PCL domains within PCL/PBA‐a alloys require either free PCL aside the covalently incorporated one or a high PCL content ≥80 wt % for samples lacking free PCL (after extraction). The impact of free PCL was found to be also beneficial for the mechanical and shape memory properties according to a higher Young's modulus for Bz‐OTs‐70 at 25 °C determined with AFM based indentation and dynamic mechanical analysis experiments. At elevated temperatures, the lower network density of Bz‐OTs‐70 and Bz‐OTs‐70‐Ext samples and the lacking crystallinity at temperatures above PCLs melting temperature seem to be responsible for a strong decrease of the local Young's moduli and global storage moduli. Furthermore, the studies revealed that the PCL content is not crucial for shape recovery, whereas shape fixity requires high PCL content. That was shown by comparing samples with varying PCL content (Bz‐OTs‐60 versus Bz‐OTs‐70) and PCLs bonding state (presence versus absence of free PCL) (Table 1). All samples exhibited a high shape recovery, whereas a PCL content below 70 wt % caused a reduced shape fixity in Bz‐OTs‐60 ranging from 60–71 % in contrast to the sample Bz‐OTs‐70 (S f≈95 %). The absence of free PCL in Bz‐OTs‐70‐Ext showed no impact on shape recovery values but resulted a considerably lower shape fixity with values ranging from 62 % in the first cycle to 45 % in the third one. The authors concluded that the network density of PBA‐a can be adjusted by covalent incorporation of PCL oligomeric chains allowing for shapeability and recovery, whereas free partially crystalline PCL stabilizes the network and fixes the new, temporary shape. Thus, in PCL/PBA‐a alloys both network density and free PCL are crucial for high shape fixity and recovery (Figure 6).32, 33, 34
Table 1.
Shape fixity and recovery values of modified samples before and after extraction (values were taken from source 33 and 32).
|
|
Shape Recovery S r [%] |
Shape Fixity S f [%] |
||||
|---|---|---|---|---|---|---|
|
Cycle |
1 |
2 |
3 |
1 |
2 |
3 |
|
Bz‐OTs‐60 |
>99 |
>99 |
>99 |
71 |
60 |
66 |
|
Bz‐OTs‐70 |
>99 |
>99 |
>99 |
95 |
95 |
96 |
|
Bz‐OTs‐70‐Ext |
97 |
>99 |
>99 |
62 |
52 |
45 |
Figure 6.
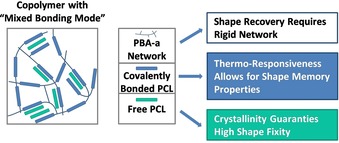
Factors influencing the shape memory effect in PCL/polybenzoxazine alloys (reprinted with permission from 32).
Comparing the properties of the PCL/epoxy and PCL/benzoxazine based SMPs, the overall shape memory properties (S f and S r) are similar. With respect to the mechanical properties, PBA‐a features a pronounced stiffness with a more than two times higher storage modulus at RT (987 MPa vs. 236 MPa, Table 2) and a shift to elevated temperatures at a low storage modulus (shapeability temperature). In contrast to the PCL/epoxy based SMP hydroxyl end group modification is required for covalent incorporation of thermoplastic oligomers into the PBA‐a network. Despite the additional manufacturing step, benzoxazine chemistry allows for a flexible material design as the epoxy based approach is limited to cycloaliphatic epoxy monomers and their need of thermolatent initiators restricts the use of high T m thermoplastics.
Table 2.
Comparison of mechanical properties of epoxy and PBA‐a based systems (values were taken from source 30 and 32).
|
TS‐SMP composition |
Storage modulus at 20 °C |
Temperature at 10 MPa |
|---|---|---|
|
PCL/Epoxy 70/30 |
236 MPa |
50.4 °C |
|
PCL/PBA‐a 70/30 |
987 MPa |
61.5 °C |
In addition, samples with only low content of incorporated PCL (ω=10–20 wt %) did not show any shape memory abilities but instead, had an impact on the toughness of the polybenzoxazines and reduced significantly the brittleness of those networks.34
Multiple‐shape memory effects of polybenzoxazine/urethane alloys
Shape memory polyurethane (SMPU) foams have been studied36 with a T g of the switching segment close to room temperature in order to obtain satisfactory shape recovery.37 In general, polyurethanes feature many beneficial properties. Applied as SMP there are, however, some disadvantages, such as a low strain recovery rate, recovery ratio, shape fixity, and recovery stress.38 N. Erden and S. C. Jana have reported alloying PU with polybenzoxazine as an approach to improve SMPUs properties without decreasing the micro‐segregated structure.36 By chemical incorporation of the bisphenol A/aniline‐based benzoxazine (BA‐a) content in the poly(benzoxazine‐urethane) based SMP the chemical interaction of the two polymeric components results an enhancement of mechanical properties. The poly(benzoxazine‐urethane) alloy consists of a mole ratio of polytetramethylene glycol (PTMG): 4,4′‐methylene diphenyl diisocyanate (MDI) of 1:5, respectively with 1,4‐butanediol (BD) acting as a chain extender.36 Erden and Jana et al. revealed that the reaction between the cyanate group of PU and the hydroxyl group of polybenzoxazine could reduce their phase segregation. Moreover, the results revealed that increased content of polybenzoxazine based hard phase could enhance the rate of shape recovery, shape recovery ratio, and recovery stress of the SMPU.36 This type of poly(benzoxazine‐urethane) alloy represents a conventional double‐shape‐memory polymer.
Prathumrat et al. have developed multiple‐shape memory polymers synthesized from bisphenol A/aniline‐based benzoxazine resin (BA‐a) and urethane prepolymer (PU).39 They studied the effect of BA‐a/PU mass ratios of 55/45, 60/40, 70/30, and 80/20 on the mechanical properties and multi‐SMP behavior. The crosslinked BA‐a/PU copolymers as a result of the reaction between PBA‐a hydroxyl groups and PUs isocyanate groups, exhibit a wide temperature range of T g and can be shaped into two temporary shapes with one programming and one recovery cycle as shown in Figure 7. With increasing BA‐a ranging from 55 to 80 wt % T g increased from 130 to 180 °C as well as the flexural strength and modulus changed in the range of 40–98 MPa and 1.0–3.7 GPa, respectively. The values of shape fixity were also reported to be as high as 70–96 % in the first temporary shape and 88–96 % in the second temporary shape. While, the shape recovery values were found to be 88–96 % in the first temporary shape and 97–99 % in the second temporary shape.39 The authors suggested that the thermally induced multiple–shape memory effect of thermosetting BA‐a/PU copolymers can be used in a wide range of applications such as construction, electronics, aerospace, medication, and textiles.
Figure 7.

Multiple‐shape memory behavior of a sample based on a benzoxazine/urethane alloy.39
Thermally induced shape memory effects of polybenzoxazine/epoxy copolymers
Although polybenzoxazine possesses a high T g, the amount of crosslink density is less compared to other thermosetting polymers with similar properties. This phenomenon is attributed to the fact that the hydrogen bonding could hinder the movement of polymer chains and support the rigidity of the polymer.40 Rimdusit et al. prepared the SMPs derived from BA‐a‐modified epoxy resin.41 In their study, aliphatic epoxy (Neopentyl glycol diglycidyl ether, NGDE), aromatic epoxy (EPON Resin 826), and Jeffamine D230 curing agent were used for blending with BA‐a monomer. The molar ratios of the curing agent and BA‐a varied from 0:1 to 1:0, while the molar ratio of BA‐a/NGDE and BA‐a/ EPON Resin 826 was kept at 1:1. The results revealed that T g value and the crosslink density of the SMP were found to increase with increasing BA‐a content. The shape fixity of SMPs was in the range of 98–99 %. Moreover, their recovery stress was increased with increasing BA‐a content up to 33 mol % with the reported recovery stress of about 38 kPa compared to 20.4 kPa of the unmodified shape memory epoxy.41
The SMP system synthesized from binary mixtures based on polybenzoxazine/ aliphatic epoxy without aromatic epoxy and curing agent was continuously developed by Tanpitaksit et al.42 This SMP system was prepared by blending NGDE with BA‐a at BA‐a molar percentage in the range of 30 to 50 %. In the epoxy and benzoxazine system the reaction between phenolic and epoxide group was expected to occur after the ring opening reaction of benzoxazine and the emerging phenolic OH‐groups.20, 42 They confirmed that (PBA‐a/NGDE) copolymers formed chemical crosslinks (Figure 8). Bisphenol A/aniline‐based polybenzoxazine (PBA‐a) and NGDE play an important role as stable and flexible network segment in SMPs. T gs from the peak of the loss tangent curves of BA‐a/NGDE copolymers increased with increasing BA‐a content, that is, ranging from 51 °C (30 mol % BA‐a) to 140 °C (50 mol % BA‐a). Moreover, the moduli at rubbery state were enhanced ranging from 40 to 172 MPa indicating the increase of crosslink density. For SMP performance, the outstanding shape fixity at room temperature of SMPs was in the range from ca. 98 to 99 %. The obtained SMPs also provided shape recovery values up to 100 % at T g+20 °C. Interestingly, the addition of BA‐a could enhance recovery stress of SMP in the range of 0.25 to 1.59 MPa as shown in Figure 9. The addition of BA‐a not only improved thermal and mechanical properties but also enhanced recovery stress. Furthermore, the results implied that thermally induced SMP based on polybenzoxazine/ aliphatic epoxy has a potential to be applied as a hinge or deployable structures application.
Figure 8.

Schematic of shape memory effects of thermoset‐shape memory polymer based on polybenzoxazine/epoxy copolymers (adapted from 42).
Figure 9.

Recovery stress of BA‐a/NGDE SMPs at various BA‐a contents (adapted from 42).
Another attractive application for SMPs is the use in medical devices such as devices for the mechanical removal of blood clots.38 Biopolymers would be beneficial for wide applications due to low cost and environmentally friendliness when compared with petroleum‐based polymers. The combination of shape memory capability and bio‐based polymers is an example of multifunctional materials. Intelligent benzoxazine based shape memory polymers from renewable resources were developed by Hombunma et al. Bio‐based benzoxazine resins were prepared from vanillin, furfurylamine, and paraformaldehyde43 via solventless technology. Vanillin‐furfurylamine‐containing polybenzoxazine (poly(V‐fa)) exhibited high thermal stability and flame retardant properties. They reported that V‐fa monomer could be copolymerized with epoxidized castor oil (ECO) by chemical crosslinking. The obtained SMP copolymer could be deformed to a temporary shape and autonomously recovered to the original shape after heating at T g+20 °C. V‐fa/ECO copolymer showed good shape memory performances with shape fixity of 98 %, shape recovery ratio of 100 %, and fast recovery time of 10 seconds under the external force as shown in Figure 10. The results suggested that SMP based on V‐fa/ECO copolymer could be suitable for potential biomedical applications due to their relatively low transition temperature close to the human body temperature of approximately 37 °C.
Figure 10.

Shape memory effects of V‐fa/ECO copolymer and shape recovery process after exposing to thermal heating at T g+20 °C.43
Thermoset‐shape memory polymers (TS‐SMPs) based on polybenzoxazine composites
Although SMP has advantages in various aspects of its structure in comparison to conventional metal based alloys, poor mechanical performance and recovery stress remain challenging.19, 44 Reinforcing the polymer matrix with particulate fillers and fibers represents a convenient approach to address the drawback.
Particle‐filled thermoset‐shape memory polymers (TS‐SMPs) based on polybenzoxazine alloys
SMP filled with particles such as carbon black, carbon nanotubes (CNT), silicon carbide (SiC), carbon nanofibers (CNF), clay, and short continuous fibers are designated as particle reinforced Shape Memory Polymer Composites (SMPC). Particle‐filled SMPCs exhibit additional functions such as a high electrical conductivity, magnetic and light responsiveness, which classifies them as multifunctional materials. Likitaporn et al. successfully prepared silicon carbide whisker (SiCw) filled BA‐a/NGDE SMPs with excellent recovery stress improvement.45 They reported a significant increase in recovery stress, storage modulus, glass transition temperature, and thermal stabilities of the rendered SMPCs upon addition of SiCw. With respect to shape memory properties, the obtained SMPCs provided an outstanding shape fixity value up to 99 %. The recovery times are various in range from 8 to 27 minutes in conventional heating. With a strong characteristic of microwave absorber, SiCw featured a much shorter recovery time under microwave heating in comparison to convection heating. Furthermore, the incorporation of SiCw in BA‐a/NGDE SMPs provided enhanced recovery stress of 11.2 MPa for the BA‐a/NGDE composites in contrast to 3.5 MPa determined for the neat SMP as shown in Figure 11. The SiCw‐filled BA‐a/NGDE SMPs are interesting shape memory materials to be used in high recovery stress and modulus such as high performance structural components.45
Figure 11.

The relationship between recovery stress and time of silicon carbide whisker‐filled BA‐a/NGDE SMPs at various silicon carbide whisker contents: (▾) 0 wt %, (▪) 5 wt %, (⧫) 10 wt %, (
 ) 15 wt %, (•) 20 wt % (adapted from 45).
) 15 wt %, (•) 20 wt % (adapted from 45).
Recently, instead of increasing the environmental temperature, thermally induced SMPs can be indirectly heated by irradiation with infrared light. This concept has been demonstrated in a laser‐activated medical device.46 In such devices, heat transfer can be increased by incorporation of conductive fillers, such as conductive ceramics, carbon black and CNT. Prasomsin et al. have developed V‐fa/ECO copolymers, which were synthesized from bio‐based materials filled with multi‐walled carbon nanotubes (MWCNTs).47 V‐fa/ECO SMPC filled with 0.5 wt % of MWCNT were deformed under a three‐point bending mode at T g+20 °C to a temporary shape. Interestingly, the permanent shape of V‐fa/ECO copolymer could be remotely recovered from a deformed temporary shape at a specified region upon exposing to NIR radiation. The shape fixity, the shape recovery ratio, and the shape recovery time improved upon incorporation of MWCNTs into the V‐fa/ECO copolymers. The V‐fa/ECO copolymer reinforced with MWCNTs showed a shape fixity as high as 90 %, a shape recovery ratio of 98 %, and a fast recovery time of 14 s as shown in Figure 12. Their results demonstrated that light‐triggered SMPCs could be developed from renewable resources and could be used for advanced smart multifunctional material applications.
Figure 12.

The shape recovery of V‐fa/ECO SMPCs filled with MWCNT using NIR laser irradiation (adapted from 47).
Improvement of recovery stress of fiber‐reinforced thermoset‐shape memory polymers (TS‐SMPs) based on polybenzoxazine alloys
Many advanced applications of SMPs sometimes require release force, which was defined as recovery stress when recovering from temporary shape to original shape. Particle based SMPCs feature improved shape memory properties regarding the strain recovery rate. For structural applications, however, particle or short fiber based SMPCs do not meet the requirements regarding the overall mechanical properties.44, 48 In contrast, continuous fiber‐reinforced SMPCs offer significant improvements in both strength and stiffness, providing excellent mechanical properties. Fiber‐reinforced SMPCs as both functional and structural materials are promising for many advanced applications such as smart textile and apparels,49 sensor and actuators.50, 51 F. Li et al. have studied systematically the SMPC performance in three‐point bending test with 5 % strain of deformation of unidirectional carbon fiber reinforced epoxy‐based SMPC with fiber mass fractions of 16 %, 23 %, 30 %, and 37 % aiming at a mechanical characterization for direct engineering application.52 The composites show good shape recovery performance, with measured recovery ratios of more than 93 % reaching 120 °C and almost 100 % after holding the specimen at 120 °C. The maximum recovery stresses increase almost proportionally to the fiber mass contents in the range of 16.5 MPa to 49.0 MPa during reheating. The composites featured good shape memory properties during cyclic loading and unloading at 120 °C.52 During the second cycle of free recovery experiments as well as the constrained displacement recovery test, respectively the maximum flexural stress during deformation process and maximum recovery stress showed a decline in stress values compared to the appropriate ones of the first cycles. The authors assume internal damages probably based on microcracks resulting from manufacturing and deformation processes as a reason for the energy dissipation. Another interesting outcome regarding the recovery stress was the dependence of the stress on the temperature and the partial load the specimens were subjected to. Higher temperatures and lower partial loads entail a higher recovery stress. Flexural stress‐strain relationships of the cyclic loading and unloading revealed that part of the energy dissipates due to internal friction within the material and the maximum stiffness of the material decreases during the first three cycles and stabilizes after in the subsequent ones. The authors correlated those observations with the microcrack formation initiated by the high shear stress between fiber and matrix, whereas the microcrack growth is levelling off with further cycles. The work of Li et al. represents a first approach to a systematic study of long‐fiber reinforced SMPCs identifying potential restrictions and negative effects depending on fiber mass content and the load scenario.
Currently, other research groups are contributing in the field of structurally interesting SMPCs based on woven glass and carbon fiber reinforced plastics. Plylaharn et al. successfully developed thermally‐induced SMPC based on woven carbon fiber and woven glass fiber reinforced BA‐a/NGDE copolymers showing excellent thermo‐mechanical properties and shape memory performances. The detailed discussion of these results will be available soon.
Conclusions
Shape memory polymers as responsive smart materials upon receiving the suitable trigger, have drawn the attention from researchers in many academic and industrial fields. As the most common components for SMPs, polybenzoxazines were used as segments representing the permanent phase with switching segments varying from urethane and epoxy prepolymers as well as partially crystalline thermoplastic oligomers such as PCL. The SMP approaches focused especially on how polybenzoxazine affects the shape recovery rate and stress. Shape recovery can be improved by i) adjusting the polymer structure and morphology or ii) reinforcing the polymeric material with appropriate fillers and reinforcing fibers and material. With respect to the polymer structure and morphology studies on polybenzoxazine alloys, blends and copolymers with partially crystalline PCL based oligomers revealed that by varying the network density of PBA‐a, and the ratio of covalently bonded and free PCL chains, SMP can be adjusted. Integrated and free PCL are crucial for a high shapeability and shape fixity, respectively, whereas the polybenzoxazine network is responsible for a high recovery. For the polybenzoxazine/epoxy‐based SMP, the SMP performance was especially good regarding the recovery stress that increased with increasing polybenzoxazine content. A multiple‐shape memory effect was discovered in SMP based on polybenzoxazine/urethane copolymers due to their broad thermomechanical transition temperature range.
With respect to the reinforcing approach, particle and fiber‐based SMP composites were reported to show improved overall mechanical properties and SMPC performance. SMPCs based on polybenzoxazine/epoxy filled with silicon carbide and CNT exhibited good shape memory performances being responsive towards various external stimuli, that is, thermal induction, microwave absorption and infrared light. Beside the recovery strain, the incorporation of conductive fillers, such as conductive ceramics, carbon black and CNT, yields an increased heat transfer, improving SMP performance. Moreover, SMPCs were successfully generated of bio‐based resources as starting materials for polybenzoxazine‐based SMPC systems (e.g., V‐fa/ECO copolymers) filled with MWCNTs. Endless and long fiber‐reinforced SMPCs play a crucial role in parts for structural applications. Recent studies have shown that SMPCs based on unidirectional carbon fiber fabrics exhibit a huge potential. There is, however, further need to describe and elucidate possible events on a molecular level (microcracks within the polymer matrix, delamination, etc.) aiming at a better understanding of how the type of reinforcement (unidirectional, fabric, non‐woven), composite lay‐up structure, fiber volume content, fiber/matrix interaction affect the overall material and in particular shape memory properties. Upcoming contributions from the research groups of J. Leng, S. Rimdusit and K. Koschek will disclose novel composite designs and insights on structurally interesting SMPCs.
Conflict of interest
The authors declare no conflict of interest.
Biographical Information
Dr. Sarawut Rimdusit is a full professor of the Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok, Thailand. He received his doctoral degree from the Department of Macromolecular Science and Engineering, Case Western Reserve University, Cleveland, Ohio, USA in 2000. His research is concentrated on multicomponent polymeric systems based on polybenzoxazines and some network forming polymers. He has published 1 book on polybenzoxazine alloys and composites (Springer), 10 book chapters in English, 4 book chapters in Japanese and more than 70 international papers related to this field.

Biographical Information
Dr. Chanchira Jubsilp is an associate professor of Department of Chemical Engineering, at Srinakharinwirot University, Thailand. She received her doctoral degrees from the Department of Chemical Engineering, Chulalongkorn University, Thailand in 2007. Her research focuses on polymer alloys and composites based on polybenzoxazine: tribological properties, curing and degradation reactions. She has published 30 peer‐reviewed articles. The recent co‐authored books are Advanced and Emerging Polybenzoxazine Science and Technology, published by Elsevier, and Novel Molecular Design and Development of Benzoxazine Resin, CMC publishing, Japan.

Biographical Information
Phattarin Mora is doctoral student of the Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Thailand. She received the Bachelor's Degree of Engineering with a major in Chemical Engineering from the Faculty of Engineering, Srinakharinwirot University, Thailand in 2014 and she graduated Master's Degree of Engineering with a major in Polymer laboratory, Chemical Engineering from Chulalongkorn University in 2016. She has co‐authored over 6 international journal articles and 1 book chapter in Japanese.

Biographical Information
Hannes Schäfer is a research fellow in the Department Polymeric Materials and Mechanical Engineering at the Fraunhofer IFAM Bremen, Germany working with benzoxazine based copolymers. He received his doctoral degree from the Faculty Biology/Chemistry University of Bremen, Germany in 2019. He received his Bachelor of Science in Chemistry in 2012 and his Master of Science in Chemistry in 2014 from the University of Bremen, Germany. He has co‐authored 7 international papers.

Biographical Information
Dr. Katharina Koschek is head of the department “Polymeric Materials and Mechanical Engineering” at Fraunhofer IFAM. She received her doctoral degree from the Department of Biology, Chemistry and Pharmacy at the Freie Universität Berlin in 2012. Her research focuses on stimuli‐responsive, bio‐based polymers and FRPs with the overall aim to develop environmental‐friendly lightweight materials, energy‐efficient manufacturing, and recycling concepts. She was co‐author in 1 book chapter, and more than 25 international papers related to this field.

Acknowledgements
The authors would like to express their sincere appreciations to the Royal Golden Jubilee (RGJ) Ph.D. Program (Grant No. PHD/0177/2560) under the Thailand Research Fund (TRF), Thailand and The 90th Anniversary of Chulalongkorn University, (Ratchadaphiseksomphot Endowment Fund) for financial support throughout the research. K. Koschek and H. Schäfer gratefully acknowledge the financial support from the Bundesministerium für Bildung und Forschung (BMBF) through the NanoMatFutur award (DuroCycleFVK 03XP0001).
P. Mora, H. Schäfer, C. Jubsilp, S. Rimdusit, K. Koschek, Chem. Asian J. 2019, 14, 4129.
References
- 1. Wei Z. G., Sandstrom R., Miyazaki S., J. Mater. Sci. 1998, 33, 3763. [Google Scholar]
- 2. Leng J., Lan X., Liu Y., Du S., Prog. Mater. Sci. 2011, 56, 1077. [Google Scholar]
- 3. Tiptipakorn S., Rimdusit S., Shape Memory Polymers, Blends and Composites, Springer Nature Singapore Pte Ltd., 2020. [Google Scholar]
- 4. Hager M. D., Bode S., Weber C., Schubert U. S., Prog. Polym. Sci. 2015, 49–50, 3. [Google Scholar]
- 5. Tiptipakorn S., Rimdusit S. in Advanced and Emerging Polybenzoxazine Science and Technology (Eds.: H. Ishida, P. Froimowicz), Elsevier, Amsterdam, 2017, p. 1029. [Google Scholar]
- 6. Chatterjee T., Dey P., Nando G. B., Naskar K., Polymer 2015, 78, 180. [Google Scholar]
- 7. Lendlein A., Jiang H., Jünger O., Langer B. R., Nature 2005, 434, 879. [DOI] [PubMed] [Google Scholar]
- 8. Chen S., Yang S., Li Z., Xu S., Yuan H., Chen S., Ge Z., Polym. Compos. 2014, 36, 439. [Google Scholar]
- 9. Xiao R., Guo J., Safranski D. L., Nguyen T. D., Soft Matter 2015, 11, 3977. [DOI] [PubMed] [Google Scholar]
- 10. Leng J., Lv H., Liu Y., Du S.., Appl. Phys. Lett. 2007, 91, 144105. [Google Scholar]
- 11. Lu H., Liu Y., Leng J., Du S., Smart Mater. Struct. 2009, 18, 085003. [Google Scholar]
- 12. Zhao Q., Qi H. J., Xie T., Prog. Polym. Sci. 2015, 49–50, 79. [Google Scholar]
- 13. Kwon H., Lee A. S., Lee J. H., Park N. K., Kim G. D., Cho B., Choi S.-C., Yu S., J. Ind. Eng. Chem. 2017, 53, 386. [Google Scholar]
- 14. Miaudet P., Derré A., Maugey M., Zakri C., Piccione P. M., Inoubli R., Poulin P., Science 2007, 318, 1294. [DOI] [PubMed] [Google Scholar]
- 15. Lendlein A., Kelch S., Angew. Chem. Int. Ed. 2002, 41, 2034; [PubMed] [Google Scholar]; Angew. Chem. 2002, 114, 2138. [Google Scholar]
- 16. Santo L., Quadrini F., Accettura A., Villadei W., Procedia Eng. 2014, 88, 42. [Google Scholar]
- 17. Liu Y., Du H., Liu L., Leng J., Smart Mater. Struct. 2014, 23, 023001. [Google Scholar]
- 18. Sokolowski W., Metcalfe A., Hayashi S., Yahia L. H., Raymond J., Biomed. Mater. 2007, 2, S23. [DOI] [PubMed] [Google Scholar]
- 19. Meng H., Li G., Polymer 2013, 54, 2199. [Google Scholar]
- 20. Rimdusit S., Jubsilp C., Tiptipakorn S., Alloys and Composites of Polybenzoxazines: Properties and Applications, New York, Springer, 2013. [Google Scholar]
- 21. Ishida H., Agag T., Handbook of benzoxazine resins, Elsevier, 2011. [Google Scholar]
- 22. Kiskan B., React. Funct. Polym. 2018, 129, 76. [Google Scholar]
- 23. Zhang S., Ran Q., Fu Q., Gu Y., Macromolecules 2018, 51, 6561. [Google Scholar]
- 24.H. Ishida, U.S. Patent 5,543,516, 1996.
- 25. Ghosh N. N., Kiskan B., Yagci Y., Prog. Polym. Sci. 2007, 32, 1344. [Google Scholar]
- 26. Takeichi T., Guo Y., Agag T., J. Polym. Sci. Chem. 2000, 38, 4165. [Google Scholar]
- 27. Arslan M., Kiskan B., Yagci Y., Macromolecules 2018, 51, 10095. [Google Scholar]
- 28. Gu S., Jana S., Polymers 2014, 6, 1008–1025. [Google Scholar]
- 29. Arnebold A., Hartwig A., Polymer 2016, 83, 40. [Google Scholar]
- 30. Lützen H., Gesing T. M., Kim B. K., Hartwig A., Polymer 2012, 53, 6089. [Google Scholar]
- 31. Ishida H., Lee Y.-H., J. Polym. Sci. Part B 2001, 39, 736. [Google Scholar]
- 32. Schäfer H., Kolberg A., Gockeln M., Kun R., Balzer B. N., Koschek K., Polym. Test. 2019, 77, 105888. [Google Scholar]
- 33. Schäfer H., Hartwig A., Koschek K., Polymer 2018, 135, 285. [Google Scholar]
- 34. Schäfer H., Koschek K., Eur. Polym. J. 2018, 108, 582. [Google Scholar]
- 35. Zheng S., Lü H., Guo Q., Macromol. Chem. Phys. 2004, 205, 1547. [Google Scholar]
- 36. Erden N., Jana S. C., Macromol. Chem. Phys. 2013, 214, 1225. [Google Scholar]
- 37. Hayashi S., Int. Prog. Urethanes, 1993, 6, 90. [Google Scholar]
- 38. Behl M., Lendlein A., Mater. Today 2007, 10, 20. [Google Scholar]
- 39. Prathumrat P., Tiptipakorn S., Rimdusit S., Smart Mater. Struct. 2017, 26, 065025. [Google Scholar]
- 40. Shen S. B., Ishida H., J. Polym. Sci. Part B 1999, 37, 3257. [Google Scholar]
- 41. Rimdusit S., Lohwerathama M., Hemvichian K., Kasemsiri P., Dueramae I., Smart Mater. Struct. 2013, 22, 075033. [Google Scholar]
- 42. Tanpitaksit T., Jubsilp C., Rimdusit S., Express Polym. Lett. 2015, 9, 824. [Google Scholar]
- 43.P. Hombunma, T. Parnklang, S. Rimdusit, Collaborative Conference on Materials Research (CCMR), 2019, 288.
- 44. Rousseau I. A., Polym. Eng. Sci. 2008, 48, 2075. [Google Scholar]
- 45. Likitaporn C., Mora P., Tiptipakorn S., Rimdusit S., J. Intell. Mater. Syst. Struct. 2018, 29, 388. [Google Scholar]
- 46. W. Small IV , Wilson T. S., Benett W. J., Loge J. M., Maitland D. J., Opt. Express 2005, 13, 8204. [DOI] [PubMed] [Google Scholar]
- 47.W. Prasomsin, T. Parnklanga, S. Rimdusit, The International Conference on Advanced and Applied Petroleum, Petrochemicals, and Polymers 2018, 2018, 125.
- 48. Lan X., Liu Y., Lv H., Wang X., Leng J., Du S., Smart Mater. Struct. 2009, 18, 024002. [Google Scholar]
- 49. Gök M. O., Bilir M. Z., Gürcüm B. H., Procedia Soc. Behav. Sci. 2015, 195, 2160. [Google Scholar]
- 50. Lu H., Yu K., Liu Y., Leng J., Smart Mater. Struct. 2010, 19, 065014. [Google Scholar]
- 51. Lan X., Liu Y., Lv H., Wang X., Leng J., Du S. Smart Mater. Struct. 2009, 18, 024002. [Google Scholar]
- 52. Li F., Scarpa F., Lan X., Liu L., Liu Y., Leng J., Composites Part A 2019, 116, 169. [Google Scholar]


