Abstract
The multinational phase 3 CheckMate 238 trial compared adjuvant therapy with nivolumab versus ipilimumab among patients with resected stage III or IV melanoma (N = 906). In this Japanese subgroup analysis of CheckMate 238 (n = 28; nivolumab, n = 18; ipilimumab, n = 10), both the 12‐ and 18‐month recurrence‐free survival rates were 56% for nivolumab and 30% for ipilimumab (hazard ratio, 0.66; 97.56% confidence interval, 0.19–2.24; P = 0.4390). No new safety signals were reported for Japanese patients. Results were consistent with those from the CheckMate 238 global population, indicating that nivolumab has the potential to be a treatment option for Japanese patients with resected melanoma who are at high risk of recurrence.
Keywords: adjuvant drug therapy, ipilimumab, Japanese patients, melanoma, nivolumab
Introduction
Patients with resected stage III or IV melanoma who are at high risk of disease recurrence constitute a population with a major unmet need, and adjuvant therapy with immune checkpoint inhibitors (such as nivolumab [an anti‐programmed death 1 antibody] and ipilimumab [an anti‐cytotoxic T‐lymphocyte antigen 4 antibody]) has demonstrated benefits over traditional treatments for these patients.1, 2, 3, 4 In the phase 3 CheckMate 238 trial, nivolumab (3 mg/kg) demonstrated a significant improvement in recurrence‐free survival (RFS) and a more tolerable safety profile compared with ipilimumab (10 mg/kg) among patients with resected stage IIIB, IIIC or IV melanoma.4 Here, we present the results of a Japanese subgroup analysis of CheckMate 238.
Case Report
Study design
In this randomized, double‐blind phase 3 trial (Clinicaltrials.gov no. NCT02388906; EudraCT no. 2014‐002351‐26), patients were enrolled from 30 March 2015 to 30 November 2015, at 130 centers in 25 countries including Japan (although patients were not stratified by region). The current report presents only data from the Japanese cohort of the study (results obtained with the global population were reported previously).4 Additional details about the study methodology may be found in Supporting Information in the online version of this article. (Bristol‐Myers Squib policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html).
Eligibility criteria and study treatment
Patients diagnosed with stage IIIB, IIIC or IV melanoma according to the 2009 classification of the American Joint Committee on Cancer (AJCC), 7th edition,5 and who were 15 years of age or older were eligible to enroll. Patients were assigned at a 1:1 ratio to receive nivolumab (3 mg/kg i.v. every 2 weeks; Bristol‐Myers Squibb, Princeton, NJ, USA) or ipilimumab (10 mg/kg i.v. every 3 weeks; Bristol‐Myers Squibb) for four doses and then every 12 weeks, along with corresponding matching placebo, for up to 1 year or until disease recurrence, unacceptable toxic effects or withdrawal of consent (Fig. S1).
Outcome measures
The primary end‐point was RFS in the intention‐to‐treat population. Secondary end‐points included overall survival (OS), safety and health‐related quality of life (HRQoL). Distant metastasis‐free survival (DMFS) was an exploratory end‐point. RFS, DMFS and HRQOL comparisons were descriptive, because the analyses were not powered to calculate differences between the groups in this Japanese subpopulation. Adverse event (AE) data were collected in each group according to the Common Terminology Criteria for Adverse Events, version 4.0.
Patients and disposition
As previously reported for the global population, 906 patients underwent randomization and 905 were treated.4 At the time of this analysis, with a minimum follow‐up of 18 months (median, 19.5), all 905 treated patients were no longer receiving study medication. A total of 397 patients had completed 1 year of treatment (275/452 patients [61%] in the nivolumab group and 122/453 patients [27%] in the ipilimumab group).4
In the Japanese subgroup, 33 patients were enrolled and 28 patients underwent randomization and were treated (Fig. S2). Demographic and baseline characteristics in this group were similar to those of the published global population (Table 1).4 The median duration of therapy for Japanese patients was 11.2 months (95% confidence interval [CI], 2.8−11.5) for nivolumab and 5.1 months (95% CI, 1.0−11.0) for ipilimumab (Fig. S3). Post‐protocol treatment including radiotherapy, surgery and systemic therapy was administrated to eight (44%) patients in the nivolumab group and seven (70%) patients in the ipilimumab group (Table S1).
Table 1.
Demographic and clinical characteristics of Japanese patients
| Nivolumab (n = 18) | Ipilimumab (n = 10) | |
|---|---|---|
| Median age, years (range) | 56.5 (25–83) | 43.0 (21–76) |
| <65 years old, n (%) | 13 (72) | 8 (80) |
| ≥65 years old, n (%) | 5 (28) | 2 (20) |
| Sex, n (%) | ||
| Male | 11 (61) | 3 (30) |
| Female | 7 (39) | 7 (70) |
| Melanoma subtype, n (%) | ||
| Cutaneous | 12 (67) | 8 (80) |
| Acral | 3 (17) | 1 (10) |
| Mucosal | 2 (11) | 1 (10) |
| Other (no ocular/uveal) | 1 (6) | 0 |
| Tumor origin, n (%) | ||
| Primary | 15 (83) | 6 (60) |
| Recurrent | 3 (17) | 4 (40) |
| Stage, n (%) | ||
| IIIB | 10 (56) | 4 (40) |
| IIIC | 8 (44) | 5 (50) |
| IV | 0 | 1 (10) |
| Lymph node involvement in stage III patients, n (%) | ||
| Microscopic | 9 (50) | 4 (40) |
| Macroscopic | 8 (44) | 4 (40) |
| Not reported | 1 (6) | 1 (10) |
| Tumor ulceration in stage III patients, n (%) | ||
| Present | 12 (67) | 5 (50) |
| Absent | 6 (33) | 4 (40) |
| M status in stage IV patients, n (%) | ||
| M1a | 0 | 1 (10) |
| M1b | 0 | 0 |
| M1c | 0 | 0 |
| ECOG PS, n (%) | ||
| 0 | 17 (94) | 10 (100) |
| 1 | 1 (6) | 0 |
| LDH status, n (%) | ||
| ≤ULN | 17 (94) | 10 (100) |
| >ULN | 1 (6) | 0 |
| >2 × ULN | 0 | 0 |
| PD‐L1 expression, n (%) | ||
| ≥1% | 7 (39) | 5 (50) |
| <1% | 8 (44) | 4 (40) |
| ≥5% | 4 (22) | 3 (30) |
| <5% | 11 (61) | 6 (60) |
| Indeterminate/unevaluable | 3 (17) | 1 (10) |
| BRAF mutation, n (%) | ||
| Positive | 10 (56) | 4 (40) |
| Negative | 5 (28) | 6 (60) |
| Not reported | 3 (17) | 0 |
ECOG PS, Eastern Cooperative Oncology Group performance status; LDH, lactate dehydrogenase; n, number of patients; PD‐L1, programmed death ligand 1; ULN, upper limit of normal.
RFS and DMFS
In the Japanese subgroup, median RFS was 19.8 months in the nivolumab group and 10.1 months in the ipilimumab group (Fig. 1a); however, these data need to be considered carefully due to the low number of patients at risk. At both 12 and 18 months, RFS rates were 56% (95% CI, 30.5–74.8) in the nivolumab group and 30% (95% CI, 7.1–57.8) in the ipilimumab group. Descriptive analysis showed that nivolumab resulted in longer RFS than ipilimumab, with recurrence or death reported by investigators in nine of 18 patients (50%) and in seven of 10 patients (70%), respectively (hazard ratio [HR] for disease recurrence or death, 0.66; 97.56% CI, 0.19–2.24; P = 0.4390). Among patients in the Japanese subgroup with stage III disease, in an exploratory analysis, the median DMFS was 20.7 months in the nivolumab group and had not been reached in the ipilimumab group (Fig. 1b). At both 12 and 18 months, the DMFS rate was 67% (95% CI, 40−83) in the nivolumab group and 50% (95% CI, 14−78) in the ipilimumab group (HR, 0.65; 95% CI, 0.18−2.32).
Figure 1.
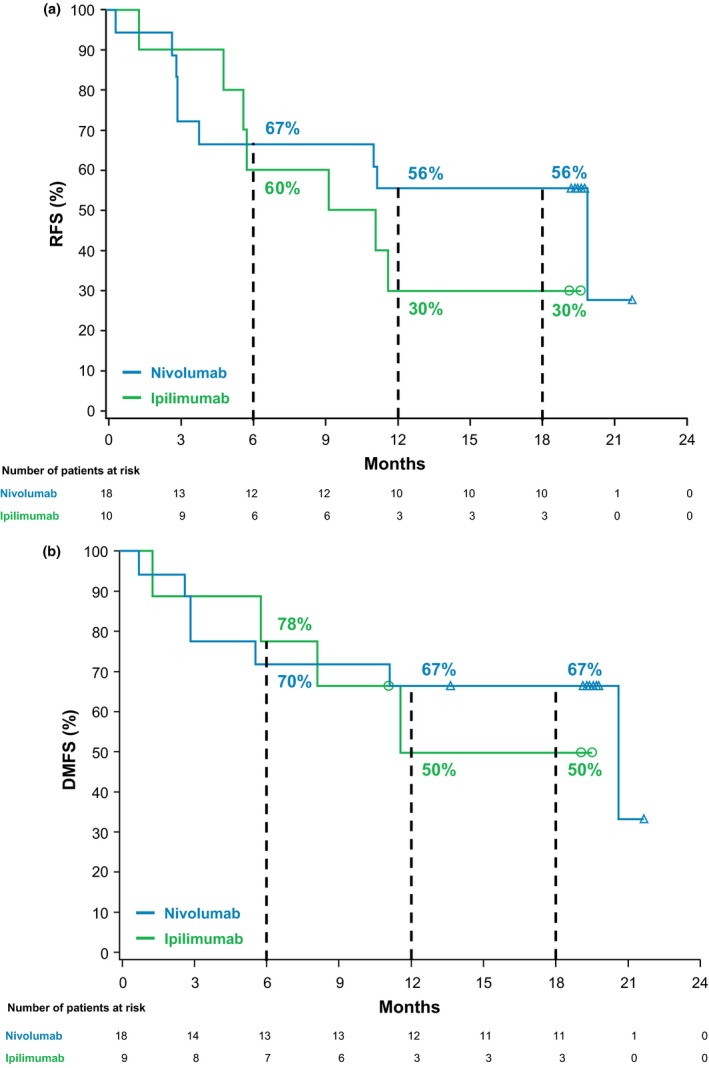
(a) Kaplan–Meier estimates of recurrence‐free survival (RFS) in Japanese patients. Median RFS was 19.8 months (95% confidence interval [CI], 2.8–not estimable [NE]) for nivolumab and 10.1 months (95% CI, 1.2–NE) for ipilimumab (hazard ratio [HR], 0.66; 97.56% CI, 0.19–2.24; P = 0.4390); (b) Kaplan–Meier estimates of distant metastasis‐free survival (DMFS) in Japanese patients with stage III disease. Median DMFS was 20.7 months (95% CI, 5.6–NE) for nivolumab and not reached (95% CI, 1.2–NE) for ipilimumab (HR, 0.65; 95% CI, 0.18–2.32).
Safety
Safety analysis in the Japanese population identified any‐grade treatment‐related AE (TRAE) in 11 (61%) patients in the nivolumab group and in 10 (100%) patients in the ipilimumab group (Table 2). The most common TRAE in the nivolumab group were rash (22%) and diarrhea (17%); these were reported in 60% and 40% of patients in the ipilimumab group, respectively. No patient in the nivolumab group reported a TRAE leading to discontinuation; in the ipilimumab group, three (30%) and two (20%) patients reported any‐grade and grade 3–4 TRAE, respectively, that led to discontinuation. Although two treatment‐related deaths were reported in the global population,4 no treatment‐related death occurred in the Japanese subgroup. Select TRAE (those AE designated by the investigator as treatment‐related and having an immunological etiology) can be found in Table S2.
Table 2.
Safety summary in Japanese patients (any‐grade AE reported in ≥10% of patients)
| AE, n (%) | Nivolumab (n = 18) | Ipilimumab (n = 10) | ||
|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Any AE | 15 (83) | 0 | 10 (100) | 5 (50) |
| Treatment‐related AE | 11 (61) | 0 | 10 (100) | 4 (40) |
| Rash | 4 (22) | 0 | 6 (60) | 0 |
| Diarrhea | 3 (17) | 0 | 4 (40) | 0 |
| Eczema | 2 (11) | 0 | 0 | 0 |
| Hyperthyroidism | 2 (11) | 0 | 1 (10) | 0 |
| Increased amylase | 2 (11) | 0 | 0 | 0 |
| Blood TSH decrease | 1 (6) | 0 | 2 (20) | 0 |
| Fatigue | 1 (6) | 0 | 2 (20) | 0 |
| Hypothyroidism | 1 (6) | 0 | 1 (10) | 0 |
| Myalgia | 1 (6) | 0 | 2 (20) | 0 |
| Pruritus | 1 (6) | 0 | 3 (30) | 0 |
| Pyrexia | 1 (6) | 0 | 2 (20) | 0 |
| Abnormal ECG | 0 | 0 | 1 (10) | 0 |
| Abnormal hepatic function | 0 | 0 | 2 (20) | 2 (20) |
| Adrenal insufficiency | 0 | 0 | 1 (10) | 1 (10) |
| Alopecia | 0 | 0 | 2 (20) | 0 |
| Anemia | 0 | 0 | 1 (10) | 0 |
| Arthralgia | 0 | 0 | 1 (10) | 0 |
| Dysgeusia | 0 | 0 | 2 (20) | 0 |
| Erythema | 0 | 0 | 1 (10) | 0 |
| Headache | 0 | 0 | 1 (10) | 0 |
| Hypophysitis | 0 | 0 | 2 (20) | 1 (10) |
| Increased ALT | 0 | 0 | 6 (60) | 1 (10) |
| Increased AST | 0 | 0 | 5 (50) | 0 |
| Increased GGT | 0 | 0 | 2 (20) | 0 |
| Insomnia | 0 | 0 | 1 (10) | 0 |
| Irregular menstruation | 0 | 0 | 1 (10) | 0 |
| Malaise | 0 | 0 | 1 (10) | 0 |
| Nausea | 0 | 0 | 1 (10) | 0 |
| Pharyngitis | 0 | 0 | 1 (10) | 0 |
| Sinobronchitis | 0 | 0 | 1 (10) | 0 |
| Soft feces | 0 | 0 | 1 (10) | 0 |
| Thyroiditis | 0 | 0 | 1 (10) | 0 |
| Any AE leading to discontinuation | 0 | 0 | 3 (30) | 2 (20) |
| Treatment‐related AE leading to discontinuation | 0 | 0 | 3 (30) | 2 (20) |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECG, electrocardiogram; GGT, γ‐glutamyltransferase; n, number of patients; TSH, thyroid‐stimulating hormone.
HRQoL
Global quality of life measures, including the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐Core 30 (QLQ‐C30), European Quality of Life‐5 Dimensions (EQ‐5D) summary index, and EQ‐5D visual analog scale (VAS), were assessed in the Japanese subpopulation during treatment and at follow‐up (Fig. S4). Although these data must be interpreted carefully due to the limited number of patients at risk, EORTC QLQ‐C30, EQ‐5D utility index and EQ‐5D VAS scores for both nivolumab and ipilimumab generally remained within the minimal important difference (MID) at all time points, except for ipilimumab at 49 weeks when the patient numbers were very low. Similarly, work impairment was relatively stable for both treatment groups, with an increase in overall work impairment at weeks 37–49 for patients treated with ipilimumab (Fig. S5). Scores for patients treated with ipilimumab extended below the MID for three of the four Work Productivity and Activity Impairment Questionnaire: General Health assessments.
Discussion
In the current report, descriptive analyses of the Japanese subpopulation of CheckMate 238 showed that nivolumab resulted in longer RFS and DMFS than ipilimumab. Among Japanese patients, 12‐month RFS rates were 56% and 30% and 12‐month DMFS rates were 67% and 50% with nivolumab and ipilimumab, respectively. Median RFS was 19.8 months (95% CI, 2.8–not estimable) for nivolumab and 10.1 months (95% CI, 1.2–not estimable) for ipilimumab; however, low patient numbers might have rendered the median estimates unreliable. Nivolumab was better tolerated than ipilimumab, with a lower rate of TRAE. Additionally, in the Japanese subgroup, no new safety signals and no treatment‐related deaths were reported in either treatment group. Quality of life remained close to baseline without any clinically meaningful changes for either treatment group based on EORTC QLQ‐C30 Global Health Status, EQ‐5D utility index and EQ‐5D VAS scores. Although the patient numbers were low, a decrease in overall work impairment was observed with ipilimumab treatment at weeks 37–49.
A specific limitation of this Japanese subgroup analysis was the small number of patients, which limited statistical analysis of the data and resulted in the descriptive analyses of RFS, DMFS and HRQoL outcomes. In addition, the type of melanoma that predominates in different regions may confound the implementation of these study results. Although not reflected in the baseline characteristic results in this study, melanoma presents differently in Asian patients compared with Caucasian patients because acral and mucosal subtypes are more predominant than cutaneous in Asian patients.6 Analysis of melanoma subtypes in the overall population demonstrated that nivolumab could be less effective in patients with acral or mucosal melanoma than in those with cutaneous melanoma,4 suggesting that data from the Japanese subpopulation be interpreted carefully, taking global data into consideration. Further research in Asian populations should be considered.
In conclusion, efficacy and safety results from this subgroup analysis of CheckMate 238 indicate that nivolumab has the potential to be a treatment option for Japanese patients with resected melanoma who are at high risk of recurrence. These results support the expanded use of nivolumab for the adjuvant treatment of melanoma in Japan.
Conflict of Interest
K. Y. has received grant support from Bristol‐Myers Squibb and Merck Sharp & Dohme. H. U. has received grant support and honoraria from ONO, Novartis, Kyowa Hakko Kirin, Merck Sharp & Dohme and Taiho; and honoraria or other personal fees from Chugai and Bristol‐Myers Squibb. S. Y. has received grant support from ONO, Bristol‐Myers Squibb and Novartis. K. O. has received grant support from Bristol‐Myers Squibb, ONO, Novartis, PPD‐SNBL and Public Health Research Foundation. J. W. has received honoraria from Bristol‐Myers Squibb, Merck, Genentech, AstraZeneca, Novartis and EMD Serono; and holds a patent on Biodesix for a PD‐1 biomarker. N. Y. has received grant support and honoraria from Bristol‐Myers Squibb, ONO, Merck Sharp & Dohme and Novartis. A. Q., V. d. P. and Y. O. are employees of Bristol‐Myers Squibb, and V. d. P. is a shareholder for Bristol‐Myers Squibb. T. T., T. I., H. U., H. I. and Y. F. do not have any conflicts to declare.
Supporting information
Table S1. Post‐protocol treatment in Japanese patients
Table S2. Any treatment‐related select adverse events† in Japanese patients
Figure S1. Study design.
Figure S2. Consolidated Standards of Reporting Trials flow diagram.
Figure S3. Duration of therapy.
Figure S4. Global quality of life.
Figure S5. Work productivity and activity impairment in Japanese patients.
Acknowledgments
This study was supported by Bristol‐Myers Squibb and ONO Pharmaceutical Company Ltd. We thank the patients and families who made this trial possible and the clinical study teams who participated in the CheckMate 238 trial. We acknowledge ONO Pharmaceutical (Osaka, Japan) for contributions to nivolumab development and Dako, an Agilent Technologies company, for collaborative development of the PD‐L1 IHC 28‐8 pharmDx assay. Professional medical writing and editorial assistance was provided by Melissa Kirk, Ph.D., and Michele Salernitano of StemScientific, an Ashfield Company, funded by Bristol‐Myers Squibb.
References
- 1. Eggermont AM, Chiarion‐Sileni V, Grob JJ et al Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): a randomised, double‐blind, phase 3 trial. Lancet Oncol 2015; 16: 522–530. [DOI] [PubMed] [Google Scholar]
- 2. Eggermont AM, Chiarion‐Sileni V, Grob JJ et al Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 2016; 375: 1845–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibney GT, Kudchadkar RR, DeConti RC et al Safety, correlative markers, and clinical results of adjuvant nivolumab in combination with vaccine in resected high‐risk metastatic melanoma. Clin Cancer Res 2015; 21: 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weber J, Mandala M, Del Vecchio M et al Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 2017; 377: 1824–1835. [DOI] [PubMed] [Google Scholar]
- 5. Balch CM, Gershenwald JE, Soong S‐J et al Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27: 6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chang JW, Guo J, Hung C‐Y et al Sunrise in melanoma management: time to focus on melanoma burden in Asia. Asia Pac J Clin Oncol 2017; 13: 423–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Post‐protocol treatment in Japanese patients
Table S2. Any treatment‐related select adverse events† in Japanese patients
Figure S1. Study design.
Figure S2. Consolidated Standards of Reporting Trials flow diagram.
Figure S3. Duration of therapy.
Figure S4. Global quality of life.
Figure S5. Work productivity and activity impairment in Japanese patients.


