Summary
Background
Consensus guidelines advocate general skincare for rosacea patients.
Objectives
Two independent studies were performed to assess whether a tinted daily SPF‐30 facial moisturizer (DFM30) improves barrier function of dry skin and the efficacy and tolerability of DFM30 on rosacea‐prone skin.
Methods
In study 1, electrical capacitance (EC) and transepidermal water loss (TEWL) were measured at baseline, 2, 4, 8, and 24 hours after a single application of DFM30 and on a control site in 21 healthy females with dry skin. Study 2 evaluated 33 females with mild to moderate rosacea and nontransient erythema. Efficacy and tolerability after once‐daily DFM30 were assessed using a chromameter, image analysis of photographs, and trained rater and patient evaluations up to day 22.
Results
In study 1, EC showed statistically significant increases at 2, 4, and 8 hours, and TEWL showed statistically significant decreases 2, 4, 8, and 24 hours after DFM30 application to healthy females compared to baseline. In study 2, covering skin redness improved significantly after DFM30 application on day 1; 33.3% showed improved covering skin redness compared to baseline. Patients reported significantly less redness on day 8 than day 3. Feelings of dryness and tightness/tension were lower 30 minutes after first application. Feeling of dryness was lower than baseline after 3 days, 1 and 3 weeks. Image analysis suggested redness was significantly lower on day 22 compared to baseline. Chromameter readings showed significantly lower erythema on the cheek compared to baseline. All patients stated that DFM30 relieves and neutralizes visible redness who also indicated that they would purchase DFM30, and the product was well tolerated.
Conclusions
These studies show that DFM30 is suitable as part of the skincare regimens advocated by ROSacea COnsensus (ROSCO) for rosacea patients. DFM30 is an effective moisturizer that improves cutaneous barrier function and the appearance of rosacea‐prone skin.
Keywords: barrier function, moisturizer, rosacea, sun protection factor
1. INTRODUCTION
Approximately 5%‐10% of the population suffers from rosacea, a chronic inflammatory skin disorder.1, 2 The chronic inflammation that underlies rosacea can result in a variety of signs and symptoms that include flushing, telangiectasia, inflammatory lesions, and ocular manifestations.3 Two features, however, seem to be diagnostic of rosacea: firstly, persistent erythema that affects the central face and that shows periodic intensification and, secondly, phyma, especially affecting the nose.3 As rosacea affects facial appearance, the condition can have a marked psychological impact and undermine health‐related quality of life.4, 5, 6, 7
Against this background, the global ROSacea COnsensus (ROSCO) panel recommended tailoring treatment according to the phenotype (eg transient and persistent erythema, inflammatory papules and pustules, telangiectasia and phyma). In addition, all rosacea patients should be educated on good general skincare, which is the main strategy to manage secondary features, such as dry appearance, dry sensation, and stinging sensation.8
The ROSCO guidelines stress that skincare regimens should include using sunscreen (sun protection factor [SPF] ≥30).8 Between 61% and 81% of patients with rosacea cite sun exposure as a contributory factor.4, 9 Skincare suggested by ROSCO include gentle cleansers, avoidance of triggers, and frequent application of quality moisturizers.8 Rosacea patients often experience dry facial skin that can exacerbate symptoms.10 Moisturizers can repair and maintain stratum corneal barrier function, enhance skin hydration, and reduce the likelihood of skin irritation. As a result, moisturizers relieve dry skin, improve softness and suppleness, and can be adjuvants to other rosacea therapies.10
This paper reports the results of two independent studies into the use of a novel tinted daily SPF‐30 facial moisturizer (DFM30), designed according to the new ROSCO guidelines, as part of the skincare regimen for rosacea patients. One study aimed to determine whether DFM30 improves barrier function in otherwise healthy women with dry skin. The other study assessed the efficacy and tolerability of a tinted daily facial moisturizer with an SPF of 30 (DFM30) in patients presenting with rosacea‐prone skin.
2. MATERIALS AND METHODS
Two independent studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, and local regulatory requirements.
The first study was performed in conformity with the standard Operating Procedures of Institut d’ Expertise Clinique. The second study adhered to the protocol and compliance with the quality system of ProDerm. Audits were performed at regular intervals to verify adherence to both study protocols.
In both studies, room humidity and temperatures were maintained within published guidelines11 and patients or healthy women rested in the room for at least 30 minutes before measurements. Safety was monitored by reporting of adverse events.
2.1. Study A (cutaneous barrier function)
The first study (performed at IEC France, Lyon) enrolled 21 healthy Caucasian females (age 18‐70 years) with dry skin on their inner forearm and an initial moisturization level that corresponded to electrical capacitance values of ≤50 arbitrary units.
Patients with rosacea often present with a defective skin barrier and therefore an increased transepidermal water loss (TEWL).12, 13 Healthy subjects with dry skin were used in order to “mimic” the damaged barrier and increased TEWL that is found in patients with rosacea.12, 13
Dryness was defined with an objective dermatological assessment of dryness as well as subjective reports from the participants. TEWL was measured using a Tewameter TM 300 (Courage & Khazaka) and electrical capacitance using a Corneometer CM 825 (Courage & Khazaka). Values for electrical capacitance are given in arbitrary units (scale from 0 to about 130), which reflects the degree of moisturization in the upper layers of the epidermis.
The test and control areas were 20 cm2 areas of dry skin on the inner forearm. A technician applied DFM30 (2 mg cm−2) to the test area on the right or left forearm according to randomization and massaged until the product penetrated completely. Electrical capacitance and TEWL were measured at baseline and 2, 4, 8, and 24 hours after a single application of DFM30.
Descriptive statistics summarized the results, and differences were assessed using the Shapiro‐Wilk test (significance defined as P < 0.01). Treated and control areas were compared using the Student t test or Wilcoxon test (both two‐tail, significance defined as P < 0.05) for normal and nonparametric distributions, respectively.
2.2. Study B (efficacy and tolerability)
The second study (performed at the proDERM Institute for Applied Dermatological Research GmbH, Schenefeld/Hamburg) planned to enrol 33 female or male patients with type I rosacea presenting with mild to moderate nontransient erythema. The study aimed to enrol at least 50% of patients with self‐reported sensitive skin. Eligible patients were between 25 and 75 years of age, with a maximum of 20% of the cohort being over 60 years. The study aimed to enrol approximately 25% of patients with Fitzpatrick type I skin, approximately 50% of patients with Fitzpatrick type II, and approximately 25% of patients with Fitzpatrick type III.
Chromameter (CR 300 or CR 400; Minolta, Device D‐Langenhagen, Germany) measurements were performed on the right cheek and on unaffected forehead, and a full‐face image was taken using VISIA‐CR BOOTH (Canfield Clinical Systems, Fairfield, NJ), which offers standardized, computer‐controlled facial photography. Image analysis of skin color (a*‐value, which corresponds to an increase in the degree of skin redness) was performed on the images using cross‐polarized light.
A trained technician ensured that patients applied DFM30 correctly and assessed tolerability (scaling, fissures, papules, pustules, edema, vesicles, weeping). Patients rated their agreement with statements about efficacy and traits immediately after application. Statements are shown in the results section. Redness, subjective efficacy (tension/tightness, feeling of dryness), subjective tolerability (itching, burning, tickling), and subjective eye status (itching, burning, sand grain feeling) were assessed, and the full‐face images were repeated 30 ± 5 minutes after DFM30 application.
Patients applied DFM30 once daily in the morning at home, according to normal use‐conditions. Patients returned to the study site on days 3, 8, and 22, when chromameter measurements were performed. A deviation of ±2 days on day 22 was permitted. Dryness was assessed by the technician on days 8 and 22. Skin redness was assessed by a trained rater and by the patients on days 3, 8, and 22. Patients assessed subjective efficacy on these days. Subjective tolerability, technician‐evaluated tolerability, and eye status were assessed on day 22. In addition, on day 22, a full‐face image was taken and patients rated their agreement with statements about product efficacy and traits.
Trained raters and patients assessed skin redness or covering of skin redness using a five‐point scale ranging from “None” to “Strong.” Raters assessed skin dryness and objective skin status (scaling, fissures, papules, pustules, edema, vesicles, weeping) using this five‐point scale. Patients used the same five‐point scale to evaluate subjective efficacy, tolerability, and eye status, and rated their agreement with statements about product efficacy and traits using a five‐point scale ranging from “Fully agree” to “Fully disagree.”
Statistical significance was defined as P < 0.05. Rater‐assessed outcomes were compared using Wilcoxon signed‐ranks test. McNemar's test was used for worsened and improved assessments. The Binomial test was used for frequencies of answers between the “agree” and “disagree” groups. Instrumental measurement parameters were compared using a paired t test.
3. RESULTS
3.1. Study A (cutaneous barrier function)
Thirty healthy females with dry skin on their inner arm were screened, of which 21 were eligible (mean age 46.0; range 20‐68 years). All those enrolled completed the study.
Figure 1 and Table 1 summarize the results. There was no statistically significant difference in electrical capacitance or TEWL between control and treated areas at baseline. Electrical capacitance showed statistically significant increases 2, 4, 8, and 24 hours after DFM30 application compared to baseline. TEWL showed statistically significant decreases 2, 4, 8, and 24 hours after DFM30 application compared to baseline.
Figure 1.
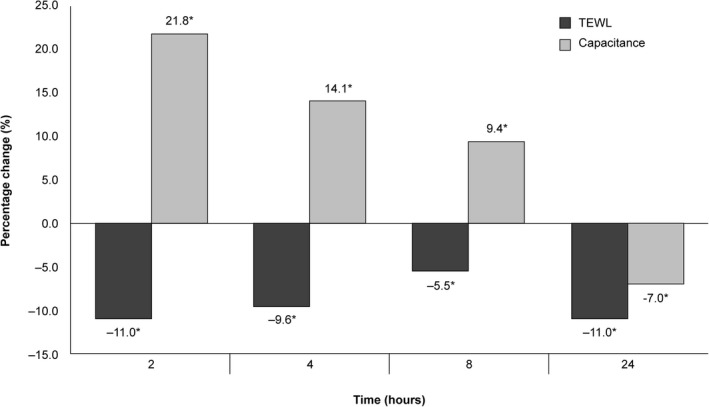
Variation in TEWL and electrical capacitance following a single application of DFM30. * indicates a statistically significant difference. Percentages indicate the change in electrical capacitance and therefore moisturization level. DFM30, daily SPF‐30 facial moisturizer; TEWL, transepidermal water loss
Table 1.
TEWL and electrical capacitance following a single application of DFM30
| Mean ± SD | P value | ||
|---|---|---|---|
| Control area | DFM30 area | ||
| TEWL (g m−2 h−1) | |||
| Initial measurement (T0) | 7.3 ± 1.4 | 7.3 ± 1.3 | 0.928 |
| 2 h | 7.1 ± 1.5 | 6.3 ± 1.4 | — |
| 4 h | 7.3 ± 1.5 | 6.6 ± 1.4 | — |
| 8 h | 7.3 ± 1.4 | 6.9 ± 1.4 | — |
| 24 h | 7.3 ± 1.4 | 6.5 ± 1.3 | — |
| Difference between 2 h and T0 | −0.2 ± 0.7 | −1.0 ± 1.0 | 0.03 |
| Difference between 4 h and T0 | 0.0 ± 0.7 | −0.7 ± 1.0 | 0.001 |
| Difference between 8 h and T0 | 0.0 ± 0.6 | −0.4 ± 0.9 | 0.042 |
| Difference between 24 h and T0 | 0.0 ± 0.7 | −0.8 ± 0.8 | 0.003 |
| Electrical capacitance (arbitrary units) | |||
| Initial measurement (T0) | 30.0 ± 7.2 | 29.8 ± 6.5 | 0.706 |
| 2 h | 29.9 ± 6.7 | 36.2 ± 6.5 | — |
| 4 h | 29.8 ± 6.5 | 33.8 ± 5.9 | — |
| 8 h | 30.1 ± 7.2 | 32.7 ± 5.3 | — |
| 24 h | 31.4 ± 7.1 | 29.1 ± 5.4 | — |
| Difference between 2 h and T0 | −0.1 ± 2.6 | 6.4 ± 3.3 | <0.001 |
| Difference between 4 h and T0 | −0.3 ± 2.3 | 4.0 ± 4.0 | <0.001 |
| Difference between 8 h and T0 | 0.0 ± 2.5 | 2.9 ± 3.6 | 0.002 |
| Difference between 24 h and T0 | 1.3 ± 2.6 | −0.7 ± 3.6 | 0.01 |
DFM30, daily SPF‐30 facial moisturizer; TEWL, transepidermal water loss.
3.2. Study B (efficacy and tolerability)
Thirty‐three females (mean age 53.7 ± 10.4 years) were enrolled of which three did not complete the study for reasons that were not associated with DFM30. Thirty patients self‐reported having sensitive skin.
No statistically significant differences were found for skin redness (Table 2) assessed by a trained rater at any time (ie baseline vs day 3, 8 or 22; day 3 vs day 8 or 22; day 8 vs day 22). However, covering skin redness assessed by a trained rater improved significantly 30 minutes after application on day 1 (P = 0.004) compared to baseline. A third (33.3%) of patients showed an improvement in covering skin redness (P = 0.002) compared to baseline.
Table 2.
Efficacy of DFM30
| Time | None (%) | Very slight (%) | Slight (%) | Moderate (%) | Strong (%) |
|---|---|---|---|---|---|
| Skin redness assessed by trained rater | |||||
| Baseline | 0.0 | 23.3 | 46.7 | 30.0 | 0.0 |
| Day 3 | 0.0 | 16.7 | 60.0 | 23.3 | 0.0 |
| Day 8 | 0.0 | 16.7 | 56.7 | 26.7 | 0.0 |
| Day 22 | 0.0 | 20.0 | 43.3 | 33.3 | 3.3 |
| Covering skin redness assessed by trained rater | |||||
| Baseline | 0.0 | 23.3 | 46.7 | 30.0 | 0.0 |
| Day 1 30 min PA | 0.0 | 56.7 | 33.3 | 10.0 | 0.0 |
| Skin redness assessed by patient | |||||
| Baseline | 0.0 | 3.3 | 50.0 | 43.3 | 3.3 |
| Day 3 | 0.0 | 10.0 | 33.3 | 46.7 | 10.0 |
| Day 8 | 0.0 | 13.3 | 50.0 | 36.7 | 0.0 |
| Day 22 | 0.0 | 23.3 | 33.3 | 36.7 | 6.7 |
| Covering skin redness assessed by patient | |||||
| Baseline | 0.0 | 3.3 | 50.0 | 43.3 | 3.3 |
| Day 1 30 min PA | 0.0 | 36.7 | 30.0 | 33.3 | 0.0 |
DFM30, daily SPF‐30 facial moisturizer; PA, postapplication.
No statistically significant differences were found for skin redness (Table 2) assessed by the patient at most times (baseline vs day 3, 8 or 22; day 3 vs day 22; day 8 vs day 22). However, rosacea patients reported significantly less redness on day 8 compared to day 3 (P = 0.016). Patients stated that covering skin redness improved significantly 30 minutes after application on day 1 (P = 0.006) compared to baseline. A third (33.3%) of rosacea patients reported an improvement in covering skin redness (P = 0.002) compared to baseline. The feeling of dryness and tightness/tension was lower 30 minutes after the first application of DFM30. After 3 days, as well as after 1 and 3 weeks use, the feeling of dryness was lower than at baseline. The feeling of tightness/tension remained comparable to baseline throughout the study, although decreased at day 1 after 30 minutes.
Image analysis suggested that skin redness (Table 3) was significantly lower on day 22 compared to baseline on the unaffected forehead. No other significant differences were found.
Table 3.
Skin redness assessed by image analysis and chromameter
| Test area | Time | Mean value | P value |
|---|---|---|---|
| Skin redness assessed by image analysis (a*‐value) | |||
| Cheek | Baseline | 21.804 | 0.889 |
| Day 22 | 21.858 | ||
| Forehead | Baseline | 16.713 | 0.030 |
| Day 22 | 16.154 | ||
| Difference between check and forehead | Baseline | 5.091 | 0.064 |
| Day 22 | 5.704 | ||
| Covering skin redness assessed by image analysis (a*‐value) | |||
| Cheek | Baseline | 21.804 | 0.431 |
| Day 1 30 min PA | 21.967 | ||
| Erythema measured by chromameter (a*‐value) | Versus baseline | Versus day 3 | Versus day 8 | |
|---|---|---|---|---|
| Cheek | ||||
| Baseline | 20.957 | — | — | — |
| Day 3 | 19.168 | <0.001 | — | — |
| Day 8 | 19.857 | 0.002 | 0.003 | — |
| Day 22 | 19.766 | <0.001 | 0.021 | 0.726 |
| Forehead | ||||
| Baseline | 16.333 | — | — | — |
| Day 3 | 16.279 | 0.851 | — | — |
| Day 8 | 16.087 | 0.327 | 0.347 | — |
| Day 22 | 15.899 | 0.198 | 0.066 | 0.416 |
| Difference between check and forehead | ||||
| Baseline | 4.624 | — | — | — |
| Day 3 | 2.889 | <0.001 | — | — |
| Day 8 | 3.771 | 0.016 | 0.007 | — |
| Day 22 | 3.867 | 0.078 | 0.007 | 0.786 |
PA, postapplication.
Chromameter readings showed significantly lower erythema at each measurement time on the cheek compared to baseline. Significantly higher a*‐values were recorded on days 8 and 22 compared to day 3 on the cheek. No significant differences were apparent on the forehead. The difference between the cheek and forehead was significantly lower compared to baseline on days 3 and 8. Significantly higher a*‐values for the difference between the cheek and forehead were recorded on days 8 and 22 compared to day 3.
After the first application of the DFM30 and on day 22, significantly more patients agreed with all statements regarding its product traits and efficacy than disagreed (Table 4). After 3 weeks, the statements that the DFM30 neutralizes the look of redness and relieves visible redness obtained highest agreement.
Table 4.
Assessment of traits and efficacy by patients
| Statement | Disagree (%) | Undecided (%) | Agree (%) | Relative frequency (%) | ||
|---|---|---|---|---|---|---|
| Disagree | Agree | P value | ||||
| Day 1 directly after application | ||||||
| The product has a soothing effect | 6.7 | 16.7 | 76.7 | 8.0 | 92.0 | <0.001 |
| The product leaves my skin soft and smooth | 0.0 | 13.3 | 86.7 | 0.0 | 100.0 | <0.001 |
| The product visibly improves my skin tone | 3.3 | 10.0 | 86.7 | 3.7 | 96.3 | <0.001 |
| The product reduces the feeling of discomfort | 6.7 | 30.0 | 63.3 | 9.5 | 90.5 | <0.001 |
| The product helps conceal skin redness immediately | 6.7 | 6.7 | 86.7 | 7.1 | 92.9 | <0.001 |
| The product can be used as an alternative to makeup due to the tinted coverage | 10.0 | 0.0 | 90.0 | 10.0 | 90.0 | <0.001 |
| The product is easy to apply | 3.3 | 0.0 | 96.7 | 3.3 | 96.7 | <0.001 |
| The product has a fast absorption but coverage remains | 3.3 | 0.0 | 96.7 | 3.3 | 96.7 | <0.001 |
| After product application, I immediately feel more confident due to my redness being less evident | 10.0 | 30.0 | 60.0 | 14.3 | 85.7 | 0.001 |
| Day 22 | ||||||
| The product has a soothing effect | 13.3 | 23.3 | 63.3 | 17.4 | 82.6 | 0.003 |
| The product leaves my skin soft and smooth | 16.7 | 23.3 | 60.0 | 21.7 | 78.3 | 0.011 |
| The product visibly improves my skin tone | 10.0 | 20.0 | 70.0 | 12.5 | 87.5 | <0.001 |
| The product neutralises the look of redness | 3.3 | 0.0 | 96.7 | 3.3 | 96.7 | <0.001 |
| The product relieves visible redness | 3.3 | 0.0 | 96.7 | 3.3 | 96.7 | <0.001 |
| The product reduces the feeling of discomfort | 23.3 | 13.3 | 63.3 | 26.9 | 73.1 | 0.029 |
| The product is suitable for sensitive skin | 6.7 | 20.0 | 73.3 | 8.3 | 91.7 | <0.001 |
| The product can be used as an alternative to makeup due to the tinted coverage | 13.3 | 3.3 | 83.3 | 13.8 | 86.2 | <0.001 |
| The product was easy to incorporate into my daily skin care regimen | 6.7 | 3.3 | 90.0 | 6.9 | 93.1 | <0.001 |
| The product improves skin texture | 16.7 | 30.0 | 53.3 | 23.8 | 76.2 | 0.027 |
| The product is easy to apply | 6.7 | 3.3 | 90.0 | 6.9 | 93.1 | <0.001 |
| When I apply the product, I feel that my skin is nourished | 20.0 | 3.3 | 76.7 | 20.7 | 79.3 | 0.002 |
| When I apply the product, I feel more confident in my skin tone | 10.0 | 13.3 | 76.7 | 11.5 | 88.5 | <0.001 |
| Overall, I like the product very much | 23.3 | 6.7 | 70.0 | 25.0 | 75.0 | 0.013 |
| Would you like to buy this product? | 0.0 | 0.0 | 100.0 | 0.0 | 100.0 | <0.001 |
Only single cases of itching, burning, and tickling were diagnosed by the trained rater throughout the study. This included 1 case of itching on day 1, and 1 case of burning and 1 case of tickling on day 22. Patients reported only isolated cases of “sand grain feeling” and no itching and burning. One patient developed a small exfoliative, dry area on her neck. Raters assessed the severity as mild and that there was a reasonable possibility that the event was related to DFM30. No action was taken and the patient recovered without treatment or sequelae.
4. DISCUSSION
The ROSCO guidelines note that education and instruction about general skin care is “essential” for all patients with rosacea to ensure the best possible treatment outcomes. Elements in skincare include sunscreen, frequent use of moisturizers and gentle over‐the‐counter cleansers, and avoiding known triggers. Indeed, general skincare is the main management strategy for the secondary features of rosacea.8 The two studies presented in this paper show that the DFM30 is suitable as part of the skincare regimens advocated by ROSCO for rosacea patients, enhances cutaneous barrier function, and improves the visible appearance of rosacea‐prone skin.
The first study enrolled healthy females presenting with dry skin on forearms. A single application of DFM30 produced statistically significant moisturization of the upper layers of the epidermis that persisted for 8 hours after the application. DFM30 also led to a statistically significant decrease in TEWL about 24 hours later, which is consistent with enhanced barrier function. These findings in otherwise healthy women were confirmed by clinical observations of patients with rosacea. Raters noted a reduction in dryness. Patients reported that the feeling of dryness and tightness/tension was noticeably lower 30 minutes after the first application. After 3, 8, and 22 days, the feeling of dryness was lower than baseline. Improved dryness and scaling is consistent with increased hydration of the stratum corneum.10 Subjects with dry skin were used in this study as patients with rosacea often present with a defective skin barrier and therefore an increased TEWL. Accordingly, subjects with dry skin on the forearms were used to replicate the damaged barrier and increased TEWL that is found in such patients.12, 13
These enhancements in skin function seem to translate into improved outcomes in patients with type I rosacea and mild to moderate nontransient erythema. DFM30 is tinted, and 30 minutes after the first application, assessments by the patient and the rater showed a camouflage effect, which was confirmed by chromameter measurements on the cheek. Image analysis, however, did not confirm a reduction in visible redness. The reasons for the discordance between the assessment methods are unclear but may be related to interference by the pigment included in the product with the measurement system.
Subjective assessment showed no reduction of skin redness after 1 and 3 weeks of application. This suggests that camouflage associated with the tinted formulation was responsible for the anti‐redness effect. However, rosacea patients reported significantly less redness on day 8 compared to day 3. Image analysis showed a significantly reduced skin redness on day 22 compared to baseline on the nonaffected forehead. As this was an exploratory study, the statistical analysis did not account for multiple observations and these may be type 1 errors.
The chromameter measurements on the cheek indicated improved erythema at all times compared to baseline. After correcting using data obtained from the control area (forehead), the results were confirmed. However, the effect on day 22 just missed significance (P = 0.078), suggesting a larger study may be warranted. Therefore, the study could be repeated in a larger number of patients using statistical analysis of multiple observations.
Patients showed a strong agreement with statements about favorable traits and attributes, suggesting that DFM30 would be an effective adjuvant to treatment for rosacea. Indeed, all the patients stated that they would like to buy DFM30. A well‐accepted product is likely to help ensure good adherence, especially during long‐term use, although further studies with longer follow‐up are needed to confirm this hypothesis.
Many patients with rosacea report heightened skin sensitivity with skincare and personal hygiene products14 and dry facial skin can exacerbate symptoms.10 Despite enrolling patients with skin sensitivity, the overall tolerability of DFM30 was very good, which should help ensure good adherence.
While these results show that DFM30 is effective and well tolerated, the studies had certain limitations and raised further suggestions for additional investigations. As mentioned above, the study could be repeated in a larger number of patients using statistical analysis of multiple observations. Each of the studies was performed at one center. They could be replicated in a larger, more diverse sample including men, more diverse skin types and the different phenotypes of rosacea. The benefit against UV/sun exposure could also be assessed in a “naturalistic” study, conducted in a natural setting, and the TEWL and skin conductance study replicated on patients with rosacea.
5. CONCLUSIONS
The two studies in this paper show that DFM30 is suitable as part of the skincare regimens advocated by ROSCO for patients with rosacea. In healthy females presenting with dry skin on their forearms, a single application of DFM30 produced a statistically and clear moisturization effect that persisted for 8 hours. DFM30 also decreased in TEWL about 24 hours later, suggesting strengthening of barrier function. This finding was confirmed by clinical observation that DFM30 improved dryness in the rosacea patients.
These improvements in skin function seem to translate into improved outcomes in patients with type I rosacea and mild to moderate nontransient erythema. DFM30 is tinted, and 30 minutes after the first application, assessments by the rosacea patients and the rater showed a camouflage effect. Patients agree that the product's traits and attributes could be appropriate for a skincare product for rosacea. All rosacea patients stated that they would like to purchase the product. Taken together, these results show that DFM30 is an effective and well‐tolerated moisturizer that improves cutaneous barrier function and the visible appearance of rosacea‐prone skin.
CONFLICTS OF INTEREST
NL and FS are employees of Galderma R&D Egerkingen, Switzerland. HB serves as an advisor, investigator or on the speaker's bureau for Galderma, Ortho Dermatologic, Bayer, L'Oreal and La Roche‐Posay. ST is an employee of Galderma Research & Development, Sophia Antipolis, France.
ACKNOWLEDGMENTS
Medical writing and editorial services were provided by MedSense Ltd and Mark Greener, funded by Galderma Research and Development.
Baldwin H, Santoro F, Lachmann N, Teissedre S. A novel moisturizer with high sun protection factor improves cutaneous barrier function and the visible appearance of rosacea‐prone skin. J Cosmet Dermatol. 2019;18:1686–1692. 10.1111/jocd.12889
Funding information
This study was supported by Galderma Research and Development.
The copyright line for this article was changed on April 26, 2019, after original online publication
REFERENCES
- 1. Wehausen B, Hill DE, Feldman SR. Most people with psoriasis or rosacea are not being treated: a large population study. Dermatol Online J. 2016;22. [PubMed] [Google Scholar]
- 2. Abokwidir M, Feldman SR. Rosacea management. Skin Appendage Disord. 2016;2:26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan J, Almeida L, Bewley A, et al. Updating the diagnosis, classification and assessment of rosacea: recommendations from the global ROSacea COnsensus (ROSCO) panel. Brit J Dermatol. 2017;176:431‐438. [DOI] [PubMed] [Google Scholar]
- 4. Blount B, Pelletier A. Rosacea: a common, yet commonly overlooked, condition. Am Fam Physician. 2002;66:435‐440. [PubMed] [Google Scholar]
- 5. Moustafa F, Lewallen RS, Feldman SR. The psychological impact of rosacea and the influence of current management options. J Am Acad Dermatol. 2014;71:973‐980. [DOI] [PubMed] [Google Scholar]
- 6. Bewley A, Fowler J, Schöfer H, Kerrouche N, Rives V. Erythema of rosacea impairs health‐related quality of life: results of a meta‐analysis. Dermatol Ther. 2016;6:237‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Linden MM, van Rappard DC, Daams JG, Sprangers M, Spuls P, Korte J. Health‐related quality of life in patients with cutaneous rosacea: a systematic review. Acta Derm Venereol. 2015;95:395‐400. [DOI] [PubMed] [Google Scholar]
- 8. Schaller M, Almeida L, Bewley A, et al. Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Brit J Dermatol. 2017;176:465‐471. [DOI] [PubMed] [Google Scholar]
- 9. Oge LK, Muncie HL, Phillips‐Savoy AR. Rosacea: diagnosis and treatment. Am Fam Physician. 2015;92:187‐196. [PubMed] [Google Scholar]
- 10. Draelos ZD, Ertel K, Berge C. Niacinamide‐containing facial moisturizer improves skin barrier and benefits subjects with rosacea. Cutis. 2005;76:135‐141. [PubMed] [Google Scholar]
- 11. Pinnagoda J, Tupkek RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermatitis. 1990;22:164‐178. [DOI] [PubMed] [Google Scholar]
- 12. James Q, Del Rosso JQ. The use of moisturizers as an integral component of topical therapy for rosacea: clinical results based on the assessment of skin characteristics study. Cutis. 2009;84:72‐76. [PubMed] [Google Scholar]
- 13. Addor F. Skin barrier in rosacea A review. An Bras Dermatol. 2016;91:59‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234‐240. [PubMed] [Google Scholar]


