Summary
In recent years, the number of emergent plant pathogens (EPPs) has grown substantially, threatening agroecosystem stability and native biodiversity. Contributing factors include, among others, shifts in biogeography, with EPP spread facilitated by the global unification of monocultures in modern agriculture, high volumes of trade in plants and plant products and an increase in sexual recombination within pathogen populations. The unpredictable nature of EPPs as they move into new territories is a situation that has led to sudden and widespread epidemics. Understanding the underlying causes of pathogen emergence is key to managing the impact of EPPs. Here, we review some factors specifically influencing the emergence of oomycete and fungal EPPs, including new introductions through anthropogenic movement, natural dispersal and weather events, as well as genetic factors linked to shifts in host range.
Keywords: climate change, disease surveillance, emergent plant pathogens, host shifts, pathogen pollution, plant pathology
Introduction
Emergent plant pathogens (EPPs) are plant pathogens that (1) are causal agents of new diseases, (2) display an unusually increased incidence, (3) are able to infect novel hosts, (4) exhibit geographic expansion, and/or (5) have alterations in pathogenesis (Anderson et al., 2004). EPPs have caused catastrophic epidemics throughout history and continue to threaten modern agriculture and ecosystem stability (Fig. 1). For instance, the Irish potato famine of the 1840s – the first severe epidemic for which an emerging microorganism was shown as the causal agent (Schumann & D'Arcy, 2000) – caused almost a million deaths in Ireland as a result of starvation and triggered mass migration (Turner, 2005); almost two centuries later, owing to a multitude of factors, the Irish population still has not reached pre‐famine numbers (Yoshida et al., 2013). Repeated human introduction of the late blight pathogen Phytophthora infestans into nonnative regions was the major cause of potato late blight epidemics. P. infestans remains the most important single biotic constraint to potato production world‐wide (Kromann et al., 2014) as a result of its high adaptability facilitated by long‐distance dispersal of asexual lineages (Cooke et al., 2012; Yoshida et al., 2013). Another example of the devastating effects of EPPs is brown spot of rice (Oryza sativa), caused by Cochliobolus miyabeanus, which was a significant factor in the deaths of over 2 million people during the Bengal famine of 1943 (Sen, 1981).
Figure 1.
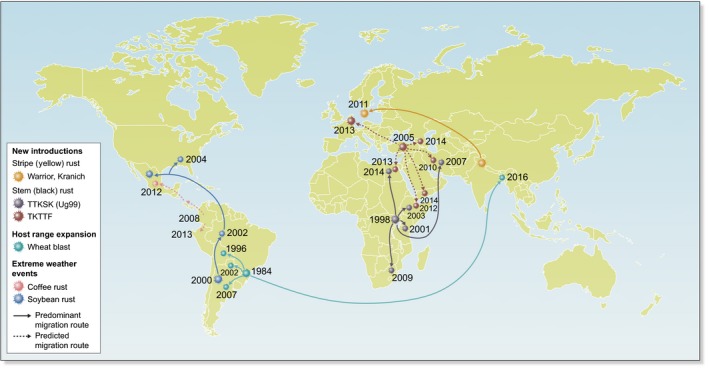
Map illustrating a selection of fungal plant pathogens classified as emergent in recent history. Dates illustrate the first record of a particular pathogen in the given location.
With the advent of modern resistance gene‐based breeding, regulated use of single‐site fungicides, and improved disease management strategies, it is hoped such catastrophic levels of human suffering can be circumvented in future. However, the timely deployment and ultimate success of such control measures are dependent on a clear understanding of the factors leading to plant disease emergence. Herein, we discuss some main driving factors in emergence, such as new introductions and genetic factors underlying shifts in host range. We focus on information gleaned from oomycete and fungal pathogens, which are responsible for over 30% of all emergent plant diseases (Anderson et al., 2004).
Biogeography of EPPs and the impact of long‐distance dispersal
Recent increases in the number of EPPs are largely attributable to alterations in the biogeography of host plants and their pathogens. The spread of plant pathogens in general has been facilitated by high volumes of trade in plants and their products, the global intensification of agriculture with the use of genetically uniform monoculture crops, and perennial crops favouring an increase in sexual recombination in pathogen populations (Anderson et al., 2004; Fisher et al., 2012). When considering fungal pathogens specifically, two main factors drive emergence: new introductions (including pathogen pollution (Cunningham et al., 2003) and natural dispersal) and weather (accounting for c. 40% each; Fig. 2) (Anderson et al., 2004), alongside alterations in agricultural practices.
Figure 2.
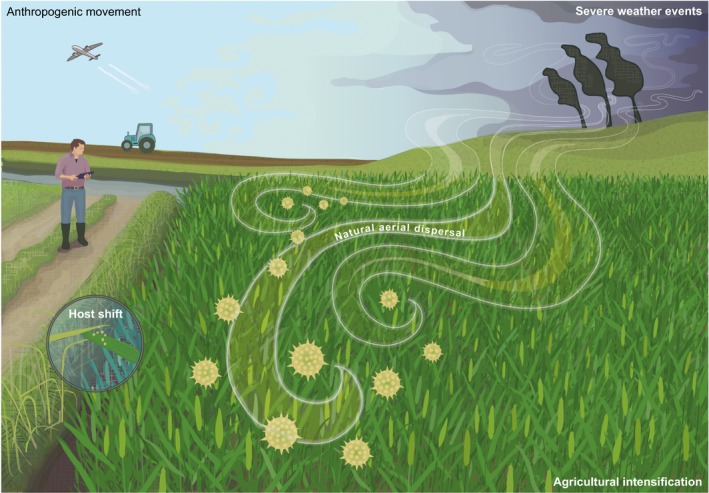
Illustration of the main factors influencing shifts in the biogeography of emergent plant pathogens. The spread of plant pathogens has been facilitated by the global intensification of agriculture, host shifts and new introductions through anthropogenic movement, natural dispersal and severe weather events.
Pathogen pollution
Anthropogenic movement of pathogens is a major cause of EPP introductions. Pathogen pollution, defined as human movement of pathogens outside their natural geographical area or host range (Cunningham et al., 2003), enables pathogens to cross geographical boundaries and infect a new host and/or settle in a new region (Anderson et al., 2004). For example, the disastrous introduction of the Chestnut blight pathogen Cryphonectria parasitica into North America, causing the loss of billions of American chestnut trees, probably occurred via the import of infected saplings from Asia (Dutech et al., 2012).
For crop pathogens, one notable example is Karnal bunt, a fungal disease of wheat (Triticum aestivum), durum, rye and triticale caused by Tilletia indica. The disease was discovered in India in the 1930s and initially restricted to South Asia and Iraq. However, in 1972 it was reported in Mexico, with further isolated outbreaks described in the southwestern United States and South Africa in the late 1990s and early 2000s (Bonde et al., 1997). Karnal bunt is a soil‐, seed‐, and air‐borne disease and is probably transmitted primarily via the import of contaminated seed. Increasing concerns have triggered a ban on grain imports from regions with Karnal bunt, with severe economic consequences for the affected countries (Gewin, 2003).
Single‐step incursions are rare, and it is probable that several external invasions are needed for the spread of EPPs into new locations. However, the unprecedented amounts of anthropogenic movement in recent years have clearly facilitated the spread of EPPs into new territories.
Natural aerial dispersal
Emergent plant pathogens also emerge in new regions via natural dispersal mechanisms. For instance, fungal pathogens that infect above‐ground plant parts frequently disperse via airborne spores, which can assist their entry into new regions. Long‐distance spore dispersal is key for the survival of certain obligate biotrophic fungi, which cannot live on soil or plant debris and must survive in a dormant state or re‐establish the population every season from external sources (Brown & Hovmoller, 2002). Spore dispersal is often essential for re‐establishment, but dispersal is successful only if there is a susceptible host in the target region and favourable environmental conditions. Unfortunately, the reduced genetic diversity of modern crops tends to increase the chances of success of global aerial dispersion of spores (Brown & Hovmoller, 2002) as it only needs one successful incursion to cause widespread damage as a result of crop uniformity.
The recent incursion into western Europe of two races of Puccinia striiformis f. sp. tritici, the causal agent of wheat yellow rust (Hubbard et al., 2015; Hovmøller et al., 2016), was attributed to long‐distance spore dispersal from their origin in sexually recombining populations in Asia (Hovmøller et al., 2016; Fig. 1). These new races, known as ‘Warrior’ and ‘Kranich’, have unprecedented degrees of genetic diversity and are virulent across many European wheat varieties, posing a serious threat to wheat production (Hubbard et al., 2015; Hovmøller et al., 2016). Versatility in disease spread assisted by human movement and/or natural wind dispersal can thus enable rapid redistribution of pathogen strains, with dire consequences in regions with high degrees of susceptibility.
Influence of weather on EPP distribution
Alterations in weather conditions associated with climate change can cause plant pathogens to re‐emerge as significant threats, as dormant pathogens or those previously under control reappear and/or adapt to new geographic regions, leading to severe outbreaks. It has been suggested that milder winters, higher temperatures and higher night temperatures could increase sporulation and infectiousness of foliar fungi (Harvell et al., 2002). For example, reductions in the diurnal thermal amplitude decreased the latency period of coffee rust in Central and South America. Together with economic factors, such as the use of susceptible cultivars as a result of high initial investment needed for cultivar replacement, variable rainfall and extreme weather conditions thus led to coffee rust outbreaks in 2008–2013 which directly affected the livelihoods of thousands of smallholder farmers (Avelino et al., 2015; Bebber et al., 2016) (Fig. 1).
Severe weather events can also influence airborne pathogen dispersal. For instance, hurricane Ivan carried spores of the fungus Phakopsora pachyrhizi, the causal agent of Asian soybean rust, into the United States in 2004 from South America, and it is now found throughout the southeastern United States and Mexico (Stokstad, 2004; Fig. 1). Climate change also provides opportunities for the re‐emergence of previously vanquished diseases. It is estimated that crop fungi and oomycetes are moving an average of 6–7 km yr–1 poleward (Bebber et al., 2013). In western Europe, earlier‐maturing wheat varieties that were bred to prevent the build‐up of wheat stem rust inoculum, which prefers warmer summer temperatures to establish disease, are likely to be at risk. Indeed, in 2013, Germany experienced its first major outbreak of wheat stem rust in decades owing to an unusually cold spring followed by early summer temperatures (Olivera Firpo et al., 2017). Stem rust has since been detected in areas of western Europe where it had been absent for many years, the most notable being in Sicily since 2016, where it constituted the largest outbreak in Europe in recent history (Bhattacharya, 2017). Additionally, unusual stem rust outbreaks in Siberia and Northern Kazakhstan in 2015–2017 caused severe yield losses (Hovmøller, 2018). As climate change accelerates, it is difficult to predict which territories EPPs will move to next, and an epidemiological perspective will be crucial to making long‐term predictions about invasion and persistence of EPPs in new areas (Gilligan & van den Bosch, 2008).
Alteration in agricultural practices
The agricultural landscape contains a very high density of genetically uniform plants that can trigger rapid pathogen cultivar specialization, and facilitate the quick emergence and spread of pathogens (McDonald & Stukenbrock, 2016). Owing to modern agricultural practices, germplasm diversity has reduced globally and high‐yielding cultivars have been specially selected and planted widely for their agronomical value (Van De Wouw et al., 2010). This favours the emergence of ‘domesticated’ crop pathogens that evolve quickly and are more virulent than their wild ancestors (McDonald & Stukenbrock, 2016). Large, dense host and pathogen populations also promote coinfection by different pathogens or different strains of the same pathogen, increasing pathogen virulence and the likelihood of horizontal gene transfer.
One example where modern agricultural practices have facilitated pathogen spread is the case of Zymoseptoria tritici, the causal agent of Septoria tritici Blotch (STB). STB causes significant wheat loses in temperate zones and is the primary wheat foliar disease in most western European countries (Fones & Gurr, 2015). High degrees of host susceptibility have enhanced the population size of Z. tritici, promoting substantial gene flow between strains (Zhan & McDonald, 2004). Furthermore, large population sizes coupled with sexual recombination can cause massive shifts in pathogen populations, which can rapidly lead to the evolution of fungicide insensitivity (Stammler & Semar, 2011; Yemelin et al., 2017). Accordingly, diversity in the agroecosystem via crop cocultivation, rotation and heterogenous planting of cultivars (McDonald & Stukenbrock, 2016) is crucial to reduce overall pathogen fitness and hinder their ability to rapidly overcome host resistance and fungicide sensitivity.
Genetic mechanisms underpinning EPP host shifts
Beyond physical transmission, the emergence of plant pathogens can also be driven by a shift in an existing pathogen's host range. For fungi and oomycetes, host jumps appear to occur even more frequently following anthropogenic movement (Slippers et al., 2005). In the agricultural environment, strong selective pressure can aid the pathogen's adaptation to new environmental factors such as host genotypes and ecological conditions. Host‐range expansion and host jumps can be facilitated by genetic events, including mutation, hybridization, sexual recombination and horizontal gene transfer among other mechanisms (Fig. 3).
Figure 3.
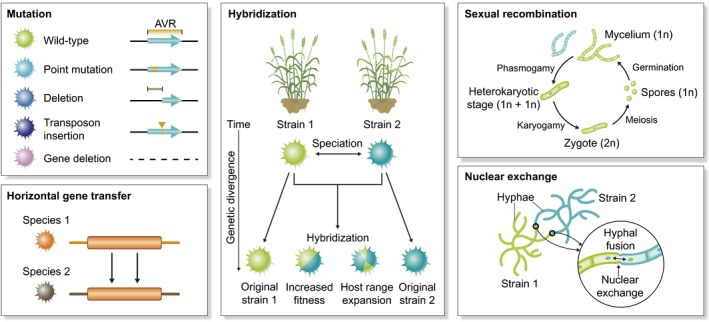
Genetic factors can facilitate host‐range expansion and/or host jumps of fungal or oomycete plant pathogens. Such genetic factors include mutation, horizontal gene transfer, hybridization, sexual recombination and nuclear exchange.
Mutation
The most prominent recent example of a host shift followed by migration is the wheat blast pathogen Magnaporthe oryzae (syn. Pyricularia oryzae) Triticum pathotype, which probably emerged from a shift of a Lolium‐ or Avena‐infecting Pyricularia population (Ceresini et al., 2018). Wheat blast was first detected on wheat in the 1980s in Brazil (Igarashi et al., 1986), from where it spread to Bolivia in 1996 and Argentina in 2007 (Ceresini et al., 2018). Now well established in South America, it is thought that seedborne inoculum on wheat grain imports from Brazil entered Southeast Asia in 2016, causing severe outbreaks particularly in Bangladesh (Islam et al., 2016; Fig. 1). M. oryzae Triticum is a diverse group with a broad host range, infecting wheat, barley (Hordeum vulgare), rye, and > 10 other grass hosts (Urashima et al., 1993; Ceresini et al., 2018). With fungicides providing only partial protection and current wheat varieties in Bangladesh displaying limited tolerance at best (CIMMYT; https://www.cimmyt.org/tag/wheat-blast/), wheat blast now poses a serious and immediate threat to wheat production across South Asia.
A key factor in the M. oryzae host jump to wheat was loss of recognition induced by mutations or deletions in the PWT3 and PWT4 avirulence genes combined with widescale planting of wheat cultivars lacking one or both of the corresponding resistance genes, Rwt3 and Rwt4, that act as a barrier for Lolium or Avena Pyricularia spp. compatibility on wheat (Inoue et al., 2017). As exemplified in this case, when existing host species are physically near novel hosts, the ease of transmission of ‘new’ pathogen strains with adaptive mutations can lead to sudden and widespread epidemics.
Hybridization and nuclear exchange
Host‐range expansion can also arise as a result of somatic hybridization. For example, nuclear exchange in multinucleated rust fungi in Australia was probably the origin of ‘Scabrum rust’, a hybrid form of the wheat stem rust pathogen (P. graminis f. sp. tritici) and the rye stem rust pathogen (P. graminis f. sp. secalis). The three formae speciales can all infect barley, causing severe stem rust epidemics in Australia (Luig & Watson, 1972; Park, 2007). The advent of human‐made crops can also provide pathogens with new hosts to exploit. For instance, the wheat–rye hybrid triticale, which had originally been resistant to powdery mildew (Menardo et al., 2015), can now be infected by a new forma specialis that arose from hybridization between wheat powdery mildew (B. graminis f. sp. tritici) and rye powdery mildew (B. graminis f. sp. secalis).
These examples demonstrate the power of hybridization and nuclear exchange as forces in the evolutionary emergence of pathogens. These ‘new’ strains with expanded host range then escape direct competition with their parental strains to exploit their new host niche.
Sexual recombination and acquisition of accessory chromosomes
Recombination events in filamentous pathogens can enhance virulence and have a direct impact on host‐range expansion (Giraud et al., 2010). Many plant pathogens undergo sexual cycles and this is especially notable in agricultural settings, where pathogens with sexual cycles are able to evolve and adapt more readily to changing environments such as new hosts. One example is Z. tritici, which has the potential for sexual reproduction at least once per growing season (Cowger et al., 2008), leading rapidly to changes in its virulence profile. New species with broader host ranges can also be created by hybrid speciation events, as in the case of Zymoseptoria pseudotritici, which has an expanded host range when compared with its wheat‐infecting Z. tritici parent (Stukenbrock et al., 2012).
Certain fungal species can also exchange ‘pathogenicity’ chromosomes that lead to host‐range expansion. This is certainly the case for the tomato pathogen Fusarium oxysporum f. sp. lycopersici. The pathogen carries effector genes in one of four accessory chromosomes (Ma et al., 2010), which are required for pathogenicity towards its tomato host (Vlaardingerbroek et al., 2016). More recently, highly variable fast‐evolving mini‐chromosomes carrying effector sequences have also been identified in the wheat‐adapted lineage of M. oryzae (Peng et al., 2018). The unstable transmission of these mini‐chromosomes between strains has been hypothesized to act as a mechanism to accelerate host adaptation for M. oryzae (Peng et al., 2018). From these examples it is clear that sexual recombination and the exchange of accessory chromosomes can play a central role in EPP outbreaks by facilitating the emergence of new pathogen strains with novel virulence combinations that can be crucial for adaptation to new hosts.
Horizontal gene transfer
Interspecific horizontal gene transfer (HGT) is also a well described mechanism for new plant pathogens to emerge in agricultural settings (Stukenbrock & McDonald, 2008). The acquisition of pathogenicity‐related factors, especially between species coinfecting the same host, can cause rapid emergence of novel pathogens. One notorious example is the transference of the proteinaceous necrotrophic effector ToxA between two wheat fungal pathogens, Parastagonospora nodorum and Pyrenophora tritici‐repentis (Friesen et al., 2006). It is hypothesized that the acquisition of ToxA from P. nodorum led to the emergence of a new highly destructive race of Pyrenophora tritici‐repentis. Recently, the same host‐specific toxin ToxA was found in Bipolaris sorokiniana (McDonald et al., 2018), a poorly understood wheat and barley pathogen that causes Helminthosporium leaf blight and common root rot and is the main constraint to cereal production in Central Asia. HGT is a potent driver for pathogen adaptation and in certain cases can have a major role in the emergence of new pathogen races (Stukenbrock & McDonald, 2008). However, for fungal EPPs, the influence of HGT is only just starting to be revealed.
Increasing preparedness for EPPs
It is crucial that we develop interconnected global surveillance systems to guarantee early warnings and predict pathogen movement within the growing season. Furthermore, understanding the causes of sudden epidemics is key to designing effective control measures. Agricultural practices have led to a ‘domestication’ of plant pathogens. The modern agricultural landscape needs to become more heterogeneous to keep EPPs under control. More regular crop rotation and cultivar turnover, smaller planted areas and an increase in the number of crop species grown per unit area are necessary to reduce the probability of pathogen adaptation and host shifts (McDonald & Stukenbrock, 2016). Accordingly, we need to couple demographic and geographical analyses to population genomic studies to elucidate how plant pathogens evolve and adapt in newly encountered agroecosystems.
Lessons from the past
Certain plant diseases have been controlled and almost eradicated thanks to effective crop protection strategies such as the use of resistant host cultivars, chemical treatment and/or removal of alternate host plants that form important roles in the life cycle of certain pathogens. For example, in Europe in the late 19th and early 20th centuries, the large‐scale removal of common barberry (Berberis vulgaris) ended severe stem rust epidemics in western Europe (Zadoks, 1967). Common barberry is essential for the pathogen (Puccinia graminis f.sp. tritici) to overwinter in temporal climates and complete sexual reproduction. However, the reintroduction of the alternate host in recent years has the potential to enhance the pathogen's genetic diversity and provide a seasonal haven for stem rust during winter (Smith et al., 2009). Accordingly, the first occurrence of a sexual population of stem rust in decades in Sweden was recently reported following repeal of the barberry exclusion law (Berlin, 2017). It is important that such lessons from the past are not forgotten and are instead used to inform and maintain historically effective crop protection strategies.
Predicting natural EPP dispersal patterns
A useful approach to predicting the dynamics of plant pathogens is to model the behaviour of a given pathogen under known conditions. Mathematical modelling can help to improve surveillance and control systems and possibly predict future outbreaks. Recently, a spore dispersal model for stem rust incorporated international field survey data, global weather data, and a dispersion model to simulate possible disease outbreak scenarios (Meyer et al., 2017). Studying the key dispersal routes for stem rust spores, the model predicted when, how often, and how many spores may be dispersed in southern/East Africa, the Middle East, and Central/South Asia for highly virulent strains such as Ug99 (Pretorius et al., 2000; Meyer et al., 2017). Such results can highlight potential dispersion routes and guide cost‐effective control strategies.
Incorporating knowledge from population genomics into disease modelling should also increase our ability to predict the evolutionary route of plant pathogens in the agricultural landscape. For wheat blast, planting wheat varieties with the Rwt3 and Rwt4 resistance genes should limit Lolium or Avena Pyricularia spp. from further jumping the barrier to wheat, as a result of the presence of the corresponding PWT3 and PWT4 avirulence genes (Inoue et al., 2017). Thus, a clear understanding of how plant pathogen genomes evolve should be incorporated into dispersal models to improve their predictive ability and value for EPP prevention.
Interconnected disease surveillance systems
One example of an effective national network for disease surveillance is the National Plant Diagnostic Network, which focuses on early detection, accurate diagnosis, and rapid communication to help control the spread of EPPs within the United States (National Plant Diagnostic Network, 2019). Such rigorous systems provide a valuable tool to manage local outbreaks; however, as pathogens shift location, EPP surveillance and reporting need international alliances to succeed. For example, the ‘Digalu’ (TKTTF) race of stem rust was initially identified in Turkey in 2007–2008 with minimal losses (Mert et al., 2012); however, once it entered Ethiopia in 2013, the race caused near‐total field losses as a result of the high degree of susceptibility of the widely grown wheat cv ‘Digalu’ (Olivera et al., 2015; Fig. 1). Interconnected screening networks testing dominant pathogen races across global germplasm collections could be used to circumvent similar outbreaks in the future.
Since 1993, the international standards for phytosanitary measures dictated by the Food and Agriculture Organisation (FAO) of the United Nations have required countries to report plant pests and diseases to the International Plant Protection Convention (FAO, 2006; International Plant Protection Convention, 2012), which provides a framework for pathogen monitoring, surveillance and management. However, the achievement of accuracy in these reports can be hampered by economic consequences, political barriers and a lack of standardization in testing procedures. Given the global reach and ease of transmission of EPPs, it is essential we develop a unified and transparent international strategy to support the accurate and timely reporting of EPPs (Carvajal‐Yepes et al., 2019).
Conclusions
As human activities continue to erode natural barriers to dispersal, human‐mediated pathogen redistribution poses an increasing threat to the stability of agricultural systems and native biodiversity (Kolar & Lodge, 2001). The versatility EPPs display in their spread emphasises the urgent need for a coordinated globalisation of disease monitoring systems to develop proactive management strategies for EPPs at high risk of movement into vulnerable areas. Fortunately, modern agricultural practices such as resistance breeding and fungicides provide us with better protection than during the catastrophic EPP outbreaks that have plagued history. However, we must remain vigilant to the threats posed by EPPs to plant health and global food security, in order to safeguard and maximise crop production as productivity demands continue to increase in the future.
Acknowledgements
We thank Matthew Heaton and Tobin Florio (JIC) for assistance with figure preparation. PC‐M is funded by the Biotechnology Biological Sciences Research Council (BBSRC: BB/M011216/1) and DGOS by the European Research Council (no. 715638), the BBSRC Programmes BB/J004553/1 and BB/P012574/1, and the John Innes Foundation.
References
- Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology and Evolution 19: 535–544. [DOI] [PubMed] [Google Scholar]
- Avelino J, Cristancho M, Georgiou S, Imbach P, Aguilar L, Bornemann G, Läderach P, Anzueto F, Hruska AJ, Morales C. 2015. The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Security 7: 303–321. [Google Scholar]
- Bebber DP, Castillo AD, Gurr SJ. 2016. Modelling coffee leaf rust risk in Colombia with climate reanalysis data. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 371: 20150458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebber DP, Ramotowski MAT, Gurr SJ. 2013. Crop pests and pathogens move polewards in a warming world. Nature Climate Change 3: 985–988. [Google Scholar]
- Berlin A. 2017. Stem rust attacks in Sweden heralds the return of a previously vanquished foe. Sweden: Swedish University of Agricultural Sciences, SLU News. [Google Scholar]
- Bhattacharya S. 2017. Deadly new wheat disease threatens Europe's crops. Nature 542: 145–146. [DOI] [PubMed] [Google Scholar]
- Bonde MR, Peterson GL, Schaad NW, Smilanick JL. 1997. Karnal bunt of wheat. Plant Disease 81: 1370–1377. [DOI] [PubMed] [Google Scholar]
- Brown JK, Hovmoller MS. 2002. Aerial dispersal of pathogens on the global and continental scales and its impact on plant disease. Science 297: 537–541. [DOI] [PubMed] [Google Scholar]
- Carvajal‐Yepes M, Cardwell K, Nelson A, Garrett KA, Giovani D, Saunders DGO, Kamoun S, Legg JP, Verdier V, Lessel J, et al 2019. A global surveillance system for crop diseases. Science 364: 1237–1239. [DOI] [PubMed] [Google Scholar]
- Ceresini PC, Lili V, Rios JA, Aucique‐p CE, Moreira SI, Alves E, Croll D, Maciel N. 2018. Wheat blast: past, present, and future. Annual Review of Phytopathology 56: 427–456. [DOI] [PubMed] [Google Scholar]
- Cooke DEL, Cano LM, Raffaele S, Bain RA, Cooke LR, Etherington GJ, Deahl KL, Farrer RA, Gilroy EM, Goss EM, et al 2012. Genome analyses of an aggressive and invasive lineage of the Irish potato famine pathogen. PLoS Pathogens 8: e1002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowger C, Brunner PC, Mundt CC. 2008. Frequency of sexual recombination by Mycosphaerella graminicola in mild and severe epidemics. Phytopathology 98: 752–759. [DOI] [PubMed] [Google Scholar]
- Cunningham AA, Daszak P, Rodríguez JP. 2003. Pathogen pollution: defining a parasitlogical threat to biodiversity conservation. Journal of Parasitology 89: S78–S83. [Google Scholar]
- Dutech C, Barrès B, Bridier J, Robin C, Milgroom MG, Ravignè V. 2012. The chestnut blight fungus world tour: successive introduction events from diverse origins in an invasive plant fungal pathogen. Molecular Ecology 21: 3931–3946. [DOI] [PubMed] [Google Scholar]
- FAO . 2006. International standards for phytosanitary measures. [WWW document] URL http://www.fao.org/3/a0450e/a0450e.pdf [accessed 9 June, 2019].
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fones H, Gurr S. 2015. The impact of Septoria tritici Blotch disease on wheat: an EU perspective. Fungal Genetics and Biology 79: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, Faris JD, Rasmussen JB, Solomon PS, McDonald BA, Oliver RP. 2006. Emergence of a new disease as a result of interspecific virulence gene transfer. Nature Genetics 38: 953–956. [DOI] [PubMed] [Google Scholar]
- Gewin V. 2003. Bioterrorism: agriculture shock. Nature 421: 106–108. [DOI] [PubMed] [Google Scholar]
- Gilligan CA, van den Bosch F. 2008. Epidemiological models for invasion and persistence of pathogens. Annual Review of Phytopathology 46: 385–418. [DOI] [PubMed] [Google Scholar]
- Giraud T, Gladieux P, Gavrilets S. 2010. Linking the emergence of fungal plant diseases with ecological speciation. Trends in Ecology and Evolution 25: 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvell CD, Mitchell CE, Ward JR, Altizer S, Dobson AP, Ostfeld RS, Samuel MD. 2002. Climate warming and disease risks for terrestrial and marine biota. Science 296: 2158–2162. [DOI] [PubMed] [Google Scholar]
- Hovmøller MS. 2018. Global rust reference center. [WWW document] URL http://wheatrust.org/. [accessed 5 November 2018].
- Hovmøller MS, Walter S, Bayles RA, Hubbard A, Flath K, Sommerfeldt N, Leconte M, Czembor P, Rodriguez‐Algaba J, Thach T et al 2016. Replacement of the European wheat yellow rust population by new races from the centre of diversity in the near‐Himalayan region. Plant Pathology 65: 402–411. [Google Scholar]
- Hubbard A, Lewis CM, Yoshida K, Ramirez‐Gonzalez RH, de Vallavieille‐Pope C, Thomas J, Kamoun S, Bayles R, Uauy C, Saunders DG. 2015. Field pathogenomics reveals the emergence of a diverse wheat yellow rust population. Genome Biology 16: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi S, Utiamada C, Igarashi L, Kazuma A, Lopes R. 1986. Ocurrence of Pyrcularia sp. in wheat (Triticum aestivum L.) in the State of Parana, Brazil. Fitopatologia Brasileira 36: 79–82. [Google Scholar]
- Inoue Y, Vy TTP, Yoshida K, Asano H, Mitsuoka C, Asuke S, Anh VL, Cumagun CJR, Chuma I, Terauchi R et al 2017. Evolution of the wheat blast fungus through functional losses in a host specificity determinant. Science 357: 80–83. [DOI] [PubMed] [Google Scholar]
- International Plant Protection Convention . 2012. IPPC strategic framework. [WWW document] URL https://www.ippc.int/static/media/files/publications/en/2013/06/03/1344410402_ippc_strategicframework_e_w_201305101054en.pdf. [accessed 9 June 2019].
- Islam MT, Croll D, Gladieux P, Soanes DM, Persoons A, Bhattacharjee P, Hossain MS, Gupta DR, Rahman MM, Mahboob MG et al 2016. Emergence of wheat blast in Bangladesh was caused by a South American lineage of Magnaporthe oryzae . BMC Biology 14: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolar CS, Lodge DM. 2001. Progress in invasion biology: predicting invaders. Trends in Ecology and Evolution 16: 199–204. [DOI] [PubMed] [Google Scholar]
- Kromann P, Miethbauer T, Ortiz O, Forbes GA. 2014. Review of potato biotic constraints and experiences with integrated pest management interventions In: Pimentel D, Peshin R, eds. Integrated pest management. Dordrecht, the Netherlands: Springer, 245–268. [Google Scholar]
- Luig NH, Watson IA. 1972. The role of wild and cultivated grasses in the hybridization of formae speciales of Puccinia graminis . Australian Journal of Biological Sciences 25: 335–342. [Google Scholar]
- Ma LJ, Van Der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B et al 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium . Nature 464: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald BA, Stukenbrock EH. 2016. Rapid emergence of pathogens in agro‐ecosystems: global threats to agricultural sustainability and food security. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences 371: 20160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MC, Ahren D, Simpfendorfer S, Milgate A, Solomon PS. 2018. The discovery of the virulence gene ToxA in the wheat and barley pathogen Bipolaris sorokiniana . Molecular Plant Pathology 19: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menardo F, Praz C, Wyder S, Bourras SA, McNally KE, Parlange F, Riba A, Roffler S, Schaefer L, Shimizu KK et al 2015. Hybridization of powdery mildew strains gives raise to pathogens on novel agricultural crop species. Nature Genetics 48: 201–205. [DOI] [PubMed] [Google Scholar]
- Mert Z, Karakaya A, Düşünceli F, Akan K, Çetin L. 2012. Determination of Puccinia graminis f. sp. tritici races of wheat in Turkey. Turkish Journal of Agriculture and Forestry 36: 107–120. [Google Scholar]
- Meyer M, Cox JA, Hitchings MDT, Burgin L, Hort MC, Hodson DP, Gilligan CA. 2017. Quantifying airborne dispersal routes of pathogens over continents to safeguard global wheat supply. Nature Plants 3: 780–786. [DOI] [PubMed] [Google Scholar]
- National Plant Diagnostic Network . 2019. National plant diagnostic network website [WWW document]. URL https://www.npdn.org/home [accessed 5 November 2018].
- Olivera Firpo PD, Newcomb M, Flath K, Sommerfeldt‐Impe N, Szabo LJ, Carter M, Luster DG, Jin Y. 2017. Characterization of Puccinia graminis f. sp. tritici isolates derived from an unusual wheat stem rust outbreak in Germany in 2013. Plant Pathology 66: 1258–1266. [Google Scholar]
- Olivera P, Newcomb M, Szabo LJ, Rouse M, Johnson J, Gale S, Luster DG, Hodson D, Cox JA, Burgin L et al 2015. Phenotypic and genotypic characterization of race TKTTF of Puccinia graminis f. sp. tritici that caused a wheat stem rust epidemic in southern Ethiopia in 2013–14. Phytopathology 105: 917–928. [DOI] [PubMed] [Google Scholar]
- Park RF. 2007. Stem rust of wheat in Australia. Australian Journal of Agricultural Research 58: 558–566. [Google Scholar]
- Peng Z, Garcia EO, Lin G, Hu Y, Dalby M, Migeon P, Tang H, Farman M, Cook D, White FF et al 2018. Effector gene reshuffling involves dispensable mini‐chromosomes in the wheat blast fungus. bioRxiv. doi: http://10.1101/359455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS. 2000. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis. f. sp. tritici in Uganda. Plant Disease 84: 203.2. [DOI] [PubMed] [Google Scholar]
- Schumann GL, D'Arcy CJ. 2000. Late blight of potato and tomato. The Plant Health Instructor; doi: 10.1094/phi-i-2000-0724-01. [DOI]
- Sen A. 1981. The great Bengal famine In: Poverty and Famines: an essay on entitlement and deprivation. Oxford, UK: Oxford University Press, 52–85. [Google Scholar]
- Slippers B, Stenlid J, Wingfield MJ. 2005. Emerging pathogens: fungal host jumps following anthropogenic introduction. Trends in Ecology and Evolution 20: 420–421. [DOI] [PubMed] [Google Scholar]
- Smith K, Draper M, Simmons K, Bennett R, Hebbar P, Royer M, Murray T. 2009. US preparations for potential introduction of Ug99 strains of wheat stem rust. Outlooks on Pest Management 20: 148–152. [Google Scholar]
- Stammler G, Semar M. 2011. Sensitivity of Mycosphaerella graminicola (anamorph: Septoria tritici) to DMI fungicides across Europe and impact on field performance. EPPO Bulletin 41: 149–155. [Google Scholar]
- Stokstad E. 2004. Plant pathologists gear up for battle with dread fungus. Science 306: 1672–1673. [DOI] [PubMed] [Google Scholar]
- Stukenbrock EH, Christiansen FB, Hansen TT, Dutheil JY, Schierup MH. 2012. Fusion of two divergent fungal individuals led to the recent emergence of a unique widespread pathogen species. Proceedings of the National Academy of Sciences, USA 109: 10954–10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukenbrock EH, McDonald BA. 2008. The origins of plant pathogens in agro‐ecosystems. Annual Review of Phytopathology 46: 75–100. [DOI] [PubMed] [Google Scholar]
- Turner RS. 2005. After the famine: plant pathology, Phytophthora infestans, and the late blight of potatoes, 1845–1960. Historical Studies in the Physical and Biological Sciences 35: 341–370. [Google Scholar]
- Urashima AS, Igarashi S, Kato H. 1993. Host range, mating type, and fertility of Pyricularia grisea from wheat in Brazil. Plant Disease 77: 1211–1216. [Google Scholar]
- Van De Wouw M, Kik C, Van Hintum T, Van Treuren R, Visser B. 2010. Genetic erosion in crops: concept, research results and challenges. Plant Genetic Resources: Characterisation and Utilisation 8: 1–15. [Google Scholar]
- Vlaardingerbroek I, Beerens B, Schmidt SM, Cornelissen BJC, Rep M. 2016. Dispensable chromosomes in Fusarium oxysporum f. sp. lycopersici . Molecular Plant Pathology 17: 1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemelin A, Brauchler A, Jacob S, Laufer J, Heck L, Foster AJ, Antelo L, Andresen K, Thines E. 2017. Identification of factors involved in dimorphism and pathogenicity of Zymoseptoria tritici . PLoS ONE 12: e0183065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Schuenemann VJ, Cano LM, Pais M, Mishra B, Sharma R, Lanz C, Martin FN, Kamoun S, Krause J et al 2013. The rise and fall of the Phytophthora infestans lineage that triggered the Irish potato famine. eLife 2: e00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks J. 1967. Epidemiology of wheat rusts in Europe. Pest Articles & News Summaries Section B. Plant Disease Control 13: 29–46. [Google Scholar]
- Zhan J, McDonald BA. 2004. The interaction among evolutionary forces in the pathogenic fungus Mycosphaerella graminicola . Fungal Genetics and Biology 4: 590–599. [DOI] [PubMed] [Google Scholar]


