Abstract
The application of behavioral economic demand theory in addiction science has proved useful for evaluating individual characteristics underlying abuse liability. Two factors that have received comparably little attention within this literature are sex and gonadal hormones. We determined cocaine and remifentanil demand in male and female rats using a within‐session procedure. Cocaine and remifentanil demand were evaluated for 15 consecutive days using a balanced, crossover design that randomized drug order. This design allowed for the evaluation of temporal and exposure effects on two independent dimensions of demand, unconstrained demand (Q 0) and demand elasticity (α). Estrous cyclicity was tracked to determine the contribution of phase to demand. No overall sex differences were observed. Increased unconstrained demand for cocaine and remifentanil was observed in females during periods in which estrogen was high (eg, estrus phase). Unconstrained remifentanil demand escalated over the 15‐day testing period, but escalation was not observed for cocaine or for demand elasticity. A significant exposure effect was also observed in which greater prior remifentanil intake increased unconstrained cocaine demand and reduced cocaine demand elasticity. These effects were directionally specific as no significant effects of prior cocaine exposure were observed on remifentanil demand measures. These data suggest that unconstrained demand and demand elasticity do not differ between male and female subjects; however, that unconstrained demand is associated with estrous cyclicity. These findings also suggest that opioid exposure enhances subsequent demand for psychomotor stimulants, which may be important when considering recent increases in nonmedical prescription opioid use in the United States.
Keywords: cocaine, estrous, hormone, opioid, rodent, self‐administration, sex, threshold
The present study used a within‐session threshold procedure to assess cocaine and remifentanil demand parameters in male and female rats. The findings indicate that unconstrained demand was significantly elevated during the estrus phase of the estrous cycle among female rats for both drugs. Additionally, we detected escalation of remifentanil intake across the 15‐day testing period as well as augmented cocaine demand following a history of remifentanil use.
1. INTRODUCTION
A growing body of research has established the utility of applying behavioral economic demand theory to characterize and assess drug abuse liability in humans and animals.1, 2, 3 Procedures developed in this tradition have generally evaluated an organism's consumption of a drug (eg, self‐administration) when the effort to obtain that drug varies across a range of prices (eg, response cost). Demand curves generated using these methods quantify the relationship between consumption and price and can be used to differentiate behavioral mechanisms underlying drug reinforcement. Specifically, theoretical and empirical data support the notion that demand can be separated into two independent behavioral mechanisms: (1) consumption of demand at unconstrained price (ie, a hedonic set point, also referred to as demand intensity) and (2) elasticity of demand (ie, sensitivity of consumption to changes in price4, 5). This literature has also supported the translational and clinical utility of these measures as reflecting distinct features of reinforcement that relate to risk factors underlying substance use disorder (see reviews in Kaplan et al6; MacKillop3). It is in this separation of behavioral mechanisms that behavioral economic demand has the potential to advance previous research on the relative reinforcing effects of drugs by accounting for the multidimensional nature of reinforcement rather than viewing reinforcement as a homogenous construct.5, 7
Demand curves are typically generated in preclinical research by allowing subjects to self‐administer a drug over multiple days on varying fixed‐ratio (FR) schedules and/or across different drug doses.5, 8, 9 One limitation of this methodology is that it can be time intensive, requiring subjects to be tested over numerous days with only one unit price determined in each session. An alternative to these between‐session approaches is the threshold procedure (see review by Bentzley et al10). The threshold procedure generates demand functions by systematically reducing the dose delivered within discrete components of a single test session. Such methods have proved valuable for a wide array of applications including examining putative pharmacotherapies (eg, Bentzley et al11), determining the effects of stress on drug self‐administration,12, 13 and evaluating the neurobiological mechanisms related to drug reinforcement.14
One important individual characteristic related to substance use and substance use disorder is sex. Clinical and preclinical research examining sex differences in drug addiction point to a consistently cited conclusion: Females are more vulnerable to the reinforcing effects of drugs than males.15, 16 These differences are typically attributed to gonadal hormones, particularly estrogen, that are thought to augment drug use behaviors.17, 18 For example, women report increased subjective drug effects during the follicular phase of the menstrual cycle during which estrogen levels are high.19, 20 Similarly, female rats reach higher cocaine breakpoints on a progressive‐ratio schedule of reinforcement during the estrus phase of the estrous cycle, when estrogen is high.21, 22, 23 Surprisingly, little is known about the extent to which sex differences and hormonal fluctuations may impact drug demand characteristics within a behavioral economic framework. One preclinical study using the between‐session demand procedure did not detect differences in unconstrained nicotine demand or demand elasticity between male and female rats,24 which is consistent with research conducted in the human laboratory and clinic.25, 26, 27 Few studies have systematically evaluated sex as it relates to drug demand in humans. Notably, however, a recent experiment found that women in the follicular, compared with luteal, phase showed elevated demand for cigarettes consistent with the research reviewed above.28
At the time of this study, no preclinical studies had systematically evaluated sex and hormonal differences in cocaine and opioid demand or evaluated the effects of estrous phase on drug demand. This represents a significant limitation given that preclinical research allows for the specific evaluation of the effects of gonadal hormones on drug abuse liability. The threshold procedure is well positioned to address these gaps given the ability to generate demand curves within a single day or session thereby allowing for high‐throughput and high‐resolution evaluation of changes in demand as a function of individual subject characteristics (eg, hormonal fluctuations).
The primary goal of the present study was to evaluate sex and estrous cycle differences in cocaine and remifentanil demand. To this end, male and female rodents were tested using a threshold procedure for 15 consecutive days for cocaine or remifentanil administration. Subjects then completed an additional 15 days of testing for the other compound. This crossover design allowed us to accomplish two secondary goals. First, we evaluated whether demand was stable over the 15‐day period. Second, we evaluated whether prior drug exposure (eg, cocaine) altered demand for other compounds from a different pharmacological class (eg, remifentanil). We predicted that female subjects would exhibit behavior consistent with greater abuse liability (ie, increased unconstrained demand and decreased demand elasticity) consistent with previous research on cocaine and opioids using alternative models.18, 29, 30 We also predicted that estrous cycle would differentially impact cocaine and remifentanil demand consistent with previous research.21, 31, 32
2. MATERIALS AND METHODS
2.1. Animals
Thirty‐two Long‐Evans rats (16 male, 16 female) were obtained at weaning and raised until early adulthood (postnatal day 75). Rats were singly housed in standard polycarbonate cages (interior dimensions: 50 × 28 × 30 cm) within a temperature‐ and humidity‐controlled colony room maintained on a 12‐hour light/dark cycle (lights on: 07:00 am). Food and water were freely available except during the brief period of lever‐press training (see Section 2.2). All animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.33 All procedures were approved by the Animal Care and Use Committee at Franklin & Marshall College.
2.2. Lever‐press training
At approximately 75 days old, rats were placed on light food restriction and maintained at 90% of free‐feeding weight. Animals were then trained to press a response lever to obtain food reinforcement (Bio‐Serv, Dustless Precision Pellets, 45 mg) in operant conditioning chambers from Med Associates Inc (Georgia, Vermont) on a fixed‐ratio 1 (FR1) schedule. All sessions terminated automatically once 50 reinforcers had been delivered or 2 hours elapsed, whichever occurred first. Once a rat earned 50 reinforcers on three consecutive training sessions, they were placed back on unrestricted feed and training was discontinued.
2.3. Surgery
Approximately 1 week following food training, rats were anesthetized using a combination of ketamine HCl (100 mg/kg, ip) and xylazine HCl (8.0 mg/kg, ip). An intravenous catheter was surgically implanted into the right jugular vein and exited the body on the dorsal surface of the scapulae. Carprofen (5.0 mg/kg, sc) was given immediately after surgery and on the following day as a postoperative analgesic, and a solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily as a prophylactic antibiotic and to maintain catheter patency. Ticarcillin was given for 7 days following surgery, including the first 3 days of behavioral testing (ie, during self‐administration training). After ticarcillin administration was discontinued, a solution of heparinized saline and gentamicin (0.05 mg/kg) was used to maintain patency. All animals were allowed 4 days to recover before self‐administration training and testing.
2.4. Self‐administration training
Half of the animals were first trained and tested with cocaine, and the other half trained and tested with remifentanil. Each group contained half male and half female subjects. A crossover design was used such that subjects completed training and testing with the other compound following completion of testing with the initial drug. Each session began with the insertion of two retractable levers into the chamber, the illumination of a stimulus light above the active (left) lever, and a priming infusion of cocaine (0.5 mg/kg per infusion) or remifentanil (5 μg/kg per infusion). During these initial training sessions, responding on the lever was reinforced on an FR1 schedule of reinforcement and each lever press resulted in a 0.5 mg/kg per infusion of cocaine or 5 μg/kg per infusion of remifentanil (infusion duration varied between 1.5 and 3.0 s based on individual body weight). Coincident with the beginning of each infusion, the lever retracted and the stimulus light was extinguished for 20 seconds to signal a time‐out period in which no drug was available. Training sessions terminated automatically once 2 hours elapsed, with a maximum of 20 infusions.
2.5. Threshold testing
Threshold testing began 5 days after self‐administration training. Details on the threshold procedure are described elsewhere.14 In each session, the active lever (left) extended into the chamber and the stimulus light above the active lever was illuminated. Responding was reinforced on an FR1 schedule of reinforcement during testing, as in training. Following each lever press, the lever retracted and the stimulus light was extinguished for 5 seconds signaling a time‐out during which drug was not available. The overall session length was 2 hours and began with a 10‐m initiation component in which 1.0 mg/kg per infusion of cocaine or 10 μg/kg per infusion of remifentanil was available. The first 10‐m (initiation) phase was designed to account for and avoid inflated estimations due to an initial “load up” phase of self‐administration and was not included in data analysis.10 This was followed by eleven 10‐m components in which the dose of cocaine or remifentanil decreased in quarter‐log units. All components were presented sequentially in decreasing dose order. Threshold testing with cocaine occurred with 1.0, 0.56, 0.32, 0.18, 0.1, 0.056, 0.032, 0.018, 0.01, 0.0056, and 0.0032 mg/kg per infusion, and remifentanil occurred with 10, 5.6, 3.2, 1.8, 1, 0.56, 0.32, 0.18, 0.1, 0.056, and 0.03 μg/kg per infusion available during each of these 10‐m intervals. When the animals transitioned to the next drug, there was a 4‐day training phase during which 0.5 mg/kg per infusion of cocaine or 5 μg/kg per infusion of remifentanil was available and animals responded for 4 days on an FR1 schedule before threshold testing resumed.
2.6. Vaginal lavage and monitoring of the estrous cycle
The estrous cycle of all female subjects was monitored daily, beginning 7 days prior to catheter implantation in order to establish a pattern of estrous cyclicity for each animal. Sampling was suspended during the surgery/recovery period and resumed on the first day of self‐administration. Vaginal samples were obtained using a standard lavage technique in which approximately 0.5 mL of physiological saline (0.9%) was used to flush the vaginal canal and allow surface cells to be collected for analysis. Samples were analyzed under 100× light microscopy for determination of estrous cycle. All lavage samples were collected less than 30 m before each self‐administration test session began. There are four phases in the rat estrous cycle: diestrus, proestrus, estrus, and metaestrus. These phases can be identified based on the proportion of three types of vaginal cells present in a given sample.34, 35 These cells are leukocytes (L), nucleated (N), and cornified (C) epithelial cells. The cycle of estrous was determined using the following guidelines:
- Diestrus
L ≥ N—or—L ≥ N > C
- Proestrus
N or N ≥ C—or—N > C
- Estrus
C—or—C > L—or—C > N
- Metaestrus
L ≥ C—or—L ≥ C > N
For the purposes of this experiment, metaestrus and diestrus (denoted as M/D herein) were combined for data analysis purposes due to the low levels of estrogen that characterize these phases.21, 22, 23
2.7. Data analysis
Individual subject estimates of unconstrained demand (Q 0) and demand elasticity (α) were determined using the exponentiated demand equation36:
where Q is the consumption; Q 0 is the derived unconstrained demand; k is a constant related to consumption range (a priori set to 2); C is the unit price (ie, responses required at each component dose to reach the component 1 dose [1 mg of cocaine/10 μg of remifentanil]); and α is the derived demand elasticity. This model differs from the exponential demand equation5 traditionally utilized in animal subjects research by removing logarithms in the equation thereby allowing for inclusion of zero consumption data without transformation. Previous research has established the benefits of the exponentiated model approach by demonstrating improved model fits and correspondence with real‐world measures of drug use as well as highlighting potential concerns related to the transformation or removal of zero values required when using the exponential equation.36, 37 Importantly, the interpretation of fitted parameters from the exponential equation is the same as the exponential model allowing for appropriate comparisons with existing literature.
The exponentiated demand equation provided an excellent fit to individual data from cocaine (median model R 2 = 0.88) and remifentanil (median model R 2 = 0.93) sessions. Parameters from test sessions with individual subject data that fit at R 2 < 0.40 were removed from estimation (47/480 of cocaine sessions and 12/480 of remifentanil sessions). However, the use of linear mixed‐effect models and maximum likelihood estimation described below allowed for the inclusion of subjects with any missing data in full model estimation. Unconstrained demand and elasticity were skewed and log‐transformed prior to inferential analysis to satisfy normality assumptions.
Figure 1 contains representative plots of individual subject data for cocaine demand. Each plot depicts a prototypic decrease in consumption with increase in price that is characteristic of behavioral economic demand functions. These plots also include estimates of unconstrained demand and elasticity as well as visually depicting how variations in these values can shift the demand function.
Figure 1.
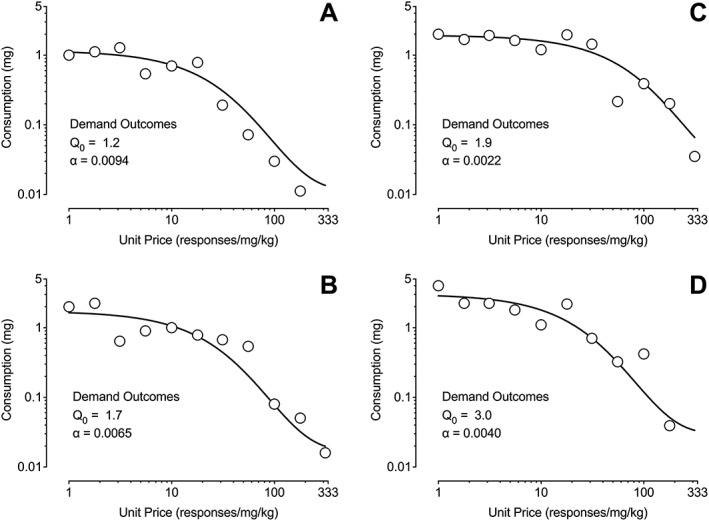
Representative individual subject plots. Depicted are behavioral economic demand curves for individual subjects. Also included are values from the exponentiated demand curve for unconstrained demand (Q 0) and demand elasticity (α)
Linear mixed‐effect models were used to evaluate unconstrained demand and elasticity measures. These models allowed for the estimation of the effect of between‐subject (eg, sex) and within‐subject (eg, estrous phase) predictors while appropriately accounting for the within‐subject repeated‐measure design. Models tested effects including (1) the linear effect of time, (2) the between‐subject effect of sex, and (3) the within‐subject effect of estrous phase in female subjects.
Additional models were used to evaluate the effects of prior drug exposure on unconstrained demand and elasticity. These models tested the relationship between average unconstrained demand throughout testing of the first drug exposure and the unconstrained demand and elasticity for the second drug tested in threshold. For example, models with subjects tested for remifentanil demand first evaluated the relationship between average unconstrained demand for remifentanil (predictor) and cocaine unconstrained demand and elasticity (outcomes). Significant effects were followed by simple slopes estimation for interpretation purposes. All analyses were conducted in R using two‐tailed tests and a type I error rate of 0.05.
3. RESULTS
3.1. Estimates over the study period
Demand estimates for unconstrained cocaine demand (top panel) and elasticity (middle panel) over the 15‐day test period are plotted in Figure 2. Significant linear effects of Day were not observed for unconstrained cocaine demand (b < 0.001, P = 0.99) or elasticity (b = 0.006, P = 0.08). Significant improvements in model fit (R 2) were observed over the 15‐day period (b = 0.005, P < 0.001; Figure 2, bottom panel).
Figure 2.
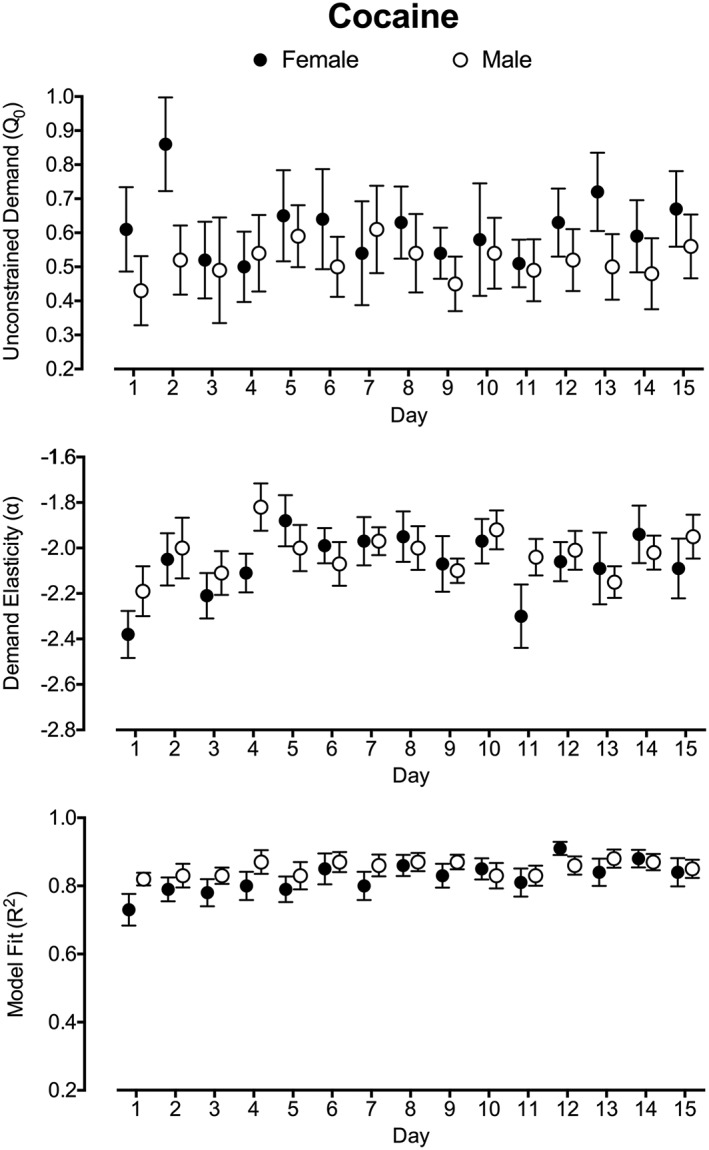
Cocaine demand parameters across the 15‐d study period. Plotted are unconstrained demand (Q 0; top panel), demand elasticity (α; middle panel), and model fit (R 2; bottom panel) over the 15‐d period. Q 0 and α are plotted as the log‐transformed value to correct for substantive variable skew in data analysis. Plotted are mean values with standard error. Filled circles represent estimates for female subjects, and open circles represent estimates for male subjects
Demand estimates for unconstrained remifentanil demand (top panel) and elasticity (middle panel) over the 15‐day test period are plotted in Figure 3. A significant linear increase in unconstrained remifentanil demand was observed over the 15‐day period (b = 0.007, P = 0.002). No effects of Day were observed for elasticity (b = 0.003, P = 0.15). A significant improvement in model fit was observed over the 15‐day period (b = 0.003, P < 0.001; Figure 3, bottom panel). Inclusion of model fit in the model predicting unconstrained demand did not change the significance or direction of the linear increase (b = 0.005, P = 0.03).
Figure 3.
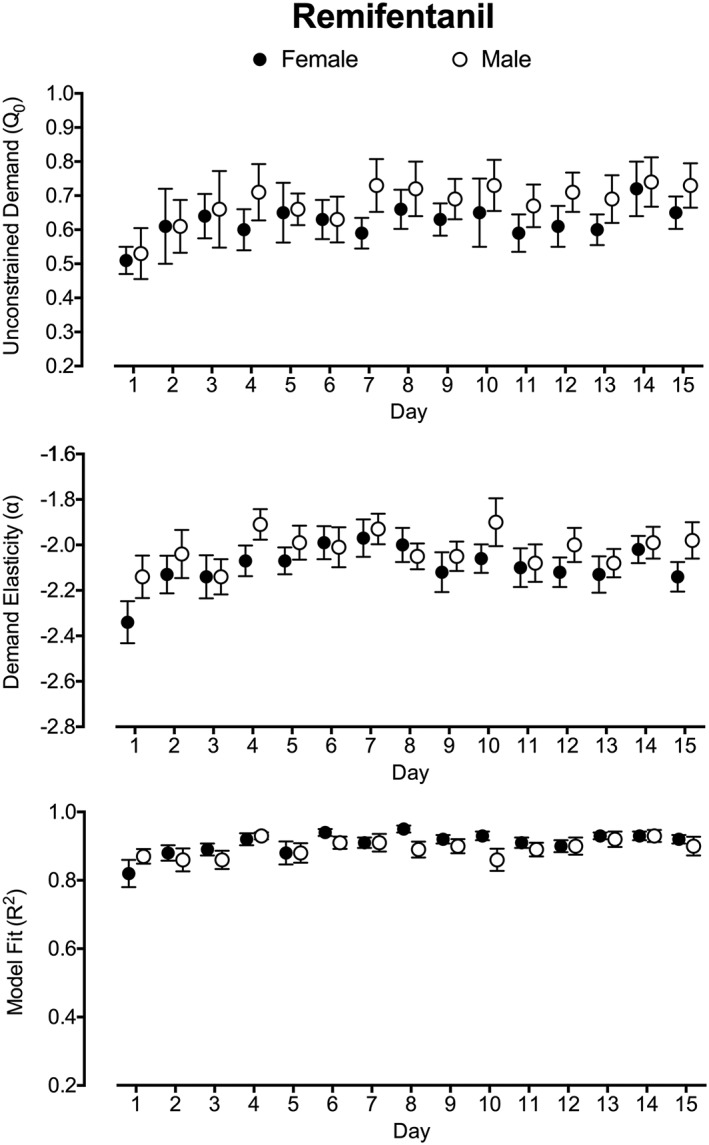
Remifentanil demand parameters across the 15‐d study period. Plotted are unconstrained demand (Q 0; top panel), demand elasticity (α; middle panel), and model fit (R 2; bottom panel) over the 15‐d period. Q 0 and α are plotted as the log‐transformed value to correct for substantive variable skew in data analysis. Plotted are mean values with standard error. Filled circles represent estimates for female subjects, and open circles represent estimates for male subjects
3.2. Sex differences
Significant sex differences were not observed for unconstrained cocaine demand (b = 0.102, P = 0.26) or elasticity (b = −0.082, P = 0.44). Similarly, significant sex differences were not observed for unconstrained remifentanil demand (b = −0.059, P = 0.32) or elasticity (b = −0.086, P = 0.29). Additional models including time or model fit as covariates did not change the direction or significance of these results.
3.3. Estrous phase effects
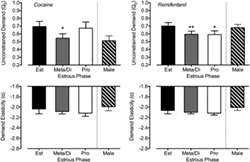
Significant effects of estrous phase were observed for unconstrained cocaine demand in which lower unconstrained demand was observed during meta/diestrus (b = −0.149, P = 0.02) as compared with estrus. Proestrus did not significantly differ from estrus (b = −0.019, P = 0.82). Estimates from this model are plotted in Figure 4 (left panel). No significant estrous phase effects were observed for cocaine demand elasticity (P values > 0.19). Additional models including time or model fit as covariates did not change the significance or direction of these results. Similarly, no differences in model fit were observed as a function of estrous phase (P values > 0.65).
Figure 4.
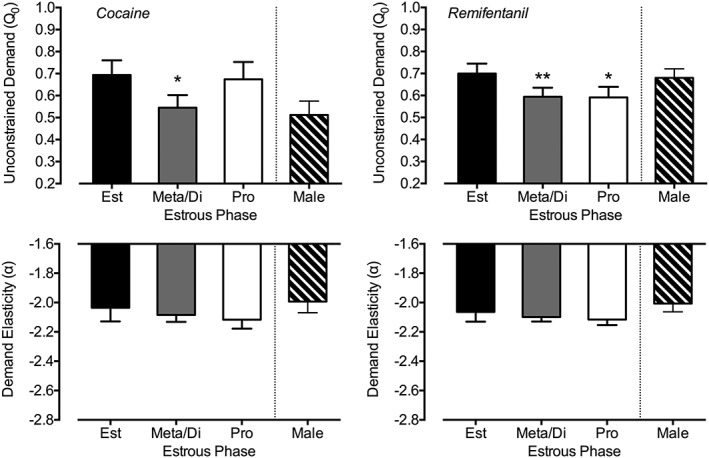
Estrous cycle effects on unconstrained demand (Q 0; top panels) and demand elasticity (α; bottom panels). Plotted are estimates from linear mixed‐effect models predicting cocaine (left panels) and remifentanil (right panels) by estrous phase. Values represent estimated value during estrus (Est; black bar), meta/diestrus (Meta/Di; gray bar), and proestrus (Pro; white bar) with error bars as standard error. Also included are values for male subjects (crossed bar) derived from mixed models evaluating sex differences. Demand values are plotted as the modeled log‐transformed values. * P < 0.05; ** P < 0.01 comparing with estrous phase in female subjects
Significant effects of estrous phase were also observed for unconstrained remifentanil demand in which lower unconstrained demand was observed during proestrus (b = −0.108, P = 0.01) and meta/diestrus (b = −0.105, P = 0.002) as compared with estrus. Estimates from this model are plotted in Figure 4 (right panel). No significant estrous phase effects were observed for remifentanil demand elasticity (P values > 0.18). Additional models including Day or model fit as covariates did not change the significance or direction of these results. Similarly, no differences in model fit were observed as a function of estrous phase (P values > 0.59).
3.4. Contribution of drug history to demand intensity and elasticity
A significant effect of remifentanil exposure on unconstrained cocaine demand was observed. Specifically, subjects exposed to remifentanil first showed a positive and significant association between average unconstrained remifentanil demand and unconstrained cocaine demand during postexposure testing (b = 0.975, P < 0.001). Notably, no differences were observed in unconstrained remifentanil demand between the two exposure groups indicating that this effect could not be explained by differences in overall intake between groups (effect of exposure: b = 0.082, P = 0.16). No relationship was observed between previous cocaine exposure and unconstrained remifentanil demand (b = −0.038, P = 0.79). These effects did not differ by sex or estrous cycle as indicated by nonsignificant interactions in moderation models and no changes in the main effect of exposure with inclusion of sex or estrous cycle as covariates. The remifentanil exposure effect is summarized in Figure 5 using simple slopes (top panel), which depict unconstrained cocaine demand at varying levels of average unconstrained remifentanil demand as a function of exposure group.
Figure 5.
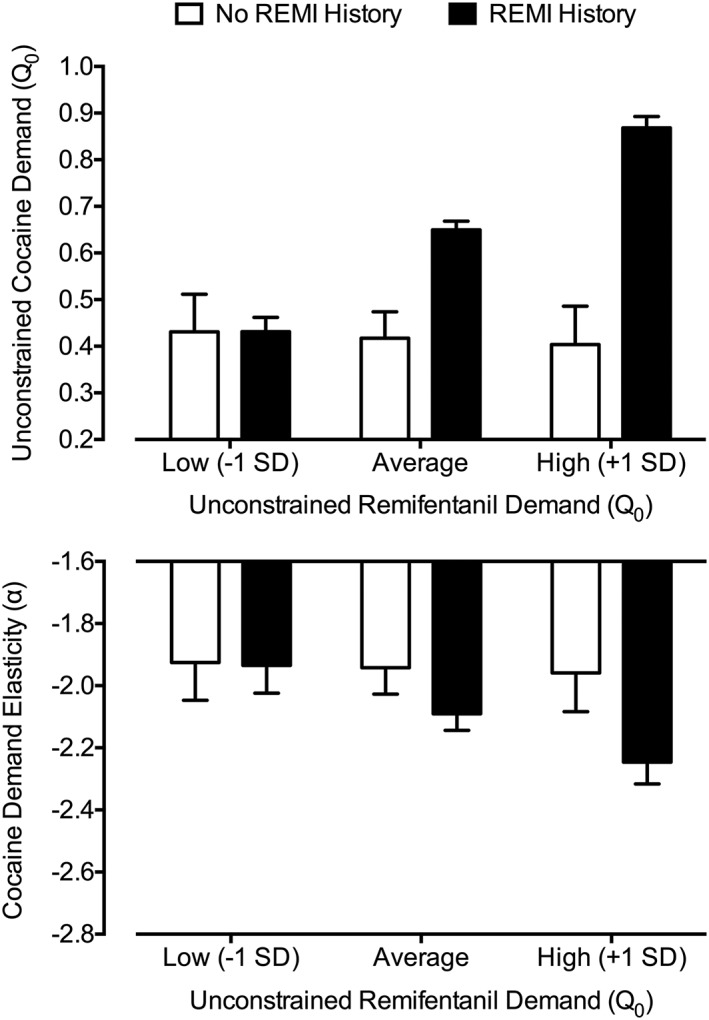
Effect of remifentanil exposure history of on cocaine demand. Plotted are simple slope estimates from linear mixed‐effect models predicting unconstrained cocaine demand (top panel) and cocaine demand elasticity (bottom panel). Estimates were generated for a subject at the average unconstrained remifentanil demand value, one standard deviation below this average (low unconstrained demand) and one standard deviation above this average (high unconstrained demand). Plots depict the modeled relationship between varying levels of unconstrained remifentanil demand and recorded cocaine values. Subjects with a prior history of remifentanil exposure first completed threshold testing for remifentanil then cocaine (a significant relationship was observed for both outcomes). Subjects with no prior history first completed threshold testing for cocaine then remifentanil (no significant relationship was observed for both outcomes). Error bars represent standard error
A significant effect of remifentanil drug history on cocaine demand elasticity was also observed. Specifically, subjects exposed to remifentanil first showed a negative and significant association between average unconstrained remifentanil demand and cocaine demand elasticity during later threshold testing (ie, greater remifentanil intake = more inelastic cocaine demand; b = −0.694, P = 0.02). Notably, no differences were again observed in remifentanil demand elasticity between the two exposure groups indicating that this effect could not be explained by behavioral differences during remifentanil testing in the first exposure group. No relationship was observed between a prior history of cocaine exposure and remifentanil demand elasticity (b = −0.212, P = 0.36). These effects did not differ by sex or estrous cycle. The remifentanil exposure effect is summarized in Figure 5 using simple slopes (bottom panel), which depict cocaine demand elasticity at varying levels of unconstrained remifentanil demand as a function of exposure group.
4. DISCUSSION
The primary goal of the present study was to evaluate sex and estrous cycle differences in cocaine and remifentanil demand. No significant sex differences were observed between male and female subjects on measures of cocaine or remifentanil unconstrained demand or elasticity. However, differences in unconstrained demand, but not elasticity, were observed as a function of estrous phase in which female subjects showed greater unconstrained demand for cocaine during estrus compared with metaestrus and diestrus. Similarly, unconstrained remifentanil demand was highest during estrus compared with other phases. These findings advance prior research by evaluating the impact of gonadal hormones on drug demand using a sensitive within‐subject demand procedure. The benefits of this approach included the ability to index day‐to‐day fluctuations in the relative reinforcing drug effects and to do so in a way that partitions these effects into distinct behavioral mechanisms of unconstrained demand and elasticity.
Although a large number preclinical studies have identified an increased vulnerability to the reinforcing effects of drugs in females, some studies report no sex differences when assessing the reinforcing effects of drugs (see Becker et al16). Of particular note, the only other preclinical study at the time of this study to systematically evaluate sex differences in drug demand found no differences between male and female rats in nicotine demand.24 A contemporaneously conducted study also found no differences in cocaine demand between male and female rats during baseline testing consistent with the results reported here.38 In the human laboratory, sex differences in cocaine demand were not found in one study,39 but others have reported more inelastic cocaine demand37 and higher unconstrained opioid demand40 in men. These findings combined with the current results highlight the complex interaction that sex differences may have with other factors such as drug class, schedule of reinforcement, and other environmental factors in determining the impact of abuse liability (see review by Becker et al16).
Despite the lack of robust sex differences, clear effects of estrous cyclicity were observed for unconstrained cocaine and remifentanil demand. These findings are consistent with prior preclinical work that has identified augmented drug effects during periods of the estrous phase in which estrogen circulation is high (see Anker and Carroll15 for review). These findings are also consistent with human laboratory work that shows greater unconstrained demand for cigarettes during the follicular compared with luteal phase.28 While the present study does support previous findings indicating that drug self‐administration behavior is elevated during the estrus phase of freely cycling females, we did not find a reduction in unconstrained cocaine demand during proestrus. Overall, progesterone levels (and its metabolites) peak in proestrus, immediately prior to estrus, and have been shown to attenuate cocaine self‐administration when progestins were administered exogenously.41, 42 One potential explanation for the difference reported here is the use of a previously untested procedure to evaluate the effects of estrous cycle (ie, the within‐session threshold procedure). As noted above, this procedure allowed us to partition drug‐taking behavior into two unique behavioral mechanisms (ie, unconstrained demand and elasticity), and it is possible that estrous phase impacts these specific mechanisms in a different way than it impacts more traditional and global metrics of drug reinforcement. Future studies may also increase the precision of detecting circulating gonadal hormone levels by collecting blood samples during threshold testing.22 In this regard, we also did not observe an effect on demand elasticity. Many factors could explain the lack of an effect on demand elasticity by estrous phase. These could include parametric manipulations such as strain differences or variations in the testing environment. These also could include testing that occurs in different modeled stages of substance use disorder, such as distinctions between early acquisition and maintenance versus behaviors following long‐access exposure and binge‐like use. Each of these and other factors are important individual difference factors that should be systematically evaluated in future research.
Overall, tracking estrous cycle is a noninvasive approach to evaluating the effects of natural variations in hormone levels on drug‐relevant behaviors. The current findings provide a clear demonstration of the benefits of this low cost addition to laboratory studies given that the evaluation of gonadal hormones rather than using biological sex alone would have revealed no significant differences. These findings also demonstrate the benefits of using data analytic methods, such as mixed‐effect models, that can fully incorporate the longitudinal structure of a research design. Specifically, such longitudinal methods, as demonstrated here, can improve precision and power by incorporating subjects with missing data, evaluating ordered temporal patterns, including continuous variables as within‐subject predictors, and efficiently parametrizing between‐ and within‐subject variances.
Many of the significant effects observed in this experiment related to unconstrained demand rather than demand elasticity. The divergence between these two outcomes is not surprising given that these metrics are thought to reflect distinct behavioral mechanisms underlying relative reinforcing efficacy. Unconstrained demand specifically represents a theoretical consumption with no cost that takes into account the totality of responding across a demand curve. This measure is thought to reflect a hedonic set point of consumption that an organism defends in the face of environmental constraints (see Bentzley et al10). As noted in Section 1, unconstrained demand has shown good construct validity in addiction science and been associated with risk and protective factors relevant to substance use disorder. As specific examples from the preclinical literature, increased levels of Q 0 have been observed during contact with a drug‐using peer43 and following long‐access drug exposure (ie, escalation procedures11), whereas decreased levels have been observed following treatment with putative pharmacotherapies (eg, oxytocin11). Similarly, at the clinical level, Q 0 has been shown to decrease following pharmacological intervention and is predictive of prospective changes in drug‐taking behavior within and outside intervention contexts (eg, Bujarski et al44, Dennhardt et al45, and Heckman et al46). This translational relevance for substance use and substance use disorder highlights the utility of measuring the distinct behavioral mechanisms underlying drug reinforcement that are offered by demand procedures.
The secondary goals of this study included evaluating temporal and exposure effects on drug demand. These aims were accomplished through the use of a longitudinal crossover design in which subjects were randomized to testing order. We detected an increase in unconstrained remifentanil demand across the 15‐day testing period representing an escalation of drug taking in the 2‐hour testing session. Escalation is typically identified using animal models in which subjects are given longer access to drug (eg, 6 h). However, escalation has also been observed using 1‐hour test sessions and in this way has been suggested to relate to context‐dependent discrimination learning.47 It is possible that such discrimination learning could explain the escalation observed here in that subjects learned to discriminate between the individual components of a session using the dose available and subsequently increased intake during the early session components to maximize responding at low unit price. However, this explanation would not fully account for the observation that escalation was observed with remifentanil and not cocaine. Increases in model fit were also observed over the 15‐day period, which could indicate that behavior was increasingly systematic and controlled by the parameters of the testing procedure. Important to note, however, is that the inclusion of model fit did not change the reported results demonstrating that these findings were not a product of increases in systematic responding or practice and training effects.
A robust and pronounced exposure effect was observed in which subjects first exposed to remifentanil showed higher levels of unconstrained demand and more inelastic demand for cocaine that was significantly associated with the extent of unconstrained remifentanil demand. A similar relationship was not found for subjects first exposed to cocaine indicating that this relationship was likely due to pharmacological exposure and directionally specific. These associations also did not differ by sex or estrous cycle suggesting a generalizability across these individual difference factors. Exposure‐dependent increases in cocaine demand following opioid use are consistent with both prior preclinical and clinical work. For example, nonhuman primates show more inelastic and higher unconstrained demand for cocaine during morphine withdrawal as well as 4 to 5 weeks after chronic exposure.9 Clinically, cocaine administration reduces the overall severity of naloxone‐precipitated withdrawal in humans.48 This reduction in opioid withdrawal symptomology may partially explain the observed results in the present study. Specifically, it has been shown that noradrenergic activity becomes hyperactive in opioid withdrawal and this hyperactivity may be attenuated through cocaine‐induced reductions in norepinephrine release via alpha‐2 autoreceptor activation.49 Our findings combined with prior literature suggest the need to further examine the mechanisms by which opioid use promotes the observed increases in psychomotor stimulant use.
The results of this study should be considered within the context of its limitations. Model fits for the exponentiated demand equation were generally higher for remifentanil than cocaine, which could indicate a greater validity for the remifentanil findings. However, as noted above, controlling for model fit in the tested models did not alter the pattern or significance of results. Strain‐dependent effects were not evaluated, and only Long‐Evans rats were used. This may be particularly relevant for the sex or estrous differences given prior research demonstrating sex by strain interactions in substance‐related behaviors (eg, Jones et al50). We also did not manipulate gonadal hormone levels and instead relied upon variations across freely cycling estrous phases. This approach provides some benefits given the noninvasive nature of the method and similarities to natural fluctuations observed in the human condition. Nevertheless, future studies should evaluate the effects of experimentally manipulated gonadal hormones on measures of drug demand.
This study represents one of the first systematic preclinical evaluations of the effects of sex and estrous cycle on cocaine and opioid demand. These data suggested minimal overt differences in demand outcomes between male and female subjects; however, these indicated that unconstrained demand, but not elasticity, was associated with estrous cyclicity. We also found evidence for the escalation of unconstrained opioid demand over a 15‐day period and that opioid exposure enhances subsequent unconstrained demand and reduces elasticity for psychomotor stimulants. Collectively, these findings contribute to the ongoing literature utilizing behavioral economic demand to understand behavioral mechanisms underlying individual differences in substance use.
DISCLOSURE/CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHORS CONTRIBUTION
RTL and JCS were responsible for the study concept and design. RTL and BPA contributed to data acquisition, and JCS assisted with data analysis. RTL and JCS drafted the manuscript. All authors provided critical revision and reviewed and approved the final version for publication.
ACKNOWLEDGEMENTS
This research was supported by Research Development Funds from Franklin & Marshall College (R.T.L.) and the National Science Foundation Grant 1247392 (J.C.S.).
Lacy RT, Austin BP, Strickland JC. The influence of sex and estrous cyclicity on cocaine and remifentanil demand in rats. Addiction Biology. 2020;25:e12716 10.1111/adb.12716.
REFERENCES
- 1. Bickel WK, Snider SE, Quisenberry AJ, Stein JS. Reinforcer pathology: the behavioral economics of abuse liability testing. Clin Pharmacol Ther. 2017;101(2):185‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hursh SR, Roma PG. Behavioral economics and empirical public policy. J Exp Anal Behav. 2013;99(1):98‐124. [DOI] [PubMed] [Google Scholar]
- 3. MacKillop J. The behavioral economics and neuroeconomics of alcohol use disorders. Alcohol Clin Exp Res. 2016;40(4):672‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bidwell LC, MacKillop J, Murphy JG, Tidey JW, Colby SM. Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addict Behav. 2012;37(11):1257‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115(1):186‐198. [DOI] [PubMed] [Google Scholar]
- 6. Kaplan BA, Foster RN, Reed DD, Amlung M, Murphy JG, MacKillop J. Understanding alcohol motivation using the alcohol purchase task: a methodological systematic review. Drug Alcohol Depend. 2018;191:117‐140. [DOI] [PubMed] [Google Scholar]
- 7. Johnson MW, Bickel WK. Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav. 2006;85(1):73‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strickland JC, Abel JM, Lacy RT, et al. The effects of resistance exercise on cocaine self‐administration, muscle hypertrophy, and BDNF expression in the nucleus accumbens. Drug Alcohol Depend. 2016;163:186‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wade‐Galuska T, Galuska CM, Winger G. Effects of daily morphine administration and deprivation on choice and demand for remifentanil and cocaine in rhesus monkeys. J Exp Anal Behav. 2011;95(1):75‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bentzley BS, Fender KM, Aston‐Jones G. The behavioral economics of drug self‐administration: a review and new analytical approach for within‐session procedures. Psychopharmacology (Berl). 2013;226(1):113‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bentzley BS, Jhou TC, Aston‐Jones G. Economic demand predicts addiction‐like behavior and therapeutic efficacy of oxytocin in the rat. Proc Natl Acad Sci U S A. 2014;111(32):11822‐11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Groblewski PA, Zietz C, Willuhn I, Phillips PE, Chavkin C. Repeated stress exposure causes strain‐dependent shifts in the behavioral economics of cocaine in rats. Addict Biol. 2015;20(2):297‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leonard MZ, DeBold JF, Miczek KA. Escalated cocaine “binges” in rats: enduring effects of social defeat stress or intra‐VTA CRF. Psychopharmacology (Berl). 2017;234(18):2823‐2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oleson EB, Richardson JM, Roberts DC. A novel IV cocaine self‐administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre‐treatments on cocaine consumption and maximal price paid. Psychopharmacology (Berl). 2011;214(2):567‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones In: Neill J, Kulkarni J, eds. Curr Top Behav Neuro. Berlin, Heidelberg: Springer; 2011:73‐96. [DOI] [PubMed] [Google Scholar]
- 16. Becker JB, McClellan ML, Reed BG. Sex differences, gender and addiction. J Neurosci Res. 2017;95(1‐2):136‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693‐699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerstetter KA, Su ZI, Ettenberg A, Kippin TE. Sex and estrous cycle differences in cocaine‐induced approach‐avoidance conflict. Addict Biol. 2013;18(2):222‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659‐674. [DOI] [PubMed] [Google Scholar]
- 20. Sofuoglu M, Dudish‐Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274‐283. [DOI] [PubMed] [Google Scholar]
- 21. Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self‐administration in rats. Psychopharmacology (Berl). 2016;233(17):3201‐3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lynch WJ. Acquisition and maintenance of cocaine self‐administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl). 2008;197(2):237‐246. [DOI] [PubMed] [Google Scholar]
- 23. Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self‐administration on a progressive ratio schedule in rats. Psychopharmacology (Berl). 1989;98(3):408‐411. [DOI] [PubMed] [Google Scholar]
- 24. Grebenstein P, Burroughs D, Zhang Y, LeSage MG. Sex differences in nicotine self‐administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav. 2013;114‐115:70‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Green R, Ray LA. Effects of varenicline on subjective craving and relative reinforcing value of cigarettes. Drug Alcohol Depend. 2018;188:53‐59. [DOI] [PubMed] [Google Scholar]
- 26. Quisenberry AJ, Koffarnus MN, Epstein LH, Bickel WK. The Experimental Tobacco Marketplace II: substitutability and sex effects in dual electronic cigarette and conventional cigarette users. Drug Alcohol Depend. 2017;178:551‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strickland JC, Stoops WW. Stimulus selectivity of drug purchase tasks: A preliminary study evaluating alcohol and cigarette demand. Exp Clin Psychopharmacol. 2017;25(3):198‐207. [DOI] [PubMed] [Google Scholar]
- 28. Farris SG, Abrantes AM, Zvolensky MJ. Emotional distress and tobacco demand during the menstrual cycle in female smokers. Cogn Behav Ther. 2018;1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self‐administered cocaine and heroin in rats. Psychopharmacology (Berl). 1999;144(1):77‐82. [DOI] [PubMed] [Google Scholar]
- 30. Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long‐ or short‐access to cocaine self‐administration. Pharmacol Biochem Behav. 2004;78(2):199‐207. [DOI] [PubMed] [Google Scholar]
- 31. Bertz JW, Jackson EL, Barron DR, Woods JH. Effects of sex and remifentanil dose on rats' acquisition of responding for a remifentanil‐conditioned reinforcer. Behav Pharmacol. 2016;27(2‐3 Spec Issue):137‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacy RT, Schorsch HK, Austin BP. Adolescent d‐amphetamine exposure enhances the acquisition of cocaine self‐administration in male and female rats. Exp Clin Psychopharmacol. 2018;26(1):18‐28. [DOI] [PubMed] [Google Scholar]
- 33. Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. Bethesda, MD: U.S. Dept. of Health and Human Services, Public Health Service; 2011. [Google Scholar]
- 34. Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80(2):84‐97. [DOI] [PubMed] [Google Scholar]
- 35. Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80(2):79‐87. [DOI] [PubMed] [Google Scholar]
- 36. Koffarnus MN, Franck CT, Stein JS, Bickel WK. A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol. 2015;23(6):504‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strickland JC, Lile JA, Rush CR, Stoops WW. Comparing exponential and exponentiated models of drug demand in cocaine users. Exp Clin Psychopharmacol. 2016;24(6):447‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kawa AB, Robinson TE. Sex differences in incentive‐sensitization produced by intermittent access cocaine self‐administration. Psychopharmacology (Berl). 2018;1‐15. 10.1007/s00213-018-5091-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruner NR, Johnson MW. Demand curves for hypothetical cocaine in cocaine‐dependent individuals. Psychopharmacology (Berl). 2014;231(5):889‐897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pickover AM, Messina BG, Correia CJ, Garza KB, Murphy JG. A behavioral economic analysis of the nonmedical use of prescription drugs among young adults. Exp Clin Psychopharmacol. 2016;24(1):38‐47. [DOI] [PubMed] [Google Scholar]
- 41. Anker JJ, Zlebnik NE, Carroll ME. Differential effects of allopregnanolone on the escalation of cocaine self‐administration and sucrose intake in female rats. Psychopharmacology (Berl). 2010;212(3):419‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self‐administration in rats. Neuropsychopharmacology. 2006;31(1):129‐138. [DOI] [PubMed] [Google Scholar]
- 43. Robinson AM, Fronk GE, Zhang H, Tonidandel S, Smith MA. The effects of social contact on cocaine intake in female rats. Drug Alcohol Depend. 2017;177:48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bujarski S, MacKillop J, Ray LA. Understanding naltrexone mechanism of action and pharmacogenetics in Asian Americans via behavioral economics: a preliminary study. Exp Clin Psychopharmacol. 2012;20(3):181‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dennhardt AA, Yurasek AM, Murphy JG. Change in delay discounting and substance reward value following a brief alcohol and drug use intervention. J Exp Anal Behav. 2015;103(1):125‐140. [DOI] [PubMed] [Google Scholar]
- 46. Heckman BW, Cummings KM, Nahas GJ, et al. Behavioral economic purchase tasks to estimate demand for novel nicotine/tobacco products and prospectively predict future use: evidence from the Netherlands. Nicotine Tob Res. 2018. 10.1093/ntr/nty042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Beckmann JS, Gipson CD, Marusich JA, Bardo MT. Escalation of cocaine intake with extended access in rats: dysregulated addiction or regulated acquisition? Psychopharmacology (Berl). 2012;222(2):257‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kosten TA. Cocaine attenuates the severity of naloxone‐precipitated opioid withdrawal. Life Sci. 1990;47(18):1617‐1623. [DOI] [PubMed] [Google Scholar]
- 49. Pitts DK, Marwah J. Chronic cocaine reduces α2‐adrenoceptor elicited mydriasis and inhibition of locus coeruleus neurons. Eur J Pharmacol. 1989;160(2):201‐209. [DOI] [PubMed] [Google Scholar]
- 50. Jones JD, Busse GD, Riley AL. Strain‐dependent sex differences in the effects of alcohol on cocaine‐induced taste aversions. Pharmacol Biochem Behav. 2006;83(4):554‐560. [DOI] [PubMed] [Google Scholar]


