To the Editor:
1.
Recently a method has been developed to assess red blood cell (RBC) deformability as a function of oxygen tension (pO2).1 This method, called oxygen gradient ektacytometry or the oxygenscan, is particularly useful for evaluating individuals affected by sickle cell disease (SCD). Sickle cell disease is caused by a single point mutation in the β‐globin gene (p.Glu7Val) leading to the production of an abnormal hemoglobin S (HbS). Abnormal hemoglobin S polymerizes under deoxygenation, which causes RBCs to take on a sickle shape. These sickled RBCs are poorly deformable and adhere to the endothelium, which contributes to painful vaso‐occlusive crises and chronic anemia.2
The oxygenscan allows the determination of maximum RBC deformability under normoxic conditions (EImax). It also allows determination of minimum RBC deformability under hypoxic conditions (EImin), and the specific pO2 level at which RBC sickling occurs (ie, the point of sickling; PoS).1 Rab et al1 recently demonstrated that hydroxyurea and blood transfusion increase EImax and EImin, and decrease PoS, indicating that these therapies are efficient in improving the rheological behavior of RBCs, both in normoxic and hypoxic conditions. Moreover, the authors have shown that this technique has low inter sample variability (coefficient of variation <5%) and is very well suited to detect the effects of drugs that alter the affinity of hemoglobin for oxygen such as Voxelotor (GBT440), a promising drug recently tested in SCD.1, 3 The joint experience of the authors of this study with the oxygenscan has prompted us to study methodological aspects and pre‐analytical factors that could influence key oxygenscan parameters. A better understanding of these aspects and factors will strongly enhance reproducibility of results and will enable inter‐laboratory comparison of results and collaboration.
The Laser Optical Rotational Red Cell Analyzer (Lorrca, RR Mechatronics, Zwaag, The Netherlands) with the oxygenscan module was used. The ektacytometer measures RBC deformability (Elongation Index, EI) as a function of continuously changing oxygen concentrations. For this study, a standardized volume (50 μL) of ethylenediaminetetraacetic acid (EDTA) blood from SCD patients was mixed with 5 mL of high viscous iso‐osmolar polyvinylpyrrolidone (PVP) suspension (viscosity ∼ 30 cP). The sample solution was inserted into the couette system of the Lorrca, which exposes the cells to shear stress (30 Pa, 37°C). At the same time, the pO2 gradually decreases from 160 mmHg to values below 20 mmHg, after which pO2 returns to normoxic values (for detailed description of the method see Rab et al 1). In this study, blood samples from 64 SCD patients were collected in three different centers to evaluate the effects of: (a) the time between blood sampling and measurement, (b) the amount of RBCs mixed with PVP, (c) the camera gain settings which controls the amount of light entering into the diaphragm of the camera, thereby changing the laser diffraction pattern, and (d) the speed of deoxygenation (Proportional integral control; PI control). The clinically most relevant parameters EImax, EImin and PoS were determined for each condition tested. Statistical analysis was done by Wilcoxon T test, a P value <0.05 was considered significant. Details of the different conditions used are mentioned in the legend of the figure.
Our results show that 24 hours of blood storage (4°C) compared to 4 hours of storage (at room temperature) significantly increased the EImax (Figure 1A). EImax also increased significantly with a lower camera gain (Figure 1G). Although the effect was less pronounced, together, this indicates that deformability measured during normoxia is influenced by the age of the sample as well as the height of the diffraction pattern.
Figure 1.
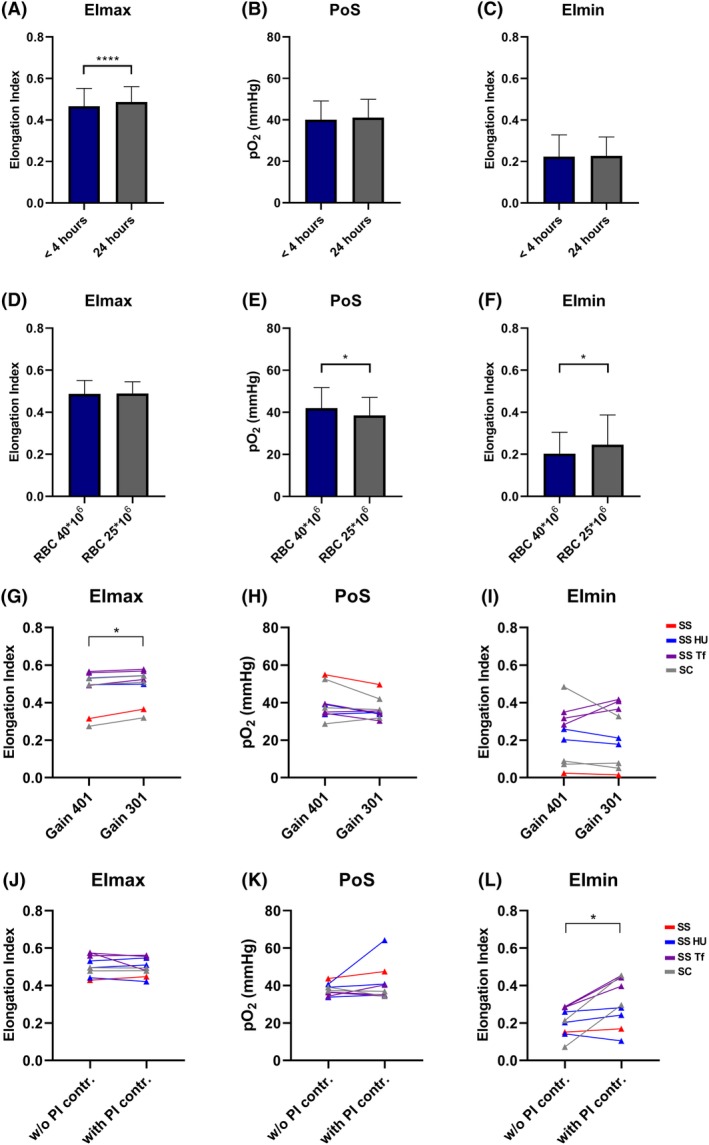
Lorrca settings and sample handling influences key oxygenscan measurements EImax, PoS and EImin. (A) RBCs of an untreated patient with HbSS (n = 1), on hydroxyurea (n = 7), on chronic transfusion therapy (n = 9), on HU and transfusion (n = 11) and 2 patients with hemoglobin SC, were measured within 4 hours after blood collection (stored at room temperature) and after 24 hours (stored at 4°C). Maximum deformability (EImax) was significantly higher when measured after 24 hours; (B) Point of Sickling (PoS) showed no difference; (C) Deformability after deoxygenation (EImin) did also not differ; (D) Different amounts of RBCs of untreated HbSS patients (n = 4), on hydroxyurea (n = 5), on chronic transfusion therapy (n = 4), on HU and transfusion (n = 3), and 1 HbSC patient and 1 HbS/δ‐thal patient were measured. EImax did not vary; (E) PoS was significantly lower when the amount of RBCs used for the oxygenscan are low (25*106 RBCs/mL PVP) compared to reference RBC count (40*106 RBCs/mL PVP); (F) EImin was significantly higher with low RBC count; (G) Adjusting the size of the diffraction pattern by changing the gain (401 increases the pattern, while 301 makes the pattern smaller) only significantly affects EImax. This was investigated with RBCs of six HbSS patients (two treated with HU, three with transfusion, and one untreated) and three HbSC patients; (H) The PoS did not differ when the gain was changed, whilst individual values are different depending on genotype and treatment; (I) EImin did not differ whilst individual values are different depending on genotype and treatment; (J) The PI control permits slower deoxygenation, adjusting its speed to the individual patient RBCs. This was investigated in seven HbSS patients (three treated with HU, three with transfusion, and pne untreated) and two HbSC patients. EImax was not different; (K) The PoS showed now difference; (L) EImin was higher when PI control is used, especially in SCD patients on transfusion therapy and HbSC patients. HU, hydroxyurea, Tf, transfusion, w/o, without. PI contr., proportional integral control. Bars represent means, error bars represent SD. ****P < .0001, *P < .05
EImin was significantly increased when less RBCs were used for the measurement (Figure 1F) and when PI control was switched on (Figure 1L). Together, this indicates that deformability under hypoxic conditions is dependent on the amount of RBCs used for measurements, and the speed at which deoxygenation occurs.
The PoS was only significantly affected by the amount of RBCs used, in a sense that a lower number of RBCs was associated with a lower oxygen concentration at which RBCs start to sickle (ie, PoS; Figure 1E). We also noted that methodological factors, that is, the camera gain and deoxygenation speed, had different effects on variance caused by the genetic background or treatment. In particular RBCs from patients on chronic transfusion and patients with HbSC behaved differently at different machine settings (see details in the legends of the figure). There was greater influence of pre‐analytical factors and run to run variability when blood from HbSC patients were used.
The present study clearly shows that key oxygenscan parameters (EImax, PoS and EImin) are dependent on methodological aspects and pre‐analytical conditions. As a consequence, intra‐ and between‐laboratories comparisons imply the need for standardization. Therefore, we believe that each laboratory using this technique should perform oxygenscan measurements in a standardized way.
The degree of anemia is highly variable in SCD. Some patients have a hematocrit lower than 20%, while others (Hemoglobin SC patients) have mild‐to‐moderate anemia. We recommend performing an RBC count prior to oxygenscan measurements to standardize the amount of RBCs used. An RBC count of 40*106/mL, for example, ensures that a sufficient amount of RBCs will be present in the couette system of the Lorrca, to render a reliable high quality diffraction pattern. Another pre‐analytical aspect is the time between collecting a sample and performing the actual measurement. Several laboratories are not able to perform measurements within 4 hours due to shipping time. Since the time between blood sampling and analysis affects EImax, we recommend that each individual laboratory performs oxygenscan measurements at a standardized time point. From a practical point of view storing samples overnight before measurements would be the preferred option, allowing for shipment of samples from other hospitals.
Our study also demonstrates that speed of deoxygenation and camera gain are important methodological parameters that influence outcome parameters of the oxygenscan module of the Lorrca. Regardless of the choice of settings we recommend that users control these methodological factors strictly in order to ensure correct interpretation of results.
We strongly believe that standardization of oxygenscan measurements will enable comparisons as well as collaborations between the different laboratories studying RBC rheology in SCD. This is particularly important now given the high number of new therapies that are currently being developed for SCD. Thus, standardized oxygenscan measurements have the potential to become an important tool in the evaluation of new treatment strategies, personalized medicine and the prediction of complications in SCD.
ACKNOWLEDGMENTS
This research has been funded in part by Eurostars grant estar18105 and by an unrestricted grant provided by RR Mechatronics.
REFERENCES
- 1. Rab MAE, van Oirschot BA, Bos J, et al. Rapid and reproducible characterization of sickling during automated deoxygenation in sickle cell disease patients. Am J Hematol. 2019;94:575‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connes P, Alexy T, Detterich J, Romana M, Hardy‐Dessources MD, Ballas SK. The role of blood rheology in sickle cell disease. Blood Rev. 2016;30:111‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vichinsky E, Hoppe CC, Ataga KI, et al. A Phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509‐519. [DOI] [PubMed] [Google Scholar]


