Abstract
Aim
To give evidence‐based recommendations on the application of ketogenic diet parenteral nutrition (KD‐PN) in emergency situations.
Method
An international group of experts (n=14) researched the literature and distributed a survey among 150 expert centers. International accepted guidelines (European Society for Clinical Nutrition and Metabolism/European Society for Paediatric Gastroenterology Hepatology and Nutrition and the American Society for Parenteral and Enteral Nutrition) and handbooks for parenteral nutrition were considered general standards of care.
Results
In the literature, we identified 35 reports of patients treated by KD‐PN. International guidelines and handbooks provided some conflicting information. Twenty‐four expert teams from nine countries responded to the survey, reflecting the limited clinical experience.
Interpretation
This paper highlights 23 consensus‐based recommendations for safe and effective KD‐PN (e.g. diet initiation, calculation, application, monitoring, and evaluation) based on the best evidence available and expert opinions.
What this paper adds
In acute settings, ketogenic diet therapy (KDT) can be administered parenterally.
Parenteral administration of KDT should be started only at the intensive care unit.
Initiate ketogenic parenteral nutrition stepwise to the highest ratio possible with the lowest level of complications.
Evaluate the risk–benefit ratio of parenteral administration continuously.
Restart enteral feeding as soon as appropriate.
What this paper adds
In acute settings, ketogenic diet therapy (KDT) can be administered parenterally.
Parenteral administration of KDT should be started only at the intensive care unit.
Initiate ketogenic parenteral nutrition stepwise to the highest ratio possible with the lowest level of complications.
Evaluate the risk–benefit ratio of parenteral administration continuously.
Restart enteral feeding as soon as appropriate.
This article's abstract has been translated into Spanish and Portuguese.
Follow the links from the http://onlinelibrary.wiley.com/doi/10.1111/dmcn.14306/abstract to view the translations.
This article is commented on by Kossoff on page https://doi.org/10.1111/dmcn.14305 of this issue.
Resumen
Manejo clínico óptimo de los niños que reciben nutrición parenteral cetogénica: una guía de práctica clínica
Objetivo
Dar recomendaciones basadas en evidencia sobre la aplicación de dieta cetogénica en la nutrición parenteral (DC‐NP) en situaciones de emergencia.
MÉtodo
Un grupo de expertos (n=14) investigó la literatura y distribuyó una encuesta en 150 centros especializados. Considerando como estándares de manejo las guías aceptadas internacionalmente (Sociedad Europea para la Nutrición Clínica y Metabolismo/Sociedad Europea de Gastroenterología Pediátrica, Hepatología y Nutrición, y la Sociedad Americana para Nutrición Enteral y Parenteral) y los manuales para la nutrición parenteral.
Resultados
En la literatura se identificaron 35 informes de pacientes tratados por DC‐NP. Las directrices y manuales internacionales proporcionaron alguna información contradictoria. Veinticuatro equipos de expertos de nueve países respondieron a la encuesta, reflejando la limitada experiencia clínica.
InterpretaciÓn
Este documento destaca 23 recomendaciones basadas en consensos para una DC‐NP segura y eficaz (por ejemplo; iniciación de la dieta, cálculo, aplicación, monitoreo y evaluación) basada en la mejor evidencia disponible y las opiniones de expertos.
Resumo
Manejo clínico ótimo de crianças recebendo nutrição cetogênica parenteral: um guia para a prática clínica
Objetivo
Oferecer recomendações baseadas em evidências da aplicação de dieta cetogênica por nutrição parenteral (DC‐NP) em situações de emergência.
MÉtodo
Um grupo internacional de especialistas (n=14) pesquisou a literatura e distribuiu um questionário em 150 centros especializados. Diretrizes internacioansi aceitas (Sociedade Européia de Nutrição Clínica e Metabolismo/ Sociedade Européia de Gastroenterologia, Hepatologia e Nutrição Pediátrica, e a Sociedade Americana de Nutrição Enteral e Parenteral) e livros sobre nutrição parenteral foram considerados padrão geral de atenção.
Resultados
Na literatura, identificamos 35 relados de pacientes tratados por DC‐NP. As diretrizes internacionais e os livros forneceram informações conflitantes. Vinte e quatro equips de especialistas de nove países responderam ao questionário, refletindo a experiência clínica limitada.
InterpretaÇÃo
Este artigo destaca 23 recomendações baseadas em consenso para DC‐NP segura e efetiva (ex: início da dieta, cálculo, aplicação, monitoramento e avaliação) com base na melhor evidência disponível e opiniões de especialistas.
Abbreviations
- FIRES
Febrile infection related epilepsy syndrome
- KD‐PN
Ketogenic diet parenteral nutrition
- KDT
Ketogenic diet therapy
Ketogenic diet therapy (KDT) is a dietary treatment with an established efficacy in children and adolescents for drug‐resistant epilepsy and metabolic disorders (e.g. glucose transporter 1 deficiency and pyruvate dehydrogenase deficiency).1, 2, 3, 4, 5 Ketogenic diets can differ in macronutritional composition: the ratio between ketone producing (fat) and non‐ketone producing (carbohydrates plus protein) nutrients varies between 3.0:1 and 4.0:1. Modified versions of KDT (e.g. the modified Atkins diet, low glycemic index diet) are designed to promote compliance without compromising the effect on seizure reduction.6 There are many ways to administer KDT. In most cases, KDT is given by an enteral diet (orally or by nasogastric tube/percutaneous endoscopic gastrostomy) that is tailored to each individual. It requires detailed calculations of the diet to ensure that both adequate energy for growth and micronutrient requirements are met while ketosis is reached. In some rare conditions such as refractory status epilepticus or febrile infection related epilepsy syndrome (FIRES), KDT needs to be started and sometimes provided through parenteral administration. Parenteral nutrition may also be indicated in the acute setting during temporary or, more rarely, chronic digestive dysfunction in pre‐ or postoperative procedures.
Recently updated international guidelines (European Society for Clinical Nutrition and Metabolism/European Society for Paediatric Gastroenterology Hepatology and Nutrition7, 8 and the American Society for Parenteral and Enteral Nutrition)9, 10 on parenteral nutrition are not applicable to KDT for the amount of carbohydrates recommended to initiate ketosis. There are currently limited data available on ketogenic diet parenteral nutrition (KD‐PN), with only 35 cases reported in the literature.11, 12, 13, 14, 15, 16, 17, 18, 19 Only the White Paper from the Charlie Foundation provides brief recommendations for KD‐PN practice.20
This paper highlights recommendations based on the best evidence combined with opinions of an international group of experts. Consensus was reached on recommendations for achieving safe and effective KD‐PN to facilitate ketosis with the highest diet ratio possible associated with the lowest level of complications. These recommendations will benefit clinical practice for multidisciplinary teams (dieticians and clinical staff in ketogenic diet expert teams and intensive care units) involved in KD‐PN.
Method
An international multidisciplinary team of experts in KDT (n=14) was established at the International Ketogenic Symposium in Banff, Canada, in 2016. A project group (n=7) was launched to address the subject using a survey of current practice in KD‐PN, a literature search, and the standard of care for parenteral nutrition.
International survey
The survey was distributed to 150 registered dieticians working in KDT throughout the world. The goal was to establish recommendations for safe international KD‐PN practice and to identify areas requiring further research. Twenty‐four centers from nine different countries (Canada, England, France, Denmark, Germany, Ireland, the Netherlands, Sweden, and USA) responded to the survey.
In addition, a literature search was compiled from 1995 to 2018 using the keywords ‘ketogenic diet’ and ‘parenteral’ in PubMed. Abstracts were reviewed to select publications in humans only. The papers were then read to identify patients treated by KD‐PN.
The project group (n=7) wrote an initial text that was reviewed by the review group (n=7). After two rounds of review, consensus agreement was reached on the final text, resulting in 23 KD‐PN recommendations. In medical practice the application of these KD‐PN recommendations has to be tailored to the medical protocol of each expert center. It is strongly recommended that centers implementing KDT parenterally have previous experience in enteral KDT.
Literature search
A literature search in PubMed identified 35 reported patients treated with KD‐PN from 1970 to 2018 (Table 1). In most cases, the use of KD‐PN was only for a few days (Table 1). The KD‐PN was then replaced by enteral administration of KDT. The use of parenteral nutrition was related to acute illnesses resulting in intestinal ileus, severe vomiting, or severe diarrhea. Few cases reported prolonged use of parenteral ketogenic diet because of conditions in which the use of the enteral route was challenging.16, 19
Table 1.
Overview of studies and case reports of ketogenic parenteral nutrition
| Number of patients | Indication | Duration | Adverse events | |
|---|---|---|---|---|
| Rosenthal et al.11 | 1 | Gastrointestinal problems during KDT | 4mo | Sepsis at 4mo |
| Roan12 | 1 | Gastrointestinal: protein losing enteropathy during KDT | 12mo (with minimal enteral nutrition) | No |
| Jung et al.13 | 10 | Gastrointestinal problems during KDT |
4.1d (mean) Range 2.6–5.6d |
All: hypertriglyceridemia n=1: increased pancreatic enzymes n=1: increased liver enzymes |
| Strzelcyk et al.14 | 1 | Status epilepticus: nasogastric tube not tolerated | 27d | Not reported |
| Lin et al.15 | 1 | SRSE: gastrointestinal (bleeding) | 8d (5 exclusive TPN) |
Hypertriglyceridemia Increased pancreatic enzymes |
| Chiusolo et al.16 | 1 | RSE: gastrointestinal problems | 8d (3 exclusive TPN) | Increased levels of amylase, lipase, liver enzymes, and triglycerides |
| Appavu et al.17 | 1 | RSE | Not available | No |
| Farias‐Moeller et al.18 | 2 | RSE |
15d 8d |
Hypertrigliceridemia and HLH Hypertrigliceridemia and pancreatitis |
| Dressler et al.19 | 17 |
Status epilepticus: n=3 Surgery: n=9 Illness with gastrointestinal: n=2 Feeding difficulties: n=3 13/17 were already on ketogenic diet |
3d (IQR 2–4d) (range 1–41d) |
n=3: transient hypertriglycerides n=5: hypercholesterol n=1: transient GGT n=1: GOT n=2: GPT n=4: lactate dehydrogenase |
KDT, ketogenic diet therapy; SRSE, super‐refractory status epilepticus; TPN, total parenteral nutrition; RSE, refractory status epilepticus; HLH, hemophagocytic lymphohistiocytosis; IQR, interquartile range; GGT, γ‐glutamyl transferase; GOT, glutamate–oxaloacetate transaminase; GPT, glutamate pyruvate transaminase.
There are currently no data allowing an evaluation of the level of efficacy of parenteral and enteral administration of KDT. The available data from the 35 reported patients show that parenteral ketogenic diet allows the patient to reach a state of ketosis, which is a hallmark of KDT treatment. There are also data reporting the experience of various teams in initiating KDT by parenteral administration and switching to enteral administration after a few days.
The use of KD‐PN may lead to side effects such as hypertriglyceridemia or an increase in pancreatic and liver enzymes (Table 1). The exact significance of these changes is not established, nor whether they are transient or long term. These parameters should be carefully monitored and actively managed. It is assumed that the use of KDT by parenteral administration is associated with the same risk as regular total parenteral nutrition such as sepsis and catheter thrombosis (the authors’ expert opinion).
Recommendations
The 23 recommendations for the application of KD‐PN are listed in Table 2.
Table 2.
Summary of 23 recommendations for application of KD‐PN
| Ketogenic parenteral nutrition should only be started in an intensive care unit |
| 1. In general, indications and contraindications (e.g. fatty acid oxidation deficiencies) for KD‐PN and enteral KDT are similar |
| 2. Start parenteral administration of KDT exclusively in case nothing by mouth >48h is expected |
| 3. Check for contraindications in case of a newly starting KDT |
| 4. Careful evaluation of the medical and nutritional status: infants born preterma and malnourished children are at high risk of complications and therefore KD‐PN should be omitted |
| 5. Change all medications to non‐carbohydrate forms if possible (pill, tablet, pharmacy compounded). Consider using toothpaste with the lowest glycerol and carbohydrate content. The carbohydrate and alcohol content of the intravenous product should be calculated into the final KD‐PN diet ratio |
| 6. Additional fasting can be performed at KDT initiation for a maximum of 24h to induce ketosis for first‐time KDT initiation. Note: hyperketosis and/or hypoglycemia might be accelerated by caloric restriction |
| 7. Obtain baseline nutritional assessment: use ideal weight/height and height/age |
| 8. Fluid: determine the net fluid volume available for parenteral nutrition. Notice the medical status, body weight, excretion, blood electrolytes, acid base status, hematocrit, specific gravity of urine, and urine electrolytes |
| 9. Calories: start with 50% of goal and build this up within a maximum of 1wk when lipids are increased. Aim for resting energy expenditure or 70–80% of calculated energy requirements. Intake of 50% of calculated energy goal might be accepted for a limited period (3–4d). If available, measure resting energy expenditure and respiratory quotient. Notice the risk of hyperalimentation |
| 10. Carbohydrates: the use of glucose/dextrose might be avoided for 3–4d at KD‐PN initiation to benefit ketosis, when this is clinically appropriate as a certain amount of carbohydrates will be delivered by glycerol metabolization from intravenous lipid emulsions. Administer sodium chloride 0.45% or other fluids required on the basis of blood electrolytes. Subsequently, on the basis of ketone and glucose levels and laboratory checks, use the lowest dextrose percentage available or start dextrose 5% solution delivering the amount of carbohydrates of the original enteral ketogenic diet as the maximum |
| 11. Protein: aim for 100% requirements as the goal, with 1.5g/kg/d as a minimum requirement. To maximize ketosis, 0.5–0.8g/kg/d temporary restriction is accepted. During critical illness, additional protein may be required to promote positive nitrogen balance |
| 12. Lipids: start with 50% of goal lipids or 1–2g/kg/d. Increase lipids every 1–2d (on the basis of triglyceride level) up to a maximum of 4g/kg/d. Run lipids continuously. Notice possible allergy or hypersensitivity of the patient to fish, egg, soybean, or peanut protein |
| 13. Diet ratio: start with 1:1 ratio and increase every 1–2d to the highest ratio possible. Aim for a diet ratio within the range 2.0:1–2.9:1 (including glycerol from lipid emulsion) within 3–4d. Aim for the highest diet ratio possible with the lowest level of complications. Consider carnitine supplementation (50mg/kg to a maximum of 1000mg/d) to benefit ketosis |
| 14. Vitamin/mineral supplementations should be age‐appropriate, and consider the weight of the patient |
| 15. Delayed treatment (after 7d) should be avoided for children already on ketogenic diet (to maintain ketosis) or when KDT is required urgently (to reach ketosis) for epilepsy control (RSE or FIRES) |
| 16. Appropriate level of ketosis might not always be achieved; when seizure burden improves this might be accepted |
| 17. Use ASPEN and ESPGHAN/ESPEN guidelines to prevent general complications of parenteral nutrition |
| 18. Side effects of parenteral nutrition during KDT that are most frequently observed are elevated lipids (up to <400mg/dL per 10mmol/L), insufficient ketosis (<1.5mmol/L), hypoglycemia (<2.5mmol/L per 45mg/dL). To a smaller degree: hyperketosis (>6.5mmol/L), hyperbilirubin (>40μmol/L), altered liver function tests, and pancreatic enzymes |
| 19. A tailored nutritional regimen must be carefully chosen and closely monitored |
| 20. KD‐PN initiation during propofol anesthesia is not recommended and should be undertaken only with caution |
| 21. Close monitoring should be completed at baseline, maintained during follow‐up, and tailored to the patient's medical and nutritional status |
| 22. Evaluation will be based on the maintenance of seizure control provided by the initial enteral prescription of the ketogenic diet; in the case of RSE or FIRES, the efficacy should be evaluated both clinically and electroencephalographically |
| 23. Transition to an enteral feed has to be tailored to the medical situation and nutritional status. Start trophic feeds as soon as possible and appropriate |
aInfants born preterm: <36wks postmenstrual age. KD‐PN, ketogenic parenteral nutrition; KDT, ketogenic diet therapy; RSE, refractory status epilepticus; FIRES, febrile infection related epilepsy syndrome; ASPEN, American Society for Parenteral and Enteral Nutrition; ESPGHAN, European Society for Paediatric Gastroenterology Hepatology and Nutrition; ESPEN, European Society for Clinical Nutrition and Metabolism.
Preparing for treatment
In general, the indications and contraindications (e.g. fatty acid oxidation deficiencies) of KDT should be similar for both parenteral and enteral dietary use.1 However, there are two main conditions when the use of KDT should be considered parenterally: when enteral feeding is not possible in a child already on KDT (with the aim of maintaining ketosis and anti‐seizure effect) or when KDT is initiated in a patient who cannot be enterally fed (with the aim of reducing their seizures). In recent years, the use of KDT for refractory status epilepticus and FIRES has been increasingly reported.21, 22, 23 In this acute setting, KDT is usually administered by an enteral route (46 of 47 pediatric cases reported by Chiusolo et al.).16 However, few patients may require parenteral administration. On the basis of the risk–benefit ratio, enteral KDT should be prioritized before considering KD‐PN (the overall risk of parenteral nutrition and side effects are reported in Table 1).
It is generally accepted that parenteral nutrition should be considered when an infant or child is not able to receive enteral feeding for more than 48 hours.9, 10, 24 When starting KDT for the first time in the acute setting, to maximize its potential for ketosis, additional fasting for 24 hours may be appropriate. This should be conducted under careful monitoring of glucose and ketone levels.
Parenteral administration of KDT should not be used in infants born preterm as they are a high‐risk group for malnutrition and complications.
A thorough nutrition assessment with anthropometric data must be completed before starting KD‐PN. Possible allergies or hypersensitivities must be identified. The nutritional implications of both parenteral nutrition and KDT should be discussed with the patient's medical team and the goals of parenteral nutrition and KDT at this critical time determined.
All medications, including those administered intravenously, should be converted to the lowest carbohydrate form. Intravenous products are often mixed with dextrose or may provide calories, which can affect ketosis negatively. Careful consideration is needed when prescribing intravenous medications (e.g. pentobarbital delivers carbohydrates from both glycerol and alcohol). Collaboration with a pharmacist is essential to determine the total carbohydrate content of all medications before initiating treatment. Other points to consider might be the carbohydrate content of toothpaste used. For most patients, switching from liquid, enterally provided anti‐seizure drugs to tablets or intravenous preparations is sufficient. The pharmacist should also carefully check the nutritional composition of the lipid brands in case of allergies.
Dietary prescription
Fluid
In medical practice, the total volume of parenteral nutrition is a factor limited by the volume required to dilute the wide range of medications used during admission to the intensive care unit.9, 10, 25 Fluid requirements vary and must be tailored to the patient's medical situation, body weight, excretion, blood electrolytes, acid–base status, hematocrit, specific gravity of urine, and urine electrolytes.24 Table 3 shows the recommendations for calculating fluid intake.26
Table 3.
Consensus recommendations for daily fluid requirement calculation
| Goal |
|---|
| 0–10kg: 100mL/kg/d |
| 10–20kg: 1000mL+50mL/kg |
| 20–40kg: 1500mL+20mL/kg |
| >40kg: use adult recommendations (or Holliday–Segar formula) |
| Minimum |
| 75% of calculated enteral fluids |
Results from our survey did not include any recommendations on fluid requirements (Appendix S1, online supporting information).
Calories
International guidelines on parenteral nutrition agree that 100% intake of energy requirements may not always be reached and requirements may vary depending on the phase of the illness: they are significantly lower during the acute and stable phases.27
If measurement of resting energy expenditure is feasible, this is preferable to using equations for calculating caloric intake. The caloric intake has to be tailored to individual circumstances. Although the patient with FIRES might be critically ill, the resting energy expenditure might be reduced in sedative coma. Repeated measurements should be obtained when the medical situation of the patient changes (e.g. fever, inflammation). However, it is important to avoid hyperalimentation because it may induce metabolic imbalance, liver damage, and risk refeeding syndrome (particularly in severely malnourished patients).27, 28 If measurement of resting energy expenditure at the intensive care unit is not feasible, the Schofield equation for weight can be used.27
In the literature, three studies reported reduced calorie intake ranging from 50% to 75% of recommended daily allowance.12, 13, 15 It seems that aiming for 50% of calories as documented in some studies may be most feasible given the low dextrose, low protein, and high fat content of the KD‐PN prescription.
Results from our international survey showed a high variety in the way energy requirements are calculated in clinical practice (e.g. 50–90% of recommended daily allowance [21%], caloric intake depending on parenteral nutrition volume [21%], measuring resting energy expenditure [17%], Schofield equation [12%]) (Appendix S1).
The caloric intake that can be achieved on the basis of a KDT composition might not always be realized.
Carbohydrates
International parenteral nutrition guidelines recommend a glucose intake that covers 40% to 60% of non‐protein calorie intake, which is not applicable in KDT and KD‐PN.8, 9, 10 The permitted quantity of carbohydrate during KDT follows from the calculation of energy and protein requirements after establishing the necessary prescription of fat.
To maintain the level of ketosis during KD‐PN, the daily carbohydrate intake from any previous oral ketogenic diet should be used as a benchmark for the maximum amount of carbohydrates prescribed in parenteral nutrition. In clinical practice, for ketosis the ultimate glucose provision depends on a diet ratio of the maximal possible lipids and minimal safe protein intake to prevent malnutrition.
To establish or maintain ketosis, the carbohydrate intake might be sacrificed, resulting in exclusion of dextrose/glucose solution during KD‐PN (i.e. at diet initiation). Special attention should be given to (young) children at risk of hypoglycemia, especially during acute illness. Because most KD‐PN preparations will be non‐inclusive of dextrose/glucose or will contain under 5% dextrose/glucose, the glucose infusion rate will be very low to 0mg/kg/min. In the case of fluid restriction, a higher percentage of dextrose/glucose might be required to establish an appropriate daily glucose administration. However, some hospital inpatient pharmacies might not be able to formulate this because of mixing and compounding devices and may require a small amount of dextrose. Intravenous lipid emulsions contain a range of 2.2% to 2.5% glycerol which will also deliver carbohydrates after metabolization. The end diet ratio will be different if these carbohydrates are considered.20 Moreover, intravenous products (i.e. sedatives such as pentobarbital) deliver not only carbohydrates by glycerol but also by alcohol.
In the literature, three studies used 5% dextrose in their formulation and therefore cycled their parenteral nutrition for a shorter period of time (for 16h and off for 8h) while cycling sodium chloride 0.45% to decrease total carbohydrate allotment.13, 14, 15 This trend was most probably adapted from each other's experience.
Results from our international survey provided conflicting information. From 24 centers, 58% did not calculate glycerol as carbohydrate into diet ratio calculations. Sixty‐four per cent of the centers used a dextrose/glucose‐free solution at initiation; 42% of the centers started parenteral nutrition by using a low dextrose/glucose solution (Appendix S1).
Protein
According to international parenteral nutrition guidelines, the protein requirement is increased by as much as 20% to 50% in critical illness, thermal injury, and catch‐up growth. Critically ill patients are at risk of malnutrition principally as a result of protein breakdown that is directly related to the seriousness of their condition, which is more profound at a very young age (infants and young children). A minimal protein intake of 1.5g/kg ideal weight is essential to prevent malnutrition.24, 28, 29
In the literature, studies recommend aiming for 1g protein/kg/d but in daily practice an intake of 0.5g/kg to 0.8g/kg seems to be more realistic.12, 15 Only the study of Dressler et al.19 reported a median protein intake of 2g/kg/d with a range of 1.5g/kg to 2g/kg in KD‐PN, owing to very young age (median age of the children 1y 10mo), resulting in a median diet ratio of 0.9:1. The White Paper from the Charlie Foundation on ketogenic diet recommends 1.5g/kg protein during stress but states an intake of 0.5g/kg to 0.8g/kg for short‐term use might be acceptable.20
Results from our international survey showed 63% of the 24 expert centers calculate more than 1g/kg into the total parenteral nutrition prescription. It is unclear whether they were able to reach the minimal amount of 1.5g/100kcal to prevent malnutrition (Appendix S1).
During KD‐PN, optimizing the diet ratio to the maximum limits the intake of adequate protein and carbohydrates. Therefore, in clinical practice, priority is given to protein intake to prevent malnutrition by the general omission of carbohydrates (when the glucose and ketone levels allow this).
Lipids
In KD‐PN the delivery of lipids is of utmost importance to ensure the optimal level of ketosis for seizure control. In clinical practice this is challenging: side effects (i.e. hyperlipidemia) might occur even with short‐term use. Lipids are an important component of parenteral nutrition as they greatly contribute to the caloric intake in a low volume and low osmolarity so are suitable for peripheral infusion. They also provide essential fatty acids.30
Intravenous lipid emulsions have varying ingredients; therefore it is important to rule out allergies and hypersensitivities before starting KD‐PN. Several intravenous lipid emulsions contain egg, olive, soybean, or peanut proteins, which are contraindicated in such conditions.31
International guidelines recommend starting with a dose of 1g/kg/d to 2g/kg/d advanced to 2.5g/kg/d to 3g/kg/d9, 10 or to start with 2g/kg/d advanced to 3g/kg/d to 4g/kg/d.30 A continuous infusion, by central intravenous line, will be better tolerated. In clinical practice the application of 4g/kg/d will be essential to maximize the diet ratio.
In the literature, the case report of KD‐PN by Roan12 and the study by Jung et al.13 reported lipids of at least 4g/kg/d. In the studies by Lin et al.15 and Dressler et al.19 the lipid intake used was 3g/kg/d. The study by Dressler et al. of 17 infants reported that, despite a median diet ratio of 0.9:1 (SD 0.28; range 0.6–1.5), 10 out of 13 children who were already on KDT were able to maintain their level of seizure control during KD‐PN and two out of four of the children with de novo KD‐PN experienced 50% and 86% seizure reduction respectively. The White Paper recommends starting with 2g/kg/d to 3g/kg/d advancing to 4g/kg/d.20
Results from our international survey showed that most centers start with 1g/kg to 3g/kg (54%), increasing with steps of 1g/kg to 1.9g/kg up to goal 3g/kg/d to 4g/kg/d and run their parenteral nutrition lipids continuously. In 66.6% of the centers, carnitine is supplemented (50mg/kg, with a maximum of 1000mg/d) to stimulate fatty acid oxidation (Appendix S1).
A 4:1 diet ratio might not be achieved, depending on the maximum lipids tolerated and the accompanying amount of glycerol, but it might still be effective in reducing seizures.
Vitamins and minerals
Parenteral vitamins and minerals are usually provided as mixtures. Vitamins and minerals both pose particular pharmacological problems. When given intravenously, some may adhere to tubing or can be degraded by light. Also, the stability of the mixture and ‘ingredients’ might have an effect. The actual amount of vitamins/minerals delivered to the patient may therefore be lower than the intended dose.32, 33, 34, 35 There are different manufacturers of vitamins and mineral mixtures, and description depends on hospital/pharmacy protocols.
In the literature, the case report of Roan12 used standard multivitamins and multi‐electrolytes; it recommended starting 2μg to 2.5μg selenium/kg/d to prevent deficiency. In the event of long‐term parenteral nutrition use, it is recommended to add 1mg vitamin K/d, and in the presence of diarrhea to add zinc (10–15mg/L of output).36 Other studies have provided supplementation according to international guidelines.32, 33 The White Paper on ketogenic diet gives no specific recommendations.20
Results from our international survey showed 20 out of 24 centers added a multivitamin (Appendix S1).
Initiation
International guidelines (European Society for Paediatric Gastroenterology Hepatology and Nutrition/European Society for Clinical Nutrition and Metabolism7 and American Society for Parenteral and Enteral Nutrition)28 have recently been evaluated. Their information on diet initiation, side effects, and monitoring gives guidance about parenteral nutrition in pediatric patients but has to be evaluated from the perspective of KDT. If a patient is unable to tolerate enteral nutrition and a ketogenic diet is needed for anticonvulsant therapy, KD‐PN needs to be considered. The time at which KD‐PN should be considered, however, is variable. The recently updated European Society for Paediatric Gastroenterology Hepatology and Nutrition/European Society for Clinical Nutrition and Metabolism and American Society for Parenteral and Enteral Nutrition guidelines recommend starting parenteral nutrition when a patient is expected to be unable to restart enteral feeding within 48 hours.24, 28 A recent publication has highlighted the benefits of delaying total parenteral nutrition (by 7d) in the critically ill child; however, this is still under debate and not yet general practice.37 Delayed treatment might not be an option under KDT because children either are already on KDT and should maintain ketosis or require KDT urgently for acute seizure control (drug‐resistant ongoing repetitive seizures, refractory status epilepticus, or FIRES).
The literature and our international survey clearly show that during KD‐PN a certain level of ketosis can be reached within a few days. Monitoring of ketosis is practiced in both urine and/or blood and checked daily or every other day. There are several ways of possible initiation and it is not clear which is most effective (Appendix S1).
Side effects and monitoring
The available international parenteral nutrition guidelines include a description of side effects9, 10, 28, 34 but do not provide specific information during KDT. When KDT is established as parenteral nutrition treatment for refractory status epilepticus or FIRES, adverse effects already known during KDT initiation might occur (e.g. hypoglycemia, constipation, hypertriglyceridemia, increased liver enzyme, increased pancreatic amylase).
On the basis of the data from KD‐PN studies11, 12, 13, 14, 15, 16, 17, 18, 19, 38 and in clinical practice, it is clear that, during KD‐PN, side effects are frequently documented even if parenteral nutrition is only given for a few days.
The mixing of drugs with parenteral nutrition has to be avoided on the basis of drug compatibility.34
Most side effects are transient and can be avoided by the use of strict protocols in the intensive care unit. This highlights the need for the slow introduction of KD‐PN based on the international parenteral nutrition guidelines with tailoring of ratio, fat, protein, and carbohydrate, and regular monitoring of liver function with quick response to hyperketosis and hypoglycemia (e.g. frequency of monitoring glucose and ketone levels tailored to the medical and nutritional status). Initiation during propofol anesthesia should be avoided owing to the risk of propofol infusion syndrome caused by the high triglyceride content of this anesthetic. If considered necessary, it should be handled with caution with strict monitoring of lipids and renal, cardiac, and liver functioning.4
Limited data have been published in this area.14, 16, 17, 18, 19 The White Paper on ketogenic diet includes brief recommendations on KD‐PN monitoring.20
Results from our international survey show a wide range of laboratory parameters (e.g. glucose, ketone levels, or bicarbonate/carbon dioxide) that should be checked during KD‐PN and its frequency (Appendix S1).
An overview of recommendations for monitoring is shown in Table 4.
Table 4.
Overview of recommendations for monitoring. (a) Recommendations on baseline checks for newly starting KDT as parenteral nutrition. (b) Recommendations on monitoring during KD‐PN
| (a) |
|---|
| Exclude contraindicated metabolic disorders (i.e. fatty acid oxidation disorders, carnitine deficiency [primary], carnitine palmitoyltransferase I or II deficiency, carnitine translocase deficiency, pyruvate carboxylase deficiency) |
| Anthropometrics (weight, height, head circumference at <1y) |
| Dietary intake and output |
| Complete blood count with platelets |
| Electrolytes to include serum bicarbonate, total protein, calcium, zinc, selenium, magnesium, phosphate |
| Trace elements; zinc, selenium, copper, CRP |
| Serum liver and kidney tests (including albumin, AST, ALT, blood urea, nitrogen, creatinine) |
| Fasting lipid profile |
| Serum acylcarnitine profile |
| Urine analysis |
| Urine calcium and creatinine |
| Antiepileptic drug levels (if applicable) |
| During initiation |
| Glucose daily/every other day |
| β‐hydroxyburyrate daily/every other day |
| (b) | ||||
|---|---|---|---|---|
| Item |
Age <1y Unstable |
Age <1y Stable |
Age >1y Unstable |
Age >1y Stable |
| Weight | Daily | Weekly | 2×wk | Weekly |
| Height | Weekly | Monthly | 1×2wks | 1×3mo |
| Head circumference | 1×2wks | Monthly | — | — |
| Intake/output | Daily | Daily | Daily | Daily |
| Seizures | Daily | Daily | Daily | Daily |
| Blood | ||||
| Blood gas and osmolarity | Daily | 2–3×wk | Daily | 2×wk |
| Sodium, potassium, chloride | Daily | 2–3×wk | Daily | 2×wk |
| Calcium, magnesium, phosphorus | Daily | 2–3×wk | Daily | 2×wk |
| Copper | Monthly | Monthly | Monthly | Monthly |
| Complete blood count | Unknown | Weekly | Unknown | Weekly |
| Albumin | Daily | 2×wk | Daily | Weekly |
| Glucosea | 1×4h | 2–4×d | 1×4–6h | 1–3×d |
| Billirubin total/direct | Daily | 2×wk | Daily | Weekly |
| ASAT, ALAT, ALP | 2×wk | 2×wk | Weekly | Weekly |
| Amylase/lipase | Unknown | Weekly | Unknown | Weekly |
| Urea | Daily | Daily | Daily | 2×wk |
| Creatinine | Daily | Daily | Daily | 2×wk |
| Triglyceridesb | Daily | Weekly | Daily | Weekly |
| β‐hydroxybutyratec | Daily | Daily | Daily | Daily |
| Carnitine free/total | Unknown | 1×6mo | Unknown | 1×6mo |
| Lipid profile | Daily when lipids off for 4h | Monthly | Unknown | Monthly |
| Hemoglobin | Weekly | Weekly | Weekly | Weekly |
| Serum ferritin | Monthly | Monthly | Unknown | Monthly |
| Vitamins A, D 25‐OH | Monthly | Monthly | Monthly | Monthly |
| Vitamins B1, B12 | Monthly | Monthly | Monthly | Monthly |
| Folic acid, Zn, Al | 1×6–12mo | 1×6–12mo | 1×6–12mo | 1×6–12mo |
| Copper, chromium, manganese | At indication | At indication | At indication | At indication |
| Selenium | Monthly | 1×6mo | Unknown | 1×6mo |
| Urine | ||||
| Sodium | Weekly | Monthly | Monthly | Monthly |
| Potassium | Weekly | Monthly | Monthly | Monthly |
| Calcium/creatinine ratio | Weekly | Monthly | Weekly | Monthly |
| Urine ketonesd (at indication) | Daily | 2×d | Daily | 2×d |
| Osmolarity | Weekly | Monthly | Monthly | Monthly |
aGlucose levels of 2–2.5mmol/L (approximately 40–45mg/dL) should be treated immediately with 2–4g of carbohydrates (20–40mL glucose 10%). Blood glucose should be rechecked 15–20min after treatment and if not improved a further dose is given until the glucose level is above 3mmol/L (approximately 55mg/dL).39 bThe upper limit for triglycerides is 10mmol/L (approximately 387mg/dL). At a level of 5mmol/L (approximately 200mg/dL), start carnitine 50mg/kg with a maximum of 1g/d.40 cThe goal is within the range 2–5mmol/L β‐hydroxybutyrate (approximately 20–52mg/dL). dIdeal range is 8–16mmol/L or 3–4+; the exact level depends on the type of urine dipstick. KDT, ketogenic diet therapy; KD‐PN, ketogenic parenteral nutrition; CRP, C‐reactive protein; AST, aspartate transaminase; ALT, alanine transaminase; ASAT, aspartate aminotransferase; ALAT, alanine aminotransferase; ALP, alkaline phosphatase.
Evaluation of risk–benefit
The evaluation of KD‐PN is based on an assessment of the efficacy, the ketosis, and side effects. When parenteral administration is used in place of an already‐commenced KDT, the evaluation will be based on the maintenance of seizure control. The seizure frequency based on a diary, as well as on the electroencephalography (EEG) recordings in some epilepsy syndromes, will be the main criteria for evaluating KD‐PN.
Careful evaluation of the medical situation (i.e. underlying disease, laboratory checks) is essential to ensure that parenteral nutrition use is safe and to determine when enteral KDT may be reintroduced.
When KD‐PN is used for a refractory status epilepticus including FIRES, appropriate criteria will be used (the ability to stop an anesthetic agent, reduction of continuous EEG discharges, cessation of seizures). When an anesthetic agent is used to treat refractory status epilepticus, the use of continuous EEG recording is required. Criteria to evaluate the efficacy should be both electroencephalographic and clinical.
As mentioned above, the measurement of ketosis by β‐hydroxybutyrate levels is helpful in evaluating whether the patient has the hallmarks of KDT. It could also be helpful to evaluate the maintenance of ketosis after the switch from enteral to parenteral administration or the appearance of ketosis after initiation of KD‐PN. The frequency of monitoring ketosis will depend on the medical and nutritional status of the patient. There are conflicting results in the literature about the direct correlation of ketosis with anti‐seizure efficacy. Therefore, this should be considered when initiating and managing parenteral ketogenic therapy: ketosis is only used as a guide with improvement in clinical symptoms/measures (e.g. seizure frequency or the ability to reduce anesthesia) and not used as the primary guide in establishing the effectiveness of ketogenic diet. If ketones are low but the patient shows clinical benefit such as freedom from seizures, no further action is required.
Weaning from parenteral to enteral ketogenic diet
On the basis of the high‐risk profile, limiting the period of parenteral nutrition is highly important. When the underlying reason for nothing by mouth is solved, the enteral route should be used immediately. Detailed information on how the patient may be weaned from parenteral nutrition is limited7, 20 but stepwise tapering (one step at the time with no diluted enteral feed) is likely to optimize tolerance of enteral feed.7
In the literature there is little information about the transition from KD‐PN to enteral ketogenic diet. Some studies only mention that enteral ketogenic diet is introduced in a stepwise fashion without giving detailed information.13, 14, 16, 17
Results from our international survey show that weaning is performed on an individual basis, with most centers using a ketogenic formula as trophic feed and 33% using medium‐chain triglyceride oil (Appendix S1).
Conclusions
After two reminders the responder rate to our international survey remained at 24 out of 150 (16%) and might reflect the limited experience centers have. Although there is a degree of variability in how international expert teams practice, KD‐PN consensual recommendations have been reached on the most important issues. Recommendations for safe and effective application of KDT‐PN have been established. Table 2 summarizes these recommendations and Figure 1 provides an overview of KD‐PN application.
Figure 1.
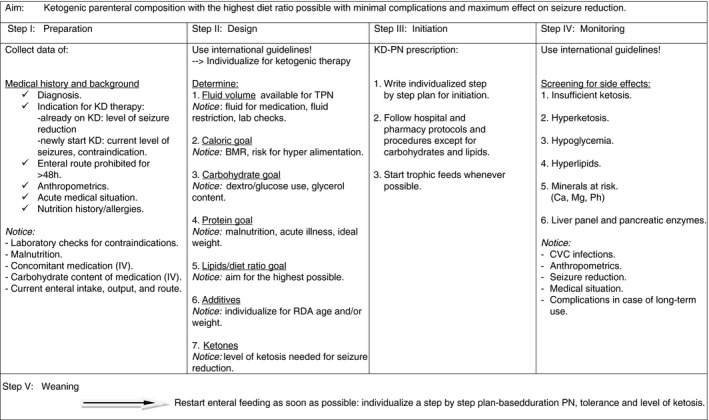
Consensus flow chart of ketogenic diet parenteral nutrition (KD‐PN) application. KD, ketogenic diet; IV, intravenous; TPN, total parenteral nutrition; BMR, basal metabolic rate; RDA, recommended daily allowance; CVC, central venous catheter; PN, parenteral nutrition.
It is important to move to the next steps by providing new data on KD‐PN application. Obviously, prospective and controlled studies are needed to prove the efficacy and safety of KD‐PN application based on these consensus recommendations. One of the first steps could be to consider an international registry collecting the cases of patients treated by KD‐PN. More data would be helpful to update these recommendations. Such an update is planned in the next 5 years.
Supporting information
Appendix S1: Outcome of international survey results.
Acknowledgements
The members of the Review Group are as follows: Eimear Forbes, Temple Street Hospital, Dublin, Ireland; Baheerathi van de Bor, UCL Great Ormond Street Hospital for Children NHS Trust, London, UK; Joanne Olieman, Erasmus MC – Sophia Children's Hospital, University Medical Center Rotterdam, the Netherlands; Venetia Simchowitz, UCL Great Ormond Street Hospital for Children NHS Trust, London, UK. Thomas Storme, Pediatric Epilepsy & Child Neurology Paris‐Diderot University, Paris, France; Joerg Klepper, Klinikum Aschaffenburg‐Alzenau, Germany; Anastasia Dressler, Universitätsklinik fur Kinder‐und Jugend heilkunde Pädiatrisches Epilepsiezentrum, Vienna, Austria. We thank Eric Kossoff for supporting us during the entire process. The authors have stated that they had no interest that could be perceived as posing a conflict or bias.
Contributor Information
Elles van der Louw, Email: e.vanderlouw@erasmusmc.nl.
Review Group:
Eimear Forbes, Baheerathi van de Bor, Joanne Olieman, Venetia Simchowitz, Thomas Storme, Joerg Klepper, and Anastasia Dressler
References
- 1. Kossoff E, Zupec‐Kania B, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open 2018; 3: 175–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klepper J. GLUT1 deficiency syndrome and ketogenic diet therapies: missing rare but treatable diseases? Dev Med Child Neurol 2015; 57: 896–7. [DOI] [PubMed] [Google Scholar]
- 3. Veggiotti P, De Giorgis V. Dietary treatments and new therapeutic perspective in GLUT1 deficiency syndrome. Curr Treat Options Neurol 2014; 16: 291. [DOI] [PubMed] [Google Scholar]
- 4. Soysal E, Gries H, Wray C. Pediatric patients on ketogenic diet undergoing general anesthesia‐a medical record review. J Clin Anesth 2016; 35: 170–5. [DOI] [PubMed] [Google Scholar]
- 5. Scholl‐Bürgi S, Höller A, Pichler K, Michel M, Haberlandt E, Karall D. Ketogenic diets in patients with inherited metabolic disorders. J Inherit Metab Dis 2015; 38: 765–73. [DOI] [PubMed] [Google Scholar]
- 6. Miranda MJ, Turner Z, Magrath G. Alternative diets to the classical ketogenic diet—can we be more liberal? Epilepsy Res 2012; 100: 278–85. [DOI] [PubMed] [Google Scholar]
- 7. Puntis JWL, Hojsak I, Ksiazyk J. ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: organisational aspects. Clin Nutr 2018; 37: 2392–400. [DOI] [PubMed] [Google Scholar]
- 8. Mesotten D, Joosten K, van Kempen A. Verbruggen S; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: carbohydrates. Clin Nutr 2018; 37: 2337–43. [DOI] [PubMed] [Google Scholar]
- 9. Corkins MR, Balint J, editors. The A.S.P.E.N. Pediatric Nutrition Support Core Curriculum, 2nd edn Silver Spring, MD: American Society for Parenteral and Enteral Nutrition, 2015. [Google Scholar]
- 10. Merritt R, editor. The A.S.P.E.N. Nutrition Support Practice Manual. Silver Spring, MD: American Society for Parenteral and Enteral Nutrition, 2005. [Google Scholar]
- 11. Rosenthal E, Weissman B, Kyllonen K. Use of parenteral medium‐chain triglyceride emulsion for maintaining seizure control in a 5‐year‐old girl with intractable diarrhea. J Parenter Enteral Nutr 1990; 14: 543–5. [DOI] [PubMed] [Google Scholar]
- 12. Roan M. Management of long‐term ketogenic parenteral nutrition. Child Obes Nutr 2011; 3: 282–7. [Google Scholar]
- 13. Jung DE, Kang HC, Lee JS, Lee EJ, Kim HD. Safety and role of ketogenic parenteral nutrition for intractable childhood epilepsy. Brain Dev 2012; 34: 620–4. [DOI] [PubMed] [Google Scholar]
- 14. Strzelczyk A, Reif PS, Bauer S, et al. Intravenous initiation and maintenance of ketogenic diet: proof of concept in super‐refractory status epilepticus. Seizure 2013; 22: 581–3. [DOI] [PubMed] [Google Scholar]
- 15. Lin JJ, Lin KL, Chan OW, et al. Intravenous ketogenic diet therapy for treatment of the acute stage of super‐refractory status epilepticus in a pediatric patient. Pediatr Neurol 2015; 52: 442–5. [DOI] [PubMed] [Google Scholar]
- 16. Chiusolo F, Diamanti A, Bianchi R, et al. From intravenous to enteral ketogenic diet in PICU: a potential treatment strategy for refractory status epilepticus. Eur J Paediatr Neurol 2016; 20: 843–7. [DOI] [PubMed] [Google Scholar]
- 17. Appavu B, Vanatta L, Condie J, Kerrigan JF, Jarrar R. Ketogenic diet treatment for pediatric super‐refractory status epilepticus. Seizure 2016; 41: 62–5. [DOI] [PubMed] [Google Scholar]
- 18. Farias‐Moeller R, Bartolini L, Pasupuleti A, Brittany Cines RD, Kao A, Carpenter JL. A practical approach to ketogenic diet in the pediatric intensive care unit for super‐refractory status epilepticus. Neurocrit Care 2017; 26: 267–72. [DOI] [PubMed] [Google Scholar]
- 19. Dressler A, Haiden N, Trimmel‐Schwahofer P, et al. Ketogenic parenteral nutrition in 17 pediatric patients with epilepsy. Epilepsia Open 2018; 3: 30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zupec‐Kania B, Partikian A, Aldaz V, et al. White Paper. Proceedings of Ketogenic Diet Therapies Symposium, March 2015. Manhattan Beach, CA: The Charlie Foundation for Ketogenic Therapies, 2016. [Google Scholar]
- 21. O'Connor SE, Ream MA, Richardson C, et al. The ketogenic diet for the treatment of pediatric status epilepticus. Pediatr Neurol 2014; 50: 101–3. [DOI] [PubMed] [Google Scholar]
- 22. Nabbout R, Mazzuca M, Hubert P, et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES). Epilepsia 2010; 51: 2033–7. [DOI] [PubMed] [Google Scholar]
- 23. Caraballo RH, Reyes G, Avaria MF, et al. Febrile infection‐related epilepsy syndrome: a study of 12 patients. Seizure 2013; 22: 553–9. [DOI] [PubMed] [Google Scholar]
- 24. Koletzko B. Parenteral nutritional support In: Koletzko B, Bhatis J, Bhutta ZA, et al. editors. Pediatric Nutrition in Practice. 2nd revised edition Basel: Karger, 2015: 158–62. [Google Scholar]
- 25. Jochum F, Moltu SJ, Senterre T, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: fluid and electrolytes. Clin Nutr 2018; 37: 2344–53. [DOI] [PubMed] [Google Scholar]
- 26. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957; 19: 823–32. [PubMed] [Google Scholar]
- 27. Joosten K, Embleton N, Yan W. Senterre T, the ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: energy. Clin Nutr 2018; 37: 2309–14. [DOI] [PubMed] [Google Scholar]
- 28. Mehta NM, Skillman HE, Irving SY, et al. Guidelines for the provision and assessment of nutrition support therapy in the pediatric critically ill patient: Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 2017; 41: 706–42. [DOI] [PubMed] [Google Scholar]
- 29. van Goudoever JB, Carnielli V, Darmaun D, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: amino acids. Clin Nutr 2018; 37: 2315–23. [DOI] [PubMed] [Google Scholar]
- 30. Lapillonne A, Fidler Mis N, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: lipids. Clin Nutr 2018; 37: 2324–36. [DOI] [PubMed] [Google Scholar]
- 31. Raman M, Almutairdi A, Mulesa L, Alberda C, Beattie C, Gramlich L. Parenteral nutrition and lipids. Nutrients 2017; 9: E388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bronsky J, Campoy C. Braegger C; ESPGHAN/ESPEN/ESPR/CSPEN working group on pediatric parenteral nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: vitamins. Clin Nutr 2018; 37: 2366–78. [DOI] [PubMed] [Google Scholar]
- 33. Mihatsch W, Fewtrell M, Goulet O, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: calcium, phosphorus and magnesium. Clin Nutr 2018; 37: 2360–5. [DOI] [PubMed] [Google Scholar]
- 34. Hartman C, Shamir R, Simchowitz V, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: complications. Clin Nutr 2018; 37: 2318–29. [DOI] [PubMed] [Google Scholar]
- 35. Domellöf M, Szitanyi P, Simchowitz V, et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: iron and trace minerals. Clin Nutr 2018; 37: 2354–9. [DOI] [PubMed] [Google Scholar]
- 36. Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract 2015; 30: 371–82. [DOI] [PubMed] [Google Scholar]
- 37. Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Joosten K, Van den Berghe G. Evidence for the use of parenteral nutrition in the pediatric intensive care unit. Clin Nutr 2017; 36: 218–23. [DOI] [PubMed] [Google Scholar]
- 38. Baumeister FA, Oberhoffer R, Liebhaber GM, et al. Fatal propofol infusion syndrome in association with ketogenic diet. Neuropediatrics 2004; 35: 250–2. [DOI] [PubMed] [Google Scholar]
- 39. Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the Pediatric Endocrine Society for evaluation and management of persistent hypoglycemia in neonates, infants, and children. J Pediatr 2015; 167: 238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuan G, Al‐Shali KZ, Hegele RA. Hypertriglyceridemia: its etiology, effects and treatment. CMAJ 2007; 176: 1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Outcome of international survey results.


