Abstract
Background
Activated clotting time (ACT)–guided heparinization is used during atrial fibrillation (AF) ablation. Differences in sensitivity to ACT assays have been identified among different direct oral anticoagulants (DOACs).
Objective
We aimed to examine ACT just before ablation (pre‐ACT) for different ablation start times (9:00, 11:00, 13:00, or 15:00) and ablation safety outcomes in minimally interrupted (min‐Int) and uninterrupted (Unint) DOAC regimens and examine differences in pre‐ACT values among four DOACs.
Methods
Consecutive patients were randomized into the min‐Int (n = 307) or Unint (n = 277) groups. DOACs examined were apixaban, dabigatran, edoxaban, and rivaroxaban.
Results
No sequential changes in pre‐ACT values were observed for each DOAC used and for all four DOACs combined in the min‐Int and Unint groups. There was no meaningful difference in pre‐ACT at each ablation start time between the groups. Clinically significant differences in overall pre‐ACT were not obtained between the groups (138 ± 24 vs 142 ± 23 seconds). The pre‐ACT (baseline) value for dabigatran was on average 29 seconds higher than that for the other three DOACs. The min‐Int and Unint groups showed similar thromboembolic (0% vs 0%) and bleeding event rates (major, 1% vs 0%; all, 3.5% vs 2.5%).
Conclusion
The pre‐ACT did not show a sequential change in the min‐Int and Unint groups. No notable differences in the time‐dependent change in pre‐ACT between the groups were observed. Variations in baseline ACT suggest the need for moderate adjustment of ACT for adequate modification of heparin dose for the other three DOACs. Both regimens provided similar acceptable AF ablation safety outcomes.
Keywords: bleeding, complication, DOAC, heparin, pulmonary vein isolation, supraventricular tachyarrhythmia, thromboembolism
1. INTRODUCTION
Atrial fibrillation (AF) is the most common sustained arrhythmia,1 and it has a significant effect on morbidity and mortality.2 Catheter ablation is now widely considered as the most effective treatment for AF.3 Specifically, catheter ablation is advocated as a class I indication for patients with paroxysmal AF who are refractory to antiarrhythmic drugs and as a class IIa indication for patients with persistent AF.4, 5 However, AF ablation is one of the most complex interventional electrophysiological procedures; thus, it is associated with several complications, with thromboembolism as the most important complication. Accordingly, periprocedural anticoagulation management has received increasing attention, with much debate regarding the optimal management strategy.
Warfarin, a vitamin K antagonist, has been commonly used as an oral anticoagulant during the periprocedural period of AF ablation. More recently, however, direct oral anticoagulants (DOACs) have been increasingly used for periprocedural anticoagulation therapy for AF ablation.6 At present, the following three perioperative DOAC anticoagulation strategies are used: uninterrupted (Unint; no discontinuation of DOACs), minimally interrupted (min‐Int; holding the morning dose on the day of ablation), and interrupted (holding doses > min‐Int holding dose). The superiority among these three anticoagulation strategies in the periprocedural period of AF ablation, however, has not been fully clarified. A meta‐analysis revealed a higher incidence of overall bleeding events associated with the Unint and min‐Int strategies compared with the interrupted strategy, but with similar safety and efficacy for all three strategies with regard to thromboembolic and major bleeding complications.7 This analysis indicates that the anticoagulation status might be different among the three anticoagulation strategies.
Activated clotting time (ACT)–guided heparinization is now widely used to prevent perioperative thromboembolic complications.4 It can be assumed that the pre‐ACT may decrease after the final administration of DOAC, leading to different ACT values just before septal perforation during the AF ablation procedure (pre‐ACT) for different AF ablation start times, such as in the morning or in the afternoon. However, no study has specifically evaluated the association between pre‐ACT values and the start time of AF ablation.
Meanwhile, several reports on hemostasis assays have indicated that dabigatran is sensitive to ACT assays, while the other three DOACs (apixaban, edoxaban, and rivaroxaban) are less sensitive to ACT assays.8, 9 Large randomized controlled studies have demonstrated identical efficacy of DOACs for the prevention of thromboembolic events, with an acceptably low risk of bleeding, indicating that these four DOACs have similar anticoagulation function.10, 11, 12, 13 Despite this comparable efficacy for the prevention of thromboembolic events, the pre‐ACT value does vary among DOACs.14 It is, thus, essential to clarify the differences in the pre‐ACT value among anticoagulation therapies using DOACs.
In accordance with these considerations, the present study aimed to clarify (a) the time‐dependent changes in pre‐ACT on the day of ablation, (b) the differences in the temporal change in pre‐ACT between the minimally interrupted and uninterrupted DOAC anticoagulation strategies, and (c) the differences in pre‐ACT (baseline ACT) values among DOACs.
2. MATERIALS AND METHODS
2.1. Patients
This single‐center study, performed at Okayama Heart Clinic (Okayama, Japan), included patients who underwent their first AF ablation between January 2017 and October 2018. The study was in compliance with the rules of the Helsinki Declaration, informed consent was obtained from all these patients, and the study was approved by the institutional ethics committee for human research.
Patients with decreased renal function (creatinine clearance rate, <30 mL/min) were excluded. The study group included 584 patients (423 men; age, 66 ± 10 years) diagnosed with paroxysmal AF (n = 370), persistent and long‐standing AF (n = 204), and atrial tachycardia (n = 10). Patients were randomly allocated to the min‐Int or Unint DOAC therapy group. In all patients, anticoagulation therapy, using a DOAC, was initiated at least 30 days before AF ablation. The anticoagulant regimen in the min‐Int and Unint groups is shown in Figure 1. The selection and dose of the DOACs were not randomized and were at the discretion of each treating physician, considering the patient's characteristics (including renal function) and drug manufacturer's directions. Dabigatran and apixaban were prescribed twice a day (morning and evening). Rivaroxaban was administered once a day in the morning, rather than in the evening, to maintain a sufficient adherence rate.15, 16 Edoxaban was prescribed once a day (morning).
Figure 1.
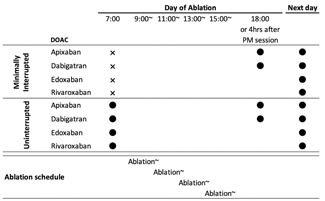
Regimen of DOAC administration in the minimally interrupted and uninterrupted DOAC anticoagulation therapy groups. ● (closed circle), administered; X, not administered; DOAC, direct oral anticoagulants
2.2. Start time of AF ablation and ACT‐guided heparin administration algorithm
AF ablation was performed at four predetermined time blocks during the day, namely, with the start times at around 9:00, 11:00, 13:00, or 15:00. The DOAC regimen (min‐Int and Unint) was implemented at 7:00 on the day of surgery. ACT was measured using the whole blood hemostasis method of two points of clot detection with the Actalyke tube (Actalyke Mini II; Helena Laboratories, Beaumont, Texas), according to the manufacturer's recommendations. The baseline ACT was determined just before the start of AF ablation (pre‐ACT). After confirmation of the absence of a risk of excessive bleeding, based on the pre‐ACT value, a heparin bolus was administered just before septal puncture, taking into consideration age, sex, and body weight, that is, 120 to 130 U/Kg for a pre‐ACT ≥150 seconds and 140 to 150 U/kg for a pre‐ACT less than 150 seconds.4, 14 After the heparin bolus, continuous heparinized saline infusion (400 U/h) was administered, via a peripheral vein, to maintain the ACT within 300 to 400 seconds to avoid thrombus formation. ACT values were obtained at baseline (pre‐ACT), at 15 minutes after pre‐ACT measurement, and at 30‐minute intervals thereafter. Additional heparin bolus was administered intravenously when the 15‐minutes ACT or subsequent 30‐minute interval ACT measurements, under continuous heparinized saline infusion, were 250 to 274 seconds at 3000 U and 275 to 299 seconds at 2000 U. The final ACT measurement was performed before catheter removal.
2.3. Endpoints
The pre‐ACT value was set as the primary endpoint of the study. The secondary endpoints were thromboembolic and bleeding complications.
2.4. Catheter ablation for AF
Details of the AF ablation procedure used have been published elsewhere.17 Briefly, extensive encircling pulmonary vein isolation (EEPVI) was performed using a double‐lasso technique, with an open‐irrigated ablation catheter (Cool Flex/FlexAbility; St. Jude Medical, Inc, St. Paul, MN) and an electroanatomic integration mapping system (Ensite NavX System; St. Jude Medical, Inc). The endpoint criteria for EEPVI were the elimination of pulmonary vein potentials and the nonrecurrence of pulmonary vein spikes in all pulmonary veins after the intravenous administration of 20 to 40 mg of adenosine triphosphate during sinus rhythm or coronary sinus. In patients with paroxysmal AF, only EEPVI was performed. In patients with persistent and longstanding persistent AF, additional ablation was combined with EEPVI at the operator's discretion. Additional ablation included left atrial linear ablation, left atrial low‐voltage area ablation, and ablation of complex fractionated atrial electrograms in the right and left atrium.
2.5. Superior vena cava isolation/cavotricuspid isthmus ablation
Superior vena cava isolation and cavotricuspid isthmus ablation were performed and confirmed in accordance with established methods.18, 19
2.6. Postablation care and follow‐up
After completing AF ablation, heparin was discontinued. Thereafter, protamine was administered intravenously (20 or 30 mg, ie, 2800 or 4200 antiheparin IU depending on whether ACT was 300‐350 seconds or >350 seconds, respectively) for hemostasis. When bleeding at the puncture site did not stop after the initial administration of protamine sulfate, additional doses (10‐30 mg, depending on the bleeding status) were administered at 4‐minute intervals until the bleeding stopped. A single DOAC dose of apixaban or dabigatran was resumed in the evening of the day of ablation or 4 hours after the completion of the postmeridiem ablation session, with confirmation of hemostasis (Figure 1). Rivaroxaban and edoxaban administrations were resumed in the morning of the day after ablation.
Data obtained over 120 days of anticoagulation therapy (from 30 days before ablation to 90 days after) were analyzed. DOAC anticoagulation therapy was continued for at least 3 months after AF ablation. The initial follow‐up visit was scheduled 2 weeks after AF ablation. All previously ineffective antiarrhythmic drugs were withdrawn after confirmation of the endpoint criteria of ablation noted above. At the follow‐up visits, surface electrocardiogram and transthoracic echocardiography were performed at our center. All patients had a telemetry electrocardiogram recorder (Omron Co, Ltd, Kyoto, Japan) to document symptomatic arrhythmias or to transfer an ECG once per week, if asymptomatic, for 6 months.
2.7. Complications and safety outcomes
Cerebrovascular accidents and transient ischemic attacks were considered thromboembolic complications once intracranial hemorrhage had been ruled out. Pulmonary embolism and deep venous embolism were also defined as thromboembolic complications. Cardiac tamponade, retroperitoneal bleeding, and groin hematoma requiring blood transfusion were defined as major bleeding episodes. Cardiac tamponade was defined by characteristic clinical features and the presence of a considerable pericardial effusion requiring drainage. Late cardiac tamponades were those occurring greater than 48 hours after the procedure. Pericardial effusion reduced hemoglobin without blood transfusion, and hematuria was defined as minor complications. Pericardial effusion was defined as an effusion identified in the pericardial space by routine follow‐up echocardiography, without hemodynamic disturbance (nontamponade). The primary safety outcome measured was a composite of bleeding and thromboembolic complications (yielding the bleeding and thromboembolic risk score). Miscellaneous nonanticoagulation‐related events were also recorded.
2.8. Statistics
Statistical analysis was performed using R (version 3.2.2., R Foundation, Vienna, Austria). Data were expressed as the mean ± standard deviation or median (with first and thrd quartiles), as appropriate for the data type and distribution. The Student t test or Mann‐Whitney U test was used to compare continuous variables between the two groups, such as min‐Int and Unint groups, as appropriate for the data distribution. Homogeneity of variance was assessed using the F‐test. The χ 2 or Fisher's exact test, with 2 × 2 contingency tables and two‐tailed tests, was used to compare categorical variables between the two groups. A one‐way analysis of variance (ANOVA) with the Bonferroni posthoc test was used to evaluate differences in continuous variables for the four ablation start times. Pearson's correlation analysis was performed to determine the relationship between pre‐ACT and ablation start time. For comparison of categorical variables among more than three groups, the χ 2 or Fisher's exact test was used, with m × n contingency tables and two‐tailed tests. The Kaplan‐Meier method, with logrank tests for between‐group comparisons, was used to compare the time‐dependent occurrence of bleeding episodes. A two‐tailed P < .05 was considered significant for all statistical analyses.
3. RESULTS
3.1. Ablation
The endpoint criteria of ablation were achieved in all patients; thus, all previously ineffective antiarrhythmic drugs were withdrawn in all patients included in the analysis.
3.2. Clinical characteristics of patients
No significant differences in clinical and echocardiogram parameters and thromboembolic and bleeding risk scores were found between the min‐Int and Unint groups, with the exception of AF duration, which was about 1 year shorter in the min‐Int group than in the Unint group (Table 1). Moreover, a significant difference in the proportion of patients using dabigatran and rivaroxaban was noted between the min‐Int and Unint groups. Procedural, fluorescent, and radiofrequency energy times were not different between the two groups.
Table 1.
Clinical, echocardiographic characteristics, DOAC administration, and procedural parameters between the minimally interrupted and uninterrupted groups
| Minimally interrupted | Uninterrupted | Total | ||
|---|---|---|---|---|
| n = 307 | n = 277 | P value | n = 584 | |
| Age, y | 65.0 ± 10.5 | 66.4 ± 10.3 | .087 | 65.7 ± 10.4 |
| Male:female | 212:95 | 211:65 | .064 | 423: 160 |
| BMI | 23.8 ± 3.4 | 24.2 ± 3.8 | .226 | 24.1 ± 3.6 |
| Type of AF | ||||
| Paroxysmal AF (n) | 199 | 171 | .513 | 370 |
| Persistent AF (n) | 61 | 65 | 126 | |
| Long‐standing persistent AF (n) | 40 | 38 | 78 | |
| Atrial tachycardia (n) | 7 | 3 | 10 | |
| AF duration (first Q:median:third Q) | 1:2:3 | 1:3:5 | <.001* | 1:2:4 |
| Hypertension n (%) | 144 (47) | 134 (48) | .740 | 278: 306 |
| Diabetes n (%) | 34 (11) | 39 (14) | .316 | 73: 556 |
| Congestive heart failure n (%) | 22 (7) | 6 (2) | .006* | 28: 556 |
| Prior stroke/TIA n (%) | 13 (4) | 16 (6) | .448 | 29: 555 |
| CHADS2 score | 0.90 ± 0.87 | 0.97 ± 0.97 | .391 | 0.93 ± 0.91 |
| CHA2DS2‐VAsc score | 1.88 ± 1.37 | 1.88 ± 1.37 | .984 | 1.88 ± 1.36 |
| HAS‐BLED score | 1.35 ± 1.05 | 1.42 ± 1.04 | .397 | 1.38 ± 1.05 |
| Left atrial diameter | 42.0 ± 6.2 | 41.8 ± 6.6 | .720 | 41.8 ± 6.5 |
| Left ventricular ejection fraction | 65.2 ± 8.0 | 65.4 ± 8.2 | .781 | 65.3 ± 8.1 |
| Antiplatelet agent n (%) | 10 (3) | 7 (3) | .632 | 17: 567 |
| DOACs | 307 | 277 | ⋯ | 584 |
| Dabigatran | 56 | 83 | .001* | 139 |
| Rivaroxaban | 109 | 65 | .002* | 174 |
| Apixaban | 59 | 58 | .607 | 117 |
| Edoxaban | 83 | 71 | .708 | 154 |
| Procedural parameters | ||||
| Procedural time, min | 98.1 ± 29.8 | 99.5 ± 31.5 | .630 | 98.6 ± 29.7 |
| Fluorescent time, min | 25.9 ± 14.0 | 26.0 ± 11.8 | .798 | 26.0 ± 13.2 |
| Application time, min | 22.8 ± 13.6 | 24.7 ± 13.3 | .116 | 23.5 ± 13.5 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; DOAC, direct oral anticoagulant; P, probability value; Q, quartile; TIA, transient ischemic attack.
Significant at the P < .01 level.
3.3. Pre‐ACT measurements
3.3.1. Time course
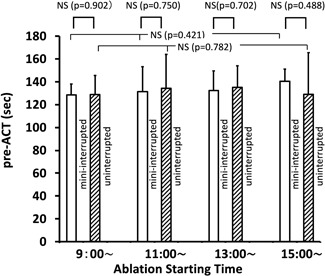
The normal reference value for ACT in the present assay system was 105 to 130 seconds. The pre‐ACT values for the four different AF ablation start times (9:00, 11:00, 13:00, and 15:00) are summarized in Table 2, for each of the four DOACs used (dabigatran, apixaban, rivaroxaban, and edoxaban), as well as for the four DOACs combined. An example of the time‐dependent change in pre‐ACT values for a patient in the apixaban group is shown in Figure 2. No significant difference in pre‐ACT values across the four different AF start times for each of the four DOACs used, as well as for all four DOACs combined, was found in both the min‐Int and Unint groups.
Table 2.
Results of pre‐ACT
| Pre‐ACT for the four different AF abiation start time and correlation with actual AF ablation time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Comparison of the pre‐ACT between the four different ablation start times | Correlation between pre‐ACT and the ablation start time | ||||||||
| AF ablation starting time | First | Second | Third | Fourth | P value | r | P value | Regression coefficient | |
| Apixaban | 9:00‐ | 11:00‐ | 13:00‐ | 15:00‐ | (s/h) | ||||
| n (min‐Int/Unint) | 24/26 | 17/12 | 11/20 | 7/3 | |||||
| Pre‐ACT | min‐Int | 128 ± 10 | 131 ± 22 | 132 ± 17 | 140 ± 11 | .421 | .23 | .08 | ⋯ |
| Unint | 129 ± 17 | 134 ± 30 | 135 ± 19 | 129 ± 37 | .782 | .10 | .47 | ⋯ | |
| P value (min‐Int vs Unint) | 0.9019 | 0.7497 | 0.7021 | 0.4877 | |||||
| Dabigatran | |||||||||
| n (min‐Int/Unint) | 21/39 | 15/12 | 15/23 | 3/9 | |||||
| Pre‐ACT | min‐Int | 169 ± 31 | 150 ± 43 | 168 ± 27 | 171 ± 21 | .343 | .03 | .83 | ⋯ |
| Unint | 164 ± 16 | 159 ± 24 | 159 ± 30 | 142 ± 24 | .076 | −.23 | .04* | −0.21 | |
| P value (min‐Int vs Unint) | 0.5352 | 0.5555 | 0.3269 | 0.0395 | |||||
| Edoxaban | |||||||||
| n (min‐Int/Unint) | 45/30 | 18/20 | 12/21 | 8/0 | |||||
| Pre‐ACT | min‐Int | 131 ± 18 | 133 ± 19 | 134 ± 15 | 139 ± 24 | .733 | .15 | .16 | ⋯ |
| Unint | 132 ± 18 | 141 ± 14 | 140 ± 24 | ⋯ | .212 | .24 | .04* | 2.3 | |
| P value (min‐Int vs Unint) | 0.3541 | 0.0682 | 0.1951 | ||||||
| Rivaroxaban | |||||||||
| n (min‐Int/Unint) | 52/30 | 28/15 | 19/15 | 9/5 | |||||
| Pre‐ACT | min‐Int | 129 ± 15 | 137 ± 26 | 132 ± 15 | 130 ± 11 | .354 | .04 | .69 | ⋯ |
| Unint | 135 ± 15 | 139 ± 17 | 139 ± 16 | 121 ± 7 | .136 | −.23 | .82 | ⋯ | |
| P value (min‐Int vs Unint) | 0.0925 | 0.8099 | 0.1899 | 0.1367 | |||||
| Total of four DOACs | |||||||||
| n (min‐Int/Unint) | 143/125 | 78/59 | 57/76 | 29/17 | |||||
| Pre‐ACT | min‐Int | 135 ± 23 | 139 ± 25 | 142 ± 25 | 142 ± 22 | .211 | .13 | .02* | 1.4 |
| Unint | 142 ± 22 | 143 ± 22 | 144 ± 25 | 134 ± 24 | .367 | .03 | .64 | ⋯ | |
| P value (min‐Int vs Unint) | 0.0015 | 0.3530 | 0.5919 | 0.2275 | |||||
| Pre‐ACT values combined for the four different ablation start times for each DOACs | ||||||||
|---|---|---|---|---|---|---|---|---|
| Apixaban | ||||||||
| n (min‐Int/Unint) | 59/58 | |||||||
| Pre‐ACT | min‐Int | 131 ± 16 | ||||||
| Unint | 132 ± 21 | |||||||
| P value (min‐Int vs Unint) | 0.858 | |||||||
| Dabigatran | ||||||||
| n (min‐Int/Unint) | 56/83 | |||||||
| Pre‐ACT | min‐Int | 164 ± 33 | ||||||
| Unint | 159 ± 23 | |||||||
| P value (min‐Int vs Unint) | 0.378 | |||||||
| Edoxaban | ||||||||
| n (min‐Int/Unint) | 83/71 | |||||||
| Pre‐ACT | min‐Int | 132 ± 18 | ||||||
| Unint | 137 ± 19 | |||||||
| P value (min‐Int vs Unint) | 0.127 | |||||||
| Rivaroxaban | ||||||||
| n (min‐Int/Unint) | 108/65 | |||||||
| Pre‐ACT | min‐Int | 132 ± 18 | ||||||
| Unint | 136 ± 16 | |||||||
| P value (min‐Int vs Unint) | 0.143 | |||||||
Abbreviations: ACT, activated clotting time; DOAC, direct oral anticoagulant; min‐Int, minimally interrupted; P, probability value; Unint, uninterrupted;
Significant at the P < .05 level.
Figure 2.
Example of the pre‐ACT value (obtained just before septal puncture) for the four different AF ablation start times in the minimally interrupted (open columns) and uninterrupted (oblique line pattern) DOAC therapy groups. The data presented are for patients treated using apixaban. ACT, activated clotting time; AF, atrial fibrillation; DOAC, direct oral anticoagulants; mini‐interrupted, minimally interrupted; NS, not significant; P, probability
Moreover, no difference in pre‐ACT values was observed between the min‐Int and Unint groups for the 9:00, 11:00, and 13:00 ablation start times, with a between‐group difference identified only for the dabigatran group at the 15:00 start time. When all four DOACs were combined, the pre‐ACT value at the 9:00 start time was significantly shorter in the min‐Int group than in the Unint group; however, this difference of only 7 seconds, on average, was, thus, not clinically meaningful (Figure 3). The pre‐ACT values combined for the four different ablation start times for each DOAC are also listed in Table 2, with no significant differences identified between the min‐Int and Unint groups. When all data were combined, no overall difference in the pre‐ACT values was identified between the min‐Int and Unint groups (Figure 4).
Figure 3.
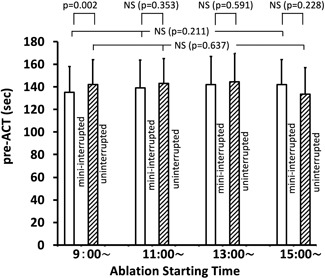
Pre‐ACT values at the four different AF ablation start times, with the data for the four DOACs combined, in the minimally interrupted (open columns) and uninterrupted (oblique line pattern) DOAC therapy groups. ACT, activated clotting time; AF, atrial fibrillation; DOAC, direct oral anticoagulants; mini‐interrupted, minimally interrupted; NS, not significant; P, probability
Figure 4.
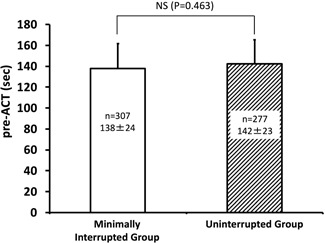
Comparison of pre‐ACT values between the minimally interrupted (n = 307; open columns) and uninterrupted (n = 277; oblique line pattern) DOAC therapy groups. ACT, activated clotting time; DOAC, direct oral anticoagulants; NS, not significant; P, probability
The correlation between pre‐ACT values and ablation start times is also listed in Table 2. Although some discrete correlations were significant, overall, the correlations coefficients were low, as were the regression coefficients for the hourly change in pre‐ACT values.
3.4. Comparison of pre‐ACT among DOACs
Because pre‐ACT (ie, baseline ACT) values were higher with the use of dabigatran than the other three DOACS, we performed a comparison of the pre‐ACT values between dabigatran therapy and the other three DOAC therapies combined, for all pre‐ACT measurements. Pre‐ACT values were significantly higher with the use of dabigatran (162 ± 25 seconds, n = 139) than for the sum of the other three DOACs (133 ± 18 seconds, n = 445; P < .0001; Figure 5). The pre‐ACT values for the other three DOACs were as follows: apixaban, 131 ± 18 seconds (n = 113); edoxaban, 134 ± 19 seconds (n = 154); and rivaroxaban, 133 ± 17 seconds (n = 174), which were not significantly different (P = 0.41 by ANOVA).
Figure 5.
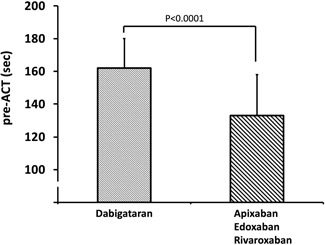
Comparison of pre‐ACT values between dabigatran and the three other DOACs combined. ACT, activated clotting time; DOAC, direct oral anticoagulants; P, probability
3.5. Time course of change in ACT during the AF ablation procedure
The time course of change in ACT values and the overall average ACT in the min‐Int and Unint groups are shown in Figure 6. The time course of change in ACT was similar in the min‐Int and Unint groups: pre‐ACT, 140 ± 23 vs 142 ± 23 seconds (P = .030); 15‐minute ACT, 314 ± 34 vs 331 ± 58 seconds (P < .001); 30‐minute ACT, 323 ± 36 vs 336 ± 49 seconds (P = .02); 60‐minute ACT, 316 ± 42 vs 324 ± 37 seconds (P = .41); 120‐minute ACT, 313 ± 32 vs 316 ± 19 seconds (P = .31); and final ACT, 323 ± 42 vs 325 ± 44 seconds (P = .476).
Figure 6.
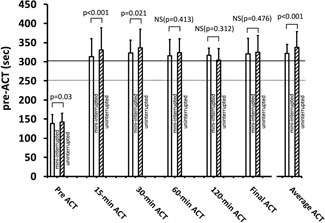
Time course of ACT during AF ablation with heparin administration in the minimally interrupted (open columns) and uninterrupted (oblique line pattern) DOAC therapy groups. ACT, activated clotting time; AF, atrial fibrillation; DOAC, direct oral anticoagulants; mini‐interrupted, minimally interrupted; NS, not significant; P, probability
The proportion (n [%]) of patients in the min‐Int and Unint group, respectively, for the different 15‐minute ACT value ranges was as follows: >300 seconds, 184 (60%) vs 201 (73%); >250 and ≤300 seconds, 95(31%) vs 57 (21%); ≤250 seconds, 28 (9%) vs 19 (7%); P = .005. Because the baseline ACT for apixaban, edoxaban, and rivaroxaban was underestimated by 29 seconds, as revealed by the comparison between these three DOACs combined and dabigatran noted above, we adjusted ACT values by the addition of 29 seconds for these three DOACs accordingly, which modified the proportional distribution, again in the min‐Int and Unint group, respectively, as follows: >300 seconds, 245 (80%) vs 228 (82%); >250 and ≤300 seconds, 53(17%) vs 42 (15%); ≤250 seconds, 9 (3%) vs 7 (3%); P = .75. An ACT greater than 450 seconds under continuous heparinization infusion was observed in 3 of the 307 patients (1.0%) in the min‐Int group and 9 of the 277 patients in the (3.2%) Unint group (P = .15). After adjustment of the ACT value, as noted above, an ACT greater than 450 seconds was identified in 12 of the 307 patients (3.9%) in the min‐Int group and 17 of the 277 patients (6.1%) in the Unint group (P = 0.25). When data of the min‐Int and Unint group were combined, an ACT greater than 450 seconds was observed in 12 of the 584 patients (2.0%) and 28 of the 584 patients (5%), using adjusted ACT values.
3.6. Complications and safety outcomes
No symptomatic thromboembolic complications were noted, either during the procedure or over the follow‐up period, in either the min‐Int or Unint group (Table 3). Major bleeding complications, including periprocedural cardiac tamponade and rate cardiac effusion, were rare in either group. The interrupted group had one cardiac tamponade (0.3%), and there were none in the uninterrupted group (P > .99). There were no other major bleeding complications in either group. Minor bleeding complications occurred rarely, with 8 (2.6%) of patients experiencing a groin hematoma in the min‐Int group and 6 (2.2%) in the Unint group (P = 0.39). Overall, there was no difference in the event‐free curve between the two groups (logrank P = .796; Figure 7), with no difference between the two groups with regard to safety outcomes.
Table 3.
Comparisons of complications and safety outcomes between the minimally interrupted and uninterrupted groups
| Minimally interrupted | Uninterrupted | ||||
|---|---|---|---|---|---|
| n = 307 | DOAC | n = 277 | DOAC | P value | |
| Complications | |||||
| Thromboembolic complications | |||||
| Stroke/TIA | 0 | 0 | 1.000 | ||
| Deep venous thrombosis | 0 | 0 | 1.000 | ||
| Pulmonary embolism | 0 | 0 | 1.000 | ||
| Bleeding complications | |||||
| Major bleeding complications | |||||
| Periprocedural cardiac tamponade | 1 (0.3%) | Dabi | 0 | 1.000 | |
| Late cardiac tamponade | 1 (0.3%) | Riva | 0 | 1.000 | |
| Retroperitoneal bleeding | 0 | 0 | 1.000 | ||
| Decreased hemoglobin, >4 g/dL | 0 | 0 | 1.000 | ||
| Blood transfusion required | 0 | 0 | 1.000 | ||
| Minor bleeding complications | |||||
| Cardiac effusion | 0 | 0 | 1.000 | ||
| Groin hematoma | 8 (2.6%) | Api, 2: Dabi, 1: Edo, 2: Riva, 3 | 6 (2.2%) | Api, 2: Edo, 1: Riva, 3 | .729 |
| Nasal bleeding | 1 (0.3%) | Riva | |||
| Hematuria | 0 | 0 | 1.000 | ||
| Overall bleeding | 11 (3.6%) | 6 (2.2%) | .309 | ||
| Other | |||||
| Gastroparesis | 3 (1.0%) | Api,1: Edo,1: Riva, 1 | 0 | .285 | |
| Prolonged hospitalization | 0 | 0 | 1.000 | ||
| Safety outcome | |||||
| 14 (4.6%) | 6 (2.2%) | .112 | |||
Abbreviations: Api, apixaban; Dabi, dabigatran; DOAC, direct oral anticoagulants; Edo, edoxaban; P, probability value; Riva, rivaroxaban; TIA, transient ischemic attack.
Figure 7.
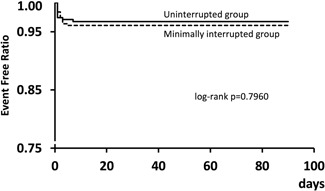
Event‐free curve in the minimally interrupted and uninterrupted groups
4. DISCUSSION
The sequential change in pre‐ACT on the day of ablation was not different for the four DOACs used, in both the min‐Int and Unint groups. There was no clinically meaningful difference in pre‐ACT values between the min‐Int and Unint groups at each of the four ablation start times, as well as overall. For both the min‐Int and Unint anticoagulation strategies, there were no thromboembolic complications, and bleeding complications were rare. Consequently, the safety outcomes for ablation were not different for the min‐Int and Unint DOAC anticoagulation strategies. Pre‐ACT values using apixaban, edoxaban, and rivaroxaban were 29 seconds shorter, on average, compared with the pre‐ACT for dabigatran, with this difference presumably due to differences in the sensitivity between these different DOACs to ACT assays.
Clinical and echocardiogram parameters, as well as AF conditions, did not differ between the min‐Int and Unint groups. In addition, our methods for catheter ablation for AF were essentially the same as those recently described as leading to improved outcomes,20, 21 with our fluoroscopic and procedure times for AF ablation being comparable to those recently reported.21, 22 These considerations validate our comparisons between the min‐Int and Unint groups.
We did not identify a significant difference in pre‐ACT values among the four different ablation start times for each DOAC, as well as for the four DOACs combined, in either the min‐Int or Unint group. The time‐dependent change in pre‐ACT for patients undergoing AF ablation has not been previously reported; thus, there are no values against which to compare our findings. We had assumed that the pre‐ACT value would shorten over time after the final administration of DOAC. Overall, however, we did not identify a time‐dependent effect on the pre‐ACT. Although the reasons for this absence of a time‐dependent effect are not entirely clear, continuous daily administration of DOAC may lead to a maintained effect, indicating that a heparin bridge may not be required, even if AF ablation is scheduled later in the afternoon.
When pre‐ACT values were compared between the min‐Int and Unint groups, a few minor statistically significant differences were found, but no clinically meaningful differences in the pre‐ACT at each of the four ablation start times and for each of the four DOACs used, as well as for the four DOACs combined, was noted. Two previous studies have examined pre‐ACT values between the minimally interrupted and uninterrupted DOAC anticoagulation therapies.15, 16 However, these studies did not include the time‐dependent pre‐ACT, prohibiting a specific comparison of our outcomes to the findings of these studies. Overall, there were a few minor statistically significant differences, but no clinically meaningful differences in the pre‐ACT in the min‐Int group compared with those in the Unint group. Previous studies have also reported significant differences in pre‐ACT between the minimally interrupted and uninterrupted anticoagulation therapies,15, 16 with the between‐group difference reported being larger than that in this study.
ACT‐guided perioperative heparinization is widely used during AF ablation.4, 23 We initially administered a heparin bolus followed by continuous infusion of heparin. Pre‐ACT value provided the anticoagulation status and informed the dose of the initial heparin bolus. Our findings of an absence of thromboembolic events and an acceptably low incidence of bleeding events further support the appropriateness of our algorithm of heparin administration based on ACT measurements. A target ACT greater than 300 seconds has been recommended in previous studies, guidelines, and meta‐analysis.4, 24 Different methods of determining heparin dosing have been reported, including the use of bolus or bolus+infusion methods and various initial bolus doses.24 The present study provided additional information regarding an algorithm for heparinization.
In this study, we did identify that pre‐ACT values were higher for dabigatran anticoagulation therapy than when using the other three DOACs, by 29 seconds on average. Several studies on hemostasis assay have reported the sensitivity of ACT values to the anticoagulation function of dabigatran, with ACT being less sensitive to the effects of the other three DOACs.8, 9, 25 Almost all patients using dabigatran had a pre‐ACT value above the upper limit of the control range, compared with patients using the other three DOACs. Large randomized control studies have shown the anticoagulation function, for the prevention of thromboembolic complications, to be clinically identical for these four DOACs, with a low incidence of bleeding events and, thus, have presumed the pre‐ACT to be identical among the four DOACs.10, 11, 12, 13 However, as stated above, we found the pre‐ACT to be higher for dabigatran than for the other three DOACs. The lower pre‐ACT with the other three DOACs was thought to be induced by a low‐sensitivity ACT assay to those DOACs. These comparisons and considerations indicate that ACT values for the other three DOACs were underestimated by approximately 29 seconds. Therefore, moderate adjustments of the ACT, measured during ablation, when using apixaban, edoxaban, or rivaroxaban were thought to be required, though the present results were not largely affected by the underestimation.
Major concerns with anticoagulation therapy are considered to cause an increased risk of thromboembolic events with interruption of the anticoagulant regimen and, conversely, an increased risk of bleeding when using a Unint regimen. We did not identify significant lengthening of the pre‐ACT value with time (until the 15:00 AF ablation start time) for either group, with no clinically meaningful difference between the min‐Int and Unint groups. Therefore, using either a min‐Int or Unint DOAC strategy would be feasible as perioperative anticoagulation management, with no evidence that a heparin bridge would be required during the procedure. In fact, in this study, both anticoagulation strategies were used without a heparin bridge, and there was no incidence of thromboembolic complications and an acceptably low incidence of bleeding events that were successfully resolved with conservative treatment. We administered heparin using our ACT‐guided algorithm. This may explain, to some degree, the absence of a clinically notable difference in the time course of ACT during AF ablation between the min‐Int and Unint groups. However, the proportion of patients with an ACT value greater than 300 seconds at 15 minutes after the start of the procedure was larger in the Unint than in the min‐Int group. Although AF ablation guidelines recommend an ACT value greater than 300 seconds during the AF ablation procedure, a lower ACT, even one between 210 and 225 seconds, may lower the risk of a thromboembolic event when using an open‐irrigated tip ablation catheter.26 Approximately less than 10% of our patients had a 15‐minute ACT value less than 300 seconds without complications, indicating that a 15‐minute ACT greater than 300 seconds may not be required. Conversely, less than 5% of all ACT measurements had an ACT greater than 450 seconds found in the present study. Numerous algorithms for ACT‐guided heparinization during the ablation procedure have been reported.24 The present study did not primarily aim to clarify the ideal protocol of ACT‐guided heparinization; thus, it does not apparently have sufficient data to clarify which algorithm including ours is ideal for heparinization during the procedure. Nevertheless, our ACT‐guided algorithm for heparinization could explain the absence of thromboembolic complications and the acceptable low incidence rate of bleeding complications in both groups.
Regarding thromboembolic and major bleeding events, a meta‐analysis reported a low incidence rate of thromboembolic (0.5%) and major bleeding (1.0‐15%) events for AF ablation, with no difference between min‐Int and Unint strategies, which is consistent with our results.7 Another meta‐analysis reported the overall rate of bleeding events to be higher for Unint and min‐Int anticoagulation (6‐8%) than interrupted anticoagulation (3.5%) strategies. Similar findings have also been recently reported.27 In our study, the incidence rate of bleeding events ranged between 2% and 4%, with no significant difference between the min‐Int and Int groups. The reasons for this small difference in our incidence of bleeding events compared to those reported in a previous study are not clear. However, our findings do concur with those of previous studies that both min‐Int and Unint DOAC anticoagulation strategies provide an acceptable low incidence of both thromboembolic and bleeding events when AF ablation is performed under ACT‐guided heparinization.
Regarding the anticoagulation status of DOACs, monitoring the inhibition of factor Xa when using apixaban, edoxaban, and rivaroxaban is theoretically reliable.28 Similarly, for dabigatran, assay for the evaluation of antifactor II has been reported.29 However, these monitoring methods are not routinely available at present. A neutralizing agent against dabigatran is now available in the clinical setting, but not for apixaban, edoxaban, and rivaroxaban. Therefore, careful monitoring for ACT‐guided heparinization during AF ablation is essential, specifically for patients using apixaban, edoxaban, and rivaroxaban anticoagulation therapy, to prevent bleeding events. The present study, thus, provided useful information regarding this issue.
4.1. Limitations
There are several limitations in this study that should be acknowledged. First, although patients were randomly allocated to the min‐Int and Unint DOAC therapy groups, due to unintended randomized error, the number of patients was not completely equivalent between these two anticoagulation strategy groups. In addition, the selection of DOAC was not randomly controlled. Consequently, the number of patients using each of the four DOACs in the min‐Int and Unint groups for each of the four different ablation start times was relatively small and was not consistent between groups. We used careful step‐by‐step statistical analyses to compensate for these limitations, as possible. Second, the study included a relatively homogeneous patient group (with all patients being Japanese). Third, as described in Section 4, we used ACT as the global measure of anticoagulation. Specific monitoring of the anticoagulation for each DOAC was not conducted. Fourth, magnetic resonance imaging (MRI) was not performed, either before or after ablation, to specifically determine the presence of a cerebral embolism. In this regard, a recent study revealed that the incidence of microembolism has been reduced with the use of recently improved catheters and that microemboli generally disappear over time.30 Careful neurological follow‐up may compensate for the lack of MRI to monitor cerebral microemboli.
The strengths of this study include the performance of all AF ablation procedures by experienced cardiologists, the absence of differences in clinical characteristics between the two groups at baseline, and statistical confirmation that increasing the number of patients would be unlikely to produce different results. Despite these strengths, a multicenter study with a larger study sample to confirm our results would be warranted.
5. CONCLUSION
There were no clinically significant differences in the time‐dependent change in the pre‐ACT value in both the min‐Int and Unint DOAC regimens, up to the start time of ablation at 15:00. Pre‐ACT did not show significant differences between the min‐Int and Unint anticoagulation groups across the four ablation start times. As a whole, pre‐ACT did not show a clinically meaningful difference between the two groups. Moreover, both strategies had comparable safety outcomes, with the absence of thromboembolic events and a rare occurrence of bleeding events. ACT for apixaban, edoxaban, and rivaroxaban was lower than for dabigatran by 29 seconds, on average. With these results, we recommend moderate adjustments of the ACT when these DOACs were used for adequate modification of heparin dose.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
HY contributed in concept/design, data analysis/interpretation, and drafting of this paper; TM contributed in critical revision of this paper; KH contributed in critical revision of this paper; SH contributed in data analysis/interpretation; HK contributed in data analysis/interpretation; MM contributed in statistics; SK contributed in data analysis/interpretation; SH contributed in drafting and critical revision of this paper; and SK contributed in concept/design, drafting of this paper, critical revision of this paper, approval of this paper, and statistics.
Yamaji H, Murakami T, Hina K, et al. Activated clotting time on the day of atrial fibrillation ablation for minimally interrupted and uninterrupted direct oral anticoagulation therapy: Sequential changes, differences among direct oral anticoagulants, and ablation safety outcomes. J Cardiovasc Electrophysiol. 2019;30:2823–2833. 10.1111/jce.14260
Disclosures: None.
REFERENCES
- 1. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119‐125. [DOI] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946‐952. [DOI] [PubMed] [Google Scholar]
- 3. Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. J Interv Card Electrophysiol. 2012;33:171‐257. [DOI] [PubMed] [Google Scholar]
- 4. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. Europace. 2018;20(20):157‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893‐2962. [DOI] [PubMed] [Google Scholar]
- 6. Potpara TS, Larsen TB, Deharo JC, et al. Oral anticoagulant therapy for stroke prevention in patients with atrial fibrillation undergoing ablation: results from the first European snapshot survey on procedural routines for atrial fibrillation ablation (ESS‐PRAFA). Europace. 2015;17:986‐993. [DOI] [PubMed] [Google Scholar]
- 7. Gorla R, Dentali F, Crippa M, et al. Perioperative safety and efficacy of different anticoagulation strategies with direct oral anticoagulants in pulmonary vein isolation. JACC: Clin Electrophysiol. 2018;4:794‐806. [DOI] [PubMed] [Google Scholar]
- 8. Favaloro EJ, Lippi G. Interference of direct oral anticoagulants in haemostasis assays: high potential for diagnostic false positives and false negatives. Blood Transfus. 2017;15:491‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dincq AS, Lessire S, Chatelain B, et al. Impact of the direct oral anticoagulants on activated clotting time. J Cardiothorac Vasc Anesth. 2017;31:e24‐e27. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981‐992. [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139‐1151. [DOI] [PubMed] [Google Scholar]
- 12. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093‐2104. [DOI] [PubMed] [Google Scholar]
- 13. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883‐891. [DOI] [PubMed] [Google Scholar]
- 14. Yamaji H, Murakami T, Hina K, et al. Adequate initial heparin dosage for atrial fibrillation ablation in patients receiving non‐vitamin K antagonist oral anticoagulants. Clin Drug Investig. 2016;36:837‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nakamura K, Naito S, Sasaki T, et al. Uninterrupted vs. interrupted periprocedural direct oral anticoagulants for catheter ablation of atrial fibrillation: a prospective randomized single‐centre study on post‐ablation thrombo‐embolic and haemorrhagic events. Europace. 2019;21:259‐267. [DOI] [PubMed] [Google Scholar]
- 16. Nagao T, Suzuki H, Matsunaga S, et al. Impact of periprocedural anticoagulation therapy on the incidence of silent stroke after atrial fibrillation ablation in patients receiving direct oral anticoagulants: uninterrupted vs. interrupted by one dose strategy. Europace. 2019;21:590‐597. [DOI] [PubMed] [Google Scholar]
- 17. Yamaji H, Murakami T, Hina K, et al. Usefulness of dabigatran etexilate as periprocedural anticoagulation therapy for atrial fibrillation ablation. Clin Drug Investig. 2013;33:409‐418. [DOI] [PubMed] [Google Scholar]
- 18. Arruda M, Mlcochova H, Prasad SK, et al. Electrical isolation of the superior vena cava: an adjunctive strategy to pulmonary vein antrum isolation improving the outcome of AF ablation. J Cardiovasc Electrophysiol. 2007;18:1261‐1266. [DOI] [PubMed] [Google Scholar]
- 19. Shah DC, Sunthorn H, Burri H, Gentil‐Baron P. Evaluation of an individualized strategy of cavotricuspid isthmus ablation as an adjunct to atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2007;18:926‐930. [DOI] [PubMed] [Google Scholar]
- 20. Di Biase L, Elayi CS, Fahmy TS, et al. Atrial fibrillation ablation strategies for paroxysmal patients: randomized comparison between different techniques. Circ Arrhythm Electrophysiol. 2009;2:113‐119. [DOI] [PubMed] [Google Scholar]
- 21. Oral H, Pappone C, Chugh A, et al. Circumferential pulmonary‐vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934‐941. [DOI] [PubMed] [Google Scholar]
- 22. Schmidt B, Tilz RR, Neven K, Julian Chun KR, Furnkranz A, Ouyang F. Remote robotic navigation and electroanatomical mapping for ablation of atrial fibrillation: considerations for navigation and impact on procedural outcome. Circ Arrhythm Electrophysiol. 2009;2:120‐128. [DOI] [PubMed] [Google Scholar]
- 23. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275‐e444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Briceno DF, Villablanca PA, Lupercio F, et al. Clinical Impact of heparin kinetics during catheter ablation of atrial fibrillation: meta‐analysis and meta‐regression. J Cardiovasc Electrophysiol. 2016;27:683‐693. [DOI] [PubMed] [Google Scholar]
- 25. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209‐219. [DOI] [PubMed] [Google Scholar]
- 26. Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Atrial fibrillation ablation using open‐irrigated tip radiofrequency: experience with intraprocedural activated clotting times ≤210 seconds. Heart Rhythm. 2014;11:963‐968. [DOI] [PubMed] [Google Scholar]
- 27. Yu HT, Shim J, Park J, et al. When is it appropriate to stop non‐vitamin K antagonist oral anticoagulants before catheter ablation of atrial fibrillation? A multicentre prospective randomized study. Eur Heart J. 2019;40:1531‐1537. [DOI] [PubMed] [Google Scholar]
- 28. Barrett YC, Wang Z, Frost C, Shenker A. Clinical laboratory measurement of direct factor Xa inhibitors: anti‐Xa assay is preferable to prothrombin time assay. Thromb Haemost. 2010;104:1263‐1271. [DOI] [PubMed] [Google Scholar]
- 29. Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Thromb Haemost. 2012;107:985‐997. [DOI] [PubMed] [Google Scholar]
- 30. Verma A, Debruyne P, Nardi S, et al. Evaluation and reduction of asymptomatic cerebral embolism in ablation of atrial fibrillation, but high prevalence of chronic silent infarction: results of the evaluation of reduction of asymptomatic cerebral embolism trial. Circ Arrhythm Electrophysiol. 2013;6:835‐842. [DOI] [PubMed] [Google Scholar]


