Abstract
Purpose
To compare the incidence of diabetic ketoacidosis (DKA) among patients with type 2 diabetes mellitus (T2DM) who were new users of sodium glucose co‐transporter 2 inhibitors (SGLT2i) versus other classes of antihyperglycemic agents (AHAs).
Methods
Patients were identified from four large US claims databases using broad (all T2DM patients) and narrow (intended to exclude patients with type 1 diabetes or secondary diabetes misclassified as T2DM) definitions of T2DM. New users of SGLT2i and seven groups of comparator AHAs were matched (1:1) on exposure propensity scores to adjust for imbalances in baseline covariates. Cox proportional hazards regression models, conditioned on propensity score‐matched pairs, were used to estimate hazard ratios (HRs) of DKA for new users of SGLT2i versus other AHAs. When I2 <40%, a combined HR across the four databases was estimated.
Results
Using the broad definition of T2DM, new users of SGLT2i had an increased risk of DKA versus sulfonylureas (HR [95% CI]: 1.53 [1.31‐1.79]), DPP‐4i (1.28 [1.11‐1.47]), GLP‐1 receptor agonists (1.34 [1.12‐1.60]), metformin (1.31 [1.11‐1.54]), and insulinotropic AHAs (1.38 [1.15‐1.66]). Using the narrow definition of T2DM, new users of SGLT2i had an increased risk of DKA versus sulfonylureas (1.43 [1.01‐2.01]). New users of SGLT2i had a lower risk of DKA versus insulin and a similar risk as thiazolidinediones, regardless of T2DM definition.
Conclusions
Increased risk of DKA was observed for new users of SGLT2i versus several non‐SGLT2i AHAs when T2DM was defined broadly. When T2DM was defined narrowly to exclude possible misclassified patients, an increased risk of DKA with SGLT2i was observed compared with sulfonylureas.
Keywords: diabetic ketoacidosis, SGLT2 inhibitor, type 2 diabetes
KEY POINTS:
In this observational study, new use of SGLT2 inhibitors was associated with an increased risk of diabetic ketoacidosis (DKA) compared with new use of sulfonylureas, DPP‐4 inhibitors, GLP‐1 receptor agonists, metformin, and insulinotropic antihyperglycemic agents using a broad definition of type 2 diabetes mellitus (T2DM).
When a more restrictive definition of T2DM was used to exclude possible misdiagnosed T1DM patients, an increased risk of DKA was observed in new users of SGLT2 inhibitors when compared with new users of sulfonylureas.
Using both definitions of T2DM, new use of SGLT2 inhibitors was associated with a reduced risk of DKA compared with new use of insulin.
1. INTRODUCTION
Diabetic ketoacidosis (DKA) is a serious acute metabolic complication of diabetes, characterized by hyperglycemia, ketosis, and metabolic acidosis.1 The underlying pathophysiology of DKA is an absolute or relative insulin deficiency, increased insulin counter‐regulatory hormones, and peripheral insulin resistance.1 DKA is often precipitated by stressful conditions such as trauma, surgery, or infection and is more common in patients with type 1 diabetes mellitus (T1DM) than in patients with type 2 diabetes mellitus (T2DM).2, 3 The incidence of DKA among patients with T1DM ranged from 8 to 56 per 1000 patient‐years in larger studies in the United States, Canada, Europe, and Israel.4 In comparison, a much lower DKA incidence of <2 per 1000 patient‐years was reported in cohort studies of adults with T2DM in clinical practice.5, 6, 7
Sodium glucose co‐transporter 2 (SGLT2) inhibitors are oral antihyperglycemic agents (AHAs) that lower blood glucose by increasing urinary glucose excretion.8 Currently, four SGLT2 inhibitors are approved in the United States and Europe for the treatment of T2DM: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin.9, 10, 11, 12, 13, 14, 15, 16 In 2015, the US Food and Drug Administration (FDA) issued a Drug Safety Communication about the risk of ketoacidosis with SGLT2 inhibitors based on postmarketing adverse event reporting, and a warning was subsequently added to the label of all SGLT2 inhibitors.17 In 2016, on the recommendation of the Pharmacovigilance Risk Assessment Committee (PRAC), the European Medicines Agency (EMA) listed DKA as a rare adverse reaction in the product information of SGLT2 inhibitors and implemented risk management measures to minimize this risk.18 Of note, patients taking SGLT2 inhibitors may present with atypical DKA (no marked hyperglycemia) because of SGLT2 inhibitors' glycosuria effect, which can delay diagnosis and treatment.18
Most clinical development programs of SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, as well as meta‐analyses of randomized controlled trials, have reported a low frequency of DKA events in patients with T2DM, with a numerically higher rate for SGLT2 inhibitors as a class or individual agent relative to placebo and active controls.19, 20, 21, 22, 23, 24, 25, 26 Real‐world studies of DKA among new users of SGLT2 inhibitors are limited, particularly in patients with T2DM. An increased risk of DKA with SGLT2 inhibitor treatment was observed in some, but not all, studies.6, 7, 27, 28 Limitations of prior studies include small sample size and number of events, specific patient population, and select treatment comparators.
The current study sought to estimate the incidence rate and further evaluate the comparative risk of DKA among patients with T2DM in routine clinical practice who were new users of SGLT2 inhibitors versus new users of other classes of AHAs using data from four large US claims databases.
2. METHODS
2.1. Study design
This was a retrospective, observational, comparative cohort study. The study protocol has been reviewed and approved by the EMA PRAC as a postauthorization safety study (PASS) and is available at https://github.com/OHDSI/StudyProtocols/tree/master/Sglt2iDka/documents.
2.2. Data source
Eligible patients were identified from four large US administrative claims databases (IBM® MarketScan® Commercial Database [CCAE], IBM® MarketScan® Multi‐State Medicaid Database [MDCD], IBM® MarketScan® Medicare Supplemental Database [MDCR], and Optum© De‐identified Clinformatics® Data Mart Database [Optum]; see Supplemental Description of Data Sources for additional details). Data from health insurance claims were used to characterize patient demographics and identify all drug exposure, medical conditions, and procedures that occurred during the enrollment period. The study period started on 1 April 2013, which coincides with the approval of the first SGLT2 inhibitor in the United States (canagliflozin was approved 29 March 2013), in all databases and ended on the last date of data availability for each database (CCAE: 31 October 2017; MDCD: 31 December 2016; MDCR: 31 December 2017; Optum: 30 September 2017).
To enable consistent analyses across multiple data sources, all four databases were converted to the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) 5.0. The OMOP CDM accommodates different data domains typically found within observational data and transforms data from diverse databases into a common data format with a standardized vocabulary for coding of health care information (such as drug utilization and condition occurrence),29 which then allows performance of analyses across multiple, disparate databases in a consistent manner.
2.3. New‐user cohort categorization
Eligible patients were required to have ≥1 diagnosis of T2DM on or before the AHA exposure index date, ≥1 prescription dispensing record for a new pre‐specified SGLT2 inhibitor or non‐SGLT2 inhibitor AHA (ie, no prior use of the respective AHA ever recorded in the database), and ≥365 days of continuous enrollment prior to the first dispensing record of the new AHA.
SGLT2 inhibitors were evaluated as a class and as individual agents (canagliflozin, dapagliflozin, and empagliflozin). Seven classes of non‐SGLT2 inhibitor AHAs were prespecified as primary comparators (sulfonylureas [SU], dipeptidyl peptidase‐4 [DPP‐4] inhibitors, glucagon‐like peptide‐1 [GLP‐1] receptor agonists, thiazolidinediones [TZD], insulin, metformin, and insulinotropic AHAs [including DPP‐4 inhibitors, GLP‐1 receptor agonists, SU, nateglinide, and repaglinide]). Since the category of insulinotropic AHAs includes several AHAs that were also evaluated separately, the results are presented mainly in the Supplemental Materials. A category of “other AHAs” (acarbose, miglitol, nateglinide, and repaglinide) was included as a secondary comparator to capture less common AHAs. Patients were categorized into a new‐user cohort at the first prescription claim recorded in the database. For those who met the new user definition for >1 AHA, patients were categorized into a new‐user cohort on the date of the first prescription of each AHA, with a different index date and different baseline characteristics for each respective AHA.
Patients with T2DM were defined by broad and narrow definitions. The broad definition required that patients had ≥1 diagnosis of T2DM and no diagnosis of T1DM or secondary diabetes mellitus (DM) on or before the AHA exposure index date. The narrow definition of T2DM additionally required that patients had no diagnosis of T1DM or secondary DM after the exposure index date, had no insulin monotherapy before the index date, and were aged ≥40 years on the index date; this definition was intended to exclude patients with T1DM or secondary DM who may have been misdiagnosed with T2DM.
2.4. Outcomes
The primary outcome of this study was the first DKA event that occurred after the index date of an AHA therapy, identified from a diagnosis code recorded in inpatient or emergency room claims. To be considered an incident event, DKA occurring after exposure to a new AHA had to occur ≥30 days after any pre‐index DKA event. Fatal DKA was defined as death at discharge from the hospitalized DKA event, whereas this information could not be assessed in the Optum database. As laboratory tests were not systematically collected in the databases used for this study, DKA cases could not be validated using lab tests or categorized as typical or atypical DKA.
2.5. Statistical analyses
Analyses were performed in SQL and R.30
2.6. Descriptive analyses
The unadjusted incidence rates of DKA were expressed as number of incident events per 1000 patient‐years and were also stratified by subgroups of age, sex, history of DKA, and history of insulin use. Distribution of the DKA risk factors and prespecified preceding events were characterized in each cohort. The mean and standard deviation (SD) are presented for continuous variables, and frequencies/proportions are presented for categorical variables. For the primary cohort follow‐up, time‐at‐risk started on the day after the first AHA exposure (index date) and ended at the first incident DKA diagnosis, disenrollment, or the end of the database coverage, whichever came first. Change of AHA treatment during follow‐up was considered in the sensitivity analyses described below.
2.7. Comparative analyses
Each new user of an SGLT2 inhibitor (class and individual agents) was matched 1:1 to a new user of a comparator AHA based on the exposure propensity score (PS). Large‐scale PS were estimated using regularized logistic regression models including all baseline covariates available from 365 days prior to the exposure index date (ie, demographics, history of all diagnoses and conditions, procedures, observations, medications, frequency of health care encounters, Charlson Comorbidity Score, and Diabetes Complication Severity Index) as candidate predictors.31 The model used a cyclic coordinate descending method with least absolute shrinkage and selection operator (LASSO), and the regularization hyperparameter was selected by optimizing the likelihood in a 10‐fold cross validation.32, 33 Conventional greedy algorithms with nearest neighbor that minimize the absolute difference between the PS were used for matching.34 The maximum matching caliper was 20% of the SD of the logit of the PS.35
Evaluations of the history of DKA and prior AHA therapies considered all available claims records prior to the exposure index date. Conditional Cox proportional hazards models were used to estimate the hazard ratio (HR) of DKA in the SGLT2 inhibitor cohort versus the comparator cohort, with each PS‐matched set treated as a separate stratum in the model. For each comparison, the P value was calibrated against an empirical null distribution estimated using 43 negative control outcomes to address potential systematic bias (Supplemental Table 1 ).36 HRs were estimated in each database separately. When the I2 was <40%, a pooled HR across the four databases was generated using a random effects approach,37 in which the standard errors of the database‐specific estimates were adjusted to incorporate variation of effects across databases, the across‐database variance was estimated by comparing the result of each database with the result of an inverse‐variance fixed‐effects meta‐analysis.
2.8. Sensitivity analyses
First, a modified DKA definition was used where cases were identified by inpatient claims only. Second, change of AHA treatment was considered for cohort follow‐up, in which censoring was applied to the time‐at‐risk at the initiation of non‐index AHAs as well as at the discontinuation of the index AHA (defined as refill gap of ≥90 days from the day the index AHA supply from the previous prescription was expected to run out).
3. RESULTS
All results have been made publicly available through an interactive web‐based application at http://data.ohdsi.org/Sglt2iDka/. This section summarizes the key findings across these results.
3.1. Study population
Of the four claims databases, CCAE provided the largest sample size for all AHA new‐user cohorts, and MDCD provided the smallest sample size for most cohorts (Supplemental Table 2 ). Canagliflozin had the largest number of new‐users in the SGLT2 inhibitor class. Using the broad definition of T2DM, the number of new users ranged from 11 141 in MDCD to 152 728 in CCAE for SGLT2 inhibitors, and from 5687 TZD users in MDCR to 329 839 metformin users in CCAE for comparator AHAs. The corresponding numbers were fewer when using the narrow definition of T2DM, ranging from 7779 users in MDCD to 130 708 users in CCAE for SGLT2 inhibitors and from 3982 TZD users in MDCD to 271 723 metformin users in CCAE for comparator AHAs. Across databases, the mean age of all new‐user cohorts ranged from 47.9 to 75.5 years and the percentage of female patients ranged from 41.0% to 72.1%.
3.2. Incidence of DKA
Using the broad definition of T2DM, the unadjusted incidence rates of DKA ranged from 2.75 to 8.84 per 1000 patient‐years among new users of all SGLT2 inhibitors and from 1.38 to 15.82 per 1000 patient‐years among new users of comparator AHAs (Table 1 and Supplemental Table 3 ). The corresponding incidence rates using the narrow definition of T2DM ranged from 1.15 to 3.91 per 1000 patient‐years in the all SGLT2 inhibitor cohorts and from 0.75 to 7.94 per 1000 patient‐years in the comparator cohorts. In general, the incidence rates were highest in MDCD, followed by Optum, and lowest in CCAE and MDCR.
Table 1.
Unadjusted incidence rate of DKA in prespecified AHA new‐user cohorts
| Database | Broad T2DM Definition | Narrow T2DM Definition | |||||
|---|---|---|---|---|---|---|---|
| N of Events | Time‐at‐Risk (PY) | Incidence Rate (per 1000 PY) | N of Events | Time‐at‐Risk (PY) | Incidence Rate (per 1000 PY) | ||
| SGLT2 inhibitor | CCAE | 638 | 227 079 | 2.81 | 218 | 189 129 | 1.15 |
| MDCD | 107 | 12 106 | 8.84 | 32 | 8180 | 3.91 | |
| MDCR | 74 | 26 944 | 2.75 | 37 | 22 187 | 1.67 | |
| Optum | 324 | 98 642 | 3.28 | 131 | 82 051 | 1.60 | |
| Canagliflozin | CCAE | 423 | 140 041 | 3.02 | 144 | 115 704 | 1.24 |
| MDCD | 98 | 10 351 | 9.47 | 29 | 6995 | 4.15 | |
| MDCR | 53 | 20 688 | 2.56 | 25 | 16 879 | 1.48 | |
| Optum | 240 | 72 659 | 3.30 | 97 | 60 110 | 1.61 | |
| Dapagliflozin | CCAE | 168 | 72 030 | 2.33 | 58 | 60 923 | 0.95 |
| MDCD | 10 | 1525 | 6.56 | 2 | 1019 | 1.96 | |
| MDCR | 18 | 4632 | 3.89 | 9 | 3864 | 2.33 | |
| Optum | 57 | 16 815 | 3.39 | 19 | 13 955 | 1.36 | |
| Empagliflozin | CCAE | 123 | 44 572 | 2.76 | 48 | 38 471 | 1.25 |
| MDCD | 5 | 625 | 8.01 | 1 | 441 | 2.27 | |
| MDCR | 9 | 3380 | 2.66 | 5 | 2991 | 1.67 | |
| Optum | 49 | 16 319 | 3.00 | 25 | 14 246 | 1.75 | |
| SU | CCAE | 542 | 231 396 | 2.34 | 193 | 190 197 | 1.01 |
| MDCD | 299 | 40 760 | 7.34 | 102 | 26 348 | 3.87 | |
| MDCR | 108 | 59 518 | 1.81 | 56 | 50 826 | 1.10 | |
| Optum | 470 | 180 646 | 2.60 | 225 | 151 094 | 1.49 | |
| DPP‐4 inhibitor | CCAE | 468 | 214 243 | 2.18 | 171 | 178 003 | 0.96 |
| MDCD | 209 | 28 941 | 7.22 | 76 | 19 578 | 3.88 | |
| MDCR | 121 | 60 529 | 2.00 | 57 | 50 533 | 1.13 | |
| Optum | 425 | 151 705 | 2.80 | 180 | 125 001 | 1.44 | |
| GLP‐1 receptor agonist | CCAE | 300 | 133 250 | 2.25 | 111 | 105 848 | 1.05 |
| MDCD | 126 | 13 558 | 9.29 | 45 | 8019 | 5.61 | |
| MDCR | 31 | 17 271 | 1.79 | 12 | 13 375 | 0.90 | |
| Optum | 196 | 67 369 | 2.91 | 73 | 53 047 | 1.38 | |
| TZD | CCAE | 138 | 51 441 | 2.68 | 53 | 43 015 | 1.23 |
| MDCD | 63 | 7287 | 8.65 | 24 | 4892 | 4.91 | |
| MDCR | 29 | 11 871 | 2.44 | 16 | 10 168 | 1.57 | |
| Optum | 137 | 48 123 | 2.85 | 58 | 40 731 | 1.42 | |
| Insulin | CCAE | 857 | 166 653 | 5.14 | 255 | 118 292 | 2.16 |
| MDCD | 741 | 46 830 | 15.82 | 212 | 26 693 | 7.94 | |
| MDCR | 205 | 53 219 | 3.85 | 98 | 35 975 | 2.72 | |
| Optum | 739 | 133 720 | 5.53 | 328 | 97 800 | 3.35 | |
| Metformin | CCAE | 1069 | 497 877 | 2.15 | 327 | 404 839 | 0.81 |
| MDCD | 627 | 81 106 | 7.73 | 143 | 49 317 | 2.90 | |
| MDCR | 144 | 104 142 | 1.38 | 68 | 90 540 | 0.75 | |
| Optum | 713 | 353 587 | 2.02 | 273 | 294 680 | 0.93 | |
| Other AHAsa | CCAE | 25 | 14 156 | 1.77 | 12 | 11 313 | 1.06 |
| MDCD | 12 | 1335 | 8.99 | 3 | 843 | 3.56 | |
| MDCR | 25 | 7160 | 3.49 | 12 | 5471 | 2.19 | |
| Optum | 52 | 14 162 | 3.67 | 20 | 11 270 | 1.77 | |
Abbreviations: AHA, antihyperglycemic agent; CCAE, IBM® MarketScan® Commercial Database; DKA, diabetic ketoacidosis; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; MDCD, IBM® MarketScan® Multi‐State Medicaid Database; MDCR, IBM® MarketScan® Medicare Supplemental Database; Optum, Optum© De‐identified Clinformatics® Data Mart Database; PY, patient‐years; SGLT2, sodium glucose co‐transporter 2; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione.
Includes acarbose, miglitol, nateglinide, and repaglinide.
Overall, the proportion of fatal DKA events was low, although it varied across databases and AHA cohorts (Supplemental Table 4 ). In general, the case fatality is higher in MDCR and MDCD than in CCAE. Among new users of SGLT2 inhibitors, the highest fatality was observed in MDCD (1.9% and 3.1% using the broad and narrow definitions of T2DM, respectively). Among new users of comparator AHAs, the highest fatality was observed in MDCR (6.3% and 8.9% using the broad and narrow definitions of T2DM, respectively).
3.3. Risk factors and preceding events
The unadjusted incidence rates of DKA were higher in patients with prior use of insulin and history of DKA (data not shown). A higher proportion of new users of SGLT2 inhibitors who had incident DKA events received a prescription for insulin prior to the index date compared with new users of comparator AHAs who had incident DKA events, except for GLP‐1 receptor agonists (Supplemental Table 5 ). Other risk factors for DKA and events that occurred prior to DKA (eg, hospitalization, surgery, and infections) were similarly distributed across the AHA new‐user cohorts.
3.4. Risk of DKA in the PS‐matched cohorts
Baseline characteristics prior to the AHA exposure index date, including demographics, comorbidities, and medications, were well balanced after PS matching for all matched cohorts (data not shown).
Using the broad definition of T2DM, a significantly increased risk of DKA was observed in new users of SGLT2 inhibitors compared with SU, DPP‐4 inhibitors, GLP‐1 receptor agonists, metformin, and insulinotropic AHAs in select individual databases and the meta‐analysis (Figure 1 and Supplemental Figure 1 A). Meta‐analytic estimates of the HR (95% confidence interval [CI]) for DKA with SGLT2 inhibitors were 1.53 (1.31‐1.79) versus SU, 1.28 (1.11‐1.47) versus DPP‐4 inhibitors, 1.34 (1.12‐1.60) versus GLP‐1 receptor agonists, and 1.31 (1.11‐1.54) versus metformin.
Figure 1.
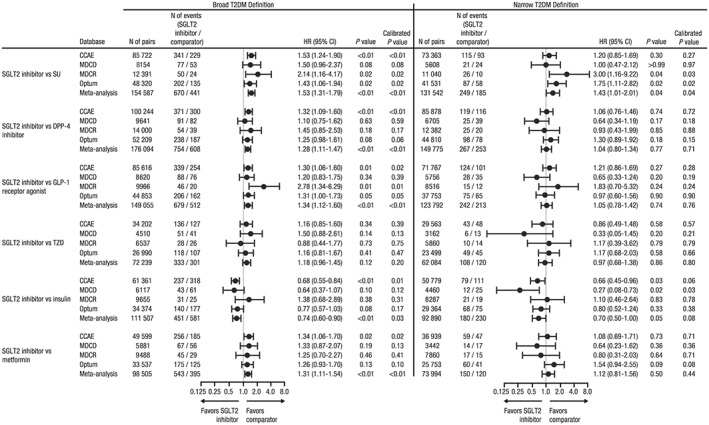
Hazard ratio of DKA for new users of SGLT2 inhibitors versus comparator AHAs.
Abbreviations: AHA, antihyperglycemic agent; CCAE, IBM® MarketScan® Commercial Database; CI, confidence interval; DKA, diabetic ketoacidosis; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; HR, hazard ratio; MDCD, IBM® MarketScan® Multi‐State Medicaid Database; MDCR, IBM® MarketScan® Medicare Supplemental Database; Optum, Optum© De‐identified Clinformatics® Data Mart Database; SGLT2, sodium glucose co‐transporter 2; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione
When using the narrow definition of T2DM to exclude patients with T1DM or secondary DM who may have been misclassified as T2DM, the risk of DKA was not significantly increased among new users of SGLT2 inhibitors compared with DPP‐4 inhibitors, GLP‐1 receptor agonists, or metformin in individual databases or the meta‐analysis. However, new users of SGLT2 inhibitors had an increased risk of DKA compared with SU in the meta‐analysis (HR [95% CI]: 1.43 [1.01‐2.01]). In all comparative analyses using the narrow T2DM definition, the associations observed in MDCD were less consistent compared with the other databases, which may reflect fewer events observed and/or unique patient characteristics captured by the database (eg, patients with a disability or low socioeconomic status).
In the meta‐analysis, with both the broad and narrow definitions of T2DM, new users of SGLT2 inhibitors had a significantly lower risk of DKA compared with insulin. Across the four databases and with both definitions of T2DM, the risk of DKA was similar for new users of SGLT2 inhibitors and TZD.
The risk of DKA for new users of individual SGLT2 inhibitor agents versus comparator AHAs was generally similar to that for the SGLT2 inhibitor class but with greater variation in HRs across databases (Supplemental Figures 2‐4 ).
3.5. Sensitivity analyses
In sensitivity analyses identifying DKA events from inpatient diagnoses only (Supplemental Table 6 ) and censoring time‐at‐risk at the regimen change (Figure 2 and Supplemental Figure 1 B), the HRs of DKA for new users of SGLT2 inhibitors versus comparator AHAs were generally consistent with the main analyses, although with fewer cases and less precision.
Figure 2.
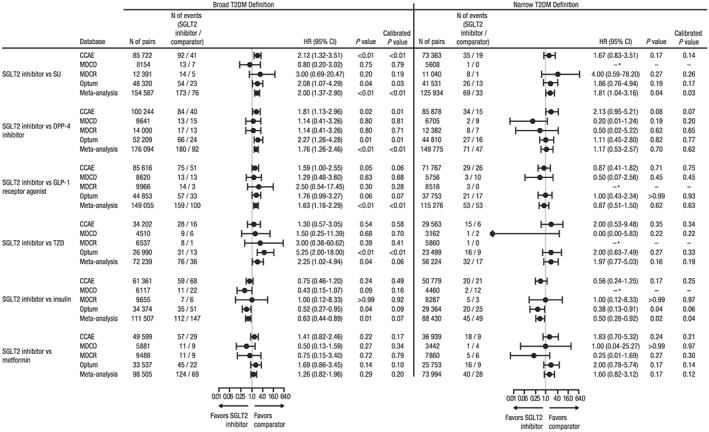
Hazard ratio of DKA for new users of SGLT2 inhibitors versus comparator AHAs with censoring at the initiation of non‐index AHAs and discontinuation of the index AHA.
Abbreviations: AHA, antihyperglycemic agent; CCAE, IBM® MarketScan® Commercial Database; CI, confidence interval; DKA, diabetic ketoacidosis; DPP‐4, dipeptidyl peptidase‐4; GLP‐1, glucagon‐like peptide‐1; HR, hazard ratio; MDCD, IBM® MarketScan® Multi‐State Medicaid Database; MDCR, IBM® MarketScan® Medicare Supplemental Database; Optum, Optum© De‐identified Clinformatics® Data Mart Database; SGLT2, sodium glucose co‐transporter 2; SU, sulfonylurea; T2DM, type 2 diabetes mellitus; TZD, thiazolidinedione.
*Either no cases in the target and/or comparator cohort or number of cases were too few for the model to converge
4. DISCUSSION
This observational, retrospective cohort study compared the risk of DKA among new users of SGLT2 inhibitors and other classes of AHAs in four US administrative claims databases. Using the broad definition of T2DM, a moderate, but statistically significant, increased risk of DKA was observed among new users of SGLT2 inhibitors compared with SU, DPP‐4 inhibitors, GLP‐1 receptor agonists, and metformin in the meta‐analysis combining four databases. Using the narrow definition of T2DM to remove younger patients and those with prior insulin monotherapy, the risk of DKA remained significantly increased among new users of SGLT2 inhibitors when compared with SU in the meta‐analysis. A trend for a higher risk of DKA for new users of SGLT2 inhibitors compared with insulinotropic AHAs (including SU, DPP‐4 inhibitors, and GLP‐1 receptor agonists) was observed in three of four databases; this trend appears to be largely driven by SU. Using both definitions of T2DM, the risk of DKA with SGLT2 inhibitors was lower compared with insulin and not significantly different compared with TZD. The association with the risk of DKA appeared generally similar for individual SGLT2 inhibitor agents; however, for the individual agent comparisons, there was more variability in HRs and 95% CIs, and the results were largely inconsistent across databases.
The different associations using the broad versus the narrow T2DM definitions suggest that the observation of an increased risk of DKA in new users of SGLT2 inhibitors might be attributed in part to use of SGLT2 inhibitors in patients who were misdiagnosed as having T2DM, such as those with T1DM or latent autoimmune diabetes. Clinical development programs of SGLT2 inhibitors and clinical observations have identified cases of DKA in patients who were diagnosed with T2DM, but presented with biochemical evidence for autoimmune diabetes.19, 38, 39, 40 In our study, the narrow definition for T2DM aimed to remove potential late‐onset autoimmune diabetes, even at the expense of excluding some true T2DM patients. However, because our definition of T2DM was based on algorithms of diagnosis codes and prescription claims, we cannot completely rule out all potential misdiagnoses.
The unadjusted incidence rates of DKA were several‐fold higher in patients with prior use of insulin and those with a history of DKA. A greater proportion of new users of SGLT2 inhibitors who had incident DKA events already had an insulin prescription before the index date compared with new users of comparator AHAs who had incident DKA events, except for GLP‐1 receptor agonists. This observation suggests that new users of SGLT2 inhibitors who subsequently developed DKA likely had more advanced diabetes with both insulin resistance and insulin deficiency at index. In our comparative analyses, the risk of DKA among new users of SGLT2 inhibitors was increased compared with new users of one AHA class that stimulates insulin release from β‐cells, but decreased compared with the new users of insulin. Together, these findings support the hypothesis that in SGLT2 inhibitor users, diminished β‐cell function could fail to suppress hepatic ketogenesis and peripheral lipolysis, subsequently leading to DKA development, particularly in the presence of acute conditions that increase insulin demand (eg, infection, surgery).
Real‐world evidence on the occurrence of DKA among patients treated with SGLT2 inhibitors remains largely in case studies and adverse event reports with limited comparative analyses. One study that used the Danish National Patient Registry found no increase in the incidence of DKA with SGLT2 inhibitors when used as monotherapy or combination therapy compared with no pharmacological therapy.6 Two retrospective cohort studies using population‐based claims databases to compare the risk of DKA with SGLT2 inhibitors and DPP‐4 inhibitors found an increased risk of DKA (HR [95% CI]: 2.2 [1.4‐3.6]) among new users of SGLT2 inhibitors in the United States,28 but not in South Korea.7 Another previous study using the CCAE claims database, which was also used in the current study, compared the incidence of DKA among over 30 000 new users of canagliflozin versus new users of non‐SGLT2 inhibitor AHAs who were 1:1 matched on PS, and showed that the HR (95% CI) of DKA were 1.91 (0.94‐4.11) and 1.13 (0.43‐3.00) using the broad and narrow definitions of T2DM, respectively.27
The current study strengthens the real‐world evidence by its use of four large claims databases, which are likely representative of routine clinical practice for US patients with insurance coverage, and comprehensive analyses comparing SGLT2 inhibitors as a class, as well as by individual agent, with multiple comparators. Nevertheless, this study is subject to the limitations of all claims database research. First, as claims data are collected for administrative purposes, no standardized methodology was implemented in the source record to validate exposures, outcomes, or baseline covariates. In particular, due to limited availability and likely biased collection of laboratory data, DKA cases identified from claims diagnoses could not be verified using lab test results, nor categorized into typical or atypical DKA. Thus, it is possible that closer scrutiny of DKA by clinicians concerned about this risk in patients taking SGLT2 inhibitors could lead to observation of more clinical diagnoses of DKA in SGLT2 inhibitor users. Second, bias due to missing data and unmeasured confounding is possible, although the use of negative control outcomes suggested little to no systematic error. Finally, sample sizes for several pairwise comparisons were relatively small; thus, the HR estimates should be interpreted with caution.
In this claims database study, an increased risk of DKA was observed for new users of SGLT2 inhibitors compared with new users of other classes of AHAs when T2DM was defined broadly. When T2DM was defined narrowly to exclude possible misdiagnosed patients, an increased risk of DKA with SGLT2 inhibitors was observed compared with SU.
Supporting information
Supplemental Table 1. Negative control outcomes
Supplemental Table 2. Baseline demographics in pre‐specified AHA new‐user cohorts
Supplemental Table 3. Unadjusted incidence rate of DKA in new users of insulinotropic AHAs†
Supplemental Table 4. Proportion of fatal DKA events across databases†
Supplemental Table 5. Selected risk factors and preceding events among patients who developed DKA after index
Supplemental Table 6. Hazard ratio of DKA identified from diagnoses in inpatient claims only for new users of SGLT2 inhibitors versus comparator AHAs
Supplemental Figure 1. Hazard ratio of DKA for new users of SGLT2 inhibitors versus insulinotropic AHAs† (A) over the entire observation period and (B) with censoring at the initiation of nonindex AHAs and discontinuation of the index AHA.
Supplemental Figure 2. Hazard ratio of DKA for new users of canagliflozin versus comparator AHAs.
Supplemental Figure 3. Hazard ratio of DKA for new users of dapagliflozin versus comparator AHAs.
Supplemental Figure 4. Hazard ratio of DKA for new users of empagliflozin versus comparator AHAs.
ACKNOWLEDGEMENTS
This study was supported by Janssen Research & Development, LLC. Medical writing support was provided by Dana Tabor, PhD, of MedErgy, and was funded by Janssen Global Services, LLC.
Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Wang L, Voss EA, Weaver J, et al. Diabetic ketoacidosis in patients with type 2 diabetes treated with sodium glucose co‐transporter 2 inhibitors versus other antihyperglycemic agents: An observational study of four US administrative claims databases. Pharmacoepidemiol Drug Saf. 2019;28:1620–1628. 10.1002/pds.4887
Prior presentations: These data were presented, in part, at the Scientific Sessions of the Quality of Care and Outcomes Research (QCOR), April 5 to 6, 2019; Arlington, Virginia. This study was supported by Janssen Research & Development, LLC.
The copyright line for this article was changed on 28 August 2019 after original online publication.
REFERENCES
- 1. Gosmanov AR, Gosmanova EO, Dillard‐Cannon E. Management of adult diabetic ketoacidosis. Diabetes Metab Syndr Obes. 2014;7:255‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335‐1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. International Diabetes Federation . IDF Diabetes Atlas. 8th ed. Brussels, Belgium: International Diabetes Federation; 2017. [Google Scholar]
- 4. Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open. 2017;7(7):e016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang ZH, Kihl‐Selstam E, Eriksson JW. Ketoacidosis occurs in both Type 1 and Type 2 diabetes—a population‐based study from Northern Sweden. Diabet Med. 2008;25(7):867‐870. [DOI] [PubMed] [Google Scholar]
- 6. Jensen ML, Persson F, Andersen GS, et al. Incidence of ketoacidosis in the Danish type 2 diabetes population before and after introduction of sodium‐glucose cotransporter 2 inhibitors—a nationwide, retrospective cohort study, 1995‐2014. Diabetes Care. 2017;40(5):e57‐e58. [DOI] [PubMed] [Google Scholar]
- 7. Kim YG, Jeon JY, Han SJ, Kim DJ, Lee KW, Kim HJ. Sodium‐glucose co‐transporter‐2 inhibitors and the risk of ketoacidosis in patients with type 2 diabetes mellitus: a nationwide population‐based cohort study. Diabetes Obes Metab. 2018;20(8):1852‐1858. [DOI] [PubMed] [Google Scholar]
- 8. Lam KS, Chow CC, Tan KC, et al. Practical considerations for the use of sodium‐glucose co‐transporter type 2 inhibitors in treating hyperglycemia in type 2 diabetes. Curr Med Res Opin. 2016;32(6):1097‐1108. [DOI] [PubMed] [Google Scholar]
- 9. INVOKANA® (canagliflozin) tablets, for oral use [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2018. [Google Scholar]
- 10. Invokana (canagliflozin hemihydrate) [package insert]. Italy: Janssen‐Cilag SpA; n.d. [Google Scholar]
- 11.FARXIGA® (dapagliflozin) [package insert]. Wilmington, DE AstraZeneca Pharmaceuticals; October 2017.
- 12. Forxiga (dapagliflozin) [package insert]. Germany: AstraZeneca GmbH; n.d. [Google Scholar]
- 13.JARDIANCE® (empagliflozin) [package insert]. Ridgefield, CT; Boehringer Ingelheim Pharmaceuticals; December 2017.
- 14. Jardiance (empagliflozin) [package insert]. Germany: Boehringer Ingelheim Pharma GmbH & Co. KG; n.d. [Google Scholar]
- 15. STEGLATRO™ (ertugliflozin) [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp; December 2017. [Google Scholar]
- 16. Steglatro (ertugliflozin) [packet insert]. Belgium: Schering‐Plough Labo NV; n.d. [Google Scholar]
- 17. Food and Drug Administration . FDA drug safety communication: FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/UCM446954.pdf. Accessed March 22, 2018.
- 18. European Medicines Agency . EMA confirms recommendations to minimise ketoacidosis risk with SGLT2 inhibitors for diabetes. Healthcare professionals should be aware of possible atypical cases. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/SGLT2_inhibitors__20/European_Commission_final_decision/WC500202393.pdf. Accessed June 19, 2017.
- 19. Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care. 2015;38(9):1680‐1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. [DOI] [PubMed] [Google Scholar]
- 21. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from Phase 2b/3 clinical trials. Diabetes Obes Metab. 2017;20(3):620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2018;380(4):347‐357. [DOI] [PubMed] [Google Scholar]
- 23. Kohler S, Zeller C, Iliev H, Kaspers S. Safety and tolerability of empagliflozin in patients with type 2 diabetes: pooled analysis of phase I‐III clinical trials. Adv Ther. 2017;34(7):1707‐1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. [DOI] [PubMed] [Google Scholar]
- 25. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT‐2 inhibitors on diabetic ketoacidosis: a meta‐analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53‐60. [DOI] [PubMed] [Google Scholar]
- 26. Tang H, Li D, Wang T, Zhai S, Song Y. Effect of sodium‐glucose cotransporter 2 inhibitors on diabetic ketoacidosis among patients with type 2 diabetes: a meta‐analysis of randomized controlled trials. Diabetes Care. 2016;39(8):e123‐e124. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Desai M, Ryan PB, et al. Incidence of diabetic ketoacidosis among patients with type 2 diabetes mellitus treated with SGLT2 inhibitors and other antihyperglycemic agents. Diabetes Res Clin Pract. 2017;128:83‐90. [DOI] [PubMed] [Google Scholar]
- 28. Fralick M, Schneeweiss S, Patorno E. Risk of diabetic ketoacidosis after initiation of an SGLT2 inhibitor. N Engl J Med. 2017;376(23):2300‐2302. [DOI] [PubMed] [Google Scholar]
- 29. Voss EA, Makadia R, Matcho A, et al. Feasibility and utility of applications of the common data model to multiple, disparate observational health databases. J Am Med Inform Assoc. 2015;22(3):553‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Core Team . R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.R-project.org/.
- 31. Genkin A, Lewis DD, Madigan D. Large‐scale bayesian logistic regression for text categorization. Technometrics. 2007;49(3):291‐304. [Google Scholar]
- 32. Tibshirani R. Regression shrinkage and selection via the lasso. J Royal Statistical Soc, Series B (Methodological). 1996;58(1):267‐228. [Google Scholar]
- 33. Suchard MA, Simpson SE, Zorych I, Ryan P, Madigan D. Massive parallelization of serial inference algorithms for a complex generalized linear model. ACM Trans Model Comput Simul. 2013;23(1):1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One‐to‐many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21(Suppl 2):69‐80. [DOI] [PubMed] [Google Scholar]
- 35. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schuemie MJ, Ryan PB, DuMouchel W, Suchard MA, Madigan D. Interpreting observational studies: why empirical calibration is needed to correct p‐values. Stat Med. 2014;33(2):209‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 38. Hine J, Paterson H, Abrol E, Russell‐Jones D, Herring R. SGLT inhibition and euglycaemic diabetic ketoacidosis. Lancet Diabetes Endocrinol. 2015;3(7):503‐504. [DOI] [PubMed] [Google Scholar]
- 39. Ahmed M, McKenna MJ, Crowley RK. Diabetic ketoacidosis in patients with type 2 diabetes recently commenced on Sglt‐2 inhibitors: an ongoing concern. Endocr Pract. 2017;23(4):506‐508. [DOI] [PubMed] [Google Scholar]
- 40. Bell DSH. Re: diabetic ketoacidosis in patients with type 2 diabetes on Sglt‐2 inhibitors: an ongoing concern. Endocr Pract. 2018;24(1):126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Negative control outcomes
Supplemental Table 2. Baseline demographics in pre‐specified AHA new‐user cohorts
Supplemental Table 3. Unadjusted incidence rate of DKA in new users of insulinotropic AHAs†
Supplemental Table 4. Proportion of fatal DKA events across databases†
Supplemental Table 5. Selected risk factors and preceding events among patients who developed DKA after index
Supplemental Table 6. Hazard ratio of DKA identified from diagnoses in inpatient claims only for new users of SGLT2 inhibitors versus comparator AHAs
Supplemental Figure 1. Hazard ratio of DKA for new users of SGLT2 inhibitors versus insulinotropic AHAs† (A) over the entire observation period and (B) with censoring at the initiation of nonindex AHAs and discontinuation of the index AHA.
Supplemental Figure 2. Hazard ratio of DKA for new users of canagliflozin versus comparator AHAs.
Supplemental Figure 3. Hazard ratio of DKA for new users of dapagliflozin versus comparator AHAs.
Supplemental Figure 4. Hazard ratio of DKA for new users of empagliflozin versus comparator AHAs.


