Abstract
Treatment of haemophilia A/B patients comprises factor VIII (FVIII) or factor IX (FIX) concentrate replacement therapy, respectively. FVIII and FIX activity levels can be measured in clinical laboratories using one‐stage activated partial thromboplastin time (aPTT)‐based clotting or two‐stage chromogenic factor activity assays. We discuss strengths and limitations of these assays, providing examples of clinical scenarios to highlight some of the challenges associated with their current use for diagnostic and monitoring purposes. Substantial inter‐laboratory variability has been reported for one‐stage assays when measuring the activity of factor replacement products due to the wide range of currently available aPTT reagents, calibration standards, factor‐deficient plasmas, assay conditions and instruments. Chromogenic activity assays may avoid some limitations associated with one‐stage assays, but their regulatory status, perceived higher cost, and lack of laboratory expertise may influence their use. Haemophilia management guidelines recommend the differential application of one or both assays for initial diagnosis and disease severity characterisation, post‐infusion monitoring and replacement factor potency labelling. Efficient communication between clinical and laboratory staff is crucial to ensure application of the most appropriate assay to each clinical situation, correct interpretation of assay results and, ultimately, accurate diagnosis and optimal and safe treatment of haemophilia A or B patients.
Keywords: chromogenic assay, factor activity assays, haemophilia A, haemophilia B, one‐stage assay
1. INTRODUCTION
The factor VIII (FVIII) and factor IX (FIX) proteins are encoded by the F8 and F9 genes, respectively, and play key roles in the intrinsic pathway of the coagulation cascade.1 FVIII is an essential cofactor for FIX. Upon tissue injury, FVIII potentiates activated FIX (FIXa) activity to form the intrinsic FXase (tenase) complex, which is responsible for the activation of factor X (FXa) generated by the coagulation cascade. FXa then combines with activated factor V (FVa) to form the FXa/FVa prothrombinase complex, which converts prothrombin to thrombin. Thrombin cleaves fibrinogen, to form fibrin monomers, and activates factor XIII (FXIIIa), which catalyses the formation of covalent bonds between fibrin monomers and a stabilized fibrin clot.
Haemophilia A and B are inherited bleeding disorders caused by defects in the F8 and F9 genes, respectively. In these patients, absent or significantly decreased FVIII or FIX activity prevents adequate clot formation, and severe deficiency may result in spontaneous bleeding into joints and muscles and severe/prolonged bleeding following traumatic injury.1 Haemophilia A and B are heterogeneous disorders due to a host of different mutations that result in differing levels of factor activity and therefore disease severity. Haemophilia severity is classified according to plasma factor activity levels, which in the majority of cases correlates well with clinical bleeding symptoms.2 Patients with FVIII or FIX activity below 1% of normal (<0.01 IU/mL) are classified as having severe haemophilia, patients with 1%‐5% (0.01‐0.05 IU/mL) activity have moderate haemophilia, and those with 6%‐39% (0.06‐0.39 IU/mL) have mild haemophilia.3 Patients with severe haemophilia A or B are primarily treated with replacement therapy comprising plasma‐derived (pd‐FVIII/FIX) or recombinant (rFVIII/FIX) concentrates, which are administered prophylactically to prevent and/or on‐demand to treat bleeding episodes.4
Either one‐stage activated partial thromboplastin time (aPTT)‐based clotting or two‐stage chromogenic factor activity assays can be used in the diagnosis of haemophilia A or B, to classify disease severity, for potency labelling of FVIII and FIX concentrates by manufacturers, to monitor post‐infusion activity levels of FVIII and FIX during treatment and to test for FVIII and FIX antibodies (inhibitors).
In this review, we discuss the use of one‐stage clotting and two‐stage chromogenic factor activity assays for the purposes outlined above, in addition to presenting the potential confounding factors that should be considered when choosing an assay for a specific patient, replacement product or clinical situation. Our aim was to increase awareness of the clinically relevant features and limitations of each assay and to foster informed communication between factor replacement product manufacturers, treating clinicians and clinical laboratory staff for the management of patients with haemophilia A or B.
2. FVIII AND FIX ACTIVITY ASSAYS
Understanding the differences in methodology between one‐stage clotting and two‐stage chromogenic factor activity assays is critical to assess the accuracy and impact of these assays on the diagnosis, potency labelling and monitoring of patients with haemophilia A or B.
2.1. One‐stage aPTT‐based factor activity assays
The one‐stage factor activity assay is based on the aPTT. The aPTT method measures the functionality of the intrinsic (or contact activation) and common coagulation pathways (Figure 1;5, 6, 7). The time required for clot formation (the aPTT) is dependent on factor levels. Normal aPTT values are dependent on the reagent used and are usually within the range of 22‐40 seconds.8
Figure 1.
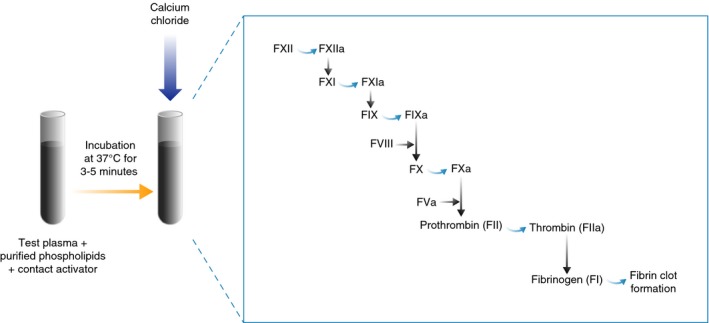
Schematic of the activated partial thromboplastin time (aPTT) method. Contact activator (glass, silica, kaolin, celite, ellagic acid or sulfatides) and phospholipid (derived from soybean, rabbit or bovine brain, human placenta or of synthetic origin) are added to the test plasma and incubated at 37°C to allow the activation of the contact system. Calcium is then added to initiate the activation of the intrinsic and common pathways and, ultimately, fibrin clot formation. The aPTT is quantified as the time (seconds) taken for the clot to form from the time point at which calcium is added and is dependent on all of the intrinsic pathway factors, including factor (F) VIII, present in the test plasma (with the exception of FII). A burst of thrombin formation occurs after sufficient levels of activated FVIII (FVIIIa) have been generated through feedback activation by thrombin, leading to the formation of a clot.5 Adapted with permission from Adcock et al72
The sensitivity of the aPTT to detect factor deficiencies is dependent on the reagents used, which may vary between manufacturers and among lots from the same manufacturer. There is no international standardisation of aPTT reagents. The Clinical Laboratory Standards Institute recommends that an aPTT reagent exhibits a sensitivity of 30% factor level in plasma.9 Sensitivity of the aPTT reflects the level to which a single factor activity within the intrinsic pathway must fall before the clotting time is above the upper limit. This prolongation may indicate the presence of an anticoagulant (either an anticoagulant drug or a non‐specific inhibitor such as a lupus anticoagulant), a deficiency of intrinsic or common coagulation pathway factors (FVIII, FIX, factor XI (FXI), factor XII (FXII) or high molecular weight kininogen [HMWK]) or the presence of a specific factor inhibitor to any of these factors. The aPTT has variable sensitivities to the common pathway factors (factor II, FV, FX and fibrinogen).10 Mixing tests can be performed to screen for the cause of prolongation. The immediate aPTT of a 1:1 mixture of test (patient) plasma and standard plasma should correct a prolonged clotting time resulting from a factor deficiency, but demonstrates no correction or partial correction in the presence of a neutralising inhibitor. It should be noted that non‐neutralising antibodies/inhibitors will correct mixing studies and should be vetted by either in vivo pharmacokinetic (PK) studies or immunoelectrophoresis methods. Some inhibitors are time‐ and temperature‐dependent, and may require incubation at 37°C for 1‐2 hours to demonstrate their inhibitory effect.
The one‐stage factor activity assay can be used to measure intrinsic pathway FVIII, FIX or FXI activity,11, 12 in addition to FXII, HMWK and PK activities. The factor being investigated is the only limiting factor in this type of assay; all reagents, except the type of deficient plasma used, and the methodology remain constant. This is accomplished by mixing the test plasma with a specific factor‐deficient plasma (ie containing normal levels of all clotting factors, but deficient in the factor under investigation). This assay is based on the assumption that the deficient factor in the test plasma is the rate‐limiting determinant of clotting time. Serial dilutions of standard reference plasma (ie containing known concentrations of FVIII, FIX or FXI) are made using a buffer, and then, aPTT assays are performed (Figure 2). The aPTT for each dilution (which correspond to a given factor concentration) is evaluated on the appropriate graphs (eg log‐linear or log‐log), and a line of best fit is drawn to form the calibration curve.13
Figure 2.
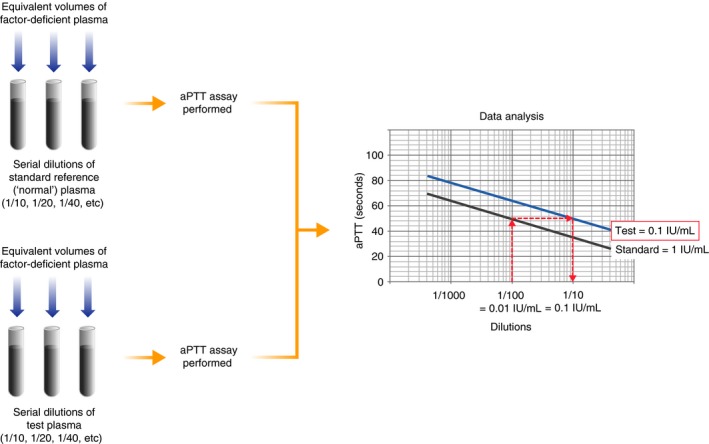
Schematic of the one‐stage activated partial thromboplastin time (aPTT)‐based factor activity assay. Standard reference plasma is serially diluted in buffer and mixed with an equal volume of factor‐deficient plasma, which is completely lacking in the factor to be measured, but contains normal (ie >50%) levels of all other clotting factors. An aPTT assay is then performed to determine the clotting time for each dilution. The clotting time data for each dilution are plotted against the calibration curve, and the difference between the lines of best fit is proportional to the reduction or increase in factor level in the test plasma. For example, as shown on the graph, if the reference plasma contains 100% (1 IU/mL) factor (F) VIII or FIX, a dilution of 1/100 is equivalent to a concentration of 1% (0.01 IU/mL), which produces an aPTT of 50 s. The test plasma that produces a clotting time of 50 s in the example shown here is equivalent to 10% (0.1 IU/mL) of the reference plasma, and therefore, the test plasma contains 10% (0.1 IU/mL) factor activity
All standards (calibrators) should be traceable against an international standard (IS), known as a primary standard.11, 13 Laboratory‐manufactured standards are usually traceable to a secondary standard that has been calibrated against the primary standard. It is recommended that factor activity assays are calibrated at a minimum of once every 6 months, although calibrations are required for new lots of reagents (both deficient plasma and APTT reagents), and some laboratories calibrate more often.5 The most common commercial factor‐deficient plasmas used in the performance of factor activity assays are immunodepleted for FVIII, FIX or FXI. The clinical impact of these method characteristics is that there are a number of sources of variation that can adversely affect accuracy, which is generally lower than screening methods such as aPTT in inter‐centre comparisons.14
To determine the factor activity of test plasma, aPTT assays are generally performed on serial dilutions of test plasma in diluent (eg buffer, saline) mixed with equivalent volumes of factor‐deficient plasma (Figure 2). The aPTT results are plotted on the same log‐linear graphic representation as the calibration curve, and a line of best fit is drawn. The difference between these two best‐fit lines is proportional to the reduction or increase in functional factor level within the test plasma, but this difference can also be non‐specific for the relevant factor. Patient results for each dilution on the calibration curve are compared with evaluate for parallelism. Non‐parallelism, in which factor activity increases or decreases disproportionally with subsequent dilutions, is an indication of a specific neutralising inhibitor or sample activation, respectively.12
2.2. Chromogenic factor activity assays
The two‐stage chromogenic factor activity assay (referred to as the “chromogenic assay”) can measure the activity of FVIII or FIX in plasma.5, 12, 15, 16 During the first stage, the test plasma is mixed with the appropriate reagents and substrates (FX, phospholipids, calcium chloride, prothrombin or thrombin [in some assays]), resulting in the rapid activation of FX (to FXa) (Figure 3A). The amount of FXa produced is proportional to the level of functional factor under investigation. During the second stage, a chromogenic substrate specific for FXa is added and FXa concentration is quantified by photometric monitoring of the cleaved coloured substrate (Figure 3B). The colour intensity is proportional to the level of FXa generated, which in turn is proportional to functional factor within the test plasma, and is determined by reading off a standard curve. In general, the recommended calibration frequency is the same as that for one‐stage assays. Some local regulatory agencies may require additional calibration verification (verification of analytical measurement range [AMR]) or may accept an AMR check in lieu of calibration. Chromogenic assays do not require the addition of factor‐deficient plasma (a significant variable), are typically performed in duplicate at one dilution and are less prone to interference by assay components and some pre‐analytical variables since the sample is diluted more than in the one‐stage assay.
Figure 3.
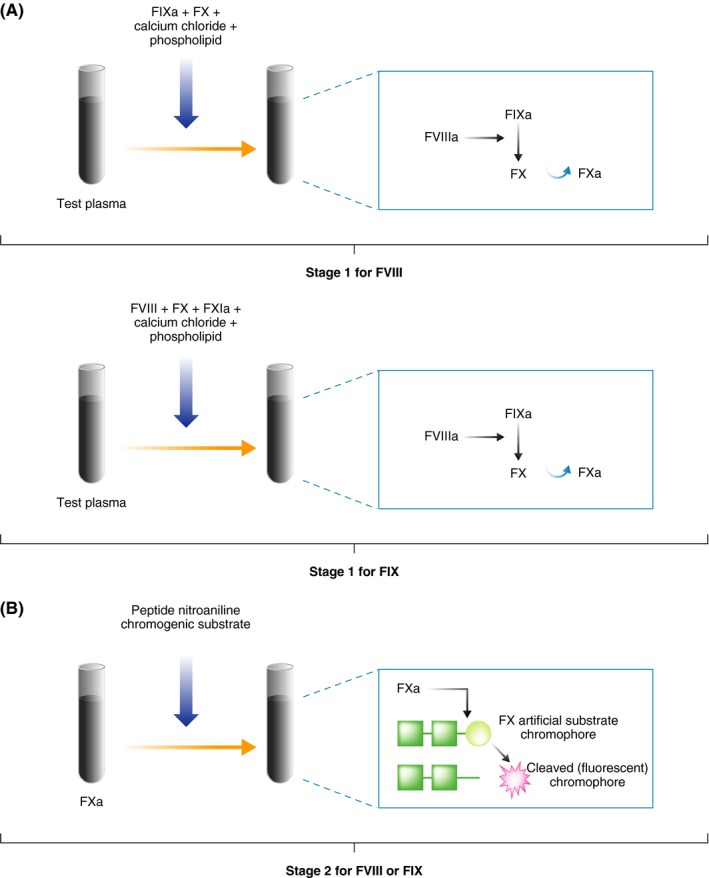
Schematic of the chromogenic factor activity assay for factor (F) VIII or FIX. A, First stage of the chromogenic assay in which activated FX (FXa) is generated. B, Second stage of the chromogenic assay in which FXa cleaves a specific chromogenic substrate. The assay should be performed at three dilutions to assess for parallelism
2.3. Application of factor activity assays
It is the current status among clinical diagnostic laboratories that the one‐stage assay is the most commonly performed factor activity assay. This was determined in an External quality Control of diagnostic Assays and Tests (ECAT) survey where 193/217 European laboratories reported using the one‐stage assay (vs 15 who reported use of the chromogenic assay),17 as well as in a global survey where one‐stage clotting assays were reported as the most frequently used for assessment of both FVIII and FIX activities in a total of 210 laboratories.18
The current predominance of the one‐stage assay over the chromogenic assay may be attributable to various factors. Because of the long‐standing use of the one‐stage assay, many laboratory scientists perceive chromogenic assays to be less rapid and more technically complex than one‐stage assays and acknowledge that there is currently a lack of standardised test protocols for the application of chromogenic assays on coagulation analysers.19 However, these issues may be resolved as more chromogenic FVIII assays and the current “Research Use Only” FIX chromogenic assays become approved by the US Food and Drug Administration (FDA) . Despite most laboratories having the appropriate instrumentation to perform chromogenic assays, some laboratory scientists and clinicians report a lack of familiarity and expertise with these assays.17 Chromogenic assays are also perceived to be more expensive than one‐stage assays. However, a recent computer‐based model analysed the costs associated with FVIII and FIX one‐stage and chromogenic assays, concluding that efficient use of reagents (ie by aliquoting and freezing prior to use) can render the cost of the two assays comparable for both single and batch samples.20 Additional cost savings are possible because the chromogenic assay is typically performed at one rather than three dilutions and does not require factor‐deficient plasma.20 Limitations of the chromogenic assay pertain to its availability and ease of setting up for emergency analyses during off‐shift hours. However, the need to perform the chromogenic assay 24/7 for analysis of unique treatment products makes the cost differential between the two assay types less of an issue.
The British Committee for Standards in Haematology, the World Federation of Haemophilia and the Nordic Haemophilia Council have recommended the use of both one‐stage and chromogenic assays for screening and diagnosis of non‐severe haemophilia A.13, 21, 22 For replacement factor potency labelling recommendations, a dichotomy exists between regulatory organisations: the European Pharmacopoeia recommends the chromogenic activity assay for FVIII and the one‐stage assay for FIX,23 whereas the FDA recommends the one‐stage assay for both FVIII and FIX.19
Chromogenic assays are imperative for the accurate diagnosis of non‐severe haemophilia A and are important for treatment monitoring in patients receiving modified factor replacement products, in addition to being more resilient to the potential and prevalent confounding effects of lupus anticoagulants and direct thrombin inhibitor oral anticoagulants.24 These additional points will be discussed in more detail below. There are a number of other factors, however, that may make the use of chromogenic assays preferable to one‐stage assays in particular circumstances.22
A wide range of aPTT reagent‐instrument combinations is used by different laboratories.17, 25 Given the large range of aPTT reagents, assay conditions, instruments, calibration standards and factor‐deficient plasmas for the one‐stage assay, the potential for substantial inter‐laboratory variability is perhaps unsurprising.17, 18, 19, 26 The limited number of available assay kits and reagents for the chromogenic assay in itself reduces this potential variability. Assay and reagent choices are often confined by the available instrumentation within the laboratory, the level of expertise of the laboratory director and technical staff and the laboratory size, but also the clinical requirements from the mix of patient samples received.
3. ASSAY DISCREPANCIES IN HAEMOPHILIA A AND B
While only the one‐stage assay is generally used for haemophilia screening or diagnosis, in some situations both assays should be used. Studies in Spain, Denmark, France, the United Kingdom, Germany and the Czech Republic reported discrepancies between one‐stage and chromogenic assays in 10%‐51% of patients with non‐severe haemophilia A and their families.24, 27, 28, 29, 30, 31 A discrepancy in FVIII activity was defined either as a one‐stage: chromogenic assay ratio of ≤0.628, 29, 30 or as a >1.5‐fold difference between assays.24, 27, 31 Normal one‐stage FVIII activities were reported in approximately 16% of patients with discrepant, non‐severe haemophilia A, indicating that one‐stage assays are not always reliable for detecting this subgroup of patients. In some patients with discrepant haemophilia, disease classification may shift depending on the assay used to measure factor activity levels27, 29 (see also Clinical scenario 1). In addition, the genotype of a patient may not always correlate with the degree of assay discrepancy or clinical phenotype,24 particularly in older patients with mild haemophilia A, in whom FVIII activity levels may increase or even normalise.
In patients with discrepant non‐severe haemophilia A, the nature of the F8 mutation can dictate whether the one‐stage or chromogenic assay produces the higher result. Evidence of normal FVIII antigen levels in patients with certain mutations suggests that a dysfunctional protein is produced.18 Most F8 mutations that have been reported to have lower activity using chromogenic activity assays compared with one‐stage assays are localised to the A1‐A2‐A3 domain interface and are associated with destabilization of the FVIIIa heterotrimer.27, 28, 31, 32, 33, 34 The prolonged incubation during the first stage of the chromogenic assay (10‐12 minutes vs <2 minutes for the one‐stage assay34) may allow the destabilized heterotrimer time to dissociate, resulting in a decrease in FVIIIa generated,18, 27, 28, 34 whereas in the one‐stage assay, FVIIIa is present only transiently.33 In situations in which the results of the one‐stage assay activity are lower than the chromogenic assay, the mutations are associated with thrombin cleavage sites, von Willebrand factor (VWF) binding sites or FIXa binding sites. The prolonged incubation in the chromogenic assay, as well as the supraphysiological factor concentrations present in chromogenic reagents, may overcome the defective binding and lead to higher measured activity in the chromogenic assay vs the one‐stage assay. Other test conditions, such as variable thrombin concentrations, phospholipids and plasma dilution factor, may also contribute to the discrepancy between the two assays.18, 34 For this type of variability, the bleeding phenotype typically correlates with the lower (ie more severe) activity level measured.27, 35, 36
The one‐stage assay may also be associated with limitations in the detection of reduced FIX levels in patients with mild haemophilia B, even when the most sensitive assay reagent is used.37 Currently, there are insufficient data to suggest that only the chromogenic FIX activity assay should be performed in the evaluation of mild haemophilia B. If only the one‐stage assay is used, the diagnosis of a subset of mild haemophilia A and B patients may therefore be missed or the severity of the disease misclassified.24 In patients with non‐severe haemophilia A, both one‐stage and chromogenic assays should be performed.
4. USE OF ONE‐STAGE AND CHROMOGENIC ASSAYS IN THE ASSESSMENT OF GENE THERAPY FOR HAEMOPHILIA A OR B
Transitioning from protein replacement therapy to gene replacement therapy has the potential to achieve sustainable levels of plasma factor activity that can serve to reduce or eliminate recurrent bleeding risk. Currently, ongoing clinical trial results have an outlook for a “curative” maintainable correction of the bleeding phenotype. However, despite significant advances in gene therapy, questions remain regarding its use in specific patient subgroups (eg factor inhibitor patients, paediatric patients and patients with underlying diseases).38, 39 The use of the one‐stage and/or chromogenic assay for the assessment of gene therapeutic products, concomitant replacement therapy or inhibitor assessment has not been totally explored and awaits further studies.
5. USE OF ONE‐STAGE AND CHROMOGENIC ASSAYS IN POTENCY ASSIGNMENT OF FVIII AND FIX CONCENTRATES
FVIII and FIX factor replacement products are assigned a potency to quantify the amount of active product and guide clinicians on treatment dosing.40, 41 The assay used for potency labelling should therefore represent the true in vivo pharmacodynamic profile of a given factor replacement product. To ensure accurate activity measures of post‐infusion factor replacement products, it is important that the assay methods used to analyse samples in the clinical laboratory align with those used to label potency of the product.42 In the presence of discrepancies between measurements, there is the risk that patients may be under‐ or over‐dosed. The risk associated with under‐dosing is the increased potential for new or continued bleeding complications, whereas over‐dosing leads to increased product usage (and hence cost) and increased risk for adverse events of thrombosis.
Potency labelling of FVIII and FIX concentrates uses either one‐stage or chromogenic assays referenced against the current World Health Organization (WHO) concentrate standard.43 The FVIII and FIX subcommittee of the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis and Haemostasis (ISTH) recommends that if either the one‐stage or chromogenic method for FVIII activity provides valid potency estimates relative to the WHO IS for concentrates, this assay can be used for potency labelling.41 If both methods provide valid potency estimates, either assay can be used for potency labelling, and if there are discrepancies between assays, the most appropriate assay for labelling must be identified. These decisions may be based on the in vitro or in vivo characteristics of the factor replacement product in relation to the assay methods.
Manufacturers conduct PK studies in order to establish the relationship between dose (based on the labelled potency) and expected FVIII/FIX recovery in patients, and however, this relationship may be assay‐dependent and/or aPTT reagent‐dependent.41 Ideally, the same assay type that was used to assign potency should be used to measure levels in patient's plasma.44 Information regarding assay specifics that allow accurate measurement of product activity may be specified in the manufacturer reference materials. Laboratories and clinicians can also attempt to determine the best method for assessing the product in the patient's plasma by reviewing the available literature.
The European Pharmacopoeia recommends the use of the chromogenic assay for FVIII potency assignments. Likewise, the majority of manufacturers in attendance at a workshop organised by the European Medicines Agency (EMA) and the European Directorate for the Quality of Medicines and HealthCare (London, November 2013) reported the use of chromogenic assays for the potency labelling of rFVIII products, due to both the European Pharmacopoeia recommendations and the variability observed for some products using one‐stage assays.40 The chromogenic activity assay has been shown to be robust for the potency labelling of new rFVIII products across assay kits,40 however, the European Pharmacopoeia recommendations do not provide specific guidance for new modified rFVIII products (eg polyethylene glycol (PEG) ylated or fusion proteins).23 The FDA recommends the one‐stage assay for FVIII potency assignment.19 Consequently, in the United States, the majority of FVIII products are currently labelled using the one‐stage assay.19 This coincides with the majority of laboratories using the one‐stage assay to monitor the treatment of patients with haemophilia.19
Both the European Pharmacopoeia and the FDA currently recommend the one‐stage assay for FIX potency labelling.23, 40 In addition, the European Pharmacopoeia, WHO and FDA FIX concentrate reference standards, used for potency assignments of FIX products, were established primarily using one‐stage assays.45 Potency values for rFVIII and rFIX products generated using the aPTT‐based factor activity assay can differ depending on the aPTT reagent used.40 Therefore, if the one‐stage assay is used to label the potency of a specific rFVIII or rFIX product, the assay performed in the clinical laboratory for post‐infusion monitoring should use an aPTT reagent or methodology that correlates with in vivo functionality. Although chromogenic assays for FIX concentrates have only been available for a relatively short period of time, the relevance of potencies determined using the chromogenic method should be considered.41 At present, the chromogenic method is not validated for potency labelling of new FIX products, and therefore, manufacturers use the one‐stage assay.46 However, evidence is emerging to indicate that the chromogenic method may generate valid potency estimates for modified rFIX products compared with the one‐stage assay.47
6. USE OF ONE‐STAGE AND CHROMOGENIC ACTIVITY ASSAYS IN MONITORING THE TREATMENT OF HAEMOPHILIA A AND B
Variability in factor activity levels measured using one‐stage and chromogenic assays has been observed for post‐infusion monitoring of both FVIII and FIX concentrates. In a National External Quality Assessment Scheme (NEQAS) study in the United Kingdom, samples from patients with moderate/severe haemophilia A who had received rFVIII products were assessed using one‐stage and chromogenic assays.47 Recovery using one‐stage assays was higher with Instrumentation Laboratory (IL) vs Siemens aPTT reagents in ReFacto® AF (Wyeth Pharmaceuticals Inc) and Advate® (Shire) samples than Kogenate® FS (Bayer) samples. Factor activity levels using chromogenic assays were 32% higher than one‐stage assay results in Kogenate® FS samples, compared with ReFacto® AF (11% higher by chromogenic assay) and Advate® (3% lower by chromogenic assay) samples, irrespective of aPTT reagents used. In a second UK NEQAS study, discrepancies were also reported between FIX activity assays (with either IL or Siemens aPTT reagents) used for post‐infusion monitoring of patients with haemophilia B who had received either BeneFIX® (Wyeth Pharmaceuticals Inc) or Replenine®‐VF (Bio Products Laboratory Ltd).47 Initially, molecule‐specific characteristics of the different factor products were thought to cause these discrepancies, however, emerging data have now shown that the diverse composition of aPTT reagents used in one‐stage assays, particularly the activator and phospholipid component, may also impact assay results as well as the calibrator used. Subsequently, regulatory agencies (ie EMA) recommended that manufacturers provide information about product recovery based on assay type within product reference materials.48, 49, 50
6.1. Effect of modified factor replacement products on assay results
A number of modified rFVIII and rFIX products have recently emerged on the market or are under development (eg B‐domain deletion or truncation [BDD], PEGylated, glycoPEGylated and fusion proteins).40, 51, 52, 53 A significant discrepancy between the potency values obtained using one‐stage and chromogenic assays has been identified for samples containing some modified rFVIII replacement therapies40 (see also Clinical scenario 2). This discrepancy can also be seen between different aPTT reagents used in one‐stage assays. Variation in potency values for some modified rFIX products has also been reported among one‐stage assays, depending on the reagents used.40
A comparison of one‐stage and chromogenic assays for testing post‐infusion samples containing BDD rFVIII (ie Nuwiq® [Octapharma Ltd], NovoEight® [Novo Nordisk A/S], N8‐GP [Novo Nordisk A/S], BAY 94‐9027 [Bayer HealthCare], Eloctate® [Biogen Idec Inc], CSL627 [CSL Behring] and BAX855 [Shire]) concluded that either one‐stage or chromogenic assays are suitable for this purpose.40 Previously observed assay discrepancies were attributed to differences in B‐domain linkers between products, with the possibility that results obtained using one‐stage assays may have more accurately reflected the lower clinical efficacy of BDD rFVIII (vs pd FVIII or full‐length rFVIII).54 A significant discrepancy between the results obtained using one‐stage and chromogenic assays was identified for samples containing a single chain rFVIII (truncated B‐domain and covalent linkage between heavy and light chain) product, CSL627 (CSL Behring).40 The difference between the two assay formats was found to be consistent and predictable, and thus, the application of a conversion factor of 2 to the one‐stage assay results was suggested.55 Some variation in one‐stage assay results would also be expected for modified rFIX products (ie Rixubis® [Shire], Alprolix® [Biogen Idec], Refixia®/Rebinyn® [Novo Nordisk A/S] and Idelvion® [CSL Behring]), depending on the reagents used.40 For example, Table 1 shows the assay results obtained for pre‐infusion and post‐infusion samples with a modified rFIX product using a one‐stage assay and a chromogenic assay. A significant discrepancy can be seen with the values obtained with each assay within the same laboratory and as compared with the results from an external laboratory.
Table 1.
FIX activity in pre‐infusion and post‐infusion samples with a rFIX modified product using one‐stage and chromogenic assays
| FIX activity (%) | |||
|---|---|---|---|
| Local laboratory | External laboratory | ||
| PTT‐Automate (BCS®‐XP) one‐stage assay | Rossix FIX (BCS®‐XP) chromogenic assay | One‐stage assay | |
| Pre‐infusion | |||
| 70 h after 3000 units of Alprolix® | 5 | 11 | 10 |
| 68 h after 3000 units of Alprolix® | 8 | 15 | 13 |
| Post‐infusion | |||
| 30 min after 3000 units of Alprolix® | 32 | 53 | 49 |
| 30 min after 3000 units of Alprolix® | 34 | 54 | 51 |
Results obtained from a single patient at two different time points pre‐infusion and post‐infusion are shown. These two methods are used in the coagulation laboratory during routine clinical practice, and the difference generally seen on commercial control material or post‐infusion samples is that results with the chromogenic method are ~20%‐30% lower.
Abbreviations: FIX, factor IX; h, hours; min, minutes; r, recombinant.
The PEG moiety of long‐acting PEGylated BDD rFVIII and rFIX products Jivi® (Bayer HealthCare) and Refixia®/Rebinyn® has been reported to interact with silica‐based aPTT assay reagents, causing activity results to be greatly under‐ or over‐estimated, respectively.51, 53 The use of ellagic acid and/or polyphenol reagents in the one‐stage assay, instead of silica‐based reagents, may correct this discrepancy, but other reagent components (phospholipid source, deficient plasma) may also play a role since reagents with the same activator give discrepant results.51
A potential solution to the problem of assay discrepancies with certain factor replacement products could be the use of product‐specific reference standards. For example, the ReFacto AF® (BDD rFVIII) laboratory standard was shown to effectively reduce the discrepancy between one‐stage and chromogenic assays, allowing an accurate assessment of FVIII activity levels in plasma samples from ReFacto AF®‐treated patients.56
Although product‐specific reference standards may facilitate accurate treatment monitoring of modified factor replacement products, the decision to use these standards may bring significant challenges.19, 40 For example, in order to ensure that the correct product‐specific standard is used, it would be essential for the clinician to communicate to the clinical laboratory team the specific factor replacement product used by each patient. Even if this information is effectively communicated to the clinical laboratory, the extra time and effort required to validate assays with multiple product‐specific standards may only be feasible for a small number of specialised laboratories. In addition, the source of the standard should be considered in this scenario, since it would cause the assay to be labelled as a laboratory‐developed test, unless the product standard has been approved by the FDA for use with the particular aPTT reagent, which is unlikely.
Another potential option for addressing systematic assay discrepancies with modified rFVIII and rFIX products is to adhere to manufacturer‐recommended postassay correction factors for the clinical interpretation of results.19, 40 However, this approach can be difficult to implement and is fraught with significant potential for error. Although reducing the burden on laboratory staff by removing the need for different assay reagents/standards to be used with different factor replacement products, laboratories would require detailed information on the specific reagents and instrument combinations for which the correction factor is valid. In addition, no data exist to confirm that the correction factor applied would be appropriate for different lots of each aPTT reagent used in the one‐stage assay. The introduction of such correction factors would necessarily represent approximations based on a relatively small number of tests and might introduce a large margin of error into single analysis results. In addition, as with the use of product‐specific reference standards, the physician would be required in each individual case to inform the clinical laboratory about the specific factor replacement product used. In this context, it is important to consider the risk of potentially serious adverse events attributable to misunderstanding and/or miscommunication.
In order to avoid incorrect measurements of post‐infusion plasma samples for modified rFVIII and rFIX products, manufacturers could also provide information on the most suitable tests (ie reagent‐instrument combinations) for treatment monitoring of specific products, and information on certified external laboratories to which patient samples can be sent for testing using the appropriate reagents for the replacement product used.40 The use of external reference laboratories may be restricted by regulatory requirements in place in certain regions (eg the United States).
In the United States, the Medical and Scientific Advisory Council (MASAC) of the National Haemophilia Foundation recommends the use of FVIII and FIX chromogenic assays for treatment monitoring, once these assays have been approved by the FDA.57 The MASAC also recommends that clinical laboratories that routinely perform factor activity assays participate in regular proficiency testing and in field surveys of new factor replacement products. Programmes for such proficiency testing are also under development in Europe (UK NEQAS, ECAT and NASCOLA [North American Specialized Coagulation Laboratory Association]). Currently, only a few external quality assessment (EQA) programmes worldwide provide assessment for some of the modified products.
Overall, in order to increase awareness of selection criteria for the appropriate choice of assay for monitoring post‐infusion assay samples for specific modified factor replacement products, in addition to any manufacturer‐provided product‐specific standards or conversion factors, clinicians and laboratories may benefit from information about the assay used for potency assignment and manufacturer recommendations on the best choice of assay.40 This information should ideally be included in the product information of the relevant FVIII or FIX product, and manufacturers should be encouraged to provide this. Each laboratory should consider performing correlations with another institution that has a validated assay for post‐infusion patient samples when new products are introduced.
6.2. Effect of a FVIII‐mimicking humanised antibody product on factor activity assay results
A novel replacement product approved for severe haemophilia A patients is a humanised monoclonal antibody that mimics the FVIII cofactor activity.58 This antibody (emicizumab; Hemlibra® [F Hoffmann‐La Roche]) forms a bridge and the appropriate orientation between human FIXa and FX on the phospholipid surface to facilitate the activation of FX to FXa. This subcutaneous injected treatment is administered weekly and remains in the blood for 28 days providing moderate levels of FVIII activity and significantly reducing breakthrough bleeding episodes, while it can also be used in the presence of inhibitors. The presence of emicizumab interferes with clotting‐based assays that rely on FVIII activity, such as the aPTT and one‐stage FVIII assays.58 Including recombinant anti‐idiotype anti‐emicizumab monoclonal antibodies in one‐stage assays has been shown to prevent interference by emicizumab, resulting in accurate measurements of both FVIII activity and inhibitor titers.59 One study did demonstrate a relationship between one‐stage FVIII and emicizumab levels; however, this required method modification which may not be readily implemented in laboratories and this relationship requires further verification.60 Current guidance states that the level of emicizumab must be measured with a chromogenic two‐stage assay using human components as emicizumab does not recognise non‐human FIXa or FX.58 The determination of the presence and the level of FVIII inhibitors can only be performed using a chromogenic two‐stage assay using bovine components. The clinician and coagulation laboratories must understand the laboratory's limitations of having to use and keep valid multiple chromogenic FVIII assays when assessing patients on emicizumab.
6.3. Haemostatic monitoring during intensive treatment (eg major surgery) or specific physical activities
The emergency treatment of acute bleeding episodes and peri‐operative treatment for patients with haemophilia requires accurate real‐time measurements of FVIII or FIX plasma levels in order to ensure patient safety.40 During such emergencies, there is the possibility that patients may receive a different factor replacement product than that which they would usually receive. Therefore, it is important to ensure that the selected assay can effectively measure the plasma levels of both products. On the whole, given the replacement products known to date and with the exception of Idelvion®, the chromogenic assays seem to be the most suitable means to accurately assess all extended half‐life products. It is therefore of paramount importance that the laboratory is informed of the specific factor placement product(s) used by a patient undergoing surgery or in an emergency situation, to ensure that the clinical laboratory uses the most appropriate assay(s) or forwards the sample to a laboratory in which the assay is available.
In addition, there is the potential for bleeding complications during surgery if the use of a specific assay results in a missed haemophilia diagnosis or an incorrect estimation of bleeding risk, as the appropriate haemostatic treatment may not be readily available during the course of the surgical procedure.18 Conversely, patients with no perceived bleeding risk may face unnecessary delays to surgery or unwarranted treatment in the case of a haemophilia misdiagnosis on the basis on assay results.18
7. EFFECT OF OTHER FACTORS ON ASSAY RESULTS
7.1. Effect of lupus anticoagulant on one‐stage assay results
It is important to be aware of the potential confounding effect that lupus anticoagulant may have on the assay employed. In aPTT‐based assays, a lupus anticoagulant can falsely suggest the presence of a FVIII and/or FIX deficiency or inhibitor.15, 61 In the presence of a lupus anticoagulant, chromogenic assays typically provide a more accurate measure of FVIII or FIX activity.15, 61, 62
The potential mechanisms for decreased interference by a lupus anticoagulant in the chromogenic assay include high dilution, lower dependence on phospholipids or the bypass of contact activation and prothrombinase complex within this assay.15, 61 It is also possible for a lupus anticoagulant to coexist with a FVIII or FIX inhibitor or to mimic a FVIII or FIX inhibitor. If the presence of a lupus anticoagulant is known or suspected, the clinical laboratory should consider measuring factor activity using the chromogenic assay, even if the activity is assessed as part of the inhibitor assay.
7.2. Effect of direct oral anticoagulants on assay results
One‐stage intrinsic factor activity assays are more likely to be affected by direct thrombin inhibitor anticoagulants than chromogenic assays.63, 64 Chromogenic FVIII and FIX activity assays are falsely reduced in the presence of direct FXa inhibitor anticoagulants, and these drugs may interfere with one‐stage assay activities.65
Rivaroxaban and other direct FXa inhibitor anticoagulants at concentrations >50 ng/mL may interfere with the one‐stage FVIII and FIX assays, and concentrations >150 ng/mL are known to interfere with the chromogenic assay, causing results to be factitiously reduced, although the magnitude of interference differs considerably between patients.64 Plasma dilutions before analysis may partially correct the rivaroxaban effect within one‐stage and chromogenic assays.
8. USE OF ASSAYS TO DETECT INHIBITORS
The cumulative incidence of inhibitors (alloantibodies) to FVIII and FIX replacement products is estimated to be 25%‐32% in haemophilia A and 4%‐5% in haemophilia B.66 These inhibitors can develop against the “replacement” FVIII/FIX products, which are recognised as foreign or “non‐self”.67, 68 FVIII/FIX inhibitors may cross‐react with the patient's own FVIII/FIX, converting a mild haemophilia phenotype into a severe phenotype and potentially resulting in excessive bleeding.67, 68 Alternatively, the inhibitor may cross‐react with FVIII/FIX replacement products, reducing the effectiveness of the replacement therapy. If an unexpected lack of response to factor replacement therapy is observed or if a patient with mild haemophilia uncharacteristically presents with excessive bleeding, the presence of an inhibitor must be considered.67, 68 In patients with acquired haemophilia A, an autoimmune disorder, FVIII inhibitors (autoantibodies) are either idiopathic (approximately half of patients) or develop in association with various other medical conditions or disorders, including pregnancy, rheumatoid arthritis and systemic lupus erythematosus, and can result in serious bleeding in patients with no personal or familial bleeding history.67, 68
The screening assay for an inhibitor is based on a mixing test using “normal” and test plasma in an aPTT reaction. If the normal plasma in a 50:50 mix fails to correct the prolonged aPTT, a factor inhibitor may be present that inhibits the FVIII or FIX in the normal plasma. Classic or Nijmegen‐modified Bethesda assays are typically used to quantify the inhibitor titre.69 Bethesda assays measure the ability of test plasma (potentially containing the factor inhibitor) to neutralise the FVIII/FIX within a normal plasma sample, through serial dilutions and analysis via one‐stage or chromogenic assays.18, 67, 68 The Nijmegen‐modified Bethesda assay includes buffer and added protein in the reaction relative to the classic version to avoid the potential for false‐positive low‐titre inhibitor results.
Both the one‐stage and chromogenic assays can be used to measure factor levels in the Bethesda assay, as well as in the Nijmegen‐modified assay.18, 70 Although correlation is good between inhibitor results from these two assays, non‐specific inhibition may be detected with the one‐stage assay.18 A study of 702 patients with haemophilia A compared different methods of detecting FVIII inhibitors.71 The one‐stage and chromogenic methods had similar sensitivity, but the one‐stage assay produced some false‐positive, low‐titre results.71 Furthermore, the chromogenic method had a greater sensitivity to detect inhibitors than the one‐stage method in the Bethesda assay.71
9. CONCLUSION
Evaluation of the strengths and limitations of one‐stage and chromogenic assays strongly supports the use of both factor assays for the initial diagnosis of non‐severe haemophilia A and determination of disease severity. The assay used may also play a significant role in monitoring patients following rFVIII or rFIX infusion. Recovery in the one‐stage assay for modified recombinant products may vary significantly depending on the aPTT reagent used. Chromogenic assays are used less frequently, but may avoid some of the limitations associated with one‐stage assays. Effective communication links should exist between product manufacturers, clinicians and clinical laboratory staff to ensure that the most suitable factor assay is performed for a particular clinical situation, the assay results are correctly interpreted and, ultimately, that each patient receives the optimal treatment regimen.
CONFLICT OF INTEREST
RM serves as an advisor to Bayer, Genentech and Novo Nordisk. KS has received speaker fees from Octapharma, Radiometer and Shire. MS has received grants and honoraria from Bayer, Bioverativ, Novo Nordisk, and Shire; an honorarium from CSL Behring; grants and honoraria from, and has served as an advisor to, Chugai Pharmaceutical. DA serves as an advisor to Novo Nordisk and Shire.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing and review of this manuscript and approved the final version.
Supporting information
ACKNOWLEDGEMENTS
The authors thank Frank Driessler for scientific accuracy review of the initial outline and final manuscript. Editorial support in the form of development of the draft outline and manuscript first draft in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact‐checking, referencing and graphic services was provided by Physicians World Europe GmbH (Mannheim, DE) with financial support from Novo Nordisk A/S (Bagsværd, DK).
Marlar RA, Strandberg K, Shima M, Adcock DM. Clinical utility and impact of the use of the chromogenic vs one‐stage factor activity assays in haemophilia A and B. Eur J Haematol. 2020;104:3–14. 10.1111/ejh.13339
Funding information
Novo Nordisk A/S (Bagsværd, DK) provided financial support for medical writing.
REFERENCES
- 1. Lee C, Berntorp E, Hoots W. Textbook of Hemophilia. 2nd ed Chichester, UK: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 2. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12(11):1935‐1939. [DOI] [PubMed] [Google Scholar]
- 3. White GC 2nd, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85(03):560. [PubMed] [Google Scholar]
- 4. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187‐197. [DOI] [PubMed] [Google Scholar]
- 5. Castellone DD, Adcock DM. Factor VIII activity and inhibitor assays in the diagnosis and treatment of hemophilia A. Semin Thromb Hemost. 2016;43:320‐330. [DOI] [PubMed] [Google Scholar]
- 6. Korte W, Clarke S, Lefkowitz JB. Short activated partial thromboplastin times are related to increased thrombin generation and an increased risk for thromboembolism. Am J Clin Pathol. 2000;113(1):123‐127. [DOI] [PubMed] [Google Scholar]
- 7. Langdell RD, Wagner RH, Brinkhous KM. Effect of antihemophilic factor on one‐stage clotting tests; a presumptive test for hemophilia and a simple one‐stage antihemophilic factor assay procedure. J Lab Clin Med. 1953;41(4):637‐647. [PubMed] [Google Scholar]
- 8. Bates SM, Weitz JI. Coagulation assays. Circulation. 2005;112(4):e53‐e60. [DOI] [PubMed] [Google Scholar]
- 9. CLSI . One‐stage prothrombin time (PT) test and activated partial thromboplastin time (APTT) test: approved guideline (H47‐A2) In: Marlar RA, ed. Vol. 28, 2nd ed.; Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2008. https://clsi.org/standards/products/hematology/documents/h47/ [Google Scholar]
- 10. Toulon P, Eloit Y, Smahi M, et al. In vitro sensitivity of different activated partial thromboplastin time reagents to mild clotting factor deficiencies. Int J Lab Hematol. 2016;38(4):389‐396. [DOI] [PubMed] [Google Scholar]
- 11. Ingerslev I. Laboratory assays in hemophilia In: Lee CA, Berntorp EE, Hoots WK. eds. Textbook of Hemophilia. Chichester, UK: Blackwell Publishing Ltd; 2010. [Google Scholar]
- 12. Kitchen S, Preston E. Assay of factor VIII and other clotting factors In: Kitchen S, Olson J, Preston E, eds. Quality in Laboratory Hemostasis and Thrombosis. 2nd ed Hoboken, NJ, USA: Wiley‐Blackwell; 2013. [Google Scholar]
- 13. Mackie I, Cooper P, Lawrie A, et al. Guidelines on the laboratory aspects of assays used in haemostasis and thrombosis. Int J Lab Hematol. 2013;35(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 14. Bolton‐Maggs PH, Favaloro EJ, Hillarp A, Jennings I, Kohler HP. Difficulties and pitfalls in the laboratory diagnosis of bleeding disorders. Haemophilia. 2012;18(Suppl 4):66‐72. [DOI] [PubMed] [Google Scholar]
- 15. Moser KA, Adcock Funk DM. Chromogenic factor VIII activity assay. Am J Hematol. 2014;89(7):781‐784. [DOI] [PubMed] [Google Scholar]
- 16. Wilmot HV, Hogwood J, Gray E. Recombinant factor IX: discrepancies between one‐stage clotting and chromogenic assays. Haemophilia. 2014;20(6):891‐897. [DOI] [PubMed] [Google Scholar]
- 17. Kitchen S, Signer‐Romero K, Key NS. Current laboratory practices in the diagnosis and management of haemophilia: a global assessment. Haemophilia. 2015;21(4):550‐557. [DOI] [PubMed] [Google Scholar]
- 18. Potgieter JJ, Damgaard M, Hillarp A. One‐stage vs. chromogenic assays in haemophilia A. Eur J Haematol. 2015;94(Suppl 77):38‐44. [DOI] [PubMed] [Google Scholar]
- 19. Peyvandi F, Oldenburg J, Friedman KD. A critical appraisal of one‐stage and chromogenic assays of factor VIII activity. J Thromb Haemost. 2016;14(2):248‐261. [DOI] [PubMed] [Google Scholar]
- 20. Kitchen S, Blakemore J, Friedman KD, et al. A computer‐based model to assess costs associated with the use of factor VIII and factor IX one‐stage and chromogenic activity assays. J Thromb Haemost. 2016;14(4):757‐764. [DOI] [PubMed] [Google Scholar]
- 21. Nordic Hemophilia Council Guideline Working Group . Nordic Hemophilia Guidelines. Vol Version 1. 23 June 2015 ed; 2015. [Google Scholar]
- 22. Kitchen S, McCraw A, Echenagucia M. Diagnosis of Hemophilia and Other Bleeding Disorders ‐ A Laboratory Manual. 2nd ed Montréal, Québec, Canada: World Federation of Hemophilia (WFH); 2010. http://www1.wfh.org/publication/files/pdf-1283.pdf. Accessed May 11, 2015. [Google Scholar]
- 23. European Pharmacopoeia . 8th ed. (8.8); 2016. https://www.edqm.eu/en/european-pharmacopoeia-8th-edition-1563.html. Accessed August 2, 2019.
- 24. Bowyer AE, Van Veen JJ, Goodeve AC, Kitchen S, Makris M. Specific and global coagulation assays in the diagnosis of discrepant mild hemophilia A. Haematologica. 2013;98(12):1980‐1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ECAT Foundation . A European External Quality Assessment Programme for Laboratories in the Field of Haemostasis Performed. ECAT Survey; 2013;1‐1‐13. [Google Scholar]
- 26. Ng VL. Prothrombin time and partial thromboplastin time assay considerations. Clin Lab Med. 2009;29(2):253‐263. [DOI] [PubMed] [Google Scholar]
- 27. Cid AR, Calabuig M, Cortina V, et al. One‐stage and chromogenic FVIII: C assay discrepancy in mild haemophilia A and the relationship with the mutation and bleeding phenotype. Haemophilia. 2008;14(5):1049‐1054. [DOI] [PubMed] [Google Scholar]
- 28. Pavlova A, Delev D, Pezeshkpoor B, Muller J, Oldenburg J. Haemophilia A mutations in patients with non‐severe phenotype associated with a discrepancy between one‐stage and chromogenic factor VIII activity assays. Thromb Haemost. 2014;111(5):851‐861. [DOI] [PubMed] [Google Scholar]
- 29. Poulsen AL, Pedersen LH, Hvas AM, Poulsen LH, Thykjaer H, Ingerslev J. Assay discrepancy in mild haemophilia A: entire population study in a National Haemophilia Centre. Haemophilia. 2009;15(1):285‐289. [DOI] [PubMed] [Google Scholar]
- 30. Provaznikova D, Houskova K, Radovska A, Salaj P, Hrachovinova I. Novel mutations associated with a discrepancy between one‐stage and chromogenic FVIII activity assays. Haemophilia. 2015;21(4):e330‐e332. [DOI] [PubMed] [Google Scholar]
- 31. Trossaert M, Boisseau P, Quemener A, et al. Prevalence, biological phenotype and genotype in moderate/mild hemophilia A with discrepancy between one‐stage and chromogenic factor VIII activity. J Thromb Haemost. 2011;9(3):524‐530. [DOI] [PubMed] [Google Scholar]
- 32. d'Oiron R, Pipe SW, Jacquemin M. Mild/moderate haemophilia A: new insights into molecular mechanisms and inhibitor development. Haemophilia. 2008;14(Suppl 3):138‐146. [DOI] [PubMed] [Google Scholar]
- 33. Oldenburg J, Pavlova A. Discrepancy between one‐stage and chromogenic factor VIII activity assay results can lead to misdiagnosis of haemophilia A phenotype. Hamostaseologie. 2010;30(4):207‐211. [PubMed] [Google Scholar]
- 34. Rodgers SE, Duncan EM, Barbulescu DM, Quinn DM, Lloyd JV. In vitro kinetics of factor VIII activity in patients with mild haemophilia A and a discrepancy between one‐stage and two‐stage factor VIII assay results. Br J Haematol. 2007;136(1):138‐145. [DOI] [PubMed] [Google Scholar]
- 35. Kitchen S, Hayward C, Negrier C, Dargaud Y. New developments in laboratory diagnosis and monitoring. Haemophilia. 2010;16(Suppl 5):61‐66. [DOI] [PubMed] [Google Scholar]
- 36. Trossaert M, Lienhart A, Nougier C, et al. Diagnosis and management challenges in patients with mild haemophilia A and discrepant FVIII measurements. Haemophilia. 2014;20(4):550‐558. [DOI] [PubMed] [Google Scholar]
- 37. Park CH, Seo JY, Kim HJ, Jang JH, Kim SH. A diagnostic challenge: mild hemophilia B with normal activated partial thromboplastin time. Blood Coagul Fibrinolysis. 2010;21(4):368‐371. [DOI] [PubMed] [Google Scholar]
- 38. Yamaguti‐Hayakawa GG, Ozelo MC. Gene therapy: paving new roads in the treatment of hemophilia. Semin Thromb Hemost. 2019;45(07):743‐750. [DOI] [PubMed] [Google Scholar]
- 39. Pipe SW. Gene therapy for hemophilia. Pediatr Blood Cancer. 2018;65(2):e26865. [DOI] [PubMed] [Google Scholar]
- 40. Dodt J, Hubbard AR, Wicks SJ, et al. Potency determination of factor VIII and factor IX for new product labelling and postinfusion testing: challenges for caregivers and regulators. Haemophilia. 2015;21(4):543‐549. [DOI] [PubMed] [Google Scholar]
- 41. Hubbard AR, Dodt J, Lee T, et al. Recommendations on the potency labelling of factor VIII and factor IX concentrates. J Thromb Haemost. 2013;11(5):988‐989. [DOI] [PubMed] [Google Scholar]
- 42. Kitchen S, Kershaw G, Tiefenbacher S. Recombinant to modified factor VIII and factor IX ‐ chromogenic and one‐stage assays issues. Haemophilia. 2016;22(Suppl 5):72‐77. [DOI] [PubMed] [Google Scholar]
- 43. Barrowcliffe TW, Hubbard AR, Kitchen S. Standards and monitoring treatment. Haemophilia. 2012;18(Suppl 4):61‐65. [DOI] [PubMed] [Google Scholar]
- 44. Van den Bossche D, Peerlinck K, Jacquemin M. New challenges and best practices for the laboratory monitoring of factor VIII and factor IX replacement. Int J Lab Hematol. 2018;40(Suppl 1):21‐29. [DOI] [PubMed] [Google Scholar]
- 45. Gray E, Pickering W, Hockley J, et al. Collaborative study for the establishment of replacement batches for human coagulation factor IX concentrate reference standards. Pharmeuropa bio/Biological Standardisation Programme, EDQM. 2008;1:19‐30. [PubMed] [Google Scholar]
- 46. Hubbard AR. Potency labeling of novel factor VIII and factor IX concentrates: past experience and current strategy. Semin Thromb Hemost. 2015;41(8):849‐854. [DOI] [PubMed] [Google Scholar]
- 47. Kitchen S, Gray E, Mertens K. Monitoring of modified factor VIII and IX products. Haemophilia. 2014;20(Suppl 4):36‐42. [DOI] [PubMed] [Google Scholar]
- 48. EMA . Idelvion® Summary of Product Characteristics; 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003955/WC500207380.pdf. Accessed August 2, 2019. [Google Scholar]
- 49. EMA . Rixubis® Summary of Product Characteristics; 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003771/WC500182066.pdf. Accessed August 2, 2019. [Google Scholar]
- 50. EMA . Alprolix® Summary of Product Characteristics; 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004142/WC500207015.pdf. Accessed August 2, 2019. [Google Scholar]
- 51. Gu JM, Ramsey P, Evans V, et al. Evaluation of the activated partial thromboplastin time assay for clinical monitoring of PEGylated recombinant factor VIII (BAY 94–9027) for haemophilia A. Haemophilia. 2014;20(4):593‐600. [DOI] [PubMed] [Google Scholar]
- 52. Oldenburg J, Albert T. Novel products for haemostasis ‐ current status. Haemophilia. 2014;20(Suppl 4):23‐28. [DOI] [PubMed] [Google Scholar]
- 53. Rosen P, Rosen S, Ezban M, Persson E. Overestimation of N‐glycoPEGylated factor IX activity in a one‐stage factor IX clotting assay owing to silica‐mediated premature conversion to activated factor IX. J Thromb Haemost. 2016;14(7):1420‐1427. [DOI] [PubMed] [Google Scholar]
- 54. Lippi G, Franchini M, Favaloro EJ. One‐stage clotting versus chromogenic assays for assessing recombinant factor VIII: two faces of a haemostasis coin. Blood Coagul Fibrinolysis. 2009;20(1):1‐3. [DOI] [PubMed] [Google Scholar]
- 55. St Ledger K, Feussner A, Kalina U, et al. International comparative field study evaluating the assay performance of AFSTYLA in plasma samples at clinical hemostasis laboratories. J Thromb Haemost. 2018;16(3):555‐564. [DOI] [PubMed] [Google Scholar]
- 56. Pouplard C, Caron C, Aillaud MF, et al. The use of the new ReFacto AF Laboratory Standard allows reliable measurement of FVIII: C levels in ReFacto AF mock plasma samples by a one‐stage clotting assay. Haemophilia. 2011;17(5):e958‐e962. [DOI] [PubMed] [Google Scholar]
- 57. MASAC statement regarding use of various clotting factor assays to monitor factor replacement therapy. National Hemophilia Foundation MASAC Document #228; 2014. http://www.hemophilia.org/sites/default/files/document/files/masac-228.pdf. Accessed March 21, 2015. [Google Scholar]
- 58. MASAC . Recommendation on the use and management of emicizumab‐kxwh (Hemlibra®) for hemophilia A with and without inhibitors; 2018. https://www.hemophilia.org/Researchers-Healthcare-Providers/Medical-and-Scientific-Advisory-Council-MASAC/MASAC-Recommendations/Recommendation-on-the-Use-and-Management-of-Emicizumab-kxwh-Hemlibra-for-Hemophilia-A-with-and-without-Inhibitors. Accessed March 28, 2019. [Google Scholar]
- 59. Nogami K, Soeda T, Matsumoto T, Kawabe Y, Kitazawa T, Shima M. Routine measurements of factor VIII activity and inhibitor titer in the presence of emicizumab utilizing anti‐idiotype monoclonal antibodies. J Thromb Haemost. 2018;16(7):1383‐1390. [DOI] [PubMed] [Google Scholar]
- 60. Adamkewicz JI, Chen DC, Paz‐Priel I. Effects and interferences of emicizumab, a humanised bispecific antibody mimicking activated factor VIII cofactor function, on coagulation assays. Thromb Haemost. 2019;119(7):1084‐1093. [DOI] [PubMed] [Google Scholar]
- 61. de Maistre E, Wahl D, Perret‐Guillaume C, et al. A chromogenic assay allows reliable measurement of factor VIII levels in the presence of strong lupus anticoagulants. Thromb Haemost. 1998;79(1):237‐238. [PubMed] [Google Scholar]
- 62. Blanco AN, Alcira Peirano A, Grosso SH, Gennari LC, Perez Bianco R, Lazzari MA. A chromogenic substrate method for detecting and titrating anti‐factor VIII antibodies in the presence of lupus anticoagulant. Haematologica. 2002;87(3):271‐278. [PubMed] [Google Scholar]
- 63. Adcock DM, Gosselin R, Kitchen S, Dwyre DM. The effect of dabigatran on select specialty coagulation assays. Am J Clin Pathol. 2013;139(1):102‐109. [DOI] [PubMed] [Google Scholar]
- 64. Tichelaar V, de Jong H, Nijland H, Kluin‐Nelemans H, Meijer K, Mulder A. Interference of rivaroxaban in one‐stage and chromogenic factor VIII: C assays. Thromb Haemost. 2011;106(5):990‐992. [DOI] [PubMed] [Google Scholar]
- 65. Adcock DM, Gosselin R. Direct Oral Anticoagulants (DOACs) in the laboratory: 2015 review. Thromb Res. 2015;136(1):7‐12. [DOI] [PubMed] [Google Scholar]
- 66. Key NS. Inhibitors in congenital coagulation disorders. Br J Haematol. 2004;127(4):379‐391. [DOI] [PubMed] [Google Scholar]
- 67. Kershaw G, Favaloro EJ. Laboratory identification of factor inhibitors: an update. Pathology. 2012;44(4):293‐302. [DOI] [PubMed] [Google Scholar]
- 68. Kershaw G, Jayakodi D, Dunkley S. Laboratory identification of factor inhibitors: the perspective of a large tertiary hemophilia center. Semin Thromb Hemost. 2009;35(8):760‐768. [DOI] [PubMed] [Google Scholar]
- 69. Adcock DM, Bethel MA, Macy PA. Coagulation Handbook. Esoterix, Inc.; 2006. http://www.coloradocoagulation.com/sites/default/files/imce/uploads/L3692.pdf. Accessed August 2, 2019. [Google Scholar]
- 70. Miller CH, Payne AB, Driggers J, Ellingsen D, Boylan B, Bean CJ. Reagent substitutions in the Centers for Disease Control and Prevention Nijmegen‐Bethesda assay for factor VIII inhibitors. Haemophilia. 2018;24(3):e116‐e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miller CH, Rice AS, Boylan B, et al. Comparison of clot‐based, chromogenic and fluorescence assays for measurement of factor VIII inhibitors in the US Hemophilia Inhibitor Research Study. J Thromb Haemost. 2013;11(7):1300‐1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Adcock DM, Strandberg K, Shima M, Marlar RA. Advantages, disadvantages and optimization of one‐stage and chromogenic factor activity assays in haemophilia A and B. Int J Lab Hematol. 2018;40(6):621‐629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


