Abstract
Chronic traumatic encephalopathy is a debilitating neurodegenerative disorder associated with repetitive traumatic brain injuries often sustained through prior contact sport participation. The frequency of this disorder in a diverse population, including amateur athletes, is unknown. Primary historical obituary and yearbook records were queried for 2566 autopsy cases in the Mayo Clinic Tissue Registry resulting in identification of 300 former athletes and 450 non‐athletes. In these cases, neocortical tissue was screened for tau pathology with immunohistochemistry, including pathology consistent with chronic traumatic encephalopathy, blinded to exposure or demographic information. Using research infrastructure of the Rochester Epidemiology Project, a comprehensive and established medical records‐linkage system of care providers in southern Minnesota and western Wisconsin, medical diagnostic billing codes pertaining to head trauma, dementia, movement disorders, substance abuse disorders and psychiatric disorders were recorded for cases and controls in a blinded manner. A total of 42 individuals had pathology consistent with, or features of, chronic traumatic encephalopathy. It was more frequent in athletes compared to non‐athletes (27 cases versus 15 cases) and was largely observed in men (except for one woman). For contact sports, American football had the highest frequency of chronic traumatic encephalopathy pathology (15% of cases) and an odds ratio of 2.62 (P‐value = 0.005). Cases with chronic traumatic encephalopathy pathology had higher frequencies of antemortem clinical features of dementia, psychosis, movement disorders and alcohol abuse compared to cases without chronic traumatic encephalopathy pathology. Understanding the frequency of chronic traumatic encephalopathy pathology in a large autopsy cohort with diverse exposure backgrounds provides a baseline for future prospective studies assessing the epidemiology and public health impact of chronic traumatic encephalopathy and sports‐related repetitive head trauma.
Keywords: chronic traumatic encephalopathy, contact sports, football, tau, traumatic brain injuries
Introduction
Chronic traumatic encephalopathy (CTE) is a debilitating and enigmatic neurodegenerative disorder associated with repetitive traumatic brain injuries, often sustained through prior contact/collision sport participation or military‐related exposure 3, 14, 15, 23, 24. The clinical presentation of CTE (“traumatic encephalopathy syndrome”) includes a spectrum of neurological and psychiatric symptoms encompassing cognitive dysfunction, emotional dysregulation, behavioral change and motor disturbance 26. Neuropathological consensus criteria for the diagnosis of CTE define the underlying pathognomonic lesion as hyperphosphorylated tau aggregates in neurons, astrocytes and cell processes around small vessels in an irregular pattern at the depths of the cortical sulci 13. At present, there exists ongoing debate and research regarding the threshold, progression, specificity and clinical salience of this tau pathology 6.
Given that approximately 28 million children (≤15 years old), 8 million high school athletes and 500 000 collegiate athletes participate in organized sports programs annually in the United States alone 19, 20, 21, research regarding whether playing specific sports (especially at the amateur level) is associated with an increased risk of developing CTE pathology is imperative. Previous studies attempting to address this issue have been limited by small sample sizes, lack of a robust control group and/or have involved mostly collegiate or professional American football players 1, 17. In the current study, participation in specific sports was assessed retrospectively in a large autopsy cohort using historical data from obituaries and high school yearbooks. Our objectives were first to assess associations of participation in these sports with presence of CTE, and second to compare clinical features between individuals with and without CTE.
Materials and methods
Study cohort
The Mayo Clinic Tissue Registry, a histological registry of all Mayo Clinic formalin‐fixed and/or paraffin‐embedded tissue, blocks and slides (totaling over 20 million tissue samples) in Rochester, Minnesota collected 2844 consecutive autopsy cases between 01/01/2005 and 06/18/2016 following next‐of‐kin consent for complete or “brain‐only” postmortem examination, including research authorization (Figure 1). Individuals without available neocortical brain tissue (N = 194) and individuals <1 year of age at death (N = 84) were excluded, resulting in 2566 cases considered for study inclusion. These exclusion criteria were elected as cortical tissue was necessary for neuropathological query, and infants less than one year in age had a low probability of experiencing repetitive mild trauma that could be determined by this study's assessment methodology. Autopsy source was not exclusionary, such that medicolegal, research, physician and family requested autopsies were available. Racial background was similar for this study population (Table 1) compared to the City of Rochester and Olmsted County, Minnesota 31. This study was approved by Institutional Review Boards at Mayo Clinic and Olmsted Medical Center.
Figure 1.
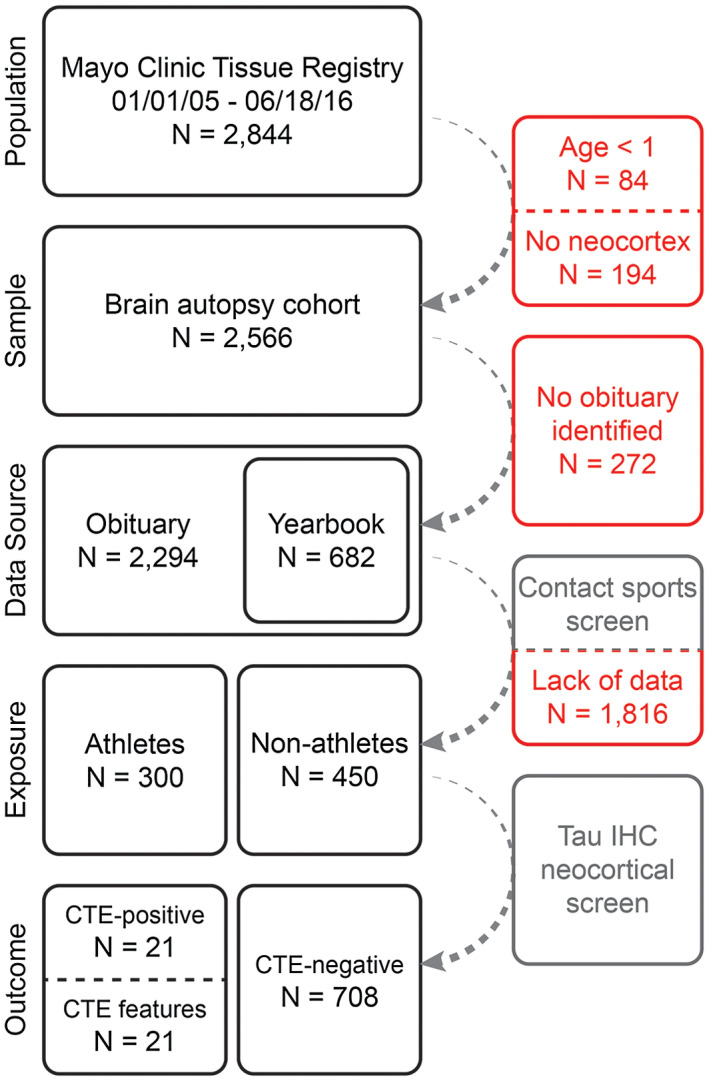
Study workflow. Obituaries were queried for all consented autopsy cases in the Mayo Clinic Tissue Registry from 2005 to 2016 (irrespective of any clinical or pathological diagnoses) with cortical brain tissue. Gray boxes denote a query while red boxes represent case exclusion. Obituary data were used to identify yearbook records. Together, contact sports participation was assessed from these historical primary sources, identifying 300 athletes (various sports) and 450 non‐athletes. Subsequent assessment via tau immunohistochemistry ascertained presence or absence of CTE pathology.
Table 1.
Characteristics according to sex and participation in any sports (ie, athlete or non‐athlete). Abbreviations: G.A.A. = Girls Athletic Association; PTSD = post‐traumatic stress disorder; AD = Alzheimer's disease; CTE = chronic traumatic encephalopathy.
| Variable | Athletes (N = 300) | Non‐athletes (N = 450) | Females (N = 273) | Males (N = 477) |
|---|---|---|---|---|
| Demographic information | ||||
| Age at death (years) | 68 (7, 99) | 64 (18, 100) | 67 (7, 100) | 66 (12, 99) |
| Sex (Male) | 232 (77.3%) | 245 (54.4%) | 0 (0.0%) | 477 (100.0%) |
| Race (white) | 292 (98.0%) | 439 (98.7%) | 268 (99.3%) | 463 (97.9%) |
| Years of education | 14 (2, 21) | 13 (8, 21) | 13 (2, 21) | 14 (6, 21) |
| Military | 98 (32.7%) | 115 (25.6%) | 7 (2.6%) | 206 (43.2%) |
| Athletic information | ||||
| Baseball/Softball | 73 (24.3%) | 0 (0.0%) | 9 (3.3%) | 64 (13.4%) |
| Youth or high school | 57 (19.3%) | 0 (0.0%) | 9 (3.3%) | 48 (10.1%) |
| Beyond high school | 12 (4.1%) | 0 (0.0%) | 0 (0.0%) | 12 (2.5%) |
| Basketball | 106 (35.3%) | 0 (0.0%) | 14 (5.1%) | 92 (19.3%) |
| Youth or high school | 98 (33.0%) | 0 (0.0%) | 14 (5.1%) | 85 (17.9%) |
| Beyond high school | 4 (1.3%) | 0 (0.0%) | 0 (0.0%) | 4 (0.8%) |
| Boxing | 7 (2.3%) | 0 (0.0%) | 0 (0.0%) | 7 (1.5%) |
| Football | 142 (47.3%) | 0 (0.0%) | 2 (0.7%) | 140 (29.4%) |
| Youth or high school | 126 (42.1%) | 0 (0.0%) | 2 (0.7%) | 124 (26.1%) |
| Beyond high school | 15 (5.0%) | 0 (0.0%) | 0 (0.0%) | 15 (3.2%) |
| G.A.A. | 41 (13.7%) | 0 (0.0%) | 41 (15.0%) | 0 (0.0%) |
| Hockey | 25 (8.3%) | 0 (0.0%) | 5 (1.8%) | 20 (4.2%) |
| Youth or high school | 17 (5.7%) | 0 (0.0%) | 5 (1.8%) | 12 (2.5%) |
| Beyond high school | 7 (2.3%) | 0 (0.0%) | 0 (0.0%) | 7 (1.5%) |
| Soccer | 8 (2.7%) | 0 (0.0%) | 3 (1.1%) | 5 (1.0%) |
| Wrestling | 39 (13.0%) | 0 (0.0%) | 0 (0.0%) | 39 (8.2%) |
| Youth or high school | 38 (12.7%) | 0 (0.0%) | 0 (0.0%) | 38 (8.0%) |
| Beyond high school | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) |
| Other † | 4 (1.3%) | 0 (0.0%) | 0 (0.0%) | 4 (0.8%) |
| Number of sports played | ||||
| 0 | 2 (0.7%) | 450 (100.0%) | 207 (75.8%) | 245 (51.4%) |
| 1 | 189 (63.0%) | 0 (0.0%) | 59 (21.6%) | 130 (27.3%) |
| 2 | 76 (25.3%) | 0 (0.0%) | 6 (2.2%) | 70 (14.7%) |
| 3 or 4 | 32 (11.0%) | 0 (0.0%) | 1 (0.4%) | 32 (6.7%) |
| Clinical information | ||||
| Alcoholism | 50 (16.7%) | 92 (20.4%) | 29 (10.6%) | 113 (23.7%) |
| Anxiety | 60 (20.0%) | 106 (23.6%) | 82 (30.0%) | 84 (17.6%) |
| Bipolar | 44 (14.7%) | 92 (20.4%) | 60 (22.0%) | 76 (15.9%) |
| Dementia | 96 (32.0%) | 113 (25.1%) | 79 (28.9%) | 130 (27.3%) |
| Depression | 95 (31.7%) | 180 (40.0%) | 116 (42.5%) | 159 (33.3%) |
| Drug abuse | 12 (4.0%) | 37 (8.2%) | 16 (5.9%) | 33 (6.9%) |
| Head injury | 37 (12.3%) | 54 (12.0%) | 31 (11.4%) | 60 (12.6%) |
| Movement disorder | 45 (15.0%) | 56 (12.4%) | 25 (9.2%) | 76 (15.9%) |
| Psychosis | 129 (43.0%) | 187 (41.6%) | 116 (42.5%) | 200 (41.9%) |
| PTSD | 8 (2.7%) | 11 (2.4%) | 11 (4.0%) | 8 (1.7%) |
| Schizophrenia | 6 (2.0%) | 3 (0.7%) | 3 (1.1%) | 6 (1.3%) |
| Suicide | 18 (6.0%) | 31 (6.9%) | 14 (5.1%) | 35 (7.3%) |
| Tobacco abuse | 94 (31.3%) | 151 (33.6%) | 64 (23.4%) | 181 (37.9%) |
| Neuropathological information | ||||
| Tau pathology | 261 (87.0%) | 344 (76.4%) | 219 (80.2%) | 386 (80.9%) |
| AD pathology | 72 (24.0%) | 105 (23.3%) | 74 (27.1%) | 103 (21.6%) |
| CTE | ||||
| CTE‐negative | 273 (91.0%) | 435 (96.7%) | 272 (99.6%) | 436 (91.4%) |
| Features of CTE | 12 (4.0%) | 9 (2.0%) | 1 (0.4%) | 20 (4.2%) |
| CTE‐positive | 15 (5.0%) | 6 (1.3%) | 0 (0.0%) | 21 (4.4%) |
The sample median (minimum, maximum) is shown for continuous variables. Information was unavailable regarding race (N = 7), level of baseball played (N = 4), level of basketball played (N = 3), level of football played (N = 1) and level of hockey played (N = 1).
Other sports included college lacrosse (N = 3 males) and amateur rodeo (N = 1 male).
Assessment of sports participation
Sports participation assessment for each subject was based on information from obituaries and high school yearbooks. Subjects were included in the study if they (1) had both obituary and yearbook information available revealing no participation in any sports, or (2) had either obituary or yearbook information available (or both) revealing participation in at least one sport. The final study sample size was 750 subjects after applying these criteria. Several differences were observed when comparing characteristics of the 750 subjects to the 1816 patients excluded because of the lack of sufficient sports participation information (Table S1).
Online obituaries (2294 [89%] cases identified) were searched using www.google.com for participant name, term “obituary,” and year of death. Personal identity was verified using the individual's date of birth and date of death. Sports participation and education data were abstracted from obituary records, including type of sport(s) and participation age/level/duration and institutional name, city, state and year of graduation. Contact sports were defined as any organized athletic activity with the potential for frequent head impact between participants or a participant and sports‐related equipment, and included baseball/softball, basketball, boxing, football, hockey, lacrosse/field hockey, soccer and wrestling. For female athlete inclusivity, participation in high school girls athletic associations was also recorded. Non‐contact sports including cross‐country, track and field, swimming, tennis and golf were not classified as sports participation for the purposes of this study.
Yearbook records (682 [27%] cases identified) were searched using the high school name, city, state and year of graduation primarily through www.classmates.com, with a smaller subset identified through Southeastern Minnesota local libraries. Individuals were verified using first name, and last name or maiden name. Sports participation, as previously defined, and extracurricular activity data were abstracted for all participants in accordance with published Rochester Epidemiology Project yearbook studies 7, 29.
Medical record query
The Rochester Epidemiology Project is a comprehensive medical records‐linkage system of care providers in southern Minnesota and western Wisconsin 28. As previously described 30, diagnostic codes were queried from the Rochester Epidemiology Project database for the following disorders: head trauma, dementia, movement, alcoholism, drug abuse, tobacco abuse, anxiety, depression, bipolar, post‐traumatic stress, psychosis, schizophrenia and suicide. Classification for the disorders was qualified according to individual lifetime presence or absence of any corresponding diagnostic code from 01 January 1976 to 18 June 2016. Military service was qualified as present or absent based upon death certificate records linked to each case in the Rochester Epidemiology Project database.
Neuropathological evaluation
According to preliminary recommendations for restricted cortical sampling in CTE, 78% of neuropathologically diagnosed CTE cases with varying degrees of severity can be identified via examination of the frontal, parietal and temporal cortices 2. To screen for CTE pathology in this series, we aimed to evaluate previously sampled formalin‐fixed, paraffin‐embedded tissue from these three regions. Blocks were selected from frontal and temporal neocortices for all 750 participants. Of additionally available archived neocortical tissue blocks, tissue was included for 565/750 individuals for neuropathological evaluation including parietal (N = 318), additional frontal (N = 112) and additional temporal (N = 135) lobes, such that a maximum of three different blocks per cases were assessed. Following deparaffinization and antigen retrieval, tau immunohistochemistry was performed using monoclonal AT8 phospho‐tau antibody (1:2500 dilution) and EnVision+ Mouse reagent kits on the same LabVision™ 480S autostainer. Subsequently, slides were hematoxylin counterstained, dehydrated and coverslipped.
Tau immunostained slides from all 750 cases were assessed independently by two evaluators (KFB and MMB), blinded to any personal identifier, sports participation information or clinical information. Cases were evaluated for Alzheimer‐type pathology (tau‐positive neuritic plaques) and aging‐related tau astrogliopathy in accordance with defined consensus neuropathological criteria 5, 9. Cases with pathology consistent with all aspects of the CTE neuropathology consensus criteria, namely (A) “hyperphosphorylated tau aggregates,” (B) “in neurons, astrocytes, and cell processes,” (C) “around small vessels” and (D) “in an irregular pattern at the depths of the cortical sulci,” were classified as “CTE‐positive” 13. “Features of CTE” was used to designate cases with multiple lesions suggestive of CTE pathology without fitting all parts of the criteria verbatim, exclusive of Alzheimer‐type pathology and aging‐related tau astrogliopathy. Cases void of any CTE pathology were classified as “CTE‐negative.” Features of CTE and CTE‐positive cases were jointly classified and analyzed as “combined CTE pathology,” which was the primary outcome measure of the study. As a result of its rarer occurrence, CTE‐positive was evaluated as a secondary outcome. All cases with tau pathology identified by either initial evaluator were also assessed by a third blinded evaluator (DWD), and a final consensus classification was determined.
Statistical analysis
Associations of participation in specific sports with occurrence of the separate outcomes of combined CTE pathology and CTE‐positive were examined using unadjusted and multivariable logistic regression models, where odds ratios (ORs) and 95% confidence intervals (CIs) were estimated. As only one female subject had features of CTE, associations were not possible to assess in females, and were therefore examined only in males. Considering commonly used guidelines regarding the number of variables that can be included in a given model 25, 32, multivariable models for the primary outcome of combined CTE pathology were adjusted for the subset of pre‐specified potential CTE risk factors (age at death and documented head injury subsequent to any sports participation) that differed between male subjects with and without combined CTE pathology with a P‐value < 0.10, and also for all individual contact sports that were associated with combined CTE pathology in unadjusted analysis with a P‐value < 0.10. When examining associations of participation in specific contact sports with CTE‐positive, multivariable models were adjusted for all contact sports that were associated with CTE‐positive in unadjusted analysis with a P‐value < 0.10.
Comparisons of demographic, clinical and neuropathological information between patients with and without CTE outcomes were made using a Wilcoxon rank sum test or Fisher's exact test. A Bonferroni correction for multiple testing was made for the primary analysis evaluating associations of participation in specific sports with CTE outcomes. Specifically, to account for the eight different sports examined (including “other” category), P‐values < 0.00625 were considered as statistically significant. P‐values < 0.05 were considered as statistically significant for all other analyses. All statistical tests were two‐sided. Statistical analyses were performed using SAS (version 9.4) and R Statistical Software (version 3.2.3). The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available as information could compromise the privacy of research participants.
Results
Patient characteristics
The final sample size was 750 subjects after applying study criteria. A summary of demographic, sports participation, clinical and neuropathological information is provided in Table 1 for the 477 males and 273 females as well as for subjects who participated in at least one sport (“athletes”) and for non‐athletes. Sixty‐six (24%) females played at least one sport with girls athletic associations (15%), basketball (5%) and baseball/softball (3%) being most common. For males, 232 (49%) played at least one contact sport; football was most common (29%), followed by basketball (19%), baseball (13%) and wrestling (8%). Characteristics are shown according to specific sports played in Table S2 (males) and Table S3 (females).
Frequency of CTE and tau pathology
Twenty‐one males (2.8%) were identified with tau pathology consistent with CTE consensus neuropathological criteria (Table 1). The burden of neocortical tau pathology varied from marked to mild (Figure 2). Additionally, 21 cases (2.8%, 20 males, 1 female) were identified and designated as “features of CTE.” Together, combined CTE pathology was observed in 5.6% of the cohort. The median age of death for these 42 cases was 73 years (range 33–91). Tau‐immunoreactive neuritic plaques, pathognomonic of Alzheimer's disease, were observed in 177 (23.6%) subjects; 15 subjects had both combined CTE pathology and Alzheimer's disease neuritic plaques. In total, 605 cases had at least one tau‐immunoreactive lesion (neurite, neurofibrillary tangle, neuritic plaque, or glial inclusion), while 145 (19.4%) study cases were entirely free of tau pathology in the cortical sections evaluated.
Figure 2.
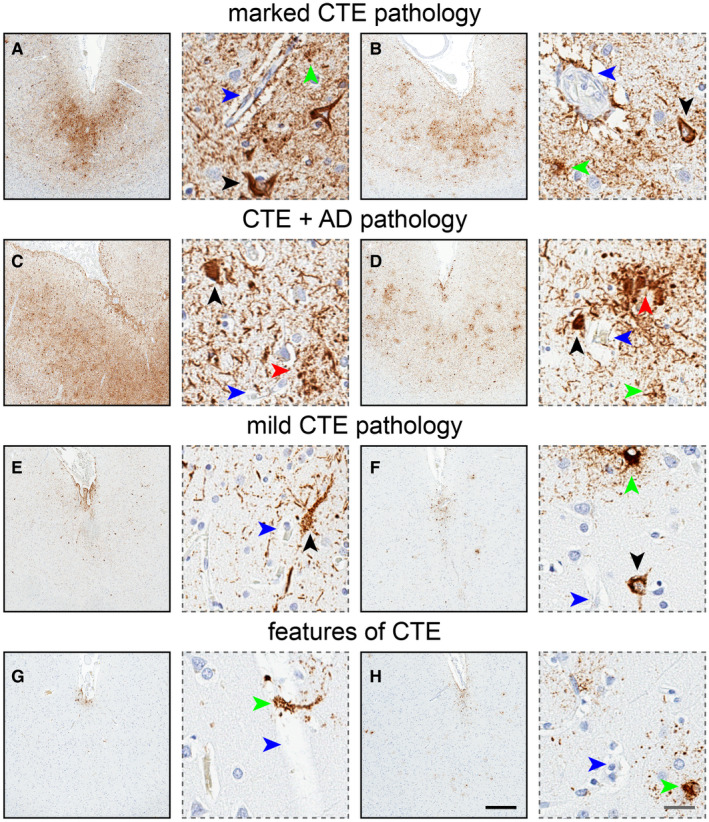
Chronic traumatic encephalopathy (CTE) pathology. CTE is characterized by focal deposition of hyperphosphorylated tau protein (brown signal) in neurons (black arrows) and astrocytes (green arrows) around penetrating blood vessels (blue arrows) at the depths of cortical sulci, as shown here in two cases (A,B). CTE pathology can be observed concomitantly with Alzheimer's disease pathology, namely tau‐immunoreactive neuritic plaques (red arrows), making CTE evaluation more complicated in cases with high Alzheimer's disease burden (C) than cases with lower Alzheimer's disease burden (D). Other CTE cases may demonstrate more subtle neuropathology yet harbor all the required components of the consensus criteria (two cases; E,F). Finally, some cases show focal and patchy tau pathology reminiscent of CTE and inconsistent of other tauopathies, yet do not meet all consensus criteria (two cases; G,H). Possibly attributable to very early pathogenesis or limited restrictive sampling, “features of CTE” delineates high probability for pathological CTE yet not in accordance with all CTE consensus standards. Black bar: 500 μm, gray bar: 25 μm.
Clinical features of CTE in males
To avoid making comparisons between subjects with and without CTE pathology that are completely confounded by sex, we made such comparisons in males only (Table 2). In comparison to CTE‐negative males, males with combined CTE pathology tended to be older at death (P = 0.006), had a lower likelihood of anxiety (P = 0.029), and had a higher likelihood of dementia (P = 0.017), movement disorders (P = 0.024), psychosis (P = 0.005) and AD neuropathology (P = 0.027). These results were relatively consistent when comparing CTE‐negative subjects to CTE‐positive subjects (Table 2). Given the differing minimum age at death between CTE‐negative males (12 years) and males with combined CTE pathology (33 years), in secondary analysis, we excluded the 26 (5.5%) CTE‐negative males with an age at death <30 years in order to address the possibility that this difference in minimum age at death influenced our results. The aforementioned statistically significant differences in characteristics that were observed between CTE‐negative males and those with combined CTE pathology remained significant in this subset of 451 males with an age at death of at least 30 years (all P ≤ 0.033).
Table 2.
Comparison of demographic, clinical and neuropathological characteristics according between subjects with and without CTE outcomes in males. Abbreviations: CTE = chronic traumatic encephalopathy; AD = Alzheimer's disease.
| Variable | CTE‐negative (N = 436) | Combined CTE pathology (N = 41) | P‐value: CTE‐negative vs. combined CTE pathology | CTE‐positive (N = 21) | P‐value: CTE‐negative vs. CTE‐positive |
|---|---|---|---|---|---|
| Demographic information | |||||
| Age at death (years) | 65 (12, 99) | 73 (33, 91) | 0.006 | 75 (39, 89) | 0.038 |
| Race (white) | 424 (98.1%) | 39 (95.1%) | 0.21 | 19 (90.5%) | 0.068 |
| Years of education | 14 (6, 21) | 16 (12, 21) | 0.36 | 16 (12, 21) | 0.41 |
| Military | 186 (42.7%) | 20 (48.8%) | 0.51 | 11 (52.4%) | 0.50 |
| Clinical information | |||||
| Alcoholism | 103 (23.6%) | 10 (24.4%) | 0.85 | 4 (19.0%) | 0.79 |
| Anxiety | 82 (18.8%) | 2 (4.9%) | 0.029 | 1 (4.8%) | 0.15 |
| Bipolar | 69 (15.8%) | 7 (17.1%) | 0.82 | 4 (19.0%) | 0.76 |
| Dementia | 112 (25.7%) | 18 (43.9%) | 0.017 | 12 (57.1%) | 0.004 |
| Depression | 145 (33.3%) | 14 (34.1%) | 1.00 | 9 (42.9%) | 0.35 |
| Drug abuse | 31 (7.1%) | 2 (4.9%) | 1.00 | 1 (4.8%) | 1.00 |
| Head injury | 57 (13.1%) | 3 (7.3%) | 0.46 | 1 (4.8%) | 0.50 |
| Movement disorder | 64 (14.7%) | 12 (29.3%) | 0.024 | 8 (38.1%) | 0.010 |
| Psychosis | 174 (39.9%) | 26 (63.4%) | 0.005 | 14 (66.7%) | 0.023 |
| PTSD | 7 (1.6%) | 1 (2.4%) | 0.52 | 1 (4.8%) | 0.30 |
| Schizophrenia | 5 (1.1%) | 1 (2.4%) | 0.42 | 1 (4.8%) | 0.24 |
| Suicide | 34 (7.8%) | 1 (2.4%) | 0.35 | 0 (0.0%) | 0.39 |
| Tobacco abuse | 162 (37.2%) | 19 (46.3%) | 0.31 | 8 (38.1%) | 1.00 |
| Neuropathological information | |||||
| AD | 88 (20.2%) | 15 (36.6%) | 0.027 | 10 (47.6%) | 0.006 |
The sample median (minimum, maximum) is shown for continuous variables. P‐values result from a Wilcoxon rank sum test or Fisher's exact test. Information was unavailable regarding race (N = 4 subjects without CTE pathology).
Participation in contact sports and CTE outcomes in males
In multivariable analysis (Table 3, Figure S1), football was the only sport significantly associated with increased odds of combined CTE pathology in males (OR = 2.62, P = 0.005). Although not significant after correcting for multiple testing, CTE‐positive was more common in multivariable analysis for subjects who boxed and those who played football (Table 3). A reasonable number of individuals played baseball or football beyond high school, and therefore for these two sports we performed subgroup analysis (Table 3). Playing football beyond high school was associated with a substantially increased odds of combined CTE pathology in multivariable analysis (OR = 13.23, P < 0.001). Findings were similar though weaker when considering CTE‐positive (OR = 6.84, P = 0.0056). Conversely, in comparison to non‐football players, odds of combined CTE pathology (OR = 1.84, P = 0.11) and CTE‐positive (OR = 2.29, P = 0.094) were not significantly increased in multivariable analysis for participants of only youth or high school football (Table 3). Results of all association analyses were relatively similar when adjusting for additional variables in multivariable analysis (Tables S4‐S7).
Table 3.
Associations of exposure to individual sports with occurrence of combined CTE pathology and CTE‐positive in males. Abbreviations: CTE = chronic traumatic encephalopathy; OR = odds ratio; CI = confidence interval.
| Sport | N | Association with combined CTE pathology | Association with CTE‐positive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) with combined CTE | Unadjusted analysis | Adjusting for age at death, baseball, boxing and football† | N (%) with CTE‐positive | Unadjusted analysis | Adjusting for boxing and football‡ | ||||||
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | ||||
| Primary analysis of individual sports | |||||||||||
| Baseball | |||||||||||
| No | 413 | 32 (7.8%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 16 (3.9%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 64 | 9 (14.1%) | 1.95 (0.88, 4.30) | 0.099 | 1.42 (0.62, 3.27) | 0.41 | 5 (7.8%) | 2.10 (0.74, 5.95) | 0.16 | 1.57 (0.52, 4.72) | 0.43 |
| Basketball | |||||||||||
| No | 385 | 30 (7.8%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 16 (4.2%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 92 | 11 (12.0%) | 1.61 (0.77, 3.34) | 0.20 | 0.95 (0.41, 2.23) | 0.91 | 5 (5.4%) | 1.33 (0.47, 3.72) | 0.59 | 0.97 (0.32, 2.95) | 0.95 |
| Boxing | |||||||||||
| No | 470 | 39 (8.3%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 19 (4.0%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 7 | 2 (28.6%) | 4.42 (0.83, 23.54) | 0.081 | 3.62 (0.63, 20.79) | 0.15 | 2 (28.6%) | 9.50 (1.73, 52.12) | 0.010 | 10.29 (1.79, 59.28) | 0.009 |
| Football | |||||||||||
| No | 337 | 20 (5.9%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 10 (3.0%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 140 | 21 (15.0%) | 2.80 (1.46, 5.35) | 0.002 | 2.62 (1.33, 5.15) | 0.005 | 11 (7.9%) | 2.79 (1.16, 6.73) | 0.022 | 2.87 (1.17, 7.01) | 0.021 |
| Hockey | |||||||||||
| No | 457 | 39 (8.5%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 20 (4.4%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 20 | 2 (10.0%) | 1.19 (0.27, 5.32) | 0.82 | 1.27 (0.27, 5.97) | 0.77 | 1 (5.0%) | 1.15 (0.15, 9.03) | 0.89 | 1.44 (0.18, 11.53) | 0.73 |
| Soccer§ | |||||||||||
| No | 472 | 41 (8.7%) | 1.00 (reference) | N/A | N/A | N/A | 21 (4.5%) | 1.00 (reference) | N/A | N/A | N/A |
| Yes | 5 | 0 (0.0%) | N/A | 1.00 | N/A | N/A | 0 (0.0%) | N/A | 1.00 | N/A | N/A |
| Wrestling | |||||||||||
| No | 438 | 38 (8.7%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 20 (4.6%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 39 | 3 (7.7%) | 0.88 (0.26, 2.98) | 0.83 | 0.86 (0.25, 3.05) | 0.82 | 1 (2.6%) | 0.55 (0.07, 4.21) | 0.56 | 0.43 (0.06, 3.38) | 0.42 |
| Other† | |||||||||||
| No | 473 | 41 (8.7%) | 1.00 (reference) | N/A | N/A | N/A | 21 (4.4%) | 1.00 (reference) | N/A | N/A | N/A |
| Yes | 4 | 0 (0.0%) | N/A | 1.00 | N/A | N/A | 0 (0.0%) | N/A | 1.00 | N/A | N/A |
| Subgroup analysis ¶ | |||||||||||
| Baseball—youth or high school only | |||||||||||
| No | 413 | 32 (7.8%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 16 (3.9%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 48 | 6 (12.5%) | 1.70 (0.67, 4.30) | 0.26 | 1.11 (0.41, 3.00) | 0.83 | 5 (10.4%) | 2.89 (1.01, 8.26) | 0.048 | 2.03 (0.66, 6.32) | 0.22 |
| Baseball—beyond high school ¶ | |||||||||||
| No | 461 | 38 (8.2%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 21 (4.6%) | 1.00 (reference) | N/A | N/A | N/A |
| Yes | 12 | 3 (25.0%) | 3.71 (0.96, 14.29) | 0.057 | 3.89 (0.98, 15.44) | 0.053 | 0 (0.0%) | N/A | 1.00 | N/A | 1.00 |
| Football—youth/high school only | |||||||||||
| No | 337 | 20 (5.9%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 10 (3.0%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 124 | 14 (11.3%) | 2.02 (0.99, 4.13) | 0.055 | 1.84 (0.87, 3.90) | 0.11 | 8 (6.5%) | 2.26 (0.87, 5.85) | 0.095 | 2.29 (0.87, 6.03) | 0.094 |
| Football—beyond high school | |||||||||||
| No | 461 | 34 (7.4%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A | 18 (3.9%) | 1.00 (reference) | N/A | 1.00 (reference) | N/A |
| Yes | 15 | 7 (46.7%) | 10.99 (3.76, 32.13) | <0.001 | 13.23 (4.23, 41.36) | <0.001 | 3 (20.0%) | 6.15 (1.60, 23.74) | 0.008 | 6.84 (1.76, 26.66) | 0.0056 |
ORs, 95% CIs and P‐values result from logistic regression models. P‐values < 0.00625 were considered as statistically significant after applying a Bonferroni adjustment for multiple testing. Information was unavailable regarding level of baseball played (N = 4) and level of football played (N = 1).
Models involving baseball—youth or high school and baseball—beyond high school were not adjusted for baseball, and models adjusting for football—youth or high school and football—beyond high school were not adjusted for football.
Models involving football—youth or high school and football—beyond high school were not adjusted for football.
Because of the lack of subjects who played soccer or other sports and who had combined CTE pathology or CTE‐positive, logistic regression analysis was not possible; P‐values result from Fisher's exact test and multivariable analysis was not performed.
Only baseball and football were examined in subgroup analysis as these were the only sports for which a reasonable number of subjects played beyond high school. When comparing individuals who did and did not play a given youth or high school sport, individuals who played that sport beyond high school were excluded to avoid combining individuals who did not play a given sport and individuals who played that sport beyond high school in to the same category.
Because of the lack of subjects who played baseball beyond high school and who had CTE‐positive, logistic regression analysis was not possible; P‐values result from Fisher's exact test and multivariable analysis was not performed.
We assessed whether playing more than one sport may be associated with CTE outcomes in males (Table S8). In multivariable analysis adjusting for age at death and football, playing two or more sports was not strongly associated with combined CTE pathology (OR = 1.30, P = 0.55) or CTE‐positive (OR = 0.96, P = 0.94). Conversely, the association of playing football with combined CTE pathology was consistent when adjusting for playing multiple sports in addition to age at death (OR = 2.37, P = 0.039), with similar results for CTE‐positive (OR = 2.77, P = 0.063). The higher increased risk of CTE outcomes in football players regardless of other sports played is illustrated in Figure 3 (combined CTE pathology) and Figure S2 (CTE‐positive). All of these findings were consistent when examining the subset of 451 males with an age at death of at least 30 years (data not shown).
Figure 3.
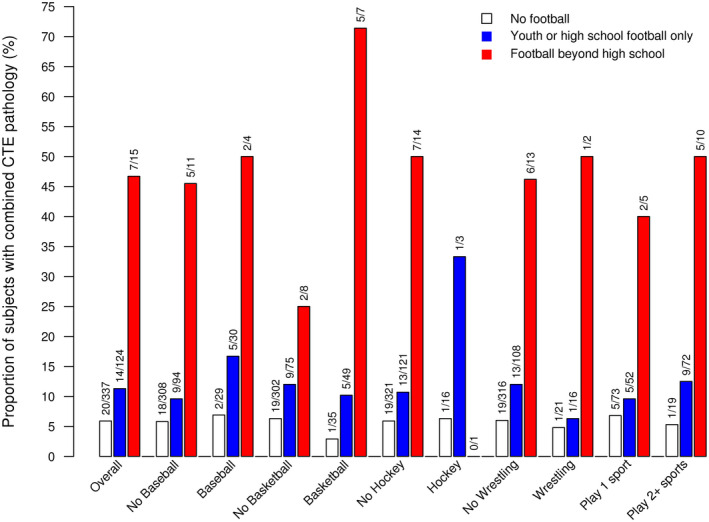
Proportion of males with combined CTE pathology in individuals who did not play football (white bars), who played youth or high school football only (blue bars), or who played football beyond high school (red bars) in the overall group and also in subgroups based on other sports played.
Discussion
Understanding the incidence/prevalence of CTE through standard epidemiologic methodologies is challenging. With a heterogeneous admixture of behavioral, mood, cognitive and motor features 18, antemortem symptomatology in autopsy‐confirmed CTE cases demonstrates significant interpersonal variability and clinical overlap with other neurodegenerative and psychiatric disorders, including Alzheimer's disease, frontotemporal dementias, Lewy body disorder, bipolar disorder and schizophrenia. Molecular neuroimaging and biofluid disease markers have not yet been sufficiently studied and validated on large study cohorts for routine clinical use. Autopsy cohorts are necessary for understanding the public health relevance of CTE as postmortem neuropathological assessment remains the diagnostic gold standard.
Because of differing sampling approaches and inclusion criteria, reported frequencies of CTE in autopsy cohorts has varied considerably, from 80%–99% in cohorts of predominantly experienced football players 15, 17, to 12%–32% in neurodegenerative disorder brain banks 1, 10 and to 6% in specific autopsy‐confirmed disorders screened for comorbidity of CTE pathology (multiple system atrophy and amyotrophic lateral sclerosis) 8, 33. Herein, review of obituaries and yearbooks was used to ascertain contact sports exposure in an autopsy cohort of 750 individuals, and standardized tau immunohistochemistry was used to screen and evaluate pathology blinded to clinical information. Combined CTE pathology was found in 42 brains, or 5.6% of the study cohort and almost exclusively in men (8.8%). Collectively, 2.8% of subjects were CTE‐positive, all of whom were male.
Importantly, we observed that participation in American football, specifically American football beyond high school, was associated with a markedly increased odds of both combined CTE pathology and CTE‐positive outcomes in males; of the 15 males who played football beyond high school, seven (46.7%) had combined CTE pathology and 3 (20.0%) CTE‐positive. Of particular interest in our study, though participation in football that did not extend beyond high school was associated with an approximate twofold increased risk of CTE outcomes, these findings were not statistically significant. Of note, of the 124 individuals who played youth or high school football and did not play beyond that point, 110 (88.7%) did not have CTE pathology. Therefore, although our findings do suggest that playing youth or high school football may be associated with a slightly elevated risk of CTE (larger studies are needed to confirm this), they also indicate that most players of youth or high school football do not experience CTE outcomes. Additionally, though there was some evidence of an association between participating in boxing and an elevated risk of CTE outcomes based on our findings, participation in baseball, basketball, hockey, soccer or wrestling were not strongly associated with occurrence of combined CTE pathology or CTE‐positive.
Of the 42 combined CTE pathology brains, 21 were classified as CTE‐positive, consistent with established consensus criteria, while 21 displayed features of CTE. The features of CTE designation recognizes neuropathological diagnostic probability of the disorder, while accounting for study limitations and limitations regarding the range of CTE pathology. Despite not meeting the consensus definition of CTE pathology, the 21 features of CTE cases were more consistent with CTE than Alzheimer's disease, pathological aging or aging‐related tau astrogliopathy alone. These findings are similar to observations by Drs. Noy, Krawitz and Del Bigio who identified 34/111 autopsy cases with lesions described as “CTE‐like” and “CTE Stage <1,” of which 53% had a history of head trauma 22. Herein, features of CTE cases may be a product of limited sectioning (one five‐micron thick section) of the tissue block and analysis of tissue from only two to three neocortical regions. More exhaustive sampling and sectioning might assist in adjudicating whether cases with features of CTE are in fact CTE‐positive. Alternatively, features of CTE may represent the earliest emergence of CTE pathology, perhaps at a latent or prodromal disease state, which marks the beginning of this progressive tauopathy below the threshold of current criteria (criteria developed from evaluation of common features in cases with advanced disease) 13. Ambiguity related to the nomenclature, assessment and relevance of very early or mild perivascular tau pathology would benefit from further validation and refinement of CTE pathological criteria.
Medical diagnoses were assessed using the Rochester Epidemiology Project research infrastructure to evaluate antemortem clinical manifestations of CTE pathology. Individuals with combined CTE pathology demonstrated rates of dementia, movement disorders and psychosis that were higher than CTE‐negative cases. Importantly, individuals with combined CTE pathology did not have higher rates of head trauma diagnoses compared to CTE‐negative subjects, suggesting that the etiology of CTE pathology is intrinsic to the repetitive traumatic brain injury sustained through contact sports participation and not single‐incident head trauma sustained later in life. Moreover, the frequency of certain disorders previously noted to associate with CTE, including illicit drug abuse, anxiety, depression, post‐traumatic stress disorder and suicide 11, 12, 24, were not observed significantly more often in subjects with combined CTE pathology compared to CTE‐negative subjects.
While this study is the largest neuropathological assessment of CTE in an autopsy series to date, there are several limitations. First, assessment of sports participation in females was more restricted compared to males. Many women in the study cohort attended higher education institutions prior to enactment of the United States Patsy T. Mink Equal Opportunity in Education Act (commonly referred to as “Title IX”) and were not provided opportunities to participate in the same types of athletic organizations as their male counterparts. No women athletes were classified as CTE‐positive and a single non‐athlete woman was assessed as features of CTE. As a result, we were unable to assess associations between participation in sports and CTE outcomes in females. The only two published cases of CTE in women to date was of a victim of domestic abuse 27 and an autistic individual with self‐injury behavior 4; while there is no reason to speculate our case experienced domestic violence or self‐injury, these variables are unknown. It seems reasonable to postulate that if more women had played contact sports in our cohort, the frequency of CTE would have correspondingly increased. Second, ascertainment of participation in contact sports was based on available information that was collected retrospectively from online obituaries and high school yearbooks, as opposed to prospective data collection or interviews. Next‐of‐kin interviews were not possible as contact information was inaccessible for this study. While interviews would be beneficial for validating exposures, these techniques are not without their own limitations including susceptibility to recall bias 16. Despite having previously demonstrated the utility of these exposure ascertainment techniques, in obituaries and yearbooks separately 1, 7, 29, we cannot rule out the possibility of sports participation in non‐athletes that was omitted in obituary and yearbook sources or the possibility of repetitive head trauma sustained from other non‐sport‐related or non‐military‐related sources. This limitation is especially relevant as 15 individuals in this study (fourteen males, one female) classified as “non‐athletes” were observed to have combined CTE pathology (nine features of CTE, six CTE‐positive). Third, yearbook and obituary sports data was limited to sport type and sport participation level; it was not possible to uniformly assess more detailed information such as position and duration of play. Finally, while the Mayo Clinic Tissue Registry serves as a comprehensive tissue archive for numerous medical research studies (not strictly neurodegenerative disorders), brain autopsies by nature are elected by the family or physician and as such represent convenience sampling (notably, autopsy randomization sampling is infeasible in practice).
In conclusion, we identified a small, yet significant, frequency of CTE pathology in this study cohort from the Mayo Clinic Tissue Registry. In assessing a variety of contact sports, we identified specific athletic activities, such as American football, that were more strongly associated with CTE pathology compared to other sports, and within football, a dose‐response relationship between exposure and CTE pathogenesis. These initial results have important implications regarding the public health relevance of CTE. As our data suggests associations between football and CTE are strongest for participation beyond high school, it may be prudent to prioritize regulatory reform mitigating head trauma at the collegiate and professional levels. Future prospective, longitudinal studies focused on specific sports positions, participation duration and sustained head impacts during participation (number, force, latency, etc.) in large population‐based cohorts will help further benefit our scientific understanding of sports‐related head trauma and the etiology of CTE.
Supporting information
Table S1. Comparison of demographic and clinical characteristics between the 750 included subjects and the 1816 subjects who were excluded because of insufficient athletic information.
Table S2. Characteristics according to sports played in females.
Table S3. Characteristics according to sports played in males.
Table S4. Association between exposure to individual sports and occurrence of combined CTE pathology in males with additional multivariable adjustments.
Table S5. Association between exposure to individual sports and occurrence of CTE‐positive in males with additional multivariable adjustments.
Table S6. Subgroup analysis of associations of exposure to sports and occurrence of combined CTE pathology in males with additional multivariable adjustments.
Table S7. Subgroup analysis of association between exposure to individual sports and occurrence of CTE‐positive in males with additional multivariable adjustments.
Table S8. Associations of playing multiple sports with occurrence of combined CTE pathology and CTE‐positive in males.
Figure S1. Proportion of males with CTE‐positive in individuals who did not play football, who played youth or high school football only, or who played football beyond high school in the overall group and also in subgroups based on other sports played.
Figure S2. Odds ratios and 95% confidence intervals from multivariable analysis displaying the associations of exposure to specific sports with the separate outcomes of combined CTE pathology and CTE‐positive in males. Baseball—beyond high school is not displayed for the CTE‐positive outcome as none of the 12 subjects who played baseball beyond high school had CTE‐positive, making estimation of an odds ratio impossible.
Acknowledgments
The authors would foremost like to thank the patients and their families for their selfless act of brain donation, without which this study would not be possible. The authors would like to thank Drs. Walter Rocca and Caterina Giannini for study access to the Rochester Epidemiology Project database and the Mayo Clinic Tissue Registry archives. This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health (R01‐AG034676). The content is solely the responsibility of the authrs and does not necessarily represent the official views of the National Institutes of Health. We are also grateful for the training and data retrieval support of Barb Abbott, Kaitlin Schwartz and Gary Larson as well as histologic support of Linda Rousseau and Ariston Librero. This study was funded through grant support by the Florida Department of Health Ed and Ethel Moore Alzheimer's Disease Research Program (#7AZ08; KFB), Mayo Clinic Younkin Scholars Program on Synaptic Biology and Memory (KFB), Mayo Clinic Alzheimer's Disease Research Center Pilot Project Grant (KFB) and the National Institutes on Aging (R01‐AG062348; KFB, DWD). All study authors declare no conflicts of interest.
References
- 1. Bieniek KF, Ross OA, Cormier KA, Walton RL, Soto‐Ortolaza A, Johnston AE et al (2015) Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 130:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darby A, Adams J, Babcock K, Alvarez V, Stein T, McKee A (2015) Detection of CTE in autopsy cohorts using restricted cortical sampling. J Neuropathol Exp Neurol 74:615. [Google Scholar]
- 3. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA et al (2012) Chronic traumatic encephalopathy in blast‐exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 4:134ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hof PR, Knabe R, Bovier P, Bouras C (1991) Neuropathological observations in a case of autism presenting with self‐injury behavior. Acta Neuropathol 82:321–326. [DOI] [PubMed] [Google Scholar]
- 5. Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National Institute on Aging‐Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iverson GL, Keene CD, Perry G, Castellani RJ (2018) The need to separate chronic traumatic encephalopathy neuropathology from clinical features. J Alzheimers Dis 61:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Janssen PHH, Mandrekar J, Mielke MM, Ahlskog JE, Boeve BF, Josephs K et al (2017) High‐school football and late‐life risk of neurodegenerative syndromes, 1956–1970. Mayo Clin Proc 92:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koga S, Dickson DW, Bieniek KF (2016) Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol 75:963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H et al (2016) Aging‐related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131:87–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T (2015) Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 130:891–893. [DOI] [PubMed] [Google Scholar]
- 11. Mahar I, Alosco ML, McKee AC (2017) Psychiatric phenotypes in chronic traumatic encephalopathy. Neurosci Biobehav Rev 83:622–630. [DOI] [PubMed] [Google Scholar]
- 12. Maroon JC, Winkelman R, Bost J, Amos A, Mathyssek C, Miele V (2015) Chronic traumatic encephalopathy in contact sports: a systematic review of all reported pathological cases. PLoS ONE 10:e0117338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I et al (2016) The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 131:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKee AC, Cantu RC, Nowinski CJ, Hedley‐Whyte ET, Gavett BE, Budson AE et al (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 68:709–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH et al (2013) The spectrum of disease in chronic traumatic encephalopathy. Brain 136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meehan W 3rd, Mannix R, Zafonte R, Pascual‐Leone A (2015) Chronic traumatic encephalopathy and athletes. Neurology 85:1504–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR et al (2017) Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R et al (2014) Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. National Collegiate Athletic Association (2018) NCAA Sports Sponsorship and Participation Rates Database. Tableau Software. Available at: www.ncaa.org/about/resources/research/ncaa-sports-sponsorship-and-participation-rates-database (accessed 16 October 2018). [Google Scholar]
- 20. National Council of Youth Sports (2008) NCYS Report on Trends and Participation in Organized Youth Sports. Available at: www.ncys.org/publications/2008-sports-participation-study.php (accessed 16 October 2018). [Google Scholar]
- 21. National Federation of State High School Associations (2018) 2017–2018 High School Athletics Participation Survey. Available at: www.nfhs.org/participationstatistics/participationstatistics/ (accessed 16 October 2018). [Google Scholar]
- 22. Noy S, Krawitz S, Del Bigio MR (2016) Chronic traumatic encephalopathy‐like abnormalities in a routine neuropathology service. J Neuropathol Exp Neurol 75:1145–54. [DOI] [PubMed] [Google Scholar]
- 23. Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH (2005) Chronic traumatic encephalopathy in a national football league player. Neurosurgery 57:128–134; discussion 34. [DOI] [PubMed] [Google Scholar]
- 24. Omalu B, Hammers JL, Bailes J, Hamilton RL, Kamboh MI, Webster G et al (2011) Chronic traumatic encephalopathy in an Iraqi war veteran with posttraumatic stress disorder who committed suicide. Neurosurg Focus 31:E3. [DOI] [PubMed] [Google Scholar]
- 25. Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR (1996) A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol 49:1373–1379. [DOI] [PubMed] [Google Scholar]
- 26. Reams N, Eckner JT, Almeida AA, Aegesen AL, Giordani B, Paulson H et al (2016) A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol 73:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts GW, Whitwell HL, Acland PR, Bruton CJ (1990) Dementia in a punch‐drunk wife. Lancet 335:918–919. [DOI] [PubMed] [Google Scholar]
- 28. Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd (2012) History of the rochester epidemiology project: half a century of medical records linkage in a US population. Mayo Clin Proc 87:1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Savica R, Parisi JE, Wold LE, Josephs KA, Ahlskog JE (2012) High school football and risk of neurodegeneration: a community‐based study. Mayo Clin Proc 87:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA (2012) Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 87:151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Pankratz JJ, Brue SM et al (2012) Data resource profile: the Rochester Epidemiology Project (REP) medical records‐linkage system. Int J Epidemiol 41:1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165:710–718. [DOI] [PubMed] [Google Scholar]
- 33. Walt GS, Burris HM, Brady CB, Spencer KR, Alvarez VE, Huber BR et al (2018) Chronic traumatic encephalopathy within an amyotrophic lateral sclerosis brain bank cohort. J Neuropathol Exp Neurol 77:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of demographic and clinical characteristics between the 750 included subjects and the 1816 subjects who were excluded because of insufficient athletic information.
Table S2. Characteristics according to sports played in females.
Table S3. Characteristics according to sports played in males.
Table S4. Association between exposure to individual sports and occurrence of combined CTE pathology in males with additional multivariable adjustments.
Table S5. Association between exposure to individual sports and occurrence of CTE‐positive in males with additional multivariable adjustments.
Table S6. Subgroup analysis of associations of exposure to sports and occurrence of combined CTE pathology in males with additional multivariable adjustments.
Table S7. Subgroup analysis of association between exposure to individual sports and occurrence of CTE‐positive in males with additional multivariable adjustments.
Table S8. Associations of playing multiple sports with occurrence of combined CTE pathology and CTE‐positive in males.
Figure S1. Proportion of males with CTE‐positive in individuals who did not play football, who played youth or high school football only, or who played football beyond high school in the overall group and also in subgroups based on other sports played.
Figure S2. Odds ratios and 95% confidence intervals from multivariable analysis displaying the associations of exposure to specific sports with the separate outcomes of combined CTE pathology and CTE‐positive in males. Baseball—beyond high school is not displayed for the CTE‐positive outcome as none of the 12 subjects who played baseball beyond high school had CTE‐positive, making estimation of an odds ratio impossible.


