Abstract
Introduction
Frequent infusions and bleeds can impact on the health‐related quality of life (HRQoL) of paediatric haemophilia B patients. rIX‐FP (IDELVION®) is a fusion protein linking recombinant factor IX with recombinant albumin, and is associated with low bleeding rates with a weekly regimen, which could improve HRQoL.
Aims
To measure the effect of rIX‐FP prophylaxis on the HRQoL of paediatric patients and treatment satisfaction in their caregivers using the Haemo‐QoL and Hemo‐SATP questionnaires, respectively.
Methods
At baseline and end‐of‐study (EOS), patients 4‐11 years old participating in the PROLONG‐9FP program answered the Haemo‐QoL questionnaire and gave information on their socio‐demographic data and physical activity. Caregivers completed the Hemo‐SatP. Minimal important differences (MID) (|Cohen's d| > 0.5) between baseline and EOS and the number of responders (patients with meaningful subject‐level improvements over time) at EOS were calculated.
Results
Twenty patients (age group I: 4‐7 years old [n = 12]; age group II: 8‐12 years old [n = 8]) completed the Haemo‐QoL questionnaire at baseline. MIDs were found in age group I representing improvement for “physical health” (d = −0.547) domain; 60% of patients were responders for “physical health.” In age group II, MIDs were seen in most domains; 71.4% patients were responders in “total score.” In caregivers, improvements were seen for most domains of the Hemo‐SatP with a small effect size. Fewer patients missed school when treated with rIX‐FP and 94.1% patients maintained their physical activity level.
Conclusion
Prophylaxis with rIX‐FP led to substantial improvements in HRQoL in paediatric patients and treatment satisfaction in caregivers.
Keywords: extended half‐life product, Haemo‐QoL, health‐related quality of life, Hemo‐SatP, minimal important difference, responder definitions, treatment satisfaction
1. INTRODUCTION
Current treatment strategies have ensured that paediatric patients with haemophilia B can now anticipate a normal life expectancy. However, many children with severe haemophilia B are infusing factor IX (FIX) 2‐3 times a week and still experience spontaneous or traumatic bleeds that prevent normal activity and may lead to disability later in life.1 Frequent infusions are one of the major obstacles to treatment adherence 2 and can also negatively affect patients’ and their caregivers health‐related quality of life (HRQoL).3, 4 Paediatric haemophilia patients frequently report pain,5 are often unable to participate in recreational activities and have high rates of school absenteeism.6, 7, 8 A large European study found that younger children show HRQoL impairments in the dimensions “family” and “treatment,” while older children reported higher impairments in social dimensions “perceived support” and “friends,”9 whereas caregivers experienced emotional strain and burden on their schedule providing treatment or managing school and work absences.10
Extending treatment periods and reduced bleeding rates by maintaining high trough levels may improve HRQoL of patients and their caregivers.11 This may be achieved with an extended half‐life recombinant FIX (rFIX) concentrate. rIX‐FP (IDELVION®) is a recombinant fusion protein linking rFIX with recombinant albumin, that has a half‐life approximately fivefold longer than conventional rFIX.12 It was demonstrated that a single dose of 50 IU/kg rIX‐FP maintained FIX activity above 5 IU/dL after 10 days and above 2 IU/dL after 14 days in paediatric patients.12 In a phase 3 paediatric study of the PROLONG9‐FP clinical programme, rIX‐FP prophylaxis resulted in low bleeding rates; with 7‐day dosing, the median annualized bleeding rate (ABR) was 3.12, the annualized spontaneous bleeding rate (AsBR) was 0 and patients had an annualized joint bleeding rate (AJBR) of 0.99.12 These low bleeding rates are consistent with the high trough levels (mean FIX activity of ~14%) that were achieved.13
Here we report the impact of rIX‐FP prophylaxis on HRQoL in paediatric patients below the age of 12 years and treatment satisfaction amongst their caregivers.
2. MATERIALS AND METHODS
2.1. Participants and study design
This was a prospective, non‐randomized, international, open‐label phase 3 study. Male patients aged 0–11 years with severe or moderate haemophilia B (FIX activity ≤2 IU/dL) who had previously received FIX products (for >150 exposure days [EDs] for patients 6–11 years old, and >50 EDs for patients <6 years old), with no personal or family history of inhibitors were eligible for enrolment. Patients were assigned a dose of 35‐50 IU/kg rIX‐FP for weekly prophylaxis, at the investigator's discretion.
The study was approved by the institutional review board/ethics committee at each participating centre, registered at clinicaltrials.gov (NCT01662531), and performed in accordance with good clinical practice, the Declaration of Helsinki and local regulatory requirements. Written informed consent was obtained from the parent/guardian of the patient and informed assent from the patient. Consent could be withdrawn at any time. The study design and results were published previously.12
2.2. Patient‐reported & observer‐reported outcomes
According to the taxonomy of the U.S. Food and Drug Administration (FDA)14 two types of clinical outcome assessments were included in the current study, a Patient‐Reported Outcome (PRO) measure and an Observer‐Reported Outcome measure (ObsRo). While a PRO is based on the direct report of the patient about the status of his health condition without interpretation by a clinician or another person, an ObsRO is based on an observation by someone other than the patient or a health professional who observes the patient in daily life and reports on a specific aspect of the patient's health.
Study subjects who were at least 4 years of age were asked to answer the age group‐specific self‐report version of the haemophilia‐specific HRQoL questionnaire (Haemo‐QoL15) at baseline and at their end‐of‐study (EOS) visit, at least 12 months later. The Haemo‐QoL is an instrument designed to assess the HRQoL of children with haemophilia. It covers 8‐12 domains depending on the age group (“physical health,” “feeling,” “view of yourself,” “family,” “friends,” “perceived support,” “other people,” “sports and school,” “dealing with haemophilia,” “treatment,” “future” and “relationships”). The version for children aged 4‐7 (I) consists of 21 items, the version for children aged 8‐12 (II) contains 64 items. Answer categories are based on a 3‐point Likert scale for age group I and on a 5‐point Likert scale for age group II ranging from “never” to “always.” Some items which were positively formulated had to be inversely recoded and scores were transformed ranging from 0 to 100 with decreasing scores indicating improvements in HRQoL. Missing values were not imputed. Domain scores were calculated when at least 75% of the items of that domain had been answered, if there were more than 25% missing items the domain and/or the total score were not calculated.
The parent/caregivers of all study subjects were asked to complete the observer‐reported haemophilia‐specific treatment satisfaction questionnaire (Hemo‐SatP 16) at baseline and at EOS. The Hemo‐SatP covers 6 domains (“ease & convenience,” “efficacy,” “burden,” “specialist/nurse,” “centre/hospital,” “general satisfaction”) and contains 35 items assessing parent's satisfaction with their children's treatment. Answer categories are based on a 5‐point Likert scale ranging from “totally agree” to “totally disagree.” Some items had to be recoded and scores were transformed ranging from 0 to 100 with decreasing scores indicating improvements in treatment satisfaction.
Socio‐demographic data and physical activity level were documented at baseline and throughout the study using single questions asked by the investigator to the caregiver/child on: number of days missed from school due to haemophilia (for school‐age children), number of days the caregiver(s) missed from work due to the need of caring for the subject, the number of important life activities missed due to haemophilia for both subject and caregiver(s) and subject's overall activity level (sedentary/moderately active/vigorous).
2.3. Clinical data
Clinical data on bleeding rates, target joints, treatment modality, infusion frequency and adverse events were collected via electronic case report forms. Data on bleeding events and dosing were collected using an e‐diary.
2.4. Statistical analysis
Socio‐demographic and clinical data for paediatric subjects are reported. Categorical variables are summarized using counts and percentages, while continuous variables are summarized by their means ± SD. Differences over time are reported as Δ means ± SD.
The magnitude of minimal important differences (MID) on a group level, defined by Guyatt as “smallest difference in score in the domain of interest that patients perceive as important and that would lead the clinician to consider a change in the patient's management’’,17 can be determined by distribution‐based and anchor‐based approaches.18, 19 In the present study, the MID from baseline to EOS was determined using a distribution‐based approach applying Cohen's d effect size calculation.17
The Cohen's d effect size is a standardized measure of change obtained by dividing the difference in mean scores from baseline to EOS by the standard deviation of baseline scores. This can be mathematically represented as: 20
Cohen has proposed the following benchmarks for interpreting effect sizes d (d = 0.2, small effect size; d = 0.5, medium effect size; d = 0.8, large effect size).21 In this study, a negative Cohen's d reflects a positive effect on HRQoL. The MID was defined as having medium to large effect sizes (absolute value of Cohen's d > 0.5).
By contrast, responder definitions (RDs) are defined as relevant thresholds identifying meaningful individual change over time.18 In this paper, RDs of HRQoL and treatment satisfaction were subject‐level improvements evaluated as the percentage of subjects who showed a decrease in mean values from baseline to EOS greater than a half of the standard deviation at baseline.19, 21
No statistical testing was performed due to lack of power by cause of small sample sizes. All statistical analyses were performed using the SPSS program version 24 (Statistical Package for Social Science, IBM®).
3. RESULTS
3.1. Patient characteristics and clinical outcomes
Twenty‐seven patients were enrolled from 17 centres in 10 countries (Australia, Austria, Canada, Czech Republic, Germany, Spain, France, Israel, Italy and Russia) between January 2013 and October 2014. Patients’ baseline demographic data for the entire cohort are shown in Table 1. Clinical study results have already been published.12
Table 1.
Baseline demographics and patient characteristics for the entire study cohort
| Baseline N = 27 | |
|---|---|
| Age in years, mean ± SD | 5.9 ± 2.93 |
| Race, n (%) | |
| White | 26 (96.3) |
| Black/African‐American | 1 (3.7) |
| BMI categories, n (%)[Link] | |
| Underweight | 4 (16.7) |
| Normal weight | 14 (58.3) |
| Overweight | 4 (16.7) |
| Obese | 2 (8.3) |
| Previous treatment regimen | |
| On‐demand | 3 (11.1) |
| Prophylaxis | 24 (88.9) |
| Previous prophylaxis interval, n (%)[Link] | |
| <2 times per week | 4 (16.7) |
| 2 to <3 times per week | 17 (70.8) |
| ≥3 times per week | 3 (12.5) |
| History of surgery, n (%) | 9 (33.3) |
BMI, body mass index; SD, standard deviation.
Categories were calculated based on the BMI‐for‐age percentiles34; for 3 patients the BMI category could not be calculated since they were younger than 2 y old.
Percentages are based on the number of patients who received prophylaxis prior to study entry.
Patients who reported HRQoL data at both baseline and EOS (n = 17) had a reduction in mean ABR and AsBR from baseline to EOS. Two patients in this cohort had target joints at baseline, both patients experienced resolution of their target joints by EOS (Table 2).
Table 2.
Clinical outcomes and socio‐demographic data for patients with HRQoL data at baseline and end‐of‐study (EOS)
| Baseline N = 17 | EOS N = 17 | |
|---|---|---|
| ABR, mean ± SD | 6.5 (12.06)[Link] | 3.5 (3.16) |
| AsBR, mean ± SD | 2.9 (8.51)[Link] | 0.6 (0.85) |
| AJBR, mean ± SD | NA | 1.4 (1.58) |
| Target joint, n (%)[Link] | 2 (11.8) | 0 |
| Patients missing no days from school due to haemophilia, n (%)[Link] | 12 (70.6) | 16 (94.1) |
| Caregiver missing no days from work due to caring for the subject, n (%)[Link] | 14 (82.4) | 16 (94.1) |
| Patients missing no important life activities due to haemophilia, n (%)[Link] | 14 (82.4) | 16 (94.1) |
| Caregivers missing no important life activities due to caring for the subject, n (%)[Link] | 15 (88.2) | 16 (94.1) |
| Patients with a moderate/vigorous level of physical activity, n (%) | 16 (94.1) | 16 (94.1) |
ABR, annualized bleeding rate; AJBR, annualized joint bleeding rate; AsBR, annualized spontaneous bleeding rate; NA, not available; SD, standard deviation.
Bleeding episodes in the 12 mo prior to study entry.
Defined as at least 3 spontaneous bleeding episodes in the same joint in a 6‐mo period.35
In the previous 30 d period.
3.2. Patient‐reported outcomes (PROs) and observer‐reported outcomes (ObsRO)
Health‐related quality of life data were available for 21 patients who had completed the Haemo‐QoL questionnaire at any time; one patient only had EOS data and three patients had only baseline data. A complete data set including clinical data and HRQoL data at both baseline and EOS is available for 17 patients (Figure 1). The study duration for patients reporting HRQoL data at both baseline and EOS (n = 17) was a mean ± SD 451.7 ± 75.82 days.
Figure 1.
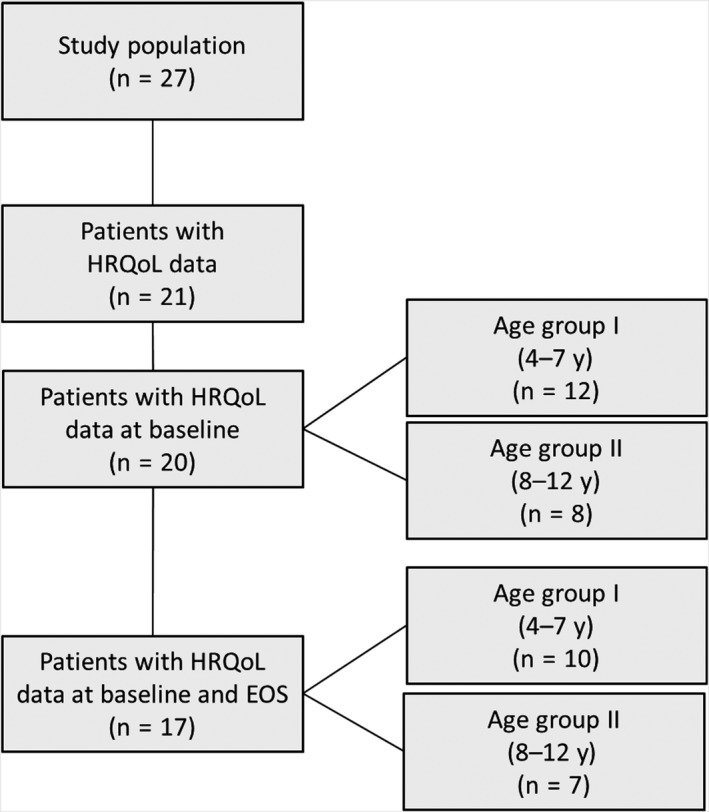
Flow chart study population
At EOS, these patients reported improvements in key socio‐demographic characteristics, as shown in Table 2. A higher proportion of patients (94% [n = 16]) did not miss a day of school due to haemophilia in the previous 30 days when treated with rIX‐FP when compared to their regimen at baseline (70.6% [n = 12]). In line with the results in patients at EOS, 94.1% (n = 16) of caregivers did not miss a day of work due to need of caring for the subject compared to 82.4% (n = 14) at baseline. The majority of patients (94.1% [n = 16]) maintained or improved their physical activity levels compared to baseline.
3.3. Impact of rIX‐FP prophylaxis on patients’ HRQoL (Haemo‐QoL)
Patients in age group I had a good HRQoL (n = 12) at baseline, with the highest impairments in the domains “family,” “physical health” and “treatment” of the Haemo‐QoL. Patients in age group II (n = 8) reported the highest impairments at baseline in social domains, namely “support,” “friends” and “family” (Table 3).
Table 3.
Patient level responders according to the Haemo‐QoL
| Baseline mean score ± SD | EOS mean score ± SD | Mean change ± SD[Link] | Responder threshold[Link] | Responder at EOS[Link] | |
|---|---|---|---|---|---|
| Age group I (4‐7 y) | [n = 12] | [n = 10] | [n = 10] | Value | % |
| Physical health | 21.9 ± 19.31 | 8.8 ± 15.65 | −11.3 ± 19.94 | 9.7 | 60.0 |
| Feeling | 13.9 ± 19.89 | 25.0 ± 27.50 | 8.3 ± 26.35 | 9.9 | 20.0 |
| View | 10.4 ± 16.71 | 10.0 ± 24.15 | 0 ± 31.18 | 8.4 | 20.0 |
| Family | 26.1 ± 20.50 | 27.5 ± 20.24 | 2.5 ± 24.86 | 10.3 | 30.0 |
| Friends | 12.5 ± 22.61 | 10.0 ± 21.08 | −5.0 ± 36.89 | 11.3 | 30.0 |
| Other | 14.6 ± 22.51 | 15.0 ± 21.08 | −2.5 ± 34.26 | 11.3 | 30.0 |
| Sport & school | 13.0 ± 18.21 | 20.8 ± 29.21 | 6.3 ± 37.73 | 9.1 | 37.5 |
| Treatment | 20.5 ± 21.85 | 30.6 ± 24.30 | 11.1 ± 25.34 | 10.9 | 11.1 |
| Total | 17.1 ± 12.30 | 20.6 ± 14.14 | 2.9 ± 21.38 | 6.2 | 33.3 |
| Age group II (8‐12 y) | [n = 8] | [n = 7] | [n = 7] | Value | % |
| Physical health | 23.2 ± 17.81 | 12.8 ± 12.35 | −11.7 ± 23.30 | 8.9 | 42.9 |
| Feeling | 24.6 ± 33.03 | 12.1 ± 8.09 | −15.0 ± 33.91 | 16.5 | 28.6 |
| View | 22.7 ± 21.77 | 10.9 ± 8.27 | −10.1 ± 20.01 | 10.9 | 42.9 |
| Family | 39.4 ± 21.29 | 23.2 ± 22.85 | −13.9 ± 22.07 | 10.6 | 57.1 |
| Friends | 43.8 ± 33.07 | 38.4 ± 31.75 | 0.9 ± 18.55 | 16.5 | 14.3 |
| Support | 47.7 ± 31.86 | 33.0 ± 34.93 | −12.5 ± 14.88 | 15.9 | 42.9 |
| Other | 14.1 ± 15.42 | 7.7 ± 13.91 | −8.3 ± 15.78 | 7.7 | 57.1 |
| Sport & school | 28.9 ± 16.26 | 19.2 ± 21.0 | −8.9 ± 19.05 | 8.1 | 57.1 |
| Dealing | 29.5 ± 13.86 | 19.4 ± 16.61 | −9.7 ± 19.96 | 6.9 | 57.1 |
| Treatment | 8.9 ± 11.45 | 11.2 ± 14.05 | 2.0 ± 6.47 | 5.7 | 14.3 |
| Total | 26.2 ± 6.32 | 17.0 ± 4.49 | −8.3 ± 8.38 | 3.2 | 71.4 |
A negative change in score signifies an improvement from baseline to end‐of‐study and includes only those participants with results at both baseline and EOS.
Responder threshold calculated as ½ SD at baseline.
SD, standard deviation.
At EOS, MIDs between baseline and EOS in age group I (n = 10) were represented by medium effect sizes observed as improvement in “physical health” and decline in “treatment” domain scores at EOS (Figure 2).
Figure 2.
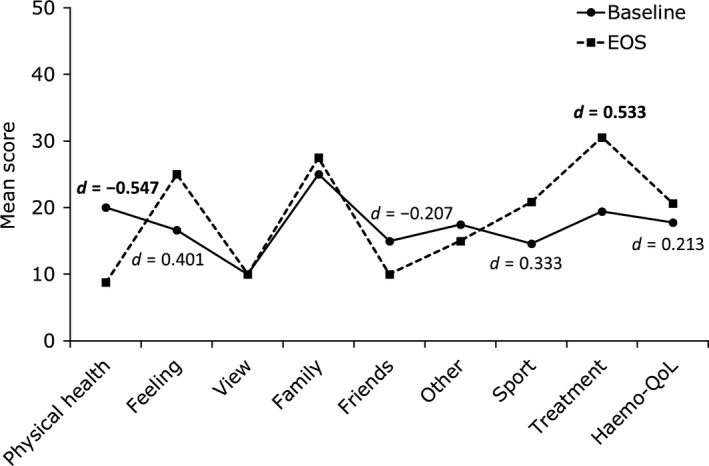
Haemo‐QoL scores and effect sizes for patients with HRQoL data at baseline and end‐of‐study (EOS) in age group I (4‐7 y) (N = 10). d = Cohen's d Effect size. Only effect sizes (|d| > 0.2) for differences in score are displayed on the graph. Bold text indicate medium or large effect sizes (|d| > 0.5), a negative effect size indicates an improvement in HRQoL
In age group II (n = 7) the MIDs were represented by medium to large effect sizes for most of the Haemo‐QoL domains. The largest improvements in HRQoL were seen in the “total score” and the Haemo‐QoL domains “dealing,’” “family” and “physical health” (Figure 3).
Figure 3.
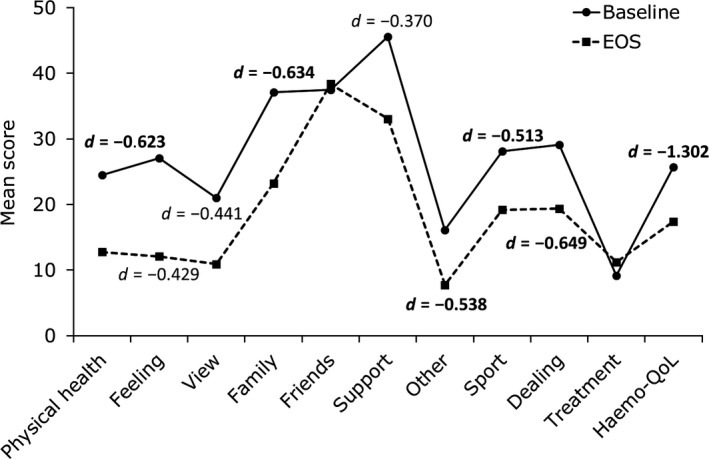
Haemo‐QoL scores and effect sizes for patients with HRQoL data at baseline and end‐of‐study (EOS) in age group II (8‐12 y) (N = 7). d = Cohen's d Effect size. Only effect sizes (|d| > 0.2) for differences in score are displayed on the graph. Bold text indicate medium or large effect sizes (|d| > 0.5), a negative effect size indicates an improvement in HRQoL
Mean changes over time in all Haemo‐QoL scores, responder thresholds and percentage of responders are presented in Table 3. In age group I, 60% of patients were responders in the domain “physical health.” The lowest number of responders was seen in the domain “treatment” (11.1%). In age group II, 71.4% of patients were responders in “total score” based on the responder definition, whilst >50% of patients were responders in the domains “family,” “sport and school,” “dealing” and “other”. The lowest number of responders were seen in the domains “treatment” and “friends” (14.3%).
3.4. Impact of rIX‐FP prophylaxis on caregiver treatment satisfaction
The Hemo‐SatP questionnaire was completed by the caregivers of 23 patients (including caregivers of children younger than 2 years). The mean study duration from baseline to EOS for caregivers was 425.7 ± 81.19 days. Caregivers showed high treatment satisfaction at baseline in the Hemo‐SatP total score (14.5 ± 11.21).
For caregivers, MIDs were represented by small effect sizes (Cohen's d) for most of the Hemo‐SatP domains (Figure 4). The largest improvement was seen in the domain “burden” with small improvements seen in “general satisfaction,” and a decline in the “specialist” domain. At subject level, 34.8% of caregivers were responders, based on the responder definition, with an improvement in the total Hemo‐SatP score (Table 4). The highest number of responders was seen in the domain “ease and convenience” with 43.5% of caregivers above the responder threshold. The lowest number of responders was seen in the “centre” domain (13.0%).
Figure 4.
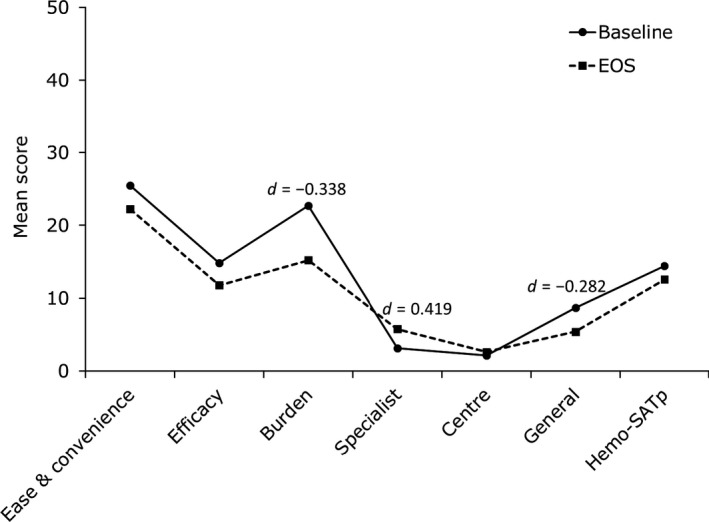
Hemo‐SatP scores and effect sizes for caregivers with treatment satisfaction data at baseline and end‐of‐study (EOS) (N = 23). d = Cohen's d Effect size. Only effect sizes (|d| > 0.2) for differences in score are displayed on the graph. A negative effect size indicates an improvement in treatment satisfaction
Table 4.
Caregiver responders according to the Hemo‐SatP
| Baseline mean score ± SD | EOS mean score ± SD | Mean change ± SD[Link] | Responder threshold[Link] | Responder[Link] | |
|---|---|---|---|---|---|
| Caregivers | [n = 23] | [n = 23] | [n = 23] | Value | % |
| Ease and convenience | 25.5 ± 18.17 | 22.2 ± 14.01 | −3.3 ± 14.90 | 9.1 | 43.5 |
| Efficacy | 14.9 ± 16.52 | 11.8 ± 11.69 | −3.1 ± 15.45 | 8.3 | 30.4 |
| Burden | 22.7 ± 22.23 | 15.2 ± 14.58 | −7.5 ± 15.36 | 11.1 | 34.8 |
| Specialist | 3.1 ± 6.31 | 5.7 ± 9.12 | 2.6 ± 10.11 | 3.2 | 21.7 |
| Centre | 2.2 ± 5.40 | 2.6 ± 6.19 | 0.4 ± 7.67 | 2.7 | 13.0 |
| General | 8.7 ± 11.58 | 5.4 ± 9.10 | −3.3 ± 14.21 | 5.8 | 34.8 |
| Total | 14.5 ± 11.21 | 12.6 ± 8.89 | −1.9 ± 10.49 | 5.6 | 34.8 |
A negative change in score signifies increased treatment satisfaction from baseline to end‐of‐study and includes only those participants with results at both baseline and EOS.
Responder threshold calculated as ½ SD at baseline.
SD, standard deviation.
4. DISCUSSION
In this phase 3 study, paediatric patients treated with rIX‐FP showed an improvement in their HRQoL. Patients in both age groups noted an improvement in the domain of “physical health.” In patients aged 8‐11 years, improvement was seen in nearly every domain with a moderate or large Cohen's d effect size and a high percentage of patients were responders (71.4%). Responders were defined as patients or caregivers that experienced an improvement in mean Haemo‐QoL or Hemo‐SatP scores from baseline to EOS greater than a half of the standard deviation at baseline. Caregivers experienced a small improvement in treatment satisfaction, with the biggest improvements occurring in the “burden,” “ease & convenience” and “general” domains.
Both age groups saw a large improvement in the domain “physical health.” In age group I, the mean change in score from baseline to EOS was (Δ) = −11. 3 ± 19.94, with 60% of patients being responders. In age group II the change in mean score was Δ = −11.7 ± 23.30, with 42.9% of patients being responders. This substantial improvement in “physical health” may be due to the reduction in bleeds from baseline to EOS. The improvement in the domain “physical health” may be due to reduced concerns about bleeds when participating in activities. In age group I, the “treatment” domain was impaired, with only 11.1% of patients being responders. Younger patients may not have appreciated the benefits associated with reduced bleeding rates. However, clinical trials can involve a greater number of clinical visits, tests and monitoring requirements, which could be perceived negatively by the child.
Improvement in a wide range of domains in age group II demonstrates that rIX‐FP treatment benefits multiple aspects of patients’ lives. Being able to remain active and participate in activities could help patients’ relationships with their peers. Lower bleeding rates, such as those seen with rIX‐FP treatment, also may improve patient's mental well‐being, impacting the “feeling” and “dealing” domains. The resolution of all target joints in the study may also have benefited patients. The presence of target joints can be a source of concern to patients, and limit their participation in activities and affect their physical HRQoL.22
Caregivers often experience a burden due to the pain experienced by their children, so a therapy that reduces the number of infusions and bleeds can be very beneficial.10 This may also have contributed to the increase in “treatment satisfaction” seen in caregivers: overall 34.8% of caregivers were responders in the “total score” domain. One of the biggest impacts of haemophilia on caregivers is the time required for care. In one study, 89% of caregivers felt that caring for a patient with haemophilia impacted on their work and employment.23 When patients were treated with rIX‐FP, caregivers missed fewer days of work from baseline to EOS (82.4 vs 94.1%). This may be due to the reduced dosing frequency and the significant reduction in bleeding rates. Reduced bleeding rates in particular could reduce unexpected clinical appointments and treatment sessions. This might be reflected in the domain “burden” that saw the largest improvement over time (Δ = −7.5 ± 15.36) with 34.8% of caregivers being responders. The highest number of responders was seen in the “ease & convenience” domain (43.5%). Again, this likely reflects the reduced infusion frequency seen with rIX‐FP.
There is minimal data on the role of extended half‐life products and HRQoL in haemophilia B patients. Carcao et al24 investigated the impact of the glycoPEGylated rFIX nonacog beta pegol (N9‐GP) on HRQoL in 25 paediatric patients with moderate or severe haemophilia receiving weekly prophylaxis with 40 IU/kg N9‐GP. HRQoL data from 12 boys between 8 and 12 years were reported, at baseline children reported a median Haemo‐QoL “total score” of 30.6, which was similar to our study (median = 26.4). They found a median change in the Haemo‐QoL “total score” of −1.6, implying a trend towards improvement.24 The median improvement in our study with rIX‐FP prophylaxis was −8.2. An improvement in HRQoL (reported for all values which were better than at baseline) was seen in 66.7% of patients (8/12) treated with N9‐GP (independently of the altitude of the score difference) compared to 85.7% of children treated with rIX‐FP (6/7).24 Both N9‐GP and rIX‐FP treatment led to improvements in HRQoL, however, due to the small sample sizes in both studies no further comparisons can be made.
Compared to this study, in Carcao et al24 no significant improvement in HRQoL was seen in patients aged 4‐7 years old. When treated with prophylaxis, paediatric haemophilia patients typically have a high HRQoL that is not significantly lower than that of their siblings or peers.9, 25 It could be that at this early developmental stage patients do not comprehend their treatment as differing to that of their healthy peers or that lower bleeding rates and reduced dosing frequencies do not impact on this younger group. This is an area that requires further investigation.
To our knowledge, there is no other data published on HRQoL in paediatric patients with haemophilia B treated with extended half‐life factors. In adults, a significant HRQoL improvement was demonstrated with a mean change in the Total Haem‐A‐QoL Score of −6.4 ± 8.5 with N9‐GP. For adolescents (n = 15), a mean change of −4.2 ± 11.4 in the Total Haemo‐QoL Score was found; this difference was not statistically significant, but implied a trend towards improvement.26
In the B‐LONG study, 73 adult haemophilia B patients were treated with rFIXFc (Alprolix®) and were followed up for 26 weeks. HRQoL was assessed using the Haem‐A‐QoL and HRQoL Responder Definitions for key domains of the Haem‐A‐QoL were calculated, as in our study. Prophylaxis with rFIXFc led to an improvement in HRQoL with a relatively high number of responders. Greater improvements were seen in patients switching from on‐demand to prophylactic treatment.27 Although this study is in adults, and not directly comparable, it will be interesting to see if the improvement in HRQoL seen with rIX‐FP is maintained in adult and adolescent patients to a similar or greater degree than seen with other long‐acting factors.
When we compare haemophilia A and B, we can see that adult patients have similar bleeding rates9 and HRQoL.27 Similar to results in our study, paediatric patients with haemophilia A that switched to BAX 855, a glycopegylated rFVIII, saw an improvement in HRQoL in all domains of the Paediatric Quality of Life Inventory. However, this did not reach significance in the youngest cohort.28 No other long‐acting FVIII has published quality‐of‐life data for paediatric patients. As stated previously, the finding that the youngest patients do not see a benefit in HRQoL seems consistent, and bears further study.
The calculation of MID and responder definitions in the field of haemophilia is a relatively new concept. It has been applied in another study evaluating the MID in HRQoL in children, adolescents and adults with haemophilia A treated with turoctocog alfa.29 They defined “HRQoL responders” as the percentage of patients with a change in scores between baseline and end‐of‐treatment greater than a threshold representing a meaningful change in each score of the Haem‐QoL or Haem‐A‐QoL. The responder threshold was categorized based on the standard error of measurement, which was calculated as the product of standard deviation at baseline by the square root of one minus the reliability coefficient, in terms of internal consistency of the dimension.30
A limitation of our analysis is that it was performed on a small number of patients, due to the small pool of paediatric patients with haemophilia B. This is a common challenge in rare bleeding disorders. The use of responder definitions and MID improves the identification of clinically relevant changes in HRQoL. An additional anchor‐based approach would have been beneficial when determining MID, as it would capture the viewpoints of patients as to what magnitude of change would be seen as clinically actionable. However, it was not possible due to the lack of an appropriate anchor in the study design.
The substantial improvements in HRQoL indicate that rIX‐FP positively impacts HRQoL in paediatric patients in a relatively short time span (12 months). However, over time there could be further improvements in HRQoL. As patients become more independent with their treatment, the reduced dosing frequency may have a greater impact on the patients’ HRQoL and treatment burden.31 The low AJBR of 0.99 and the resolution of target joints seen in this study may lead to reduced arthropathy in later years. Arthropathy is a large contributor to lower HRQoL in adults.32 Patients in the trial may also experience fewer bleeds throughout their lifetime, patients with zero bleeds experience better or normalized HRQoL scores when compared to patients experiencing frequent bleeds.33 Patients treated with rIX‐FP through to adulthood may notice substantially higher HRQoL than their peers who have experienced less comprehensive bleed prevention, or have experienced a high treatment burden.
In conclusion, 7‐day prophylaxis with rIX‐FP improved HRQoL in paediatric patients, reduced school absenteeism and enabled patients to maintain their levels of physical activity. Caregivers also experienced an improvement in their “burden” related to their child's treatment. The long‐term impact of the low bleeding rates achievable with rIX‐FP prophylaxis may lead to more significant improvements in HRQoL later in life.
DISCLOSURES
This study was funded by CSL Behring. Sylvia von Mackensen is the developer of the Haemo‐QoL and Hemo‐SatP questionnaires and has consulted for CSL Behring. Gili Kenet has received honoraria from CSL Behring. Jinesh Shah is an employee of CSL Behring, is a paid contractor with CSL Behring and owns stock in CSL Behring. Wilfried Seifert is an employee of CSL Behring.
AUTHOR CONTRIBUTIONS
Sylvia von Mackensen contributed to the conception and design of the work, analysis and interpretation of the data. Jinesh Shah provided substantial contributions to the analysis and verification of results and the interpretation of data. All authors were involved in drafting the work or revising it critically and gave final approval of the version to be published.
ACKNOWLEDGEMENTS
The authors would like to thank the PROLONG‐9FP clinical study investigators for their contribution. Editorial support for the writing of this manuscript was provided by Hannah Johnston and Kate Holliday of Meridian HealthComms and was funded by CSL Behring. CSL Behring reviewed and provided feedback on the paper. The authors had full editorial control of the paper and provided their final approval of all content.
von Mackensen S, Shah J, Seifert W, Kenet G. Health‐related quality of life in paediatric haemophilia B patients treated with rIX‐FP. Haemophilia. 2019;25:45–53. 10.1111/hae.13624
REFERENCES
- 1. Franchini M. Current management of hemophilia B: recommendations, complications and emerging issues. Expert Rev Hematol. 2014;7:573‐581. [DOI] [PubMed] [Google Scholar]
- 2. Hacker MR, Geraghty S, Manco‐Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7:392‐396. [DOI] [PubMed] [Google Scholar]
- 3. Cavazza M, Kodra Y, Armeni P, et al. Social/economic costs and quality of life in patients with haemophilia in Europe. Eur J Health Econ. 2016;17:53‐65. [DOI] [PubMed] [Google Scholar]
- 4. Torres‐Ortuño A, Cuesta‐Barriuso R, Nieto‐Munuera J. Parents of children with haemophilia at an early age: assessment of perceived stress and family functioning. Haemophilia. 2014;20:756‐762. [DOI] [PubMed] [Google Scholar]
- 5. Rambod M, Forsyth K, Sharif F, Khair K. Assessment and management of pain in children and adolescents with bleeding disorders: a cross‐sectional study from three haemophilia centres. Haemophilia. 2016;22:65‐71. [DOI] [PubMed] [Google Scholar]
- 6. Monahan PE, Baker JR, Riske B, Soucie JM. Physical functioning in boys with hemophilia in the U.S. Am J Prev Med 2011;41:S360‐S368. [DOI] [PubMed] [Google Scholar]
- 7. Hernandez G, Baumann K, Guelcher C, et al. Understanding the impact of hemophilia B on activity of US children with hemophilia (CWH) from their caregivers: the bridging hemophilia experiences results and opportunities into solutions (B‐HERO‐S Study). Haemophilia. 2016;22:1. [Google Scholar]
- 8. Baumann K, Hernandez G, Witkop M, et al. Impact of mild to severe hemophilia on engagement in recreational activities by US men, women, and children with hemophilia B: the bridging hemophilia B experiences, results and opportunities into solutions (B‐HERO‐S) study. Eur J Haematol. 2017;98:25‐34. [DOI] [PubMed] [Google Scholar]
- 9. Gringeri A, Von Mackensen S, Auerswald G, et al. Health status and health‐related quality of life of children with haemophilia from six West European countries. Haemophilia. 2004;10:26‐33. [DOI] [PubMed] [Google Scholar]
- 10. DeKoven M, Karkare S, Kelley LA, et al. Understanding the experience of caring for children with haemophilia: cross‐sectional study of caregivers in the United States. Haemophilia. 2014;20:541‐549. [DOI] [PubMed] [Google Scholar]
- 11. Carcao M. Changing paradigm of prophylaxis with longer acting factor concentrates. Haemophilia. 2014;20:99‐105. [DOI] [PubMed] [Google Scholar]
- 12. Kenet G, Chambost H, Male C, et al. Long‐acting recombinant fusion protein linking coagulation factor IX with albumin (rIX‐FP) in children: results of a phase 3 trial. Thromb Haemost. 2016;116:659‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Castaman G, Gill JC, Roberts J, Jochems J, Li Y, Seifert W. High and sustained observed trough FIX activity levels with prophylactic dosing of IDELVION (rFIX‐FP) in patients with hemophilia B. Res Prac Thromb Haemost. 2017;1:781. [Google Scholar]
- 14. Food and Drug Administration . Guidance for industry on patient‐reported outcome measures: use in medical product development to support labeling claims. Fed Reg. 2009;74:1‐39. [Google Scholar]
- 15. Von Mackensen S, Bullinger M, Haemo‐QoL Group . Development and testing of an instrument to assess the quality of life of children with haemophilia in Europe (Haemo‐QoL). Haemophilia 2004;10(Suppl. 1):17‐25. [DOI] [PubMed] [Google Scholar]
- 16. von Mackensen S, Gringeri A. Quality of Life in Hemophilia In: Preedy VR, Watson RR, eds. Handbook of Disease Burdens and Quality of Life Measures. New York, NY: Springer New York, 2010: 1895‐1920. [Google Scholar]
- 17. Cumming G. Cohen's d needs to be readily interpretable: comment on Shieh (2013). Behav Res Methods. 2013;45:968‐971. [DOI] [PubMed] [Google Scholar]
- 18. Wyrwich KW, Norquist JM, Lenderking WR, Acaster S. Methods for interpreting change over time in patient‐reported outcome measures. Qual Life Res. 2013;22:475‐483. [DOI] [PubMed] [Google Scholar]
- 19. Norman GR, Sloan J, Wyrwich KW. Interpretation of changes in health‐related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582‐592. [DOI] [PubMed] [Google Scholar]
- 20. Wyrwich KW, Monika B, Neil A, et al. Estimating clinically significant differences in quality of life outcomes. Qual Life Res. 2005;14:285‐295. [DOI] [PubMed] [Google Scholar]
- 21. Cohen J. Statistical Power Analysis for the Behavioral Sciences (Revised Edition). New York, NY: Academic Press; 1977:1‐463. [Google Scholar]
- 22. Klamroth R, Pollmann H, Hermans C, et al. The relative burden of haemophilia A and the impact of target joint development on health‐related quality of life: results from the ADVATE Post‐Authorization Safety Surveillance (PASS) study. Haemophilia. 2011;17:412‐421. [DOI] [PubMed] [Google Scholar]
- 23. Cutter S, Molter D, Dunn S, et al. Impact of mild to severe hemophilia on education and work by US men, women, and caregivers of children with hemophilia B: the bridging hemophilia B experiences, results and opportunities into solutions (B‐HERO‐S) study. Eur J Haematol. 2017;98:18‐24. [DOI] [PubMed] [Google Scholar]
- 24. Carcao M, Kearney S, Santagostino E, et al. Insight into health‐related quality of life of young children with haemophilia B treated with long‐acting nonacog beta pegol recombinant factor IX. Haemophilia. 2017;23:e222‐e224. [DOI] [PubMed] [Google Scholar]
- 25. Neuner B, von Mackensen S, Holzhauer S, et al. Health‐related quality of life in children and adolescents with hereditary bleeding disorders and in children and adolescents with stroke: cross‐sectional comparison to siblings and peers. Biomed Res Int. 2016;2016:1579428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chowdary P, Kearney S, Regnault A, Hoxer CS, Yee DL. Improvement in health‐related quality of life in patients with haemophilia B treated with nonacog beta pegol, a new extended half‐life recombinant FIX product. Haemophilia. 2016;22:e267‐e274. [DOI] [PubMed] [Google Scholar]
- 27. Wyrwich KW, Krishnan S, Auguste P, et al. Changes in health‐related quality of life with treatment of longer‐acting clotting factors: results in the A‐LONG and B‐LONG clinical studies. Haemophilia. 2016;22:866‐872. [DOI] [PubMed] [Google Scholar]
- 28. Mullins ES, Stasyshyn O, Alvarez‐Román MT, et al. Extended half‐life pegylated, full‐length recombinant factor VIII for prophylaxis in children with severe haemophilia A. Haemophilia. 2017;23:238‐246. [DOI] [PubMed] [Google Scholar]
- 29. Santagostino E, Lentz SR, Busk AK, Regnault A, Iorio A. Assessment of the impact of treatment on quality of life of patients with haemophilia A at different ages: insights from two clinical trials on turoctocog alfa. Haemophilia. 2014;20:527‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wyrwich KW, Tierney WM, Wolinsky FD. Further evidence supporting an SEM‐based criterion for identifying meaningful intra‐individual changes in health‐related quality of life. J Clin Epidemiol. 1999;52:861‐873. [DOI] [PubMed] [Google Scholar]
- 31. Khair K, Meerabeau L, Gibson F. Self‐management and skills acquisition in boys with haemophilia. Health Expect. 2015;18:1105‐1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Forsyth AL, Witkop M, Lambing A, et al. Associations of quality of life, pain, and self‐reported arthritis with age, employment, bleed rate, and utilization of hemophilia treatment center and health care provider services: results in adults with hemophilia in the HERO study. Patient Prefer Adherence. 2015;9:1549‐1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Neufeld EJ, Recht M, Sabio H, et al. Effect of acute bleeding on daily quality of life assessments in patients with congenital hemophilia with inhibitors and their families: observations from the dosing observational study in hemophilia. Value Health. 2012;15:916‐925. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention (CDC) . BMI percentile calculator for child and teen. 2017.
- 35. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935‐1939. [DOI] [PubMed] [Google Scholar]


