Abstract
Acyldepsipeptides are a unique class of antibiotics that act via allosterically dysregulated activation of the bacterial caseinolytic protease (ClpP). The ability of ClpP activators to kill non-growing bacteria represents a new opportunity to combat deep-seated biofilm infections. However, the acyl depsipeptide scaffold is subject to rapid metabolism. Herein, we explore alteration of the potentially metabolically reactive α,β unsaturated acyl chain. Through targeted synthesis, a new class of phenyl urea substituted depsipeptide ClpP activators with improved metabolic stability is described. The ureadepsipeptides are potent activators of S. aureus ClpP and show activity against Gram-positive bacteria, including S. aureus biofilms. These studies demonstrate that a phenyl urea motif can successfully mimic the double bond, maintaining potency equivalent to acyldepsipeptides, but with a decreased metabolic liability. While removal of the double bond from acyldepsipeptides generally has a significant negative impact on potency, structural studies revealed that the phenyl urea depsipeptides can retain potency through the formation of a third hydrogen bond between the urea and the key Tyr63 residue in the ClpP activation domain. Ureadepsipeptides represent a new class of ClpP activators, with improved drug-like properties, potent antibacterial activity, and the tractability to be further optimized.
Keywords: ClpP, Biofilm, Antibiotic, acyldepsipeptide, ureadepsipeptide
Graphical Abstract
The Centers for Disease Control estimates that methicillin resistant Staphylococcus aureus (MRSA) is responsible for nearly 120,000 severe infections per year in the United States with 20,000 of these infections resulting in death. 1 MRSA is classified as a serious threat, requiring further study and expedited development of novel therapeutics that can treat the most recalcitrant forms of MRSA infection. The ability of S. aureus to form biofilms contributes heavily to the pathology of infection with this organism. Within a biofilm, the bacterial population can withstand unfavorable conditions for growth and evade elimination by the host immune system, which is unable to penetrate the protective extracellular matrix.2 When treated with antibiotics, bacteria within the biofilm commonly enter a dormant persister state, in which many druggable targets are down-regulated. Once drug pressure is relieved, these persisters can then re-establish an active infection. The lack of actively transcribed targets within the dormant population limits therapeutic options for the successful treatment of an S. aureus biofilm infection. 2-6
Acyldepsipeptides are a unique class of antibiotics that act via allosterically dysregulated activation of the bacterial caseinolytic protease (ClpP).7-8 ADEP4, an optimized acyldepsipeptide, has recently shown promise as an antimicrobial agent capable of acting on dormant bacteria and eradicating S. aureus biofilm infections.9 Although ClpP is not essential and ClpP activating antibiotics are prone to resistance development, combining ClpP activators with other antibiotics prevents resistance development and kills high-density stationary phase cultures of bacteria.10 ADEP4 tested in combination with rifampin sterilized deep-seated mouse thigh infections and biofilms grown in vitro. These findings indicate that the development of this class of ClpP activators or analogous small molecule ClpP modulators could provide a valuable new therapeutic option for recalcitrant S. aureus infections.11 ClpP offers the advantage of being a unique target that does not carry the risk of target mediated cross-resistance to existing therapeutics. Previously described ADEPs suffer from moderate to high clearances, a problem that must be solved if they are to become a viable therapeutic option.8
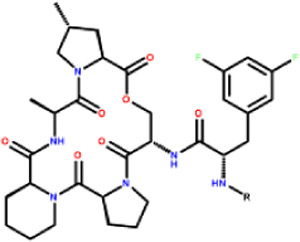
The acyldepsipeptide scaffold was first discovered by Eli Lilly in 1985 from soil sample isolate Streptococcus hawaiiensis (NRRL 15010). It consists of a five member amino acid cyclic depsipeptide core, a phenylalanine linker, a variable hydrophobic tail region, and demonstrated potent antibacterial activity against a broad range of Gram-positive bacteria. 12 ADEPs are currently being studied for their activity against a broad variety of pathogenic bacteria including M. tuberculosis, VRE and C. difficile. 10, 13-14 Optimization of this class by researchers at Bayer enhanced the antimicrobial activity and yielded lead ADEP4. ADEP4 has nanomolar activity against S. aureus and is the current standard for the acyldepsipeptide class.15
Elegant studies have demonstrated the novel antibacterial mechanism of acyldepsipeptides, which target ClpP.8, 16 ClpP is a highly conserved serine protease that plays a critical role in bacterial virulence and homeostasis. 17 This is accomplished through the degradation of short-term regulatory proteins as well as misfolded or damaged proteins. Alone, the peptidase activity of ClpP is limited to substrates with fewer than six residues. 18 To form a fully functional Clp protease, ClpP must associate with a chaperone. These regulatory proteins activate ClpP in an ATP dependent manner and confer substrate specificity.19 Chaperones bind ClpP via loop regions containing distinctive Ile-Gly-Phe motifs.20 Once bound, substrate proteins are fed into the interior of ClpP. Here the proteins are exposed to and degraded by 14 serine proteolytic triads ringing the periphery of the ClpP interior space.21 ADEPs bind to a groove between the head regions of adjacent ClpP subunits mimicking the Ile-Gly-Phe motif of the Clp chaperones (ClpC, ClpX). 22-24 This opens the central pore and orders the serine catalytic triad leading to uncontrolled proteolysis and eventual cell death.7, 22-23
Recent SAR studies on the acyldepsipeptide scaffold have focused on the depsipeptide core, 25 including methyl substitution on the lactone core which rigidifies the scaffold and increases potency 26-27. It has also been determined that methylation of the southern pyrrolidine ring improves potency.28 Modification of the acyl side chain has been limited by the belief that the α,β unsaturated acyl (acryl) chain is required for activity. 15 The acryl side chain is an electrophilic center, found in many covalent drugs29 and may contribute to the rapid metabolism of the series, limiting its utility. Here, we explored whether replacement of the acryl side chain was tolerated and the impact on metabolic stability.
Alterations of the ADEP α,β unsaturated acryl chain using protein crystallography, computer-guided design and synthesis led to the discovery of a new class of phenyl urea substituted depsipeptide (UDEP) ClpP activators. Structural and biophysical studies demonstrate that ureadepsipeptides can maintain potency equivalent to ADEP4 through an additional hydrogen bond interaction within the active site. The UDEPs activate S. aureus ClpP, have excellent potency against Gram-positive bacteria including S. aureus biofilms, and improved metabolic stability compared to ADEP4.
Results and Discussion:
We began our exploration of acryl replacements using crystallography experiments, computer-aided design and targeted synthesis of isosteric side chains. S. aureus ClpP (SaClpP) was crystallized with ADEP4, and structural data was collected to a resolution of 2.2 Å (Supplementary Table 1). Results show the electron density of ADEP4 was not well defined (Supplementary Figure 1). This was likely due to poor solubility of ADEP4 in the crystallization matrix, rather than ligand flexibility. To fully understand the acyldepsipeptide binding pose within S. aureus ClpP, crystallization efforts were repeated with a more soluble analog of ADEP4, compound 2, which has a shortened acyl side chain and lacks fluorination on the phenylalanine motif. This analog proved to be a useful tool, with clear electron density visible in the Fo-Fc map for 2, in which the acyl side chain interactions could be studied in detail. (Supplementary Table 1 and Supplementary Figure 1). Docking experiments with these structures were performed using Glide to allow for identification of candidate isosteric sidechain replacements that could more fully occupy the alkyl binding pocket, while maintaining binding and reducing metabolism. 30,31
One of the potentially suitable side chains identified in these experiments was phenyl urea. We hypothesized that the urea substitution could mimic the geometry afforded by the α, β-unsaturated bond in 2. This substitution would replace the α, β-unsaturated acyl motif, which is a Michael acceptor and likely contributes to the metabolic instability of the ADEP scaffold. Docking experiments indicated a phenyl ring could replace the alkyl chain, which would take better advantage of the area available in the hydrophobic pocket. Compound 5 was synthesized with the urea substitution, and a phenyl group was incorporated to replace the alkyl chain. Compound 5 was active in the casein degradation assay (59.8%) and had whole cell MIC activity (6.25μM) against both MSSA and MRSA S. aureus (Table 1), but was inactive against a ClpP deficient strain, indicating activity remained on target as the result of ClpP activation. The metabolic stability (t1/2 1.24h) in mouse microsomal studies increased 6.2-fold compared to ADEP4 (t1/2 0.2h). These data indicated that it was possible to replace the acryl side chain to improve the metabolic stability of the series.
Table 1.
Series 1 – Evaluation of non-fluorinated analogs
 |
Metabolic Stability t1/2 (hours) Mouse Liver Microsomes |
Casein Degradation (μM) |
Minimal Inhibitory Concentration (μM) |
||||
|---|---|---|---|---|---|---|---|
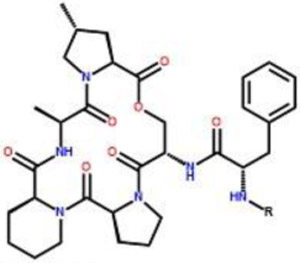
| Compound | R | EC50 | % Activation | ATCC 29213 | USA 300 | USA 300 Δ ClpP |
|
| 1 | 0.18 ± 0.01 | 0.34 ± 0.04 | 95.8 ± 2.3 | 0.8 | 0.8 | >25 | |
| 2 | 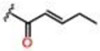 |
0.19 ± 0.02 | 10.6 ± 0.76 | 69.3 ± 2.2 | 12.5 | 25 | >25 |
| 3 | 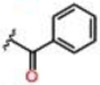 |
0.35 ± 0.02 | Inactive | Inactive | >25 | >25 | >25 |
| 4 | 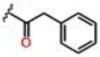 |
0.38 ± 0.03 | 48.9 ± 4.3 | 40.3 ± 3.9 | >25 | >25 | >25 |
| 5 | 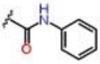 |
1.24 ± 0.06 | 4.38 ± 0.37 | 59.8 ± 1.7 | 6.25 | 6.25 | >25 |
| 6 | 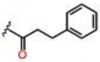 |
0.20 ± 0.01 | 31.8 ± 4.1 | 33.2 ± 4.1 | >25 | >25 | >25 |
| 7 | 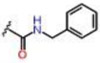 |
0.71 ± 0.04 | Inactive | Inactive | >25 | >25 | >25 |
| 8 | 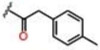 |
1.07 ± 0.06 | 41.6 ± 2.6 | 48.5 ± 2.6 | >25 | >25 | >25 |
| 9 | 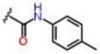 |
1.62 ± 0.13 | 0.90 ± 0.1 | 76.4 ± 2.2 | 0.8 | 0.8 | >25 |
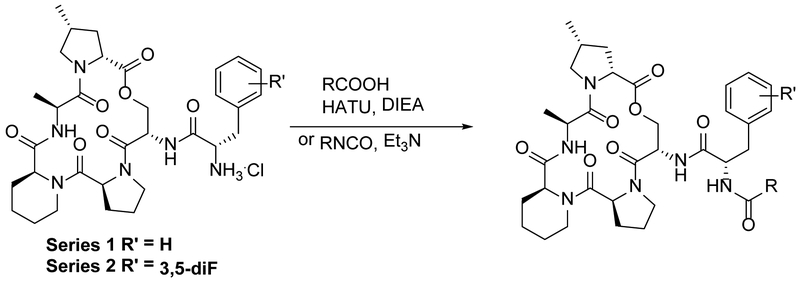
To further develop the SAR of the binding pocket, two series of close structural orthologs were examined to evaluate the effect of the urea and placement of the phenyl ring being used to mimic the acyl chain. Identical sets of compounds were generated to compare the modified side chains in combination with either the non-fluorinated phenylalanine (Series 1, Table 1), present in 2 and naturally occurring ADEPs, or the difluorinated phenylalanine motif present in ADEP4 (Series 2, Table 2). These two series were synthesized following the approach reported by Hinzen, generating a common macrocyclic peptide lactone intermediate followed by incorporation of either a phenylalanine or a 3,5-difluoro phenylalanine. e motif before final amino terminus capping with the acryl isostere motif (Scheme 1).15 Of the acryl replacements reported in this study, only the urea analogs required alteration of the synthetic route in the last step by reaction of the terminal peptide amine with a phenyl isocyanate.
Table 2.
Series 2 – Evaluation of difluorinated analogs
 |
Metabolic Stability t1/2 (hours) Mouse Liver Microsomes |
Casein Degradation (μM) |
Minimal Inhibitory Concentration (μM) |
||||
|---|---|---|---|---|---|---|---|
| Compound | R | EC50 | % Activation | ATCC 29213 | USA 300 | USA 300 Δ ClpP |
|
| ADEP4 | 0.2 ± 0.02 | 0.20 ± 0.02 | 103 ± 2.5 | 0.1 | 0.2 | >25 | |
| 10 | 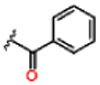 |
0.19 ± 0.02 | Inactive | Inactive | >25 | >25 | >25 |
| 11 | 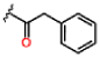 |
0.26 ± 0.02 | 30.0 ± 12.8 | 70.7 ± 14.5 | >25 | >25 | >25 |
| 12 | 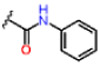 |
1.01 ± 0.08 | 0.61 ± 0.12 | 68.8 ± 2.3 | 3.1 | 3.1 | >25 |
| 13 | 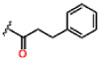 |
0.23 ± 0.02 | 3.04 ± 0.38 | 52.6 ± 2.2 | >25 | 25 * | >25 |
| 14 | 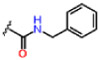 |
0.66 ± 0.02 | 7.10 ± 1.17 | 53.7 ± 3.2 | 25 | 25 | >25 |
| 15 | 1.42 ± 0.07 | 8.84 ± 0.80 | 63.4 ± 2.3 | 25 | 25 | >25 | |
| 16 | 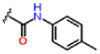 |
1.72 ± 0.11 | 0.33 ± 0.03 | 100.7 ± 3.0 | 0.1 | 0.2 | >25 |
>75% inhibition at 12.5 μM
Scheme 1.
The chemical synthesis approach for depsipeptide analogs.
All analogs were profiled for their ability to activate S. aureus ClpP (SaClpP) digestion of β-casein. As we have found that the magnitude of activation can vary amongst chemical series with similar EC50 values, EC50 and % activation values were compared to corresponding values for ADEP4, to report binding and activation potential, respectively. Antimicrobial activity was tested against MSSA and MRSA, a S. aureus ΔClpP knockout strain to confirm on-target activity (Table 1 and 2), and a panel of Gram-positive and Gram-negative bacterial strains (Supplementary Table 2). All analogs tested lacked Gram-negative activity. The ability of the analogs to resist hepatic metabolism was established using mouse liver microsomes.
Comparison of analogs with or without the urea demonstrated phenyl urea compounds (Series 1 5, 9; Series 2 12, 16) were able to maintain good whole cell and enzymatic activity, while their isosteric 2-phenylacetamide counterparts (Series 1 4, 8; Series 2 11, 15) had relatively poor activity. In most cases the series 1 and 2 activities were similar with the only exception being the benzyl urea, 7, which lacked activity. However, its difluorinated counterpart 14, had moderate ClpP activation and weak whole cell activity. This indicated that the added binding affinity derived from difluorination of the phenylalanine ring can, in some cases, overcome this disfavored side chain substitutions.
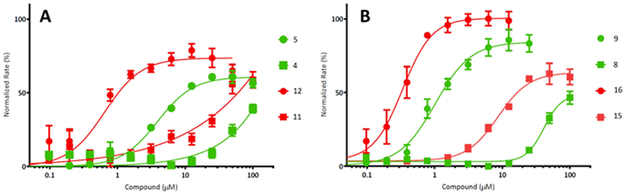
The effect of the position of the phenyl ring in the side chain was also explored using differently sized linkers. Benzamide analogs (3, 10) with short linkers were not accommodated in the active site, as both difluorinated and non-fluorinated compounds were inactive in enzymatic and whole cell assays. Similarly, placing the phenyl ring at the end of the longer linker resulted in poor activity. Phenyl propanamides (6 and 13) and benzyl ureas (14 and 7) had weak or no whole cell activity with only moderate enzymatic activity observed for the fluorinated compounds (6 and 14). As seen with the original isosteric phenyl urea 5, its difluorinated counterpart 12 had reasonable enzymatic and whole cell activity. However, when these compounds were modeled in the active site they did not extend as far into the pocket as the acryl chain. Subsequent addition of a para methyl group to the phenyl ring (9, 16) mimicked the longer acyl chain of ADEP4 and enhanced activity to sub-micromolar concentrations for EC50 and MIC. 100% activation of SaClpP (compared to the Vmax of ADEP4) was achieved with the difluorinated compound 16. (Figure 1).
Figure 1.
Casein-BODIPY ClpP mediated degradation assay, a comparison of matched urea and non-urea pairs. These graphs show the rate of SaClpP casein-BODIPY degradation (normalized to the maximum rate of SaClpP degradation in the presence of ADEP4) as a function of experimental compound concentration. A) Compounds represented in this graph have a non-fluorinated phenylalanine motif. Compounds 5 and 12 (round markers) have urea substitutions while compounds 4 and 11 (square markers) retain the acyl side chains. Compounds 5 and 4 (green) contain phenyl side chains. Compounds 12 and 11 (red) have a para methyl addition to the phenyl side chain. B) Compounds represented in this graph have a 3,5 difluorinated phenylalanine motif. Compounds 9 and 16 (round markers) have urea substitutions while compounds 8 and 15 (square markers) retain the acyl side chains. Compounds 9 and 8 (green) contain phenyl side chains. Compounds 15 and 16 (red) have a para methyl addition to the phenyl side chain.
A comparison of the non-fluorinated phenylalanine (Series 1) and the difluorinated phenylalanine motif (Series 2) present in ADEP4 showed universally higher potency for the difluorinated compounds. Series 2 compounds generally had lower EC50 values (1.6-10.4 fold), higher activation (7.2-53.7%), and slightly better MICs (2-4 fold) than their Series 1 counterparts.
The goal of this series was to replace the metabolically labile acryl bond with an alternative substitution to increase the metabolic stability of the series while maintaining activity. The α,β unsaturated acryl analogs ADEP4, 1 and 2 were all rapidly metabolized by mouse microsomes with half-lives ≤ 0.2 hours confirming the metabolic liability posed by this portion of the ligand. Similarly, rapid metabolism was seen in the analogs with only a phenyl ring substitution: benzamides 3 and 10 (t1/2 0.35 and 0.19 h respectively), phenacetamides 11 and 4 (t1/2 0.26 and 0.38 h, respectively) and phenyl propamides 6 and 13 (t1/2 0.20 and 0.23 h, respectively). Phenyl urea substitution successfully stabilized the depsipeptide scaffold as seen in compounds 12 and 5 which showed a 5-fold improvement in stability over ADEP4 (t1/2 1.01 and 1.24h respectively). Addition of a para-methyl group further increased the metabolic stability, as seen in compounds 16 and 9 (t1/2 1.72 and 1.62 h respectively). Notably, the urea depsipeptide 16 shows an 8.6-fold improvement over ADEP4 in a mouse microsomal model and has biological activity equivalent to ADEP4. Interestingly, the addition of the para-methyl group also stabilized phenyl compounds without the urea as seen with compounds 15 and 8 (t1/2 1.42 and 1.07 h respectively. Benzyl ureas 14 and 7 (t1/2 0.66 and 0.71 h respectively) were the least stable of the phenyl ureas substituted series. Plasma stability studies indicated that all analogs exhibited high stability to serum esterases (Supplementary Table 3). These data demonstrate that isosteric phenyl urea substitution for the acryl side successfully improves microsomal stability of the depsipeptides.
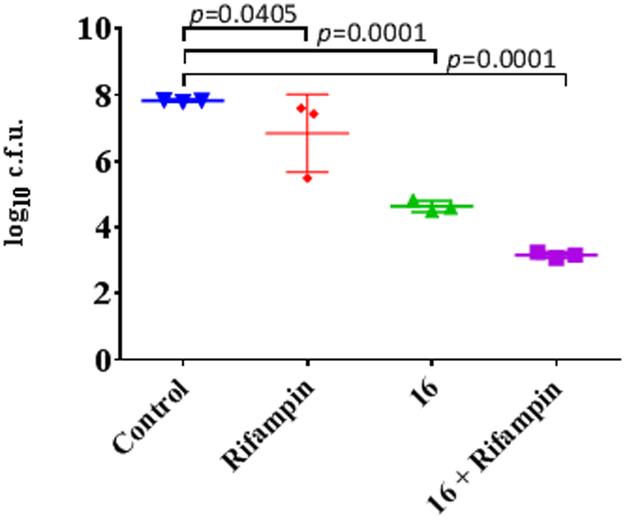
Lead 16 was selected for evaluation against S. aureus biofilm growth, as a key target of ClpP activation therapy. (Figure 2) Treatment of mature S. aureus biofilms with rifampin alone yielded limited reduction in growth over 24 hours (p= 0.0405). In contrast, treatment with 16 yielded a significant reduction when compared with the control (p<0.0001). Compound 16 shows comparable activity to ADEP4 over 24 hours. Eradication of stationary phase cultures as previously published occurs between 3 and 5 days. 9 Furthermore, when S. aureus biofilms were exposed to 16 in combination with rifampin, there was a significant additional reduction in growth when compared to treatment with 16 alone (p <0.0001). This data suggests that 16 could be a viable lead for the treatment of S. aureus biofilm infections.
Figure 2.
In vitro S. aureus biofilm killing by UDEPs and in combination with rifampin. Assays were performed at 10 times the MIC and statistical analysis completed with 1way ANOVA and Dunnett’s multiple comparisons test.
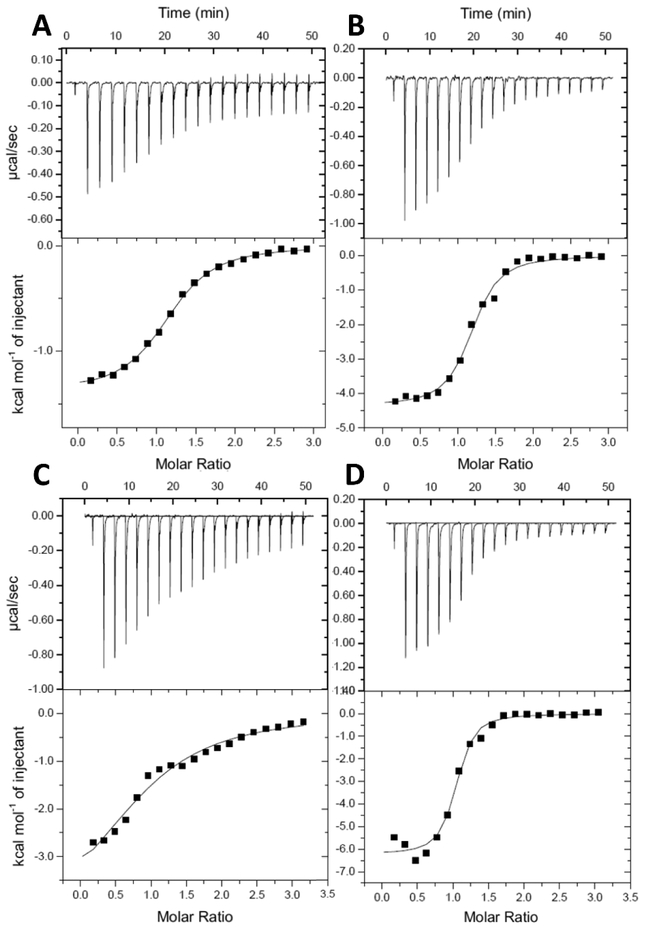
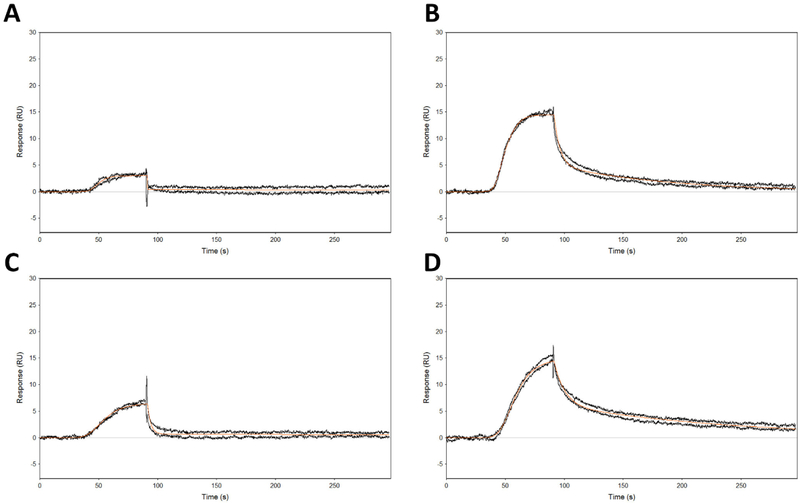
To better understand the mode of binding of the ureadepsipeptides, Isothermal Titration Calorimetry (ITC) and Surface Plasmon Resonance (SPR) testing was performed using matched structural pairs: series 1 phenyl acetamide 8 and phenyl urea 9; series 2 phenyl acetamide 15 and phenyl urea 16. ITC experiments (Figure 3 and Table 3) indicated stoichiometric binding occurred in all cases. The phenyl ureas both had superior binding affinities compared to the isosteric phenyl acetamides (9 Kd 1.7μM vs. 8 Kd 13.8μM; and 16 Kd 1.2μM vs. 15 Kd 38.7μM), consistent with their superior ClpP activation potential and MIC activities (Figure 1 and 2). A breakdown of the relative energy binding contributions revealed that the phenyl ureas increased affinity was driven by increased enthalpic and decreased entropic contributions (9 ΔH −4.3 Kcal mol−1 vs. 8 ΔH −1.6 Kcal mol−1; and 16 ΔH −4.1 Kcal mol−1 vs. 15 ΔH −2.8 Kcal mol−1) consistent with the addition of a new receptor ligand hydrogen bonding interaction. In the SPR studies, the phenyl acetamide 15 bound with a weak KD of 60 μM and a short residence time of 1.9 s, while the corresponding urea depsipeptide 16 bound with a KD of 0.233 μM and a much longer half-life time of 114 s. This relationship of increased potency and extended half-life was mirrored in the series 1 phenyl acetamide and urea substituted pair 8 and 9 (8 KD of 5 μM, T1/2 0.02 s, 9 KD 0.0234 μM T1/2 65 s) (Figure 4 and Table 4). Combined, these biophysical data suggest that urea substitution directly contributes to improved potency, ligand binding, and target residency.
Figure 3.
Isothermal titration calorimetry results. Top panels show the change in enthalpy per injection of SaClpP into experimental compounds. Bottom panels show the integrated enthalpies and the results of nonlinear regression fitting of the integrated enthalpies. A) SaClpP injected into compound 8. B) SaClpP injected into compound 9.C) SaClpP injected into compound 15. D) SaClpP injected into compound 16.
Table 3.
Isothermal titration calorimetry results
| Compound | Kd (μM) |
ΔH (kcal mol−1) |
−TΔS (kcal mol−1) |
N |
|---|---|---|---|---|
| 9 | 1.66 ± 0.3 | −4.33 ± 0.04 | −3.55 ± 0.13 | 1.09 ± 0.06 |
| 8 | 13.8 ± 8.4 | −1.61 ± 0.26 | −5.09 ± 0.6 | 1.12 ± 0.05 |
| 16 | 1.19 ± 0.1 | −4.11 ± 0.01 | −1.88 ± 0.06 | 0.99 ± 0.06 |
| 15 | 38.7 ± 10.9 | −2.76 ± 0.68 | −1.23 ± 0.6 | 1.01 ± 0.07 |
Figure 4.
Surface plasmon resonance sensograms. A) Compound 8. B) Compound 9.C) Compound 15. D) Compound 16
Table 4.
Surface plasmon resonance calculations
| Compound |
ka (M−1s−1) |
kd (s−1) |
KD (μM) |
t1/2 (s) |
|---|---|---|---|---|
| 8 | (5.62 ± 0.01) × 106 | 28.1 ± 0.09 | 5.00 ± 0.02 | 0.02 |
| 9 | (4.56 ± 0.09) × 105 | 0.0106 ± 0.00002 | 0.0234 ± 0.0005 | 65.2 |
| 15 | (6.00 ± 1) × 103 | 0.362 ± 0.002 | 60.0 ± 10 | 1.91 |
| 16 | (2.55 ± 0.07) × 104 | (6.49 ± 0.01) × 103 | 0.233 | 114 |
To examine the structural basis of SaClpP activation by the urea leads, the crystal structure of SaClpP in complex with 5 and 16 were solved. Diffraction data were collected to 2.4 Å and 2.2 Å respectively. This data was compared to the 2.2 Å 2 acyldepsipeptide structure discussed earlier. The asymmetric unit of all ClpP structures in this study contained a single tetradecamer composed of stacked heptameric rings, with characteristic extended ClpP barrel conformation. Depsipeptide binding allosterically favors this active conformation driving nonspecific protein degradation. 7 Clear electron density was present in the simulated annealing omit map in all 14 binding pockets in each structure (Supplementary Figure 1). Ligand binding poses closely mirrored those of previously reported ClpP-acyldepsipeptide structures (E. coli 23, B. subtilis 22, M. tuberculosis 32, E. faecium 10, H. sapiens 33 and N. meningitidis 34) with one key difference found in the ureadepsipeptide scaffold interactions.
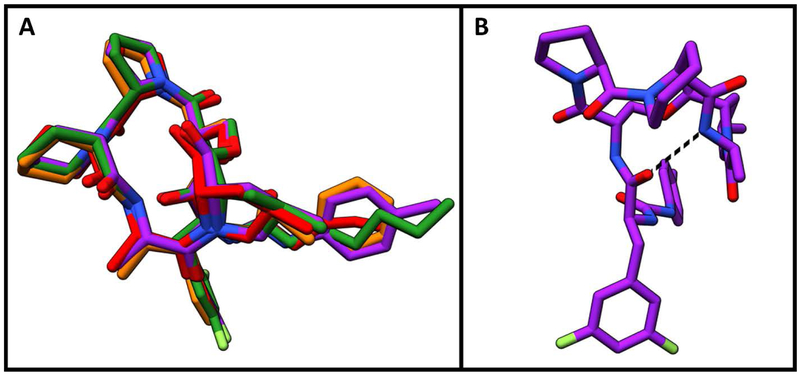
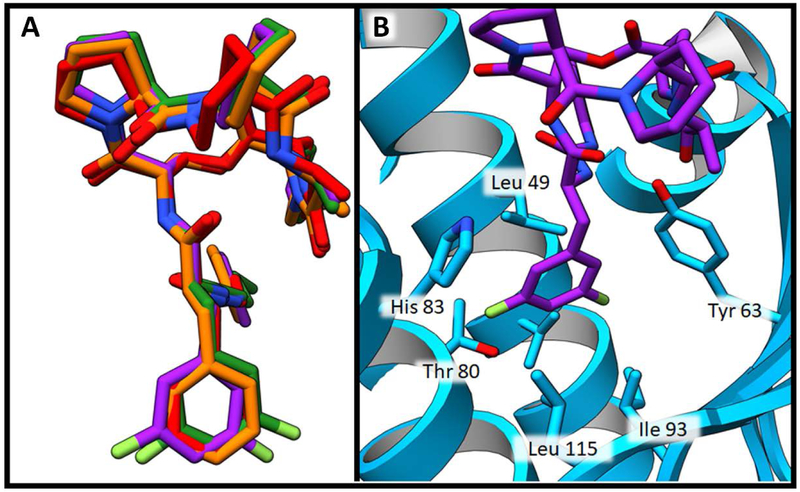
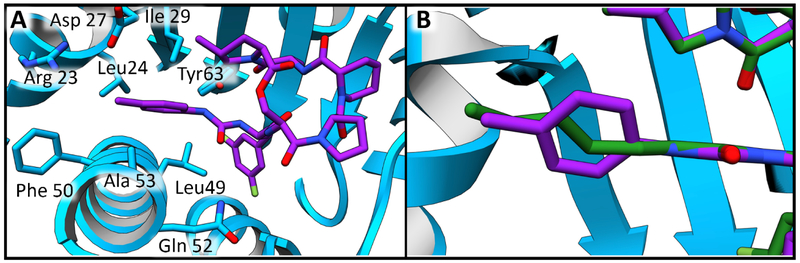
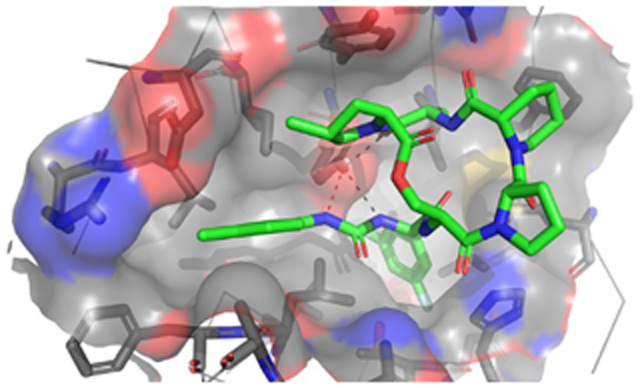
As anticipated, the identical lactone cores of acyl depsipeptides ADEP4, 2 and urea depsipeptides 5 and 16 adopted the same binding pose (Figure 5 A). The carbonyl of the phenylalanine linker formed an internal hydrogen bond with the secondary amide of the lactone core stabilizing the active conformation. 15 Such intramolecular hydrogen bonding interactions are common in natural products as they can mask polar groups, allowing for better uptake into the target organism. The capability for this intramolecular interaction is retained in the urea depsipeptides (Figure 5 B). The phenylalanine motif of the phenyl urea binds similarly to the acyl depsipeptides, (Figure 6 A) in a deep desolvated pocket (formed by residues His 83, Leu 49, Tyr 63, Ile93, Leu 115 and Thr80) that helps drive binding affinity (Figure 6 B).
Figure 5.
Depsipeptide lactone core binding positions A) Structure overlay demonstrating similarity of lactone core binding positions. Compound 2 is shown in red, compound 5 is shown in orange, compound 16 is shown in purple and ADEP4 is shown in green. B) Intramolecular hydrogen bond within the lactone core of compound 16 is modeled with dashed black line.
Figure 6.
Depsipeptide phenylalanine motif binding pocket. A) Structure overlay demonstrating similarity of phenylalanine binding positions. Compound 2 is shown in red, compound 5 is shown in orange, compound 16 is shown in purple and ADEP4 is shown in green. B) Binding pocket of compound 16 shown in purple. SaClpP is shown in blue ribbon structure. SaClpP side chains within 4 Å of the phenyl ring of this ligand are shown as blue sticks.
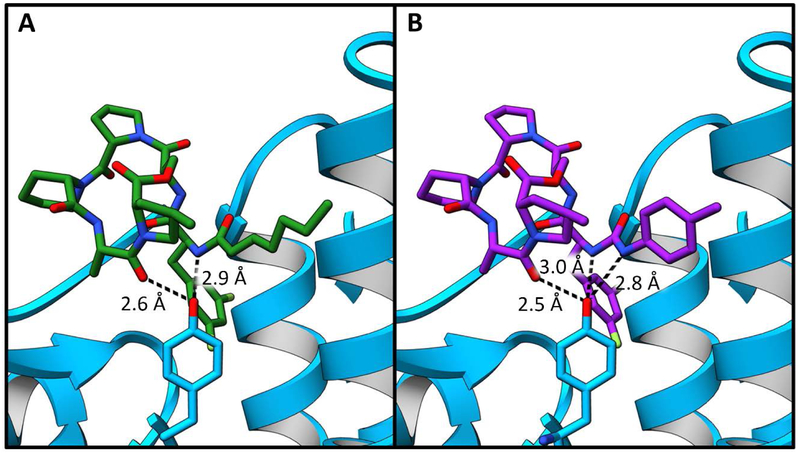
One key structural difference was revealed when comparing the acryl and urea hydrophobic side chain interactions. Tyr63 generally forms two hydrogen bonding interactions – one with the terminal amide of the aliphatic tail and the other with the carbonyl group of the alanine portion of the lactone core (Figure 7 A). Studies have shown that this residue plays a critical role in ClpP regulation. 35 Unique to all depsipeptide structures to date, ureadepsipeptides demonstrate the formation of a third hydrogen bond with Tyr63 (Figure 7 B). The urea NH is sufficiently close and at an appropriate angle to the Tyr63 phenolic oxygen to form a hydrogen bond. The value of this additional hydrogen bond is shown through comparison of isosteric compounds 15 (ADEP) and 16 (UDEP) in which 16 is significantly more potent in all whole cell, biochemical and biophysical assays.
Figure 7.
Tyr 63 interactions. SaClpP is represented in blue ribbons with Tyr 63 shown in blue sticks. A) SaClpP bound to ADEP4 shown here as a green stick figure. Hydrogen bonding interactions between ADEP4 and Tyr 63 are represented with black dashed lines. B) SaClpP bound to compound 16, which is shown here as a purple stick figure. Hydrogen bonding interactions between compound 16 and Tyr 63 are represented with black dashed lines.
Comparison of the binding modes of acryl portion of ADEP4 and 2, and the urea side chain of compounds 5 and 16 demonstrates binding in a hydrophobic groove of SaClpP comprised of residues Gln 52, Leu 49, Ala 53, Phe 50, Arg 23, Asp 27, Leu 24, Ile 29, and Tyr 63 (Figure 8 A). The terminal carbon of ADEP4 side chain extends 2.8 Å beyond the phenyl ring of compound 5. In compound 16 the addition of a methyl group in the para phenyl urea position allows a closer match to the occupancy of the ADEP acyl within the groove (Figure 8 B). This extension further into the pocket is important as it improves the potency so that 16 is comparable to ADEP4. Ongoing studies with additional modifications around this ring system may further improve potency and drug-like properties of the UDEPs.
Figure 8.
Hydrophobic side chain binding pocket. A) The binding pocket of compound 16 shown in purple sticks. Residues within 4 Å of the urea side chain are shown in blue sticks. B) An overlay of ADEP4 shown in green sticks and compound 16 shown in purple sticks. Image focused on the acyl and urea side chain of the compounds.
Conclusion
Although the acyldepsipeptide class has shown promise as an antimicrobial agent capable of acting on dormant bacteria, they are limited as a therapeutic by rapid metabolism. Prior studies have demonstrated that removal of the metabolically labile α,β-unsaturated acyl chain results in a significant loss of antimicrobial activity. 15 Using structure-guided design, we were able to replace this double bond with a more metabolically stable urea. This resulted in a novel class of ureadepsipeptides that are effective ClpP activators, with improved metabolic stability (8.6-fold) and antimicrobial activity equivalent to ADEP4. When replacing the acryl side chain with a phenyl urea substitution the urea replicates the geometry of the unsaturated double bond and these portions of the molecules align well, which is critical for ADEP binding. Structural studies revealed that the UDEPs can retain potency through the formation of a third hydrogen bond between the urea NH and a key Tyr63 residue in the active site. It was also found necessary to fully occupy the alkyl binding pocket to achieve sub micromolar activity. In the design of 16, a para methyl phenyl substitution was introduced to the phenyl urea core to mimic the more potent, longer chain acyldepsipeptides, resulting in a 32-fold increase in potency compared to 12. This activity is comparable to ADEP4 and was demonstrated to be on target, as it was dependent on the presence of ClpP within the cell. Combination of 16 with rifampin effectively killed biofilms indicating that ureadepsipeptides could be potential drug candidates for S. aureus biofilm infections. The ureadepsipeptides offer increased metabolic stability over traditional acyldepsipeptides suggesting that they should have improved pharmacokinetic properties. The synthetic approach and late stage introduction of the urea motif allows for further rapid ureadepsipeptides SAR expansion. Ongoing work is aimed at creating ureadepsipeptides with a long pharmacological half-life and potent antibacterial activity to treat recalcitrant Gram-positive infections.
Experimental
General Synthesis Pathway for Amide Analogs
For macrocyclic series 1 or series 2 amine hydrochloride salts were dissolved in DMF and cooled to 0°C, corresponding acid (1.3 eq), HATU (1.3 eq.) and DIEA (3.9 eq) were then added, and the reaction mixture was then allowed to warm to room temperature and stirred overnight. The reaction solution was diluted with ethyl acetate, washed with a 1N hydrochloric acid, aq. saturated sodium hydrogen carbonate solution and aq. saturated sodium chloride solution. The organic phase was dried over sodium sulfate and evaporated. Target compounds were obtained after reverse phase chromatography using a water to acetonitrile gradient. Analytical data for all compounds in available in the supplementary data section.
General Synthesis Pathway for Urea Analogs
Isocyanate (1.2 eq.), macrocyclic series 1 or series 2 amine hydrochloride salts (1 eq.) and triethylamine (3.6 or 4.8 eq.) were dissolved in THF/DMF (v/v = 1:1, 2 mL) in a microwave safe tube and subject to microwave irradiation at 200 °C for 10 min. Solvents were removed under reduced pressure, and the crude residue was purified by reverse-phase flash column chromatography using water to acetonitrile gradient. Fractions containing the desired compound were pooled and dried to afford urea analogs. Analytical data for all urea analogs in available in the supplementary data section.
In vitro Microsomal Metabolic Stability
The stability of compounds to mouse microsomal degradation was determined as described previously, 36 and the rate of degradation was monitored by LC-MS analysis using multiple sampled time points. Metabolic stability was evaluated via the half-life calculated from least squares fit of the concentration remaining at various time points based on first-order kinetics.
Plasma Stability
Compound stocks were prepared at a concentration of 10 mM in DMSO. The internal standard was 4 μg/mL warfarin in acetonitrile. Compound stocks were mixed with mouse plasma (Fisher Scientific) or pooled human plasma (Innovative Research Inc) to prepare a final concentration of 10 μM in a 2 mL 96- well deep well plate (pION Inc., Woburn, MA, catalog #110023); this was the master plate. From the master plate, 70 μL was taken from each well and added into four storage plates (pION Inc., Woburn, MA, catalog #110323) in triplicate, each for a different time point. The storage plates were then incubated at 37 °C and shaken at 100 rpm. Samples were taken at 0 min, 3h, 24 h, and 48h. At each time point, 210 μL of internal standard was added to quench the reaction. The plates were then centrifuged at 4000 rpm for 20 min, and supernatant was analyzed by Ultra High Pressure Liquid Chromatography with Mass Spectrometry (UPLC-MS; Waters Inc., Milford, MA). The compound was detected by selected ion recording (SIR), and quantitation was based on the peak area ratio of the test compound vs. the internal standard.
Bacterial Strains and Growth Conditions
Strains were routinely grown with Mueller-Hinton broth or agar at 37°C. Liquid cultures were incubated with shaking at 225 RPM. Bacterial strains were obtained through ATCC, BEI Resources, or from academic labs : Staphylococcus aureus ATCC 29213, S. pyogenes- ATCC 700294, Streptococcus pneumoniae- R6, B. subtilis ATCC 23857, E. faecalis ATCC 33186, S. aureus USA 300, S. aureus USA 300 ΔClpP, Klebsiella pneumoniae- ATCC 700603, Acinitobacter baumannii- ATCC 19606, Pseudomonas aeruginosa- ATCC 15692, Escherichia coli- K12, E. coli- K12 ΔtolC.
Minimal Inhibitory Concentration Assays
MICs were determined using the microbroth dilution method according to Clinical Laboratory Standards Institute (CLSI) standards and were read by visual inspection. Compounds were dissolved at 10 mM in dimethylsulfoxide and stored at −80 °C. Two-fold serial dilutions of antibiotic in 100 μL of Mueller-Hinton broth media were first prepared in 96-well round bottom microtiter plates (Nunc, USA). An equivalent volume (100 μL) of bacterial broth inoculum (containing approximately 105 cfu/mL bacteria) was added to each well to give final concentrations of drug starting at 200 μM. Plates were incubated aerobically at 37 °C overnight. The MIC was recorded as the lowest concentration of drug to visibly inhibit growth. Assays were performed in triplicate.
Biofilm Killing Assays
Overnight, stationary cultures of UAMS-1 were diluted 1:20 in BHI broth. 100 μl of this culture was added to each well of a tissue-culture treated polystyrene 96-well plate. Plates were incubated at 37 °C for 24 hours. Medium was carefully removed and wells were gently washed twice with PBS. 100 μl of fresh medium containing 10 times the MIC of antibiotics was carefully added to each well. Plates were incubated for 24 hours. Medium was carefully removed and wells were gently washed twice with PBS. 100 μl of PBS was then added to each well and biofilms were disrupted by sonication. Serial dilutions of each well were performed and 10 μl of each dilution was spotted onto BHI plates and incubated overnight at 37 °C for cfu count. Biofilm data was examined in GraphPad Prism. Statistical analysis completed with 1way ANOVA and Dunnett’s multiple comparisons test.
ClpP Expression and Purification
The sequence of S. aureus NCTC 8325 was synthesized into the NcoI and XhoI sites of the pET28a vector by GenScript. ClpP with a C-terminal 6 Histidine tag was overexpressed in BL21(DE3) E. coli at 18 °C for 8 hours. The resulting protein was then purified in a two-step process incorporating affinity chromatography with a HisTrap High Performance column (GE Healthcare) and size exclusion chromatography with a Sephacryl (16/60) column in buffer containing 50 mM Tris pH 8, 100 mM NaCl and 2 mM DTT. Purity was assessed through SDS PAGE analysis. This protein was also analyzed by analytical ultracentrifugation and shown to exist as a mixture of the tetradecameric and heptameric forms as a function of protein concentration with concentrations >10 uM favoring the tetradecamer, consistent with other studies. 23
Casein-BODIPY Degradation Assay
Casein-BODIPY degradation assays were conducted in triplicate using 20 μl per well volume in a 384 well microtiter plate. Each well contained 1 μM SaClpP, 1 μM BODIPY labeled casein (Invitrogen E6638), and experimental compounds with concentrations ranging from 0-200 μM in assay buffer (50 mM Tris pH 8, 100 mM NaCl, 2 mM DTT, and 2% DMSO). All molar concentrations of SaClpP were calculated with a monomer of SaClpP (MW 22.58 kDa). Fluorescence was measured with excitation and emission at 485 and 528 nm, respectively, at 1 min intervals over a period of 30 min using a Pherastar FS plate reader (BMG Labtech; Cary, NC) incubated at 37°C. Initial velocity (the rate of fluorescence increase over time) for each reaction was calculated. The resulting data was log transformed and a dose response curve was fitted to log (agonist) vs. response-variable slope using the four parameters symmetrical equation in GraphPad Prism Version 7.00 37, 100% activation was computed using Vmax of ADEP4 as a reference for 100% activation.
Surface Plasmon Resonance
Direct binding of experimental compounds with SaClpP (Monomeric MW 21513.57 Da) was measured via Surface Plasmon Resonance (SPR) using a FortѐBio Pioneer FE instrument. The Ni-NTA biosensor was conditioned with a pulse of 0.5 M EDTA, followed by 50 μM NiCl2 at flowrate of 25 μl/min for 5 min. SaClpP was tethered (3300 RU) to a Ni-NTA biosensor using the capture couple method (using primary amines) and standard Ni-NTA-His interactions to enhance the baseline stability. Experimental compounds were diluted to a final concentration of 1 μM in 1.04X buffer to closely match the DMSO concentration in the running buffer (50 mM HEPES pH 8.0, NaCl 200 mM, 0.01% Triton X-100, 4% DMSO v/v). Analyte or test compound Injections were performed with a flowrate of 125 μl/min with an association time of 90 sec and a dissociation time of 300 sec, twice over both SaClpP and reference surface (without any ligand) using Taylor dispersion based OneStep dynamic injection method 38. The surface heterogeneity originating from the immobilization and complex binding mode with positive cooperativity of ADEP4 to ClpP produced nonlinear kinetics that did not fit to simple 1:1 kinetic model. Thus, the double referenced data was fitted with a two-site simple kinetic model fit using Qdat data analysis tool. The measurement of absolute KD values using this technique was hampered by the cooperativity of activator binding to the tetradecamer ClpP which is immobilized to SPR chip surface breaking the symmetry of binding sites. The primary advantage of this technique is that it allows for close comparative study of the dissociation rates amongst an analog series, which was used to calculate residence times.
Isothermal Titration Calorimetry
Isothermal titration calorimetry (ITC) experiments were performed at 25 °C using a MicroCal iTC200 (GE Healthcare). SaClpP was dialyzed at 8 °C overnight into a solution containing 50 mM Tris pH 8.0 and 100 mM NaCl. Concentration of SaClpP was determined after dialysis by absorbance at 280 nm using a molar extinction coefficient of 7680 M−1 cm−1. To minimize heat of dilution effects resulting from differences in buffer composition between experimental compounds and protein, compounds were dissolved in dialysate buffer. DMSO concentrations were normalized to 1% in protein and compound samples. Because aqueous solubility of the experimental compounds were highly variable, 39.6 μl SaClpP was loaded into the syringe and 204 μl experimental compounds were loaded into the cell. Injections were carried out by serial injection of SaClpP; first, one injection of 0.4 μl followed by 19 incremental injections of 2 μl, at 150 second intervals. Data from the first injection was excluded, due to pre-equilibration mixing between contents of cell and syringe at the syringe tip. All experiments were completed in triplicate. Data collection, analysis, and plotting were performed using Origin 7 SR4 (version 7.0552). Peak areas were integrated, normalized, and then fitted by non-linear regression using the one-site model. Binding isotherms provided the equilibrium association or binding constant (Ka), the change in enthalpy (ΔH), and the stoichiometry of binding (N). Binding stoichiometry was 1:1 within experimental error. The change in free energy (ΔG) and change in entropy (ΔS) were determined using the equation:
| (Equation 1.) |
where R is the universal gas constant, T is the temperature in degrees Kelvin, and other parameters are as defined.
Crystallization and Data Collection
SaClpP was concentrated to 20mg ml−1 in a buffer containing 50mM Tris (pH 8), 100mM NaCl, and 2mM DTT. Sparse matrix screens in a 400nl sitting drop were conducted using JCSG Core I-IV and Index Suites. An optimized condition of 100mM Sodium Acetate (pH 4.5), 30% MPD and 20mM CaCl2 yielded cubic crystals (0.2-0.4mm) that formed over 72 hours at 18°C. Resulting crystals were soaked in a solution of the experimental compound at a molar ratio of 3:1 for nine hours. Ethylene glycol was added to crystallization buffer (25% v/v) as a cryo protectant and crystals were flash frozen in liquid nitrogen prior to data collection at 100K. X-ray data was collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advanced Photon Source, Argonne National Laboratory. Resulting data were indexed, scaled, and merged using the HKL2000 program suite. 39
Structure Determination and Refinement
The SaClpP crystal occupies the P 1 21 1 space group. Initial phases were established through molecular replacement using PHASER Software. 40 A single heptamer from S. aureus ClpP (PDB:3STA) was used as a search model with handle (residues 123-172), N-terminal loop regions (residues 1-21) and C-terminal (residues 182-192) removed to minimize model bias. 41 Two stacked heptameric rings were found in the asymmetric unit. Model building was then completed using COOT. 42 Refinement was completed using standard protocols in Refmac and phenix.refine. 43-44 Clear electron density was present in difference maps for the C-terminal and handle regions. In the N-terminal loop regions, electron density was not sufficient to identify the positions of all amino acids in the region.
Supplementary Material
Supplementary Table 1 Data reduction and refinement
Supplementary Figure 1 Ligand Discovery Electron Density Maps
Supplementary Figure 2 Correlation between MIC and EC50
Supplementary Table 2 Gram positive minimal inhibitory concentrations
Supplementary Table 3. Plasma stability
Acknowledgements
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R44AI122426 and R01AI110578, and by ALSAC, St. Jude Children’s Research Hospital.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health or other funding agencies.
Crystallographic support was provided by the St. Jude X-ray Center. Data were collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID beamline at the Advanced Photon Source, Argonne National Laboratory. SER-CAT is supported by its member institutions (see www.ser-cat.org/members.html), and equipment grants (S10_RR25528 and S10_RR028976) from the National Institutes of Health. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38.
Footnotes
PDB ID Codes
SaClpP-ADEP4 PDB 6PMD
SaClpP-Compound 2 PDB 5VZ2
SaClpP-Compound 5 PDB 5W18
SaClpP-Compound 16 PDB 6PKA
References
- 1.Kourtis AP; Hatfield K; Baggs J; Mu Y; See I; Epson E; Nadle J; Kainer MA; Dumyati G; Petit S; Ray SM; Emerging Infections Program, M. a. g.; Ham D; Capers C; Ewing H; Coffin N; McDonald LC; Jernigan J; Cardo D, Vital Signs: Epidemiology and Recent Trends in Methicillin-Resistant and in Methicillin-Susceptible Staphylococcus aureus Bloodstream Infections - United States. MMWR Morb Mortal Wkly Rep 2019, 68 (9), 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L; Costerton JW; Stoodley P, Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004, 2 (2), 95–108. [DOI] [PubMed] [Google Scholar]
- 3.Conlon BP; Rowe SE; Lewis K, Persister cells in biofilm associated infections. Adv Exp Med Biol 2015, 831, 1–9. [DOI] [PubMed] [Google Scholar]
- 4.Lewis K, Persister cells: molecular mechanisms related to antibiotic tolerance. Handb Exp Pharmacol 2012, (211), 121–33. [DOI] [PubMed] [Google Scholar]
- 5.Fauvart M; De Groote VN; Michiels J, Role of persister cells in chronic infections: clinical relevance and perspectives on anti-persister therapies. J Med Microbiol 2011, 60 (Pt 6), 699–709. [DOI] [PubMed] [Google Scholar]
- 6.Hoiby N; Bjarnsholt T; Moser C; Bassi GL; Coenye T; Donelli G; Hall-Stoodley L; Hola V; Imbert C; Kirketerp-Moller K; Lebeaux D; Oliver A; Ullmann AJ; Williams C; Biofilms, E. S. G. f.; Consulting External Expert Werner, Z., ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect 2015, 21 Suppl 1, S1–25. [DOI] [PubMed] [Google Scholar]
- 7.Gersch M; Famulla K; Dahmen M; Gobl C; Malik I; Richter K; Korotkov VS; Sass P; Rubsamen-Schaeff H; Madl T; Brotz-Oesterhelt H; Sieber SA, AAA+ chaperones and acyldepsipeptides activate the ClpP protease via conformational control. Nat Commun 2015, 6, 6320. [DOI] [PubMed] [Google Scholar]
- 8.Brotz-Oesterhelt H; Beyer D; Kroll HP; Endermann R; Ladel C; Schroeder W; Hinzen B; Raddatz S; Paulsen H; Henninger K; Bandow JE; Sahl HG; Labischinski H, Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med 2005, 11 (10), 1082–7. [DOI] [PubMed] [Google Scholar]
- 9.Conlon BP; Nakayasu ES; Fleck LE; LaFleur MD; Isabella VM; Coleman K; Leonard SN; Smith RD; Adkins JN; Lewis K, Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503 (7476), 365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown Gandt A; Griffith EC; Lister IM; Billings LL; Han A; Tangallapally R; Zhao Y; Singh AP; Lee RE; LaFleur MD, In Vivo and In Vitro Effects of a ClpP-Activating Antibiotic against Vancomycin-Resistant Enterococci. Antimicrob Agents Chemother 2018, 62 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye F; Li J; Yang CG, The development of small-molecule modulators for ClpP protease activity. Mol Biosyst 2016, 13 (1), 23–31. [DOI] [PubMed] [Google Scholar]
- 12.Michel KH; Ralph EK; Eli L; Co L A54556 Antibiotics and process for production thereof. 1985-01-08, 1985. [Google Scholar]
- 13.Lavey NP; Shadid T; Ballard JD; Duerfeldt AS, Clostridium difficile ClpP Homologues are Capable of Uncoupled Activity and Exhibit Different Levels of Susceptibility to Acyldepsipeptide Modulation. ACS Infect Dis 2019, 5 (1), 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Famulla K; Sass P; Malik I; Akopian T; Kandror O; Alber M; Hinzen B; Ruebsamen-Schaeff H; Kalscheuer R; Goldberg AL; Brotz-Oesterhelt H, Acyldepsipeptide antibiotics kill mycobacteria by preventing the physiological functions of the ClpP1P2 protease. Mol Microbiol 2016, 101 (2), 194–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hinzen B; Raddatz S; Paulsen H; Lampe T; Schumacher A; Habich D; Hellwig V; Benet-Buchholz J; Endermann R; Labischinski H; Brotz-Oesterhelt H, Medicinal chemistry optimization of acyldepsipeptides of the enopeptin class antibiotics. ChemMedChem 2006, 1 (7), 689–93. [DOI] [PubMed] [Google Scholar]
- 16.Kirstein J; Hoffmann A; Lilie H; Schmidt R; Rubsamen-Waigmann H; Brotz-Oesterhelt H; Mogk A; Turgay K, The antibiotic ADEP reprogrammes ClpP, switching it from a regulated to an uncontrolled protease. EMBO Mol Med 2009, 1 (1), 37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu AY; Houry WA, ClpP: a distinctive family of cylindrical energy-dependent serine proteases. FEBS Lett 2007, 581 (19), 3749–57. [DOI] [PubMed] [Google Scholar]
- 18.Woo KM; Chung WJ; Ha DB; Goldberg AL; Chung CH, Protease Ti from Escherichia coli requires ATP hydrolysis for protein breakdown but not for hydrolysis of small peptides. J Biol Chem 1989, 264 (4), 2088–91. [PubMed] [Google Scholar]
- 19.Wang J; Hartling JA; Flanagan JM, The structure of ClpP at 2.3 A resolution suggests a model for ATP-dependent proteolysis. Cell 1997, 91 (4), 447–56. [DOI] [PubMed] [Google Scholar]
- 20.Kim YI; Levchenko I; Fraczkowska K; Woodruff RV; Sauer RT; Baker TA, Molecular determinants of complex formation between Clp/Hsp100 ATPases and the ClpP peptidase. Nat Struct Biol 2001, 8 (3), 230–3. [DOI] [PubMed] [Google Scholar]
- 21.Frees D; Gerth U; Ingmer H, Clp chaperones and proteases are central in stress survival, virulence and antibiotic resistance of Staphylococcus aureus. Int J Med Microbiol 2014, 304 (2), 142–9. [DOI] [PubMed] [Google Scholar]
- 22.Lee BG; Park EY; Lee KE; Jeon H; Sung KH; Paulsen H; Rubsamen-Schaeff H; Brotz-Oesterhelt H; Song HK, Structures of ClpP in complex with acyldepsipeptide antibiotics reveal its activation mechanism. Nat Struct Mol Biol 2010, 17 (4), 471–8. [DOI] [PubMed] [Google Scholar]
- 23.Li DH; Chung YS; Gloyd M; Joseph E; Ghirlando R; Wright GD; Cheng YQ; Maurizi MR; Guarne A; Ortega J, Acyldepsipeptide antibiotics induce the formation of a structured axial channel in ClpP: A model for the ClpX/ClpA-bound state of ClpP. Chemistry & biology 2010, 17 (9), 959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin A; Baker TA; Sauer RT, Pore loops of the AAA plus ClpX machine grip substrates to drive translocation and unfolding. Nat Struct Mol Biol 2008, 15 (11), 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li YX; Lavey NP; Coker JA; Knobbe JE; Truong DC; Yu HT; Lin YS; Nimmo SL; Duerfeldt AS, Consequences of Depsipeptide Substitution on the CIpP Activation Activity of Antibacterial Acyldepsipeptides. Acs Med Chem Lett 2017, 8 (11), 1171–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carney DW; Schmitz KR; Truong JV; Sauer RT; Sello JK, Restriction of the Conformational Dynamics of the Cyclic Acyldepsipeptide Antibiotics Improves Their Antibacterial Activity. J Am Chem Soc 2014, 136 (5), 1922–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvanitis M; Li G; Li DD; Cotnoir D; Ganley-Leal L; Carney DW; Sello JK; Mylonakis E, A Conformationally Constrained Cyclic Acyldepsipeptide Is Highly Effective in Mice Infected with Methicillin-Susceptible and -Resistant Staphylococcus aureus. PLoS One 2016, 11 (4), e0153912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socha AM; Tan NY; LaPlante KL; Sello JK, Diversity-oriented synthesis of cyclic acyldepsipeptides leads to the discovery of a potent antibacterial agent. Bioorgan Med Chem 2010, 18 (20), 7193–7202. [DOI] [PubMed] [Google Scholar]
- 29.Baillie TA, Targeted Covalent Inhibitors for Drug Design. Angew Chem Int Ed Engl 2016, 55 (43), 13408–13421. [DOI] [PubMed] [Google Scholar]
- 30.Friesner RA; Murphy RB; Repasky MP; Frye LL; Greenwood JR; Halgren TA; Sanschagrin PC; Mainz DT, Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 2006, 49 (21), 6177–96. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Z; Parsons JB; Tangallapally R; Zhang W; Rock CO; Lee RE, Discovery of novel bacterial elongation condensing enzyme inhibitors by virtual screening. Bioorg Med Chem Lett 2014, 24 (11), 2585–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz KR; Carney DW; Sello JK; Sauer RT, Crystal structure of Mycobacterium tuberculosis ClpP1P2 suggests a model for peptidase activation by AAA+ partner binding and substrate delivery. Proc Natl Acad Sci U S A 2014, 111 (43), E4587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong KS; Mabanglo MF; Seraphim TV; Mollica A; Mao YQ; Rizzolo K; Leung E; Moutaoufik MT; Hoell L; Phanse S; Goodreid J; Barbosa LRS; Ramos CHI; Babu M; Mennella V; Batey RA; Schimmer AD; Houry WA, Acyldepsipeptide Analogs Dysregulate Human Mitochondrial ClpP Protease Activity and Cause Apoptotic Cell Death. Cell Chem Biol 2018, 25 (8), 1017-+. [DOI] [PubMed] [Google Scholar]
- 34.Goodreid JD; Janetzko J; Santa Maria JP Jr.; Wong KS; Leung E; Eger BT; Bryson S; Pai EF; Gray-Owen SD; Walker S; Houry WA; Batey RA, Development and Characterization of Potent Cyclic Acyldepsipeptide Analogues with Increased Antimicrobial Activity. J Med Chem 2016, 59 (2), 624–46. [DOI] [PubMed] [Google Scholar]
- 35.Ni T; Ye F; Liu X; Zhang J; Liu H; Li J; Zhang Y; Sun Y; Wang M; Luo C; Jiang H; Lan L; Gan J; Zhang A; Zhou H; Yang CG, Characterization of Gain-of-Function Mutant Provides New Insights into ClpP Structure. Acs Chem Biol 2016, 11 (7), 1964–72. [DOI] [PubMed] [Google Scholar]
- 36.North EJ; Scherman MS; Bruhn DF; Scarborough JS; Maddox MM; Jones V; Grzegorzewicz A; Yang L; Hess T; Morisseau C; Jackson M; McNeil MR; Lee RE, Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem 2013, 21 (9), 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swift ML, GraphPad Prism, Data Analysis, and Scientific Graphing. Journal of Chemical Information and Computer Sciences 1997, 37 (2), 411–412. [Google Scholar]
- 38.Quinn JG, Modeling Taylor dispersion injections: determination of kinetic/affinity interaction constants and diffusion coefficients in label-free biosensing. Anal Biochem 2012, 421 (2), 391–400. [DOI] [PubMed] [Google Scholar]
- 39.Otwinowski Z; Minor W, Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 1997, 276, 307–26. [DOI] [PubMed] [Google Scholar]
- 40.McCoy AJ; Grosse-Kunstleve RW; Adams PD; Winn MD; Storoni LC; Read RJ, Phaser crystallographic software. J Appl Crystallogr 2007, 40 (Pt 4), 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J; Ye F; Lan L; Jiang H; Luo C; Yang CG, Structural switching of Staphylococcus aureus Clp protease: a key to understanding protease dynamics. J Biol Chem 2011, 286 (43), 37590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Emsley P; Lohkamp B; Scott WG; Cowtan K, Features and development of Coot. Acta Crystallogr D Biol Crystallogr 2010, 66 (Pt 4), 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afonine PV; Grosse-Kunstleve RW; Echols N; Headd JJ; Moriarty NW; Mustyakimov M; Terwilliger TC; Urzhumtsev A; Zwart PH; Adams PD, Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 2012, 68 (Pt 4), 352–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vagin AA; Steiner RA; Lebedev AA; Potterton L; McNicholas S; Long F; Murshudov GN, REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol Crystallogr 2004, 60 (Pt 12 Pt 1), 2184–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1 Data reduction and refinement
Supplementary Figure 1 Ligand Discovery Electron Density Maps
Supplementary Figure 2 Correlation between MIC and EC50
Supplementary Table 2 Gram positive minimal inhibitory concentrations
Supplementary Table 3. Plasma stability