Abstract
In contrast to hematological malignancies, meaningful improvements in survival statistics for patients with malignant brain tumors have not been realized in >40 years of clinical research. Clearly, a new medical approach to brain cancers is needed. Recent research has led to a new concept that needs to destroy all cancer subclones to control the cancer progression. However, this new concept fails to distinguish the difference between dominating subclones and dormant subclones. Here, we address the issue of clonal switch and emphasize that there may be one or more than one dominant clones within the tumor mass at any time. Destructing one dominant clone triggers activating other dormant subclones to become dominating subclones, causing cancer progress and post-treatment cancer recurrence. We postulate the concept of subclonal switchboard signaling and the pathway that involved in this process. In the context of stem cell and development, there is a parallel with the concept of quiescent/dormant cancer stem cells (CSC) and their progeny, the differentiated cancer cells; these 2 populations communicate and co-exist. The mechanism with which determines to extend self-renewal and expansion of CSC is needed to elucidate. We suggest eliminating the “dominating subclonal switchboard signals” that shift the dormant subclones to dominating subclones as a new strategy.
Background
The long-term survival for patients with solid cancers has remained almost zero even though multiple billion dollars have been spent since U.S. President Richard Nixon declared war on cancer in 1971 [1]. Over 1.4 million people in the United States were found to have cancer in 2007, and the national cost of the disease was over $206 billion in 2006, accounting one-third of healthcare dollars (total: $686 billion) spent in the United States [2]. An estimated 18,820 new cases of brain cancer were diagnosed in the United States of America in 2006, and >12,000 would die of the disease (data from the National Cancer Institute of the United States of America). Our current forms of therapy for these diseases are brain surgery, followed by administration of toxic drugs and exposure to radiation, which lead that the patients face challenges because of both the effects of treatment and potential neurological dysfunction. Overall, the cost of care per patient was $67,887, with accrued mean monthly healthcare costs that were 20 times higher than demographically similar individuals without cancer ($6,364 vs. $277) [3]. Malignant gliomas are, for all practical purposes, incurable and new therapeutic approaches are desperately needed. Darren J. Burgess suggested that developing therapies that would target all subclones should be the future direction in Research Highlights [3]. However, we would argue that we should block the subclonal switchboard signals (SSS) that shift dominating subclones on disease progression and post-treatment. Here, we discuss the SSS hypothesis and its implication for new therapies.
The Hypotheses
Burgess' conclusion was based on 2 Nature articles on genetic complexity and heterogeneity of cancer. Anderson and colleagues found that the classic model of the linear clonal evolution could not explain their data because multiple subpopulations (also known as subclones) co-exist [4]. Burgess proposed a hypothesis, “genetic heterogeneity and the branching evolutionary trajectories,” to explain the Darwinian perspective of evolving these leukemia-initiating cells [3]. Surprisingly, Notta and colleague reported that these co-existed subclones of many leukemia patient samples co-evolve during disease progression and post-treatment relapse by shifting subclonal dominance [5]. Most importantly, this shifting subclonal dominance can reproduce by using transplantation assays.
Here, we provide a new hypothesis that this shifting subclonal dominance is controlled by the subclonal SSS (Fig. 1). Using experimental models [3,5], we can decipher these SSS, so we can specifically block their signal transduction and stop the subclonal switchboard function. However, we must be ready to co-exist with the cancer cells in our body. These cancer stem cells (CSC) may be not detrimental as long as we can keep them in surveillance.
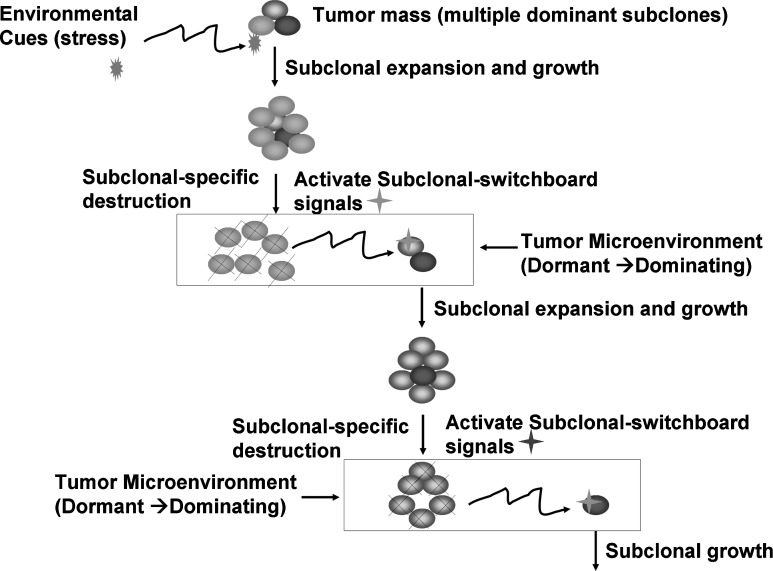
FIG. 1.
Subclonal switchboard signals (SSS) as mechanisms for leading to shift dominating subclones as triggered by environmental cues (stress) for cancer progression and post-treatment. A cancer subclone may gain a mutation that, in the appropriate environment cue, leads to dominating subclonal activation due to positive selection. Showed lettering and lines/arrows in black color is the current concept of treatment strategy for cancer-dominant subclonal cells (cancer stem cells) that may acquire a mutation, in the suitable environment, triggering to dominating subclonal expansion and growth. When this dominating subclone is specifically destroyed, it sends out dominating subclonal-SSS to a dormant/quiescent subclonal cell, which gets activated for dominating subclonal expansion and growth.
Emerging evidence supports the SSS concept. Cancer cells have been traditionally treated as invading aliens, which must be completely destroyed and removed. We may, however, argue for the need to view cancer differently from traditionally. We and others have found that similarities and overlapping mechanisms between induced cell plasticity and cancer formation shed new light on the emerging picture of p53 sitting at the crossroads between 2 intricate cellular potentials: stem cell versus cancer cell generation [6] and regulating the quiescence and self-renewal of hematopoietic stem cells [7]. We may over-react toward cancer cells, “the invading aliens,” which lead to over-treating and injure our own body by using aggressive multi-modalities (surgery, radiation, and chemotherapies) [8]. Perhaps, we ought to consider that cancer cells share similar citizenship, demanding to survive on the Earth because their survivorship is driven by their evolutional driving force [9]. Sustaining the biodiversity and heterogeneity may balance organisms or organs out of the hostile environment [10,11]. As such, managing tumor growth rather than eliminating it should be a new guideline for treating tumors. The eliminating-cancer treatments drive producing populations of drug-resistant tumor cells upon eliminating the drug-sensitive cells while managing tumor growth to treat tumors with minimum doses of drug so as to modulate the survival of some drug-sensitive cells [12,13]. This treatment paradigm may help the drug-sensitive cells out-compete the resistant ones upon completion of drug treatment, thereby keeping tumors alive but small and manageable [1,14].
“Keeping tumors alive but small and manageable” sounds a reasonable strategy. However, how can we manage tumor growth rather than eliminate it? We argue that managing SSS may be an effective strategy.
Implications of the Hypothesis
Understanding the mechanisms for SSS will provide new insights to develop anti-cancer therapies. The SSS may be activated upon eliminating a drug-sensitive dominant subclonal population, and SSS may activate a neighboring dormant subclone within a microenvironment (tumor) or traffic out of the tumor microenvironment (Fig. 2). Upon characterization of SSS, we can better detect and control the outbreaks of SSS-driven cancers with defensive strategies for disruptions of SSS signal transduction during different stages of tumorigenesis and cancer progression.
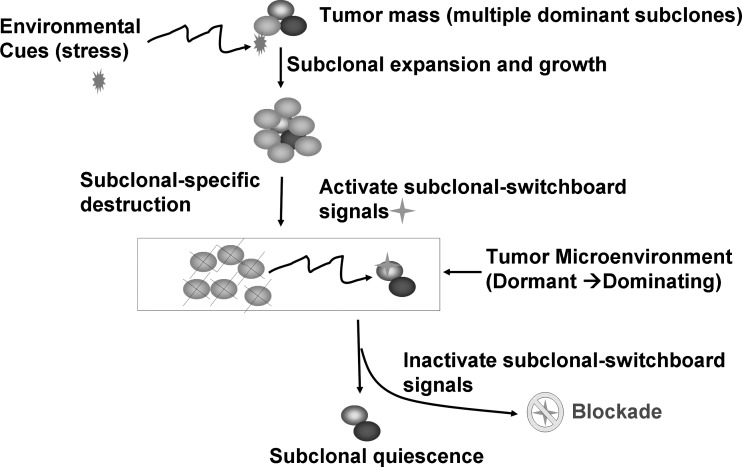
FIG. 2.
Blockade of the dominating subclonal SSS as a new therapeutic strategy to suppress the dominating subclone shift to control cancer progression and post-treatment cancer recurrence. Showed is the proposed new treatment paradigm that should target the subclonal-SSS. Blocking the dominating subclonal SSS leads to subclonal quiescence, so keeping tumors alive but small and manageable (dormant/quiescent subclone).
A defining feature of SSS system is the presence of multiple structural elements specializing in distinct biological functions. As a general rule, these elements can stably maintain their identity over long periods despite fluctuations in their external physiological environment and internal regulatory networks [15]. Tumor–tissue barrier (physical boundary) may maintain the intra-tumor pressure during tumor development [16]. The quiescent/dormant CSC and their progeny, the differentiated cancer cells, may communicate and co-exist within the tumor microenvironment. Leakage of the tumor–tissue barrier via treatments (surgery, radiation, and chemotherapy) may lead to change the intra-tumor pressure that may act as a physical SSS signal to wake up a dormant subclone. How this is achieved at the molecular level is a central but poorly understood question. An interesting finding was that the matrix elasticity (physical signal) activates the stem cell differentiation signal pathway, directing the organ-specific stem cell lineage specification [17–19]. The nature of the chemical-based SSS signals and the pathways that involved in the signaling process are not well understood. Some examples of the SSS signals may be cytokines [20,21], growth factors [22], angiogenesis factors [23,24], and the balance of their expression levels [25]. Indeed, irradiation of mouse bone marrow stromal cell lines induces release of significant levels of transforming growth factor (TGF)-beta into the tissue culture medium despite the lack of a detectable increase in TGF-beta mRNA [26]. TGF-beta regulates the coexistence and interconvertibility of CSCs that sustain tumor growth through their ability to self-renew and to generate differentiated progeny [27].
It is possible that SSS is maintained by modifying host gene products. This model predicts, for example, that many host gene products changes after the host is treated by an anti-tumor agent. This is in line with the recognition that there is a combined effect of 2 distinct inputs: positive and negative. The positive inputs from the SSS may be to activate biological events that favor the host, and the negative inputs may be detrimental to the host.
We can design a comprehensive strategy that allows the systematic identification of those inputs from the SSS. Specifically, we can fundamentally define the SSS by execution of the following experiments:
1. assess the time-course of dominant subclonal formation in vivo;
2. determine the biochemical nature of SSS molecules upon dominant subclonal destruction;
3. activate the dormant (quiescent) cancer subclone using SSS molecules.
The SSS hypothesis has broad impact on many areas of biology and medicine, including developmental biology, stem cell biology, cancer biology, aging, epigenetics, functional genomics, systems biology, regenerative medicine, molecular diagnostics, and drug discovery. For example, it implies that cancers and neurodegenerative disorders may be governed by the same SSS mechanism, which escapes the host surveillance system for their subclonal reproduction. The organizational structure of normal epithelium may be such a host surveillance system [12], and the tumor microenvironment may evolve in the SSS production and traffic [28]. Noninvasive monitoring of intra-tumor SSS behavior in vivo is potentially useful for evaluating the efficacy of individual treatment responses with prognostic value in the clinic [29]. However, understanding the SSS-evolved host surveillance for cancer suppression mechanisms is essential to defining the first steps of tumorigenesis and developing rational cancer prevention strategies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Drake N. Forty years on from Nixon's war, cancer research “evolves”. Nat Med. 2011;17:757. doi: 10.1038/nm0711-757. [DOI] [PubMed] [Google Scholar]
- 2.Li SC. Loudon WG. A novel and generalizable organotypic slice platform to evaluate stem cell potential for targeting pediatric brain tumors. Cancer Cell Int. 2008;8:9. doi: 10.1186/1475-2867-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgess DJ. Cancer genetics: initially complex, always heterogeneous. Nat Rev Genet. 2011;12:154–155. doi: 10.1038/nrg2965. [DOI] [PubMed] [Google Scholar]
- 4.Anderson K. Lutz C. van Delft FW. Bateman CM. Guo Y. Colman SM. Kempski H. Moorman AV. Titley I, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469:356–361. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 5.Notta F. Mullighan CG. Wang JC. Poeppl A. Doulatov S. Phillips LA. Ma J. Minden MD. Downing JR. Dick JE. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469:362–367. doi: 10.1038/nature09733. [DOI] [PubMed] [Google Scholar]
- 6.Li SC. Jin Y. Loudon WG. Song Y. Ma Z. Weiner LP. Zhong JF. From the Cover: increase developmental plasticity of human keratinocytes with gene suppression. Proc Natl Acad Sci USA. 2011;108:12793–12798. doi: 10.1073/pnas.1100509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai T. Liu Y. Bae N. Nimer SD. The p53 tumor suppressor protein regulates hematopoietic stem cell fate. J Cell Physiol. 2011;226:2215–2221. doi: 10.1002/jcp.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gatenby RA. Silva AS. Gillies RJ. Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–4903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andre N. Pasquier E. For cancer, seek and destroy or live and let live? Nature. 2009;460:324. doi: 10.1038/460324c. [DOI] [PubMed] [Google Scholar]
- 10.Kunin V. Ouzounis CA. The balance of driving forces during genome evolution in prokaryotes. Genome Res. 2003;13:1589–1594. doi: 10.1101/gr.1092603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatenby RA. Application of competition theory to tumour growth: implications for tumour biology and treatment. Eur J Cancer. 1996;32A:722–726. doi: 10.1016/0959-8049(95)00658-3. [DOI] [PubMed] [Google Scholar]
- 12.Gatenby RA. Gillies RJ. Brown JS. Evolutionary dynamics of cancer prevention. Nat Rev Cancer. 2011;10:526–527. doi: 10.1038/nrc2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatenby RA. Frieden BR. Inducing catastrophe in malignant growth. Math Med Biol. 2008;25:267–283. doi: 10.1093/imammb/dqn014. [DOI] [PubMed] [Google Scholar]
- 14.Gatenby RA. A change of strategy in the war on cancer. Nature. 2009;459:508–509. doi: 10.1038/459508a. [DOI] [PubMed] [Google Scholar]
- 15.Perfahl H. Byrne HM. Chen T. Estrella V. Alarcon T. Lapin A. Gatenby RA. Gillies RJ. Lloyd MC, et al. Multiscale modelling of vascular tumour growth in 3D: the roles of domain size and boundary conditions. PloS ONE. 2011;6:e14790. doi: 10.1371/journal.pone.0014790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucher Y. Salehi H. Witwer B. Harsh GR., 4th Jain RK. Interstitial fluid pressure in intracranial tumours in patients and in rodents. Br J Cancer. 1997;75:829–836. doi: 10.1038/bjc.1997.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engler AJ. Carag-Krieger C. Johnson CP. Raab M. Tang HY. Speicher DW. Sanger JW. Sanger JM. Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engler AJ. Griffin MA. Sen S. Bonnemann CG. Sweeney HL. Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engler AJ. Sen S. Sweeney HL. Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Fukuro H. Mogi C. Yokoyama K. Inoue K. Change in expression of basic fibroblast growth factor mRNA in a pituitary tumor clonal cell line. Endocr Pathol. 2003;14:145–149. doi: 10.1385/ep:14:2:145. [DOI] [PubMed] [Google Scholar]
- 21.Razmkhah M. Jaberipour M. Erfani N. Habibagahi M. Talei AR. Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-beta1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011;266:116–122. doi: 10.1016/j.cellimm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Nazarenko I. Hedren A. Sjodin H. Orrego A. Andrae J. Afink GB. Nister M. Lindstrom MS. Brain abnormalities and glioma-like lesions in mice overexpressing the long isoform of PDGF-A in astrocytic cells. PloS ONE. 2011;6:e18303. doi: 10.1371/journal.pone.0018303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollard PJ. Spencer-Dene B. Shukla D. Howarth K. Nye E. El-Bahrawy M. Deheragoda M. Joannou M. McDonald S, et al. Targeted inactivation of fh1 causes proliferative renal cyst development and activation of the hypoxia pathway. Cancer Cell. 2007;11:311–319. doi: 10.1016/j.ccr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Legros L. Bourcier C. Jacquel A. Mahon FX. Cassuto JP. Auberger P. Pages G. Imatinib mesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood. 2004;104:495–501. doi: 10.1182/blood-2003-08-2695. [DOI] [PubMed] [Google Scholar]
- 25.Scheel C. Eaton EN. Li SH. Chaffer CL. Reinhardt F. Kah KJ. Bell G. Guo W. Rubin J, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greenberger JS. Epperly MW. Jahroudi N. Pogue-Geile KL. Berry L. Bray J. Goltry KL. Role of bone marrow stromal cells in irradiation leukemogenesis. Acta Haematol. 1996;96:1–15. doi: 10.1159/000203708. [DOI] [PubMed] [Google Scholar]
- 27.Schober M. Fuchs E. Tumor-initiating stem cells of squamous cell carcinomas and their control by TGF-beta and integrin/focal adhesion kinase (FAK) signaling. Proc Natl Acad Sci USA. 2011;108:10544–10549. doi: 10.1073/pnas.1107807108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HO. Silva AS. Concilio S. Li YS. Slifker M. Gatenby RA. Cheng JD. Evolution of tumor invasiveness: the adaptive tumor microenvironment landscape model. Cancer Res. 2011;71:6327–37. doi: 10.1158/0008-5472.CAN-11-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmer GM. Boruta RJ. Viglianti BL. Lan L. Spasojevic I. Dewhirst MW. Non-invasive monitoring of intra-tumor drug concentration and therapeutic response using optical spectroscopy. J Control Release. 2011;142:457–464. doi: 10.1016/j.jconrel.2009.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]