Abstract
Background
High-resolution ultrasound (US) is the primary tool used to identify locoregional recurrences in differentiated thyroid cancer. Although small thyroid bed (TB) nodules are a commonly reported sonographic finding, their natural history, regardless of whether they are benign or malignant, has not been well characterized. This study was designed to determine the likelihood, magnitude, and rate of growth of small TB nodules identified on routine surveillance neck US after thyroidectomy for differentiated thyroid cancer as well as to identify ultrasonographic and clinical predictors of growth.
Methods
This retrospective review identified 191 patients with at least one TB nodule (≤11 mm) on the first postoperative US performed at a comprehensive cancer center. Change in size of each TB nodule was determined using serial US studies over time. Clinicopathologic and sonographic characteristics were analyzed as possible predictors for growth of the TB nodules.
Results
Over a median clinical follow-up of 5 years, 9% (17/191) of patients had increase in size of at least one TB nodule. Median size of the TB nodules was 5 mm (range: 2–11 mm). Suspicious US features were seen in 63% (121/191) of patients with TB nodules identified on initial US and in 31% (21/67) of those with TB nodules detected on subsequent follow-up US. The rate of growth was 1.3 mm/year in those nodules showing an increase in size and thus demonstrated a significant increase in size only after several years of follow-up. The negative predictive values associated with the absence of any suspicious US features (0.97), the absence of abnormal cervical lymph nodes (0.94), and the lack of a rising serum thyroglobulin (0.93) provided clinically useful information regarding the likelihood that nodules would not increase in size.
Conclusion
Most TB nodules do not show clinically significant growth over several years of follow-up. Thus, TB nodules can be followed up with cautious observation and serial ultrasonography using an approach similar to that recommended by the American Thyroid Association thyroid cancer guidelines for the management of small abnormal cervical lymph nodes.
Introduction
As the risk of locoregional recurrence of papillary thyroid cancer (PTC) in either cervical lymph nodes or in the thyroid bed (TB) ranges from 15% to 25% (1–3), careful structural evaluation of the neck is a key component of the follow-up paradigm in differentiated thyroid cancer (DTC). In recent years, high-resolution cervical ultrasound (US) has become the primary tool in the identification of locoregional thyroid cancer recurrence (4–6). According to the Revised American Thyroid Association (ATA) thyroid cancer guidelines, cervical US to evaluate the TB, central, and lateral cervical nodal compartments should be performed 6–12 months after initial surgery and then periodically, depending on the patient's risk for recurrence and thyroglobulin (Tg) status (recommendation 48a) (7).
In addition to identifying normal and abnormal cervical lymph nodes, ultrasonography often finds small discrete nodules in the postoperative TB. Most of these TB nodules are benign lesions due to postoperative fibrosis or suture granulomas; however, some represent persistent thyroid cancer (8,9). Although the ultrasonographic appearance of cervical lymphadenopathy in DTC patients is useful in distinguishing benign from malignant cervical lymph nodes (microcalcifications, cystic changes, rounded shape, abnormal vascularity, and loss of echogenic fatty hilum) (10–16), these sonographic features do not accurately predict which small TB nodules are malignant (9,17–19). Further, the natural history and rate of growth of these TB nodules (either benign or malignant) has not been well characterized.
Although the revised ATA guidelines allow for observation without fine-needle aspiration (FNA) of small abnormal cervical lymph nodes (7), there are no specific recommendations regarding the management of small TB nodules. Because these nodules are small and located within TB scar tissue, attempted surgical resection could be associated with more harm than good. Further, treatment with radioactive iodine (RAI) would not be warranted if the TB nodules are benign and may or may not be effective even if they represent persistent thyroid cancer. Therefore, it is important to determine how many of these small TB nodules (whether benign or malignant) increase in size over time and to identify clinical and ultrasonographic features that may be predictive of nodule growth.
To that end, this study was designed to describe the structural changes over time in small TB nodules identified on routine surveillance neck ultrasonography after total thyroidectomy for DTC. Specifically, the aims of the study were to determine how many of these nodules increased in size over time, to describe the magnitude and rate of increase in size, and to identify specific ultrasonographic and clinical predictors of TB nodule growth.
Materials and Methods
Subjects
After obtaining approval from our institutional review board, we retrospectively reviewed the US reports of 1531 patients with DTC who had two or more cervical US studies during their follow-up at Memorial-Sloan Kettering Cancer Center between August 1998 and June 2009. Of the 1531 patients initially reviewed, 521 patients (34%) had TB nodules identified on a first postoperative US.
To be eligible for the study, patients had to have at least one small TB nodule (≤11 mm in their largest diameter) on the first posttotal thyroidectomy sonogram available at our institution and had to have at least two additional US studies during follow-up. A total of 191 patients met these inclusion criteria and are the basis of this study. Patients were excluded from the study for the following reasons: fewer than two additional US examinations done during follow-up (117 patients), TB nodules greater than 11 mm (69 patients), additional neck surgery after total thyroidectomy before the first neck US (33 patients), not enough data on follow-up or follow-up at an outside institution (68 patients), less than total thyroidectomy was performed as the initial surgery (39 patients), pediatric patient (one patient), and medullary or anaplastic thyroid cancer (three patients). Patients receiving additional administered activities of RAI during follow-up were included in the study. Patients with other abnormal findings on the initial postoperative US (such as central or lateral compartments cervical lymph nodes) were included.
Ultrasound evaluation of TB nodules
Ultrasound examinations were performed according to standard protocol that included grayscale and color Doppler US assessment of the TB and cervical lymph nodes in all compartments. Examinations were performed by technologists and supervised by experienced attending radiologists, who reviewed images in real time. The US studies were performed with Siemens Acuson Sequoia (Siemens Medical, Mountain View, CA) units using 15 to 8 MHz linear 15L8w transducers.
US reports included size, shape, echogenicity, internal vascularity, and presence of microcalcifications of TB nodules and cervical lymph nodes. Size was defined as the largest diameter among the three dimensions provided, as previously considered in other reports on TB nodules (17). Echogenicity was characterized relative to strap muscles. Microcalcifications were defined as multiple punctate bright echoes with or without acoustic shadowing. Avascular nodules had absent flow. Thyroid bed nodules considered to have increased vascularity included those with either peripheral or intranodular vascular flow. Hypoechogenicity, internal vascular flow, and/or microcalcifications in TB nodules were classified as suspicious sonographic features, as previously reported in the literature (17).
Other imaging modalities used to characterize TB nodules
In some cases, RAI avidity of TB lesions was assessed by diagnostic RAI whole-body scanning. When 131I uptake was seen in the TB region, TB nodules were presumed to show RAI avidity and were classified as such. The same reasoning was applied for 18fluorodeoxyglucose (FDG) uptake in the TB area if a FDG-positron emission tomography scan was performed in the evaluation of the patient.
Pathologically proven disease
Nine of 191 patients had proven thyroid carcinoma either by cytology examination (US-guided FNA) or by histology after surgical reintervention in the central compartment. The remaining 182 patients had serial US follow-up without obtaining a definitive cytologic or histologic diagnosis.
Laboratory studies
All Tg values were measured using the Dynotest-TgS immunoradiometric assay (Brahms, Inc., Berlin, Germany; functional sensitivity 0.6 ng/mL normalized to Certified Reference Material [CRM] 457).
Clinical follow-up
Patients were usually followed every 6 months during the first year and at 6–12-month intervals thereafter at the discretion of the attending physician based on risk of recurrence of the individual patient and the clinical course of the disease, with Tg measurement on suppressive therapy and neck US. A diagnostic whole-body scan and a stimulated Tg value were ordered when deemed appropriate by the attending physician.
Clinical outcomes
Patients were considered to have no clinical evidence of disease (NED) at final follow-up if they had a suppressed serum Tg <0.6 ng/mL, no detectable TgAb, and no structural evidence of disease (20). Patients with suppressed Tg values ≥0.6 ng/mL, stimulated Tg values ≥2 ng/mL, or any evidence of disease on cross-sectional imaging (US, computed tomography scan, or magnetic resonance imaging), functional imaging (RAI scan or 18-FDG-positron emission tomography scan), or biopsy-proven disease (cytology or histology) were considered as having persistent disease. A recurrence was defined as new biochemical (suppressed Tg ≥0.6 ng/mL and/or stimulated Tg ≥2 ng/mL), structural, or functional evidence of disease that was detected after a period of NED. Patients dying of unrelated conditions had the final endpoint determined based on data available before their demise. Response to therapy, based on restratification 6–24 months after RAI therapy, was included as a clinical endpoint (20).
Outcome of TB nodules
Each TB nodule was classified as either showing increase in size or no increase in size (multiple TB nodules were often present within a single individual). Growth was defined as increase of ≥3 mm in the largest dimension when compared with the size of the nodule at initial detection. A 3 mm increase was selected, because this is the minimal size change that can be reproducibly determined by high-resolution US while minimizing operator-dependent differences in size assessment (21). US images of the cases showing structural progression were submitted for a blinded review to S.F., who is recognized specialist in thyroid US.
Statistical methods
Continuous data are presented as means and standard deviations with median values (ranges). Categoric comparisons were performed with Fisher's exact test. Analysis was performed using SPSS software (Version 18.0.1; SPSS, Inc., Chicago, IL). A p-value of ≤0.05 was considered statistically significant.
Results
Clinicopathologic features of the entire cohort
Table 1 presents the clinicopathologic features of the 191 DTC patients with small TB nodules (≤11 mm) detected on routine follow-up neck ultrasonography after total thyroidectomy that were included in this study. As expected, 96% had PTC and 78% were female. Initial therapy included central neck dissection (with or without lateral neck dissection) in 47% of the patients and RAI remnant ablation in 84% of the patients. At the time of final follow-up (mean: 7 years; median: 5 years), 59% were without evidence of disease, 17% had persistent biochemical evidence of disease without structural correlate, 11% were rendered NED after additional therapy, and 13% had persistent structural disease (of which, 96% was locoregional and 4% was pulmonary metastases). No patients died of thyroid cancer.
Table 1.
Description of the Cohort
| Age (years) | |
| Mean ± SD | 40 ± 14 |
| Median | 39 |
| Range | 16–78 |
| Gender | |
| Female | 78% |
| Histology | |
| Papillary | 96% |
| Poorly differentiated | 2% |
| Follicular | 2% |
| Tumor size (cm) | |
| Mean ± SD | 1.9 ± 1.3 |
| Median | 1.6 |
| Range | 0.2–9.0 |
| Extent of neck dissection at thyroidectomy | |
| Central only | 34% |
| Lateral only | 10% |
| Both central and lateral compartments | 13% |
| No lymph node dissection | 43% |
| AJCC stage | |
| I | 72% |
| II | 4% |
| III | 14% |
| IVa | 10% |
| ATA initial risk classification | |
| Low | 27% |
| Intermediate | 65% |
| High | 8% |
| Response to therapy classification | |
| Excellent | 1% |
| Acceptable | 67% |
| Incomplete | 24% |
| Cannot classify | 8% |
| RAI ablation | |
| Yes | 84% |
| No | 16% |
| 131I activity for ablation (mCi) | |
| Mean ± SD | 126 ± 45 |
| Median | 146 |
| Range | 28–350 |
| Follow-up duration (years) | |
| Mean ± SD | 7 ± 5 |
| Median | 5 |
| Range | 2–37 |
| Basal thyroglobulin at the time of first postoperative ultrasound (ng/mL) | |
| Mean ± SD | 4.8 ± 3.0 |
| Median | 0.6 |
| Range | <0.6–480 |
| Clinical status at final follow-up | |
| No evidence of disease | 59% |
| No evidence of disease after additional therapy | 11% |
| Biochemical persistent/recurrent disease | 17% |
| Structural persistent/recurrent disease | 13% |
| Disease-specific death | 0% |
| Number of ultrasounds on follow-up | |
| Mean ± SD | 5 ± 2 |
| Median | 4 |
| Range | 3–16 |
| Time from initial surgery to first postoperative ultrasound done at MSKCC (months) | |
| Mean ± SD | 33 ± 46 |
| Median | 18 |
| Range | 3–312 |
| Increase in size of at least one thyroid bed nodule over follow-up | 9% |
SD, standard deviation; AJCC, American Joint Committee on Cancer; ATA, American Thyroid Association; RAI, radioactive iodine; MSKCC, Memorial Sloan-Kettering Cancer Center.
A median of four US examinations (range: 3–16) were performed in these patients. Over a median clinical follow-up of 5 years, only 9% of the patients had an increase in size of 3 mm or more in at least one of their TB nodules.
Ultrasonographic features of the small TB nodules
Each of the 191 patients included in the study had at least one small TB nodule (range: 1–6 nodules) detected on the initial postoperative neck ultrasonographic examination. In addition, 35% of these patients (67/191) had at least one additional TB nodule (range: 1–9 additional nodules) detected on follow-up US. The specific ultrasonographic features are presented in Table 2. Median size of the TB nodules was about 5 mm (range: 2–11 mm). Suspicious US features (microcalcification, hypoechogenicity, or increased vascularity) were identified in 63% (121/191) of patients with TB nodules seen on initial US and in 31% (21/67) of patients with TB nodules detected on subsequent follow-up US.
Table 2.
Description of Thyroid Bed Nodules Identified on the First Postoperative Ultrasound and Thyroid Bed Nodules Identified on Follow-Up Ultrasounds
| Variable | Thyroid bed nodules detected on first postoperative US (n = 191 patients) | New thyroid bed nodules identified on follow-up US (n = 67/191 patients) |
|---|---|---|
| Number of nodules | ||
| Median (range) | 1 (1–6) | 1 (1–9) |
| Size of nodules (mm) | ||
| Median (range) | 5.7 (2–11) | 4.5 (2–11) |
| Avascular | 45% (86/190) | 22% (15/67) |
| Hypoechogenicity | 70% (85/121) | 57% (12/21) |
| Increased vascularity | 39% (47/121) | 38% (8/21) |
| Microcalcifications | 15% (18/121) | 19% (4/21) |
| Any suspicious sonographic featurea | 63% (121/191) | 31% (21/67) |
| RAI avidity in thyroid bed | 23% (9/40) | 20% (1/5) |
| FDG avidity in thyroid bed | 24% (5/21) | 29% (2/7) |
| Follow-up after detection of nodules (years) | ||
| Mean | 3.8 ± 2.1 | 2.7 ± 2.1 |
| Median | 3.0 | 2.1 |
| Range | 0.8–11.6 | 0–9.5 |
| Increase in size of at least one of the nodules | 8% (16/191) | 3% (2/67) |
Suspicious features defined as microcalcifications, hypoechogenicity, or increased vascularity in any thyroid bed nodule.
US, ultrasound; RAI, radioactive iodine; FDG, fluorodeoxyglucose.
Thyroid bed nodules showing an increase in size
After a median follow-up of 3 years from the time that the TB was detected, only 8% (16/191) of patients with TB nodules detected on the initial US examination demonstrated an increase in size of 3 mm or more. Similarly, over a median follow-up of 2.1 years, only 3% (2/67) of patients with TB nodules detected on subsequent US examination demonstrated an increase in size of the same magnitude. Because one patient had evidence of growth of a TB nodule present at the first postoperative US as well as a TB nodule detected at the subsequent US, a total of 17 patients (17/191, 9%) demonstrated change in size of at least one TB nodule detected at either initial or follow-up US.
Among the 17 patients who demonstrated an increase in size of at least one of their TB nodules, only three patients were evaluated with US-guided FNA. In each of these three cases, the FNA confirmed PTC. Two of these patients underwent central compartment neck dissection, and the third patient (whose TB nodule increased from 6 to 9 mm in the first 6 months of observation and then remained stable on all US examinations since then) has been followed with observation for 5 years.
The 14 remaining patients with TB nodule documented growth were observed with serial US, without receiving additional RAI therapy or surgery. However, early in the course of follow-up, before documented increase in size of TB nodules, a second administered activity of RAI was given to 6 of 17 patients for other evidence of persistent disease. No significant decrease in the size of the TB nodules was noted in response to the additional RAI therapy.
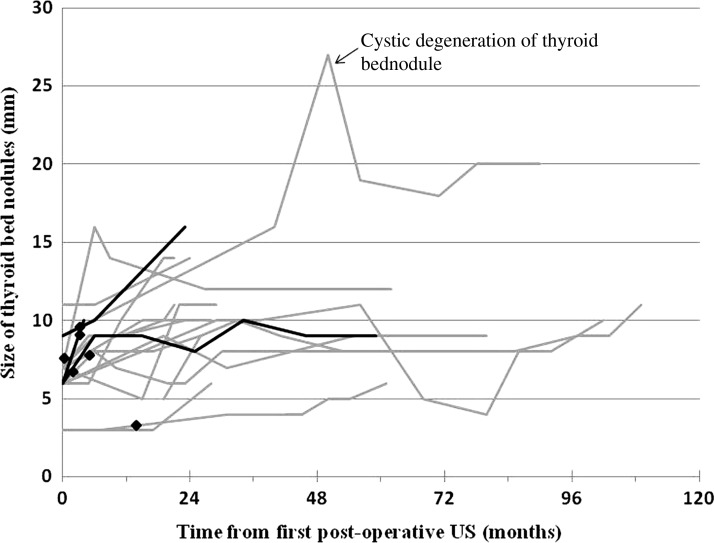
Figure 1 depicts the size of the TB nodules in the 17 patients who demonstrated at least one TB nodule increasing in size during follow-up. Many of the TB nodules showed only minor growth over several years of follow-up, often showing a waxing and waning growth pattern. In fact, the median rate of growth was 1.3 mm/year (range: 0.4–3.7 mm/year) in those few patients who had documented increase in the size of the TB nodules. The three patients who had TB nodules that were proven to be malignant are represented by darker lines. As several of the TB nodules in these 17 patients appeared to be increasing in size at the time of the last follow-up US, longer follow-up will be required to determine the ultimate outcome in this small subset of patients.
FIG. 1.
Thyroid bed nodules showing increase in size in 17 of 191 patients. There was growth of a least one of the thyroid bed nodules in 17 patients. Black lines represent patients with thyroid bed nodules proven to be thyroid cancer (n = 3). Gray lines represent patients with thyroid bed nodules that were not biopsied (n = 14). Radioactive iodine therapy is depicted as black diamonds.
However, one of the TB nodules showed a clinically significant increase in size from 9 mm at the first US, to 16 mm 40 months later, and then peaked at 27 mm before demonstrating cystic degeneration and decreasing to about 20 mm. Although not cytologically proven, the US characteristics of this lesion are highly suspicious for malignancy in this 26-year-old woman with longstanding persistent well-differentiated thyroid cancer who received multiple neck dissections and RAI therapies.
Factors predictive of TB nodule growth
Table 3 describes clinical and sonographic features predictive of growth in TB nodules detected on the first postoperative US or on subsequent US. The number of TB nodules detected at the first US (2.1 vs. 1.5, p = 0.01), presence of abnormal neck lymph nodes on US (17% vs. 6%, p = 0.02), any suspicious sonographic features (12% vs. 3%, p = 0.03), microcalcifications (28% vs. 10%, p = 0.05), and increased nodular vascularity (21% vs. 7%, p = 0.02) were all factors more likely to be present in nodules showing increase in size. An increasing Tg value over time was also more likely to be observed in association with growing TB nodules (24% vs. 7%, p = 0.05). Conversely, avascular TB nodules were statistically less likely to demonstrate growth than vascular nodules (3% vs. 13%, p = 0.02).
Table 3.
Clinical Features Predictive of Growth in Small Thyroid Bed Nodules Sonographically Detected Postthyroidectomy
| Size of thyroid bed nodule(s) | |||
|---|---|---|---|
| Variable | No increase (n = 174 patients) | Increase (n = 17 patients) | p-value |
| Age at diagnosis (years) | 40 ± 14 | 37 ± 14 | NS |
| Tumor size (cm) | 1.9 ± 1.3 | 2.1 ± 1.4 | NS |
| Ablation dose (mCi) | 126 ± 46 | 129 ± 39 | NS |
| Basal Tg at first US (ng/mL) | 2.3 ± 19 | 3.8 ± 6.8 | NS |
| RAI cumulative dose (mCi) | 196 ± 150 | 216 ± 132 | NS |
| Time to last follow-up (months) | 81 ± 57 | 90 ± 44 | NS |
| Number of ultrasounds on follow-up | 5 ± 2 | 6 ± 3 | NS |
| Number of nodules at first US | 1.5 ± 0.9 | 2.1 ± 0.9 | 0.01 |
| Mean nodule size at first US (mm) | 6 ± 2 | 6 ± 1 | NS |
| Time from first US to last US (months) | 45 ± 24 | 54 ± 32 | NS |
| Gender | |||
| Male (n = 43) | 88% | 12% | |
| Female (n = 148) | 92% | 8% | NS |
| Central neck dissection | |||
| Yes (n = 84) | 93% | 7% | |
| No (n = 97) | 90% | 10% | NS |
| Lateral neck dissection | |||
| Yes (n = 42) | 82% | 18% | |
| No (n = 139) | 91% | 9% | NS |
| Surgery location | |||
| Outside institution (n = 90) | 89% | 11% | |
| MSKCC (n = 100) | 93% | 7% | NS |
| RAI ablation | |||
| Yes (n = 161) | 91% | 9% | |
| No (n = 30) | 90% | 10% | NS |
| Preparation for RAI ablation | |||
| rhTSH (n = 95) | 93% | 7% | |
| THW (n = 27) | 89% | 11% | NS |
| AJCC | |||
| I (n = 136) | 89% | 11% | |
| II (n = 8) | 100% | 0% | |
| III (n = 27) | 96% | 4% | |
| IV (n = 19) | 95% | 5% | NS |
| ATA risk | |||
| Low (n = 51) | 90% | 10% | |
| Intermediate (n = 122) | 93% | 7% | |
| High (n = 14) | 79% | 21% | NS |
| Tg Ab present at first US | |||
| Yes (n = 24) | 100% | 0% | |
| No (n = 167) | 90% | 10% | NS |
| Increasing Tg over time | |||
| Yes (n = 17) | 76% | 24% | |
| No (n = 173) | 93% | 7% | 0.05 |
| Response to therapy | |||
| Acceptable (n = 128) | 94% | 6% | |
| Incomplete (n = 45) | 82% | 18% | |
| No RAI (n = 15) | 93% | 7% | |
| Excellent (n = 2) | 100% | 0% | NS |
| Presence of abnormal neck lymph nodes | |||
| Yes (n = 52) | 83% | 17% | |
| No (n = 139) | 94% | 6% | 0.02 |
| Any suspicious sonographic feature | |||
| Yes (n = 121) | 88% | 12% | |
| No (n = 70) | 97% | 3% | 0.03 |
| Microcalcifications | |||
| Yes (n = 18) | 72% | 28% | |
| No (n = 103) | 90% | 10% | 0.05 |
| Hypoechogenicity | |||
| Yes (n = 85) | 91% | 9% | |
| No (n = 36) | 81% | 19% | NS |
| Increased vascularity | |||
| Yes (n = 47) | 79% | 21% | |
| No (n = 74) | 93% | 7% | 0.02 |
| Avascular nodule | |||
| Yes (n = 86) | 97% | 3% | |
| No (n = 104) | 87% | 13% | 0.02 |
| RAI avid thyroid bed | |||
| Yes (n = 10) | 89% | 11% | |
| No (n = 31) | 87% | 13% | NS |
| FDG avid thyroid bed | |||
| Yes (n = 5) | 60% | 40% | |
| No (n = 16) | 75% | 25% | NS |
FDG, 18fluorodeoxyglucose; NS, not significant; Tg, thyroglobulin; rhTSH, recombinant human TSH; THW, thyroid hormone withdrawal.
From a clinical standpoint, none of the specific clinical or US features have a high-enough positive predictive value (range: 0.47–0.56) to reliably determine which nodules are likely to grow (Table 4). However, negative predictive values (NPVs) associated with the absence of any suspicious US features (NPV = 0.97), the absence of abnormal cervical lymph nodes (NPV = 0.94), and the lack of a rising serum Tg (NPV = 0.93) do provide reassuring information that a TB nodule is statistically less likely to increase in size. Further, the NPV associated with a combination of the absence of any suspicious US features, the absence of abnormal cervical lymph nodes, and the lack of a rising serum Tg was 0.96.
Table 4.
Predictors of Increase in Size of Thyroid Bed Nodules
| Clinical feature | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Any suspicious feature in thyroid bed nodulea | 0.80 | 0.52 | 0.12 | 0.97 |
| Microcalcifications in thyroid bed nodule | 0.74 | 0.56 | 0.28 | 0.90 |
| Increased vascularity in thyroid bed nodule | 0.75 | 0.54 | 0.21 | 0.93 |
| Avascular thyroid bed nodule | 0.19 | 0.47 | 0.30 | 0.87 |
| Other abnormal cervical lymph nodes | 0.74 | 0.53 | 0.17 | 0.94 |
| Rising serum Tg | 0.77 | 0.55 | 0.24 | 0.93 |
| Combination of any suspicious features, other abnormal cervical lymph nodes, and rising serum Tg | 0.88 | 0.31 | 0.11 | 0.96 |
Suspicious features defined as microcalcifications, hypoechogenicity, or increased vascularity in any thyroid bed nodule.
PPV, positive predictive value; NPV, negative predictive value.
Thyroid bed nodules proven to be DTC
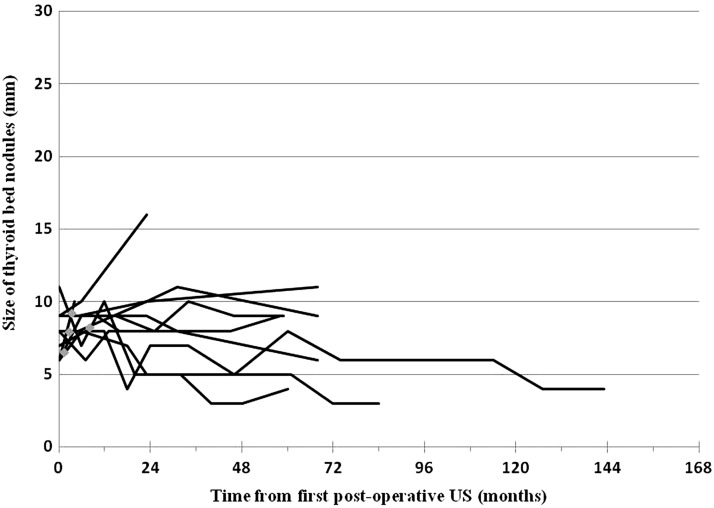
Definitive pathological diagnosis for a TB nodule was obtained in only 9 of 191 patients by either US-guided FNA (n = 5) or histologic confirmation after central compartment neck dissection (n = 4). In all nine cases, FNA of the TB nodule was performed by the referring clinician before referral to our center. The clinical characteristics of these nine patients are given in Table 5. Structural progression was seen in 33% (3/9) of patients and at least one suspicious ultrasonographic feature was identified in 78% (7/9) of patients, leading to FNA or surgical resection. Figure 2 depicts the follow-up over time according to size of the lesions, with the first postoperative US as a starting point. Interestingly, most of the biopsy-proven malignant TB nodules demonstrated no significant increase in size over many years of follow-up.
Table 5.
Clinical and Sonographic Features of Nine Patients in Whom Thyroid Bed Nodules Were Pathologically Proven to Be Thyroid Cancer
| Age (years) | |
| Mean ± SD | 32 ± 9 |
| Median | 33 |
| Range | 19–46 |
| Gender | |
| Female | 67% |
| Tumor size (cm) | |
| Mean ± SD | 2.9 ± 2.1 |
| Median | 1.9 |
| Range | 0.7–6.5 |
| Extent of neck dissection at thyroidectomy | |
| Central only | 44% |
| Lateral only | 0% |
| Both central and lateral compartments | 11% |
| No lymph node dissection | 44% |
| AJCC stage | |
| I | 78% |
| II | 11% |
| III | 11% |
| IVa | 0% |
| ATA risk of recurrence | |
| Low | 11% |
| Intermediate | 78% |
| High | 11% |
| Response to therapy classification | |
| Excellent | 0% |
| Acceptable | 22% |
| Incomplete | 78% |
| RAI ablation | |
| Yes | 89% |
| No | 11% |
| 131I activity for ablation (mCi) | |
| Mean ± SD | 132 ± 51 |
| Median | 106 |
| Range | 100–248 |
| Follow-up duration (years) | |
| Mean ± SD | 8.0 ± 2.4 |
| Median | 7.4 |
| Range | 5.0–13.2 |
| Basal thyroglobulin at the time of first postoperative ultrasound (ng/mL) | |
| Mean ± SD | 4.0 ± 4.0 |
| Median | 2.0 |
| Range | 0.6–10.0 |
| Number of ultrasounds on follow-up | |
| Mean ± SD | 8 ± 4 |
| Median | 8 |
| Range | 4–16 |
| Additional therapy over the course of follow-up | |
| RAI therapy alone | 33% |
| Surgery alone | 0% |
| Both RAI and surgerya | 44% |
| No | 22% |
| Total RAI activity (mCi) | |
| Mean ± SD | 346 ± 248 |
| Median | 318 |
| Range | 100–938 |
| Increase in size of at least one thyroid bed nodule over follow-up | 33% |
| Any suspicious sonographic feature of thyroid bed nodules | 78% |
In four patients (44%), additional treatment included central neck dissection. This latter intervention interrupted the follow-up of thyroid bed nodules. Structural progression was thus assessed in those cases in the period leading to additional neck surgery.
FIG. 2.
Thyroid bed nodules proven to be differentiated thyroid cancer in nine patients. Thyroid bed nodules were proven to be thyroid cancer either by cytology (US-guided fine needle aspiration) in five patients or by histology after central compartment neck dissection in four patients. Radioactive iodine therapy is depicted as gray diamonds. US, ultrasound.
Discussion
Our data demonstrate for the first time the behavior of TB nodules over time in a cohort of patients followed for DTC after total thyroidectomy. Small TB nodules were seen in 34% (521/1531) of thyroid cancer patients undergoing postoperative thyroid US. However, nodule size increase was seen in only 9% (17/191) of patients included in this study. Further, the rate of growth was quite slow, demonstrating a median of 1.3 mm/year in those patients who had an increase in the size of TB nodules. Interestingly, in documented malignant TB nodules, only three of the nine patients showed structural disease progression over a median of 7.4 years of follow-up, emphasizing the indolent course of well-differentiated thyroid cancer in many patients. These findings suggest that most of these small TB nodules can be followed with observation without routine FNA using an approach similar to that recommended by the ATA for management of small (less than 5–8 mm) abnormal cervical lymph nodes (recommendation 48c) (7).
Rather than defining ultrasonographic characteristics of malignant TB nodules (17) or identifying predictors of TB nodule growth, this study identified several important features (absence of other coexistent abnormal cervical lymph nodes, suspicious US features, and rising serum Tg) that have a high NPV for selecting which TB nodules are unlikely to grow. In the absence of these suspicious clinical features, only 4% of TB nodules will show a gradual increase in size over years of follow-up. Therefore, cautious observation without a need for definitive FNA diagnosis appears to be the appropriate follow-up approach for these patients. In our practice, we usually reserve US-guided FNA evaluation of TB nodules for patients with structurally progressive TB nodules (usually greater than 1 cm) in whom a positive FNA finding would lead to a change in management.
Figures 1 and 2 also point out that minor differences in size (both increase and decrease) can be seen in TB nodules that are followed with serial US over the years, even when modern equipment is used by very experienced operators. It is unclear whether these size variations are true changes in the size of the nodule or whether technical differences in measurements are also playing a role. Regardless of the reason, it is important to note that minor variations in size are often seen in the follow-up of TB nodules. Therefore, changes in management usually require a clear trend in serial measurements taken at several points in time.
To our surprise, the lack of vascularity in TB nodules did not achieve as high NPV value as could have been expected (only 0.87). This finding could be related to the difficulty assessing vascularity of very small nodules. Previous studies have shown that very small lymph nodes are less likely to demonstrate vascular flow by power Doppler US than larger lesions (10,22). As with all retrospective studies, our data have important limitations. Although the US studies were performed by a limited group of experienced radiologists at a specialized center, operator-dependent variation in size measurements of TB nodules is an intrinsic variable of US images review that we were unable to control. However, the criterion for growth (≥3 mm in largest diameter) was intentionally chosen to minimize the impact of these variations. In addition, all of the important management issues (such as the need for additional imaging, intensity of follow-up, magnitude of TSH suppression, and indications for additional surgery or RAI) were made by individual treating physicians based on the specific characteristics of each patient. This risk-adapted approach to the management of thyroid cancer by definition leads to more intense testing and follow-up in high-risk patients. As with any retrospective study, these clinical management decisions can have a significant impact on the types and quantity of data available for analysis.
Conclusion
Small TB nodules are commonly seen in the routine follow-up of DTC patients after total thyroidectomy. As most of these TB nodules fail to show clinically significant growth over several years of follow-up, serial US evaluations done at yearly intervals appear to be a very reasonable approach to management. US-guided FNA, RAI therapy, or surgical intervention should be reserved for the few small TB nodules that demonstrate a clinically significant increase in size over time. The absence of suspicious sonographic features combined with the absence of other abnormal cervical lymph nodes and rising serum Tg can provide clinicians with a strong predictor for clinical quiescence over several years of follow-up. This cautious observation approach is consistent with the updated ATA guideline recommendations regarding a conservative management approach to ultrasonographically detected small abnormal cervical lymph nodes in patients with DTC.
Disclosure Statement
S.F., L.E.H., and J.A.F. have nothing to disclose. R.M.T. is a consultant to and has received honoraria from the Genzyme Corporation. G.R. has received research support from Genzyme Corporation.
References
- 1.Mazzaferri EL. Kloos RT. Clinical review 128: current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86:1447–1463. doi: 10.1210/jcem.86.4.7407. [DOI] [PubMed] [Google Scholar]
- 2.Hay ID. Thompson GB. Grant CS. Bergstralh EJ. Dvorak CE. Gorman CA. Maurer MS. McIver B. Mullan BP. Oberg AL. Powell CC. van Heerden JA. Goellner JR. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940–1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26:879–885. doi: 10.1007/s00268-002-6612-1. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–428. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 4.Frasoldati A. Pesenti M. Gallo M. Caroggio A. Salvo D. Valcavi R. Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer. 2003;97:90–96. doi: 10.1002/cncr.11031. [DOI] [PubMed] [Google Scholar]
- 5.Pacini F. Molinaro E. Castagna MG. Agate L. Elisei R. Ceccarelli C. Lippi F. Taddei D. Grasso L. Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 6.Torlontano M. Attard M. Crocetti U. Tumino S. Bruno R. Costante G. D'Azz G. Meringolo D. Ferretti E. Sacco R. Arturi F. Filetti S. Follow-up of low risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab. 2004;89:3402–3407. doi: 10.1210/jc.2003-031521. [DOI] [PubMed] [Google Scholar]
- 7.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 8.Langer JE. Luster E. Horii SC. Mandel SJ. Baloch ZW. Coleman BG. Chronic granulomatous lesions after thyroidectomy: imaging findings. AJR Am J Roentgenol. 2005;185:1350–1354. doi: 10.2214/AJR.04.0920. [DOI] [PubMed] [Google Scholar]
- 9.Kim J. Lee J. Shong Y. Hong S. Ko M-S. Lee D. Choi C. Kim S. Ultrasound features of suture granulomas in the thyroid bed after thyroidectomy for papillary thyroid carcinoma with an emphasis on their differentiation from locally recurrent thyroid carcinomas. Ultrasound Med Biol. 2009;35:1452–1457. doi: 10.1016/j.ultrasmedbio.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Fish S. Langer J. Mandel S. Sonographic imaging of thyroid nodules and cervical lymph nodes. Endocrinol Metab Clin North Am. 2008;37:401–417. doi: 10.1016/j.ecl.2007.12.003. ix. [DOI] [PubMed] [Google Scholar]
- 11.Rosario PW. de Faria S. Bicalho L. Alves MF. Borges MA. Purisch S. Padrao EL. Rezende LL. Barroso AL. Ultrasonographic differentiation between metastatic and benign lymph nodes in patients with papillary thyroid carcinoma. J Ultrasound Med. 2005;24:1385–1389. doi: 10.7863/jum.2005.24.10.1385. [DOI] [PubMed] [Google Scholar]
- 12.Takashima S. Sone S. Nomura N. Tomiyama N. Kobayashi T. Nakamura H. Nonpalpable lymph nodes of the neck: assessment with US and US-guided fine-needle aspiration biopsy. J Clin Ultrasound. 1997;25:283–292. doi: 10.1002/(sici)1097-0096(199707)25:6<283::aid-jcu1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Ying M. Ahuja A. Metreweli C. Diagnostic accuracy of sonographic criteria for evaluation of cervical lymphadenopathy. J Ultrasound Med. 1998;17:437–445. doi: 10.7863/jum.1998.17.7.437. [DOI] [PubMed] [Google Scholar]
- 14.Lyshchik A. Higashi T. Asato R. Tanaka S. Ito J. Hiraoka M. Insana MF. Brill AB. Saga T. Togashi K. Cervical lymph node metastases: diagnosis at sonoelastography—initial experience. Radiology. 2007;243:258–267. doi: 10.1148/radiol.2431052032. [DOI] [PubMed] [Google Scholar]
- 15.Kuna S. Bracic I. Tesic V. Kuna K. Herceg G. Dodig D. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J Ultrasound Med. 2006;25:1531–1537. doi: 10.7863/jum.2006.25.12.1531. [DOI] [PubMed] [Google Scholar]
- 16.Leboulleux S. Girard E. Rose M. Travagli J. Sabbah N. Caillou B. Hartl D. Lassau N. Baudin E. Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–3594. doi: 10.1210/jc.2007-0444. [DOI] [PubMed] [Google Scholar]
- 17.Shin J. Han B-K. Ko E. Kang S. Sonographic findings in the surgical bed after thyroidectomy: comparison of recurrent tumors and nonrecurrent lesions. J Ultrasound Med. 2007;26:1359–1366. doi: 10.7863/jum.2007.26.10.1359. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH. Lee HK. Lee DH. Choi CG. Gong G. Shong YK. Kim SJ. Ultrasonographic findings of a newly detected nodule on the thyroid bed in postoperative patients for thyroid carcinoma: correlation with the results of ultrasonography-guided fine-needle aspiration biopsy. Clin Imaging. 2007;31:109–113. doi: 10.1016/j.clinimag.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Frates MC. Ultrasound in recurrent thyroid disease. Otolaryngol Clin North Am. 2008;41:1107–1116. doi: 10.1016/j.otc.2008.05.007. viii. [DOI] [PubMed] [Google Scholar]
- 20.Tuttle RM. Tala H. Shah J. Leboeuf R. Ghossein R. Gonen M. Brokhin M. Omry G. Fagin JA. Shaha A. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20:1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y. Miyauchi A. Inoue H. Fukushima M. Kihara M. Higashiyama T. Tomoda C. Takamura Y. Kobayashi K. Miya A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. doi: 10.1007/s00268-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 22.Wu CH. Chang YL. Hsu WC. Ko JY. Sheen TS. Hsieh FJ. Usefulness of Doppler spectral analysis and power Doppler sonography in the differentiation of cervical lymphadenopathies. AJR Am J Roentgenol. 1998;171:503–509. doi: 10.2214/ajr.171.2.9694484. [DOI] [PubMed] [Google Scholar]