Abstract
Background
The number of new cases of dementia is projected to rise significantly over the next decade. Thus, there is a pressing need for accurate tools to detect cognitive impairment in routine clinical practice. The Addenbrooke's Cognitive Examination III (ACE‐III), and the mini‐ACE are brief, bedside cognitive screens that have previously reported good sensitivity and specificity. The quality and quantity of this evidence has not, however, been robustly investigated.
Objectives
To assess the diagnostic test accuracy of the ACE‐III and mini‐ACE for the detection of dementia, dementia sub‐types, and mild cognitive impairment (MCI) at published thresholds in primary, secondary, and community care settings in patients presenting with, or at high risk of, cognitive decline.
Search methods
We performed the search for this review on 13 February 2019. We searched MEDLINE (OvidSP), Embase (OvidSP), BIOSIS Previews (ISI Web of Knowledge), Web of Science Core Collection (ISI Web of Knowledge), PsycINFO (OvidSP), and LILACS (BIREME). We applied no language or date restrictions to the electronic searches; and to maximise sensitivity we did not use methodological filters. The search yielded 5655 records, of which 2937 remained after we removed duplicates. We identified a further four articles through PubMed 'related articles'. We found no additional records through reference list citation searching, or grey literature.
Selection criteria
Cross‐sectional studies investigating the accuracy of the ACE‐III or mini‐ACE in patients presenting with, or at high risk of, cognitive decline were suitable for inclusion. We excluded case‐control, delayed verification and longitudinal studies, and studies which investigated a secondary cause of dementia. We did not restrict studies by language; and we included those with pre‐specified thresholds (88 and 82 for the ACE‐III, and 21 or 25 for the mini‐ACE).
Data collection and analysis
We extracted information on study and participant characteristics and used information on dementia and MCI prevalence, sensitivity, specificity, and sample size to generate 2×2 tables in Review Manager 5. We assessed methodological quality of included studies using the QUADAS‐2 tool; and we assessed the quality of study reporting with the STARDdem tool.
Due to significant heterogeneity in the included studies and an insufficient number of studies, we did not perform meta‐analyses.
Main results
This review identified seven studies (1711 participants in total) of cross‐sectional design, four examining the accuracy of the ACE‐III, and three of the mini‐ACE. Overall, the majority of studies were at low or unclear risk of bias and applicability on quality assessment. Studies were at high risk of bias for the index test (n = 4) and reference standard (n = 2). Study reporting was variable across the included studies. No studies investigated dementia sub‐types. The ACE‐III had variable sensitivity across thresholds and patient populations (range for dementia at 82 and 88: 82% to 97%, n = 2; range for MCI at 88: 75% to 77%, n = 2), but with more variability in specificity (range for dementia: 4% to 77%, n = 2; range for MCI: 89% to 92%, n = 2). Similarly, sensitivity of the mini‐ACE was variable (range for dementia at 21 and 25: 70% to 99%, n = 3; range for MCI at 21 and 25: 64% to 95%, n = 3) but with more variability specificity (range for dementia: 32% to 100%, n = 3; range for MCI: 46% to 79%, n = 3). We identified no studies in primary care populations: four studies were conducted in outpatient clinics, one study in an in‐patient setting, and in two studies the settings were unclear.
Authors' conclusions
There is insufficient information in terms of both quality and quantity to recommend the use of either the ACE‐III or mini‐ACE for the screening of dementia or MCI in patients presenting with, or at high risk of, cognitive decline. No studies were conducted in a primary care setting so the accuracy of the ACE‐III and mini‐ACE in this setting are not known. Lower thresholds (82 for the ACE‐III, and 21 for the mini‐ACE) provide better specificity with acceptable sensitivity and may provide better clinical utility. The ACE‐III and mini‐ACE should only be used to support the diagnosis as an adjunct to a full clinical assessment. Further research is needed to determine the utility of the ACE‐III and mini‐ACE for the detection of dementia, dementia sub‐types, and MCI. Specifically, the optimal thresholds for detection need to be determined in a variety of settings (primary care, secondary care (inpatient and outpatient), and community services), prevalences, and languages.
Plain language summary
How accurate are the Addenbrooke's Cognitive Examination III (ACE‐III) and mini‐ACE for the screening of dementia and mild cognitive impairment (MCI)?
Why is recognising dementia important?
The number of people being diagnosed with dementia is expected to increase significantly over the next 10 years. There is therefore an increasing need for tools that can assess memory and learning to aid the diagnosis of dementia and MCI. The ACE‐III and mini‐ACE are currently used in clinical practice, but the evidence for their accuracy to identify dementia has not been fully established.
What was the aim of this review?
The aim of this review was to find out how accurate the ACE‐III and mini‐ACE are in identifying dementia and MCI across a range of healthcare settings. The test is performed on a patient who is suspected to have dementia.
What was studied in this review?
The ACE‐III has 21 questions, with a total score of 100. The test is performed with the patient who presented with, or is suspected to have, dementia. The questions cover five different areas of brain function, and a higher score indicates better function. The mini‐ACE is shorter, with only five questions, and a total score of 30. The thresholds describe the score at which a diagnosis of dementia should be considered and these are usually 82 or 88/100 for the ACE‐III and 21 or 25/30 for the mini‐ACE.
The ACE‐III and mini‐ACE are not used on their own to make a diagnosis of dementia, but help clinicians when used in addition to other clinical information and investigations.
What are the main results of the review?
This review included seven studies with a total of 1711 patients; four studies examined the ACE‐III, and three examined the mini‐ACE. We did not combine the study information statistically due to significant differences between the studies.
The ability of both the ACE‐III and the mini‐ACE to identify patients with either dementia or MCI was variable (between 70% and 99% of people were correctly identified as having dementia and between 64% and 95% for MCI). However, there was more variability between the studies in the number of false positives identified by the tests (between 0% and 96% of people were incorrectly identified as having dementia and between 8% and 54% of people were incorrectly identified as having MCI). At the lower test thresholds, there were fewer false positive diagnoses of dementia (between 64% and 100% of people correctly identified as not having dementia or MCI).
How reliable are the results of this review?
There were some issues with the methods used by studies: the way in which patients were identified and enrolled into the studies, and the way in which the ACE‐III and mini‐ACE were carried out were not well described. The studies were small and did not study enough people to be confident about the results. These issues mean that the accuracy of the ACE‐III and mini‐ACE may have appeared better than it actually was.
Who do the results of this review apply to?
The average age in all the studies was over 60 years. The proportion of people with dementia was different between studies (range: 15% to 55.6%). All of the studies were conducted in a specialist setting, so we do not know if the ACE‐III or mini‐ACE could be used in general practice or the community. Four studies were in the UK, two were in China, and one in Japan.
What are the implications of this review?
Overall, the quality, size, and number of included studies has not allowed a definitive conclusion on whether the ACE‐III or the mini‐ACE should be used to identify dementia or MCI. These findings can only be used in a hospital setting, as none of the studies investigated community or general populations. The ACE‐III or mini‐ACE should only be used as part of a clinical assessment when making a diagnosis of dementia, and should not be relied upon alone. More research is needed to investigate the ACE‐III and mini‐ACE in different healthcare settings, languages, and cultures.
How up to date is this review?
The review authors searched for and included studies up to April 2019.
Summary of findings
Summary of findings'. 'Summary of Test Accuracy Findings.
| Patient population | Patients presenting with cognitive decline but no known diagnosis of dementia. | |||||
| Index test | The ACE‐III and mini‐ACE, including different languages. | |||||
| Reference standard | Undifferentiated dementia: DSM‐IV and DSM‐5, ICD‐10 and ICD‐11; Alzheimer’s disease: NINCDS/ADRDA, ICD‐10 and ICD‐11, DSM‐IV and DSM‐5, NIA/AA; vascular dementia: NINDS‐AIREN, DSM‐IV and DSM‐5, ICD‐10 and ICD‐11; frontotemporal dementia: Lund‐Manchester criteria, NINDS; Lewy body dementia: International consensus criteria; MCI: NIA/AA, DSM‐IV and DSM‐5, Mayo, Petersen; post‐stroke dementia: DSM‐IV and DSM‐5, ICD‐10 and ICD‐11. | |||||
| Target condition | Dementia (all‐cause and sub‐types), MCI. | |||||
| Included studies | 7 studies (1711 patients) | |||||
| Quality concerns | The majority of studies were identified to be at low or unclear risk of bias on the QUADAS‐2 assessment. More studies were labelled at high risk of bias for the index test (n = 4) and reference standard (n = 2) due to lack of information on the conduct of the index test or reference standard. All studies were low or unclear risk of applicability. Studies were at unclear risk mainly due to inadequate reporting. | |||||
| Heterogeneity | There was significant heterogeneity between studies in terms of patient population, study setting, language and culture, and reference standard. | |||||
| Study ID | Comparison | Test threshold | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
| ACE‐III | ||||||
| Jubb 2015 | Dementia vs. none High education Low education |
81 82 88 84 79 |
79 82 97 87 78 |
96 77 50 91 100 |
96 82 71 95 100 |
78 77 92 78 70 |
| Lees 2017 | Dementia vs. none | 82 ‐ approach 1 82 ‐ approach 2 82 ‐ approach 3 82 ‐ approach 4 |
87 81 93 89 |
5 10 11 4 |
52 48 45 51 |
25 33 67 25 |
| Li 2019 | Dementia vs. none MCI vs. none MCI vs. none high education Dementia vs. none high education Dementia vs. none low education |
74/75 88/89 89/90 82/83 70/71 |
94 75 82 88 94 |
83 89 85 92 84 |
72 89 88 86 78 |
97 75 78 93 96 |
| Takenoshita 2019 | Dementia vs. none MCI vs. none |
75/76 88/89 |
82 77 |
90 92 |
87 95 |
85 68 |
| Mini‐ACE | ||||||
| Hobson 2016 | Dementia vs. none Dementia vs. none MCI vs. none |
21 25 25 |
70 96.5 91 |
100 84.3 53 |
100 67 56 |
91 99 90 |
| Larner 2019 | Dementia vs. none Dementia vs. none MCI vs. none MCI vs. none |
21 25 21 25 |
95 99 64 95 |
64 32 79 46 |
32 20 62 49 |
99 100 80 95 |
| Yang 2019 | Dementia vs. none MCI vs. none |
21/22 25/26 |
96 88 |
87 72 |
78 80 |
98 82 |
|
Conclusions This review identified 7 studies of cross‐sectional design, 4 examining the screening accuracy of the ACE‐III, and 3 of the mini‐ACE. We identified no studies in primary care populations, 4 studies were conducted in outpatient clinics, 1 study in an in‐patient setting, and 2 were unclear. We did not perform meta‐analysis due to significant heterogeneity. The majority of studies investigated published thresholds, but 3 studies determined optimal cut‐offs. Sensitivity of the mini‐ACE for the detection of dementia and MCI across thresholds and patient populations was generally high (range: 64% to 99%) but with more variable specificity (range: 32% to 100%). The ACE‐III also had good sensitivity across thresholds and patient populations (range: 75% to 97%), but specificity varied between populations, being significantly poorer in the post‐stroke rehabilitation setting (range: 5% to 11%) compared to an outpatient memory clinic (range: 50% to 77%). | ||||||
|
Implications Overall, there is insufficient information in terms of both quality and quantity to recommend the use of either the mini‐ACE or ACE‐III for the detection of dementia in a clinical setting. Of the thresholds published in the index study, the lower thresholds (21 for the mini‐ACE, and 82 for the ACE‐III) provide better specificity with acceptable sensitivity and may provide better utility in a secondary care setting. Further research is needed to determine the clinical utility of the mini‐ACE and ACE‐III in the detection of dementia, dementia sub‐types, and MCI. Specifically, the optimal thresholds for detection need to be determined in a variety of settings (secondary care (inpatient and outpatient), primary care, community services), prevalences, cultures, and languages. | ||||||
Background
Dementia is an emerging public health concern; 46 million people currently live with dementia worldwide (Alzheimer's Society 2016). As the population ages, this figure is only expected to rise further, and thus sensitive screening tests are becoming increasingly important to distinguish healthy older adults from those with early cognitive impairment (Alzheimer's Society 2016; Prince 2015). Early identification of people with dementia is important to facilitate the early introduction of current available therapies, and to instigate important holistic patient and carer support through the provision of allied health professional and support services (Aminzadeh 2007; de Vugt 2013). Sensitive screening tests are therefore required to support early referral for specialist assessment and management. Screening tests can be used to target high‐risk groups who are more likely to develop dementia (i.e. those over 65 years of age) and those who are presenting with memory complaints, or to screen wider, unselected populations who are not presenting with memory problems. The Addenbrooke’s Cognitive Examination‐III (ACE‐III), and its shorter counterpart, the mini‐ACE, are two such cognitive screening tests that are widely available for use across a variety of healthcare settings (Hsieh 2013; Hsieh 2015). The ACE‐III and mini‐ACE have reported good sensitivity and specificity in the literature (Hsieh 2013; Hsieh 2015), but to date have not been included in systematic reviews or meta‐analyses. The ACE‐III is freely available for clinical and research purposes, which is important in light of recent concerns over copyright of similar cognitive screening tools (e.g. MoCA, MMSE). In this review we will evaluate the validity of the ACE‐III and mini‐ACE to screen for dementia and mild cognitive impairment across all healthcare settings. Given that widespread cognitive screening in unselected populations is not currently standard practice, we have focused this review on the diagnostic test accuracy for those presenting with cognitive symptoms or in high risk groups. For consistency with previous Cochrane Reviews, we use the term 'diagnostic test accuracy' throughout the review.
Target condition being diagnosed
Dementia currently affects 850,000 people in the UK alone, and this is projected to rise by 40% over the next decade as the population ages (Alzheimer's Society 2016). Dementia is characterised by a progressive loss of memory or cognitive function, resulting in impaired ability to perform activities of daily living (Creavin 2016; Davis 2015). The most typical presentation of dementia is that of progressive memory loss. Dementia can present in a multitude of ways, however, from language deficits to loss of executive functioning (Robinson 2015). Dementia is an overarching term that encompasses several forms, including Alzheimer’s disease, vascular dementia, frontotemporal dementia and Lewy body dementia (Robinson 2015). As knowledge and understanding has evolved, it has become increasingly difficult to distinguish between these dementia subtypes, as there is considerable clinical and pathological overlap between them (Attems 2014; Mandal 2006). Alzheimer’s disease is the most common dementia subtype, accounting for 62% of all cases (Alzheimer's Society 2016). Alzheimer’s disease is notably characterised by the development of amyloid plaques, tau deposits, and neurofibrillary tangles, resulting in a progressive deterioration in cognitive function (Takahashi 2017). Vascular dementia is the second most common form, comprising 17% of all dementia cases (Alzheimer's Society 2016). It is associated with vascular risk factors and events (i.e. transient ischaemic attack, acute stroke), resulting in chronic small vessel disease and leading to sustained cerebral hypoperfusion and thus cognitive impairment (Dichgans 2017). Deterioration in cognitive function would characteristically result in a step‐wise decline in cognition, although a slow progression similar to that seen with Alzheimer’s disease is also seen in vascular dementia secondary to small vessel disease, rather than discrete vascular events (Dichgans 2017). Ten per cent of dementia is mixed between subtypes, and the remainder comprises rarer forms: frontotemporal (2%), Parkinson’s disease (2%), and Lewy body dementia (4%) (Alzheimer's Society 2016). It is important to distinguish between these dementia subtypes as this can affect both the approach to diagnosis and treatment. Furthermore, identifying and stratifying the subtypes of dementia allows therapies to be tailored on an individual and personalised basis. Acetylcholinesterase inhibitors and N‐methyl‐D‐aspartate (NMDA) receptor antagonists are now established therapies for the treatment of mild to moderate Alzheimer’s disease (NICE 2011). The evidence base for the use of acetylcholinesterase inhibitors in vascular dementia is considerably smaller; however, the use of donepezil and rivastigmine are supported in a number of Cochrane Reviews (Birks 2013; Malouf 2004).
Mild cognitive impairment (MCI) is characterised by subjective and objective evidence of cognitive decline but, importantly, the patient's functional status is maintained (Petersen 2004). Up to 60% of people with MCI will develop dementia within 10 years (Korolev 2016; Petersen 2004). However, it is unclear why 40% of people with MCI do not progress to dementia (Korolev 2016; Petersen 2004). Tools that can identify and distinguish MCI and predict those that are likely to develop dementia in the future are therefore becoming increasingly important for patients, clinicians, and researchers (Petersen 2004).
Despite the emergence of a number of novel biomarkers, the detection and diagnosis of dementia is still achieved by thorough clinical assessment, and exclusion of important, potentially reversible causes of cognitive decline (Health Quality Ontario 2014; Panegyres 2016; Robinson 2015). Cognitive assessment tools are a key component of this process, and allow physicians to identify not only the presence of cognitive impairment, but its severity, and the key cognitive domains affected (Panegyres 2016; Velayudhan 2014). Radiological and biochemical investigations are adjunctive in the assessment of dementia, and are primarily used to exclude important structural and reversible causes of cognitive decline, for instance tumours, hydrocephalus, and subdural haematoma (Harper 2014; Health Quality Ontario 2014; Panegyres 2016). Pathological changes (such as hippocampal atrophy and small vessel disease) are identified on brain imaging, but formal cognitive testing remains the primary tool for the identification and diagnosis of dementia and specific cognitive deficits (Harper 2014; Health Quality Ontario 2014; NICE 2018; Panegyres 2016; Robinson 2015). There are now several validated cognitive assessment tools available for screening, diagnosis and monitoring of cognitive disorders (Velayudhan 2014); thus, standard assessment practice is currently highly variable across the UK (Care Quality Commission 2014; Walker 2017). Choice of cognitive assessment tool is dependent on clinician and area, which introduces significant variations in dementia assessment practices nationally (Care Quality Commission 2014; Walker 2017), and indeed worldwide due to lack of standardisation of tests across languages, literacy levels, and cultures (Kalaria 2010). Furthermore, there is a lack of consistent international guidance on the assessment and management of dementia, which has the potential to introduce further geographical disparities in care (Ngo 2015). Concerns have been raised regarding the widespread use of common assessment tools, particularly for the assessment of mild cognitive impairment, where the sensitivity is low (Nasreddine 2005). Clarity is therefore urgently required on the most appropriate and valid cognitive assessment tool for the early identification and monitoring of cognitive disorders.
Cognitive impairment is frequently not identified in routine assessments in primary care; cognitive decline is not recognised in up to 76% of patients (Chodosh 2004; Ganguli 2004; Lin 2013; Valcour 2000). The majority of these patients will be diagnosed in the later stages of disease (Lin 2013). Early identification of dementia can often be the gateway to accessing crucial support and care services available to patients and their carers (Aminzadeh 2007; de Vugt 2013).
Index test(s)
The Addenbrooke’s Cognitive Examination (ACE) was originally designed as a brief, bedside cognitive screen that was specifically developed to incorporate tests of memory, and visuospatial and executive function, with the ability to detect early dementia and differentiate Alzheimer’s disease from frontotemporal and Parkinson’s dementia (Larner 2014; Mioshi 2006; Noone 2015; Velayudhan 2014). A number of limitations were identified with the ACE, and it was updated to improve sensitivity, ease of administration, and to facilitate translation and cross‐cultural use as the Addenbrooke’s Cognitive Examination Revised (ACE‐R) (Mioshi 2006). The ACE‐R demonstrated significantly better sensitivity and specificity than the ACE (Larner 2014; Mioshi 2006), but further weaknesses were identified, including ceiling effects to several questions, confounding to verbal repetition by poor hearing, and difficulty translating for cross‐cultural use (Hsieh 2013; Velayudhan 2014). The ACE‐III was developed to address these limitations (Hsieh 2013). The ACE‐III has subsequently been translated into a number of languages, including Portuguese, Spanish, and Egyptian Arabic (Mirza 2017). The ACE‐III has also been recommended for use in cognitive screening in the most recent guidance published by the Alzheimer's Society on cognitive assessment (Alzheimer's Society 2015).
The ACE‐III is a brief, bedside, cognitive screening test that takes approximately 15 to 20 minutes to deliver; it encompasses five major cognitive domains: attention, memory, language, visuospatial function, and verbal fluency (Hsieh 2013; Noone 2015; Velayudhan 2014). It is composed of 21 cognitive tasks and has a total score of 100, where the common cut‐offs for dementia and MCI are considered at scores lower than 82 and 88, respectively (Hsieh 2013; Velayudhan 2014). Studies have demonstrated good sensitivity (93% to 100%) and specificity (96% to 100%) at these cut‐offs, but pooled estimates are lacking (Noone 2015; Velayudhan 2014). The mini‐ACE was derived as a shorter version of the ACE‐III, and takes under five minutes to perform, but maintains good sensitivity (61%, 85%), and specificity (100%, 87%), at established thresholds of 21 and 25 respectively (Hsieh 2015). Furthermore, the mini‐ACE can be used to distinguish between Alzheimer's disease and other forms of dementia (i.e. frontotemporal dementia, primary progressive aphasia, and corticobasal syndrome) (Hsieh 2015). The mini‐ACE is a 30‐point scale covering four cognitive domains: orientation, memory, verbal fluency and visuospatial function. It can be used in a variety of clinical settings and is easily translated (Hsieh 2015). The mini‐ACE is designed to be used as a brief screening tool to facilitate referral for formal neuropsychological testing and cognitive assessment (Hsieh 2015). Although patients remain functionally independent with MCI, it is important to distinguish those with MCI from dementia to avoid the associated psychological harm and stigma that can be associated with an incorrect dementia diagnosis. Furthermore, as patients with MCI are at high risk of developing dementia, identifying MCI can facilitate further monitoring and lifestyle changes that may reduce the risk of subsequent dementia.
The ACE‐III and mini‐ACE are screening tests for the diagnosis of dementia or MCI and should not be used in isolation to make a diagnosis of dementia.
Clinical pathway
Patients presenting with cognitive decline are encountered in a variety of healthcare settings, including general practice, inpatient settings, outreach, and community services (Creavin 2016; Davis 2015; Robinson 2015). National screening for dementia is not currently recommended for all people aged over 65 (NICE 2018). However, the Government’s Commissioning for Quality and Innovation (CQUIN) has recently expressed support for targeted screening of at‐risk groups in accident and emergency departments and general practice (Alzheimer's Research UK 2017). This identifies patients presenting in these settings who are more likely to be at risk of dementia, and prompts further questioning and investigation (Alzheimer's Research UK 2017). Cognitive assessment tools are becoming increasingly important as part of this targeted screening approach in identifying who should be referred for further specialist assessment.
Patients with dementia typically present with a progressive history of declining cognitive function over a period of months to years, which eventually results in loss of daily function for that individual (Creavin 2016; Davis 2015; Robinson 2015). Current guidance from the National Institute for Health and Care Excellence (NICE) advocates early referral to a specialist memory service when a diagnosis of dementia is suspected (NICE 2018). Brief cognitive assessments, specifically designed for community and general practice, are available to assist community practitioners in deciding where referral may be appropriate (NICE 2018; Velayudhan 2014). A diagnosis of dementia should only be made following a comprehensive, specialist assessment (NICE 2018). Therefore all patients with a diagnosis of suspected dementia should undergo formal cognitive testing at the initial specialist assessment, and this should include measures of: attention and orientation; short‐ and long‐term memory; praxis; language; and executive function (NICE 2018). Cognitive assessment should be undertaken alongside a full history, collateral history, mental state examination, physical examination, medication review, laboratory investigations, and brain imaging (NICE 2018; Robinson 2015). A diagnosis of dementia requires deficits in at least two cognitive domains, with an impact on the patient’s ability to carry out activities of daily living (Robinson 2015).
Patients with MCI typically present with cognitive decline or change in memory, and can be identified in primary, secondary, and community care settings. The key factor which distinguishes MCI from dementia is the absence of functional impact on day‐to‐day living (Petersen 2004). In order to confirm a diagnosis of MCI, patients must have both subjective and objective cognitive decline, in addition to remaining functionally independent (Petersen 2004). Cognitive assessment tools form an integral component in identifying any objective cognitive deficits. It is important to distinguish MCI from dementia, as it has clinically relevant consequences for therapeutic management. Where patients with mild dementia would be eligible for initiation of acetylcholinesterase inhibitors, there is currently no evidence to support their use in the treatment of MCI (NICE 2018). Ensuring the correct identification and diagnosis of individuals is a crucial step in the clinical pathway for these patients.
Although a clinical pathway for dementia assessment and management has been long established in the UK, there is considerable variability in dementia assessment internationally (Samsi 2014). Not all countries have a dedicated dementia pathway, and this was highlighted as an area for development in the World Alzheimer Report 2013 (Alzheimer's Disease International 2013). In particular, despite the presence of international guidelines, procedures for dementia assessment are still driven by local factors, (culture, politics, resources) (Prince 2016; Zhao 2016). Stigma, and lack of awareness, training, and knowledge of practitioners remain significant barriers to dementia assessment and treatment in many countries (Aminzadeh 2012; Prince 2016; Zhao 2016). In contrast to the focus on dementia care delivery in secondary healthcare services in the UK, in other countries the responsibility for the assessment and management of cognitive disorders falls in primary care (e.g. Canada, Switzerland), and in some cases patients are able to self‐refer to specialist services (e.g. Switzerland) (Aminzadeh 2012; Prince 2016). In keeping with the UK, many countries have developed national standards and guidelines for the diagnosis and management of cognitive disorders (Prince 2016). Where diagnoses are largely made in primary and not specialist care services, however, concerns remain around uncoordinated services, and under‐utilisation of diagnostic tools and imaging for the assessment of cognitive decline (Aminzadeh 2012; Prince 2016). In countries with large geographical areas and remote communities, delivery of dementia services and timely diagnosis are a particular challenge, with an increasing move towards telemedicine to improve access to dementia services for these populations (Chen 2013; Prince 2016).
In South Korea, there has been a recent push to increase diagnosis rates through the National Dementia Early Detection programme (Banerjee 2016; Prince 2016). In contrast to the UK, all older adults are offered dementia screening, and this has been made widely available through a screening app (Check Dementia) (Banerjee 2016; Prince 2016). If a person screens positive, they are typically referred to a government centre or seek private healthcare for further assessment and diagnosis (Banerjee 2016; Prince 2016). However, only 25% of those screened are diagnosed with dementia, and a proportion of patients do not seek further assessment after screening (Banerjee 2016; Prince 2016)
In lower‐ to middle‐income countries, access to dementia services and the establishment of defined care pathways remains an issue (Prince 2016; Zhao 2016). Specialist services are frequently limited to the major cities and to those who can afford private healthcare, leading to significant inequalities in dementia care (Prince 2016; Zhao 2016). Typically, patients would see their family doctor in the first instance but doctors are frequently less well equipped in terms of knowledge, skills, and resources to practise effective dementia assessment and care, unlike those in higher‐income countries (Prince 2016; Zhao 2016 ). Furthermore, in lower‐ to middle‐income countries, dementia is still seen as a normal consequence of the ageing process, thus presenting a significant barrier to further assessment and treatment. In many cases, neuropsychological tests and imaging methods are not used (Prince 2016; Zhao 2016).
Alternative test(s)
There are numerous cognitive assessment tools available for the screening and diagnosis of dementia, and these have been assessed in a number of previous reviews (Tsoi 2015; Velayudhan 2014). The Mini Mental State Examination (MMSE) is amongst one of the more widely used tests, but its use has been limited in recent years due to lack of availability, and concerns about lack of sensitivity (Tsoi 2015). The findings of a recent Cochrane Review do not support the use of the MMSE to identify patients with MCI who could develop dementia (Arevalo‐Rodriguez 2015), but Creavin and colleagues stated it can be used to support the diagnosis of dementia in primary care (Creavin 2016). The Montreal Cognitive Assessment (MoCA) has recently been evaluated in a Cochrane Review for the diagnosis of dementia (Davis 2015). The MoCA was able to correctly identify dementia in 94% of cases, across all settings, but was limited by a high rate of false positive diagnoses (Davis 2015). Furthermore, the evidence supporting the use of MoCA was only in secondary care settings, which limits the generalisability of these findings to primary care (Davis 2015). The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) is a questionnaire based on informant responses to support a diagnosis of dementia (Harrison 2016). The IQCODE has good sensitivity, but was found to lack sufficient specificity for diagnosing dementia across several healthcare settings (Harrison 2016). A full list detailing the currently available Cochrane diagnostic test accuracy (DTA) reviews for neuropsychological assessments in dementia is available in Table 2.
1. Cochrane reviews of DTA studies for neuropsychological assessment tools in dementia.
| Cognitive Test | Available | Community | Primary | Secondary |
| Mini‐Cog | Y | x | x | ‐ |
| IQCODE | Y | x | x | x |
| AD‐8 | Y | x | x | x |
| MMSE | Y | x | x | x |
| MoCA | Y | x | ‐ | x |
IQCODE = Informant Questionnaire on Cognitive Decline in the Elderly; MMSE = Mini Mental State Examination; MoCA = Montreal Cognitive Assessment. Y = yes, x indicates the review has been conducted in this setting, ‐ indicates the review has not been conducted in this setting.
Rationale
A diagnosis of dementia still carries much stigma and fear in modern society (Aminzadeh 2007; de Vugt 2013). Despite increasing research, accurate diagnostic tests and curative treatments remain elusive. Given the absence of an available cure, the consequences of a dementia diagnosis are profound and have an enormous impact on the patient, their family, and support network (Aminzadeh 2007; Davis 2015; de Vugt 2013). A high specificity will minimise the number of false positive diagnoses. A false positive diagnosis of dementia could cause serious psychological harm, and lead to unnecessary further investigations and treatments for a patient and their carers (de Vugt 2013). Sensitivity is also important to minimise the rate of false negative diagnoses, which can prevent or delay access to available treatments and support services, and potentially worsen the dementia state and carer strain, and evoke loss of confidence in care services (de Vugt 2013). Given the lack of current therapeutic options available in dementia, high specificity and minimising false positive diagnoses take precedence over sensitivity. If clinical practitioners had access to a screening test with high sensitivity and specificity, it would reduce the negative consequences outlined above, and facilitate the timely delivery of support and available treatments (de Vugt 2013).
In summary, there have been a number of reviews of the ACE and ACE‐R (Crawford 2012; Larner 2014; Tsoi 2015), but no comprehensive review of later versions of the ACE (ACE‐III and mini‐ACE) has been carried out to date. Therefore, a Cochrane Review is required to assess the validity of the ACE‐III and mini‐ACE across all the available evidence, cut‐off scores, settings in which the tools have been validated, and the quality of the evidence to date. In particular, the ACE‐III and mini‐ACE have shown promising results in a number of studies, and so may prove more sensitive and specific tests for the early detection of cognitive disorders, with the ability to distinguish between dementia subtypes (Hsieh 2013; Hsieh 2015). Correct and early identification and stratification of patients with dementia can result in better clinical outcomes, through the early initiation of available therapeutics and support services for patients and carers (Creavin 2016; Davis 2015; de Vugt 2013).
Objectives
To assess the diagnostic test accuracy of the Addenbrooke’s Cognitive Examination‐III (ACE‐III) and the mini‐ACE, for the screening of all‐cause dementia, dementia subtypes (Alzheimer’s disease, vascular dementia, frontotemporal dementia, Lewy body dementia), and mild cognitive impairment, across all healthcare settings at all pre‐specified thresholds.
Secondary objectives
To identify the quality and quantity of the research evidence on the diagnostic test accuracy of the ACE‐III and mini‐ACE for the assessment of all‐cause dementia, dementia subtypes (Alzheimer’s disease, vascular dementia, frontotemporal dementia, Lewy body dementia), and mild cognitive impairment, across all healthcare settings at all reported thresholds.
To identify sources of heterogeneity (age, sex, education, severity or stage of the target condition, operator characteristic of the index test and reference standard) in the included studies.
To identify gaps in the evidence where further research is required.
Methods
Criteria for considering studies for this review
Types of studies
We considered cross‐sectional studies for inclusion in this review, where the index test was administered alongside expert confirmation for reference. We considered comparative studies between dementia subtypes (i.e. Alzheimer’s disease and frontotemporal dementia), or comparing the index tests with an alternative (i.e. the Mini Mental State Examination (MMSE), the Montreal Cognitive Assessment (MoCA)) for inclusion if an appropriate reference standard was present, but we only included data on the ACE‐III and mini‐ACE.
We excluded case control studies in this review due to the high risk of bias in these studies. We did not consider delayed verification or longitudinal studies for inclusion.
We considered nested case control studies for inclusion, where cases and controls are selected from the cohort population, which has a lower risk of bias than a traditional case‐control study.
We did not include studies with a small number of cases (fewer than 10), due to their associated high risk of bias.
Participants
We included patients presenting with cognitive decline, undergoing cognitive testing in primary or secondary care. In the secondary care setting we included participants recruited in both outpatient (clinic) and in‐patient (ward) settings. We also included studies conducted in patient populations with a high risk of cognitive decline, but not necessarily presenting with cognitive symptoms. We excluded studies which included participants with a comorbidity associated with cognitive impairment (motor neurone disease (MND), multiple sclerosis (MS), Parkinson’s disease, brain injury/tumour/infection), where these participants comprised more than 20% of the study population. In addition, we excluded studies which included participants with known substance abuse or medication use known to affect cognition where these participants comprised more than 20% of the study population.
Index tests
We considered only the ACE‐III and mini‐ACE for inclusion. Though there are other versions, such as the Addenbrooke’s Cognitive Examination and the Addenbrooke’s Cognitive Examination Revised (ACE‐R), the ACE‐III and mini‐ACE have superseded these versions and thus represent the most up‐to‐date versions of the tool. Threshold scores of 82 and 88 for the ACE‐III (Velayudhan 2014), and 21 and 25 for the mini‐ACE (Hsieh 2015), have been reported consistently in the literature, and are currently used conventionally in clinical practice. We therefore investigated the summary sensitivity and specificity values at these predefined thresholds. The ACE‐III and mini‐ACE have been translated into several languages and we considered all versions for inclusion. The ACE‐III and mini‐ACE tools are available at dementia.ie/images/uploads/site‐images/ACE‐III_Administration_(UK).pdf and s3‐eu‐west‐1.amazonaws.com/pstorage‐karger‐594308543098/6990263/450784_sm1.pdf, respectively.
Target conditions
The target conditions to be detected by the ACE‐III or mini‐ACE were as follows: all‐cause dementia (undifferentiated); specific dementia subtypes (Alzheimer’s disease, vascular dementia, frontotemporal dementia, Lewy body dementia); and mild cognitive impairment (MCI). We included all‐cause dementia as a target condition, as it was anticipated that some studies will not have differentiated between dementia subtypes. In addition, the ACE‐III and mini‐ACE were being evaluated as screening tests, therefore understanding the ability of the test to identify undifferentiated cognitive impairment for onward specialist referral for subtype and classification would be of relevance to primary care practitioners.
Reference standards
At present, there is no 'gold standard' test for the confirmation of MCI, dementia, or subtype. In current practice, dementia and MCI are confirmed by an appropriately qualified clinical specialist or expert (i.e. neurologist or psychiatrist), using internationally developed and validated criteria. The reference standard for this review was a clinical confirmation of dementia or MCI using disease‐specific reference standards developed by a consensus group or accredited body, as follows.
Undifferentiated dementia: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition and Fifth Edition (DSM‐IV and DSM‐5) (American Psychiatric Association 2000; American Psychiatric Association 2013), International Classification of Diseases 10th Revision and 11th Revision (ICD‐10 and ICD‐11) (World Health Organization 2010; World Health Organization 2018).
Alzheimer’s disease: NINCDS/ADRDA (McKhann 1984), ICD‐10 and ICD‐11 (World Health Organization 2010; World Health Organization 2018), DSM‐IV and DSM‐5 (American Psychiatric Association 2000; American Psychiatric Association 2013), National Institute on Aging and the Alzheimer's Association (NIA/AA) (McKhann 2011).
Vascular dementia: NINDS‐AIREN (Román 1993), DSM‐IV and DSM‐5 (American Psychiatric Association 2000; American Psychiatric Association 2013), ICD‐10 and ICD‐11 (World Health Organization 2010; World Health Organization 2018).
Frontotemporal dementia: Lund‐Manchester criteria (Lund 1994), NINDS (Rascovsky 2011).
Lewy body dementia: international consensus criteria (McKeith 2006)
MCI: NIA/AA (McKhann 2011), DSM‐IV and DSM‐5 (American Psychiatric Association 2000; American Psychiatric Association 2013), Mayo (Petersen 2013), Petersen (Petersen 2004).
Post‐stroke dementia (DSM‐IV and DSM‐5 (American Psychiatric Association 2000; American Psychiatric Association 2013), ICD‐10 and ICD‐11 (World Health Organization 2010; World Health Organization 2018).
The presence of the disease had to be confirmed using one of these recognised criteria by an appropriately qualified specialist, expert, or consensus group in order for us to consider a study eligible for inclusion in this review. Imaging and biochemical investigations are often used alongside clinical assessment to confirm dementia or MCI but we excluded studies which relied on imaging and biochemical investigations alone (without clinical assessment) from this review.
Studies using a histopathological diagnosis of dementia as a reference standard were not suitable for inclusion as this is a post‐mortem diagnosis.
Search methods for identification of studies
We devised search methods in accordance with the guidance given in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1; and we developed the search strategy in conjunction with the Information Specialist at the Cochrane Dementia and Cognitive Improvement Group (CDCIG).
Electronic searches
We searched MEDLINE (OvidSP), Embase (OvidSP), BIOSIS (Ovid), Web of Science Core Collection (ISI Web of Knowledge), PsycINFO (Ovid) and LILACS (Bireme), using a structured search strategy appropriate for each database. We used controlled vocabulary, such as MeSH terms and Emtree, where appropriate. We did not restrict the search by date, sampling frame, setting, or language. The search strategies used can be seen in Appendix 1.
Searching other resources
We reviewed the reference lists of all included studies. We also searched the following databases.
Database of Abstracts of Reviews of Effects (DARE): www.york.ac.uk/inst/crd/crddatabases.html (updated to 2015)
Aggressive Research Intelligence Facility (ARIF): www.arif.bham.ac.uk (updated to 2018)
We used the 'related articles' feature of PubMed to search for additional studies. We searched citation databases, such as Science Citation Index and Scopus, using key studies to identify any additional relevant studies. We searched grey literature, including conference proceedings, theses, and PhD abstracts. We did not perform handsearching, in accordance with the generic protocol (Davis 2013). We contacted research groups involved in previously published or ongoing research on the ACE‐III or mini‐ACE to identify any relevant, unpublished data.
Data collection and analysis
Selection of studies
The eligibility criteria are as follows.
Inclusion criteria
Primary, secondary, and community care services
Patients presenting with cognitive decline or screening in a high‐risk population
Cross‐sectional, comparative, or nested case‐control studies
Studies utilising the ACE‐III or mini‐ACE as the index test
Presence of a referenced standard as specified above
Exclusion criteria
Patients with a diagnosis of dementia at presentation
Patients with comorbidity associated with cognitive impairment, motor neurone disease (MND), multiple sclerosis (MS), Parkinson’s disease, brain injury, tumour, infection
Patients with presence of substance abuse, or medication use known to affect cognition
Case‐control studies, longitudinal or delayed‐verification studies
Small sample size (fewer than 10 participants)
Studies utilising older versions of the tool (ACE, ACE‐R)
Absence of a reference standard as specified above
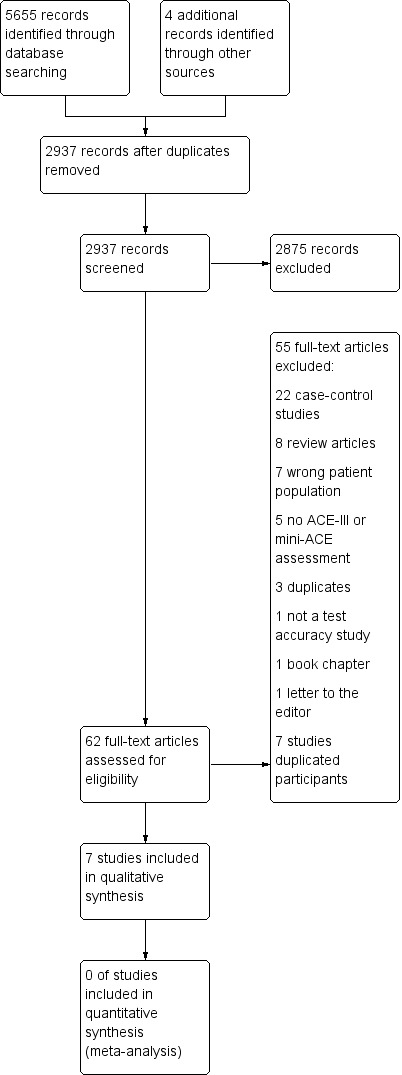
Two review authors (LCB, APB) independently screened eligible articles based on title and abstract. After this, two authors (LCB, APB) independently reviewed full texts for inclusion in the review. We resolved disagreements by discussion; and if they remained unresolved, we referred them to an arbitrator within the study team (TJQ). Where disagreements were resolved, our default position was to include the study in the review. The study selection process is detailed in a PRISMA flow diagram (Figure 1).
1.
Study flow diagram
Data extraction and management
We developed a study‐specific proforma, and extracted data on the following: study characteristics (setting, type, number of participants, diagnostic criteria, language, index test); demographics of the participants (age, gender, diagnosis, comorbidities); study quality assessment; and heterogeneity. The data that we collected with the study proforma are detailed in Appendix 2.
Two review authors (LCB, APB) independently extracted data. Test accuracy data were cross‐tabulated in two‐by‐two tables of index test results (positive or negative) against the target condition (positive or negative). We resolved disagreements between authors on data extraction by discussion. We extracted the results directly into tables in Review Manager 5 software (Review Manager 2014).
Assessment of methodological quality
Two authors (LCB, APB) independently assessed methodological quality, using the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS‐2) (Whiting 2011). The tool consists of four domains: patient selection; index tests; reference standard; and patient flow. We assessed each domain in terms of risk of bias, and the first three domains were considered in terms of applicability. We piloted the QUADAS‐2 tool on the first five studies included in the review. Where there was poor agreement between the two review authors, we revised and re‐piloted the tool. We resolved disagreements between authors on study quality by discussion. We graded studies as being at high, medium or low risk of bias, and presented a narrative summary for each study (Characteristics of included studies). The QUADAS‐2 tool is available in Appendix 3, and the anchoring statements in Appendix 4. The use of the reference standard and index tests are not completely independent of one another, and this introduces a risk of incorporation bias; we assessed included studies for the presence of incorporation bias.
The STARDdem tool has been recently developed to report the quality of study reporting in dementia (Table 3) (Noel‐Storr 2014). In addition to reporting methodological quality, this review also reported on the quality of study reporting using this checklist (www.ncbi.nlm.nih.gov/pmc/articles/PMC4115600/table/T3/?report=objectonly).
2. STARDdem reporting quality.
| Study ID | Yes | No |
| Hobson 2016 | 1,2,3,4,6,7,9,12,15,16,18,21,23,25 | 5,8,10,11, 13,14,17,19,20,22,24 |
| Jubb 2015 | 1,2,3,4,5,6,7,8,9,10,11,12,14,15,16,18,21,23,25 | 5,13,17,19,20,22,24 |
| Larner 2019 | 1,2,3,4,5,6,7,9,12,14,18 | 8,10,11,13,15,16,17,19,20,21,22,23,24,25 |
| Lees 2017 | 1,2,3,4,6,7,8,9,10,11,12,14,15,16,21,22,25 | 5,13,17,18,19,20,23,24 |
| Li 2019 | 1,2,3,6,7,8,9,11,12,15,17,18,23,24,25 | 4,5,10,13,14,16,19,20,21,22 |
| Takenoshita 2019 | 1,2,3,4,6,7,9,12,13,14,15,18,23,24,25 | 5,8,10,11,16,17,19,20,21,22 |
| Yang 2019 | 1,2,3,6,7,8,9,11,12,15,17,18,25 | 4,5,10,13,14,16,19,20,21,22,23,24 |
Statistical analysis and data synthesis
The target condition comprised three categories: 1) undifferentiated (all‐cause) dementia; 2) specific dementia subtypes (Alzheimer’s disease, vascular dementia, frontotemporal dementia, Lewy body dementia); and 3) MCI. The index test comprised two categories: ACE‐III; or the mini‐ACE. The setting also comprised three categories: primary; secondary; and community care. Due to insufficient studies at each of these levels we were unable to perform meta‐analysis, and we have provided a descriptive summary of the numerical results.
For all included studies (cross‐sectional), we extracted data in binary two‐by‐two tables (binary test results cross‐classified with the binary reference standard) and we used this to calculate sensitivities and specificities, with 95% confidence intervals. We have presented individual study results graphically by plotting estimates of sensitivities and specificities in a forest plot. All analyses were performed with Review Manager 5 software (Review Manager 2014). As outlined above, data are presented at predefined thresholds of 82 and 88 for the ACE‐III (Velayudhan 2014), and 21 and 25 for the mini‐ACE (Hsieh 2015). Each study included in this review can contribute to one or more thresholds, and we excluded from this review studies which do not report any of these thresholds. We undertook graphical presentations for all predefined thresholds reported in the included studies.
We did not undertake summary and univariate analyses due to insufficient studies for each of the test thresholds and settings, and significant heterogeneity between the included studies. We present results for each individual study in tables and forest plots (Table 1, Figures 4 to 11).
Investigations of heterogeneity
As anticipated in the protocol, there were insufficient studies for heterogeneity analysis. In line with previous Cochrane DTA reviews of neuropsychological tests, we anticipated there would be a number of sources of heterogeneity in the studies identified for review (Creavin 2016; Davis 2013; Davis 2015; Harrison 2016). We explored the key factors, as outlined below, in a pre‐specified heterogeneity analysis.
Case mix
The case mix of the populations included in the studies could introduce significant heterogeneity in terms of age, dementia diagnosis, specific versus unselected populations, and the severity or stage of the dementia diagnosis. The test properties are likely to differ in younger compared to older populations: studies where less than 20% of the population is under 65 years of age are not likely to be representative of this population. The majority of studies enrolled adults from an unselected population; some studies, however, enrolled a specific or limited population. There were insufficient studies to conduct sensitivity analyses; data were therefore collected on the type of study population enrolled and summarised in the Characteristics of included studies and Table 1.
Reference standard criteria
An important source of heterogeneity, and a key component of methodological quality, is the process by which the cases of dementia or MCI are confirmed and sub‐classified. We collected data on this process, including which reference standard or criteria were used; whether it was by consensus meeting, individual assessment, or algorithm; and whether imaging or biochemical investigations were included. We assessed the quality of this process at study level using the QUADAS‐2 tool.
Technical features of the index tests
Several thresholds have been reported in the literature for both the ACE‐III and mini‐ACE; we have, however, selected for analysis the two most consistent levels which are currently used in clinical practice. Data were collected for all of the predefined thresholds for each test.
We investigated heterogeneity informally through visual examination of forest plots of sensitivities and specificities. There were insufficient data present for formal investigation of the sources of heterogeneity through subgroup or regression analyses.
Sensitivity analyses
We did not undertake sensitivity analyses due to insufficient studies for analysis.
Assessment of reporting bias
We did not examine reporting bias in this review, as current quantitative methods for exploring reporting bias are not well established for studies of DTA. Specifically, we did not consider funnel plots of the diagnostic odds ratio versus the standard error of this estimate.
Results
Results of the search
In total, the search identified 5659 records. After de‐duplication we were left with 2937 references to assess, of which we obtained 62 full‐text articles to further screen against the inclusion and exclusion criteria for the review.
This review includes seven studies with a total of 1711 patients included in analyses. The inclusion and exclusion of studies is summarised in the PRISMA flow diagram (Figure 1).
Methodological quality of included studies
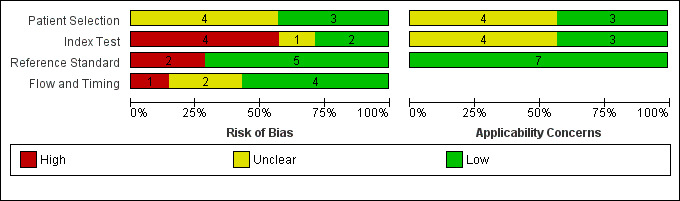
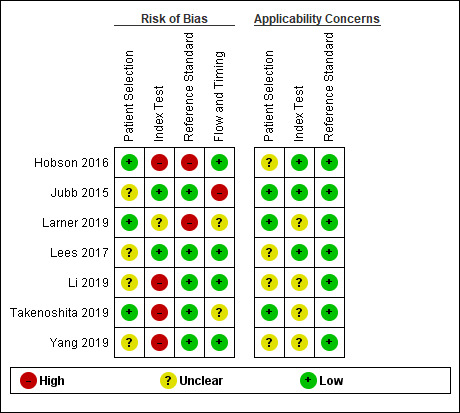
We assessed the risk of bias for included studies using the QUADAS‐2 tool (Appendix 3); and the quality of study reporting using the STARDdem tool. The anchoring statements used in conjunction with the QUADAS‐2 tool can be seen in Appendix 4. The quality assessment and study characteristics can be seen in the Characteristics of included studies. Summary figures for the outcomes of the risk of bias can be seen in Figure 2 and Figure 3.
2.
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
3.
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
We classified the majority of domains as unclear or low risk of bias for all of the studies included; we rated no study as low risk of bias across all four of the categories. Where there was insufficient information to deem a study at low or high risk of bias, we contacted study authors for more information or clarification. We contacted all seven study authors to provide further information; of these, three authors responded to queries (Hobson 2016; Lees 2017; Takenoshita 2019).
Patient selection/sampling
We assessed four studies to be at unclear risk of bias (Jubb 2015; Lees 2017; Li 2019; Yang 2019); and three to be at low risk of bias (Hobson 2016; Larner 2019; Takenoshita 2019). All seven studies recruited from secondary care settings: four were in outpatient clinics (memory clinic/neurology) (Hobson 2016; Jubb 2015; Larner 2019; Takenoshita 2019); one from in‐patient stroke rehabilitation units (Lees 2017); and two were unclear (Li 2019; Yang 2019).
In terms of applicability, we found three studies to be low risk of bias, recruiting from out‐patient cognitive disorder clinics where patients were presenting with cognitive decline (Jubb 2015; Larner 2019; Takenoshita 2019). We found the remaining four studies to be at unclear risk of bias as they did not explicitly state they recruited patients presenting with cognitive decline (Li 2019; Yang 2019), or recruited from populations at high risk of cognitive impairment (patients with chronic kidney disease and type two diabetes (Hobson 2016), and post stroke (Lees 2017)).
ACE‐III and mini‐ACE application
The screening accuracy of the ACE‐III was investigated by four studies (Jubb 2015; Lees 2017; Li 2019; Takenoshita 2019), and three investigated the accuracy of the mini‐ACE (Hobson 2016; Larner 2019; Yang 2019). In this domain, we determined two studies to be at low risk of bias (Jubb 2015; Lees 2017), one at unclear risk of bias (Larner 2019), and four at high risk of bias (Hobson 2016; Li 2019; Takenoshita 2019; Yang 2019). Test accuracy data at the published cut‐off values were presented in four studies, and these were pre‐specified in the introduction or methods (Hobson 2016; Jubb 2015; Larner 2019; Lees 2017); but five studies investigated optimal cut‐offs, where test thresholds were calculated by applying Receiver Operating Characteristic (ROC) analysis using their own study data (Jubb 2015; Larner 2019; Li 2019; Takenoshita 2019; Yang 2019).
For three studies there was low concern in terms of applicability in the conduct of the index test (Hobson 2016; Jubb 2015; Lees 2017); we felt, however, that the remaining four studies provided insufficient information for this to be assessed and have therefore assessed them as unclear applicability (Larner 2019; Li 2019; Takenoshita 2019; Yang 2019).
Reference standard application
In this domain, we classified five studies at low risk of bias (Jubb 2015; Lees 2017; Li 2019; Takenoshita 2019; Yang 2019), and two studies at high risk of bias (Hobson 2016; Larner 2019). All seven studies used an appropriate reference standard for the diagnosis of cognitive impairment (i.e. DSM‐IV, Petersen, DSM‐V), and two studies used recently published guidelines for the diagnosis of post‐stroke and vascular dementia respectively (Lees 2017; Takenoshita 2019).
We classified all seven studies at low risk of applicability concerns given that the appropriate reference standards were used to diagnose dementia.
Flow and timing
In this domain, we classified four studies at low risk of bias (Hobson 2016; Lees 2017; Li 2019; Yang 2019), one at high risk (Jubb 2015), and two at unclear risk (Larner 2019; Takenoshita 2019). Dropout rates were reported in three studies (Hobson 2016; Jubb 2015; Lees 2017), and on contact with the author in one study (Takenoshita 2019), but three did not specify (Larner 2019; Li 2019; Yang 2019). An appropriate time interval between the index test and the reference standard was reported in two studies (within one week) (Li 2019; Yang 2019), and for one study the authors provided this information on request (days) (Lees 2017).
Reporting quality
We used the STARDdem tool to assess reporting quality (Appendix 5). A summary of the reporting quality can be found in Table 3. Areas found to have consistently low reporting across included studies were: the participant sampling procedure; the training and expertise of the persons delivering the index test; methods and estimates of test reproducibility; the number of participants who did not undergo the index test or reference standard and reasons; the time interval between the index test and the reference standard; a cross‐tabulation of the results of the index test and the reference standard; adverse events; estimates of statistical uncertainty; and how missing data, outliers or indeterminate data were handled.
Findings
We have summarised the study characteristics for included studies in Characteristics of included studies, and the findings in the Table 1. We did not perform meta‐analysis of included studies due to too few studies at pre‐specified test thresholds for each of the index tests (less than three), and significant differences in patient populations limiting the interpretation of results. The sensitivity and specificity findings from each study at published thresholds are summarised in Figures 4 to 10.
ACE‐III
Target condition
All‐cause dementia was the target condition in three studies, and post‐stroke cognitive impairment in one study. In addition, two studies also investigated diagnostic test accuracy in MCI. None of the studies investigated specific dementia sub‐types.
Setting
All four studies were conducted in secondary care settings — we identified no studies in primary or community care settings. Of these, three studies were conducted in a memory clinic or in a Neurology department (Jubb 2015; Li 2019; Takenoshita 2019), and one in the stroke rehabilitation setting (Lees 2017). All four studies had a relatively high prevalence of dementia (range: 32.4% to 55.9%).
Threshold
Only one study investigated diagnostic test accuracy for dementia at a threshold of 88, (sensitivity: 97% (95% confidence interval (CI) 84% to 100%); specificity: 50% (95% CI 30% to 70%)) (Figure 4) (Jubb 2015).
4.

Forest plot of ACE‐III for the detection of dementia at a threshold of 88.
Two studies investigated diagnostic test accuracy at a threshold of 82 for dementia (sensitivity: 82% (95% CI 65% to 93%); specificity: 77% (95% CI 56% to 91%) (Figure 5)) (Jubb 2015), and post‐stroke cognitive impairment (sensitivity: 89% (95% CI 71% to 98%), specificity: 4% (95% CI 0% to 21%) (Figure 6)) (Lees 2017).
5.
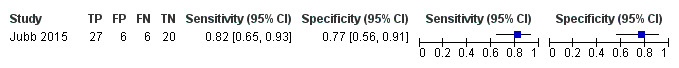
Forest plot of 6 ACE‐III for the detection of dementia at a threshold of 82.
6.

Forest plot of 8 ACE‐III for the detection of post‐stroke cognitive impairment at a threshold of 82.
In two studies, at a threshold of 88 the sensitivity of the ACE‐III for the detection of MCI was 75% to 77%, and specificity was 89% to 92% (Figure 7) (Li 2019; Takenoshita 2019).
7.

Forest plot of ACE‐III for the detection of MCI at a threshold of 88.
Mini‐ACE
Setting
All three studies were conducted in secondary care settings — we identified no studies in primary or community care settings. Of these, two studies were conducted in a memory clinic or in a Neurology department (Larner 2019; Yang 2019), and one in a clinic for chronic kidney disease (Hobson 2016). The prevalence of dementia was lower in these studies than for the ACE‐III (range: 15% to 32%).
Target condition
All‐cause dementia and MCI were the target conditions in all three studies of the mini‐ACE. No study investigated diagnostic test accuracy of dementia sub‐types.
Threshold
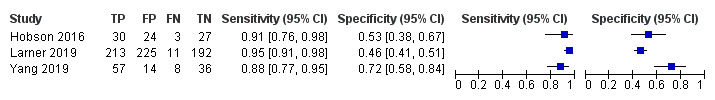
In two studies, at a threshold of 25 to detect dementia, sensitivity was 96% to 99% and specificity was 32% to 85% (Figure 8) (Hobson 2016; Larner 2019).
8.

Forest plot of Mini‐ACE for the detection of dementia at a threshold of 25.
In three studies, at a threshold of 25 to detect MCI, sensitivity was 88% to 95%, and specificity was 46% to 72% (Figure 9) (Hobson 2016; Larner 2019; Yang 2019).
9.
Forest plot of Mini‐ACE for the detection of MCI at a threshold of 25.
In three studies, at a threshold of 21 to detect dementia, sensitivity was 70% to 96%, and specificity was 64% to 100% (Figure 10) (Hobson 2016; Larner 2019; Yang 2019).
10.

Forest plot of Mini‐ACE for the detection of dementia at a threshold of 21.
Only one study investigated the diagnostic test accuracy for the detection of MCI at a threshold of 21 (sensitivity: 64%; specificity: 79%) (Figure 11) (Larner 2019).
11.

Forest plot of Mini‐ACE for the detection of MCI at a threshold of 21.
Discussion
Summary of main results
This review identified seven studies, four examining the diagnostic test accuracy of the ACE‐III, and three of the mini‐ACE. There was significant heterogeneity between studies in terms of the study populations, which precluded meta‐analysis. Of the included studies, five had relatively small sample sizes, with two studies enrolling larger samples of more than 300 participants. Risk of bias was generally unclear to low across the majority of the domains; and the quality of study reporting was variable, particularly with reference to the conduct of the index test and reference standard, and the dropout or flow of participants. We determined optimal thresholds from study data in three studies, and classified them at high risk of bias. The sensitivity of the ACE‐III varied across thresholds and patient populations (range: 75% to 97%), but specificity was more variable between populations, being significantly poorer in the post‐stroke rehabilitation setting (range: 5% to 11%) compared to an outpatient memory clinic (range: 50% to 77%). Similarly, sensitivity of the mini‐ACE for the detection of dementia and MCI varied across thresholds and patient populations (range: 64% to 99%) but with more variability in specificity (range: 32% to 100%).
Strengths and weaknesses of the review
The strengths of this review are the use of a robust and pre‐specified protocol in accordance with guidance published on undertaking a diagnostic test accuracy review of cognitive assessment tools (Davis 2013). The review was conducted in accordance with this protocol. An extensive search was undertaken by Information Specialists at Cochrane across a range of databases. Despite this, only seven identified studies were suitable for inclusion. This was less likely to be as a result of a restricted search or extensive exclusion criteria, and more likely due to the lack of cross‐sectional studies examining the diagnostic test accuracy properties of the ACE‐III and mini‐ACE. Furthermore, the number of studies was reduced significantly as a result of the recent publication of data from several studies in one manuscript (Larner 2019). The small number of studies identified is in keeping with previous Cochrane Reviews of the IQCODE (Harrison 2016), and the MoCA (Davis 2015). This review is also strengthened by the independent article screening, quality assessment, and data extraction by two study authors (LB and APB). The quality assessment tool (QUADAS‐2) and study reporting criteria (STARDdem) are specific to diagnostic test accuracy studies and those reporting research in dementia. Furthermore, where domains in the risk assessment were found to be unclear, we contacted the study authors to provide additional information on this.
Weaknesses of this review include the small number of studies identified which precluded meta‐analysis of the individual study findings to generate pooled estimates. In addition, there was significant heterogeneity between the study populations in which accuracy of the tools were investigated, which limits the generalisability of the findings. No studies were conducted in primary or community settings, and all of the studies investigated populations either at high risk of cognitive impairment, or where the prevalence of dementia or MCI is likely to be higher. Three of the studies in this review calculated optimal thresholds using their own study data, limiting the interpretation of these studies due to a higher risk of bias.
Applicability of findings to the review question
The results of the studies included in this review have limited generalisability given that they were all conducted in secondary care settings and in limited geographical locations (UK, China, Japan). The sensitivity of the ACE‐III and mini‐ACE was generally high across these settings at both thresholds for the detection of MCI or dementia, but specificity was more variable. Specificity could be improved by using low thresholds of detection, but many of the studies used their own study data to calculate these thresholds leading to a high risk of bias. A lack of specificity could result in a higher number of false positive diagnoses, with a risk of significant psychological harm to patients from misdiagnosis. Given there are currently few treatment options available for people living with dementia, the priority for sensitivity may be lower than for a specific test which is able to exclude a diagnosis of dementia.
Authors' conclusions
Implications for practice.
Overall, there is insufficient information in terms of both quality and quantity to recommend the use of either the ACE‐III or mini‐ACE for the detection of dementia in patients presenting with cognitive decline or in high‐risk groups. As there are no studies in a community or primary care setting, the test properties of either the ACE‐III or mini‐ACE in a low prevalent setting remain unknown. In secondary care where the prevalence of dementia or MCI is likely to be higher, particularly in high‐risk groups or those presenting with symptoms of cognitive decline, the ACE‐III and mini‐ACE have good sensitivity for the detection of cognitive impairment, but specificity remains highly variable at different thresholds and in different patient populations. Thus, the ACE‐III or mini‐ACE should only be used by clinicians for the screening of cognitive impairment as an adjunct to clinical history, neuroimaging, and laboratory testing. It is also important to note that the published thresholds of 82 and 88 for the ACE‐III, and 21 and 25 for the mini‐ACE were originally generated from case‐control studies to detect cognitive impairment, and thus have been developed in studies with a high risk of bias. Clinicians may want to consider the need for further or additional neuropsychological testing where there remains diagnostic uncertainty, given the lack of specificity of these tools for excluding other causes of cognitive decline. Of the thresholds published in the index study, the lower thresholds (21 for the mini‐ACE, and 82 for the ACE‐III) provide better specificity with acceptable sensitivity and may provide better utility in a secondary care setting.
Implications for research.
Further research is needed to determine the clinical utility of the ACE‐III and mini‐ACE in the detection of dementia, dementia sub‐types, and MCI. Specifically, the optimal thresholds for detection need to be determined in a variety of settings (primary care, secondary care (inpatient and outpatient), community services), prevalences, cultures, and languages. Five of the studies included in this review certainly highlighted that the previously published thresholds may not be applicable to all populations, settings, and languages, and may require adjustment depending on patient characteristics, and disease prevalence. Studies should follow the STARDdem reporting guidelines for diagnostic test accuracy studies in dementia. Ideally, studies should be cohort in design with the ACE‐III or mini‐ACE conducted on the same day as — but independent of — the reference standard to reflect clinical practice. Studies could also take a delayed verification approach, with prospective application of the reference standard with or without histopathological confirmation, which provides more accurate estimates of test properties. Practically, however, delayed verification studies are problematic, with significant losses to follow‐up as identified in previous Cochrane Reviews (Harrison 2016).
Acknowledgements
LCB is a Research Fellow supported by the Dunhill Medical Trust.
TGR is a Senior Investigator for the National Institute for Health Research.
We would like to acknowledge peer reviewers Dimity Pond and Susan Shenkin for their comments and feedback.
Appendices
Appendix 1. Sources searched and search strategies
The search strategy uses two concepts: index test/s and populations of interest. The search was devised and then tested on a set of known studies. All known studies were identified by the search.
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (Ovid SP) Date of search: 13 February 2019 |
1. Addenbrooke* Cognitive Exam*.ti,ab. 2. ACE.ti,ab. 3. ACE‐r.ti,ab. 4. Mini‐Addenbrooke* Cognitive Exam*.ti,ab. 5. mini‐ACE.ti,ab. 6. ACE‐III.ti,ab. 7. or/1‐6 8. ((cognit$ or memory or cerebr$ or mental$) adj3 (declin$ or impair$ or los$ or deteriorat$ or degenerat$ or complain$ or disturb$ or disorder$)).ti,ab. 9. (forgetful$ or confused or confusion).ti,ab. 10. MCI.ti,ab. 11. AMCI.ti,ab. 12. ARCD.ti,ab. 13. SMC.ti,ab. 14. CIND.ti,ab. 15. BSF.ti,ab. 16. AAMI.ti,ab. 17. MD.ti,ab. 18. LCD.ti,ab. 19. QD.ti,ab. 20. AACD.ti,ab. 21. MNCD.ti,ab. 22. MCD.ti,ab. 23. ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 24. minor neurocognitive disorder.ti,ab. 25. Cognitive Dysfunction/ 26. Cognition Disorders/ 27. or/8‐26 28. exp DEMENTIA/ 29. major cognitive disorder.ti,ab. 30. alzheimer*.ti,ab. 31. dement*.ti,ab. 32. ((lewy adj2 bod*) or LBD or DLB).ti,ab. 33. (FTLD or frontotemp*).ti,ab. 34. or/28‐33 35. 27 or 34 36. 7 and 35 |
1056 |
| Embase 1974 to 2019 (Ovid SP) Date of search: 13 February 2019 |
1 Addenbrooke* Cognitive Exam*.ti,ab. 2 ACE.ti,ab. 3 ACE‐r.ti,ab. 4 Mini‐Addenbrooke* Cognitive Exam*.ti,ab. 5 mini‐ACE.ti,ab. 6 ACE‐III.ti,ab. 7 or/1‐6 8 ((cognit$ or memory or cerebr$ or mental$) adj3 (declin$ or impair$ or los$ or deteriorat$ or degenerat$ or complain$ or disturb$ or disorder$)).ti,ab. 9 (forgetful$ or confused or confusion).ti,ab. 10 MCI.ti,ab. 11 AMCI.ti,ab. 12 ARCD.ti,ab. 13 SMC.ti,ab. 14 CIND.ti,ab. 15 BSF.ti,ab. 16 AAMI.ti,ab. 17 MD.ti,ab. 18 LCD.ti,ab. 19 QD.ti,ab. 20 AACD.ti,ab. 21 MNCD.ti,ab. 22 MCD.ti,ab. 23 ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 24 minor neurocognitive disorder.ti,ab. 25 cognitive defect/ 26 or/8‐25 27 exp dementia/ 28 major cognitive disorder.ti,ab. 29 alzheimer*.ti,ab. 30 dement*.ti,ab. 31 ((lewy adj2 bod*) or LBD or DLB).ti,ab. 32 (FTLD or frontotemp*).ti,ab. 33 or/27‐32 34 26 or 33 35 7 and 34 |
2077 |
| PSYCINFO 1806 to 2019 (Ovid SP) Date of search: 13 February 2019 |
1 Addenbrooke* Cognitive Exam*.ti,ab. 2 ACE.ti,ab. 3 ACE‐r.ti,ab. 4 Mini‐Addenbrooke* Cognitive Exam*.ti,ab. 5 mini‐ACE.ti,ab. 6 ACE‐III.ti,ab. 7 or/1‐6 8 ((cognit$ or memory or cerebr$ or mental$) adj3 (declin$ or impair$ or los$ or deteriorat$ or degenerat$ or complain$ or disturb$ or disorder$)).ti,ab. 9 (forgetful$ or confused or confusion).ti,ab. 10 MCI.ti,ab. 11 AMCI.ti,ab. 12 ARCD.ti,ab. 13 SMC.ti,ab. 14 CIND.ti,ab. 15 BSF.ti,ab. 16 AAMI.ti,ab. 17 MD.ti,ab. 18 LCD.ti,ab. 19 QD.ti,ab. 20 AACD.ti,ab. 21 MNCD.ti,ab. 22 MCD.ti,ab. 23 ("N‐MCI" or "A‐MCI" or "M‐MCI").ti,ab. 24 minor neurocognitive disorder.ti,ab. 25 exp Cognitive Impairment/ 26 or/8‐25 27 exp DEMENTIA/ 28 major cognitive disorder.ti,ab. 29 alzheimer*.ti,ab. 30 dement*.ti,ab. 31 ((lewy adj2 bod*) or LBD or DLB).ti,ab. 32 (FTLD or frontotemp*).ti,ab. 33 or/27‐32 34 26 or 33 35 7 and 34 |
423 |
| 4. Web of Science core collection (ISI Web of Science) Date of search: 13 February 2019 |
TOPIC:(Addenbrooke* Cognitive Exam* OR ACE OR Mini‐Addenbrooke* Cognitive Exam* OR mini‐ACE OR ACE‐III) AND TOPIC: (memory OR MCI OR neurocognitive disorder* OR dement* OR alzheimer* OR Cognitive Dysfunction OR Cognition Disorder* OR forget* or confused or confusion) | 1313 |
| 5. BIOSIS (ISI Web of Science) Date of search: 13 February 2019 |
TOPIC:(Addenbrooke* Cognitive Exam* OR ACE OR Mini‐Addenbrooke* Cognitive Exam* OR mini‐ACE OR ACE‐III) AND TOPIC: (memory OR MCI OR neurocognitive disorder* OR dement* OR alzheimer* OR Cognitive Dysfunction OR Cognition Disorder* OR forget* or confused or confusion) Timespan: All years. Indexes: BCI. |
753 |
| 6. LILACS (BIREME) Date of search: 13 February 2019 |
Addenbrooke$ Cognitive Exam$ OR ACE OR Mini‐Addenbrooke$ Cognitive Exam$ OR mini‐ACE OR ACE‐III [Words] and memory OR MCI OR neurocognitive disorder$ OR dement$ OR Alzheimer$ OR Cognitive Dysfunction OR Cognition Disorder$ OR forget$ or confused or confusion | 33 |
| TOTAL | 5655 | |
| TOTAL after de‐duplication | 2937 | |
Appendix 2. Study data to be included in the data collection proforma
Bibliographic details of primary paper: author, title of study, year, and journal.
Details of index test: method of ACE‐III and mini‐ACE administration, including who administered and interpreted the test, and their training. Thresholds used to define positive and negative tests.
Reference standard: reference standard used. Method of reference standard administration, including who administered the test and their training.
Study population: number of subjects. Age. Gender. Other characteristics. Settings: community, primary care, secondary care outpatients, and secondary care inpatients and residential care. Participant recruitment. Sampling procedures. Time between index test and reference standard. Proportion of people in sample with dementia. Subtype and stage of dementia if available. MCI definition used (if applicable). Attrition and missing data.
Appendix 3. QUADAS‐2 tool
| DOMAIN | PARTICIPANT SELECTION | INDEX TEST | REFERENCE STANDARD | FLOW AND TIMING |
| Description | Describe methods of participant selection: describe included participants (prior testing, presentation, intended use of index test and setting): | Describe the index test and how it was conducted and interpreted | Describe the reference standard and how it was conducted and interpreted. | Describe any participants who did not receive the index test(s) and/or reference standard or who were excluded from the 2x2 table (refer to flow diagram): describe the time interval and any interventions between index test(s) and reference standard: |
| Signalling questions (yes/no/unclear) | Was a consecutive or random sample of participants enrolled? | Were the index test results interpreted without knowledge of the results of the reference standard? | Is the reference standard likely to correctly classify the target condition? | Was there an appropriate interval between index test(s) and reference standard? |
| Was a case‐control design avoided? | If a threshold was used, was it prespecified? | Were the reference standard results interpreted without knowledge of the results of the index test? | Did all participants receive a reference standard? | |
| Did the study avoid inappropriate exclusions? | Did all participants receive the same reference standard? | |||
| Were all participants included in the analysis? | ||||
| Risk of bias: (high/low/unclear) |
Could the selection of participants have introduced bias? | Could the conduct or interpretation of the index test have introduced bias? | Could the reference standard, its conduct, or its interpretation have introduced bias? | Could the participant flow have introduced bias? |
| Concerns regarding applicability: (high/low/unclear) |
Are there concerns that the included participants do not match the review question? | Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Are there concerns that the target condition as defined by the reference standard does not match the review question? |
Appendix 4. QUADAS‐2 anchoring statements
We have adapted the core anchoring statements provided for use with the QUADAS‐2 tool. The original anchoring statements were determined from a two day multi‐disciplinary group meeting, designed for use with the QUADAS‐2 tool to support decisions concerning methodological quality for studies included in systematic reviews. Some of the original anchoring statements are less applicable to DTA reviews of neuropsychological assessments (ref MMSE review, etc.). Thus, two authors (LCB, APB) adapted the original anchoring statements specifically for this review, and these revised statements were reviewed by the co‐authors. The tool and anchoring statements will be piloted against the first five studies included in this review and if there is poor inter‐rater agreement of study methodological quality, the statements will be revised and re‐piloted until good agreement between raters is achieved.
Domain 1: participant selection
Was a consecutive or random sample of participants enrolled?
The method of sampling should be stated or described. Non‐random sampling, sampling based on volunteers, or selecting participants from a clinic or research population is more likely to introduce a high risk of bias and should be classified as such, whereas consecutive or random sampling are least likely to introduce bias, and should be classified as low risk.
Weighting: high risk
Was a case control design avoided?
Case control designs are associated with a high risk of bias and should be excluded from this review. However, nested case control studies (where the study population is drawn from a larger pool of patients from an interventional or cohort study) are associated with a lower risk of bias, and are considered for inclusion in this review. Nested‐case control studies should be classified as a high risk of bias, and any study which increases or decreases the proportion of patients with the target condition (i.e. enrichment from secondary care settings) should be classified as high risk of bias.
Weighting: high risk
Did the study avoid inappropriate exclusions?
Studies which do not explicitly detail exclusion criteria will be classified as unclear risk of bias, but study authors will be contacted for this information. Studies which clearly detail all exclusions, and are felt to be appropriate by review authors will be classified as low risk of bias. Exclusion criteria must be justified for studies which exclude difficult to diagnose groups. It is anticipated that there will be common exclusion criteria (e.g. substance misuse, other degenerative disease) for included studies, which are listed in the protocol. Community studies with extensive exclusion criteria should be classified at high risk of bias. Post‐hoc exclusions will be classified as high risk of bias.
Weighting: high risk
Domain 2: index test
Could the conduct or interpretation of the ACE‐III/mini‐ACE have introduced bias?
Studies will be considered low risk where the investigators conducting the ACE‐III/mini‐ACE were blinded to the participant’s diagnosis or were independent from the study and without knowledge of the reference standard. Studies which explicitly state this do not require further information on the blinding or independence of the process and will be classified as low risk of bias. Studies will be classified as low risk of bias if the ACE‐III or mini‐ACE were conducted prior to the reference standard.
Weighting: high risk
Were the ACE‐III/mini‐ACE thresholds pre‐specified?
A study will be classified as high risk of bias where the authors set the optimal cut off point post‐hoc using their own study data. Studies that do not use defined thresholds, and use an alternative methods of analysis will be classified as not applicable.
Weighting: high risk
Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study?
For studies to be classified at low risk of bias, information on the method of administration (i.e. appropriately qualified/trained), and the language of assessment should be provided. If a translated version of the ACE‐III or mini‐ACE is used, details of the scale and on the validation process will be needed to be classified at low risk of bias.
Weighting: low risk
Domain 3: reference standard
Is the reference standard likely to correctly classify the target condition?
Studies using reference standards listed in the protocol or a recognised/validated reference standard will be considered at low risk of bias. Studies using a reference standard not recognised by the authors or the Cochrane Dementia and Cognitive Improvement Group, will be classified at high risk of bias.
Weighting: high risk
Were the reference standard results interpreted without knowledge of the results of the ACE‐III/mini‐ACE?
For a study to be classified as low risk of bias, the investigators would need to have interpreted the reference standard results independently to those of the ACE‐III or mini‐ACE. Studies which explicitly state this do not require further information on the blinding or independence of the process and will be classified as low risk of bias. If the ACE‐III or mini‐ACE were used as part of the clinical dementia/MCI assessment as reference standard, this will be considered to be at high risk of bias.
Weighting: high risk
Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study?
The method of dementia assessment will need to be described to be considered at low risk of bias. Information should be provided on: the training and expertise of the assessor, whether it was by individual, consensus, or algorithm, and the use of neuropsychological, laboratory and neuroimaging assessments.
Weighting: high risk if not described
Domain 4: patient flow and timing
Was there an appropriate interval between the ACE‐III or mini‐ACE and the reference standard?
Ideally, the reference standard and ACE‐III or mini‐ACE would be completed on the same day or visit, to minimise changes or fluctuations in cognition over time. However, dementia is slowly progressive and an irreversible condition so delay is unlikely to introduce significant bias. However, patients with MCI can revert to normal cognition, progress, or remain stable over time. Therefore, a time delay could affect the measured cognitive status of these individuals, however the duration over which this might occur is not known. We have therefore set an arbitrary cut off of one month for studies assessing MCI. Longitudinal and delayed verification studies are excluded from this review.
Weighting: low risk
Did all subjects receive the same reference standard?
Where the clinical assessment or reference standard differs between participants in a study, this will be classified at high risk of bias. Participants who score test positive on the ACE‐III or mini‐ACE who are subject to further testing above other participants will be classified at high risk of bias.
Weighting: high risk
Were all participants included in the final analysis?
Attrition will vary with study design, but drop‐out rates and missing data should be reported and accounted for. Where attrition is higher than expected (greater than 20% of study cohort), these studies will be classified at high risk of bias.
Weighting: high risk
Applicability
Were those included representative of the general population?
The included participants should match the intended population as described in the review protocol. The setting of the included study will need to be taken into account, and the prevalence of the disease within that setting. Included participants should be presenting with cognitive decline, but the disease status should not be known at the time of administering the ACE‐III or mini‐ACE. Studies will be classified as low applicability where they included a highly selected population, or sub‐group.
Was the ACE‐III or mini‐ACE performed consistently and in a manner similar to its use in clinical practice?
Variation in the length, structure, language, and/or administration of the ACE‐III or mini‐ACE not in line with the original description of the ACE‐III or mini‐ACE may affect the applicability. Included studies will be judged against the original description of the ACE‐III or mini‐ACE.
Was the clinical diagnosis of dementia or MCI (reference standard) made in a manner similar to current clinical practice?
Although studies may have utilised a validated reference standard for the diagnosis of dementia or MCI, there is a risk that the reference standard may over‐ or under‐diagnose the proportion of participants with the disease. If there are concerns that the reference standard diagnosed a smaller or larger than anticipated proportion of participants given the specified clinical population, this would be rated as poor applicability.
Appendix 5. STADRDdem reporting criteria
| Section | Number | Description |
| Title/abstract/keywords | 1 | Identify the article as a study of diagnostic accuracy |
| Introduction | 2 | State the research questions or study aims, such as estimating diagnostic accuracy or comparing accuracy between tests across participant groups |
| Methods | ||
| Participants | 3 | The study population: the inclusion and exclusion criteria, setting and locations where data were collected |
| 4 | Participant recruitment: was recruitment based on presenting symptoms, results from previous tests, or the fact that the participants had received the index test or the reference standard? | |
| 5 | Participant sampling: was the study population a consecutive series of participants defined by the selection criteria in items 3 and 4? If not, specify how participants were further selected. | |
| 6 | Data collection: Was data collection planned before the index test and reference standard were performed (prospective study) or after (retrospective study)? | |
| Test methods | 7 | The reference standard and its rationale |
| 8 | Technical specifications of materials and methods involved including how and when measurements were taken, and/or cite references for index tests and reference standard | |
| 9 | Definition of and rationale for the units, cut‐offs, and/or categories of the results of the index tests and the reference standard | |
| 10 | The number, training, and expertise of the persons executing and reading the index tests and the reference standard | |
| 11 | Whether or not the readers of the index tests and the reference standard were blinded (masked) to the results of the other test and describe other clinical information to the readers | |
| Statistical methods | 12 | Methods for calculating or comparing measures of diagnostic accuracy, and the statistical methods used to quantify uncertainty |
| 13 | Methods for calculating test reproducibility if done | |
| Results | ||
| participants | 14 | When the study was performed, including beginning and end dates of recruitment |
| 15 | Clinical and demographic characteristics of the study population | |
| 16 | The number of participants satisfying the criteria for inclusion who did or did not undergo the index test and/or the reference standard, describe why participants failed to undergo either test | |
| Test results | 17 | Time interval between the index tests and the reference standard and any treatment administered in between |
| 18 | Distribution of severity of disease in those with the target condition; other diagnoses in participants without the target condition | |
| 19 | A cross‐tabulation of the results of the index tests (including indeterminate and missing results) by the results of the reference standard, for continuous results, the distribution of the test results by the results of the reference standard. | |
| 20 | Any adverse events from performing the index tests or the reference standard | |
| Estimates | 21 | Estimates of diagnostic accuracy and measures of statistical uncertainty |
| 22 | How indeterminate results, missing data, and outliers of the index tests were handled | |
| 23 | Estimates of variability of diagnostic accuracy between subgroup of participants, readers, or centres if done | |
| 24 | Estimates of test reproducibility if done | |
| Discussion | ||
| 25 | Discuss the clinical applicability of the study findings | |
Appendix 6. Summary of included studies
| Study ID | Population and setting | Sample size (n) | Age (years) | Index test | Language | Test thresholds | Target condition(s) and prevalence (%) | Reference standard | Comparisons |
| Hobson 2016 | Outpatient clinic Chronic kidney disease and type 2 diabetes, aged>60 |
118 | Range: 76 to 80 | Mini‐ACE | English | 21, 25 | Dementia ‐ 34 MCI ‐ 29 |
DSM‐V Petersen |
Dementia vs. none (MCI + no cognitive impairment) MCI vs. no cognitive impairment |
| Jubb 2015 | Outpatient memory clinic, UK | 69 | Range: 75 to 85 | ACE‐III | English | 82, 88. optimal | Dementia ‐ 55.9 | DSM‐IV (dementia) NINCDS/ADRDRA (Alzheimer's disease) NINDS‐AIREN (vascular dementia) Petersen (MCI) |
Dementia vs. none High (>11 years) vs. low education |
| Larner 2019 | Outpatient memory clinic, UK | 755 | Median: 60 | Mini‐ACE | English | 21, 25, optimal | Dementia ‐ 15 MCI ‐ 29 |
DSM‐V Petersen |
Dementia vs. none (MCI + no cognitive impairment) MCI vs. no cognitive impairment |
| Lees 2017 | Inpatient stroke rehabilitation units, UK | 86 | Median: 74 | ACE‐III | English | 82 | Post‐stroke dementia ‐ 53 | Guidelines (Brainin 2014) | Dementia vs. none |
| Li 2019 | Department of Neurology, China | 176 | Mean: 74.1 | ACE‐III | Chinese | Optimal | Dementia ‐ 32.4 MCI ‐ 36.4 |
DSM‐V (dementia) Petersen criteria (MCI) NINCDS/ADRDA (Alzheimer's disease) ICD‐10 (vascular dementia), DSM‐V (frontotemporal dementia. lewy‐body dementia, and Parkinson's disease dementia) |
Dementia vs. none (MCI + no cognitive impairment) MCI vs. no cognitive impairment High (> 12 years) vs. low education |
| Takenoshita 2019 | Outpatient memory clinic, Japan | 389 | Range: 72 to 79 | ACE‐III | Japanese | Optimal | Dementia ‐ 48.5 MCI ‐ 35.2 |
DSM‐V (dementia) Petersen criteria (MCI) NINCDS/ADRDA (Alzheimer's disease) ICD‐10 (vascular dementia), DSM‐V (frontotemporal dementia, Lewy‐body dementia, and Parkinson's disease dementia) |
Dementia vs. none (MCI + no cognitive impairment) MCI vs. no cognitive impairment |
| Yang 2019 | Department of Neurology, China | 169 | Range: 72 to 75 | Mini‐ACE | Chinese | Optimal | Dementia ‐ 37.9 MCI ‐ 32 |
DSM‐V (dementia), Petersen criteria (MCI), NINCDS/ADRDA (Alzheimer's disease), ICD‐10 (vascular dementia), DSM‐V (frontotemporal dementia. Lewy‐body dementia, and Parkinson's disease dementia) | Dementia vs. none (MCI + no cognitive impairment) MCI vs. no cognitive impairment |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
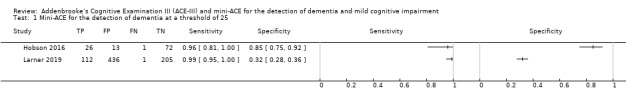
Mini‐ACE for the detection of dementia at a threshold of 25.
2. Test.
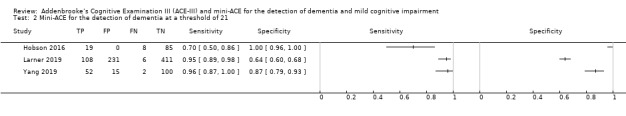
Mini‐ACE for the detection of dementia at a threshold of 21.
3. Test.
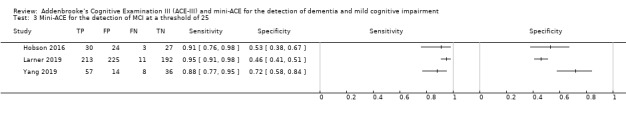
Mini‐ACE for the detection of MCI at a threshold of 25.
4. Test.

Mini‐ACE for the detection of MCI at a threshold of 21.
5. Test.

ACE‐III for the detection of dementia at a threshold of 88.
6. Test.

ACE‐III for the detection of dementia at a threshold of 82.
7. Test.
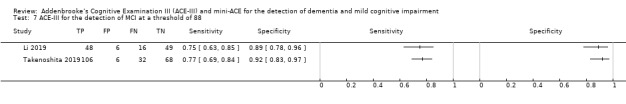
ACE‐III for the detection of MCI at a threshold of 88.
8. Test.

ACE‐III for the detection of post‐stroke cognitive impairment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hobson 2016.
| Study characteristics | |||
| Patient sampling | 118 patients attending outpatient clinic appointments who were aged over 60 years and had a diagnosis of chronic kidney disease (CKD) (eGFR < 60 ml/min/1.73 m²), and a diagnosis of diabetes. Participants who had a pre‐existing diagnosis of stroke, cognitive impairment, or dementia were excluded from the study. The sampling procedure was not well described and it is not clear if this was a consecutive or random sample of patients. The following additional information was provided by the study author: a consecutive sample of patients attending a renal diabetic clinic were enrolled. Patients were excluded if they had had a stroke or pre‐existing neurocognitive disorder. All patients were screened for cognitive impairment as part of routine clinical management. |
||
| Patient characteristics and setting | This study included 118 patients over the age of 60 with diagnoses of CKD and diabetes. Participants were a community‐based sample attending an outpatient clinic appointment. The type of clinic and geographical location were not specified. All patients were screened with ACE‐III and MMSE, and the mini‐ACE scores were derived from the ACE‐III assessment. The diagnosis of dementia and MCI was based upon patient, informant, clinical case review, neuropsychological assessment, and application of the DSM‐V and Petersen criteria respectively. In addition, MCI was diagnosed on the basis of patients', caregivers', informants', or clinicians' observed or reported symptoms of cognitive impairment, ability to perform activities of daily living, in the absence of delirium or dementia. Further information provided by the author on request: the diagnosis of dementia was reached by consensus by all of the authors in this study, who also clinically managed all of the patients participating in the study. 27 participants had a diagnosis of dementia, 33 had a diagnosis of MCI, and 52 had no diagnosis of cognitive impairment. The prevalence of dementia in this sample was 24%. There were no significant differences in baseline characteristics between participant groups. Age in the non‐cognitively impaired group was 76.4 ± 7.4 years, in the MCI group 78.1 ± 10.1 years, and in the dementia group 79.8 ± 5.4 years. Gender male:female, non‐cognitively impaired group 27:13, MCI 18:12, dementia 16:10. Education for the non‐cognitively impaired group was 10.9 ± 1.9 years, for the MCI group 10.7 ± 1.8 years, and for the dementia group 10.5 ± 2.5 years. Total mini‐ACE score for the non‐cognitively impaired group was 27.1 ± 1.95, MCI 22.5 ± 1.91, dementia: 17.2 ± 4.5. Sources of the referrals were not specified. |
||
| Index tests | The mini‐ACE was the index test, but scores were derived from the ACE‐III. Basic details of the mini‐ACE were provided, but no details on the administration or training of those conducting and interpreting the test. The test thresholds were pre‐specified at 21 and 25. Additional information provided by the author on the conduct of the index test: the assessments were completed by the physician in clinic due to a lack of clinical staff with suitable training and expertise in delivering cognitive assessment in the real clinic. |
||
| Target condition and reference standard(s) | Target condition: dementia and MCI Reference standards: DSM‐V (dementia) or Petersen criteria (MCI) |
||
| Flow and timing | Of the original sample of 118 patients, 112 were included in the final analysis. 6 patients were unable to complete the ACE‐III (4 due to visual impairment, 1 due to learning difficulties, and 1 patient declined participation). The time interval between the reference standard and the index test was unclear. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. Further information provided by the author: the time interval between the mini‐ACE/ACE‐III and the diagnosis was not reached on the same day due to the assembly of patient and significant other reports, review of records, tests and getting the multidisciplinary team together. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Jubb 2015.
| Study characteristics | |||
| Patient sampling | 69 patients presenting to an outpatient memory service for the investigation of a memory or cognitive complaint, over a period of 16 months. Participants were aged between 75 and 85 years, not established on anti‐dementia medication, had capacity to consent to the study, were not distressed by the assessment process, and had not completed the ACE‐III as part of their clinical assessment. Exclusion criteria for the study were: unable to complete the ACE‐III or there was significant evidence for an alternative cause for their cognitive impairment that was not degenerative or vascular in pathology (i.e. substance misuse, head injury, epilepsy, severe mood disorder). Participants were identified through initial clinical assessment appointments but it was not clear if this was a random or consecutive sample. |
||
| Patient characteristics and setting | 69 patients were recruited from a memory clinic at the Leeds and Yorkshire NHS Foundation Trust Memory Service. Sources of referrals were not reported. A diagnosis of dementia was made by an old age psychiatrist or specialist registrar using clinical history or informant report, neuroimaging, brief cognitive assessment, mood assessment, and dementia screening blood tests. If the diagnosis remained unclear, participants underwent a comprehensive neuropsychological assessment with a clinical psychologist. 33 participants had a diagnosis of dementia, 26 had no diagnosis of dementia. The prevalence of dementia in the sample was 55.9%. There was a significant difference in the proportion of male and female participants between the groups, but no other differences were significant in the participant demographics. Age of the participants with no dementia was 79.5 ± 2.8 years and for those with dementia was 80.4 ± 2.7 years. In the group without dementia 73.1% were male; and in the group with dementia 51.5% were male. Within the dementia group the sub‐types were as follows: Alzheimer's disease (57.6%), cerebrovascular disease mixed with Alzheimer's disease (30.3%), vascular dementia (12.1%), MCI (76.9%). Those without cognitive impairment made up 23.1% of the total sample. In the dementia group, the average number of years of education were 13.1 ± 3.4, and in those without dementia was 12.1 ± 2.5 years. ACE‐III scores for the participants without dementia were 87.3 ± 5.9, and for those with dementia were 70.4 ± 12.5. |
||
| Index tests | The index test was the ACE‐III which was administered by either an experienced clinical psychologist or 1 of 3 postgraduate researchers who were trained in the administration of the ACE‐III. The ACE‐III scores were checked for consistency to improve the test reliability. Test thresholds of 82 and 88 were pre‐specified, but optimal cut‐offs were also calculated using study data. | ||
| Target condition and reference standard(s) | Target conditions: dementia, Alzheimer's disease, vascular dementia, Alzheimer's disease with cerebrovascular disease, and MCI. Reference standards: DSM‐IV (dementia), NINCDS/ADRDRA (Alzheimer's disease), NINDS‐AIREN (vascular dementia), and Petersen (MCI). |
||
| Flow and timing | 69 patients agreed to the initial contact, but of these 6 declined to participate, 1 lacked capacity to consent, 1 participant withdrew from the process, 1 participant had an unclear diagnosis, and 1 participant put suboptimal effort into completing the ACE‐III. Therefore, the total sample was 59 patients. The time interval between the reference standard and the index test was unclear. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| High | |||
Larner 2019.
| Study characteristics | |||
| Patient sampling | 755 consecutive new outpatient referrals to a dedicated cognitive function clinic based at a regional neuroscience centre, located in the northwest of the UK. Patients were seen between June 2014 and December 2018. There were no specific exclusion criteria, excepting patients with an established diagnosis of dementia. |
||
| Patient characteristics and setting | 755 new outpatient referrals were recruited from a dedicated cognitive function clinic in the northwest of the United Kingdom. The diagnosis was made by an experienced clinician using diagnostic criteria. The results of the mini‐ACE were not used in the final diagnosis. No further information on the diagnostic process was provided in this publication; however the process was detailed as follows in a previous report (Williamson 2018): the diagnosis was made by an experienced clinician, based upon patient interview, collateral history (if available), neuroimaging, and neuropsychological assessment. 114 patients were diagnosed with dementia, 22 with MCI, and the remaining 419 patients with subjective memory complaints. The median age of the whole sample was 60 years, and 47% of the sample were female. No further information on participant characteristics was provided. The prevalence of dementia in the sample was 15%, and 29% for MCI. The sources of the referrals were not specified. |
||
| Index tests | The index test was the mini‐ACE. There was no information on the training or expertise of the person administrating the mini‐ACE. There were no details provided on the administration of the mini‐ACE. Test thresholds of 21 and 25 were pre‐specified but optimal cut‐offs were also calculated using study data. | ||
| Target condition and reference standard(s) | Target condition: dementia and MCI Reference standards: DSM‐IV (dementia) or Petersen criteria (MCI). |
||
| Flow and timing | 755 patients were recruited. Dropout rates were not reported. No information on the time interval between the test and the reference standard; however in a previous report (Williamson 2018) the tests were completed on the same day. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Did all patients receive a reference standard? | Unclear | ||
| Unclear | |||
Lees 2017.
| Study characteristics | |||
| Patient sampling | 86 patients who were admitted to 1 of 2 University Hospital stroke rehabilitation units. Participants were recruited who had a confirmed diagnosis of stroke at a minimum of 2 weeks post‐event. Patients were excluded if the clinical team felt that cognitive assessment was inappropriate. Patients with depression or delirium were not excluded from the study. Additional information from author: study sampling was sequential, but assessors did not go to the ward every day, and not everyone who was eligible agreed to participate, but everyone was asked if they would like to participate. The authors were not able to provide information on numbers excluded as a result of advice from the clinical team. |
||
| Patient characteristics and setting | 51 patients at 2 weeks post confirmed acute stroke were recruited over a 6‐month period from 2 stroke rehabilitation units. The geographical location was not specified. Participants underwent a multidisciplinary team assessment of cognition, and the final diagnosis was made by an experienced consultant in Geriatric Medicine based upon clinical psychology and occupational therapy assessments. 27 patients were diagnosed with post‐stroke cognitive impairment. The prevalence of dementia in the sample was 53%. The median age of the total sample was 74 years (interquartile range (IQR): 67 to 84 years), and 55% of the total sample were female. The median National Institutes of Health Stroke Scale was 9 (IQR: 6 to 13). 76% of the participants had an ischaemic stroke, and of 35% had a total anterior circulation stroke. The median time since stroke was 36 days (IQR: 20 to 55). 16% of patients were diagnosed with delirium, 8% had a pre‐existing diagnosis of dementia, and 12% of participants had pre‐stroke depression. |
||
| Index tests | The index test was the ACE‐III, in addition to the MMSE, and MoCA. The index test was performed by 1 of 2 psychology graduates who were trained in the use of the scales. The tests were administered as paper and pencil using verbal instructions in the first instance, and then further assistance if required. In total, 4 approaches were taken to completing the cognitive assessments, and test accuracy data are provided for each of the 4 approaches. The first approach excluded patients whose testing was incomplete and assigned a score of zero to partially completed items. At a threshold of 82, test sensitivity for approach 1 was 87% (95% CI 66% to 97%), and specificity was 5% (95% CI 1% to 25%). The second approach excluded patients with incomplete testing, and adapted partially completed assessments by excluding non‐completed items from the total score. At a test threshold of 82, the sensitivity for approach 2 to detect cognitive impairment was 81% (95% CI 59% to 95%), and specificity was 10% (95% CI 1% to 31%). The third approach excluded all patients with either incomplete or partially incomplete tests. At a cut‐off of 82, the sensitivity of approach 3 to detect cognitive impairment was 93% (95% CI 66% to 100%), and specificity was 11% (95% CI 1% to 35%). The final approach was the most inclusive which included all patients, assigning a score of zero to any incomplete items. The sensitivity to detect cognitive impairment at a threshold of 82 for approach 4 was 90% (95% CI 73% to 98%), and specificity was 5% (95% CI 1% to 22%). Participants were re‐approached 1 week later if they were unable to complete the test at the first trial. The thresholds for the ACE‐III were pre‐specified at 82 and 88. | ||
| Target condition and reference standard(s) | Target condition: post‐stroke cognitive impairment. Reference standard: guidelines on the diagnosis of post‐stroke cognitive impairment (Brainin 2014). Additional information from the author: no requests were made from the clinical team conducting the reference standard for results of the index tests. |
||
| Flow and timing | 86 patients were admitted to the stroke rehabilitation units. 51 patients were included in the final analysis. 75 patients were eligible for the study, 24 were excluded due to: lack of capacity/no representative for consent (13), refused assessment (6), inappropriate to approach (2), discharged before test complete (2), language barrier (1). No information on the time interval between the test and the reference standard. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. Additional information from the author: the reference standard was performed weekly at the multi‐disciplinary team meetings and so the longest time from index test to reference standard was a few days. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Li 2019.
| Study characteristics | |||
| Patient sampling | 176 participants were recruited from the Department of Neurology, Sichuan Provincial People’s Hospital, Chengdu, China. Inclusion criteria were: Chinese speaking, aged over 60 years, reasonable vision, hearing, and ability to communicate. Exclusion criteria were: history of major depression, schizophrenia, epilepsy, significant head injury, substance abuse, alcoholism, or other severe physical disorders. The sampling procedure was not well described and it was unclear if this was a consecutive or random sample. | ||
| Patient characteristics and setting | This study included 176 Chinese‐speaking participants over the age of 60, who were recruited from the Department of Neurology in Chengdu, China. The diagnosis of dementia was based upon demographic information, history or informant report, presentation at interview, general and neurological examination, neuropsychological examination, neuroimaging, screening blood tests. Daily and social function was evaluated using the Clinical Dementia Rating Scale. The Common Objects Memory Test was used to assess cognitive deficits. The diagnoses were based on the DSM‐V criteria for dementia, and Petersen criteria for MCI. The healthy group had no memory complaints, and normal activities of daily living. Diagnoses were made by 1 of 2 neurologists who checked each other's decisions, and disputes were resolved by consensus. 55 participants were healthy, 64 had a diagnosis of MCI, and 57 had a diagnosis of mild dementia. The prevalence of dementia in the sample was 32.4%, and for MCI 36.4%. All participants with dementia were classified as mild severity, defined as Clinical Dementia Rating Scale of 1. The mean age of the MCI and mild dementia groups were significantly older than the healthy participants, and the healthy group had significantly more years of education than the MCI and dementia groups. The total age of the sample was 74.14 ± 6.68 years; healthy: 72.2 ± 6.48 years, MCI: 75.12 ± 6.41 years, mild dementia: 74.89 ± 6.90 years. The total sample comprised 55.7% men. 60% of healthy participants were male, 56.3% of the MCI group, and 50.9% of the mild dementia group. The mean years of education for each of the groups were: healthy: 12.49 ± 4.25 years, MCI: 11.14 ± 3.64 years, mild dementia: 9.68 ± 4.27 years. The mean ACE‐III scores for each of the groups were: healthy: 90.25 ± 4.74, MCI: 81.98 ± 6.45, mild dementia: 65.18 ± 9.65. The sources of participants were not specified. |
||
| Index tests | The index test was the ACE‐III which was translated and adapted culturally for a Chinese‐speaking population using forward and backward translation methods. The translation and adaptation procedures were well described but there was no information on the training or expertise of the assessor. Test thresholds were not pre‐specified, and the authors calculated optimal thresholds based on their study data. The authors also investigate the accuracy of the Chinese versions of the MoCA and MMSE. | ||
| Target condition and reference standard(s) | Target condition: dementia and MCI Reference standards: DSM‐V (dementia), Petersen criteria (MCI), NINCDS/ADRDA (Alzheimer's disease), ICD‐10 (vascular dementia), DSM‐V (frontotemporal dementia), Lewy body dementia, and Parkinson's disease dementia. |
||
| Flow and timing | 176 patients were recruited. Dropout rates were not reported. The index test was completed within 1 week of the reference standard. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Takenoshita 2019.
| Study characteristics | |||
| Patient sampling | 389 participants were recruited from the memory clinic at Okayama University Hospital in Japan between January 2013 and March 2017. Inclusion criteria were: received general, physical and neurological examinations; laboratory testing; underwent the MMSE and the Hasegawa Dementia Scale‐revised; and neuroimaging. The exclusion criteria were: the presence of delirium or the existence of psychiatric diseases. Additional information provided by the author: they confirmed this was a cross‐sectional study and that a consecutive sample of patients was enrolled. |
||
| Patient characteristics and setting | The study recruited 389 participants from an outpatient memory clinic at Okayama University Hospital between January 2013 and March 2017. Participants underwent general, physical and neurological examinations, laboratory testing, MMSE, Hasegawa Dementia Scale‐revised, and neuroimaging. Neuropsychological assessment was conducted by clinical psychologists, and the Clinical Dementia Rating Scale by a clinician. The diagnosis was by consensus between 2 or more geriatric psychiatrists and 2 or more experienced clinical psychologists. 178 patients were diagnosed with dementia, 137 with MCI, and 74 were healthy. The prevalence of dementia in the sample was 48.5%, and 35.2% for MCI. All participants with a diagnosis of dementia were classified as mild severity, defined as a Clinical Dementia Rating Scale of 0.5 to 1. In the dementia group, 131 patients were diagnosed with Alzheimer’s disease dementia, 21 with dementia with Lewy bodies, 9 with frontotemporal dementia, 4 with vascular dementia, and 13 with another or unknown subtype. Participants in the MCI (75.3 ± 8.3 years) and dementia (78.6 ± 7.2 years) groups were significantly older than those in the healthy group (72.1 ± 7.1 years). 40.5% of the healthy group were male, compared to 51.8% of the MCI group, and 37.1% of the dementia group. The dementia group had significantly fewer years of education (12.0 ± 2.4 years), compared to the MCI (13.0 ± 2.7 years), and healthy (12.9 ± 2.3 years) groups. The mean ACE‐III scores were: healthy: 93.5 ± 3.4; MCI: 82.7 ± 7.2; dementia: 66.0 ± 11.4. The sources of the referrals were not specified. |
||
| Index tests | The index test was the ACE‐III which was translated and adapted culturally for a Japanese‐speaking population. The translation and adaptation procedures were not well described. Test thresholds were not pre‐specified, and the authors calculated optimal thresholds based on their study data. Additional information provided by the study author: the ACE‐III and other tests were performed by 3 Certified Public Psychologists, who had more than 10 years of clinical experience in psychological testing in dementia, and they received thorough training before conducting the ACE‐III. The ACE‐III was conducted without the knowledge of the patient diagnoses. | ||
| Target condition and reference standard(s) | Target condition: dementia and MCI Reference standards: National Institute on Aging‒Alzheimer’s Association (probable Alzheimer's disease and MCI), McKeith Criteria (Lewy body dementia), FTDC criteria (fronto‐temporal dementia), American Heart Association/American Stroke Association guidelines (vascular dementia). |
||
| Flow and timing | 389 patients were recruited. Dropout rates were not reported. The time interval between the index and the reference standard was unclear. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. Additional information from the author: there were fewer than 1% dropouts from the study. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Unclear | |||
Yang 2019.
| Study characteristics | |||
| Patient sampling | 169 patients were recruited from the Department of Neurology, Sichuan Provincial People’s Hospital, Chengdu, China. Inclusion criteria were: Chinese speaking, aged over 60 years, reasonable vision, hearing, and ability to communicate. Exclusion criteria were: major depression, schizophrenia, epilepsy, significant head injury, substance abuse, alcoholism, or other disorders
which might influence task performances. The sampling procedure was not well described and it was unclear if this was a consecutive or random sample. |
||
| Patient characteristics and setting | This study included 169 Chinese‐speaking participants over the age of 60, who were recruited from the Department of Neurology in Chengdu, China. The diagnosis of dementia was based upon demographic information, history or informant report, presentation at interview, general and neurological examination, neuropsychological examination, neuroimaging, screening blood tests. Daily and social function was evaluated using the Clinical Dementia Rating Scale. The Common Objects Memory Test was used to assess cognitive deficits. The diagnoses were based on the DSM‐V criteria for dementia, and Petersen criteria for MCI. The healthy group had no memory complaints, and normal activities of daily living. Diagnoses were made by 1 of 2 neurologists who checked each other's decisions, and disputes were resolved by consensus. All participants with dementia were classified as mild severity, defined as Clinical Dementia Rating Scale of 1. 54 patients had a diagnosis of dementia, 64 had a diagnosis of MCI, and 51 were healthy. The prevalence of dementia in the sample was 32%, and 37.8% for MCI. Of the patients diagnosed with dementia, 24 were diagnosed with Alzheimer's disease, 14 with vascular dementia, 10 with mixed dementia, 3 with Lewy body dementia, 2 with frontotemporal dementia, and 1 with Parkinson's disease dementia. There were no differences in age, sex, or years of education across the 3 groups. The mean ages were: healthy: 72.8 ± 6.4 years; MCI: 75.1 ± 6.4 years; and dementia: 75.1 ± 7.0 years. 39% of the healthy participants were female, compared to 43.8% of participants in the MCI group, and 48.1% of participants in the dementia group. The healthy group had a mean of 11.8 ± 4.0 years of education, compared to 11.1 ± 3.6 years in the MCI group, and 10.3 ± 3.7 years in the dementia group. The mean mini‐ACE scores were: healthy: 27.4 ± 1.8; MCI: 24.1 ± 2.4; dementia: 16.5 ± 4.8. The sources of referrals were not specified. |
||
| Index tests | The index test was the ACE‐III which was translated and adapted culturally for a Chinese‐speaking population using forward and backward translation methods. The translation and adaptation procedures were well described but there was no information on the training or expertise of the assessor. Test thresholds were not pre‐specified, and the authors calculated optimal thresholds based on their study data. | ||
| Target condition and reference standard(s) | Target condition: dementia and MCI Reference standards: DSM‐V (dementia), Petersen criteria (MCI), NINCDS/ADRDA (Alzheimer's disease), ICD‐10 (vascular dementia), DSM‐V (frontotemporal dementia) Lewy body dementia, and Parkinson's disease dementia. |
||
| Flow and timing | 169 patients were recruited. Dropout rates were not reported The index test was completed within 1 week of the reference standard. Information on the true positive and negative values were not provided in the original publication and were calculated from sensitivity and specificity data reported in the publication. |
||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Were sufficient data on ACE‐III or mini‐ACE application given for the test to be repeated in an independent study? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Were sufficient information on the method of dementia/MCI assessment given for the assessment to be repeated in an independent study? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive a reference standard? | Yes | ||
| Low | |||
Differences between protocol and review
We included studies examining the screening accuracy of the ACE‐III or mini‐ACE in high‐risk populations, but not necessarily in patients presenting with cognitive decline. Although patients with traumatic and hereditary forms of brain injury were excluded, we have included one study with patients who were post‐stroke which can be considered a form of acquired brain injury. We did not conduct meta‐analysis, meta‐regression, and sensitivity analyses due to too few studies identified and heterogeneity between included studies.
Contributions of authors
LCB developed the draft and final versions of the manuscript.
TGR, VJH, AB, RBP, TJQ, and CPN all reviewed and contributed to the draft and final versions of the manuscript.
LCB and APB independently screened all studies on title and abstract, and at full text.
LCB and APB independently quality assessed all included studies using QUADAS‐2 and the STARDdem criteria.
LCB and ABP independently extracted data from the publications.
TQ mediated disagreements between LCB and APB in quality assessment and inclusion of relevant studies.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Dunhill Medical Trust, Other.
Funding of LCB as a clinical research fellow.
-
NIHR, UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Lucy C Beishon: none known. Angus P Batterham: none known. Terry J Quinn: none known. Ronney B Panerai: none known. Christopher P Nelson: none known. Thompson Robinson: none known. Victoria J Haunton: none known.
New
References
References to studies included in this review
Hobson 2016 {published data only}
- Hobson P, Rohoma KH, Wong SP, Kumwenda MJ. The utility of the Mini‐Addenbrooke's Cognitive Examination as a screen for cognitive impairment in elderly patients with chronic kidney disease and diabetes. Dementia and Geriatric Cognitive Disorders Extra 2016;6(3):541‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jubb 2015 {published data only}
- Jubb MT, Evans JJ. An investigation of the utility of the Addenbrooke’s Cognitive Examination III in the early detection of dementia in memory clinic patients aged over 75 years. Dementia and Geriatric Cognitive Disorders 2015;40(3‐4):222‐32. [DOI] [PubMed] [Google Scholar]
Larner 2019 {published data only}
- Larner AJ. MACE for diagnosis of dementia and MCI: examining cut‐offs and predictive values. Diagnostics (Basel) 2019;9(2):pii: E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 2017 {published data only}
- Lees RA, Hendry K, Broomfield N, Stott D, Larner AJ, Quinn TJ. Cognitive assessment in stroke: feasibility and test properties using differing approaches to scoring of incomplete items. International Journal of Geriatric Psychiatry 2016;32(10):1072‐8. [DOI] [PubMed] [Google Scholar]
Li 2019 {published data only}
- Xiaojia L, Lili Y, Jia Y, Nengwei Y, Fang Y. Validation study of the Chinese version of Addenbrooke’s Cognitive Examination III for diagnosing mild cognitive impairment and mild dementia. Journal of Clinical Neurology (Seoul, Korea) 2019;15(3):313‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takenoshita 2019 {published data only}
- Shintaro T, Seishi T, Hidenori Y, Megumi Y, Mayumi Y, Nao I, et al. Validation of Addenbrooke’s Cognitive Examination III for detecting mild cognitive impairment and dementia in Japan. BMC Geriatrics 2019;19(1):1120‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2019 {published data only}
- Yang L, Li X, Yin J, Yu N, Liu J, Ye F. A validation study of the Chinese Version of the Mini‐Addenbrooke’s Cognitive Examination for screening mild cognitive impairment and mild dementia. Journal of Geriatric Psychiatry and Neurology 2019;32(4):205‐10. [DOI] [PubMed] [Google Scholar]
Additional references
Alzheimer's Disease International 2013
- Alzheimer's Disease International. Ideas and advice on developing and implementing a national dementia plan. Alzheimer's Disease International 2013:13‐4.
Alzheimer's Research UK 2017
- Alzheimer's Research UK. Population screening and targeted case finding policy statement. www.alzheimersresearchuk.org/wp‐content/uploads/2015/01/ARUK‐Screening‐and‐Case‐Finding‐Policy‐Statement‐Jan‐16.pdf (accessed 31 August 2018).
Alzheimer's Society 2015
- Alzheimer's Society. Helping you to assess cognition. a practical toolkit for clinicians. Alzheimer's Society 2015:1‐40.
Alzheimer's Society 2016
- Alzheimer's Society. Demography. https://www.alzheimers.org.uk/info/20091/position_statements/93/demography (accessed 31 August 2018).
American Psychiatric Association 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
American Psychiatric Association 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐V). 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Aminzadeh 2007
- Aminzadeh F, Byszewski A, Molnar F J, Eisner M. Emotional impact of dementia diagnosis: exploring persons with dementia and caregivers' perspectives. Aging & Mental Health 2007;11(3):281‐90. [DOI] [PubMed] [Google Scholar]
Aminzadeh 2012
- Aminzadeh F, Molnar FJ, Dalziel WB, Ayotte D. A review of barriers and enablers to diagnosis and management of persons with dementia in primary care. Canadian Geriatrics Journal 2015;15(3):85‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arevalo‐Rodriguez 2015
- Arevalo‐Rodriguez I, Smailagic N, Roque I Figuls M, Ciapponi A, Sanchez‐Perez E, Giannakou A, et al. Mini‐Mental State Examination (MMSE) for the detection of Alzheimer's disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010783.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Attems 2014
- Attems J, Jellinger K A. The overlap between vascular disease and Alzheimer's disease — lessons from pathology. BMC Medicine 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Banerjee 2016
- Banerjee S, Farina N, Hughes L, Woong K, Sim K. UK‐Korea Initiative: Optimizing the Impacts of National Dementia Strategies. https://www.bsms.ac.uk/_pdf/cds/korea‐presentations/optimizing‐the‐impacts‐of‐national‐dementia‐strategies‐uk‐final‐submitted‐25‐4‐16.pdf.
Birks 2013
- Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD004744.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brainin 2014
- Brainin M, Tuomilehto J, Heiss WD, Bornsteim NM, Bath PM, Teuschl Y, et al. Post‐stroke cognitive decline: an update and perspectives for clinical research. European Journal of Neurology 2014;22(2):229‐e16. [DOI] [PubMed] [Google Scholar]
Care Quality Commission 2014
- Care Quality Commission. Cracks in the Pathway. www.cqc.org.uk/sites/default/files/20141009_cracks_in_the_pathway_final_0.pdf (accessed 31 August 2018):4‐48.
Chen 2013
- Chen S, Boyle LL, Conwell Y, Chiu H, Li L, Xiao S. Dementia care in rural China. Mental Health in Family Medicine 2013;10(3):133‐41. [PMC free article] [PubMed] [Google Scholar]
Chodosh 2004
- Chodosh J, Petitti DB, Elliott M, Hays RD, Crooks VC, Reuben D B, et al. Physician recognition of cognitive impairment: evaluating the need for improvement. Journal of the American Geriatrics Society 2004;52(7):1051‐9. [DOI] [PubMed] [Google Scholar]
Crawford 2012
- Crawford S, Whitnall L, Robertson J, Evans JJ. A systematic review of the accuracy and clinical utility of the Addenbrooke's Cognitive Examination and the Addenbrooke's Cognitive Examination ‐ Revised in the diagnosis of dementia. International Journal of Geriatric Psychiatry 2012;27(7):659‐69. [DOI] [PubMed] [Google Scholar]
Creavin 2016
- Creavin ST, Wisniewski S, Noel‐Storr AH, Trevelyan CM, Hampton T, Rayment D, et al. Mini‐Mental State Examination (MMSE) for the detection of dementia in clinically unevaluated people aged 65 and over in community and primary care populations. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD011145.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2013
- Davis DH, Creavin ST, Noel‐Storr A, Quinn TJ, Smailagic N, Hyde C, et al. Neuropsychological tests for the diagnosis of Alzheimer's disease dementia and other dementias: a generic protocol for cross‐sectional and delayed‐verification studies. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010775.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2015
- Davis DH, Creavin ST, Yip JL, Noel‐Storr AH, Brayne C, Cullum S. Montreal Cognitive Assessment for the diagnosis of Alzheimer’s disease and other dementias. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD010775.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Vugt 2013
- Vugt ME, Verhey FR. The impact of early dementia diagnosis and intervention on informal caregivers. Progress in Neurobiology 2013;110:54‐62. [DOI] [PubMed] [Google Scholar]
Dichgans 2017
- Dichgans M, Leys D. Vascular cognitive impairment. Circulation Research 2017;120(3):573‐91. [DOI] [PubMed] [Google Scholar]
Ganguli 2004
- Ganguli M, Rodriguez E, Mulsant B, Richards S, Pandav R, Bilt J V, et al. Detection and management of cognitive impairment in primary care: The Steel Valley Seniors Survey. Journal of the American Geriatrics Society 2004;52(10):1668‐75. [DOI] [PubMed] [Google Scholar]
Harper 2014
- Harper L, Barkhof F, Scheltens P, Schott JM, Fox NC. An algorithmic approach to structural imaging in dementia. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(6):692‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2016
- Harrison JK, Stott DJ, McShane R, Noel‐Storr AH, Swann‐Price RS, Quinn TJ. Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) for the early diagnosis of dementia across a variety of healthcare settings. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD011333.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Quality Ontario 2014
- Health Quality Ontario. The appropriate use of neuroimaging in the diagnostic work‐up of dementia. Ontario Health Technology Assessment Series 2014;14(1):1‐64. [PMC free article] [PubMed] [Google Scholar]
Hsieh 2013
- Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dementia and Geriatric Cognitive Disorders 2013;36:242‐50. [DOI] [PubMed] [Google Scholar]
Hsieh 2015
- Hsieh S, McGrory S, Leslie F, Dawson K, Ahmed S, Butler CR, et al. The Mini‐Addenbrooke's Cognitive Examination: a new assessment tool for dementia. Dementia and Geriatric Cognitive Disorders 2015;39(1‐2):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalaria 2010
- Kalaria RN, Maestre GE, Arizaga R, Friedland RP, Galasko D, Hall K, et al. Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurology 2008;7(9):812‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korolev 2016
- Korolev IO, Symonds LL, Bozoki AC, Herholz K. Predicting progression from mild cognitive impairment to Alzheimer's dementia using clinical, MRI, and plasma biomarkers via probabilistic pattern classification. PLoS One 2016;11(2):e0138866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larner 2014
- Larner AJ, Mitchell AJ. A meta‐analysis of the accuracy of the Addenbrooke's Cognitive Examination (ACE) and the Addenbrooke's Cognitive Examination‐Revised (ACE‐R) in the detection of dementia. International Psychogeriatrics / IPA 2014;26(4):555‐63. [DOI] [PubMed] [Google Scholar]
Lin 2013
- Lin JS, O'Connor E, Rossom RC, Perdue LA, Eckstrom E. Screening for cognitive impairment in older adults: a systematic review for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2013;159(9):601‐12. [DOI] [PubMed] [Google Scholar]
Lund 1994
- The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. Journal of Neurology, Neurosurgery, and Psychiatry 1994;57(4):416‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malouf 2004
- Malouf R, Birks J. Donepezil for vascular cognitive impairment. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandal 2006
- Mandal PK, Pettegrew JW, Masliah E, Hamilton R L, Mandal R. Interaction between Abeta peptide and alpha synuclein: molecular mechanisms in overlapping pathology of Alzheimer's and Parkinson's in dementia with Lewy body disease. Neurochemical Research 2006;31(9):1153‐62. [DOI] [PubMed] [Google Scholar]
McKeith 2006
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. Journal of Alzheimer's Disease 2006;9(3 Suppl):417‐23. [DOI] [PubMed] [Google Scholar]
McKhann 1984
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34(7):939‐44. [DOI] [PubMed] [Google Scholar]
McKhann 2011
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Clifford JR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging‐Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer's & Dementia 2011;7(3):263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mioshi 2006
- Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE‐R): a brief cognitive test battery for dementia screening. International Journal of Geriatric Psychiatry 2006;21(11):1078‐85. [DOI] [PubMed] [Google Scholar]
Mirza 2017
- Mirza N, Panagioti M, Waheed MW, Waheed W. Reporting of the translation and cultural adaptation procedures of the Addenbrooke's Cognitive Examination version III (ACE‐III) and its predecessors: a systematic review. BMC Medical Research Methodology 2017;17(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasreddine 2005
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 2005;53(4):695‐9. [DOI] [PubMed] [Google Scholar]
Ngo 2015
- Ngo J, Holroyd‐Leduc JM. Systematic review of recent dementia practice guidelines. Age and Ageing 2015;44:25‐53. [DOI] [PubMed] [Google Scholar]
NICE 2011
- NICE. Donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease. www.nice.org.uk/guidance/ta217/chapter/1‐guidance (accessed 31 August 2018).
NICE 2018
- NICE. Dementia: assessment, management and support for people living with dementia and their carers. www.nice.org.uk/guidance/ng97 (accessed 31 August 2018). [PubMed]
Noel‐Storr 2014
- Noel‐Storr AH, McCleery JM, Richard E, Ritchie CW, Flicker L, Cullum SJ. Reporting standards for studies of diagnostic test accuracy in dementia. The STARDdem Initiative. Neurology 2014;83(4):364‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noone 2015
- Noone P. Addenbrooke's Cognitive Examination‐III. Occupational Medicine 2015;65(5):418‐20. [DOI] [PubMed] [Google Scholar]
Panegyres 2016
- Panegyres PK, Berry R, Burchell J. Early dementia screening. Diagnostics (Basel) 2016;6(1):pii: E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2004
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine 2004;256(3):183‐94. [DOI] [PubMed] [Google Scholar]
Petersen 2013
- Petersen RC, Aisen P, Boeve BF, Geda YE, Ivnik RJ, Knopman DS, et al. Mild cognitive impairment due to Alzheimer disease in the community. Annals of Neurology 2013;74(2):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prince 2015
- Prince M, Wimo A, Guerchet M, Ali GC, Wu Y, Prina M. World Alzheimer Report 2015. The global impact of dementia. Alzheimer's Disease International. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed 31 August 2018).
Prince 2016
- Prince M, Comas‐Herrera A, Knapp M, Guerchet M, Karagiannidou M. World Alzheimer Report 2016: Improving healthcare for people living with dementia: coverage, quality and costs now and in the future. Available at www.alz.co.uk/research/WorldAlzheimerReport2016.pdf.
Rascovsky 2011
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134(Pt 9):2456‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2015
- Robinson L, Tang E, Taylor J P. Dementia: timely diagnosis and early intervention. BMJ 2015;350:h3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Román 1993
- Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH. Vascular dementia: diagnostic criteria for research studies. Report of the NINDS‐AIREN International Workshop. Neurology 1993;43(2):250‐60. [DOI] [PubMed] [Google Scholar]
Samsi 2014
- Samsi K, Manthorpe J. Care pathways for dementia: current perspectives. Clinical Interventions in Aging 2014;9:2055‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2017
- Takahashi RH, Nagao T, Gouras GK. Plaque formation and the intraneuronal accumulation of beta‐amyloid in Alzheimer's disease. Pathology International 2017;67(4):185‐93. [DOI] [PubMed] [Google Scholar]
Tsoi 2015
- Tsoi KK, Chan JY, Hirai HW, Wong SY, Kwok TC. Cognitive tests to detect dementia: a systematic review and meta‐analysis. JAMA Internal Medicine 2015;175(9):1450‐8. [DOI] [PubMed] [Google Scholar]
Valcour 2000
- Valcour VG, Masaki KH, Curb JD, Blanchette PL. The detection of dementia in the primary care setting. Archives of Internal Medicine 2000;160(19):2964‐8. [DOI] [PubMed] [Google Scholar]
Velayudhan 2014
- Velayudhan L, Ryu SH, Raczek M, Philpot M, Lindesay J, Critchfield M, et al. Review of brief cognitive tests for patients with suspected dementia. International Psychogeriatrics / IPA 2014;26(8):1247‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2017
- Walker IF, Lord PA, Farragher TM. Variations in dementia diagnosis in England and association with general practice characteristics. Primary Health Care Research & Development 2017;18(3):235‐41. [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Williamson 2018
- Williamson J, Larner A. MACE for the Diagnosis of Dementia and MCI: 3‐Year Pragmatic Diagnostic Test Accuracy Study. Dementia and Geriatric Cognitive Disorders 2018;45:300‐307. [DOI] [PubMed] [Google Scholar]
World Health Organization 2010
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). Geneva (Switzerland): World Health Organization, 2010. [Google Scholar]
World Health Organization 2018
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). Geneva (Switzerland): World Health Organization, 2018. [Google Scholar]
Zhao 2016
- Zhao M, Lv X, Tuerxun M, He J, Luo B, Chen W, et al. Delayed help seeking behaviour in dementia care: preliminary findings from the Clinical Pathway for Alzheimer’s Disease in China (CPAD) study. International Psychogeriatrics / IPA 2016;28(2):211‐9. [DOI] [PubMed] [Google Scholar]


