Abstract
Aim
To assess glycaemic control and factors associated with poor glycaemic control at initiation of second‐line therapy in the DISCOVER programme.
Materials and methods
DISCOVER (NCT02322762 and NCT02226822) comprises two similar prospective observational studies of 15 992 people with type 2 diabetes (T2D) initiating second‐line glucose‐lowering therapy in 38 countries across six regions (Africa, Americas, South‐East Asia, Eastern Mediterranean, Europe and Western Pacific). Data were collected using a standardized case report form. Glycated haemoglobin (HbA1c) levels were measured according to standard clinical practice in each country, and factors associated with poor glycaemic control (HbA1c >8.0%) were evaluated using hierarchical regression models.
Results
HbA1c levels were available for 80.9% of patients (across‐region range [ARR] 57.5%‐97.5%); 92.2% (ARR 59.2%‐99.1%) of patients had either HbA1c or fasting plasma glucose levels available. The mean HbA1c was 8.3% (ARR 7.9%‐8.7%). In total, 26.7% of patients had an HbA1c level ≥9.0%, with the highest proportions in South‐East Asia (35.6%). Factors associated with having HbA1c >8.0% at initiation of second‐line therapy included low education level, low country income, and longer time since T2D diagnosis.
Conclusions
The poor levels of glycaemic control at initiation of second‐line therapy suggest that intensification of glucose‐lowering treatment is delayed in many patients with T2D. In some countries, HbA1c levels are not routinely measured. These findings highlight an urgent need for interventions to improve monitoring and management of glycaemic control worldwide, particularly in lower‐middle‐ and upper‐middle‐income countries.
Keywords: glycaemic control, observational study, type 2 diabetes
1. INTRODUCTION
Early achievement of sustained glycaemic control is a key component of the effective management of patients with type 2 diabetes (T2D), given the well‐established increased risk of diabetes‐related complications associated with hyperglycaemia.1, 2, 3 The UK Prospective Diabetes Study demonstrated that an absolute reduction in glycated haemoglobin (HbA1c) level of 1.0% is associated with risk reductions of 21%, 14% and 37% for diabetes‐related death, myocardial infarction and microvascular complications, respectively.1 In a 10‐year post‐interventional follow‐up of the study cohort, early attainment of glycaemic control was associated with long‐term microvascular benefits,4 and there is also evidence that early attainment of tight glycaemic control is predictive of long‐term glycaemic control.5
On the basis of this evidence, most clinical guidelines advocate a target HbA1c level of either <7.0% or ≤6.5% depending on additional patient‐specific factors such as age, duration of diabetes, comorbidities, and risk of hypoglycaemia.6, 7, 8, 9, 10 Treatment intensification is recommended when patients remain above their HbA1c targets for >3 months after the last intervention. Despite these recommendations, available data, mainly from Europe and North America, indicate poor attainment of glycaemic targets and infrequent implementation of timely treatment intensification.11, 12, 13, 14, 15, 16 Moreover, real‐world data on the management of T2D are scarce in many low‐ and middle‐income countries, in which the rising disease prevalence is a concern.
DISCOVER is a 3‐year, global, prospective, observational study programme designed to describe the disease management patterns and a broad range of associated outcomes, including glycaemic control, in patients with T2D initiating a second‐line glucose‐lowering treatment (defined as adding a glucose‐lowering drug or switching between therapies) after first‐line (defined as the first pharmacological treatment given for the disease) oral therapy in routine clinical practice.17, 18 The aim of the present analysis was to describe the level of glycaemic control in participants in DISCOVER at initiation of second‐line glucose‐lowering therapy. Factors associated with poor glycaemic control were also assessed.
2. MATERIALS AND METHODS
The methods for the DISCOVER study programme have been reported in detail elsewhere17, 18 and are summarized below.
2.1. Study design
The global DISCOVER study programme comprises two similar, 3‐year, non‐interventional, prospective studies conducted simultaneously in 38 countries; DISCOVER (NCT02322762) in 37 countries and J‐DISCOVER (NCT02226822) in Japan. Included countries are divided into regions according to the World Health Organization (WHO) categories: Africa (Algeria and South Africa); the Americas (Argentina, Brazil, Canada, Colombia, Costa Rica, Mexico and Panama); South‐East Asia (India and Indonesia); Europe (Austria, Czech Republic, Denmark, France, Italy, Netherlands, Norway, Poland, Russia, Spain, Sweden and Turkey); Eastern Mediterranean (Bahrain, Egypt, Jordan, Kuwait, Lebanon, Oman, Saudi Arabia, Tunisia and United Arab Emirates); and the Western Pacific region (Australia, China, Japan, Malaysia, South Korea and Taiwan). The study protocols were approved by the appropriate clinical research ethics committees in each participating country, and the relevant institutional review boards at each site. The protocols comply with the Declaration of Helsinki, the International Conference on Harmonization of Good Clinical Practice, and the local regulations for clinical research.
2.2. Site and investigator selection
Characteristics of physicians and practices involved in the management of patients with T2D were assessed in each participating country, before the start of the study, by combining data from peer‐reviewed articles, information from reports published by organizations such as the WHO, and insights from key local diabetes experts who acted as national coordinating investigators.19 The proportions of different types of physicians (primary care physicians, diabetologists, endocrinologists, cardiologists and other specialists) and practices (primary care centres, specialized diabetes centres and different types of hospitals), as well as the location of practices (urban vs rural and geographical distribution within a country), treating patients with T2D in each country were collated. A list of sites that would match these characteristics as closely as possible was then established for each country, and all sites were invited to participate in the study.19
2.3. Patient recruitment
Full inclusion and exclusion criteria are shown in Table S1. Patients aged >18 years (>20 years in Japan) with T2D, who were initiating a second‐line glucose‐lowering therapy were eligible for inclusion if they were not pregnant, were not undergoing dialysis, did not have a history of renal transplant, and if their first‐line therapy was not an injectable agent, a herbal remedy, or a natural medicine alone. The study protocol stated that investigating physicians should invite consecutive eligible patients to participate in the study. All participating patients provided signed informed consent.
2.4. Data collection
Data were collected at initiation of second‐line glucose‐lowering therapy using a standardized case report form and transferred to a central database via a web‐based data capture system. Some data were extracted from existing electronic health records in Canada, Denmark, France, Norway and Sweden; in these countries, an abbreviated case report form was used.
Variables collected included: physician and site characteristics; patient socio‐economic demographics; physiological characteristics including height, weight and seated blood pressure; laboratory test results including HbA1c level and/or fasting plasma glucose (FPG) at the time of treatment change; change in glucose‐lowering therapies and reason(s) for change; comorbidities, including diabetes‐related microvascular and macrovascular diseases; and co‐medications. In line with the observational nature of the study, clinical variables, such as HbA1c levels, were measured and recorded in accordance with routine clinical practice; data collection was not mandatory for any of the clinical variables.
2.5. Statistical analysis
For the present analysis, patients from China (n = 1293) were excluded because complete data were not available at the time of publication; therefore, the total number of patients included in the analysis was 14 699 (91.9% of the total DISCOVER population). Descriptive data are presented as numbers and percentages for categorical variables. For continuous variables, mean (SD), median (interquartile range [IQR]), and across‐region ranges (ARRs) are reported, where appropriate.
Factors associated with poor glycaemic control were assessed in patients with available HbA1c levels using hierarchical logistic regression models, with country as a random effect. HbA1c was modelled as a dichotomous variable (≤7.0% vs >7.0%, ≤8.0% vs >8.0%, and ≤9.0% vs >9.0%) with the following additional variables included in the models: age; sex; education level; smoking status; body mass index (BMI); systolic blood pressure (SBP); time since diagnosis of T2D (used as a proxy for diabetes duration); use of co‐medications (angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, diuretics, β‐blockers, statins and acetylsalicylic acid); first‐line glucose‐lowering therapy; history of microvascular complications (including nephropathy [presence of chronic kidney disease and/or albuminuria], retinopathy [history of retinopathy or retinal laser photocoagulation], and neuropathy [autonomic neuropathy, peripheral neuropathy and erectile dysfunction]); and history of macrovascular complications (including coronary artery disease [history of coronary artery disease, angina, myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting], cerebrovascular disease [stroke, transient ischaemic attack, carotid artery stenting and carotid endarterectomy], peripheral artery disease [history of peripheral artery disease including revascularization procedures, diabetic foot, and amputation], heart failure, and implantable cardioverter defibrillator use). Gross national income was also included in the models, using 2016 data from the World Bank (Classification of DISCOVER countries by gross national income in 2016 is shown in Figure S1).20 Complete data were available for 81.7% of patients included in the model. Separate models were also used to assess the association between receiving education on diabetes management in the past year and having poor glycaemic control. These models did not include data from Japan because data for this variable were not collected in this country. Multiple imputation was used in multivariable analyses to account for unreported data and missing values. Imputation was carried out using IVEware (University of Michigan). All other statistical analyses were carried out using the SAS statistical software system (SAS Institute, Inc., Cary, North Carolina).
3. RESULTS
The demographics and characteristics of the DISCOVER cohort (N = 15 992) at initiation of second‐line therapy have been reported previously.21 Characteristics of patients with available HbA1c data (N = 11 891) are presented in Table 1. Overall, most patients were Asian (43.1%) or white (30.0%), and 56.0% of participants were men. The mean (SD) age was 57.7 (12.1) years and the mean (SD) BMI and time since diagnosis of T2D were 29.4 (6.0) kg/m2 and 5.7 (5.3) years, respectively.
Table 1.
Patient characteristics, overall and according to glycated haemoglobin category
| Total | HbA1c <7.0% | HbA1c 7.0% to <8.0% | HbA1c 8.0% to <9.0% | HbA1c ≥9.0% | |
|---|---|---|---|---|---|
| (N = 11 891) | (n = 2071) | (n = 3840) | (n = 2804) | (n = 3176) | |
| Men, n (%) | 6657 (56.0) | 1134 (54.8) | 2129 (55.5) | 1585 (56.5) | 1809 (57.0) |
| Gender data missing | 4 | 2 | 2 | 0 | 0 |
| Self‐reported ethnicity, n (%) | |||||
| White | 3403 (30.0) | 581 (29.8) | 1195 (32.6) | 829 (30.8) | 798 (26.2) |
| Black | 128 (1.1) | 22 (1.1) | 42 (1.1) | 22 (0.8) | 42 (1.4) |
| Asian | 4892 (43.1) | 992 (50.8) | 1653 (45.1) | 1016 (37.7) | 1231 (40.4) |
| Hispanic | 661 (5.8) | 110 (5.6) | 192 (5.2) | 154 (5.7) | 205 (6.7) |
| Arabic | 2019 (17.8) | 209 (10.7) | 509 (13.9) | 610 (22.7) | 691 (22.7) |
| Mixed | 110 (1.0) | 14 (0.7) | 33 (0.9) | 19 (0.7) | 44 (1.4) |
| Other | 142 (1.3) | 23 (1.2) | 40 (1.1) | 42 (1.6) | 37 (1.2) |
| Missing | 536 | 120 | 176 | 112 | 128 |
| Time in formal education, n (%) | |||||
| No formal education | 322 (3.0) | 27 (1.5) | 93 (2.7) | 82 (3.2) | 120 (4.2) |
| Primary (1–6 y) | 1609 (14.9) | 230 (12.6) | 460 (13.2) | 391 (15.1) | 528 (18.3) |
| Secondary (7–13 y) | 5348 (49.6) | 950 (51.9) | 1803 (51.9) | 1247 (48.3) | 1348 (46.7) |
| Higher (>13 y) | 3497 (32.5) | 625 (34.1) | 1118 (32.2) | 863 (33.4) | 891 (30.9) |
| Missing | 1115 | 239 | 366 | 221 | 289 |
| Age, y | 57.7 (12.1) | 60.5 (12.3) | 59.3 (12.0) | 57.5 (12.0) | 54.1 (11.3) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Time since diagnosis, years | 5.7 (5.3) | 5.4 (5.2) | 6.0 (5.4) | 5.8 (5.1) | 5.5 (5.3) |
| Missing | 325 | 81 | 116 | 61 | 67 |
| HbA1c, % | 8.3 (1.7) | 6.4 (0.4) | 7.5 (0.3) | 8.4 (0.3) | 10.5 (1.4) |
| FPG, mmol/L | 9.5 (3.1) | 7.1 (1.6) | 8.3 (1.8) | 9.6 (2.3) | 12.2 (3.6) |
| Missing | 3207 | 624 | 1108 | 722 | 753 |
| BMI, kg/m2 | 29.4 (6.0) | 29.0 (6.2) | 29.1 (5.8) | 29.8 (5.7) | 29.7 (6.2) |
| Missing | 790 | 114 | 226 | 184 | 266 |
| Tobacco smoking, n (%) | |||||
| Non‐smoker | 7771 (67.0) | 1284 (63.8) | 2456 (65.8) | 1918 (69.9) | 2113 (68.1) |
| Ex‐smoker | 2088 (18.0) | 466 (23.1) | 717 (19.2) | 433 (15.8) | 472 (15.2) |
| Current smoker | 1737 (15.0) | 263 (13.1) | 562 (15.0) | 392 (14.3) | 520 (16.7) |
| Missing | 295 | 58 | 105 | 61 | 71 |
| SBP, mm Hg | 132.6 (16.4) | 131.6 (16.6) | 132.6 (16.1) | 133.0 (15.9) | 132.9 (17.2) |
| Missing | 513 | 73 | 158 | 109 | 173 |
| History of microvascular diseasea, n (%) | 2567 (21.6) | 444 (21.4) | 812 (21.2) | 575 (20.5) | 736 (23.2) |
| Missing | 11 | 1 | 6 | 2 | 2 |
| History of macrovascular diseaseb, n (%) | 1732 (14.6) | 354 (17.2) | 623 (16.3) | 385 (13.8) | 370 (11.7) |
| Missing | 35 | 8 | 14 | 5 | 8 |
| Received education on diabetes management in the past year, n (%) | 6722 (75.0) | 1057 (77.0) | 2007 (72.7) | 1694 (75.3) | 1964 (75.9) |
| NAc | 1865 | 531 | 789 | 304 | 241 |
| Missing | 1059 | 168 | 292 | 250 | 349 |
| Comedications, n (%) | |||||
| ASA | 2042 (17.2) | 331 (16.0) | 662 (17.2) | 526 (18.8) | 523 (16.5) |
| Statins | 5460 (45.9) | 988 (47.7) | 1834 (47.8) | 1307 (46.6) | 1331 (41.9) |
| ACE inhibitors/ARBs | 4727 (39.8) | 844 (40.8) | 1617 (42.1) | 1149 (41.0) | 1117 (35.2) |
| Diuretics | 1421 (12.0) | 275 (13.3) | 476 (12.4) | 350 (12.5) | 320 (10.1) |
| β‐blockers | 1702 (14.3) | 332 (16.0) | 555 (14.5) | 444 (15.8) | 371 (11.7) |
| First‐line therapy, n (%) | |||||
| MET monotherapy | 6961 (58.5) | 1232 (59.5) | 2398 (62.5) | 1610 (57.4) | 1721 (54.2) |
| SU monotherapy | 805 (6.8) | 139 (6.7) | 231 (6.0) | 217 (7.7) | 218 (6.9) |
| DPP‐4 inhibitor monotherapy | 1122 (9.4) | 304 (14.7) | 453 (11.8) | 206 (7.3) | 159 (5.0) |
| Other monotherapy | 445 (3.7) | 146 (7.0) | 167 (4.4) | 73 (2.6) | 59 (1.9) |
| MET + SUs | 1525 (12.8) | 137 (6.6) | 323 (8.4) | 399 (14.2) | 666 (21.0) |
| MET + DPP‐4 inhibitors | 425 (3.6) | 61 (2.9) | 115 (3.0) | 116 (4.1) | 133 (4.2) |
| MET + otherd | 131 (1.1) | 14 (0.7) | 49 (1.3) | 41 (1.5) | 27 (0.9) |
| Other combinations | 476 (4.0) | 38 (1.8) | 103 (2.7) | 142 (5.1) | 193 (6.1) |
| Missing | 1 | 0 | 1 | 0 | 0 |
| Second‐line therapy, n (%) | |||||
| MET monotherapy | 194 (1.6) | 99 (4.8) | 52 (1.4) | 21 (0.7) | 22 (0.7) |
| SU monotherapy | 322 (2.7) | 93 (4.5) | 96 (2.5) | 57 (2.0) | 76 (2.4) |
| DPP‐4 inhibitor monotherapy | 531 (4.5) | 210 (10.1) | 194 (5.1) | 78 (2.8) | 49 (1.5) |
| Other monotherapy | 348 (2.9) | 96 (4.6) | 106 (2.8) | 81 (2.9) | 65 (2.0) |
| MET + SU | 2227 (18.7) | 280 (13.5) | 684 (17.8) | 585 (20.9) | 678 (21.3) |
| MET + DPP‐4 inhibitors | 3250 (27.3) | 607 (29.3) | 1294 (33.7) | 760 (27.1) | 589 (18.5) |
| MET + otherc | 1164 (9.8) | 260 (12.6) | 431 (11.2) | 236 (8.4) | 237 (7.5) |
| Other combinations | 3131 (26.3) | 394 (19.0) | 904 (23.5) | 843 (30.1) | 990 (31.2) |
| Insulin | 723 (6.1) | 32 (1.5) | 78 (2.0) | 143 (5.1) | 470 (14.8) |
| Missing | 1 | 0 | 1 | 0 | 0 |
Data are reported as mean (SD), unless otherwise stated. Percentages are calculated for all patients with data available; missing data are excluded.
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; DPP‐4, dipeptidyl peptidase‐4; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; MET, metformin; NA, not available; SBP, systolic blood pressure; SU, sulphonylurea.
Includes nephropathy (presence of chronic kidney disease and/or albuminuria), retinopathy (history of retinopathy or retinal laser photocoagulation), and neuropathy (autonomic neuropathy, peripheral neuropathy, and erectile dysfunction).
Includes coronary artery disease (history of coronary artery disease, angina, myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting), cerebrovascular disease (stroke, transient ischaemic attack, carotid artery stenting, and carotid endarterectomy), peripheral artery disease (history of peripheral artery disease including revascularization procedures, diabetic foot, and amputation), heart failure, and implantable cardioverter defibrillator use.
These patients are from Japan, where data on diabetes education were not collected.
Excluding insulin.
3.1. Patterns of glycaemic control by country and region
Overall, HbA1c data were available for 11 891 patients (80.9%; ARR 57.5%‐98.2%), with substantial variation between countries (Tables 2 and 3). FPG data were available for 70.3% of patients (ARR 36.2%‐84.5%), and 13 546 patients (92.2%) had either HbA1c or FPG data available (ARR 59.2%‐99.1%; Table 2). Among countries, the proportions of patients with either HbA1c or FPG measurements available ranged from 36.8% to 100.0% (Table 3). Reasons for changing therapy among patients with or without available HbA1c or FPG data, as well as according to country income, are shown in Table S2. In both patient populations, lack of efficacy was the most commonly stated reason for changing therapy, although this was more common in patients with HbA1c or FPG measurements than in patients without available measurements (90.5% vs 67.7% of patients). Physician preference, patient request and side effects were more commonly stated as reasons for changing therapy in patients without available HbA1c or FPG measurements than in patients with available measurements (16.5% vs 5.1%, 4.2% vs 1.3% and 6.2% vs 4.7%, respectively). Efficacy was the most commonly stated reason for choosing a second‐line therapy in both patient populations.
Table 2.
Glycated haemoglobin and fasting plasma glucose data and availability, by region
| Overall | Africa | Americas | South‐East Asia | Europe | Eastern Mediterranean | Western Pacific | |
|---|---|---|---|---|---|---|---|
| (N = 14 699) | (n = 812) | (n = 2002) | (n = 3360) | (n = 3479) | (n = 2182) | (n = 2864) | |
| Mean (SD) HbA1c, %, | 8.3 (1.7) | 8.6 (1.9) | 8.5 (1.9) | 8.6 (1.7) | 8.1 (1.6) | 8.7 (1.6) | 7.9 (1.6) |
| Mean (SD) FPG, mmol/L | 9.5 (3.1) | 9.7 (3.5) | 9.8 (3.4) | 9.3 (3.0) | 9.3 (3.0) | 10.2 (3.3) | 8.9 (2.8) |
| Availability of data, n (%) | |||||||
| With HbA1c data | 11 891 (80.9) | 467 (57.5) | 1531 (76.5) | 2052 (61.1) | 3003 (86.3) | 2046 (93.8) | 2792 (97.5) |
| HbA1c and FPG data | 8684 (59.1) | 280 (34.5) | 1180 (58.9) | 1706 (50.8) | 2306 (66.3) | 1774 (81.3) | 1438 (50.2) |
| HbA1c data only | 3207 (21.8) | 187 (23.0) | 351 (17.5) | 346 (10.3) | 697 (20.0) | 272 (12.5) | 1354 (47.3) |
| With FPG data only | 1655 (11.3) | 14 (1.7) | 211 (10.5) | 1030 (30.7) | 285 (8.2) | 70 (3.2) | 45 (1.6) |
| No HbA1c or FPG data | 1153 (7.8) | 331 (40.8) | 260 (13.0) | 278 (8.3) | 191 (5.5) | 66 (3.0) | 27 (0.9) |
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin.
Table 3.
Availability of glycated haemoglobin and fasting plasma glucose data, by country
| Region | Country | With HbA1c and FPG data | With HbA1c data only | With FPG data only | No HbA1c or FPG data |
|---|---|---|---|---|---|
| Overall | – | n = 8684 (59.1) | n = 3207 (21.8) | n = 1655 (11.3) | n = 1153 (7.8) |
| Africa | Algeria | 207 (70.6) | 75 (25.6) | 8 (2.7) | 3 (1.0) |
| South Africa | 73 (14.1) | 112 (21.6) | 6 (1.2) | 328 (63.2) | |
| Americas | Argentina | 222 (74.2) | 35 (11.7) | 23 (7.7) | 19 (6.4) |
| Brazil | 309 (70.7) | 95 (21.7) | 14 (3.2) | 19 (4.3) | |
| Canada | 230 (59.6) | 50 (13.0) | 4 (1.0) | 102 (26.4) | |
| Colombia | 140 (68.0) | 38 (18.4) | 10 (4.9) | 18 (8.7) | |
| Costa Rica | 52 (40.9) | 50 (39.4) | 8 (6.3) | 17 (13.4) | |
| Mexico | 179 (39.3) | 70 (15.4) | 143 (31.4) | 63 (13.8) | |
| Panama | 48 (52.2) | 13 (14.1) | 9 (9.8) | 22 (23.9) | |
| South‐East Asia | India | 1599 (50.9) | 327 (10.4) | 962 (30.6) | 251 (8.0) |
| Indonesia | 107 (48.4) | 19 (8.6) | 68 (30.8) | 27 (12.2) | |
| Europe | Austria | 156 (74.6) | 39 (18.7) | 1 (0.5) | 13 (6.2) |
| Czech Republic | 357 (78.6) | 83 (18.3) | 4 (0.9) | 10 (2.2) | |
| Denmark | 2 (4.9) | 30 (73.2) | 0 (0.0) | 9 (22.0) | |
| France | 204 (77.3) | 40 (15.2) | 4 (1.5) | 16 (6.1) | |
| Italy | 327 (90.6) | 29 (8.0) | 4 (1.1) | 1 (0.3) | |
| Netherlands | 143 (88.3) | 13 (8.0) | 3 (1.9) | 3 (1.9) | |
| Norway | 3 (3.8) | 65 (82.3) | 1 (1.3) | 10 (12.7) | |
| Poland | 160 (49.4) | 84 (25.9) | 39 (12.0) | 41 (12.7) | |
| Russia | 276 (46.9) | 70 (11.9) | 199 (33.8) | 43 (7.3) | |
| Spain | 195 (86.7) | 16 (7.1) | 10 (4.4) | 4 (1.8) | |
| Sweden | 16 (6.8) | 198 (83.9) | 0 (0.0) | 22 (9.3) | |
| Turkey | 467 (87.1) | 30 (5.6) | 20 (3.7) | 19 (3.5) | |
| Eastern Mediterranean | Bahrain | 55 (78.6) | 15 (21.4) | 0 (0.0) | 0 (0.0) |
| Egypt | 489 (83.9) | 45 (7.7) | 45 (7.7) | 4 (0.7) | |
| Jordan | 208 (76.8) | 40 (14.8) | 7 (2.6) | 16 (5.9) | |
| Kuwait | 48 (94.1) | 3 (5.9) | 0 (0.0) | 0 (0.0) | |
| Lebanon | 277 (79.6) | 54 (15.5) | 2 (0.6) | 15 (4.3) | |
| Oman | 21 (67.7) | 10 (32.3) | 0 (0.0) | 0 (0.0) | |
| Saudi Arabia | 402 (77.5) | 80 (15.4) | 7 (1.3) | 30 (5.8) | |
| Tunisia | 191 (89.3) | 14 (6.5) | 9 (4.2) | 0 (0.0) | |
| United Arab Emirates | 83 (87.4) | 11 (11.6) | 0 (0.0) | 1 (1.1) | |
| Western Pacific | Australia | 92 (55.1) | 66 (39.5) | 1 (0.6) | 8 (4.8) |
| Japan | 691 (37.0) | 1174 (62.8) | 0 (0.0) | 4 (0.2) | |
| Malaysia | 257 (76.9) | 32 (9.6) | 37 (11.1) | 8 (2.4) | |
| Korea, South | 163 (69.1) | 68 (28.8) | 2 (0.8) | 3 (1.3) | |
| Taiwan | 235 (91.1) | 14 (5.4) | 5 (1.9) | 4 (1.6) |
Data are reported as n (%).
Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin.
The overall mean (SD; ARR) HbA1c level at initiation of second‐line therapy was 8.3 (1.7; 7.9‐8.7)% (Table 2). Mean HbA1c levels were highest in the Eastern Mediterranean region and lowest in the Western Pacific region (8.7% and 7.9%, respectively). The overall proportions of patients with HbA1c <8.0%, ≥8.0 to <9.0% and ≥9.0% were 49.8%, 23.6% and 26.7%, respectively. These proportions varied across countries and regions (Figure 1).
Figure 1.
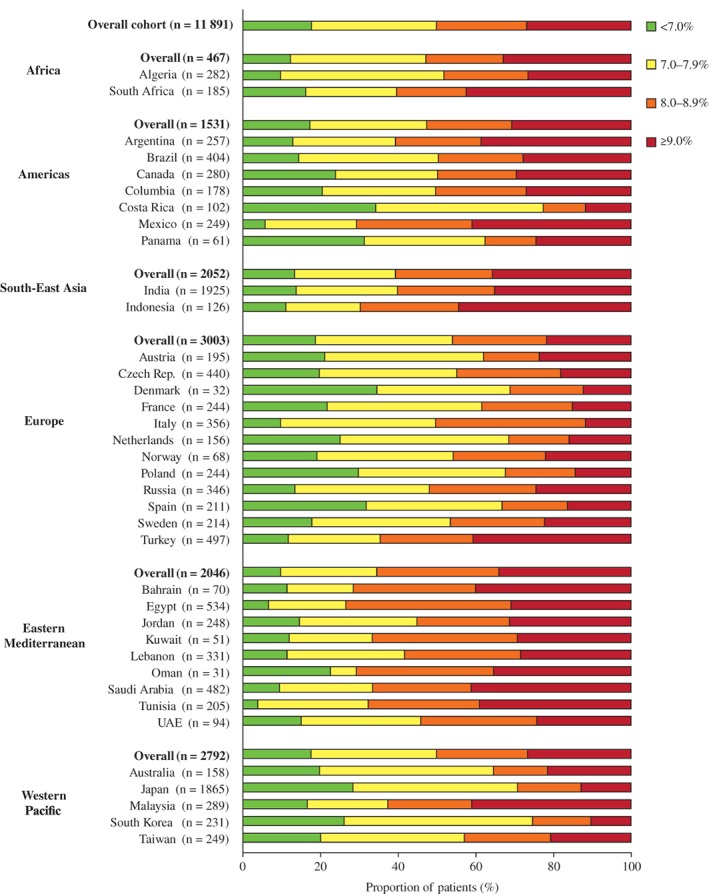
Proportions of patients in different glycated haemoglobin ranges at initiation of second‐line therapy. UAE, United Arab Emirates
As expected in a population of patients initiating second‐line glucose‐lowering therapy, the overall proportion of patients with HbA1c <7.0% among patients with available values was low (17.4%, ARR 9.6%‐25.8%; Figure 1). The mean (SD) HbA1c among these patients was 6.4 (0.4)% (Table 1), and the reasons for changing therapy are presented in Table S3. As in the overall cohort, the majority of patients (72.3%) with HbA1c <7.0% changed first‐line therapy owing to lack of efficacy (ARR 56.9%‐85.8%). The most common reasons for choosing a second‐line therapy were expected efficacy (39.7%, ARR 15.4%‐70.8%) and tolerability (22.7%, ARR 5.3%‐36.2%). The proportion of patients with HbA1c <7.0% was particularly low in Africa (12.2%), South‐East Asia (13.4%), and the Eastern Mediterranean region (9.6%), and was highest in Europe and the Western Pacific region (18.7% and 25.8%, respectively). The proportion of patients with HbA1c ≥9.0% varied substantially across regions, with the highest proportions in South‐East Asia (35.6%) and the Eastern Mediterranean region (33.9%). In total, 19 countries had >25% of patients with HbA1c ≥9.0% at initiation of second‐line therapy.
3.2. Factors associated with poor glycaemic control at initiation of second‐line therapy
Figure 2 shows the factors associated with poor glycaemic control, defined as HbA1c >8.0%, at initiation of second‐line therapy. In this model, young patients were more likely to have poor glycaemic control at the time of treatment intensification than old patients, and the odds of having poor glycaemic control decreased with each 10‐year age increment. The following factors were also associated with poor glycaemic control: male sex; having a low level of education versus >13 years of formal education; being a current smoker; having high SBP (per 10 mm Hg increment); having a time since T2D diagnosis of >10 years; not taking statins; receiving sulphonylurea (SU) monotherapy, an SU or dipeptidylpeptidase‐4 (DPP‐4) inhibitor in combination with metformin or another combination of two or more agents as first‐line treatment, versus metformin; and having a history of microvascular complications. Additionally, patients in lower‐middle‐income countries were more likely to have HbA1c levels >8.0% than patients from high‐income countries. Results of analyses using thresholds of 7.0% or 9.0% to define poor glycaemic control (Figure S2) were similar to those of the primary analysis. There was no significant association between receiving education on diabetes management in the past year and the likelihood of having HbA1c levels >8.0%, when assessed in patients for whom this information was collected (Figure S3).
Figure 2.
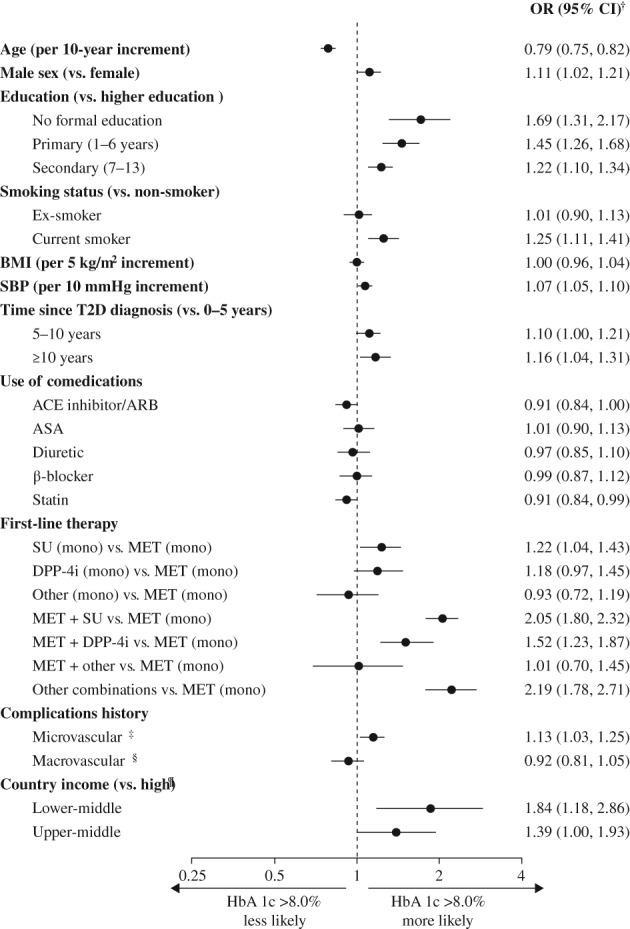
Multivariate analysis of factors associated with poor glycaemic control defined as glycated haemoglobin (HbA1c) >8.0%. †The plot shows odds ratios, adjusted for all variables in the figure, using a hierarchical logistic model as described in the methods. HbA1c is modelled as a dichotomous variable. ‡Includes nephropathy (presence of chronic kidney disease and/or albuminuria), retinopathy (history of retinopathy or retinal laser photocoagulation), and neuropathy (autonomic neuropathy, peripheral neuropathy, and erectile dysfunction). §Includes coronary artery disease (history of coronary artery disease, angina, myocardial infarction, percutaneous coronary intervention, and coronary artery bypass grafting), cerebrovascular disease (stroke, transient ischaemic attack, carotid artery stenting, and carotid endarterectomy), peripheral artery disease (history of peripheral artery disease including revascularization procedures, diabetic foot, and amputation), heart failure, and implantable cardioverter defibrillator use. ¶Categorized using the 2016 World Bank classification. ACE, angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; ASA, acetylsalicylic acid; BMI, body mass index; CI, confidence interval; DPP‐4, dipeptidyl peptidase‐4; MET, metformin; mono, monotherapy; OR, odds ratio; SBP, systolic blood pressure; SU, sulphonylureas; T2D, type 2 diabetes
4. DISCUSSION
The present analysis of data from the DISCOVER study programme revealed consistently high HbA1c levels at initiation of second‐line therapy across countries and regions worldwide. Approximately 50% of patients with available HbA1c measurements had HbA1c >8.0%, and >25% had HbA1c >9.0%. Overall, <20% of patients had HbA1c <7.0%. Many patients did not have available HbA1c measurements, despite a decision to initiate second‐line therapy having been made, which highlights an opportunity for improvement of the quality of care of patients with T2D.
Although previous studies have reported poor levels of glycaemic control among patients with T2D,22, 23, 24 these have mostly been conducted in populations of patients with more advanced disease than in the present study. For example, the A1chieve study25 was a global, prospective, observational study of patients with T2D who were initiating insulin therapies in routine clinical practice. That study included patients from 30 countries across four continents (Asia, Africa, South America and Europe); 21 of these countries were included in DISCOVER. In A1chieve, the mean baseline HbA1c was higher than that in DISCOVER participants (9.5% vs 8.3%), which is likely to reflect the more severe diabetic phenotype in patients who are initiating insulin therapies. Similarly, the IMPROVE study, which included >50 000 patients across eight countries with a mean diabetes duration of 6.9 years, reported a mean HbA1c of 9.4%.26 In the multinational, prospective International Diabetes Management Practice Study (IDMPS) survey, the mean HbA1c and mean diabetes duration were 7.8% and 8.4 years, respectively, among patients with T2D.11 The IDMPS study cohort comprised 9901 patients with T2D from Asia (Korea, China, Indonesia, India, Hong Kong, Taiwan, Malaysia and Thailand), Eastern Europe (Romania, Bulgaria, Turkey, Tunisia and Bosnia), Latin America (Argentina, Ecuador, Venezuela and Columbia), and Africa (Tunisia), many of whom were receiving insulin therapy. In the context of these studies of patients with presumably more severe diabetes than patients in the present study, the poor overall glycaemic control among DISCOVER patients is concerning.
The mean HbA1c at initiation of second‐line therapy and the proportion of patients with HbA1c ≥9.0% were generally higher in lower‐middle‐ and upper‐middle‐income countries than in high‐income countries. Particularly concerning regions were parts of Asia and Africa, as well as the Middle‐Eastern region. Consistent with this finding, results from the multivariate analysis showed that living in a lower‐middle‐income country was strongly associated with poor glycaemic control (HbA1c >8.0%) relative to living in a high‐income country; this result was also seen when a threshold of 9.0% was used in the analysis. This was not unexpected, given that low income is likely to translate into reduced expenditure on healthcare. Indeed, many of the countries included in DISCOVER have very low diabetes‐related healthcare expenditure compared with high‐income countries.27 Likely consequences of this low expenditure on care for patients with T2D include a lack of resources for HbA1c monitoring, which may lead to delays in intensifying second‐line glucose‐lowering therapies. Consistent with this hypothesis, over one‐third of patients in the South‐East Asia and African regions lacked HbA1c measurements in the present study. These findings are consistent with those from the IDMPS survey, which revealed that 36% of patients with T2D in developing regions had never had their HbA1c levels measured.11 Similarly, the authors of a study conducted in Brazil commented that kits for HbA1c measurement are not routinely provided by the National Brazilian Health Care System.28 Aside from HbA1c monitoring, patients in lower‐middle and upper‐middle‐income countries may also encounter problems with the availability and affordability of glucose‐lowering therapies compared with patients in high‐income countries.29 Indeed, in the present study, physicians cited cost and access to treatment as reasons for choosing second‐line therapy for 7.2% and 5.1% of patients, respectively, and these proportions were higher in middle‐income countries than in high‐income countries.
Mean levels of HbA1c were also well above guideline‐recommended values in many high‐income countries. As with lower‐middle and upper‐middle‐income countries, this finding may reflect delays in treatment intensification but for different reasons. A possible contributing factor is conservative management of patients by clinicians, as has been documented previously.16, 30 In addition, the current stepwise approach to treatment intensification that is advocated by major treatment guidelines may lead to prolonged periods of hyperglycaemia in between steps.15, 31 A recent analysis of patients in the United States showed no improvements in overall glycaemic control and an increase in the proportion of patients with HbA1c ≥9.0% between 2006 and 2013, despite increased utilization of newer and costlier glucose‐lowering agents among these patients.32 These data, combined with the present data, highlight a pressing need to re‐evaluate existing treatment pathways for patients with T2D in order to improve glycaemic control.
Other factors associated with poor glycaemic control in multivariate analyses included younger age, male sex, low education level, and use of combination glucose‐lowering therapies as first‐line diabetes treatment. The inverse relationship between age and glycaemic control, while somewhat counter‐intuitive, might be explained by older patients being monitored more closely by physicians than younger patients, owing to their increased comorbidity and heightened risk of complications. Authors of other studies have also hypothesized that older patients might be more motivated to look after their health and adhere to their medications than young patients.33 Patients with a high level of education are likely to have better means to fund treatment or private medical care than less educated patients, and there is some evidence of a correlation between education level and the quality of diabetes care and outcomes.34 This hypothesis is also consistent with the association seen in our data between lower country income and poor glycaemic control. As might be expected, having a time since diagnosis of T2D of at least 10 years compared with 0 to 5 years was also strongly correlated with poor glycaemic control. This finding is consistent with other observational studies that have demonstrated a positive relationship between disease duration and poor glycaemic control.35, 36 The trend is likely to reflect the continual decline in β‐cell function that is characteristic of T2D. These findings emphasize the importance of intensifying treatment in a timely manner once HbA1c is no longer controlled by first‐line therapy.
The positive association between use of combination glucose‐lowering therapy as first‐line treatment and poor glycaemic control is probably explained by the fact that patients with high HbA1c levels at the time of diagnosis require more intensive pharmacological treatment than patients with lower HbA1c levels, as per clinical guideline recommendations.6 However, these intensive treatments may fail to control glycaemia adequately, which is why HbA1c levels could remain high and require initiation of second‐line therapy. As described previously,21, 37 our findings also showed a positive association between having a history of microvascular complications and having HbA1c levels >8.0%. This finding is consistent with evidence that intensive glycaemic control for a prolonged period decreases the incidence of microvascular complications.37 However, longitudinal data from DISCOVER are required to confirm a relationship between changes in HbA1c trajectories and the incidence of diabetes complications in the present study cohort.
An interesting finding in the present study was that close to 20% of patients in the study cohort had HbA1c <7.0%. This was somewhat unexpected, given that this is a population of patients who are initiating second‐line glucose‐lowering therapy. The finding that the majority of these patients cited efficacy as the reason for changing treatment was also surprising, although it is notable that this proportion of patients was lower than in the overall population of patients with available HbA1c or FPG measurements. Similarly, although almost half of patients with HbA1c <7.0% cited efficacy as a reason for choosing a second‐line therapy, this was lower than in the overall patient population. It could be the case that the patients with HbA1c <7.0% in the present study were early in their disease trajectory and therefore had been set HbA1c targets below 7.0% by their physicians, consistent with guideline recommendations for patients with few comorbidities and low risk of hypoglycaemia.7
Within the study cohort, there were large numbers of patients without available data on the extent of glycaemic control. As highlighted previously, this was particularly evident in lower‐middle and upper‐middle‐income countries in which physicians may not monitor HbA1c levels routinely, owing to the high cost of this practice compared with obtaining other measures of glycaemic control. Many patients who lacked HbA1c data in the present cohort had FPG data instead, which suggests that FPG may be used as an alternative to HbA1c to monitor glycaemia and to support treatment change decisions in some countries. While there is some evidence to suggest a good correlation between HbA1c and FPG measurements within a certain range,38 this practice is contradictory to treatment guidelines. Overall, 7.8% of the cohort had neither HbA1c nor FPG data available, and this proportion was particularly high (40.8%) in the African region. It is concerning that in some countries, 10% to 20% of patients switched glucose‐lowering therapy in the absence of FPG or HbA1c measurements to direct this decision. Although one might expect that this would be due to concerns about cost or tolerability, it is notable that the proportions of patients for whom these factors were recorded as reasons for changing therapy were low, despite being slightly higher in patients without FPG or HbA1c measurements than in patients for whom these measurements were available.
Key strengths of the DISCOVER study programme include the large numbers of patients and inclusion of many lower‐middle and upper‐middle‐income countries which have rarely or never been studied before.17 The use of a standardized electronic case report form for data collection allows comparison of results within and across countries and regions. As DISCOVER is a longitudinal study, data collected during follow‐up will provide valuable insights into the relationship between glycaemic control and clinical outcomes in patients with T2D across the globe. The results reported in this manuscript provide context for the interpretation of these follow‐up data. There are also potential limitations of DISCOVER. Although study sites were selected with the intention of providing a patient population that was as representative of T2D care in each country as possible,19 attainment of a truly representative sample is inherently difficult to achieve in large international studies. Reasons for this include infrastructure challenges, and the fact that some primary care centres are not set up for or willing to participate in observational research. Such practical constraints resulted in urban locations and secondary care centres being over‐represented in this study. Moreover, levels of education seen in our patient population are higher on average than would be expected. This potential selection bias is likely to lead to an over‐estimation of the quality of diabetes care, since better‐educated patients in urban locations would be expected to receive better healthcare than less educated patients in rural locations.19 Thus, the level of glycaemic control at initiation of second‐line treatment across the DISCOVER countries may be even worse than the findings reported in the present study. Despite these limitations, the efforts made to maximize representativeness resulted in the inclusion of a heterogeneous patient population, as well as a diverse range of sites and physicians. Overall, ethnicity and sex distributions of DISCOVER patients were in agreement with corresponding data from the 2017 Atlas of the International Diabetes Federation.19 The high proportion of missing data in several countries, which might have reduced the precision of the multivariate analysis where imputation was used to compensate for unreported data, should also be acknowledged. However, this is likely to be reflective of routine clinical care; for example, HbA1c is not routinely measured in some clinical settings.
In conclusion, data from the DISCOVER study confirmed that therapeutic inertia is a global phenomenon with consistently high HbA1c levels at initiation of second‐line glucose‐lowering therapy, particularly in lower‐middle and upper‐middle‐income countries. Globally, there are large numbers of patients with very poor glycaemic control (HbA1c ≥9.0%) at initiation of second‐line glucose‐lowering therapy, suggesting that treatment is not intensified in a timely manner as recommended by clinical guidelines. Factors associated with poor glycaemic control included low education level, low country income, and longer time since diagnosis of diabetes. Despite guideline recommendations, HbA1c was not routinely measured in all countries, perhaps owing to the higher cost of HbA1c measurements in lower‐middle‐income countries than in high‐income countries. These findings suggest a need for better monitoring of glycaemic control in patients with T2D worldwide, as well as interventions to improve HbA1c control at early stages of the disease.
CONFLICT OF INTEREST
K.K., M.B.G., L.J, M.K, S.P., M.V.S., I.S., H.W. and A.N. are members of the DISCOVER Scientific Committee, and received support from AstraZeneca to attend DISCOVER planning and update meetings. N.A., P.F. and S.K. are employees of AstraZeneca. N.H. is a former employee of AstraZeneca. J.C.R. is an employee of Evidera. In addition, K.K. has received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche and Sanofi, and research support from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Roche and Sanofi, and also acknowledges support from the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care – East Midlands (NIHR CLAHRC – EM) and the National Institute of Health Research (NIHR) Leicester Biomedical Research Centre. M.B.G. has received honoraria from Merck‐Serono. L.J. has received honoraria from Eli Lilly, Bristol‐Myers Squibb, Novartis, Novo Nordisk, Merck, Bayer, Merck Sharp & Dohme, Takeda, Sanofi, Roche, Boehringer Ingelheim and AstraZeneca, and research support from Roche, Sanofi, Merck Sharp & Dohme, AstraZeneca, Novartis, Eli Lilly and Bristol‐Myers Squibb. M.K. has received honoraria from Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Intarcia, Janssen, Novartis, Novo Nordisk, Glytec Systems, Merck (Diabetes) and Sanofi, and research support from AstraZeneca and Boehringer Ingelheim. S.P. has received honoraria from AstraZeneca. M.V.S. has received honoraria from Eli Lilly, Merck Sharp & Dohme, Sanofi, Novo Nordisk, Boehringer Ingelheim and AstraZeneca, and research support from Sanofi. I.S. has received honoraria from Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Kowa, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho and Takeda Pharmaceutical, and research support from Astellas Pharma, AstraZeneca, Daiichi Sankyo, Eli Lilly, Japan Foundation for Applied Enzymology, Japan Science and Technology Agency, Kowa, Kyowa Hakko Kirin, Midori Health Management Center, Mitsubishi Tanabe Pharma, Novo Nordisk, Ono Pharmaceutical, Sanofi, Suzuken Memorial Foundation and Takeda Pharmaceutical. F.T. has received research support from AstraZeneca. H.W. has received honoraria from Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kowa, Merck Sharp & Dohme, Novo Nordisk, Novartis, Ono Pharmaceutical, Sanofi, Sanwa Kagaku Kenkyusho, Takeda, Astellas Pharma, Mitsubishi Tanabe Pharma, AstraZeneca, Kyowa Hakko Kirin and Kissei Pharma, and research support from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eli Lilly, Kissei Pharma, Merck Sharp & Dohme, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Novartis, Novo Nordisk, Pfizer, Sanofi, Sanwa Kagaku Kenkyusho, Takeda, Terumo Corp, Astellas Pharma, Abbott, Ono Pharmaceutical, Kyowa Hakko Kirin, Kowa, Johnson & Johnson, Taisho Toyama Pharmaceutical, Nitto Boseki, Bayer, Bristol‐Myers Squibb and Benefit one Health care. A.N. has received honoraria from Novo Nordisk, Medtronic, AstraZeneca and Eli Lilly, and research support from Novo Nordisk, Sanofi‐Aventis, Artsana and Dexcom.
AUTHOR CONTRIBUTIONS
The general content of the manuscript was agreed upon by all authors. The first draft of the manuscript was developed by K.K., and all authors contributed to the development of subsequent drafts. All authors approved the final version of the manuscript before its submission. An AstraZeneca team reviewed the manuscript during its development and was allowed to make suggestions; however, the final content was determined by the authors. K.K. is the guarantor of this work.
Supporting information
Table S1. Inclusion and exclusion criteria.
Table S2. Reasons for changing therapy in patients with and without available HbA1c or FPG data, and according to country income.
Table S3. Reasons for changing therapy in patients with HbA1c <7.0%.
Figure S1. Classification of DISCOVER countries by GNI per capita (2016).
Figure S2. Multivariate analysis of factors associated with poor glycaemic control defined as (A) HbA1c >9.0% and (B) HbA1c >7.0%.
Figure S3. Multivariate analysis of factors associated with poor glycaemic control defined as HbA1c >8.0%, excluding data from Japan.
ACKNOWLEDGMENTS
The DISCOVER study programme is funded by AstraZeneca. DISCOVER is a non‐interventional study, and no drugs were supplied or funded. The authors would like to thank all investigators and patients participating in the DISCOVER study programme. Medical writing support was provided by Lucy Ambrose DPhil of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Khunti K, Chen H, Cid‐Ruzafa J, et al. Glycaemic control in patients with type 2 diabetes initiating second‐line therapy: Results from the global DISCOVER study programme. Diabetes Obes Metab. 2020;22:66–78. 10.1111/dom.13866
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/dom.13866.
Funding information The DISCOVER study programme is funded by AstraZeneca. DISCOVER is a non‐interventional study, and no drugs were supplied or funded. Medical writing support was provided by Lucy Ambrose DPhil of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
REFERENCES
- 1. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 3. Chinese Diabetes Society . Chinese guideline for type 2 diabetes prevention (2013). Chin J Diabetes. 2014;22:2‐42. [Google Scholar]
- 4. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577‐1589. [DOI] [PubMed] [Google Scholar]
- 5. Abdul‐Ghani MA, Puckett C, Triplitt C, et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add‐on therapy in subjects with new‐onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes Metab. 2015;17:268‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association . Standards of medical care in diabetes – 2017. Diabetes Care. 2017;40(Suppl 1):S1‐S132. [DOI] [PubMed] [Google Scholar]
- 7. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE/ACE comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21:438‐447. [DOI] [PubMed] [Google Scholar]
- 8. Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156:218‐231. [DOI] [PubMed] [Google Scholar]
- 9. International Diabetes Federation . Global guideline for type 2 diabetes. 2012. https://www.idf.org/e-library/guidelines/79-global-guideline-for-type-2-diabetes.html. Accessed July 31, 2019.
- 10. Davies MJ, D'Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669‐2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan JC, Gagliardino JJ, Baik SH, et al. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care. 2009;32:227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Juarez DT, Ma C, Kumasaka A, Shimada R, Davis J. Failure to reach target glycated A1C levels among patients with diabetes who are adherent to their antidiabetic medication. Popul Health Manag. 2014;17:218‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kibirige D, Atuhe D, Sebunya R, Mwebaze R. Suboptimal glycaemic and blood pressure control and screening for diabetic complications in adult ambulatory diabetic patients in Uganda: a retrospective study from a developing country. J Diabetes Metab Disord. 2014;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stark Casagrande S, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988‐2010. Diabetes Care. 2013;36:2271‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411‐3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khunti K, Gomes MB, Pocock S, et al. Therapeutic inertia in the treatment of hyperglycaemia in patients with type 2 diabetes: a systematic review. Diabetes Obes Metab. 2018;20:427‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ji L, Bonnet F, Charbonnel B, et al. Towards an improved global understanding of treatment and outcomes in people with type 2 diabetes: rationale and methods of the DISCOVER observational study program. J Diabetes Complications. 2017;31:1188‐1196. [DOI] [PubMed] [Google Scholar]
- 18. Katakami N, Mita T, Takahara M, et al. Rationale and design for the J‐DISCOVER study: DISCOVERing the treatment reality of type 2 diabetes in a real‐world setting in Japan ‐ A protocol. Diabetes Ther. 2018;9:165‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rathmann W, Medina J, Kosiborod M, et al. The DISCOVER study: diversity of sites, physicians, and patients. Pharmacoepidemiol Drug Saf. 2018;27:228. [Google Scholar]
- 20. World Bank . Classification of country income (2016). http://databank.worldbank.org/data/download/site-content/OGHIST.xls. Accessed July 31, 2019.
- 21. Kosiborod M, Gomes MB, Nicolucci A, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol. 2018;17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Pablos‐Velasco P, Parhofer KG, Bradley C, et al. Current level of glycaemic control and its associated factors in patients with type 2 diabetes across Europe: data from the PANORAMA study. Clin Endocrinol (Oxf). 2014;80:47‐56. [DOI] [PubMed] [Google Scholar]
- 23. Paul SK, Klein K, Thorsted BL, Wolden ML, Khunti K. Delay in treatment intensification increases the risks of cardiovascular events in patients with type 2 diabetes. Cardiovasc Diabetol. 2015;14:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shaya FT, Yan X, Lin PJ, et al. US trends in glycemic control, treatment, and comorbidity burden in patients with diabetes. J Clin Hypertens. 2010;12:826‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shah SN, Litwak L, Haddad J, Chakkarwar PN, Hajjaji I. The A1chieve study: a 60 000‐person, global, prospective, observational study of basal, meal‐time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract. 2010;88(Suppl 1):S11‐S16. [DOI] [PubMed] [Google Scholar]
- 26. Valensi P, Benroubi M, Borzi V, et al. The IMPROVE study–a multinational, observational study in type 2 diabetes: baseline characteristics from eight national cohorts. Int J Clin Pract. 2008;62:1809‐1819. [DOI] [PubMed] [Google Scholar]
- 27. International Diabetes Federation . IDF Diabetes Atlas. 8th ed. 2017. http://diabetesatlas.org/resources/2017-atlas.html. Accessed July 31, 2019.
- 28. Gomes MB, Gianella D, Faria M, et al. Prevalence of Type 2 diabetic patients within the targets of care guidelines in daily clinical practice: a multi‐center study in Brazil. Rev Diabet Stud. 2006;3:82‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chow CK, Ramasundarahettige C, Hu W, et al. Availability and affordability of essential medicines for diabetes across high‐income, middle‐income, and low‐income countries: a prospective epidemiological study. Lancet Diabetes Endocrinol. 2018;6:798‐808. [DOI] [PubMed] [Google Scholar]
- 30. Khunti K, Davies MJ. Clinical inertia‐Time to reappraise the terminology? Prim Care Diabetes. 2017;11:105‐106. [DOI] [PubMed] [Google Scholar]
- 31. Bianchi C, Daniele G, Dardano A, Miccoli R, Del Prato S. Early combination therapy with oral glucose‐lowering agents in type 2 diabetes. Drugs. 2017;77:247‐264. [DOI] [PubMed] [Google Scholar]
- 32. Lipska KJ, Yao X, Herrin J, et al. Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006‐2013. Diabetes Care. 2017;40:468‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. El‐Kebbi IM, Cook CB, Ziemer DC, Miller CD, Gallina DL, Phillips LS. Association of younger age with poor glycemic control and obesity in urban African Americans with type 2 diabetes. Arch Intern Med. 2003;163:69‐75. [DOI] [PubMed] [Google Scholar]
- 34. Flatz A, Casillas A, Stringhini S, Zuercher E, Burnand B, Peytremann‐Bridevaux I. Association between education and quality of diabetes care in Switzerland. Int J Gen Med. 2015;8:87‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alzaheb RA, Altemani AH. The prevalence and determinants of poor glycemic control among adults with type 2 diabetes mellitus in Saudi Arabia. Diabetes Metab Syndr Obes. 2018;11:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yousefzadeh G, Shokoohi M, Najafipour H. Inadequate control of diabetes and metabolic indices among diabetic patients: a population based study from the Kerman Coronary Artery Disease Risk Study (KERCADRS). Int J Health Policy Manag. 2014;4:271‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Group AC , Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 38. Guan X, Zheng L, Sun G, et al. The changing relationship between HbA1c and FPG according to different FPG ranges. J Endocrinol Invest. 2016;39:523‐528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion and exclusion criteria.
Table S2. Reasons for changing therapy in patients with and without available HbA1c or FPG data, and according to country income.
Table S3. Reasons for changing therapy in patients with HbA1c <7.0%.
Figure S1. Classification of DISCOVER countries by GNI per capita (2016).
Figure S2. Multivariate analysis of factors associated with poor glycaemic control defined as (A) HbA1c >9.0% and (B) HbA1c >7.0%.
Figure S3. Multivariate analysis of factors associated with poor glycaemic control defined as HbA1c >8.0%, excluding data from Japan.


