Abstract
Objectives
A substantial proportion of individuals with stroke are of working age. After stroke, it is important to return to work (RTW), both for the individual's satisfaction with life and economically for society. The current comprehensive, long‐term study aimed at investigating in what time period the RTW continues after stroke and what factors could predict RTW.
Materials and methods
All individuals registered in the registry Riksstroke with stroke in Sweden at ages 18‐58 years during 2011 were eligible for participation. RTW was based on sickness absence data from the Social Insurance Agency covering 1 year prestroke to 5 years post‐stroke. Time to RTW was analyzed with Kaplan‐Meier curves. Potential predictors of RTW were analyzed with Cox regression and logistic regression.
Results
For RTW analyses, 1695 participants were included. Almost 50% RTW within 3 months, 70% within 1 year, and 80% within 2 years post‐stroke. However, the RTW continued for several years, with a total of 85% RTW. Predictors of favorable time to RTW were male sex, ischemic stroke, and long university education compared with primary school education. Predictors of unfavorable times to RTW were higher stroke severity, defined by the level of consciousness, and older ages. Participants with self‐expectations of RTW 1 year post‐stroke had higher odds of RTW within 5 years.
Conclusions
The RTW continues for a longer time after stroke than previously known. Both self‐expectations and demographical, socioeconomic, stroke‐related factors were important predictors of RTW. This knowledge could assist healthcare professionals to individualize the rehabilitation post‐stroke.
Keywords: follow‐up study, rehabilitation, return to work, stroke
1. INTRODUCTION
Stroke is one of the most common causes of disability worldwide.1 Although the risk of stroke increases with older age, approximately one out of five individuals that experience a stroke in Sweden are of working age,2 and globally, that proportion is even larger.3 In a working‐age person, the consequences of stroke might include reduced participation in society, and more specifically, at work. The ability be return to work (RTW) was shown to be important for life satisfaction and for well‐being after a stroke.4, 5 Furthermore, it is economically important for the society that the RTW is optimized, because indirect costs (productive years lost due to sickness absence or death after stroke) represent 21% of the total costs after a stroke in Sweden.6 To optimize RTW, studies are needed to map the current patterns and facilitators/barriers of RTW, as they are today.
The frequency of individuals that RTW in previous studies has varied. Two review articles found RTW rates from approximately 10%‐70%.7, 8 A recent study in a small stroke population in Sweden showed that the RTW can occur at just over 3 years post‐stroke.9 However, most previous studies about the RTW after a stroke have had relatively short follow‐up times (up to 2 years) 7 and used self‐reported outcome measures.
In previous research, different factors have been identified as facilitators or barriers of RTW. Older ages, female sex, and more severe stroke were negatively associated with RTW.10 Furthermore, work‐related factors11, 12 and socioeconomic factors have had partial impacts on self‐reported RTW within 1 year post‐stroke previously.13 To improve patient care after a stroke, studies are needed to increase the knowledge about predictors important for RTW, from a long‐term perspective after stroke.
The expectations of RTW in an individual on sickness absence have been shown to play an important role in the outcome of RTW.14 However, previous research has mainly focused on individuals with musculoskeletal diseases14 and little is known about the importance of the expectations of RTW in persons with stroke.
The aim was to investigate in what time period stroke survivors continue to RTW, possible predictors of RTW including demographical, stroke‐related, and socioeconomic factors, as well as self‐reported expectations of RTW, in a comprehensive national population with a long‐term perspective.
2. MATERIALS AND METHODS
2.1. Study population
All individuals that were registered in Riksstroke (the Swedish Stroke Registry) after a stroke in Sweden during 2011 were eligible for inclusion. Additional inclusion criteria were as follows: age 18‐58 years when the stroke occurred (to ensure the participants were of working age at the end of follow‐up, because old‐age retirement is most common at 65 years and uncommon before 63 years of age in Sweden), and no previous stroke recorded in the registry. Exclusion criteria were as follows: subarachnoid hemorrhage (SAH), and living in a nursing home when the stroke occurred. In addition, for inclusion in the RTW analyses, participants must not have received sickness compensation for more than 50% of extent 1 year prior to the stroke.
2.2. Data collection
Data were linked from several different registries with the Swedish personal identification number of each participant.
Riksstroke provided background data (age, sex, living alone, smoking, diabetes, atrial fibrillation, and hypertension), clinical data (stroke severity, stroke type, and reperfusion treatment), and data from a 1‐year follow‐up questionnaire (expectations of RTW). Riksstroke is a Swedish quality registry that covers all hospitals that admit patients with acute stroke. The Riksstroke coverage rate was >90% of all patients with stroke treated at a hospital.15
Statistics Sweden includes individuals with a personal identification number in Sweden. The registry provided socioeconomic data (country of birth, income, educational level) for the current study.
The date of death during the follow‐up period was provided by the National Board of Health and Welfare.
The Social Insurance Agency provided sickness absence data for 1 year prior to the stroke and up to 5 years after the stroke. The Social Insurance Agency is a public authority that provides financial compensation to individuals in all kinds of occupations, parental leave, or unemployment, during sickness absence. The authority starts to pay sickness benefits after 2 weeks of sickness absence (the employer provides sick pay for the first 2 weeks) and uses a model with criteria that become more stringent as the absence from work increases. When an individual is considered unlikely to RTW due to sickness, they could be granted sickness compensation (early retirement) instead of sickness benefit. Both sickness benefit and sickness compensation can be granted with four different extents, 25%, 50%, 75%, or 100%. The Social Insurance Agency had a previous regulation (valid between 2008 and 2016), which stated that individuals were eligible to sickness benefits for a maximum of 914 days16 (this did not apply to sickness compensation); after that, the individual might RTW and could not start a new case of sickness benefit for at least 87 days. Some exceptions to that regulation were granted, for example, when an individual had severe sickness.
2.3. Variables
For the analyses, we categorized the age at the time of stroke into three age groups: 18‐44 years, 45‐52 years, and 53‐58 years, since the effect of age was not linearly associated with log odds of RTW.
The country of birth was designated Sweden, Nordic countries (except for Sweden), European countries (except for the Nordic countries), and countries outside of Europe. For descriptive purposes, the countries outside of Europe are presented according to the corresponding continent, in a characteristics table.
Educational levels were grouped into four levels: primary school (≤9 years), secondary school (10‐12 years), a short university education (13 years), and a long university education (≥14 years), which included post‐graduate education.
Income was based on the individual's portion of disposable household income, calculated before the stroke (in 2010 or 2009). Income is expressed in Swedish krona (SEK), and 1 SEK was worth approximately 0.111 USD (January 17, 2019). For the analyses, income was divided into tertiles of low, middle, and high income.
The stroke type was classified according to the International Classification of Diseases, 10th revision (ICD‐10) codes. I61 was intracerebral hemorrhage (ICH), and I63 was ischemic stroke (IS). When an individual was classified as I64 (unspecified stroke), they were designated as an IS in the present study. Riksstroke data do not distinguish between large vessel distribution and lacunar infarcts.
The level of consciousness at admission was rated based on the Reaction Level Scale (RLS)17 and was used as a proxy for stroke severity. The three levels were as follows: alert (RLS 1), drowsy (RLS 2‐3), and unconscious (RLS 4‐8). The majority of individuals in the Riksstroke registry had missing scores from the National Institute of Health Stroke Scale (NIHSS); therefore, the variable was not used. Neither severity of paresis nor side of stroke is registered in Riksstroke.
Return to work was defined as not being registered with more than 50% extent of sickness benefit or sickness compensation for at least 2 months. When a registration at the Social Insurance Office ended within a month of the date of a participant's death, the participant's status was classified as death instead of RTW.
A separate predictor analysis with expectations of RTW 1 year post‐stroke as predictor of interest was performed. The question is Have you RTW? with the possible answers No, No but I am planning to RTW, Yes but in less extent than before stroke, Yes to the same extent as before stroke, No I did not work before the stroke, and I do not know. Expectations of RTW were defined as answering No but I am planning to RTW to the question and answering No was classified as no expectations of RTW. Participants that gave other answers to the question were excluded from the logistic regression analysis since the aim was to compare the participants expecting to RTW to the participants not expecting to RTW.
2.4. Statistical methods
All analyses were performed with IBM SPSS 25. The level of significance was set to P < .05.
The time to RTW was graphically presented with Kaplan‐Meier curves. Reasons for censoring were as follows: the end of follow‐up time (set to 1825 days) and death before RTW. Death violates the non–informative‐censoring assumption; and therefore, death was treated with a worst‐case scenario approach to obtain a more conservative estimate. Thus, censoring due to death was set at end of follow‐up (1825 days), instead of the commonly used date of death.
Potential predictors of the time to RTW (sex, age groups, stroke severity, stroke type, country of birth, income groups, and educational level) were analyzed with multiple Cox regression and presented with hazard ratios (HRs), 95% confidence intervals (95% CIs), and P‐values. Potential predictors were chosen, based on clinical reasoning. The adjusted hazard ratios should be interpreted under the assumption that the other predictors in the model are constant. Kaplan‐Meier curves and log (−log(survival curves)) were performed to check for major violations of the proportional hazard assumption. In case of major violations of the assumption, the corresponding variable was excluded as predictor in the regression model, and instead, the model was adjusted by employing a stratification of the variable in the model.
Whether self‐expectations of RTW 1 year post‐stroke could predict RTW after 5 years was analyzed with logistic regression and presented with odds ratios (ORs), 95% confidence intervals (95% CIs), and P‐values. Both unadjusted results and results adjusted for age, sex, and stroke severity were presented. To test the goodness of fit of the regression model, the Hosmer and Lemeshow test and receiver operating characteristics curves (ROC curves) were performed.
2.5. Ethics
The study was approved by the regional ethical review board in Gothenburg (Dnr922‐17). The Data Inspection Board in Sweden state that data that are handled in quality registries are considered an exception to the general rule of requiring written informed consent, to promote improvements in care and treatment, which is of general interest. Therefore, the current study did not obtain consent from the participants. Nevertheless, the participants were informed that their data could be used for research, when their data were reported to Riksstroke, and they had the right to withdraw their data at any time.
2.6. Availability of data
Data that support the findings of this study cannot be made available from the authors for more than what has been applied and approved for by the Ethical Review Board. The analyses were based on data from different registries (Riksstroke, Statistics Sweden, the National Board of Health and Welfare, and the Social Insurance Agency of Sweden), and the data can be made available upon reasonable request to each of the registry managers.
3. RESULTS
Of the 25 108 stroke events registered in Riksstroke in 2011, 2302 events occurred in persons that were 18‐58 years old at the time of the stroke (Figure 1). Of these, 1968 participants fulfilled the inclusion criteria. The excluded participants were significantly older than the included (P = .04), but there was no significant sex difference (P = .851). The majority of the participants were men, and the mean age at time of stroke was 50 years of age (Table 1).
Figure 1.
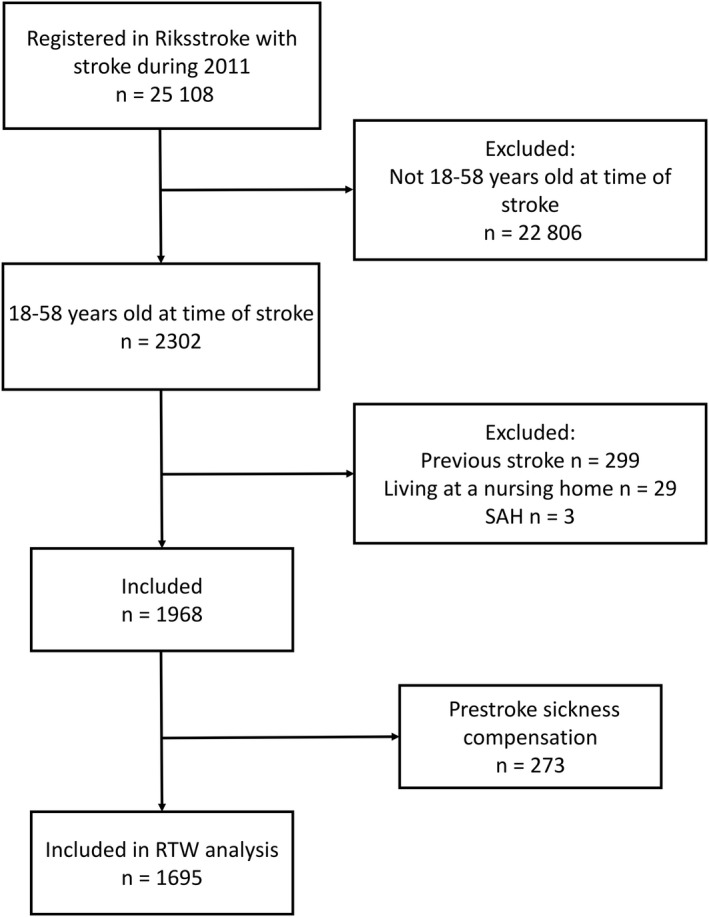
Flowchart of the study population. Abbreviations: RTW, return to work; SAH, subarachnoid hemorrhage
Table 1.
Characteristics of the study population
| Characteristics | Participants |
|---|---|
| Total, n | 1968 |
| Age, mean (SD) | 49.61 (7.912) |
| Age group, n (%) | |
| 18‐44 y | 419 (21.3) |
| 45‐52 y | 640 (32.5) |
| 53‐58 y | 909 (46.2) |
| Sex, n (%) | |
| Men | 1252 (63.6) |
| Women | 716 (36.4) |
| Living alone, n (%; 17 missing) | |
| Yes | 570 (29.2) |
| Smoker prestroke, n (%; 90 missing) | |
| Yes | 602 (32.1) |
| Atrial fibrillation diagnosis, n (%; four missing) | |
| Yes | 104 (5.3) |
| Diabetes diagnosis, n (%; two missing) | |
| Yes | 282 (14.3) |
| Medication for hypertension prestroke, n (%; six missing) | |
| Yes | 602 (30.7) |
| Stroke type, n (%) | |
| ISa | 1623 (82.5) |
| ICH | 345 (17.5) |
| Level of consciousness, n (%; 34 missing) | |
| Alert | 1694 (87.6) |
| Drowsy | 151 (7.8) |
| Unconscious | 89 (4.6) |
| Reperfusion treatment, n (%) | |
| Thrombolysis (three missing) | 235 (12.0) |
| Thrombectomy (four missing) | 43 (2.2) |
| Educational level, n (%; 71 missing) | |
| Primary school | 445 (23.5) |
| Secondary school | 983 (51.8) |
| Short university education | 115 (6.1) |
| Long university education | 354 (18.7) |
| Income, median (min‐max; 49 missing) | 204 351 (0‐4 324 420) |
| Income tertiles, n (%; 49 missing) | |
| Low (≤160 294 SEK) | 639 (33.3) |
| Middle (160 440‐246 735 SEK) | 640 (33.4) |
| High (≥246 882 SEK) | 640 (33.4) |
| Country of birth, n (%; 58 missing) | |
| Sweden | 1523 (79.7) |
| Nordic countries outside of Sweden | 107 (5.6) |
| European countries outside of the Nordic countries | 120 (6.3) |
| Countries outside of Europe | 160 (8.1) |
| Africa | 38 (23.8) |
| Asia | 95 (59.4) |
| South and Central America | 20 (12.5) |
| North America | 7 (4.4) |
Abbreviations: ICH, intracerebral hemorrhage; IS, ischemic stroke; SDs, standard deviations.
Fifteen of the participants with IS were classified as unspecified stroke.
3.1. Time to RTW
Of the 1968 participants, 273 (13.9%) had received prestroke sickness compensation for more than 50%; of these, eight participants were on 75% and 265 participants on 100% sickness compensation. Therefore, these participants were not included in the RTW analyses. Fifty‐eight (2.9%) participants received sickness compensation on 25% or 50% prior to stroke, and these participants were included in the following analyses.
Among the 1695 participants included (Figure 2), 1448 (85.4%) were able to RTW within 5 years. Forty‐eight per cent could RTW within the first 3 months, 72% within the first year, and 79% within the first 2 years post‐stroke. The last participant to RTW did so after 1727 days. Of the 247 participants that did not RTW, 99 (40.1%) died before the end of follow‐up; the 148 survivors remained on sickness benefit or sickness compensation through the study.
Figure 2.
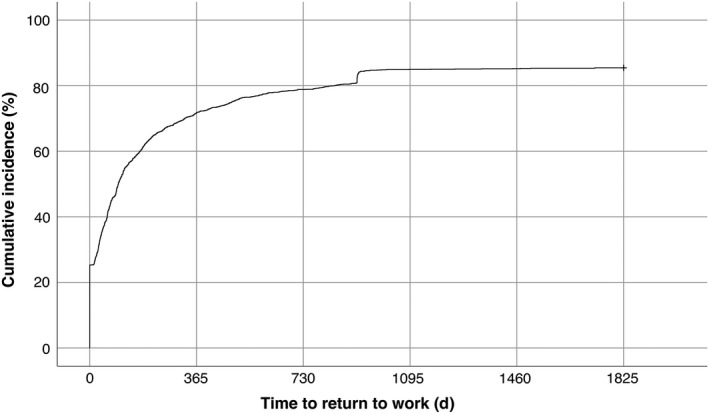
Kaplan‐Meier curve of time to return to work. Censoring due to death was set at end of follow‐up
The notch in the curve in Figure 2 corresponds to the 26 participants that did RTW at 914 days (approximately 2.5 years) post‐stroke. Of these participants, 15 were non‐registered in the Social Insurance Agency for a period of approximately 3 months (80‐100 days) before they were registered with a new case of sickness benefit.
3.2. Predictors of time to RTW
Male sex (compared with female sex) and IS (compared with ICH) were significant favorable predictors of time to higher RTW. Additionally, higher stroke severity and the oldest age group (compared with the youngest) were unfavorable predictors (Table 2). Long university education was significantly favorable compared with primary school. Country of birth and income did not fulfill the proportional hazard assumption; therefore, they were omitted from the analysis, but stratified for in the Cox model. Kaplan‐Meier curves (Figure 3) showed that the lowest income tertile had the fastest RTW the first year post‐stroke, but after the first year, the highest income tertile displayed a higher RTW and ended up on a higher RTW frequency compared with the other income groups. Participants born outside of Europe displayed the shortest time to RTW the first year; after that, Sweden, Nordic countries, and countries outside of Europe as country of birth seem to have approximately the same RTW rate. Participants born in a European country outside of the Nordic countries seem to have the lowest RTW rate throughout the follow‐up. The participants born in a country outside of Europe had significantly more long university education than the participants born in Sweden (P = .015).
Table 2.
Cox regression modeling time to RTW, adjusted for income and country of birth. Hazard ratios (HRs) with 95% confidence intervals (CIs)
| Predictors | HR | 95% CI | P‐value |
|---|---|---|---|
| Educational level | |||
| Primary school, ref | .006 | ||
| Secondary school | 1.062 | 0.919‐1.227 | .415 |
| Short university education | 1.223 | 0.994‐1.590 | .056 |
| Long university education | 1.227 | 1.096‐1.544 | .003 |
| Male sex | 1.148 | 1.022‐1.288 | .020 |
| Age | |||
| 18‐44 y, ref | .135 | ||
| 45‐52 y | 0.915 | 0.792‐1.058 | .231 |
| 53‐58 y | 0.866 | 0.752‐0.997 | .045 |
| Stroke severity | |||
| Alert, ref | <.001 | ||
| Drowsy | 0.495 | 0.390‐0.629 | <.001 |
| Unconscious | 0.217 | 0.138‐0.342 | <.001 |
| Stroke type, IS | 1.409 | 1.200‐1.655 | <.001 |
Bold values means P < 0.05.
1591 participants were included, 104 missing.
Abbreviations: CI, confidence interval; HR, hazard ratio; IS, ischemic stroke.
Figure 3.
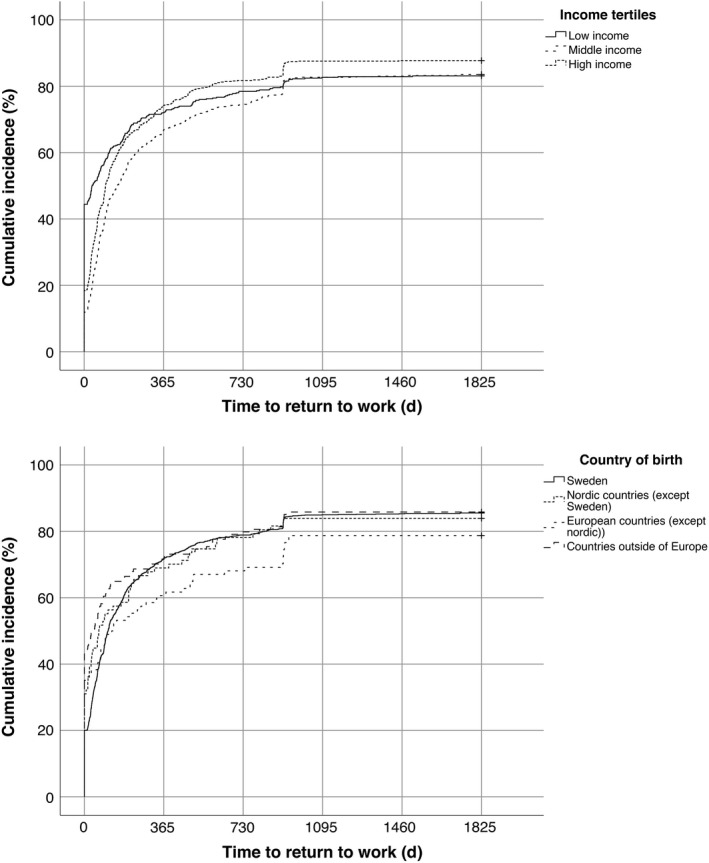
Kaplan‐Meier curves of time to return to work, divided according to income and country of birth. Censoring due to death was set at end of follow‐up
3.3. Self‐expectations of RTW
Of the 1695 participants, 1038 (61%) responded to the RTW question administered at 1 year. However, of these 817 selected a response excluding them from the analysis (eg, that they had already RTW by the end of the year), leaving 221 respondents to be included in the logistic regression analysis of self‐expectations prediction of RTW. There was no significant difference between the participants included and those not included in the logistic regression regarding sex (P = .649) or age (P = .226). However, the included participants had experienced significantly more severe stroke (P < .001), compared with those not included. Self‐expectations of RTW were reported in 109 participants, and 112 had no self‐expectations of RTW 1 year after stroke. The odds of RTW within 5 years were over 3‐fold higher among participants that expected to RTW 1 year post‐stroke, compared with the participants that did not expect to RTW, in both the unadjusted and the adjusted models (Table 3).
Table 3.
Logistic regression modeling higher RTW. Unadjusted and adjusted (age, sex, and stroke severity) odds ratios (ORs) and 95% confidence intervals (CIs)
| Predictor | Unadjusted | Adjusted | ||
|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95%CI) | P‐value | |
| Expecting to RTW | 3.720 (2.019‐6.855) | <.001 | 3.718 (1.938‐7.133) | <.001 |
Unadjusted model: n = 221. Area under the ROC curve = 0.656.
Adjusted model: n = 215. Hosmer‐Lemeshow P = .424. Area under the ROC curve = 0.743.
Abbreviations: CI, confidence interval; ORs, odds ratio; RTW, return to work.
4. DISCUSSION
The majority of participants RTW within the first 2 years post‐stroke, but the RTW continued for more than 4 years post‐stroke, with a total of 85% RTW within the study period. Male sex, an ischemic stroke, and long university education compared with a primary school education were all predictors of favorable time to RTW. Predictors of unfavorable times to RTW were higher stroke severity and older ages. The participants with self‐expectations of RTW 1 year post‐stroke had higher odds of RTW within 5 years.
With participants continuing to RTW for several years post‐stroke, the present study could strengthen what was indicated in a smaller previous Swedish study9 that the RTW after a stroke could continue for more than 1‐2 years. However, the majority still RTW within the first 2 years after stroke in the present study. It remains unknown whether this pattern is the same in other parts in the world, due to the lack of studies that investigated the time to RTW with a long‐term perspective.7 It is important to know that the RTW after a stroke could be a protracted process; this knowledge could contribute to policy making, and it is also important for healthcare professionals that meet with the patient. More accurate information and rehabilitation efforts can help optimize the RTW process without placing unreasonable pressure on the affected individual.
In the present study, 85% of participants were able to RTW. This frequency is higher than those found in most previous studies, although frequency varies widely.7 Methodological reasons for the disparate frequencies include different follow‐up times and different definitions of RTW. The present study had a long follow‐up time and a definition of RTW differing from self‐reported RTW. For example, the notch in the Kaplan‐Meier curve was caused by a few participants being defined as RTW at exactly 914 days post‐stroke. Some of these cases might be explained by the regulation valid at the time, which specified a maximum time of sickness benefit,16 after which a break of at least 87 days was required. Thus, in the current study, these participants were designated as RTW. It is also likely that different health care and health insurance systems, and different views on work and sickness led to disparate RTW patterns in different countries.
Older ages (53‐58 years old) comprised an unfavorable factor compared with the youngest ages (18‐44 years old), and male sex was a favorable factor for the time to RTW. The fact that men were able to RTW at a higher rate than women after a stroke has repeatedly been shown.18, 19 Women were shown to have worse functional outcomes than men after a stroke,20 but the differences in the time to RTW could perhaps also be explained by a segregated labor market, where jobs for men and women have different characteristics.11 The fact that men were more likely to RTW emphasizes the need for an awareness of possible gender prejudices from both the employer and the Social Insurance Agency. Age has been a more inconsistent predictor for RTW; it has been described as an insignificant predictor in some studies.21 Consistent with the present study, other studies have also reported younger age as favorable for RTW22, 23; however, in one study, the youngest age group (25‐34 years) had the lowest RTW rate, compared with persons of 35‐44 and 45‐55 years of age.13
Among the socioeconomic factors, a long university education was more favorable for the time to RTW than a primary school education. The country of birth and income could not be included as predictors, because they were time‐dependent; their favorability as predictors changed over short and long periods after the stroke. Participants with lower incomes seemed to RTW soonest within the first year post‐stroke. In contrast, participants with higher incomes had a higher RTW over the long term. Participants born in a country outside Europe seemed to RTW soonest in the short term, and only those born in a European country outside of the Nordic countries seemed to have a lower RTW in the long term. A potential explanation for the rapid RTW among participants born in a country outside of Europe might be that those participants also had significantly more long university education than the participants born in Sweden. The present study differs somewhat from previous studies. Other studies showed that a higher income and a higher educational level were associated with an increased RTW,24 while being born in a European country (except for the Nordic countries) or in a country outside Europe was a negative predictor of RTW.13 Both the present study and the previous study that investigated country of birth as a predictor of RTW included relatively few participants born outside of Sweden, which could potentially affect the results and explain the contradictory findings.
Higher stroke severity was associated with lower odds of RTW in the present study. This factor is also one of the most consistently associated with RTW in previous studies.24 The odds of RTW were higher among those with an IS compared to those with ICH. Previous studies have also shown that the ICH was an unfavorable factor for the time to RTW compared with the IS.25 This observation might be explained by the fact that the ICH is generally more severe and has worse outcome than the IS.26
The odds of RTW within 5 years were 3‐fold higher among participants that expected to RTW than among participants that did not expect to RTW at 1 year post‐stroke. Similar findings of self‐expectations of RTW were reported in numerous studies that investigated individuals with musculoskeletal diseases.14 The importance of self‐expectations of RTW also for persons with stroke could be a useful therapy target during rehabilitation post‐stroke. One study that investigated factors that influenced expectations of RTW showed that many of the factors were amendable,27 which indicates an opportunity for improvement. Important factors for expectations of RTW included sense of self (eg, pride and identity as a worker), the work context (eg, flexibility in adjusting the work environment and stress), and the disability management (eg, getting a diagnosis/treatment and the influence of their doctor, and physical/occupational therapist).27 Furthermore, feelings (depression, frustration, uncertainty) and wants (wanting to contribute and do the right thing) were also important when creating expectations of RTW.27
Only 99 of the 1695 participants died by the end of follow‐up. Therefore, the conservative approach of censoring all death at the end of follow‐up likely had only a minor impact on the results of the analysis.
4.1. Limitations
The definition of RTW should be discussed. Although the use of sickness absence data could provide an objective outcome without the risk of selection bias, it also has some limitations. It could be that a participant ends their registration with sickness benefit or sickness compensation without actually RTW, the fact that the Social Insurance Agency has deemed the individual to have working capacity does not guarantee a successful RTW. For example, some participants that were counted as RTW after 914 days (discussed above) might not have actually achieved an RTW. Unemployment is not taken under consideration. Furthermore, the current study defined RTW as working half‐time or more, and did not analyze RTW to different extent separately.
To use the level of consciousness as a proxy for stroke severity could weaken the results. However, the variable has been shown to be a useful proxy for stroke severity before.28 The level of consciousness would be expected to be a good predictor of failure to return to work, as depressed level of consciousness is most often observed with ICH and top of the basilar infarction, both known to carry a worse prognosis. The severity of paresis and large vessel distribution infarction might be more useful variables in gauging likelihood of return to work, but they are not included in Riksstroke.
The question used to assess the expectations of RTW was not originally formulated to investigate expectations of RTW; rather, the question was created to investigate RTW in general after stroke. This difference in intent might limit the results from the logistic regression analysis. Furthermore, only a minority of participants chose the responses that made them eligible for inclusion in the logistic regression. Consequently, there could be a risk of selection bias. However, the participants included and not included in the analysis only differed significantly in terms of stroke severity. The majority of participants that were not included in the analysis had responded to the survey, but they responded for example that they had already achieved a RTW at 1 year post‐stroke.
The present study is carried out in Sweden within the frame of the public Swedish social insurance system. Differences in Social Insurance between countries could potentially effect the generalizability of the results.
5. CONCLUSIONS
The RTW was most frequent the first 2 years, but continued for several years after a stroke at working age. Demographical, stroke‐related factors, and socioeconomic factors were important predictors of the time to RTW. Furthermore, participants that had self‐expectations of RTW at 1 year post‐stroke had more than 3‐fold higher odds of RTW within 5 years, compared with participants that did not expect to RTW. This information might facilitate individualized and optimized rehabilitation after a stroke, to increase the RTW.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to thank the Riksstroke Collaboration http://www.riksstroke.org/eng/ for providing data to the current study and a special thanks to statistician Fredrik Jonsson (Riksstroke/Umeå) for his dedicated contribution.
Westerlind E, Persson HC, Eriksson M, Norrving B, Sunnerhagen KS. Return to work after stroke: A Swedish nationwide registry‐based study. Acta Neurol Scand. 2020;141:56–64. 10.1111/ane.13180
Funding information
The study was funded by the Norrbacka‐Eugenia foundation, the Swedish Science Council (VR2017‐00946), the Swedish Heart and Lung foundation, Promobilia, the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (ALFGBG‐718711), and the Sparbanksstiftelsen Färs & Frosta.
DATA AVAILABILITY STATEMENT
Data that supports the findings of this study cannot be made available from the authors for more than what has been applied and approved for by the Ethical Review Board. The analyses were based on data from different registries (Riksstroke, Statistics Sweden, the National Board of Health and Welfare, and the Social Insurance Agency of Sweden) and the data can be made available upon reasonable request to each of the registry managers.
REFERENCES
- 1. Krishnamurthi RV, Feigin VL, Forouzanfar MH, et al. Global and regional burden of first‐ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1(5):e259‐e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty‐four‐year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44(9):2388‐2393. [DOI] [PubMed] [Google Scholar]
- 3. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vestling M, Tufvesson B, Iwarsson S. Indicators for return to work after stroke and the importance of work for subjective well‐being and life satisfaction. J Rehabil Med. 2003;35(3):127‐131. [DOI] [PubMed] [Google Scholar]
- 5. Roding J, Glader EL, Malm J, Lindstrom B. Life satisfaction in younger individuals after stroke: different predisposing factors among men and women. J Rehabil Med. 2010;42(2):155‐161. [DOI] [PubMed] [Google Scholar]
- 6. Ghatnekar O, Persson U, Asplund K, Glader EL. Costs for stroke in Sweden 2009 and developments since 1997. Int J Technol Assess Health Care. 2014;30(2):203‐209. [DOI] [PubMed] [Google Scholar]
- 7. Edwards JD, Kapoor A, Linkewich E, Swartz RH. Return to work after young stroke: a systematic review. Int J Stroke. 2018;13(3):243‐256. [DOI] [PubMed] [Google Scholar]
- 8. Harris C. Return to work after stroke: a nursing state of the science. Stroke. 2014;45(9):e174‐e176. [DOI] [PubMed] [Google Scholar]
- 9. Westerlind E, Persson HC, Sunnerhagen KS. Return to work after a stroke in working age persons; A six‐year follow up. PLoS One. 2017;12(1):e0169759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang YC, Kapellusch J, Garg A. Important factors influencing the return to work after stroke. Work. 2014;47(4):553‐559. [DOI] [PubMed] [Google Scholar]
- 11. Palstam A, Westerlind E, Persson HC, Sunnerhagen KS. Work‐related predictors for return to work after stroke. Acta Neurol Scand. 2019;139(4):382‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka H, Toyonaga T, Hashimoto H. Functional and occupational characteristics predictive of a return to work within 18 months after stroke in Japan: implications for rehabilitation. Int Arch Occup Environ Health. 2014;87(4):445‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glader EL, Jonsson B, Norrving B, Eriksson M. Socioeconomic factors' effect on return to work after first stroke. Acta Neurol Scand. 2017;135(6):608‐613. [DOI] [PubMed] [Google Scholar]
- 14. Young AE, Besen E, Choi Y. The importance, measurement and practical implications of worker's expectations for return to work. Disabil Rehabil. 2015;37(20):1808‐1816. [DOI] [PubMed] [Google Scholar]
- 15. Soderholm A, Stegmayr B, Glader EL, Asplund K. Validation of hospital performance measures of acute stroke care quality. Riksstroke, the Swedish stroke register. Neuroepidemiology. 2016;46(4):229‐234. [DOI] [PubMed] [Google Scholar]
- 16. The Swedish Social Insurance Agency . Sjukpenning, rehabilitering och rehabiliteringsersättning ‐ Vägledning 2015:1 Version 10. 2019.
- 17. Starmark JE, Stalhammar D, Holmgren E, Rosander B. A comparison of the Glasgow coma scale and the reaction level scale (RLS85). J Neurosurg. 1988;69(5):699‐706. [DOI] [PubMed] [Google Scholar]
- 18. Busch MA, Coshall C, Heuschmann PU, McKevitt C, Wolfe CDA. Sociodemographic differences in return to work after stroke: the South London stroke register (SLSR). J Neurol Neurosurg Psychiatry. 2009;80(8):888‐893. [DOI] [PubMed] [Google Scholar]
- 19. Hannerz H, Holbaek Pedersen B, Poulsen OM, Humle F, Andersen LL. A nationwide prospective cohort study on return to gainful occupation after stroke in Denmark 1996–2006. BMJ Open. 2011;1(2):e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Synhaeve NE, Arntz RM, van Alebeek ME, et al. Women have a poorer very long‐term functional outcome after stroke among adults aged 18–50 years: the FUTURE study. J Neurol. 2016;263(6):1099‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen LP, Biering K, Johnsen SP, Andersen G, Hjollund NH. Self‐rated health and return to work after first‐time stroke. J Rehabil Med. 2016;48(4):339‐345. [DOI] [PubMed] [Google Scholar]
- 22. Hackett ML, Glozier N, Jan S, Lindley R. Returning to paid employment after stroke: the Psychosocial Outcomes In StrokE (POISE) cohort study. PLoS One. 2012;7(7):e41795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonner B, Pillai R, Sarma PS, Lipska KJ, Pandian J, Sylaja PN. Factors predictive of return to work after stroke in patients with mild‐moderate disability in India. Eur J Neurol. 2016;23(3):548‐553. [DOI] [PubMed] [Google Scholar]
- 24. Ashley KD, Lee LT, Heaton K. Return to work among stroke survivors. Workplace Health Saf. 2019;67(2):87‐94. [DOI] [PubMed] [Google Scholar]
- 25. Endo M, Sairenchi T, Kojimahara N, et al. Sickness absence and return to work among Japanese stroke survivors: a 365‐day cohort study. BMJ Open. 2016;6(1):e009682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sennfalt S, Norrving B, Petersson J, Ullberg T. Long‐term survival and function after stroke. Stroke. 2019;50(1):53‐61. [DOI] [PubMed] [Google Scholar]
- 27. Young AE, Choi Y, Besen E. An exploration of the factors considered when forming expectations for returning to work following sickness absence due to a musculoskeletal condition. PLoS One. 2015;10(11):e0143330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bray BD, Campbell J, Cloud GC, et al. Derivation and external validation of a case mix model for the standardized reporting of 30‐day stroke mortality rates. Stroke. 2014;45(11):3374‐3380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data that support the findings of this study cannot be made available from the authors for more than what has been applied and approved for by the Ethical Review Board. The analyses were based on data from different registries (Riksstroke, Statistics Sweden, the National Board of Health and Welfare, and the Social Insurance Agency of Sweden), and the data can be made available upon reasonable request to each of the registry managers.
Data that supports the findings of this study cannot be made available from the authors for more than what has been applied and approved for by the Ethical Review Board. The analyses were based on data from different registries (Riksstroke, Statistics Sweden, the National Board of Health and Welfare, and the Social Insurance Agency of Sweden) and the data can be made available upon reasonable request to each of the registry managers.


