Abstract
Background
The results of the randomized, phase 3 ET743‐SAR‐3007 trial demonstrated that trabectedin had a significantly longer progression‐free survival (PFS) compared with dacarbazine in patients with advanced leiomyosarcoma/liposarcoma after the failure of prior chemotherapy. Patients randomized to trabectedin received a 24‐hour intravenous infusion either in an inpatient or outpatient setting. Herein, the authors reported the safety, efficacy, and patient‐reported outcomes based on first infusion site of care.
Methods
Patients were randomized 2:1 to trabectedin (at a dose of 1.5 mg/m2) or dacarbazine (1 g/m2 over 20‐120 minutes) with overall survival (OS) as the primary endpoint and PFS, time to disease progression, objective response rate, duration of response, safety, and patient‐reported symptom scoring as secondary endpoints. The setting of the trabectedin infusion was based on institutional preference and categorized based on the setting of the first infusion.
Results
Of the 378 patients who were treated with trabectedin, 100 (27%) and 277 (73%), respectively, first received trabectedin in the inpatient and outpatient setting. No differences were observed with regard to PFS or OS based on site of care. The median PFS was 4.1 months versus 4.2 months (hazard ratio, 0.90; P = .49) for inpatients versus outpatients, respectively, and the median OS was 14.3 months versus 13.7 months (hazard ratio, 0.89; P = .40), respectively. Grade 3/4 adverse events (classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events [version 4.0]) were reported in 87 inpatients (87%) compared with 219 outpatients (79%); grade 3/4 serious adverse events were reported in 43 inpatients (43%) and 92 outpatients (33%). Extravasation occurred in 0 inpatients and 5 outpatients (2%), whereas the incidence of catheter‐related complications was similar between groups (16% vs 15%).
Conclusions
Although the majority of patients who were randomized to trabectedin received outpatient therapy, the outcomes of the current study suggested equivalent safety and efficacy in either setting.
Keywords: extravasation, inpatients, leiomyosarcoma, liposarcoma, outpatients, soft‐tissue sarcoma, trabectedin
Short abstract
The majority of patients randomized to trabectedin in the ET743‐SAR‐3007 trial have received the drug in the outpatient setting. The results of the current analysis suggest that trabectedin has equivalent efficacy and comparable safety when administered in an inpatient or outpatient setting.
Introduction
Soft‐tissue sarcomas (STSs) are rare, heterogeneous malignancies arising from tissues of mesenchymal origin.1 Despite evolving treatments, options remain limited for patients with advanced, metastatic, or unresectable disease. First‐line chemotherapy typically includes anthracycline‐based treatment (with or without ifosfamide2) or gemcitabine plus docetaxel.3, 4 After the failure of first‐line treatment, regimens are less well defined but commonly comprise ifosfamide, doxorubicin, gemcitabine with or without docetaxel, and dacarbazine; the newer agents eribulin, pazopanib, and trabectedin also have been indicated for certain STS subtypes.1
Trabectedin is an antineoplastic alkaloid with a multimodal mechanism of action,5 and was approved in the European Union in 2007 based on a phase 2 trial in which patients with previously treated, advanced liposarcoma or leiomyosarcoma (LPS/LMS) were randomized to receive 1 of 2 schedules of the drug. This trial established the 24‐hour infusion of trabectedin as the standard schedule (compared with a 3‐hour infusion).6 Trabectedin was approved in the United States in 2015 based on the randomized phase 3 ET743‐SAR‐3007 trial and demonstrated a significant improvement in median progression‐free survival (PFS) for patients with previously treated, advanced LPS/LMS who were treated with trabectedin compared with those receiving dacarbazine (4.2 months vs 1.5 months, respectively) and a safety profile consistent with that of previous studies.7
Trabectedin is a vesicant with the potential for extravasation from the blood vessels and subsequent damage to surrounding tissue. Damage may include blistering, severe pain, and/or tissue necrosis8 and can require surgical intervention in severe cases (eg, debridement, skin reconstruction). Device dislodgement and/or infections at the site of central venous access may be the cause of some of the complications that have been documented among patients treated with trabectedin.9, 10 To the best of our knowledge, there are few published data to date regarding trabectedin‐associated extravasation, all of which have involved retrospective case studies or very small case series. Evidence‐based guidance for managing extravasation in patients who receive cytotoxic treatments also is unclear.8, 11
Given the importance of the early detection of extravasation during infusion, a subgroup analysis of the ET743‐SAR‐3007 patient population was conducted. The objective of the current study was to evaluate the safety, efficacy, and patient‐reported outcomes (PROs) of patients receiving trabectedin based on the first infusion site of care (inpatient vs outpatient).
Materials and Methods
Patients
Inclusion and exclusion criteria for eligibility in the ET743‐SAR‐3007 trial have been reported previously.7 Briefly, eligible patients were aged ≥15 years with histologically proven unresectable, locally advanced or metastatic LPS/LMS who previously were treated with at least: 1) a regimen containing an anthracycline and ifosfamide; or 2) an anthracycline and ≥1 additional cytotoxic chemotherapy regimen(s). Patients had measurable disease according to Response Evaluation Criteria in Solid Tumors (version 1.1); an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤1; and adequate renal, hepatic, and bone marrow function. Patients with known central nervous system metastases, chronic liver disease, myocardial infarction within 6 months of study enrollment, and/or New York Heart Association class II or greater heart failure were excluded. Review boards at all participating institutions approved the trial, which was conducted according to the Declaration of Helsinki of the World Medical Association. All patients provided written informed consent to participate. This study is registered at https://www.ClinicalTrials.gov (identifier NCT01343277).
Study Design
The ET743‐SAR‐3007 trial was conducted between May 27, 2011, and January 5, 2015. Patients were randomized 2:1 to trabectedin at a dose of 1.5 mg/m2 as a 24‐hour intravenous infusion or to dacarbazine at a dose of 1 g/m2 as a 20‐minute to 120‐minute intravenous infusion, with study drug administered on day 1 of each 21‐day cycle. Patients assigned to receive trabectedin were pretreated with intravenous dexamethasone at a dose of 20 mg approximately 30 minutes before each dose and received trabectedin via a central venous catheter. Treatment setting (inpatient vs outpatient) was determined at the discretion of the investigator based on institutional preference or standard of care. The setting of administration was collected for the first infusion with the assumption that this remained unchanged for subsequent doses.
The primary endpoint was overall survival (OS); secondary endpoints included PFS, objective response rate (ORR), time to disease progression, duration of response (DOR), safety, and patient‐reported symptom scoring. In addition, the clinical benefit rate (CBR; complete responses plus partial responses plus stable disease for ≥18 weeks) and duration of stable disease were analyzed to evaluate prolonged disease control.
Efficacy Evaluations
Investigators assessed tumor response using radiographic imaging of the chest, abdomen, and pelvis every 6 weeks for the first 36 weeks on study and every 9 weeks thereafter until disease progression, subsequent anticancer therapy, end of the study, or patient death occurred. As per protocol, a final analysis of OS data was planned to be conducted after 376 death events had occurred, with an interim analysis conducted after approximately 50% of those deaths had occurred. The final analyses of PFS, ORR, time to disease progression, and DOR were to occur at the time of the interim OS analysis.
Safety Evaluations
Safety assessments were based on reported adverse events (AEs), clinical laboratory tests, vital sign measurements, physical examination, multigated acquisition scan or echocardiogram, and concomitant medication use. Toxicity grades were classified according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Patient‐Reported Outcomes
The MD Anderson Symptom Inventory (MDASI) scores were used to assess patients' perceived symptom burden and the impact of treatment on symptom changes or stability. The MDASI is a 19‐item self‐reported questionnaire designed to measure cancer symptom or treatment‐related symptom severity and the degree to which those interfere with daily function. Thirteen items pertain to symptom severity “at its worst” (eg, pain, shortness of breath), and 6 items measure how much those symptoms have interfered with 6 daily activities (general activity, mood, work, relations with others, walking, and enjoyment of life); all are rated on a rating scale from 0 to 10, with increasing scores indicating greater symptom severity or burden. For this subanalysis, the percentages of patients reporting severe symptoms (MDASI score ≥7) are reported.
Statistical Analyses
The current exploratory analysis included participants in the ET743‐SAR‐3007 trial who were randomized to treatment with trabectedin and received ≥1 dose of study drug. The inpatient versus outpatient effectiveness comparisons for OS and PFS were performed using the log‐rank test. A Cox proportional hazards model was used to estimate the hazard ratios (HRs) for OS and PFS. The ORR and CBR were evaluated using the Fisher exact test. Demographic, baseline disease characteristics, safety, and PRO data were summarized descriptively. Progression‐free survival, ORR, DOR, and CBR analyses were performed using interim data (clinical cutoff date of September 16, 2013), in which 189 death events had occurred in the overall study population. The final OS and safety analyses were performed using data collected after 381 death events had occurred in the overall study population (clinical cutoff date of January 5, 2015).
Results
By the clinical cutoff date for the final OS analysis (January 5, 2015), a total of 577 patients had been randomized (384 to trabectedin and 193 to dacarbazine) and 550 had been treated (378 with trabectedin and 172 with dacarbazine). Among patients who received trabectedin, 100 patients (27%) and 277 patients (73%), respectively, were treated in the inpatient and outpatient settings; treatment setting was not recorded for 1 patient (Fig. 1).
Figure 1.
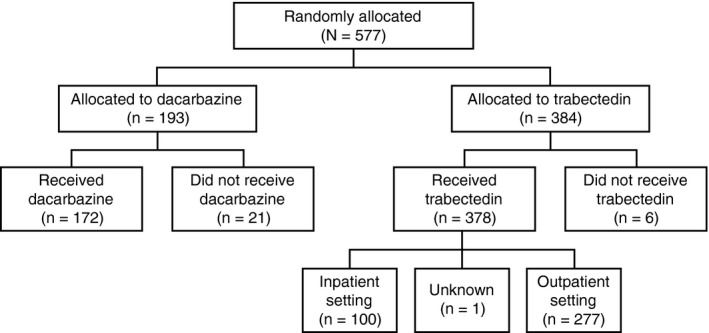
Patient disposition of inpatient versus outpatient subgroups of patients who received trabectedin in the ET743‐SAR‐3007 study.
Baseline demographics and disease characteristics were generally balanced across both the inpatient and outpatient subgroups, similar to the overall study population. Patients were predominantly women (67 patients [67%] and 189 patients [68%], respectively, in the inpatient vs outpatient subgroups) and white (77 patients [77%] and 217 patients [78%], respectively, in the inpatient vs outpatient subgroups). The inpatient subgroup was found to have a higher population of elderly patients (aged ≥65 years) compared with the outpatient subgroup (36 patients [36%] vs 57 patients [21%], respectively) and with the overall study population (93 patients [25%]), indicating potential selection bias in the choice of treatment setting. No major differences in tumor histology, ECOG PS, or lines of prior chemotherapy received were observed. In the inpatient and outpatient subgroups, patients primarily were characterized as having LMS (77 patients [77%] and 199 patients [72%], respectively), having received ≥2 prior lines of chemotherapy (85 patients [85%] and 246 patients [89%], respectively), and having a similar likelihood of an ECOG PS of 0 or 1 (Table 1). Treatment exposure also was found to be similar between the inpatient and outpatient subgroups, with a median of 4 treatment cycles administered in each (Table 2).
Table 1.
Patient Demographics and Baseline Disease Characteristics
| Characteristic | Inpatients n = 100 | Outpatients n = 277 | All Patientsa n = 377 |
|---|---|---|---|
| Age, y | |||
| 18 to <65 | 64 (64) | 220 (79) | 284 (75) |
| ≥65 | 36 (36) | 57 (21) | 93 (25) |
| Mean (SD) | 59 (11.5) | 56 (10.8) | 57 (11.1) |
| Median (range) | 60 (27‐81) | 56 (18‐81) | 57 (18‐81) |
| Sex | |||
| Women | 67 (67) | 189 (68) | 256 (68) |
| Men | 33 (33) | 88 (32) | 121 (32) |
| Race | |||
| White | 77 (77) | 217 (78) | 294 (78) |
| Black or African American | 16 (16) | 32 (12) | 48 (13) |
| Otherb | 4 (4) | 19 (7) | 23 (6) |
| Asian | 3 (3) | 8 (3) | 11 (3) |
| American Indian or Alaska Native | 0 (0) | 1 (0.4) | 1 (0.3) |
| Histology | |||
| Leiomyosarcoma | 77 (77) | 199 (72) | 276 (73) |
| Uterine | 35 (35) | 105 (38) | 140 (37) |
| Nonuterine | 42 (42) | 94 (34) | 136 (36) |
| Liposarcoma | 23 (23) | 78 (28) | 101 (27) |
| Dedifferentiated | 10 (10) | 38 (14) | 48 (13) |
| Myxoid ± round cell | 9 (9) | 33 (12) | 42 (11) |
| Pleomorphic | 4 (4) | 7 (3) | 11 (3) |
| Baseline ECOG performance status score | |||
| 0 | 44 (44) | 139 (50) | 183 (49) |
| 1 | 56 (56) | 138 (50) | 194 (52) |
| No. of lines of prior chemotherapy | |||
| 1 | 15 (15) | 31 (11) | 46 (12) |
| 2 | 47 (47) | 125 (45) | 172 (46) |
| 3 | 20 (20) | 77 (28) | 97 (26) |
| 4 | 12 (12) | 26 (9) | 38 (10) |
| ≥4 | 6 (6) | 18 (7) | 24 (6) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Values are presented as n (%) unless otherwise specified.
All patients from the ET743‐SAR‐3007 study who were randomized to the trabectedin group and for whom inpatient versus outpatient status was recorded.
“Other” included race categories of other, unknown, and not reported.
Table 2.
Treatment Exposure
| Inpatients n = 100 | Outpatients n = 277 | |
|---|---|---|
| Total no. of treatment cycles | ||
| Mean (SD) | 7 (6.9) | 6 (6.1) |
| Median (range) | 4 (1‐44) | 4 (1‐41) |
| Cumulative dose, mg/m2 | ||
| Mean (SD) | 9.2 (8.76) | 8.5 (8.19) |
| Median (range) | 5.95 (1.5‐54.5) | 5.70 (1.5‐61.5) |
| Dose intensity per cycle, mg/m2 | ||
| Mean (SD) | 1.25 (0.23) | 1.30 (0.20) |
| Median (range) | 1.29 (0.7‐1.6) | 1.34 (0.7‐1.6) |
Efficacy
Analyses by infusion setting demonstrated similar PFS and OS results across each subgroup. The median PFS was 4.1 months and 4.2 months, respectively, in the inpatient and outpatient subgroups (HR, 0.90; P = .49) (see Supporting Fig. 1); the median OS was 14.3 months and 13.7 months, respectively (HR, 0.89; P = .40). The CBR demonstrated no significant differences: 33 inpatients (38%) (95% CI, 27.5%‐39.5%) versus 84 outpatients (33%) (95% CI, 27.7%‐49.0%; odds ratio, 1.22 [95% CI, 0.71‐2.08; P = .44]). Likewise, the ORR demonstrated no significant differences: 12 inpatients (14%) (95% CI, 5.2%‐12.5%) versus 21 outpatients (8%) (95% CI, 7.3%‐22.9%; odds ratio, 1.76 [95% CI, 0.75‐3.95; P = .15]).
Safety
Treatment‐emergent AEs were found to occur at similar frequencies in both the inpatient and outpatient settings, with nausea, fatigue, anemia, vomiting, and transaminase increases among the most commonly reported for each group of patients (Table 3). Of note, that among AEs reported for ≥20% of patients, anemia, hypokalemia, cough, and pain in the extremities were reported with the most notably different frequencies between the inpatient and outpatient subgroups. Grade 3/4 AEs occurred in 87 patients (87%) and 219 patients (79%), respectively, who received trabectedin as inpatients versus outpatients. For both subgroups, grade 3/4 AEs most commonly included transaminase increases and hematologic toxicities as well as nausea and fatigue (Table 4); anemia, asthenia, and a decreased platelet count were reported with the most notably different frequencies between inpatients versus outpatients.
Table 3.
Most Commonly Reported Adverse Events (≥20% of Patients)
| Adverse Event | Inpatients n = 100 | Outpatients n = 277 |
|---|---|---|
| Nausea | 73 (73) | 212 (77) |
| Fatigue | 63 (63) | 199 (72) |
| Anemia | 58 (58) | 99 (36) |
| Alanine aminotransferase increased | 54 (54) | 133 (48) |
| Vomiting | 47 (47) | 126 (46) |
| Aspartate aminotransferase increased | 43 (43) | 99 (36) |
| Decreased appetite | 38 (38) | 103 (37) |
| Neutropenia | 37 (37) | 81 (29) |
| Constipation | 34 (34) | 107 (39) |
| Diarrhea | 34 (34) | 97 (35) |
| Peripheral edema | 33 (33) | 75 (27) |
| Cough | 30 (30) | 56 (20) |
| Headache | 29 (29) | 66 (24) |
| Neutrophil count decreased | 28 (28) | 68 (25) |
| Dyspnea | 28 (28) | 66 (24) |
| Blood alkaline phosphatase increased | 28 (28) | 59 (21) |
| Pyrexia | 25 (25) | 48 (17) |
| Hypokalemia | 25 (25) | 28 (10) |
| White blood cell count decreased | 24 (24) | 73 (26) |
| Thrombocytopenia | 24 (24) | 49 (18) |
| Platelet count decreased | 22 (22) | 41 (15) |
| Blood creatine phosphokinase increased | 20 (20) | 37 (13) |
| Pain in extremity | 20 (20) | 29 (11) |
Values are presented as n (%).
Table 4.
Grade 3 to 4a Adverse Events Occurring in ≥5% of Patients
| Inpatients n = 100 | Outpatients n = 277 | |
|---|---|---|
| Alanine aminotransferase increased | 29 (29) | 82 (30) |
| Neutropenia | 27 (27) | 63 (23) |
| Anemia | 26 (26) | 41 (15) |
| Neutrophil count decreased | 23 (23) | 54 (20) |
| White blood cell count decreased | 20 (20) | 55 (20) |
| Aspartate aminotransferase increased | 16 (16) | 41 (15) |
| Platelet count decreased | 15 (15) | 24 (9) |
| Thrombocytopenia | 14 (14) | 25 (9) |
| Leukopenia | 10 (10) | 27 (10) |
| Nausea | 10 (10) | 16 (6) |
| Fatigue | 9 (9) | 23 (8) |
| Vomiting | 8 (8) | 15 (5) |
| Dehydration | 6 (6) | 12 (4) |
| Pulmonary embolism | 6 (6) | 6 (2) |
| Asthenia | 6 (6) | 1 (0.4) |
| Blood creatine phosphokinase increased | 5 (5) | 17 (6) |
| Febrile neutropenia | 5 (5) | 13 (5) |
| Dyspnea | 5 (5) | 11 (4) |
| Hypokalemia | 5 (5) | 9 (3) |
| Catheter site infection | 5 (5) | 6 (2) |
| Hypoalbuminemia | 5 (5) | 2 (0.7) |
Values are presented as n (%).
Toxicity was classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Catheter‐related complications of any grade were reported at similar percentages for patients treated in the inpatient (16 patients [16%]) versus outpatient (42 patients [15%]) setting. Among inpatients, complications were classified as grade 1 and grade 2, respectively, for 8 patients (8%) and 2 patients (2%); among outpatients, the corresponding values were 13 patients (5%) and 15 patients (5%), respectively. Grade 3 catheter‐related complications are shown in Table 5; none were reported as grade ≥4. The 2 most frequently observed catheter‐related complications were catheter site infection (5 patients in the inpatient group vs 14 patients in the outpatient group; 5% each) and catheter site pain (7 patients in the inpatient group [7%] vs 12 patients in the outpatient group [4%]) (Table 5). Infusion site extravasations were observed in 5 outpatients (2%) compared with 0 inpatients (Table 5). Although the numbers were relatively small, patient‐level narratives did not reveal any further details that may have contributed to the increased number of outpatient extravasation events. Other catheter‐related complications that were observed in only ≥1% of the outpatient subgroup were device thrombosis (5 patients [2%]) and soft‐tissue necrosis (4 patients [1%]) (Table 5). Conversely, catheter site swelling was observed more frequently in the inpatient setting (3 patients [3%]) compared with the outpatient setting (0 patients [0%]). With the exception of catheter site infection and pain, the majority of complications were reported in small percentages for both subgroups, ranging from 0.4% to 3% (Table 5).
Table 5.
Adverse Events From Catheter‐Related Complicationsa
| Inpatients n = 100 | Outpatients n = 277 | |||
|---|---|---|---|---|
| Total | Grade 3 | Total | Grade 3 | |
| Catheter‐related complications | 16 (16) | 6 (6) | 42 (15) | 14 (5) |
| Catheter site infection | 5 (5) | 5 (5) | 14 (5) | 6 (2) |
| Catheter site pain | 7 (7) | 0 (0) | 12 (4) | 3 (1) |
| Catheter site inflammation | 1 (1) | 1 (1) | 7 (3) | 0 (0) |
| Infusion site extravasation | 0 (0) | 0 (0) | 5 (2) | 2 (1) |
| Thrombosis in device | 0 (0) | 0 (0) | 5 (2) | 1 (0.4) |
| Soft‐tissue necrosis | 0 (0) | 0 (0) | 4 (1) | 4 (1) |
| Catheter site erythema | 1 (1) | 0 (0) | 3 (1) | 0 (0) |
| Catheter site pruritus | 1 (1) | 0 (0) | 3 (1) | 0 (0) |
| Catheter site cellulitis | 1 (1) | 0 (0) | 2 (1) | 0 (0) |
| Catheter site–related reaction | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Device breakage | 0 (0) | 0 (0) | 1 (0.4) | 1 (0.4) |
| Device component issue | 2 (2) | 1 (1) | 1 (0.4) | 0 (0) |
| Device occlusion | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Infusion site erythema | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Infusion site pain | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Injection site bruising | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Injection site hemorrhage | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Injection site reaction | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Medical device complication | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) |
| Catheter site edema | 1 (1) | 0 (0) | 0 (0) | 0 (0) |
| Catheter site swelling | 3 (3) | 0 (0) | 0 (0) | 0 (0) |
Values are presented as n (%).
Toxicity was classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). No grade 4 or 5 catheter‐related complications were reported for either subgroup.
Patient Management After Infusion Site Extravasation
Of the 5 patients for whom infusion site extravasation was reported, the majority were managed conservatively. One patient was a 72‐year‐old white woman with nonuterine LMS who was randomized to treatment with trabectedin at a dose of 1.5 mg/m2. On day 2 after the fourth cycle, a grade 3 infusion site extravasation was noted and had progressed to necrosis by day 12. The patient was hospitalized and initially received intravenous antibiotics (cefazolin) and pain management. An initial surgical excision with debridement of the left anterior chest wall was performed as corrective treatment. At the time of further dissection, the chest wall and soft tissues were found to demonstrate additional necrosis in adjacent soft tissues. The device subsequently was removed, and the surrounding tissue was debrided extensively. A complex closure was performed with placement of a Jackson‐Pratt drain, and the patient was discharged on day 20. During a follow‐up visit on day 22, there was good wound healing noted after the prior surgical removal of necrotic tissues, and the drain was removed. Although no action was taken with the study drug due to soft‐tissue necrosis, trabectedin was discontinued permanently due to the infusion site extravasation. The events of soft‐tissue necrosis and infusion site extravasation resolved by day 110.
Patient‐Reported Outcomes
Compliance in completing the MDASI questionnaire at each treatment cycle ranged from 91% to 100% of patients in the trabectedin group overall. At the end of treatment, pain, fatigue, and feeling sad were among the most frequently high‐scoring symptoms reported using the MDASI PRO tool; however, no clinically meaningful differences were observed in patients reporting severe symptoms (MDASI score ≥7) when comparing inpatient with outpatient treatment settings (see Supporting Table 1).
Discussion
The objective of the current subanalysis of the ET743‐SAR‐3007 trial was to evaluate the safety, efficacy, and PROs in patients treated with trabectedin in the inpatient versus outpatient settings, with particular attention given to extravasation. Although the analysis was not preplanned, the data estimated what could be expected in either the inpatient or outpatient clinical setting from both a safety and efficacy perspective. No statistically significant difference was observed in terms of efficacy; however, the 2 most clinically meaningful safety differences were the incidences of extravasation (2%) and soft‐tissue necrosis (1%) observed in the outpatient infusion setting but not in the inpatient subgroup. Although the incidences were very low, a review of individual patient‐level data could not identify any contributing factors in terms of demographics, procedural errors, or prior infusion‐related events. The data set in the current study did not collect information regarding the specific type of central venous access.
Efforts to reduce extravasation at the trabectedin infusion site have been studied at the Dana‐Farber Cancer Institute.12 This was a quality initiative prompted by 3 cases of trabectedin infusion‐related extravasations that led to significant patient morbidity requiring surgical intervention. The interventions were based on proposed risk factors for extravasation (see Supporting Table 2), including patient‐derived, infusion‐related, and staff‐based risk factors. Steps taken to mitigate these presumed risks were initiated and demonstrated a decreased frequency in the reporting of trabectedin infusion‐related events. These, in addition to ensuring that the central venous catheter is functioning properly, provide suggested guidance regarding safety optimization, mitigation, and management of extravasation in the outpatient setting.
The current analysis was strengthened by the collection of data from a large prospective trial with a significant number of patients undergoing 24‐hour infusion between inpatient (100 patients) and outpatient (277 patients) settings. Although the setting of infusion was based on physician preference and/or institutional practice (ie, a patient‐agnostic decision) and not stratified, patient demographics were relatively well balanced. The main demographic difference was the higher number of patients aged ≥65 years in the inpatient subgroup. Although this introduced some degree of bias, it clearly illustrated the need to individualize patient treatment to further mitigate presumed risk. Another limitation of the current subanalysis was that the site of administration was documented only for the first infusion cycle, with the assumption that all subsequent cycles took place according to the cycle 1 administration site.
Conclusions
The current subanalysis from the phase 3 ET743‐SAR‐3007 trial, which compared inpatient with outpatient 24‐hour continuous infusion of trabectedin, demonstrated no significant differences with regard to safety, efficacy, and PROs. The risk of infusion‐related extravasation and soft‐tissue necrosis was <2% in the current study. It is envisioned that these findings will provide physicians and patients with a better opportunity to assess the individual risks and benefits of the inpatient versus outpatient infusion of trabectedin.
Funding Support
Supported by Janssen Research & Development LLC.
Conflict of Interest Disclosures
Robin L. Jones has received honoraria and consulting fees from Johnson and Johnson for work performed as part of the current study and has received honoraria and consulting fees from Adaptimmune, Blueprint Medicines, Clinigen, Eisai, Epizyme, Daiichi‐Sankyo, Deciphera Pharmaceuticals Inc, Immune Design, Janssen Oncology, Lilly, Merck, Pfizer, PharmaMar, Tracon, and UpToDate for work performed outside of the current study. Robert G. Maki has received grants from PharmaMar and Janssen to his institution for work performed as part of the current study and the conduct of studies using trabectedin and has acted as a paid member of the Data Safety Monitoring Board for Aadi Bioscience, Deciphera Pharmaceuticals Inc, and Karyopharm; acted as a paid consultant for Arcus, Eisai, and Foundation Medicine; acted as paid consultant for and received travel fees from Bayer; has received a grant from Morphotek; has received a grant from and acted as a paid consultant for GlaxoSmithKline; has received a grant to his institution from and acted as a paid consultant for Janssen/PharmaMar, Lilly, ImClone, Novartis, Pharmacia, Pfizer, Ciba Geigy, Sarcoma Alliance for Research through Collaboration, and Immune Design; has received clinical trials support to his institution from Beta Cat, Daiichi‐Sankyo, Forma Therapeutics, Genentech, Immunocore, Janssen/PharmaMar, Lilly/ImClone, Presage Biosciences, Regeneron, and Tracon Pharmaceuticals; has received honoraria from the American Association for Cancer Research, the American Society of Clinical Oncology, and Medical Oncology Exam Committee membership for American Board of Internal Medicine, and has received royalties from Springer, UpToDate, and Wiley for work performed outside of the current study. Shreyaskumar R. Patel reports grants from Janssen, personal fees from PharmaMar (travel for advisory board), and personal fees from M.J. Hennessey/OncLive (presentation at an educational meeting) for work performed as part of the current study and has acted as a paid consultant for Bayer, Daiichi Sankyo, Eli Lilly, Epizyme, Immune Design, Janssen, and Novartis Oncology; has received grants from Blueprint Medicines; and has received personal fees from CytRx and EMD Serono for work performed outside of the current study. George Wang, Tracy A. McGowan, and Waleed S. Shalaby are employees of Janssen and hold stock in Johnson and Johnson, of which Janssen is a wholly‐owned subsidiary. Roland E. Knoblauch is an employee of Janssen Oncology. Margaret von Mehren received support to the Fox Chase Cancer Center for the conduct of the current study from and has acted as a member of the advisory board and a member of the scientific steering committee for the current study for Janssen, and has acted as a paid consultant and member of the Data and Safety Monitoring Board for Eisai. George D. Demetri has received grants, personal fees, and nonfinancial support (for medical writing) from Janssen and grants, personal fees, and travel support from PharmaMar for work performed as part of the current study; has received grants, personal fees, nonfinancial support, and travel support from Daiichi‐Sankyo, Epizyme, Novartis, and Roche; has received grants, personal fees, and travel support from Adaptimmune, Bayer, Loxo Oncology, and Pfizer; has received personal fees and travel support from EMD Serono, M.J. Hennessey/OncLive, and WIRB‐Copernicus Group; has received personal fees from Sanofi; has received grants and personal fees from Ignyta; has received grants, personal fees, and nonfinancial support from AbbVie; has received personal fees from Mirati Therapeutics, Polaris Pharmaceuticals, and ZioPharm Oncology; has received a grant from GlaxoSmithKline; has received travel support from and holds equity in Blueprint Medicines and equity options in Merrimack Pharmaceuticals; holds equity and equity options in G1 Therapeutics; has received travel support and equity options from Caris Life Sciences; has equity options in Bessor Pharma and Erasca Pharmaceuticals; has received personal fees from and holds equity in Champions Oncology; and has a patent issued and licensed to PharmaMar for trabectedin use for cancer (patent from PharmaMar; no funds from this and no license to the Dana‐Farber Cancer Center or to Dr. Demetri) and a patent issued and licensed to Novartis for imatinib use in gastrointestinal stromal tumor and receives royalties due to patent for work performed outside of the current study.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, providing final approval of the version to be submitted, and agreeing to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of it are appropriately investigated and resolved. Robin L. Jones: Conception and design of the work, acquisition or collection of the data, and analysis or interpretation of the data. Robert G. Maki: Conception and design of the work, acquisition or collection of the data, and analysis or interpretation of the data. Shreyaskumar R. Patel: Conception and design of the work. George Wang: Conception and design of the work and analysis or interpretation of the data. Tracy A. McGowan: Conception and design of the work and analysis or interpretation of the data. Waleed S. Shalaby: Acquisition or collection of the data and analysis or interpretation of the data. Roland E. Knoblauch: Conception and design of the work, acquisition or collection of the data, and analysis or interpretation of the data. Margaret von Mehren: Conception and design of the work, acquisition or collection of the data, and analysis or interpretation of the data. George D. Demetri: Conception and design of the work, acquisition or collection of the data, and analysis or interpretation of the data.
Supporting information
We thank Gianna Paone, MS, MPH, of Janssen Scientific Affairs LLC for medical writing support.
References
- 1. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): soft tissue sarcoma. Version 2.2018. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed May 7, 2018.
- 2. Judson I, Verweij J, Gelderblom H, et al; European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group . Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first‐line treatment of advanced or metastatic soft‐tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15:415‐423. [DOI] [PubMed] [Google Scholar]
- 3. Seddon B, Strauss SJ, Whelan J, et al. Gemcitabine and docetaxel versus doxorubicin as first‐line treatment in previously untreated advanced unresectable or metastatic soft‐tissue sarcomas (GeDDiS): a randomised controlled phase 3 trial. Lancet Oncol. 2017;18:1397‐1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of Sarcoma Alliance for Research through Collaboration Study 002 [corrected]. J Clin Oncol. 2007;25:2755‐2763. [DOI] [PubMed] [Google Scholar]
- 5. D’Incalci M, Galmarini CM. A review of trabectedin (ET‐743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157‐2163. [DOI] [PubMed] [Google Scholar]
- 6. Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188‐4196. [DOI] [PubMed] [Google Scholar]
- 7. Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol. 2016;34:786‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Theman TA, Hartzell TL, Sinha I, et al. Recognition of a new chemotherapeutic vesicant: trabectedin (ecteinascidin‐743) extravasation with skin and soft tissue damage. J Clin Oncol. 2009;27:e198‐e200. [DOI] [PubMed] [Google Scholar]
- 9. Martella F, Salutari V, Marchetti C, et al. A retrospective analysis of trabectedin infusion by peripherally inserted central venous catheters: a multicentric Italian experience. Anticancer Drugs. 2015;26:990‐994. [DOI] [PubMed] [Google Scholar]
- 10. Verboom MC, Ouwerkerk J, Steeghs N, et al. Central venous access related adverse events after trabectedin infusions in soft tissue sarcoma patients: experience and management in a nationwide multi‐center study. Clin Sarcoma Res. 2017;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haslik W, Hacker S, Felberbauer FX, et al. Port‐a‐Cath extravasation of vesicant cytotoxics: surgical options for a rare complication of cancer chemotherapy. Eur J Surg Oncol. 2015;41:378‐385. [DOI] [PubMed] [Google Scholar]
- 12. Polson K, Sullivan C, Houston M, et al. Optimizing safety in outpatient administration of trabectedin, a novel anticancer vesicant drug via continuous intraveneous infusion. Poster presented at: 16th Annual Meeting of the Connective Tissue Oncology Society; November 11‐13, 2010; Paris, France.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


