Abstract
Objectives
The aim of the study was to examine baseline neurocognitive impairment (NCI) prevalence and factors associated with NCI among patients enrolled in the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study.
Methods
The NAMACO study is an ongoing, prospective, longitudinal, multicentre and multilingual (German, French and Italian) study within the Swiss HIV Cohort Study. Between 1 May 2013 and 30 November 2016, 981 patients ≥ 45 years old were enrolled in the study. All underwent standardized neuropsychological (NP) assessment by neuropsychologists. NCI was diagnosed using Frascati criteria and classified as HIV‐associated or as related to other factors. Dichotomized analysis (NCI versus no NCI) and continuous analyses (based on NP test z‐score means) were performed.
Results
Most patients (942; 96.2%) had viral loads < 50 HIV‐1 RNA copies/mL. NCI was identified in 390 patients (39.8%): 263 patients (26.8%) had HIV‐associated NCI [249 patients (25.4%) had asymptomatic neurocognitive impairment (ANI)] and 127 patients (13%) had NCI attributable to other factors, mainly psychiatric disorders. There was good correlation between dichotomized and continuous analyses, with NCI associated with older age, non‐Caucasian ethnicity, shorter duration of education, unemployment and longer antiretroviral therapy duration.
Conclusions
In this large sample of aging people living with HIV with well‐controlled infection in Switzerland, baseline HIV‐associated NCI prevalence, as diagnosed after formal NP assessment, was 26.8%, with most cases being ANI. The NAMACO study data will enable longitudinal analyses within this population to examine factors affecting NCI development and course.
Keywords: aging, HIV‐associated neurocognitive disorder, neurocognitive impairment, neuropsychological testing
Introduction
The availability of safe and effective antiretroviral therapy (ART) for HIV infection has enabled people living with HIV (PLWH) to age. As the population of older PLWH increases, so does the prevalence of comorbidities. One such comorbidity, neurocognitive impairment (NCI), has been described since the start of the HIV epidemic, but the spectrum post‐ART has changed. HIV‐associated dementia (HAD), previously seen in advanced HIV disease, is now uncommon 1, 2, while the prevalence of milder NCI, which may be asymptomatic, has increased 3. Moreover, the presentation of NCI has changed. In the pre‐ART era, HAD was subcortical, rapidly progressive, and characterized by psychomotor slowing, motor dysfunction and extrapyramidal signs. In the current ART era, NCI is both cortical and subcortical, relatively stable over time, and characterized by impairments in working memory and executive function 4, 5, 6.
To reflect this shift, the diagnostic nomenclature of HIV‐associated NCI was revised in 2007 to define stages of impairment according to the so‐called Frascati criteria 7. In increasing severity, the stages are: asymptomatic neurocognitive impairment (ANI), corresponding to mild to moderate neurocognitive deficits without repercussions in activities of daily living (ADL); mild neurocognitive disorders (MNDs), corresponding to mild to moderate neurocognitive deficits with repercussions in ADL; and HIV‐associated dementia (HAD), corresponding to moderate to severe neurocognitive deficits with repercussions in ADL 7. Diagnosing NCI as specifically HIV‐associated requires the exclusion of confounding factors, such as psychiatric disorders and organic cerebral pathology 7.
Despite these definitions, the NCI literature can be confusing. Firstly, ANI, MNDs and HAD may be referred to collectively as HIV‐associated neurocognitive disorders (HANDs) if ADL have been assessed 4, or as mild or moderate NCI if the assessment is based on NP testing alone 8. Secondly, some patient populations make up cohorts designed from the start to study NCI 3, 9, or else subcohorts of long‐standing cohorts 10, whereas other populations are pooled from larger studies designed originally to answer different clinical questions 8, 11. Thirdly, the level of HIV control is not uniform: some cohorts group patients together, regardless of HIV viral load or whether or not they are on ART, while others exclude patients with detectable viraemia.
In Switzerland, the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study was set up as a longitudinal study to examine NCI within a well‐characterized and well‐treated cohort of PLWH, the Swiss HIV Cohort Study (SHCS) cohort 12. In the current study, we present the characteristics of NAMACO study patients and the baseline prevalence of NCI.
Methods
The NAMACO study
The NAMACO study is an ongoing, prospective, longitudinal, multicentre and multilingual (German, French and Italian) study included within an observational open research cohort study, the SHCS. Since 1988, the SHCS has collected data at twice‐yearly standardized SHCS clinic visits on the demography, behaviour, medical history (HIV and non‐HIV), HIV infection parameters, treatments and comorbidities of all enrolled patients aged ≥ 18 years 12.
The NAMACO study was set up to examine the cognitive and neurological impact of HIV infection in an aging HIV‐positive population and has been established in collaboration with physicians, neuropsychologists, neurologists and study nurses.
Standard protocol approvals, registrations and patient consents
The ethics committees of each cantonal hospital centre approved the NAMACO study protocol and all patient participants signed informed consent prior to being included.
Patient population and study design
The NAMACO study had the following inclusion criteria: HIV‐positive status, age ≥ 45 years, enrolment in the SHCS, engagement in care at one of seven cantonal university‐affiliated hospital centres (Basel, Bern, Geneva, Lausanne, Lugano, St Gallen and Zurich) and sufficient oral fluency in the local language to enable neuropsychological (NP) tests to be performed. SHCS‐affiliated infectious disease physicians received a list of eligible patients attending their clinics and were responsible for inviting patients to participate in the NAMACO study between 1 May 2013 and 30 November 2016. Enrolment was discontinued at 981 patients. Nonenrolment of eligible patients was related to manpower at each site; as age and language were the only limiting criteria beyond the need to be seen at a university‐affiliated SHCS centre, patients were not, in principle, disproportionately selected on the basis of neurocognitive symptoms or previous neurological history.
While all SHCS patients are asked European AIDS Clinical Society (EACS) screening questions on memory, reasoning and attention difficulties 13 at their twice‐yearly cohort visits, NAMACO study patients also undergo a standardized NP assessment at baseline and again at 2 (2016–2018) and 4 years (2018–2020). For all NAMACO study patients, regardless of their EACS screening question responses, NP assessment is performed by either a neuropsychologist or a neurologist trained in behavioural neurology and working under the supervision of a neuropsychologist.
As part of the study, patients with NCI are invited to undergo further investigations according to EACS guidelines 14, namely, full neurological examination, cerebrospinal fluid (CSF) collection via lumbar puncture and cerebral magnetic resonance imaging (Fig. 1), to examine the benefit of these interventions as diagnostic tools and factors associated with NCI.
Figure 1.
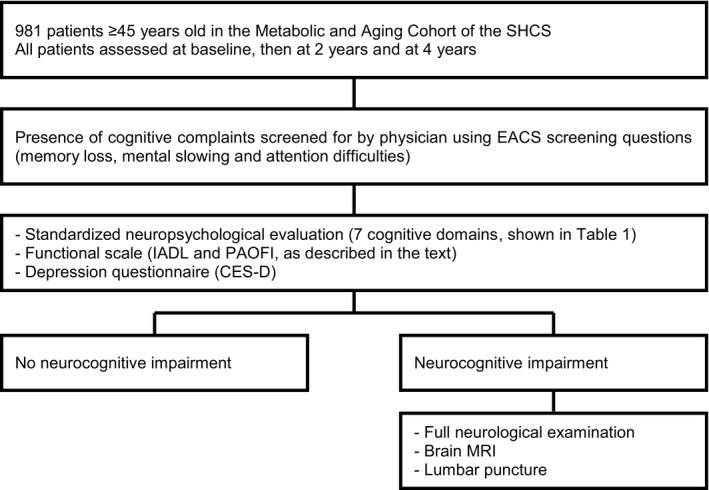
Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study design. SHCS, Swiss HIV Cohort Study; EACS, European AIDS Clinical Society; IADL, Instrumental Activities of Daily Living; PAOFI, Patients’ Assessment of Own Functioning Inventory; CES‐D, Center for Epidemiologic Studies Depression scale; MRI, magnetic resonance imaging.
The current study was a cross‐sectional analysis investigating the neurocognitive status of patients at the time of enrolment (baseline).
Neurocognitive evaluation
Cognitive complaints
For each of the three EACS screening questions mentioned above, the response options are never, hardly ever, or yes, definitely. Patients answering ‘yes, definitely’ to at least one question were considered to have cognitive complaints.
NP assessment
The NP test battery covered seven cognitive domains known to be affected in HIV‐associated NCI (Table 1). The complete battery required no more than 90 min to perform if the patient had no deficits. The raw score for each NP test was converted to a demographically adjusted standard score (z‐score).
Table 1.
The seven cognitive domains examined and the neuropsychological tests performed in the standardized neuropsychological assessment of all patients enrolled in the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study
| Standardized neuropsychological assessment | |
|---|---|
| Cognitive domain | Neuropsychological tests |
| Motor skills | Finger Tapping (dominant and nondominant hands) |
| Grooved Pegboard (dominant and nondominant hands) | |
| Speed of information processing | WAIS‐IV, Coding |
| Colour Trails 1 | |
| Attention and working memory | WAIS‐IV Digit Span (forward and backward) |
| Executive function | Category Fluency |
| 5‐point Figural Fluency | |
| Victoria Stroop (trial 3 and/or 3/1) | |
| Colour Trails 2 | |
| Verbal episodic memory | Hopkins Verbal Learning Test – Revised |
| Language | Category Fluency |
| Victoria Stroop (trial 1) | |
| Sensory and perceptual skills | Grooved Pegboard (dominant and nondominant hands) |
| Victoria Stroop (trial 1) | |
WAIS‐IV, Wechsler adult intelligence scale 4th edition.
The cognitive domains assessed were based on those used in the International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) and Strategic Timing of Antiretroviral Treatment (START) Study Group 8 and were in accordance with the guidelines published by Antinori et al. 7. The tests are sensitive in detecting NCI, rapid and easy to perform, standardized for several age and education groups, and available in different languages. The Hopkins Verbal Learning Test‐Revised is particularly appropriate for longitudinal studies as it provides six parallel forms to avoid the practice effect at retest which has been described as a reason for improvement in NP function over time 15, 16. In addition to the tests used in the START study, we chose to include the five‐point Figural Fluency and the Victoria Stroop test, to enrich the assessment of executive function, and the Wechsler adult intelligence scale 4th edition (WAIS‐IV) Digit Span Subtest to assess attention and working memory (Table 1).
Self‐administered assessments
As functional scales are required to differentiate between ANI and MNDs, Lawton's Instrumental Activities of Daily Living (IADL) were assessed 17, 18 to quantify the impact of NCI, if present, on daily function. Three supplementary questions were added, inspired by the Patients’ Assessment of Own Functioning Inventory questionnaire (a subjective measure of cognitive function), on professional work quality and productivity and on relatives’ comments about cognitive decline (Appendix 1). Functional impairment was defined as difficulties being reported in at least two items out of 11 (Fig. 2).
Figure 2.
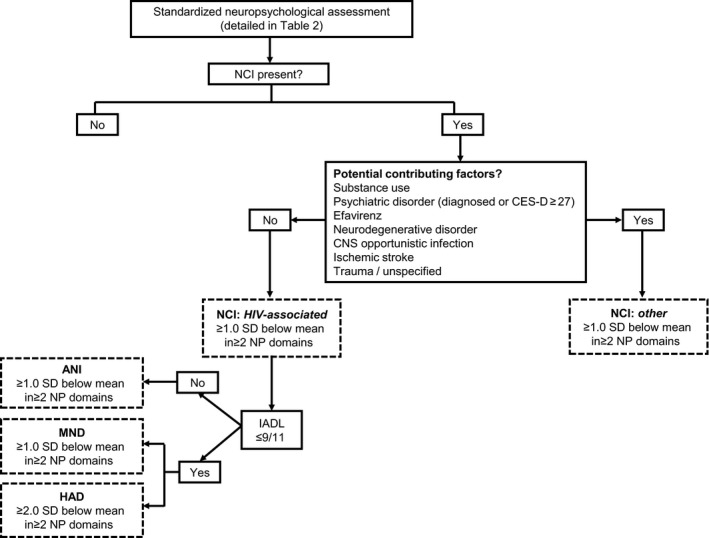
Neurocognitive assessment algorithm of the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study. Neurocognitive impairment was classified according to the Frascati criteria 7 as shown in the boxes with dashed outlines. CNS, central nervous system; CES‐D, Center for Epidemiologic Studies Depression scale; NCI, neurocognitive impairment; NCI, HIV‐associated neurocognitive disorder; ANI, asymptomatic neurocognitive impairment; MND, mild neurocognitive disorder; HAD, HIV‐associated dementia; IADL, Instrumental Activities of Daily Living; SD, standard deviation; NP, neuropsychological.
Depression severity was graded using the Center for Epidemiologic Studies Depression (CES‐D) scale by way of a questionnaire rating mood. Although the CES‐D scale has commonly been used to assess depression among PLWH, it does have limitations in this setting as some items in the questionnaire may be related to having HIV infection rather than depression (items on positive affect and somatic symptoms such as restless sleep and poor appetite) 19, 20. In the current study, CES‐D scores between 16 and 26 were classed as mild depression and scores ≥ 27 were classed as severe depression in order to apply a more stringent depression definition.
NCI definitions and associations
Using the Frascati criteria 7, patients were classified into five categories as follows (Fig. 2): normal NP examination (no NCI), ANI, MND, HAD and non‐HIV‐associated NCI (‘other’). In patients in the ‘other’ category, NCI was attributed to confounding conditions such as substance use, psychiatric disorders (including severe depression: CES‐D scores ≥ 27), ART toxicity, neurodegenerative disorders, opportunistic central nervous system (CNS) infection, stroke history and trauma, rather than to HIV infection (Fig. 2). Patients with CES‐D scores of 16–26 (moderate to severe low mood) were assigned to the ‘other’ category if the examining neuropsychologist considered the NP profile to be related to depression rather than to HIV infection alone.
To identify potential factors associated with NCI, we examined: demographic factors (age, sex, ethnicity, education and employment), HIV‐related factors (viral load, CD4 T‐cell count at baseline and nadir CD4 count, duration of current ART, CPE score at baseline, ART drug class, namely, nucleoside reverse transcriptase inhibitor, protease inhibitor, integrase strand transfer inhibitor or efavirenz, and mode of HIV acquisition), cardiovascular risk factors [diabetes, hypertension, and total and high‐density lipoprotein (HDL) cholesterol], other comorbidities (hepatitis B and C, and syphilis), depression score, cognitive complaints and toxin consumption (smoking, alcohol use, past injecting drug use, current cannabis use and current noninjecting cocaine use). Efavirenz use was analysed individually given the higher rates of neuropsychiatric adverse events associated with this treatment than with other antiretroviral treatments 21; treatment with dolutegravir was not assessed at baseline as this treatment became available in Switzerland 1 year into the NAMACO study. All these variables were obtained from the SHCS database extraction closest in time to each patient's neurocognitive assessment. For the current study, comorbidities and toxin consumption were analysed as binary (presence or absence) rather than continuous data.
Statistical analysis
Descriptive analyses are presented as mean and standard deviation (SD) for symmetric continuous variables, as median and interquartile range (IQR) for asymmetric continuous variables and as percentages for categorical variables.
NCI was analysed as a dichotomized and as a continuous variable. For the dichotomized analysis, NCI was dichotomized as follows: no impairment (no NCI) versus impairment (ANI, MND, HAD and other). In this setting, a multivariable logistic regression model was applied to identify factors associated with NCI occurrence. For the continuous analysis, NCI was considered as a continuous variable based on NP test mean z‐scores calculated per domain. The mean of the mean z‐scores was calculated for each patient for five of the seven domains; the two domains of language and sensory‐perceptual skills were not included in the overall mean z‐score calculation as these domains (1) shared NP tests used for other domains and (2) were impaired in relatively few patients. For the continuous analysis, a multivariable linear model was applied to identify factors associated with a decreased mean z‐score (worse NP performance).
In our analysis, the primary objective was to examine the prevalence and factors associated with NCI of any aetiology, HIV‐associated and other. We then conducted a sensitivity analysis, removing patients with other NCI, to examine only those with specifically HIV‐associated NCI (ANI, MND and HAD).
All analyses were conducted using r Core Team version 2014 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
Results
NAMACO patients
Of the 981 patients enrolled, 782 (79.7%) were male, 899 (91.7%) were Caucasian and 627 patients (63.9%) were aged > 50 years [mean (± SD) age 54.5 ± 7.5 years] (Table 2). At baseline, most patients (96.2%) had viral loads < 50 HIV‐1 RNA copies/mL; the median CD4 count was 634 cells/μL (IQR 468, 814 cells/μL) and the median nadir CD4 count was 180 cells/μL (IQR 74, 270 cells/μL) (Table 2).
Table 2.
Demographic, comorbidity and HIV‐related data for 981 HIV‐positive patients enrolled in the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study
| Parameter | |
|---|---|
| Age (years) [mean (SD)] | 54.5 (7.5) |
| Sex (male) [n (%)] | 782 (79.7) |
| Ethnicity [n (%)] | |
| Caucasian | 899 (91.7) |
| Black/other* | 81 (8.3) |
| Education (years) [mean (SD)] | 13 (2.8) |
| Employment [n (%)] | |
| Unemployed (working < 25% of potential work time) | 373 (38.0) |
| Employed part‐time (25–75%) | 81 (8.3) |
| Employed full‐time (80–100%) | 527 (53.7) |
| Cognitive complaints† [n (%)] | 244 (25.1) |
| CES‐D [median (IQR)] | 10 (4–17) |
| Drug use [n (%)] | |
| History of injecting drug use | 137 (14.0) |
| Current cannabis use | 103 (10.5) |
| Current noninjecting cocaine use | 16 (1.6) |
| At‐risk alcohol consumption‡ | 161 (16.5) |
| Cardiovascular risk factors | |
| Cigarette smoking [n (%)] | 356 (36.4) |
| Diabetes [n (%)] | 59 (6.0) |
| Hypertension [n (%)] | 398 (40.6) |
| Cholesterol [mean (SD)] | 5.1 (1.1) |
| HDL cholesterol [mean (SD)] | 1.4 (0.45) |
| Coinfections [n (%)] | |
| Hepatitis B virus | 460 (46.9) |
| Hepatitis C virus | 171 (17.4) |
| Syphilis | 249 (25.4) |
| HIV parameters | |
| HIV viral load < 50 copies/mL [n (%)] | 942 (96.2) |
| CD4 count (cells/μL) [median (IQR)] | 634 (468–814) |
| Nadir CD4 count (cells/μL) [median (IQR)] | 180 (74–270) |
| ART duration (years) [median (IQR)] | 12.7 (6.5–18) |
| Current CPE score ≥ 7 [n (%)] | 756 (78.9) |
| ART drug class [n (%)] | |
| Nucleoside reverse transcriptase inhibitor | 940 (98.1) |
| Protease inhibitor | 419 (43.7) |
| Integrase strand transfer inhibitor | 259 (27.0) |
| Efavirenz | 203 (21.2) |
| Likely mode of HIV acquisition [n (%)] | |
| Men who have sex with men | 506 (51.6) |
| Heterosexual | 325 (33.1) |
| Injecting drug use | 118 (12.0) |
| Other/unknown | 32 (3.3) |
IQR, interquartile range; CES‐D, Center for Epidemiologic Studies Depression scale; HDL, high‐density lipoprotein; ART, antiretroviral therapy; CPE, central nervous system penetration effectiveness; SD, standard deviation.
*59 patients (6%) black; 22 (2.2%) other (non‐black, non‐Caucasian).
†Answering, ‘Yes, definitely’ to at least one of the three questions.
‡≥ 3 alcoholic drinks per day or ≥ 6 alcoholic drinks in one sitting at least once a week.
The percentage of NAMACO patients who were male or Caucasian was slightly higher than that of SHCS patients eligible for NAMACO but not enrolled or followed up at non‐NAMACO centres (Table S1).
Prevalence of NCI and cognitive domains affected
Of all 981 patients, 390 (39.8%) presented with NCI, of whom 263 patients (26.8% of 981) had HIV‐associated NCI and 127 patients (13.0% of 981) had non‐HIV‐associated NCI. Examining patients with HIV‐associated NCI, 249 patients (25.4% of 981) had ANI, eight patients (0.8%) had MNDs and six patients (0.6%) had HAD. Examining the 127 patients with non‐HIV‐associated NCI, the majority had a psychiatric disorder (mostly depression) (79 of 127 patients; 62.2%), a history of substance use (26 patients; 20.5%), a history of trauma, stroke or unspecified pathology (32 patients; 25.2%) or previous opportunistic infections (nine patients; 7.1%).
Taking the 981 patients together, the seven cognitive domains examined were impaired as follows: motor skills in 396 patients (41%), speed of information processing in 325 patients (33.1%), attention and working memory in 324 patients (33%), executive function in 240 patients (24.8%), verbal episodic memory in 169 patients (17.2%), language in 68 patients (7%) and sensory and perceptual skills in 56 patients (5.8%) (Fig. 3). These proportions were similar in patients with HIV‐associated NCI and in those with mild to moderate and severe depression (Fig. 3).
Figure 3.
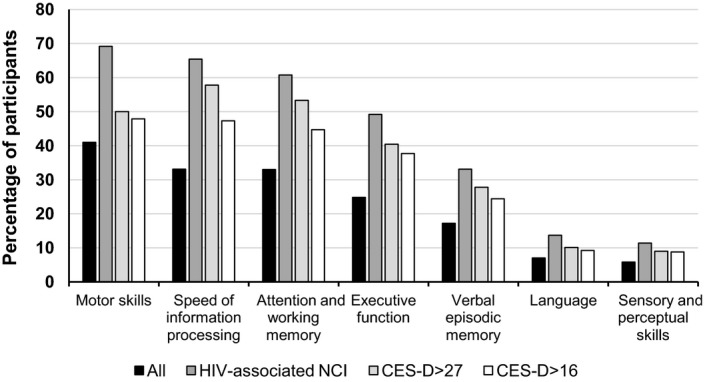
Percentage of patients with deficits in each of the seven cognitive domains examined by neuropsychological testing. The category ‘All’ refers to all 981 patient participants; the category ‘HIV‐associated NCI’ comprised 263 patients diagnosed with an HIV‐associated neurocognitive impairment (NCI); the category ‘CES‐D > 27’ comprised 90 patients with a Center for Epidemiologic Studies Depression (CES‐D) score > 27 and the category ‘CES‐D > 16’ comprised 262 patients with a CES‐D score > 16.
Individual factors associated with NCI
The patient variables associated with NCI were roughly the same whether the outcome was dichotomized (presence versus absence of NCI) or considered as a continuous variable (z‐scores). The main risk factors for NCI were older age, non‐Caucasian ethnicity, shorter duration of education, unemployment, presence of cognitive complaints, high depression score, longer ART duration and heterosexual mode of HIV acquisition (Table 3). The ART duration effect had a significant quadratic form in both dichotomized and continuous models: the chance of NCI increased significantly when ART duration increased (positive linear term), stabilizing with long ART durations (negative quadratic term); mean z‐scores decreased significantly when ART duration increased (negative linear term), stabilizing with long ART durations (positive quadratic term).
Table 3.
Demographic and clinical associations with neurocognitive impairment in multivariate models applied to 981 HIV‐positive patients. Impairment is presented as a dichotomous variable (multivariate logistic model, left‐hand columns) and as a continuous variable (multivariate linear model, right‐hand columns)
| Dichotomized analysis | Continuous analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio* | P‐value | 95% CI | Effect† | P‐value | 95% CI | |||
| Age (years) | 1.03 | 0.020 | 0.00 | 0.06 | −0.01 | 0.001 | −0.01 | 0.00 |
| Sex (male) | 1.24 | 0.393 | −0.28 | 0.72 | −0.09 | 0.060 | −0.19 | 0.00 |
| Ethnicity (ref: Caucasian) | 8.80 | <0.0001 | 1.41 | 3.03 | −0.64 | <0.0001 | −0.76 | −0.52 |
| Education | 0.85 | <0.0001 | −0.23 | −0.09 | 0.04 | <0.0001 | 0.03 | 0.06 |
| Employment | ||||||||
| Part‐time (25–75%) (ref: unemployed) | 0.57 | 0.071 | −1.17 | 0.04 | 0.17 | 0.004 | 0.05 | 0.29 |
| Full‐time (80–100%) (ref: unemployed) | 0.45 | <0.0001 | −1.18 | −0.40 | 0.15 | <0.0001 | 0.08 | 0.23 |
| Cognitive complaints‡ | 1.53 | 0.024 | 0.05 | 0.79 | −0.10 | 0.005 | −0.17 | −0.03 |
| CES‐D | 1.05 | <0.0001 | 0.03 | 0.06 | −0.01 | <0.0001 | −0.01 | −0.01 |
| Drug use | ||||||||
| History of injecting drug use | 1.42 | 0.468 | −0.60 | 1.30 | −0.02 | 0.809 | −0.19 | 0.15 |
| Current cannabis use | 0.67 | 0.170 | −0.99 | 0.17 | 0.12 | 0.032 | 0.01 | 0.22 |
| Current noninjecting cocaine use | 1.31 | 0.709 | −1.23 | 1.61 | −0.01 | 0.922 | −0.25 | 0.23 |
| Alcohol consumption at risk§ | 1.05 | 0.821 | −0.39 | 0.48 | 0.00 | 0.993 | −0.08 | 0.08 |
| Cardiovascular risk factors | ||||||||
| Cigarette smoking | 1.00 | 0.999 | −0.36 | 0.36 | −0.04 | 0.292 | −0.10 | 0.03 |
| Diabetes | 1.73 | 0.105 | −0.11 | 1.22 | −0.12 | 0.064 | −0.25 | 0.01 |
| Hypertension | 0.82 | 0.240 | −0.53 | 0.13 | 0.01 | 0.736 | −0.05 | 0.07 |
| Cholesterol | 0.98 | 0.843 | −0.17 | 0.14 | 0.01 | 0.563 | −0.02 | 0.04 |
| HDL cholesterol | 1.07 | 0.745 | −0.33 | 0.46 | 0.03 | 0.358 | −0.04 | 0.11 |
| Coinfections | ||||||||
| Hepatitis B virus | 1.08 | 0.812 | −0.53 | 0.67 | −0.06 | 0.327 | −0.17 | 0.06 |
| Hepatitis C virus | 1.39 | 0.065 | −0.02 | 0.69 | −0.04 | 0.189 | −0.11 | 0.02 |
| Syphilis | 0.57 | 0.007 | −0.98 | −0.16 | 0.07 | 0.051 | 0.00 | 0.15 |
| HIV parameters | ||||||||
| HIV viral load < 50 copies/mL | 0.63 | 0.285 | −1.30 | 0.39 | −0.02 | 0.862 | −0.19 | 0.16 |
| CD4 count at enrolment | 0.97 | 0.312 | −0.10 | 0.03 | 0.00 | 0.734 | −0.01 | 0.01 |
| Nadir CD4 count | 1.01 | 0.896 | −0.13 | 0.14 | −0.02 | 0.190 | −0.04 | 0.01 |
| ART duration | 1.13 | 0.021 | 0.02 | 0.23 | −0.03 | 0.002 | −0.05 | −0.01 |
| ART duration2 | 0.99 | 0.005 | −0.01 | 0.00 | 0.00 | 0.001 | 0.00 | 0.00 |
| Current CPE score ≥ 7 | 1.20 | 0.409 | −0.24 | 0.61 | −0.06 | 0.157 | −0.14 | 0.02 |
| ART drug class | ||||||||
| Nucleoside reverse transcriptase inhibitor | 0.99 | 0.982 | −1.17 | 1.23 | 0.06 | 0.604 | −0.17 | 0.29 |
| Protease inhibitor | 1.34 | 0.126 | −0.08 | 0.67 | −0.04 | 0.314 | −0.11 | 0.03 |
| Integrase strand transfer inhibitor | 0.87 | 0.505 | −0.53 | 0.26 | −0.01 | 0.827 | −0.08 | 0.07 |
| Efavirenz | 0.90 | 0.651 | −0.55 | 0.34 | −0.02 | 0.671 | −0.10 | 0.07 |
| Likely mode of HIV acquisition | ||||||||
| Heterosexual (ref: MSM) | 1.97 | 0.002 | 0.24 | 1.12 | −0.12 | 0.008 | −0.20 | −0.03 |
| Injecting drug use (ref: MSM) | 0.62 | 0.364 | −1.50 | 0.54 | 0.03 | 0.790 | −0.16 | 0.22 |
| Other/unknown (ref: MSM) | 3.07 | 0.013 | 0.24 | 2.02 | −0.13 | 0.149 | −0.30 | 0.05 |
CI, confidence interval; SD, standard deviation; IQR, interquartile range; CES‐D, Center for Epidemiologic Studies Depression scale; HDL, high‐density lipoprotein; ART, antiretroviral therapy; CPE, central nervous system penetration effectiveness; MSM, men who have sex with men.
P‐values < 0.05 are shown in bold text.
Odds ratios with P‐values from a multivariate logistic model based on a binary outcome of neurocognitive impairment: no impairment (0) versus impairment (> 0).
†Difference in mean z‐score.
‡Answering, ‘Yes, definitely’ to at least one of the three questions.
§≥ 3 alcoholic drinks per day or ≥ 6 alcoholic drinks in one sitting at least once a week.
Slight differences between the two models were as follows: a significant protective effect of cannabis was observed in the continuous model, losing significance when examined as a dichotomized outcome; unknown mode of HIV acquisition was a significant risk factor in the dichotomized model, losing significance when examined as a continuous outcome; syphilis was a significant protective factor in the dichotomized model, just not reaching significance when examined as a continuous outcome.
In a sensitivity analysis excluding non‐HIV‐associated NCI (‘other’) (analysis of 854 patients), we obtained substantially similar results (Table S2). The differences concerned the depression score, presence of cognitive complaints and mode of HIV acquisition, which did not reach significance as risk factors for NCI, while hepatitis B virus coinfection was a significant risk factor.
Finally, the percentage of NCI in the seven participating centres was examined according to language. Although NCI prevalence was observed to be higher in French‐speaking compared to German‐speaking regions, this was related to one French‐speaking centre having a higher NCI prevalence than all the other centres and to one German‐speaking centre having a particularly low NCI prevalence. All NCI rates examined were adjusted for age, sex, origin, duration of education, mode of HIV transmission and presence of cognitive complaints to exclude a patient population effect on NCI. Adjusting for further patient variables, or examining only HIV‐associated NCI, did not alter this centre effect.
Discussion
In this study, examining a very large and highly characterized cohort of aging HIV‐positive individuals with well‐controlled infection, we observe a baseline prevalence of HIV‐associated NCI (HANDs) of 26.8%. For non‐HIV‐associated NCI, the majority of confounding factors were psychiatric disorders, mainly depression. Older age, non‐Caucasian ethnicity, shorter duration of education, being unemployed and longer ART duration were the main factors associated with both HIV‐ and non‐HIV‐associated NCI. These associations were observed using two different statistical models, considering NCI as a dichotomized and as a continuous variable.
The majority of our patients with NCI had ANI, the clinical relevance of which has been a matter of debate. Some researchers argue that NCI prevalence is increasing merely through the overestimation of impairment among asymptomatic individuals who, without formal NP testing, would pass as cognitively normal 22, 23. This is reflected in the EACS guidelines which recommended screening PLWH regardless of symptoms until 2014 14 and then, from 2015, recommended assessing only symptomatic patients 24. The Mind Exchange Program recommends screening for cognitive impairment within 6 months of diagnosis but with a different tool from the three questions recommended by EACS 25. Other national guidelines vary in recommendations for screening and the screening tool used, but all recommend a comprehensive assessment with exclusion of confounding factors before a diagnosis of HIV‐associated NCI is made 26.
The clinical relevance of ANI is suggested by virtue of NCI being a dynamic state, with patients changing NCI stage with time. Among 347 patients (121 with ANI and 226 neurocognitively normal) enrolled in the CNS HIV Antiretroviral Therapy Effects Research (CHARTER) study, having ANI was found to convey a two‐ to six‐fold increase in the risk of developing symptomatic NCI 27. Again in the CHARTER study, of 436 patients followed up over a mean of 35 months, 60.8% were found to remain neurocognitively stable while the others either declined or improved 28. Similarly, among 197 patients enrolled in the Multicenter AIDS Cohort Study (MACS), 77% remained neurocognitively stable, 13% deteriorated and 10% improved over a 4‐year period 5. We would argue that, if NCI stage can change over 2 to 4 years, it is worth conducting a cohort study where the baseline assessment is sensitive enough to identify patients with mild or very mild impairment. We would further argue that, as older individuals are more likely to have NCI than younger individuals, even with controlled viraemia 3, 29, and as the population of older PLWH is increasing, studies of NCI should include the widest possible spectrum of impairment to gain understanding of how NCI is likely to evolve. That said, although NP testing remains the gold standard of NCI diagnosis, it is time‐consuming and costly in resource‐limited environments 30. We therefore agree with current clinical guidelines on NCI assessment until research on asymptomatic NCI justifies change.
Our results differ from those of some other studies in terms of factors associated with NCI; perhaps this is related to differences in data collection methods, sample size and the population analysed. Unlike an Italian study of 245 asymptomatic HIV‐positive patients 31, we did not observe an association between NCI and cardiovascular risk factors. However, the Italian patient population was younger (mean age 46 years) with a higher percentage of smokers (54.3%). Nadir CD4 count has been reported as a predictor of NCI among patients in the CHARTER study, the Dutch TREVI Cohort study and the AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) study 11, 32, 33. The fact that we did not observe this association is possibly related to the relatively high nadir CD4 count in our population (median 180 cells/μL); among the French Aquitaine Cohort of 400 patients, nadir CD4 count (median 260 cells/μL) was also not associated with NCI 34. Finally, the negative association we observed between NCI and syphilis is contrary to the findings of previous studies and is difficult to explain when CNS inflammation has been described in the context of neurosyphilis 35, 36. In our multivariable models, dichotomized and continuous, each element was examined as an independent variable. It is possible that the protective effect of syphilis is linked to a variable that we did not enter into our model; whether the effect is real will become clearer as the 2‐year and 4‐year NAMACO study data are analysed.
Depression was a common confounder in our patients with NCI. This is not surprising as the depression prevalence among PLWH is estimated to be two to four times that of the general population 37. In depressed individuals, executive function, speed of information processing, attention and working memory and verbal episodic memory have been reported to be impaired 38, 39 and this may have influenced the profile of cognitive domains impaired among NAMACO study patients overall. Nevertheless, disentangling cognitive disorders as attributable to HIV infection or to depression is not straightforward, especially when the profile of NCI observed with both conditions is similar, that is, of a subcortical nature. This underpins the value of incorporating the clinical judgement of neuropsychologists in categorizing patients with NCI as having HIV‐associated or other NCI, particularly those with CES‐D scores of 16–26, that is, below the diagnostic cut‐off of 27. The association between depression and neurocognitive performance is the subject of a separate study within the NAMACO study population (G Santos, I Locatelli, I Nadin, R Du Pasquier, KEA Darling, M Cavassini, unpublished data).
This study has limitations. There is a possible recruitment bias among NAMACO study participants compared to other SHCS patients aged ≥ 45 years. Men who have sex with men (MSM) and Caucasians, in particular, are slightly over‐represented in the NAMACO study, and this is probably related to the requirement for NAMACO study participants to speak the local language to enable performance of the neurocognitive tests that require sufficient verbal knowledge. Patient recruitment depended on SHCS‐affiliated infectious disease physicians who had a list of eligible patients. Although the study protocol had clear inclusion criteria, we cannot exclude recruitment bias related to physicians (degree of involvement with study, personal judgement as to which patients would be suitable participants or who ‘should’ be assessed) or to patients (more/less willing to participate if cognitively symptomatic). We presented NCI diagnosis according to the Frascati criteria to enable comparison between our patient population and other cohorts. However, we acknowledge that applying other diagnostic criteria might have classified our patients differently. The low number of patients with MNDs in particular is surprising and may be related to the relatively low sensitivity of functional impairment scales which differentiate between MNDs and ANI. In our future analysis within the NAMACO study, notably the 2‐year follow‐up, we will focus on patient z‐scores rather than on Frascati‐based NCI labels. In order to dovetail the NAMACO study with a database that extensively characterizes each patient in terms of ART history and other HIV‐ and non‐HIV‐related variables, patients needed to be enrolled in the SHCS and followed up regularly in larger hospital centres. Against these limitations, the NAMACO study did not focus on enrolling only patients with cognitive complaints and has a large patient cohort designed specifically to study NCI. Moreover, this study reflects the cognitive status of PLWH aged ≥ 45 years in the whole of Switzerland, which means that the cognitive battery had to be administered in three languages: German, French and Italian. Such an achievement required NP test validation in three languages. To the best of our knowledge, this is the first neuropsychological study (in any field, not only HIV) to assess patients in parallel in the three linguistic regions of Switzerland. In addition to the large cohort, the link to the SHCS makes the NAMACO study database a high‐quality resource with which to examine factors important in determining NCI prevalence, incidence and course.
In conclusion, we observed that over a quarter of aging patients (≥ 45 years old) enrolled in the NAMACO study had HIV‐associated NCI, a proportion that may increase as our patient cohort ages, despite optimal viral suppression. Among patients with non‐HIV‐associated NCI, psychiatric disorders, particularly depression, were prevalent. The longitudinal analysis of NAMACO study participants at 2 and 4 years from baseline will shed light on how NCI develops and may be modified in our cohort.
Disclaimer
The opinions expressed in this article are those of the authors and do not necessarily represent those of ViiV. ViiV had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
MC, RDP, PT and A. Calmy designed the study. MM, IL, IN and GS finalized the neuropsychologist database. IL performed the statistical analysis. MC and RDP supervised the study. MM and KEAD wrote the manuscript. All investigators contributed to data collection and interpretation, reviewed drafts of posters and the manuscript, and approved the final manuscript.
Supporting information
Table S1. Patients enrolled in the Swiss HIV Cohort Study (SHCS) who were recruited to the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study compared to patients who were eligible but not recruited and patients followed up at non‐NAMACO centres.
Table S2. Demographic and clinical associations with neurocognitive impairment in multivariate models applied to 854 HIV‐positive patients (excluding the category ‘other’).
Acknowledgements
We thank all the patients participating in the NAMACO study. We thank all the infectious disease physicians and the study nurses working in the centres for their dedicated patient work and contribution to the NAMACO study. We thank the neuropsychologists Samanta Simioni, Severin Früh, Stefanie Clarke and Stefania Rossi, for their work in NAMACO. Finally, we thank Dr Kevin Robertson for his advice regarding the selection of cognitive tests and his encouragement to launch the study. The NAMACO study group: director: Matthias Cavassini; co‐director: Renaud Du Pasquier; neuropsychologists: Mélanie Métral, Samanta Simioni, Peter Brugger, Klemens Gutbrod, Andreas U. Monsch, Ursi Kunze, Marianne Schneitter, Isaure Nadin, Severin Früh, Marc Schwind, Riccardo Pignatti and Stefanie Clarke; neurologists: Frédéric Assal, Tobias Derfuss, Sebastian von Arx, Günter Eisele, Leonardo Sacco, Manuel Bertschi, Thomas Hundsberger and Renaud Du Pasquier; infectious disease specialists: Alexandra Calmy, Thanh Doco Lecompte, Christoph Hauser, Alexia Cusini, Rainer Weber, Helen Kovari, Barbara Hasse, Philip Tarr, Marcel Stoeckle, Christoph Fux, Enos Bernasconi, Caroline Di Benedetto, Alessandra Bruno, Patrick Schmid, Katharine Darling and Matthias Cavassini; SHCS data centre: Alexandra Scherrer; data management unit: Alexandra Scherrer, Yannick Vallet and Deolinda Alves; statistician: Isabella Locatelli; pharmacologist: Laurent Decosterd; neuro‐imaging specialists: Cristina Granziera, Gunnar Krueger, Reto Meuli and Maria Vargas.
Conflicts of interest: KEAD's institution has received research funding unrelated to this publication from Gilead and sponsorship for specialist meetings from MSD. A. Cusini has received travel grants and meeting expenses from MSD, BMS, Gilead and Astellas paid to her institution. PET's institution has received research grants and advisory fees from ViiV and Gilead. A. Calmy's institution has received unrestricted education grants from AbbVie, Gilead, MSD and ViiV. CDB has received sponsorship for specialist meetings from Janssen‐Cilag and AbbVie. RDP is a board member at Gilead. MC's institution has received a research grant from ViiV and Gilead and offered expert testimony for AbbVie, MSD, Gilead and Sandoz. The other authors report no conflicts of interest.
Financial disclosure: The NAMACO study is supported by the Swiss National Science Foundation (grant number 163348) and the Swiss HIV Cohort Study (grant number 148522, project 811). Additional funding has been provided by the Swiss HIV Cohort Foundation and ViiV Healthcare.
Instrumental Activities of Daily Living (IADL) scale
| PATIENT ID:__________ | DATE OF VISIT: ___________ | VISIT N: ____ |
| A. Ability to use telephone | |
| 1. Operates telephone on own initiative; looks up and dials numbers | 1 |
| 2. Dials a few well‐known numbers | 1 |
| 3. Answers telephone but does not dial | 1 |
| 4. Does not use telephone at all | 0 |
| B. Shopping | |
| 1. Takes care of all shopping needs independently | 1 |
| 2. Shops independently for small purchases | 0 |
| 3. Needs to be accompanied on any shopping trip | 0 |
| 4. Completely unable to shop | 0 |
| C. Food preparation | |
| 1. Plans, prepares, and serves adequate meals independently | 1 |
| 2. Prepares adequate meals if supplied with ingredients | 0 |
| 3. Heats and serves prepared meals or prepares meals but does not maintain adequate diet | 0 |
| 4. Needs to have meals prepared and served | 0 |
| D. Housekeeping | |
| 1. Maintains house alone with occasional assistance (heavy work) | 1 |
| 2. Performs light daily tasks such as dishwashing, bed making | 1 |
| 3. Performs light daily tasks but cannot maintain acceptable level of cleanliness | 1 |
| 4. Needs help with all home maintenance tasks | 1 |
| 5. Does not participate in any housekeeping tasks | 0 |
| E. Laundry | |
| 1. Does personal laundry completely | 1 |
| 2. Launders small items, rinses socks, stockings, etc. | 1 |
| 3. All laundry must be done by others | 0 |
| F. Models of transportation | |
| 1. Travels independently on public transportation or drives own car | 1 |
| 2. Arranges own travel via taxi, but does not otherwise use public transportation | 1 |
| 3. Travels on public transportation when assisted or accompanied by another | 1 |
| 4. Travel limited to taxi or automobile with assistance of another | 0 |
| 5. Does not travel at all | 0 |
| G. Responsibility for own medications | |
| 1. Is responsible for taking medication in correct dosages at correct time | 1 |
| 2. Takes responsibility if medication is prepared in advance in separate dosages | 0 |
| 3. Is not capable of dispensing own medication | 0 |
| H. Ability to handle finances | |
| 1. Manages financial matters independently (budgets, writes checks, pays rent and bills, goes to bank); collects and keeps track of income | 1 |
| 2. Manages day‐to‐day purchases, but needs help with banking, major purchases, etc. | 1 |
| 3. Incapable of handling money | 0 |
Source: see Lawton and Brody 17.
Supplementary questions on job performance
| I. Job performance | |
| 1. Unable to perform some aspects of previous job (not due to medical symptoms) | 0 |
| L. Job performance | |
| 1. Reduced efficiency or productivity; or more errors or difficulties meeting expectations; or greater effort to perform the same activities | 0 |
Scoring (TOTAL): if the patient receives a score of 0 for at least two of the items above (A–L), then s/he is considered to be functionally impaired.
Source: see Lawton and Brody 7.
Supplementary question concerning social entourage
| M. Entourage | |
| 1. Comments made by entourage (close family, friends, colleagues, etc.) regarding decline in cognitive function | 0 |
Contributor Information
KEA Darling, Email: katharine.darling@chuv.ch.
Swiss HIV Cohort Study:
Matthias Cavassini, Renaud Du Pasquier, Mélanie Métral, Samanta Simioni, Peter Brugger, Klemens Gutbrod, Andreas U. Monsch, Ursi Kunze, Marianne Schneitter, Isaure Nadin, Severin Früh, Marc Schwind, Riccardo Pignatti, Stefanie Clarke, Frédéric Assal, Tobias Derfuss, Sebastian von Arx, Günter Eisele, Leonardo Sacco, Manuel Bertschi, Thomas Hundsberger, Renaud Du Pasquier, Alexandra Calmy, Thanh Doco Lecompte, Christoph Hauser, Alexia Cusini, Rainer Weber, Helen Kovari, Barbara Hasse, Philip Tarr, Marcel Stoeckle, Christoph Fux, Enos Bernasconi, Caroline Di Benedetto, Alessandra Bruno, Patrick Schmid, Katharine Darling, Matthias Cavassini, Alexandra Scherrer, Alexandra Scherrer, Yannick Vallet, Deolinda Alves, Isabella Locatelli, Laurent Decosterd, Cristina Granziera, Gunnar Krueger, Reto Meuli, and Maria Vargas
Data availability statement
Anonymized data will be shared by request from any qualified investigator.
References
- 1. McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol 2005; 4: 543–555. [DOI] [PubMed] [Google Scholar]
- 2. Bhaskaran K, Mussini C, Antinori A et al Changes in the incidence and predictors of human immunodeficiency virus‐associated dementia in the era of highly active antiretroviral therapy. Ann Neurol 2008; 63: 213–221. [DOI] [PubMed] [Google Scholar]
- 3. Heaton RK, Clifford DB, Franklin DR Jr et al HIV‐associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75: 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaton RK, Franklin DR, Ellis RJ et al HIV‐associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacktor N, Skolasky RL, Seaberg E et al Prevalence of HIV‐associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology 2016; 86: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sacktor N. Changing clinical phenotypes of HIV‐associated neurocognitive disorders. J Neurovirol 2018; 24: 141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Antinori A, Arendt G, Becker JT et al Updated research nosology for HIV‐associated neurocognitive disorders. Neurology 2007; 69: 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright EJ, Grund B, Cysique LA et al Factors associated with neurocognitive test performance at baseline: a substudy of the INSIGHT strategic timing of antiretroviral treatment (START) trial. HIV Med 2015; 16 (Suppl 1): 97–108. [DOI] [PubMed] [Google Scholar]
- 9. Bagkeris E, Burgess L, Mallon PW et al Cohort profile: the pharmacokinetic and clinical observations in people over fifty (POPPY) study. Int J Epidemiol 2018; 47: 1391–1392e. [DOI] [PubMed] [Google Scholar]
- 10. Becker JT, Kingsley LA, Molsberry S et al Cohort profile: recruitment cohorts in the neuropsychological substudy of the multicenter AIDS cohort study. Int J Epidemiol 2015; 44: 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robertson KR, Smurzynski M, Parsons TD et al The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS 2007; 21: 1915–1921. [DOI] [PubMed] [Google Scholar]
- 12. Schoeni‐Affolter F, Ledergerber B, Rickenbach M et al Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 13. Simioni S, Cavassini M, Annoni JM et al Cognitive dysfunction in HIV patients despite long‐standing suppression of viremia. AIDS 2010; 24: 1243–1250. [DOI] [PubMed] [Google Scholar]
- 14. European Aids Clinical Society Guidelines Version 7.1. Available at http://www.eacsociety.org/files/guidelines_english_71_141204.pdf. 2014. (accessed 1 July 2019).
- 15. Heilbronner RL, Sweet JJ, Attix DK, Krull KR, Henry GK, Hart RP. Official position of the American Academy of Clinical Neuropsychology on serial neuropsychological assessments: the utility and challenges of repeat test administrations in clinical and forensic contexts. Clin Neuropsychol 2010; 24: 1267–1278. [DOI] [PubMed] [Google Scholar]
- 16. Grund B, Wright EJ, Brew BJ et al Improved neurocognitive test performance in both arms of the SMART study: impact of practice effect. J Neurovirol 2013; 19: 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist 1969; 9: 179–186. [PubMed] [Google Scholar]
- 18. Heaton RK, Marcotte TD, Mindt MR et al The impact of HIV‐associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004; 10: 317–331. [DOI] [PubMed] [Google Scholar]
- 19. Gay CL, Kottorp A, Lerdal A, Lee KA. Psychometric limitations of the center for epidemiologic studies‐depression scale for assessing depressive symptoms among adults with HIV/AIDS: a Rasch analysis. Depress Res Treat 2016; 2016: 2824595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simoni JM, Safren SA, Manhart LE et al Challenges in addressing depression in HIV research: assessment, cultural context, and methods. AIDS Behav 2011; 15: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gazzard B, Balkin A, Hill A. Analysis of neuropsychiatric adverse events during clinical trials of efavirenz in antiretroviral‐naive patients: a systematic review. AIDS Rev 2010; 12: 67–75. [PubMed] [Google Scholar]
- 22. Gisslen M, Price RW, Nilsson S. The definition of HIV‐associated neurocognitive disorders: are we overestimating the real prevalence? BMC Infect Dis 2011; 11: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Underwood J, De Francesco D, Leech R et al Medicalising normality? Using a simulated dataset to assess the performance of different diagnostic criteria of HIV‐associated cognitive impairment. PLoS ONE 2018; 13: e0194760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. European AIDS Clinical Society Guidelines Version 8.0. Available at http://www.eacsociety.org/files/guidelines_8_0-english_web.pdf. 2015. (accessed 1 July 2019).
- 25. Mind Exchange Working G . Assessment, diagnosis, and treatment of HIV‐associated neurocognitive disorder: a consensus report of the mind exchange program. Clin Infect Dis 2013; 56: 1004–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Underwood J, Winston A. Guidelines for evaluation and management of cognitive disorders in HIV‐positive individuals. Curr HIV/AIDS Rep 2016; 13: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant I, Franklin DR Jr, Deutsch R et al Asymptomatic HIV‐associated neurocognitive impairment increases risk for symptomatic decline. Neurology 2014; 82: 2055–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heaton RK, Franklin DR Jr, Deutsch R et al Neurocognitive change in the era of HIV combination antiretroviral therapy: the longitudinal CHARTER study. Clin Infect Dis 2015; 60: 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coban H, Robertson K, Smurzynski M et al Impact of aging on neurocognitive performance in previously antiretroviral‐naive HIV‐infected individuals on their first suppressive regimen. AIDS 2017; 31: 1565–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carroll A, Brew B. HIV‐associated neurocognitive disorders: recent advances in pathogenesis, biomarkers, and treatment. F1000Research 2017; 6: 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fabbiani M, Ciccarelli N, Tana M et al Cardiovascular risk factors and carotid intima‐media thickness are associated with lower cognitive performance in HIV‐infected patients. HIV Med 2013; 14: 136–144. [DOI] [PubMed] [Google Scholar]
- 32. Ellis RJ, Badiee J, Vaida F et al CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011; 25: 1747–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van den Dries LWJ, Wagener MN, Jiskoot LC et al Neurocognitive impairment in a chronically well‐suppressed HIV‐infected population: the Dutch TREVI Cohort study. AIDS Patient Care STDS 2017; 31: 329–334. [DOI] [PubMed] [Google Scholar]
- 34. Bonnet F, Amieva H, Marquant F et al Cognitive disorders in HIV‐infected patients: are they HIV‐related? AIDS 2013; 27: 391–400. [DOI] [PubMed] [Google Scholar]
- 35. Marra CM, Deutsch R, Collier AC et al Neurocognitive impairment in HIV‐infected individuals with previous syphilis. Int J STD AIDS 2013; 24: 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ho EL, Maxwell CL, Dunaway SB et al Neurosyphilis increases human immunodeficiency virus (HIV)‐associated central nervous system inflammation but does not explain cognitive impairment in HIV‐infected individuals with syphilis. Clin Infect Dis 2017; 65: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep 2015; 17: 530. [DOI] [PubMed] [Google Scholar]
- 38. McDermott LM, Ebmeier KP. A meta‐analysis of depression severity and cognitive function. J Affect Disord 2009; 119: 1–8. [DOI] [PubMed] [Google Scholar]
- 39. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta‐analysis. Psychol Med 2014; 44: 2029–2040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patients enrolled in the Swiss HIV Cohort Study (SHCS) who were recruited to the Neurocognitive Assessment in the Metabolic and Aging Cohort (NAMACO) study compared to patients who were eligible but not recruited and patients followed up at non‐NAMACO centres.
Table S2. Demographic and clinical associations with neurocognitive impairment in multivariate models applied to 854 HIV‐positive patients (excluding the category ‘other’).
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.


