Abstract
Gradual rewarming from hypothermic to normothermic is a novel perfusion modality with superior outcome to sudden rewarming to normothermic. However, the identification of an oxygen carrier that could function at a temperature range from 4 to 7°C or whether it is necessary to use oxygen carrier during kidney rewarming, remains unresolved. This study was designed to test the use of a hemoglobin‐based oxygen carrier (HBOC) during gradual kidney rewarming as an alternative to simple dissolved oxygen. In this study, 10 rat kidneys were randomly divided into the control and the HBOC group. In the control group, no oxygen carrier was used during rewarming perfusion and the perfusion solution was oxygenated only by applying diffused carbogen flow. The protocol mimicked a donor after circulatory death (DCD) kidney transplantation, where after 30 minutes warm ischemia and 120 minutes cold storage in University of Wisconsin solution, the DCD kidneys underwent gradual rewarming from 10 to 37°C during 90 minutes with or without HBOC. This was followed by 30 minutes of warm ischemia in room temperature to mimic the anastomosis time and 120 minutes of reperfusion at 37°C to mimic the early post‐transplant state of the graft. The HBOC group demonstrated superior kidney function which was highlighted by higher ultrafiltrate production, better glomerular filtration rate and improved sodium reabsorption. There was no significant difference between the 2 groups regarding the hemodynamics, tissue injury, and adenosine triphosphate levels. In conclusion, this study suggests better renal function recovery in DCD kidneys after rewarming with HBOC compared to rewarming without an oxygen carrier.
Keywords: donor after circulatory death kidney graft, hemoglobin‐based oxygen carrier, oxygen carrier, perfusion, rewarming perfusion
1. INTRODUCTION
One of the challenges in organ transplant is improving the organ preservation method especially in the grafts with inferior quality such as donor after circulatory death (DCD).1, 2 Machine perfusion has been developed as an alternative preservation method to cold storage (CS) with promising results in organ quality improvement.3, 4, 5 Different perfusion protocols have been studied by many groups and among the emerged perfusion protocols, normothermic machine perfusion has gained more attention as it provides the feasibility of assessing organ viability and function before transplant.6, 7 It has also been shown that gradual rewarming from hypothermic to normothermic is superior to sudden normothermic perfusion after CS.4, 8 Although gradual rewarming solves the problem strategically, it remains to be established whether there is a need for oxygen carrier during gradual rewarming for providing a sufficient amount of oxygen. Red blood cells (RBCs) have been mainly used in normothermic perfusion, however there is major biophysical limits such as hemolysis and rheological complications when submitted to temperatures below normothermic (37°C).9 Artificial oxygen carriers, either with hemoglobin‐based or perfluorocarbon‐based, are the relatively new solutions to this problem. After disappointing results with perfluorocarbons10, 11, HBOCs with the ability of functioning in wide range of temperature, from hypothermic to normothermic, seem to be an option for gradual rewarming and a potential alternative to blood in normothermic perfusion.12 HBOC‐201 (Hemopure) is a second generation glutaraldehyde‐polymerized hemoglobin of bovine origin that can function as an oxygen bridge in order to preserve oxygen carrying capacity in the lack of RBCs.12 HBOCs have been used in subnormothermic and normothermic perfusion and the data suggested improved organ quality.12, 13, 14 In this study, our aim was to investigate the viability and efficacy of HBOC‐based perfusion solution in gradual rewarming perfusion in a rat kidney model.
2. MATERIALS AND METHODS
2.1. Experimental animals and kidney procurement
Male Lewis rats weighing 290‐350 g were used in this study. Animals received care according to the National Research Council guidelines on animal experiments. The study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the Massachusetts General Hospital. Anesthesia and surgical details of recovery are provided elsewhere.15 Briefly, the rats were anesthetized using 5% of isoflurane and heparin administration was performed by injection of 1 mL of 0.9% of NaCl with 500 IU of heparin via the dorsal penile vein. Next, the ureter was cannulated with regard to monitor ultrafiltrate production during gradual rewarming and reperfusion. For controlled warm ischemia, during cessation of circulation the animal was kept at 37°C for 30 minutes, then renal artery was cannulated using a 20‐G intravenous catheter and the kidney was flushed through the renal artery with 10 mL of 0.9% of NaCl at room temperature followed by 10 mL University of Wisconsin (UW) preservation solution at 4°C. Afterwards, the kidneys were removed and stored in cold UW (4°C) during CS preservation for 120 minutes.
2.2. Experimental design and perfusion solutions
2.2.1. Rewarming and reperfusion
A total number of 10 rat kidneys were divided into 2 experimental groups (group 1‐Control, group 2‐HBOC). In both groups, after DCD procurement (n = 5 per group), grafts were preserved for 120 minutes during CS in UW media. Then, grafts underwent gradual rewarming perfusion from 10 to 37°C for 90 minutes. Temperature of the perfusion solution was set at 10°C at the beginning of rewarming in both groups, which is the minimum allowed by the equipment. Temperature was stable at 10°C for the first 15 minutes of the rewarming phase, and was then gradually increased to 37°C over 60 minutes by gradually increasing the temperature of thermostat and arterial flow. The temperature was kept stable at 37°C for the final 30 minutes of rewarming perfusion. Pressure was also observed during the rewarming phase, with the range of 20‐40 mm Hg in the hypothermic phase, 40‐80 mm Hg in transaction from hypothermia to normothermia and 80‐100 mm Hg in the normothermic phase of the rewarming protocol.
Afterwards, the grafts were flushed with 10 mL of cold saline and stored in a petri dish covered with wet gauze at room temperature for 30 minutes, in order to mimic surgical implantation period. Subsequently, the kidney grafts were reperfused at 37°C for 120 minutes. The flow was set at 8 mL/min in the beginning of reperfusion, and afterwards was gradually increased with keeping the pressure in physiological range (80‐110 mm Hg) (Figure 1).
Figure 1.
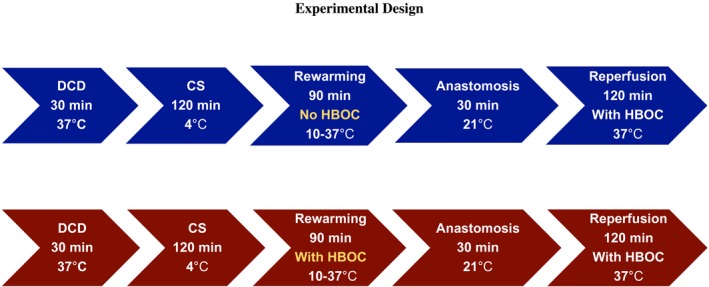
Experimental design. Illustrates the experimental design of the study and the duration in each section of the experiments [Color figure can be viewed at https://www.wileyonlinelibrary.com]
2.3. Gradual rewarming perfusion media
2.3.1. HBOC group
The perfusion solution consisted of Williams Medium E (Sigma‐Aldrich, St Louis, MO, USA) supplemented with heparin (1000 U/L APP Pharmaceuticals, Schaumberg, IL, USA), albumin 15% (Sigma‐Aldrich), creatinine (1000 µmol/L) and 25% HBOC‐201 v/v (provided by Hemoglobin Oxygen Therapeutics, Souderton, PA, USA). The solution was oxygenated with a carbogen mixture of 95% of O2 and 5% of CO2 in the arterial flow and saturation SaO2 > 97% (Table 1). In all the experiments before connecting the kidney, the perfusion solutions were checked to be in physiological osmolarity, oncotic pressure range, and contain adequate oxygen amount (>400 mm Hg).
Table 1.
Rewarming media
| Control | HBOC | P value | |
|---|---|---|---|
| pH | 7.5 ± 0.07 | 7.42 ± 0.14 | .886 |
| PCO2 (mm Hg) | 36.2 ± 31.4 | 32.2 ± 12.5 | .629 |
| PO2 (mm Hg) | 590 ± 96.0 | 452 ± 5.0 | .200 |
| BE (mmol/L) | 2 ± 5.0 | −3 ± 5.0 | .057 |
| HCO3− (mmol/L) | 26.2 ± 4.1 | 25.8 ± 2.6 | .000 |
| Na+ (mmol/L) | 147 ± 0.75 | 149 ± 2.0 | .016 |
| K+ (mmol/L) | 5.0 ± 0.2 | 5 ± 0.1 | .730 |
| Cl− (mmol/L) | 115 ± 2.5 | 115 ± 3 | .690 |
| HGB (g/dL) | – | 3.0 | – |
| SO2 (%) | – | 100% | – |
| Lactate (mmol/L) | 0.03 ± 0.0 | 2.6 ± 0.1 | .016 |
Represents and compares the biochemical composition of the perfusion solution in the control and HBOC groups, used in rewarming phase.
2.3.2. Control group
The same media was used in the control group, except no HBOC was used during rewarming perfusion and the HBOC volume was replaced with Williams Medium E solution. The composition of both perfusion media is available in (Table 1).
For reperfusion assessment of the simulated transplant, the perfusion formula with HBOC was used in both groups.
The perfusion device was flushed, cleaned, and the system was primed with fresh HBOC media for reperfusion during the 30 min simulated anastomosis time.
2.3.3. HBOC
This acellular hemoglobin was produced by purifying bovine hemoglobin and was polymerized to decrease the potential side effects and alleviate the risk of toxicities. HBOC with a molecular weight of 250 kDa and in vivo half‐life time of 20 hours, can be stored at 2‐30°C for up to 3 years.12, 13 The oxygen affinity of HBOC is regulated by chloride ion concentration which means that oxygen‐HBOC dissociation curve shifts to right and HBOC releases oxygen to tissue more freely compared to other carriers like human hemoglobin with HBOC P50 around 40 mm Hg (±6 mm Hg) at 37°C compared to 27 mm Hg of human hemoglobin.13 In general, the effects of temperature on oxygen affinity of HBOC are similar to the effects of temperature on native (corpuscular) Hb, which means that oxygen affinity increases by temperature decrease (low in hypothermic and higher in normothermic temperature). However, across all temperatures HBOC demonstrates high tendency to release oxygen which is still better than Hb in erythrocytes.16
2.3.4. Perfusion system
The perfusion device is a flow‐controlled system for rodent organ perfusion. The device consists of a Roller pump (Cole Parmer, Cat. No. HV‐07522‐20), Oxygenator containing silicon tubing and providing Carbogen and heat exchanger (Radnoti, Cat. No. 130144), Bubble trap (Radnoti, Cat. No. 130149), Pressure probe (Living Systems, Cat. No. PM‐P‐1), Circulating controlled rate chiller (Neslab, Cat. No. RTE‐111) and Organ chamber (Radnoti, Cat. no 158360) (Figure 2, Part I). (Figure 2, Part II) shows a cannulated kidney graft in the organ chamber during perfusion.
Figure 2.
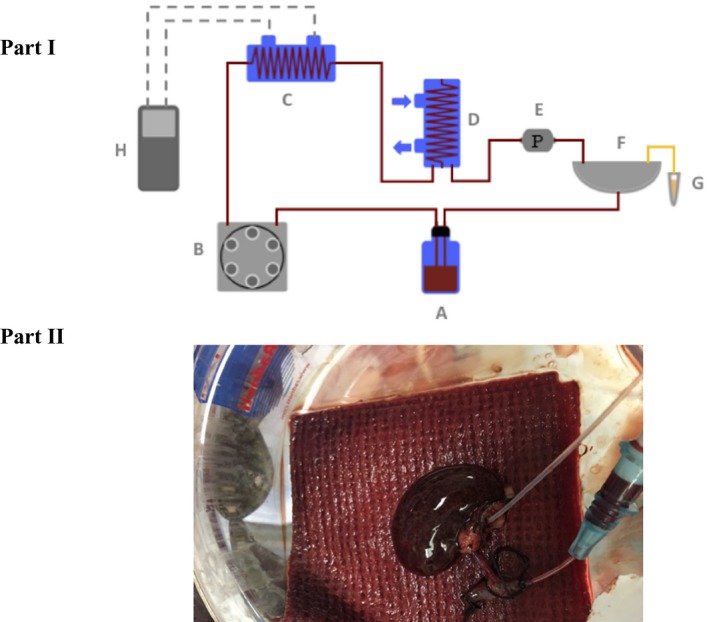
Graphic representation of the rodent kidney perfusion system. Part I: A, Solution reservoir; B, Roller pump; C, Heat exchanger and oxygenator containing silicone tubing and providing carbogen; D, Bubble trap; E, Pressure probe; F, Organ chamber; G, Urine Eppendorf; H, Thermostat which regulates the temperature. Part II: This picture shows a cannulated rat kidney during machine perfusion [Color figure can be viewed at https://www.wileyonlinelibrary.com]
2.3.5. Perfusion profile
During rewarming and reperfusion phase in both groups, flow and pressure were recorded at 30 minutes intervals and subsequently the resistance was calculated. pH and pO2 in the perfusate samples were measured every 30 minutes. Lactate concentration in the perfusate samples was measured and recorded every 30 minutes during 120 minutes of reperfusion. I‐Stat analyzer (Abbott, Lake Bluff, IL, USA) was used to measure pO2 in the arterial and venous sides, pH and lactate levels, and in general to monitor hemodynamic, chemistry, and electrolytes profile in the perfusate and ultrafiltrate samples. In order to monitor potential edema formation in the organs, the kidney grafts were weighed before, and after both rewarming and reperfusion phases. Consequently, the weight gain percentage was calculated (increase/original weight × 100).
To measure methemoglobin (Met‐Hb) level in the perfusate samples, we used RAPIDPoint 500 (Siemens, Washington, D.C., USA). Met_Hb was measured in the samples before, during and after perfusion.
2.3.6. Ultrafiltrate production and renal function
The produced ultrafiltrate was collected in Eppendorf tubes and was measured in milliliters (mL) in 30 minute intervals during reperfusion phase in the both HBOC and control groups. Glomerular filtration rate (GFR) (ultrafiltrate creatinine × ultrafiltrate volume/perfusate creatinine) and fractional sodium re‐absorption ((perfusate sodium‐ultrafiltrate sodium)/(perfusate sodium) ×100) were calculated accordingly.4
2.3.7. Oxygen consumption
Oxygen consumption during reperfusion was calculated in the HBOC group during rewarming and in the both groups during reperfusion using the following formula:
([{ApO2−VpO2} × K /760] × total flow) + ([{AsO2−VsO2} × Hb × c × 0.0001] × flow)/Kidney weight × 100. In which pO2 was in mm Hg, sO2 in %, Hb in g/dL, Renal artery flow in mL/min and kidney weight in g. c the oxygen binding capacity of HBOC (1.26) and K was a constant (0.0225).16
2.3.8. Tissue energy state
The tissue samples to determine energy cofactors adenosine monophosphate (AMP), adenosine diphosphate (ADP) and adenosine triphosphate (ATP) were taken at the end of reperfusion (t = 120 minutes) in order to prevent inducing injury to the renal grafts during rewarming and reperfusion. Method for the extraction and measurement has been described previously.17 Briefly, after extracting the metabolites using a mixture of methanol/chloroform and 3 freeze‐thaw cycles, cold water (4°C) was added to the extracts and the extracts were centrifuged. Then, the analysis was started by transferring the top phase of the mixture into the sample vial. The frozen tissue samples were analyzed using a chromatography‐mass spectrometry system (AB Sciex, Foster City, CA, USA).
Energy charge was calculated as:
Energy charge = [2ATP + ADP]/[ATP + ADP + AMP]
2.3.9. Renal injury & histological evaluation
Lactic acid dehydrogenase (LDH) was measured in the perfusate samples using Elisa kit (# MBS041480 MyBioSource, Inc., San Diego, CA, USA) during reperfusion in the both HBOC and control groups. Of note, we did not measure lactate and LDH in the rewarming phase as HBOC interference with these assays and subsequently the HBOC group data would include false positive data. We did the measurements in the reperfusion as both groups had the same perfusion environment regarding HBOC level. Biopsies were obtained from the renal tissues at the end of 120 minutes reperfusion and stored in 10% of formalin for histological evaluation. Paraffin‐embedded slides of kidney biopsies were prepared for hematoxylin and eosin (H&E).
2.4. Statistical analysis
Continuous data were presented as the median and interquartile range (IQR). Mann‐Whitney U test was used to compare groups. A P value of less than .05 was considered significant. Analyses were performed using SPSS software version 25.0 for Windows (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Rewarming
3.1.1. Perfusion profile in rewarming phase
Temperature profile in the control and HBOC groups during rewarming is shown in (Figure 3A). Renal resistance slightly decreased toward the end of rewarming perfusion with no significant difference between the HBOC and control groups (Figure 3B). Both groups showed normal pH within physiological range (7.34‐7.45) through the 90 minutes of rewarming perfusion with no significant difference (Figure 3C).
Figure 3.
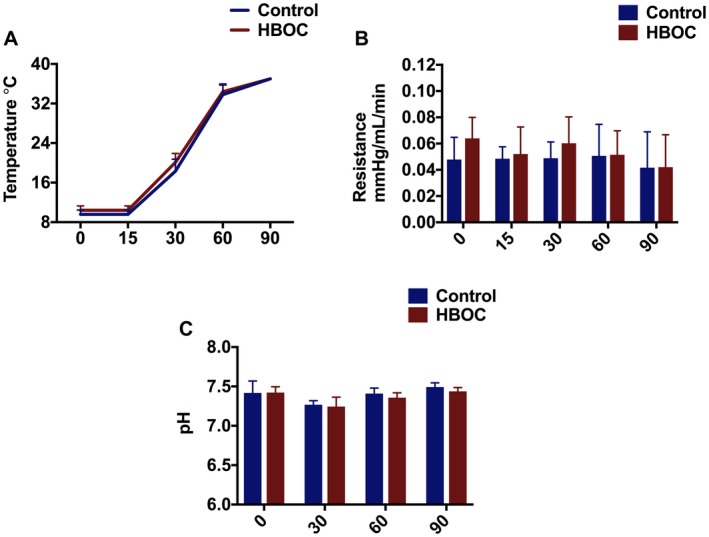
Kidney profile during gradual rewarming. A, Temperature. B, Renal Resistance, which slightly reduced during gradual rewarming in the both HBOC and control groups. C, pH was in the physiologic range during gradual rewarming. D, Oxygen consumption [Color figure can be viewed at https://www.wileyonlinelibrary.com]
3.1.2. Perfusion profile during reperfusion
The resistance in renal artery was higher at the beginning of reperfusion in the HBOC group. It decreased gradually and there was no significant difference during the rest of reperfusion between the 2 groups (Figure 4A). pH was within physiological range in the both HBOC and control groups with no significant difference (Figure 4B). No significant difference was found in the lactate concentration in both groups during 120 minutes of reperfusion (Figure 4C). The kidney grafts weigh scan revealed no significant edema level in both groups with a median of (10.5% vs. 12.5%) in the rewarming and (2.5% vs. 7.4%) in the reperfusion phase. The comparison between groups (control & HBOC) and the phases (rewarming & reperfusion) did not reveal a significant difference (Figure 4D).
Figure 4.
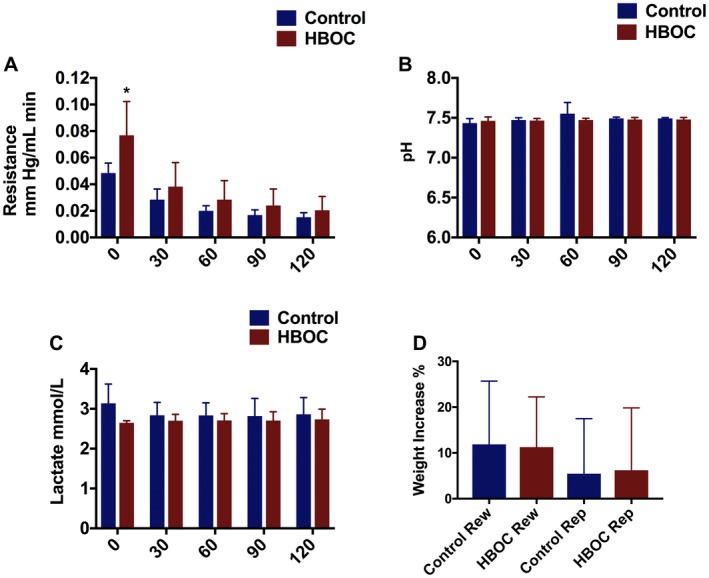
Kidney profile during reperfusion. A, Resistance was high in the both groups in the beginning of the reperfusion with significant difference, and then was reduced in both groups toward the end of reperfusion with no difference. B, pH was in normal range in the both groups with no significant difference. C, There was no significant difference in the lactate level between both groups. D, This graph indicates the weight gain during rewarming and reperfusion phase in both groups with no significant difference. *P ≤ 0.05 [Color figure can be viewed at https://www.wileyonlinelibrary.com]
We observed gradual rise in Met_Hb level during rewarming (Figure 5A) and more drastically in normothermic reperfusion, with no significant difference between control and HBOC group during reperfusion phase (Figure 5B).
Figure 5.
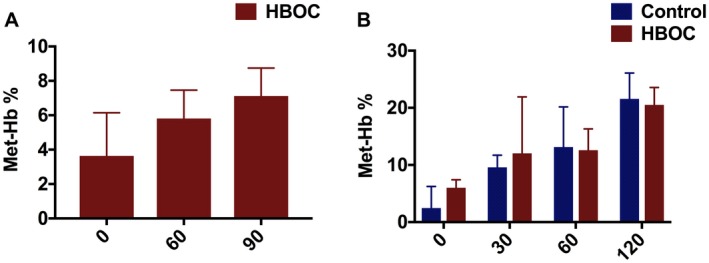
Kidney Met‐Hb during rewarming and reperfusion. A, Represents Met‐Hb level in the HBOC group during rewarming. B, Indicates a gradual increase in Met‐Hb level in the both HBOC and control groups during 120 minutes of reperfusion [Color figure can be viewed at https://www.wileyonlinelibrary.com]
3.1.3. Functional parameters during reperfusion
Ultrafiltrate production gradually decreased in the control group during 120 minutes of reperfusion while it gradually increased in the HBOC group with the statistical difference at t = 90 (P = .05) (Figure 6A). GFR rate was higher in the HBOC group during the 120 minutes of reperfusion with the statistical difference at t = 90 (P = .32) compared to the control group (Figure 6B). Fractional sodium re‐absorption was improved in the HBOC group compared to the control group during 120 minutes of reperfusion and this reached statistical significance at t = 90 (P = .017) and t = 120 (P = .032) (Figure 6C). It was notable that re‐absorption completely ceased in the control group after 90 minutes of reperfusion, but was stable throughout in the HBOC group.
Figure 6.
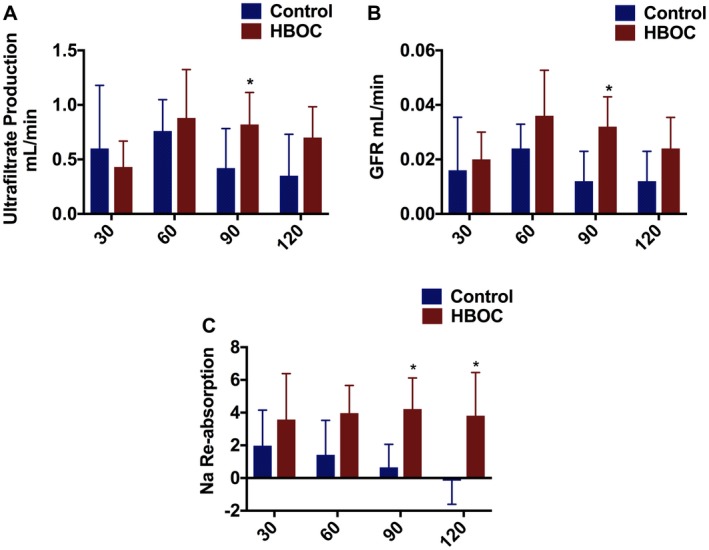
Kidneys undergoing gradual rewarming show improved function and recovery compared to controls during reperfusion. A, The volume of ultrafiltrate production was measured every 30 minutes and was improved in the HBOC group and this was significant at t = 90. B, GFR was superior in the HBOC group with significant differences at t = 90. C, Fractional sodium re‐absorption was gradually increased in the HBOC group while it was decreased in the control group with significant differences at t = 90 and t = 120. *P ≤ 0.05 [Color figure can be viewed at https://www.wileyonlinelibrary.com]
3.1.4. Oxygen consumption and energy state
Oxygen consumption was gradually increased parallel to an increase in the temperature over 90 minutes rewarming phase in the group with HBOC (Figure 7A). Oxygen consumption could not be calculated in the control group during rewarming due to absence of an oxygen carrier, therefore the difference of pO2 in the arterial and venous was calculated (Figure 7B).
Figure 7.
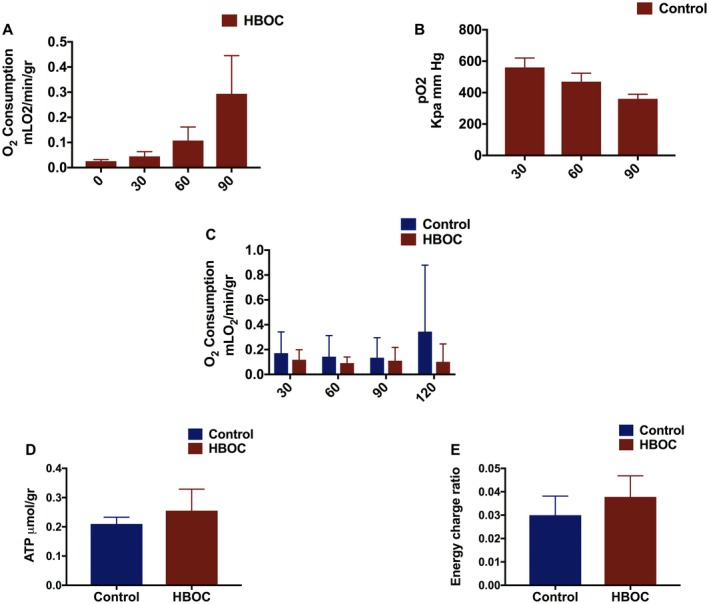
Graphical presentation of oxygen concentration, ATP level, and energy charge ratio in the rewarming and control groups. A, This graph demonstrates oxygen consumption during 90 minutes rewarming in the HBOC group. B, The difference between arterial pO2 and venous pO2 during rewarming phase in the control groups is shown in this graph. C, Oxygen consumption remained the same in the both groups during reperfusion. D, E, ATP and energy charge ratio trended higher in the HBOC group with no significant difference between the groups [Color figure can be viewed at https://www.wileyonlinelibrary.com]
Oxygen consumption was also observed in the both groups during 120 minutes of reperfusion with no difference found between the control and HBOC groups (Figure 7C). After 120 minutes of reperfusion, the HBOC group trended higher in terms of ATP and energy charge, however, the difference did not reach statistical difference (Figure 7D,E).
3.1.5. Renal parenchymal injury & histological evaluation
There was no difference in LDH between the 2 groups during reperfusion (Figure 8, Part I). Light microscopy performed on tissue samples obtained at the end of the 120 minutes reperfusion also did not show significant differences between the HBOC group and the control group. Overall only slight changes of normal structural appearance like epithelial shedding was observed in both groups (Figure 8, Part II, A & B).
Figure 8.
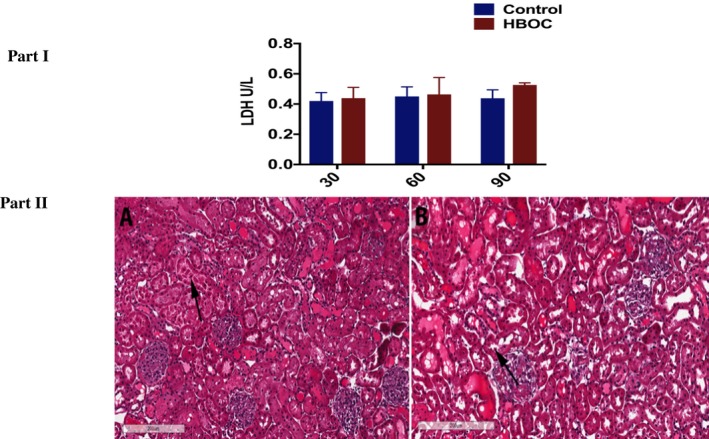
Renal Injury and H & E staining of kidney tissue from HBOC and control groups at the end of reperfusion. Part I: No difference was observed in LDH levels between the 2 groups. Part II: A, HBOC. B, Control. The arrow in the both groups shows slight epithelial shredding [Color figure can be viewed at https://www.wileyonlinelibrary.com]
4. DISCUSSION
The aim of our study was to assess whether the supplement of HBOC during gradual rewarming in DCD kidneys recovers and improves renal function, as viability testing was evaluated during 120 minutes of reperfusion at 37°C. Our study indicates that 90 minutes gradual rewarming with HBOC after CS could improve renal function compared to gradual rewarming alone. The improvements in renal function are highlighted by a higher trend of ultrafiltrate production, better GFR and improved sodium reabsorption during reperfusion phase. Although the kidneys in the HBOC group showed higher trend of ATP content and elevated energy charge status, these findings did not reach a significant difference.
It has been established that combination of warm and cold ischemia in DCD kidneys leads to severe ischemia reperfusion injury and subsequently increases the rate of delayed graft function after transplant and shortens long‐term graft survival.18 Urine production is the first sign of graft function after transplant and the urine volume may correlate with graft survival post‐transplant.19 Alongside urine volume, the quality of urine also plays an important role, for instance, GFR as a marker of renal glomerular function is used as a predictor factor of patient survival after kidney transplant.20 HBOC group demonstrated better graft function with higher trend of ultrafiltrate production and improved GFR compared to control group. Fractional sodium re‐absorption is another functional parameter as a marker of renal tubular function.21 Tubular function is an energy demanding process and sufficient oxygenation is required to provide adequate energy.21 HBOC group also showed improved tubular function and recovery indicated by superior sodium reabsorption.
HBOC has originally been developed as an alternative to RBCs in emergency care and trauma in the acutely anemic patients; however, the use in human trials has been limited due to hypertensive response as a result of nitric oxide (NO) depletion and vasoconstriction.22 We therefore closely observed the resistance during both rewarming and reperfusion by monitoring pressure and flow, HBOC caused higher resistance in the beginning of the both rewarming and reperfusion phases, however, it quickly decreased with no sign of sustained hypertension. The use of HBOC in liver normothermic perfusion also did not report higher pressure and resistance13, 14 which is in line with our findings. In the context of machine perfusion, these findings could be useful for translating to clinical studies as HBOC will be flushed out before graft implantation in recipient.
Fontes et al have tested HBOC in a preclinical porcine model during prolonged subnormothermic perfusion with the outcome indicating consistent oxygen delivery and adequate graft function after transplant.12 The use of HBOC in 2 discarded human liver studies reported the feasibility and safety of applying HBOC‐based perfusion media during normothermic machine perfusion (NMP) as an alternative to RBCs.13, 14 A recent study by de Vries et al described the use of HBOC in a pretransplant combined dual oxygenate hypothermic machine perfusion (D‐HOPE) with controlled oxygenated rewarming (COR) to NMP in initially declined human liver grafts.16 The study presented promising results for further future use of HBOCs in clinical studies with the outcome of 100% patient survival in the first 3 months post‐transplant.
Although HBOC has been used in a number of liver perfusion studies with improved perfusion parameters, the liver studies were either preclinical or clinical with small population and limited information about HBOC in human kidney perfusion. Hence, further studies could assess the effects of HBOC‐based perfusion solution in a comprehensive clinical trial model.
Due to the lack of NADH‐dependent enzyme (methemoglobin reductase) in HBOC, the possibility of Met‐Hb production is a disadvantage associated with HBOC use and the gradual rise in Met_Hb level has been previously reported.16 Our experiments also revealed a slight increase in the Met‐Hb during rewarming and more extreme during reperfusion. In spite of higher Met‐Hb formation, the risk of Met‐Hb transfer after perfusion to the organ recipient's body is negligible as the perfusion solutions get flushed out of the organ prior to transplantation.14
This study as a preclinical kidney rewarming model presents supportive information about the feasibility of HBOC use in renal perfusion and the effective outcome on renal function. However, lack of transplantation validation is a limitation of our study and the next phase would be to validate this data in a transplant model. The other limitation is that although positive effect of HBOC on kidney function could be due to better oxygen carrying capacity of HBOC, nevertheless, we did not specifically study the O2 carrying capacity in this study and this could be further investigated in future studies.
5. CONCLUSION
This study is the first report on using HBOC‐201 in the gradual rewarming kidney model with improved kidney function. Altogether, this proposes the possibility of using HBOCs in the future in different perfusion setups as a potential oxygen carrier in different temperatures.
CONFLICT OF INTEREST
The authors of this manuscript have conflicts of interest to disclose: Dr. Uygun is inventor on pending patents relevant to this study. Dr. Uygun has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. Dr. Uygun's interests are managed by the MGH and Partners HealthCare in accordance with their conflict of interest policies. Drs. Tessier and Uygun have several IP disclosures on extended organ preservation that may be relevant to this study. The HOBC‐201 used in this study was provided by HBO2 Therapeutics LLC.
AUTHOR CONTRIBUTIONS
All authors read and approved the final version of the manuscript.
Study design, experiments performance, data collection, statistical analysis/interpretation, drafting the article: Mahboub
Experiments performance: Aburawi
Study design: Karimian
ATP analysis: Lin, Karabacak
Data collection: Fontan, Tessier
Critical revision of article: Markmann, Yeh
Study design/concept, critical revision of article, approval of article: Uygun
ACKNOWLEDGMENTS
Support from the US National Institutes of Health (grants R01DK096075, R01DK107875, and R01DK114506) and the Shriners Hospitals for Children is gratefully acknowledged. We would like to acknowledge the Mass Spectrometry Core Facility at Shriners Hospital for Children for processing our samples and performing the ATP analysis. We thank HBO2 Therapeutics LLC for providing HBOC‐201, and for providing information on the effect of temperature on oxygen affinity in HBOC.
Mahboub P, Aburawi M, Karimian N, et al. The efficacy of HBOC‐201 in ex situ gradual rewarming kidney perfusion in a rat model. Artif Organs. 2020;44:81–90. 10.1111/aor.13534
REFERENCES
- 1. Mahboub P, Bozorgzadeh A, Martins PN. Potential approaches to improve the outcomes of donation after cardiac death liver grafts. World J Transplant. 2016;6:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson PT, Aquil S, McLean K, McAlister VC, Sener A, Luke PP. First Canadian experience with donation after cardiac death simultaneous pancreas and kidney transplants. Can J Surg J Can Chir. 2017;60:323–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Op den Dries S, Karimian N, Porte RJ. Normothermic machine perfusion of discarded liver grafts. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2013;13:2504. [DOI] [PubMed] [Google Scholar]
- 4. Mahboub P, Ottens P, Seelen M, ‘t Hart N, Van Goor H, Ploeg R, et al. Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS One 2016;11:e0152006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Westerkamp AC, Mahboub P, Meyer SL, Hottenrott M, Ottens PJ, Wiersema‐Buist J, et al. End‐ischemic machine perfusion reduces bile duct injury in donation after circulatory death rat donor livers independent of the machine perfusion temperature. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2015;21:1300–11. [DOI] [PubMed] [Google Scholar]
- 6. Kaths JM, Hamar M, Echeverri J, Linares I, Urbanellis P, Cen JY, et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2018;18:580–9. [DOI] [PubMed] [Google Scholar]
- 7. Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: Better conditioning and repair? Transpl Int Off J Eur Soc Organ Transplant. 2015;28:657–64. [DOI] [PubMed] [Google Scholar]
- 8. Gallinat A, Lu J, von Horn C, Kaths M, Ingenwerth M, Paul A, et al. Transplantation of cold stored porcine kidneys after controlled oxygenated rewarming. Artif Organs. 2018;42:647–54. [DOI] [PubMed] [Google Scholar]
- 9. Fontes PA. The evolution of oxygen carrier solutions for machine perfusion. Transplantation. 2017;101:2657–8. [DOI] [PubMed] [Google Scholar]
- 10. Hosgood SA, Nicholson H, Nicholson ML. Oxygenated kidney preservation techniques. Transplantation. 2012;93:455–9. [DOI] [PubMed] [Google Scholar]
- 11. Hosgood SA, Nicholson ML. The role of perfluorocarbon in organ preservation. Transplantation. 2010;89:1169–75. [DOI] [PubMed] [Google Scholar]
- 12. Fontes P, Lopez R, van der Plaats A, Vodovotz Y, Minervini M, Scott V, et al. Liver preservation with machine perfusion and a newly developed cell‐free oxygen carrier solution under subnormothermic conditions. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. 2015;15:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laing RW, Bhogal RH, Wallace L, Boteon Y, Neil DAH, Smith A, et al. The use of an acellular oxygen carrier in a human liver model of normothermic machine perfusion. Transplantation. 2017;101:2746–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matton APM, Burlage LC, van Rijn R, de Vries Y, Karangwa SA, Nijsten MW, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2018;24:528–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van denEijnden M, Leuvenink H, Ottens PJ, ‘t Hart N, van Oeveren W, Morariu AM, et al. Effect of brain death and non‐heart‐beating kidney donation on renal function and injury: an assessment in the isolated perfused rat kidney. Exp Clin Transplant Off J Middle East Soc Organ Transplant. 2003;1:85–95. [PubMed] [Google Scholar]
- 16. de Vries Y, Matton APM, Nijsten MWN, Werner MJM, Berg AP, Boer MT, et al. Pretransplant sequential hypo‐ and normothermic machine perfusion of suboptimal livers donated after circulatory death using a hemoglobin‐based oxygen carrier perfusion solution. Am J Transplant. 2019;19:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruinsma BG, Avruch JH, Sridharan GV, Weeder PD, Jacobs ML, Crisalli K, et al. Peritransplant energy changes and their correlation to outcome after human liver transplantation. Transplantation. 2017;101:1637–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao H, Alam A, Soo AP, George A, Ma D. Ischemia‐reperfusion injury reduces long term renal graft survival: Mechanism and beyond. EBioMedicine. 2018;2:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khosroshahi HT, Oskui R, Shoja MM, Tubbs RS, Ardalan MR. Time‐dependent variations in urine output after renal transplantation. Transplant Proc. 2007;39:932–33. [DOI] [PubMed] [Google Scholar]
- 20. Santos J, Martins LS. Estimating glomerular filtration rate in kidney transplantation: Still searching for the best marker. World J Nephrol. 2016;4:345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. von Horn C, Minor T. Isolated kidney perfusion: The influence of pulsatile flow. Scand J Clin Lab Invest. 2018;78:131–5. [DOI] [PubMed] [Google Scholar]
- 22. Taverne YJ, de Wijs‐Meijler D, te Lintel Hekkert M, Moon‐Massat PF, Dubé GP, Duncker DJ, et al. Normalization of hemoglobin‐based oxygen carrier‐201 induced vasoconstriction: targeting nitric oxide and endothelin. J Appl Physiol Bethesda Md 1985. 2017;122:1227–37. [DOI] [PubMed] [Google Scholar]


