Abstract
The extracellular electrogram is caused by transmembrane currents that flow into extracellular space during propagation of the electrical impulse. Electrograms are usually recorded in unipolar or bipolar mode that have different characteristics, but provide complementary information. Both recording modes have specific advantages, but also suffer from disadvantages. Techniques to circumvent some of the weaknesses are reviewed. The origin of remote and fractionated deflections and their relation with electrode characteristics are discussed. Epicardial and endocardial sites of origin and breakthrough sites as well as the effect of fatty tissue on extracellular electrograms are presented. Induction of tachycardia to assess the arrhythmogenic area is not always possible because of hemodynamic instability of the patient. Techniques to assess sites with high reentry vulnerability without induction of arrhythmias are outlined such as activation‐repolarization mapping and decremental stimulation. Pitfalls of substrate mapping and techniques to avoid them as omnipolar mapping and characterization of complex electrograms by entropy are presented. Technical aspects that influence electrogram morphology as electrode size, filtering, contact force, and catheter position are delineated. Data from the various publications suggest that a combination of unipolar and bipolar electrogram analysis techniques is helpful to optimize determination of target sites for ablation.
Keywords: analysis techniques, bipolar, extracellular electrogram, target site, unipolar
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- AF
atrial fibrillation
- CFAE
complex fractionated atrial electrograms
- CS
coronary sinus
- cv
conduction velocity
- Cx43
connexin 43
- LAD
left anterior descending artery
- RSPV
right superior pulmonary vein
- SCV
superior caval vein
1. INTRODUCTION
Cardiomyocytes are coupled electrically by connexins, of which C × 43 is the major connexin in ventricular myocardium. Connexins are small protein channels through which ions and small molecules can flow between the cardiomyocytes. Axial current will flow between activated and non‐activated cardiomyocytes because of the voltage difference between them (approximately 100 mV) and the electrical coupling mediated by the connexins. As the axial current flows along the cells, some of it leaks across the surface membrane as transmembrane current. The amount of transmembrane current in any region must be equal and opposite to the change in axial current. This results in a total transmembrane current that is proportional to the second derivative of the voltage. The transmembrane currents flow through the membrane into extracellular space. Because the extracellular fluid has resistance, an extracellular (dipole) potential field is generated. This field is positive ahead of the activation front and negative at the back. The change in the extracellular potential with time at a certain site is the extracellular electrogram.
2. UNIPOLAR RECORDING MODE
In the unipolar recording mode, a different electrode is positioned at the site where the electrical potential must be determined. The indifferent electrode is at zero potential; this electrode is positioned at a large distance from the heart or connected to the Wilson's Central Terminal. Unipolar electrograms have the following major characteristics: (a) interpretation of the morphology of the signals is usually straightforward, (b) electrogram morphology of a passing wave front is independent of the direction of the front, (c) the electrogram is rather sensitive to electrical signals generated at a distance from the electrode, such as remote activation caused by activity of distant heart cells or electrical disturbances such as 50/60 Hz interference. Elimination or reduction of electrical disturbance (“noise rejection”) is important to select accurate local activation time for both unipolar and bipolar electrograms. In low‐noise condition (<0.02 mV baseline noise), small local potentials (<0.05 mV) can well be identified in both unipolar and bipolar electrograms.1 Mapping systems and special electrodes that are available today allow acquisition of bipolar recordings with a noise level of 0.01 mV (peak‐to‐peak).2
2.1. Morphology of the unipolar electrogram
The unipolar electrogram recorded at the origin of activation is negative because the electrode is always located in the negative part of the extracellular potential field during propagation of the activation front. At a recording site where the activation front passes the electrode, the deflection is biphasic. During the approaching phase of the front the electrode is located in the positive part of the potential field, which results in a positive deflection of the electrogram. The amplitude of the electrogram becomes zero at the time the front is exactly over the electrode. Then, when the front has passed the electrode, the electrogram becomes negative because the electrode is located in the negative part of the potential field. This sequence results in a biphasic deflection for the electrogram, a positive deflection followed by a negative one. At a site where activation comes to an end, the electrode is located in the positive part of the potential field and therefore the signal will be positive.
2.2. Activation time
The activation time is defined as the time cardiomyocytes underneath the electrode depolarize. This coincides with the upstroke of the action potential of the cells, which occurs within a millisecond in healthy myocardium. The time of steepest upstroke of the action potential is taken as the activation time. Simultaneous recordings of action potentials and unipolar extracellular electrograms at the same spot have shown that the upstroke of the action potential coincides with the point of steepest down stroke in the unipolar electrogram. This is not only valid for healthy myocardium, but also for ischemic myocardial tissue. Only part of the unipolar electrogram (the down stroke) reflects the local event (depolarization) at the recording site. Large parts of unipolar deflections are in fact remote, caused by the wave front that approaches, and retreats the recording site; only the steep negative deflection reflects depolarization of cardiomyocytes underneath the electrode (Figure 1, upper tracing).
Figure 1.
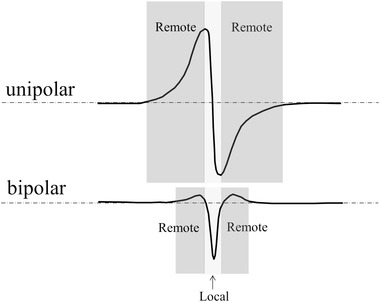
Unipolar and bipolar electrogram recorded during passage of an activation front. Parts of the extracellular electrograms reflect remote (dark gray) and local (light gray) activation. The remote part is smallest for the bipolar recording
An electrogram is termed local if it is generated by activation directly underneath the electrode. If activation propagates through a bundle and the electrode terminal is at some distance from the bundle, the electrogram becomes therefore remote. The amplitude of an extracellular unipolar electrogram decreases if distance increases. It can be shown that at large distances (>1.5 bundle diameter), this decrease of amplitude will be approximately inversely proportional to the power of 2 with the distance. The distance between the positive and negative peak of the unipolar electrogram will increase with distance, which results in less steep signals for remote deflections. The amplitude of the bipolar electrograms will also decrease with distance, albeit that the decrease is faster. If multiple deflections are present with similar steepness of the down stroke, selection of activation times is less straightforward. However, by the simultaneous recording of electrograms from adjacent sites, the right activation time can often be assessed, as discussed later.
2.3. Activation in two‐ and three‐dimensions
Activation in two‐dimensional (2D) myocardial tissue will affect electrogram morphology because of the anisotropic characteristics of myocardial tissue.3 If a sub‐epicardial sheet of myocardial tissue (2D) is stimulated at the center, isochronal lines will be elliptically shaped (Figure 2). The unipolar electrogram at the site of stimulation (a) is negative, because it is recorded at the origin of activation. At a distance from the site of stimulation where activation runs parallel to the fiber direction, the electrogram is biphasic. If the distance is large, the positive and negative deflections caused by the passing wave front are virtually equal (site b). If the recording site is close to the site of stimulation, the positive deflection will be less high than the negative one, because the approaching front is less strong (due to its small size during onset) than the front that has passed the recording site. A similar effect occurs for positions near the site where activation comes to an end; here, the negative deflection will be smaller than the positive one because activation is passing the recording site shortly.
Figure 2.
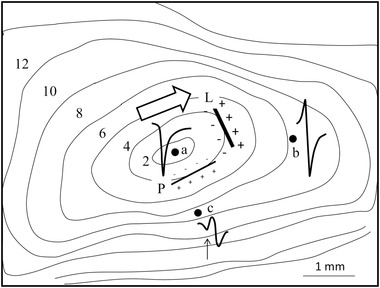
Isochronal map of a sub‐epicardial sheet of myocardium superfused in a tissue bath and stimulated at the center (site a). The bold arrow shows major fiber direction in the middle part of the tissue. Three electrograms are shown. The electrogram at the site of stimulation (site a) is negative. At site b, at a distance from the site of stimulation, activation passes the electrode resulting in a biphasic deflection with similar amplitudes for the positive and negative deflection. At site c, activation is passing during propagation perpendicular to the fiber direction. The biphasic deflection of the passing front is preceded by a negative one (arrow), which is remote and caused by activation running away from the recording site in the fiber direction. Numbers are activation times with respect to the onset of stimulation. The two straight lines schematically mark two dipole fields at time 4 ms for activation parallel and perpendicular to fiber direction. Electrode diameter: 0.05 mm; inter‐electrode distance: 0.15 mm; reference electrode: border of tissue bath; filter setting: 0.1‐30 kHz. (Adapted from Spach MS et al., Circ Res 1979;45:188‐204)
For recording sites where activation proceeds perpendicular to the fiber direction, electrogram morphology is more complex. The tight electrical coupling between myocardial cells at the long axis of the cells compared to the side‐by‐side connections results in a stronger activation front (and extracellular potential field) during propagation in the fiber direction. This difference in strength of the activation fronts is schematically indicated in Figure 2 by the bold lines “L” and “P” at isochronal line 4 (4 ms after stimulation at the center). The line marked “L” shows the (strong) potential field of activation moving in the fiber direction (bold arrow). Electrode “c” is located in the negative zone of this potential field. Line “P” marks the activation front (and potential field) moving perpendicular to the fiber direction. This potential field is weak and electrode “c” is located in the positive potential zone of this front. Because of its strength, the negative contribution of the front running in the fiber direction is (initially) greater than the contribution of the front running perpendicular to the fiber direction, despite its larger distance. This results in the initial negative deflection for the electrogram at site “c” (arrow). If front “P” is closer to site “c” the distance effect becomes greater and a biphasic deflection of a passing wave front ensues.
Fiber direction of the ventricles rotates from epicardium to endocardium over about 120 degrees. This rotation will affect the epicardial activation pattern and morphology of unipolar electrograms. If stimulating the epicardium of three‐dimensional (3D) myocardium, the elliptically shaped epicardial activation pattern remains similar, but the crowding of isochronal lines for activation perpendicular to the fiber direction decreases for distances further away from the site of stimulation. From the site of stimulation activation will spread out centrifugally. Epicardially, activation will spread slowly perpendicular to the fiber direction. At the same time activation moves toward the endocardium. At the depth where fiber direction has rotated over 90 degrees, activation will move fast in the direction in which activation moves slowly at the epicardium. After a while this sub‐epicardial front will overtake the epicardial activation front and by moving toward the epicardium activate the epicardium earlier than the epicardial traveling front.
2.4. Change in signal amplitude with distance in 3D
The mathematical relationship between distance and electrogram amplitude for myocardial activation is complex. For a myocardial bundle it can be shown that for large distances the amplitude decreases with the power of 2 with distance. Podziemski et al determined this relationship in 3D atrial tissue. The investigators used activation wave fronts that traveled along a line of conduction block.4 In this way, they were able to measure the amplitude of electrograms at several distances from activated tissue distal from the line of block. The authors fitted the data to an exponential function, but it can be shown that the amplitude‐distance curve they composed fits well with the inverse of the distance to the power of 2 for large distances.
2.5. Initially negative deflections
As outlined before, an initially, sharp negative deflection points to a recording site where activation arises. Such a signal is expected to occur at a site where normal or abnormal impulse formation occurs. This feature of the unipolar electrogram is helpful to determine the arrhythmogenic site of focal cardiac arrhythmias. It is, however, important to check the initial deflections for (tiny) R‐waves. As long as an R‐wave is present, the electrode is not at the site of origin of activation.
In a large number of cases, the signal recorded at the origin of activation is more complex, as illustrated in Figure 3. It shows the epicardial activation map of a patient with symptomatic ectopic ventricular activity. The electrogram recorded at the earliest activated site indeed starts with a sharp negative deflection, but the initially down stroke is followed by a second negative deflection. This second deflection is due to fiber rotation from epicardium to endocardium as outlined before. The fiber direction is perpendicular to the left anterior descending artery (LAD) at the epicardium and more or less parallel to the LAD at the endocardium. Therefore, conduction velocity parallel to the LAD is low at the subepicardium, but fast in subendocardial layers. The fast activation in the fiber direction at subendocardial layers is (initially) somewhat delayed compared to the epicardial activation. It is related with a strong potential field and will induce a delayed large negative deflection in the signal at the epicardial recording electrode, because activation moves away from that site.
Figure 3.
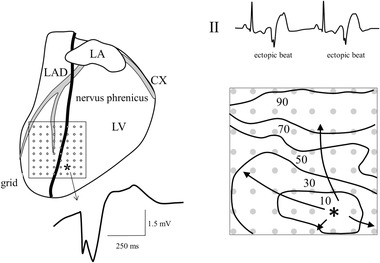
Epicardial mapping in a patient with symptomatic ectopic ventricular activity (see tracing II). Anti‐arrhythmic surgery was carried out because epicardial catheter mapping revealed that the location of ectopic activity was located close to the phrenic nerve. The right lower panel shows the isochronal map of an ectopic beat derived from extracellular electrograms recorded by an 8 × 8 electrode grid (left panel). Numbers are activation times after earliest activation. The earliest activated site of the ectopic complex is indicated by an asterisk. The electrogram at that site is initially negative, as illustrated by the tracing. CX = circumflex; LA = left atrium; LAD = left anterior descending artery; RA = right atrium; LV = left ventricle; electrode diameter: 1 mm; inter‐electrode distance: 5 mm; reference electrode: surgical wound; filter setting: 0 Hz – 400 Hz
Initially, negative electrograms may also arise at sites that reveal a tissue discontinuity. Such sites are intrinsically present in the heart, for instance at Purkinje‐muscle junctions where the Purkinje strands are connected to ventricular myocardium. The Purkinje strand consists of a small number of cells compared to ventricular myocardial tissue it is connected with. This small number of cells of the Purkinje strand has to deliver current to activate the large number of cells of the connected myocardial tissue. Thus, less current per myocardial cell is available, which results in conduction delay at the interface. The current to load mismatch at these tissue discontinuities may also lead to conduction block.5 Activation block at Purkinje‐muscle junctions is usually avoided because specific transitional cells take care of the transition of activation from Purkinje to muscle. A recording electrode positioned at the Purkinje‐muscle interface will reveal an electrogram with a negative deflection only (Q‐wave) if propagation is from Purkinje to muscle. An initially positive deflection (R‐wave) caused by the approaching front is missing. The reason is that the small number of cells in the Purkinje strand generates too little current to obtain an initially positive deflection. When the interface has been passed, a strong activation front will develop in the muscular tissue and generate a large negative deflection.
Several heart diseases too are associated with tissue discontinuities. In the Wolff‐Parkinson‐White (WPW) syndrome an aberrant myocardial bundle traverses the atrio‐ventricular groove and connects atrial with ventricular myocardium. These bundles usually consist of myocardial tissue, have a small diameter, and are connected to substantial myocardial tissue. At the site where ventricular myocardium is activated by activation in the aberrant bundle, an electrogram with initially negative deflection arises. Again, the R‐wave of the (small) approaching wave front in the aberrant bundle is virtually invisible. This feature of the unipolar electrogram can be used to track down the location of the aberrant bundle; the site where the bundle is connected with the bulk mass of myocardium.6
Tissue discontinuities may also arise in infarcted myocardium. Collateral flow may give rise to surviving myocardial strands within the infarcted zone. These strands may traverse the infarcted zone paving the way for re‐entry, which is often the mechanism of infarct related ventricular tachycardias.7 At the site where activation leaves the bundle and activates remaining healthy myocardium, the recorded electrogram will be initially negative. The surviving bundles usually have a small diameter and the R‐wave they generate in the electrogram at the exit site will be virtually invisible.
The initially negative deflection of the unipolar recording can also be helpful to detect tissue discontinuities where slow conduction suddenly speeds up, such as the proximal part of the His bundle. At the site where the common AV bundle connects with the His bundle, a QS deflection is recorded.8
3. BIPOLAR RECORDING MODE
3.1. Characteristics
For bipolar recordings, the different electrode is, as in the unipolar mode, positioned at the site of interest. The indifferent electrode is, however, positioned close to the different one. Wave fronts that pass the electrodes induce similar (unipolar) signals at both poles. The main difference is that these signals are shifted in time. The degree of the shift depends on the direction of the wave front and is virtually zero if activation moves perpendicular to the line between the poles. Because the recording amplifier subtracts the two signals, the output signal is in fact proportional to the first derivative of the unipolar electrogram (Figure 4). Prerequisite is that the time difference (dt) between the signals is small, which is the case if the distance between the poles is small (dt = dx/cv; dx is the distance between the poles, cv the conduction velocity).
Figure 4.
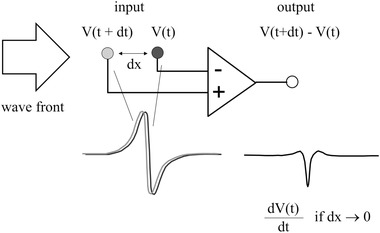
Schematics of the bipolar recording mode during passage of a broad wave front indicated by the open arrow. Input signals at the + and – input of the amplifier are similar (gray and black tracings), but shifted in time. The output signal is the difference between the two input signals and is proportional to the first derivative of the unipolar electrogram if the distance (dx) between the poles is small
A bipolar electrogram is sharper than a unipolar one, because differentiation of a signal promotes the high frequency components in the signal. In contrast to a unipolar electrogram, morphology and amplitude of a bipolar electrogram depend on the direction of the wave front.
3.2. Activation times and remote signals
The activation time in a bipolar electrogram is more difficult to assess than in a unipolar electrogram. In Figure 5, the simultaneous recording of unipolar and bipolar electrograms is shown for different directions of the activation front. The setup for the bipolar recording is as illustrated in Figure 4. The activation time in the unipolar electrogram is independent of the direction of the activation front and is at the point of steepest down stroke in the signal (upper arrow). Activation time in the bipolar electrogram is at the peak negative point (middle arrow) if the activation front comes from the left (open arrow A). However, if the activation front comes from the opposite direction (open arrow B), the signal inverts and the activation time is at the peak positive point (lower arrow). It becomes even worse if the plane of the wave front is parallel to the line between the poles (open arrow C and D). Then, the signals induced at the poles are nearly the same (without delay) and the bipolar electrogram is virtually zero and no activation time can be assessed in that case. In practical circumstances where the electrode size of the tip and the ring of a catheter are different and/or wave fronts are not plain, cancellation will not be 100%.
Figure 5.
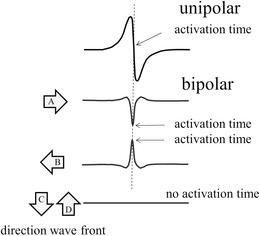
Simultaneous recording of unipolar and bipolar electrograms from the same position. A unipolar electrogram is independent on the direction of the activation front and the activation time is at the point of steepest negative dV/dt in the signal. Setup for the bipolar recordings is as indicated in Figure 4. If the activation front comes from the left (A), the bipolar electrogram is mainly negative (as in Figure 4) and the activation time is at peak negative. If the activation front comes from the right (B), the bipolar electrogram inverts and becomes mainly positive. The activation time is now at peak positive. Finally if the activation front is parallel to the line between the poles (C and D), the signal output is virtually zero and an activation time cannot be determined
A great advantage of bipolar recordings is that distant electrical activity, being caused by a remote wave front or by interference of the mains, is suppressed because the induced signal is nearly the same at both poles. Therefore, the bipolar electrogram is more suitable to detect whether a deflection is local or remote than the unipolar electrogram. The amplitude of the bipolar electrogram generated by activation in a myocardial bundle (one‐dimensional) is inversely proportional to the power of three of the distance. Although the bipolar electrogram is sharper than the unipolar one, recordings made in bipolar mode also have remote parts as illustrated in Figure 1 (lower tracing).
The direction dependence of the bipolar electrogram can be used to assess the direction of wave fronts. Main positive or negative deflections correspond with opposite directions of the activation fronts. This feature of the bipolar electrogram can be used to assess the site of origin of focal activation as will be discussed later.
3.3. Simultaneous recording of unipolar and bipolar electrograms
Because unipolar and bipolar electrograms have complementary information, it is often helpful to record them simultaneously. The bipolar recording reveals which deflection is local in the unipolar electrogram. Figure 6 shows a tracing of a sinus complex followed by an ectopic one in a patient with ectopic beats arising in the left ventricular outflow tract. The sinus complex shows that the first deflection of the unipolar recordings (deflection a) is remote, because a deflection at the same time has a very low amplitude in the bipolar recording. The second deflection in the unipolar recordings (deflection b) is local because a prominent deflection arises in the bipolar recording at the same time. For the ectopic complex, the bipolar recording shows that the negative deflections in the unipolar recordings are local because they occur at virtually the same time (deflection c). In addition, the steepest and earliest deflection occurs in unipolar tracing 1 and it is at the tip electrode of the catheter from which radiofrequency current was delivered. From the bipolar recording, it is not evident whether the earliest activation arises at the proximal or distal pole, thus activation time can best be detected from the unipolar electrogram.
Figure 6.
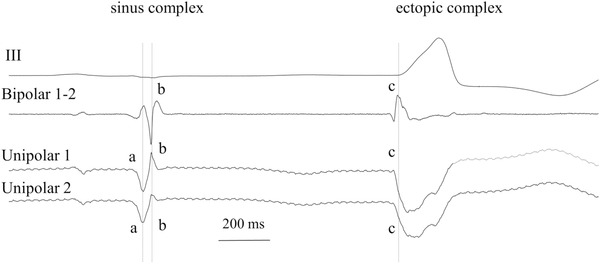
Simultaneous recording of two unipolar and one bipolar electrogram together with lead III at the LVOT in a patient with symptomatic ectopic beats arising at the outflow tract. To determine “local” deflection(s) in the unipolar electrogram, deflections in the unipolar electrogram are compared with bipolar deflections at the same instant. Deflection a in the unipolar recordings of the sinus complex, is virtually missing in the bipolar one, indicating that this deflection is remote. Deflection b is present in both unipolar and bipolar tracings, indicating that this deflection marks a local event. In the same way, deflection c is the local one in the ectopic complex. The (initially) negative deflection is sharpest and earliest at pole 1 of the unipolar recording, indicating that this pole should be used for ablation. Electrode size: 2 × 1 mm; inter‐electrode distance: 2 mm; reference electrode: WCT; filter setting: 0.1‐250 Hz
4. CIRCUMVENTING DRAWBACKS OF UNIPOLAR AND BIPOLAR RECORDINGS
Although unipolar and bipolar electrograms are the basis for electrophysiological recordings, both have some drawbacks. These can often be circumvented by using multiple adjacent electrograms for analysis. Modern mapping systems allow the recording and analysis of a large number (even thousands) of electrograms in a short time interval by using multi‐pole catheter‐electrodes.
4.1. Omnipolar recording
One of the major problems with bipolar electrograms is their direction dependence. Bipolar signals are often used for voltage‐guided approaches to determine compromised myocardial areas. The orientation of the poles with respect to the direction of the wave front will affect the correct interpretation of the amplitude of the bipolar electrogram. The omnipolar technique circumvents this problem and provides a measure of the largest possible bipolar electrogram.9 Within a square area defined by four electrode terminals that record unipolar electrograms, the omnipolar technique allows the calculation of bipolar electrograms in any direction, from which the direction that gives the maximal amplitude can be determined. Haldar et al applied this technique in a dog model of AF and showed that the omnipolar approach is able to identify the largest possible bipolar electrogram in sinus rhythm as well as AF.10 In addition, the wave front direction can be estimated by taking into account wave front orientation, collision, and fractionation, yielding a velocity vector field.11
4.2. LaPlacian technique
Interpretation of unipolar electrograms and their local activation times can be hampered by remote activity or intervening repolarization waves, especially during complex arrhythmias. LaPlacian electrograms allow detection of the moment of activation without these interferences. The calculation involves spatial and temporal differentiation of the extracellular voltage recorded in a grid electrode. The LaPlacian approximates the second spatial derivative of the unipolar electrogram (if the poles are close together) and is proportional to the transmembrane current. Coronel and co‐workers showed that during ventricular fibrillation wave fronts are better recognizable in the LaPlacian signal.12
5. ORIGIN OF REMOTE DEFLECTIONS
As illustrated in Figure 2, remote deflections may arise because of anisotropy of myocardial tissue. Differences in the strength of the potential field for activation parallel and perpendicular to the fiber directions are the important factor for these deflections. There are, however, other ways remote deflections may arise in unipolar, but also bipolar electrograms. Recordings from the atrium can be disturbed by activation in the ventricle. The ventricular mass is much larger than that of the atrium and therefore, activation in the ventricle can be recorded at a large distance, that is, in the atrium. It is important to trace such remote deflections, because they may result to false interpretations if considered to be local. The simultaneous recording of ventricular activity, for instance by means of a surface ECG, may be helpful to cope with this problem.
Activity in various adjacent heart structures may generate remote deflections that are preferentially visible if activation is asynchronous. The superior caval vein (SCV) is located close to the right superior pulmonary vein (RSPV).13 The myocardial mass of the SCV is such that enough current can be generated to produce a (remote) signal in recordings of the RSPV. Similar in this respect are overlaying structures as present at the coronary sinus (CS). Atrial tissue overlays the CS, but is electrically isolated from the CS at most sites by adipose tissue.14 Recordings made from sites in the CS reveal deflections from atrial tissue and myocardium in the CS. Distinguishing remote from local activity may be difficult in such overlaying structures because activation in the two tissues is very close together and both deflections can be sharp.
5.1. Fractionated electrograms
Increased collagen deposition is associated with a large number of cardiac diseases and will affect conduction as well as electrogram morphology. Often myocardial and collagen bundles intermingle, which may result in tortuous routes for activation. Because activation in various bundles is often asynchronous, electrograms become fractionated. Such fractionated electrograms often arise in infarcted myocardium.15 Increased collagen deposition may give rise to structural and functional conduction block. Current to load mismatch imposed by the mixture of myocardial and collagen fibers is an important factor in generating functional conduction block.
A major problem with fractionated electrograms is the selection of the local component (and activation time), the more so because multiple local components can be present in a signal. If multiple recordings are available from neighboring sites, a reliable detection of the local deflection may be possible as illustrated in Figure 7. Panel (A) shows an infarcted papillary muscle that was superfused in a tissue bath.16 Tissue consisted of parallel oriented myocardial bundles, separated over variable distances by collagen bundles, which resulted in asynchronous activation causing fractionated electrograms. Electrograms were recorded with a line‐electrode harboring eight terminals with 200 µm inter‐electrode distance. The line‐electrode was perpendicular to the fiber direction of the papillary muscle. Five of the electrograms (a‐e) are shown in panel (B). All deflections along the dashed vertical line marked 6 occur at the same time, suggesting that these deflections are generated by the same activation front in one of the myocardial bundles of the muscle. Panel (C) shows the amplitude and the maximal negative slope of the five deflections. The largest value of amplitude and slope of complex 6 is present in tracing c. Amplitude and slope decrease in tracings d and e as well as b and a. This means that the activated myocardial bundle that generates deflection 6 is located underneath electrode c and at a distance for the other electrodes (a and b and d and e). Deflections 1‐4 in tracing c are remote. They have counterparts in adjacent tracings at the same time, but amplitude and slope of these corresponding deflections are greater in tracings b and a. This supports the remote character of the deflections 1‐4 in c. Deflection 5 in tracing c is, however, caused by local activity once more. For this deflection the amplitude and slope decrease in the corresponding deflections in tracings b and d. The amplitude of deflection 5 is much smaller than that of deflection 6 in tracing c, suggesting that deflection 5 is caused by activation in a bundle with a smaller diameter or at a larger distance from the surface. The cause of two local deflections is related to the size (surface area) of the electrode. If the electrode surface covers two bundles, activation in both bundles will be local.
Figure 7.
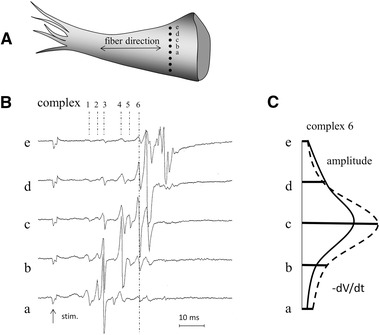
Panel (A) shows a papillary muscle with a line electrode (black dots are the eight electrode terminals) positioned perpendicular to the long axis of the muscle. Unipolar electrograms recorded simultaneously at five sites (a‐e) are shown in panel (B). Numbers indicate six different complexes. Note that deflections along the dashed lines of complex 6 in the different recordings occur at the same time. Amplitude and dV/dt of these deflections are shown in panel (C). They are largest in tracing c, which suggests that the bundle that generates this deflection is located underneath electrode c. Deflections 1‐4 in tracing c have counterparts in adjacent tracings that have larger amplitude and dV/dt, thus these deflections are remote in tracing c. Deflection 5 is, however, also local because its amplitude and dV/dt are largest in tracing c. Stim., stimulus artifact; electrode diameter: 0.1 mm; inter‐electrode distance: 0.2 mm; reference electrode: at border tissue bath; filter setting: 0.01‐1 kHz
6. DETERMINING TARGET SITES
6.1. Activation maps
Activation sequence mapping is frequently applied to detect the target site for ablation. This is done during tachycardia to assess a temporal sequence of activation. Electrograms are recorded sequentially or simultaneously at a large number of sites. From the recorded electrograms, the activation map during tachycardia is determined. For this purpose, activation times have to be determined. As outlined, the point of steepest down stroke in the unipolar electrogram marks the local activation time. This corresponds with the peak negative or peak positive point in the bipolar electrogram. In several studies the onset of the bipolar electrogram has been used to select the activation time. This is, however, not correct because as with the unipolar electrogram, the first and last part of the bipolar electrogram are remote (see Figure 1). In healthy myocardium, this selection of activation time may, although wrong, not be detrimental, but in diseased myocardium activation maps based on such activation times may result in erroneous conclusions. The activation map constructed from activation times based on the onset of the bipolar electrograms may for instance suggest a re‐entry mechanism, whereas in fact two colliding wave fronts are present as illustrated in Figure 8.
Figure 8.
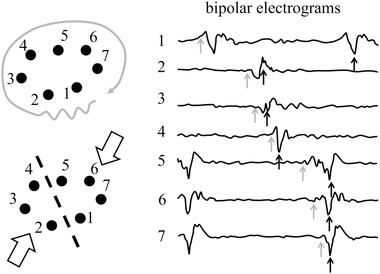
Right panel: Bipolar electrograms recorded at seven sites during an atrial arrhythmia. The position of the electrodes is indicated by the black dots in the left panels. Gray arrows indicate “activation times” if the onset of the bipolar deflections is (erroneously) chosen as activation time. The corresponding activation pattern at the left suggests re‐entrant activation (left upper panel). Black arrows indicate activation times if the largest positive or negative deflection is chosen as activation time. Now the activation map shows that recordings are made at a site where two activation fronts collide (left lower panel). Electrode size: 1 mm; inter‐electrode distance: 4 mm; reference electrode: WCT; filter setting: 30‐500 Hz
Determining activation times from unipolar recordings is not always without difficulties, because of deflections caused by remote activity. Complex filtering and statistical approaches have been proposed to enhance the accuracy of activation times.17, 18 The filtering study by Cabo et al suggests that inclusion of frequencies between 500 and 1000 Hz improves detection performance.17 The statistical approaches suggest that incorporating multiple features (voltage, first and second derivative, and ratio) of unipolar deflections enhance prediction of activation time of complex fractionated electrograms.
Other investigators optimized detection of local activation by looking for the maximal unipolar negative dV/dt within a window demarcated by the beginning and the end of the bipolar complex.19 This prevents detection of activation times in deflections that are remote in the unipolar electrogram. These authors also tested the onset of the bipolar electrogram and the moment when the cumulative area of the signal reached 50% of the total area between begin and end of the signal. They indicated that these parameters have a less solid physiological meaning.
Because induction of tachycardia can be problematic due to hemodynamic intolerance of the rhythm, Child et al suggested another method to determine the origin of re‐entry tachycardias.20 Their method assesses localized regions of high susceptibility to conduction block and re‐entry. The method requires unipolar electrograms, because both activation and repolarization at multiple sites have to be determined. A re‐entry vulnerability index is determined at all recording sites that is related to the difference between repolarization and activation times for adjacent sites. The lower the index value, the higher the vulnerability for re‐entry.
6.2. High resolution multi‐channel systems
Some systems for electrophysiological mapping that are available today allow automatic collection of electrograms and construction of activation patterns and scar maps containing thousands of points. The Rhythmia system for instance uses a 64 pole steerable basket catheter that enables continuous collection of electrograms, while the basket is in contact with the surface of the heart. Splines contain low noise electrode terminals at distances of 2 mm, allowing the automated construction of high density maps. Only electrograms that are close to the shell of the cardiac chamber are used, which results in data for contact maps. Automatic acceptance or rejection of signals is based on stability and phase of the respiratory cycle. The large number of electrograms that is detected within a short time allows the construction of multiple electrical maps to assess reliability of early activated sites and lines of block. Although annotation of activation times is made after comparison with neighboring electrograms, manual adaptation is still possible. The system records unipolar electrograms, while bipolar electrograms are calculated. A special algorithm (lumipoint) allows the selection of a window of interest to identify late potentials, regions that exhibit discontinuous activation and detection of critical isthmus sites and abnormal potentials.21 The lumipoint algorithm displays an activation histogram over the entire tachycardia cycle length with a normalized score. Takigawa and collaborators showed that the algorithm can be used to identify reentrant versus focal atrial tachycardia.22
6.3. Decrement evoked potentials
Decrement evoked potential mapping is another technique that has recently been developed to determine critical sites of VT circuits without induction of tachycardia. This technique uses decremental extrastimuli to identify isolated near‐field potentials.23 If late potentials or fractionated potentials are identified during mapping, a pacing train with decremental extrastimuli is applied. If the local potential on the mapping catheter delays, this is annotated as a decrement evoked potential (DEEP). A minimum delay of 10 ms is required for an electrogram to be categorized as DEEP.23 The investigators found that potentials with decremental properties within scar or at scar border zones are able to detect targets for ablation that are more likely to participate in VT circuits than targets determined during conventional substrate mapping. Application of DEEP mapping identified diastolic pathways with greater specificity than mapping of late potentials. In a prospective multicenter study, the viability of the decrement evoked potential approach to identify functional substrate modification for VT therapy has been confirmed.24
6.4. Bipolar electrogram morphology to assess ablation sites
Although the unipolar electrogram is physically sound to assess activation time, its detection can be bothersome in some cases. The area revealing a QS morphology in the unipolar electrogram may be too large or remote deflections may hamper an accurate estimation. Therefore, some investigators suggested the use of bipolar electrogram morphology to pin‐point the origin. Van Huls van Taxis et al used reversed polarity of adjacent bipolar electrograms as a criterion to target the ablation site of idiopathic right ventricular outflow tract arrhythmias.25 Four electrode terminals (1‐4) of a catheter were used and bipolar recordings were made between each successive pair, yielding three bipolar electrograms. In fact this concept uses the direction dependence of the bipolar electrogram. In case the focus is located between terminals 2 and 3, the direction of the activation fronts running to the most distal electrode (3‐4) is opposite to the direction of the front toward the most proximal pair (1‐2) and these signals are inverse. In case the focus is not between terminals 2 and 3, signals will deflect in the same direction. This method is, however, not applicable to determine exit sites of infarct related ventricular tachycardias.
Sorgente et al used the negative concordance between the unipolar and bipolar electrogram as an additional criterion to localize the site of origin of focal ventricular arrhythmias.26 If a catheter is close to the origin of activation, unipolar electrograms at both the proximal and distal pole are virtually negative. Possible R‐waves due to the approaching front will be very small because of the close proximity of the terminals to the origin. If the tip (distal pole) electrode of the catheter is located at the origin, the bipolar electrogram (proximal subtracted from the distal) will be negative, because the negative deflection caused by activation at the distal electrode occurred earlier than activation at the proximal electrode. Thus both the unipolar and bipolar electrograms are negative (concordant). If the proximal electrode is, however, over the origin, the negative deflection at the proximal pole occurs earlier than the negative deflection at the distal pole and the bipolar electrogram becomes positive. Thus, in case the tip is not located at the origin, the unipolar and bipolar electrograms are discordant.
6.5. Epicardial or endocardial origin of activation
To optimize ablation, it is important to know whether the origin of a tachycardia arises at (or near) the epicardium or endocardium. Several experimental studies show that small R‐waves arise in the unipolar electrogram at the endocardium if activation originates at the epicardium and the other way around. Figure 9 shows electrograms recorded with a needle electrode inserted in the left ventricular wall in case stimulation is performed at the epicardium of a Langendorff‐perfused porcine heart. Note that at the epicardium, the electrogram is initially negative; no R‐wave is present. For recording sites toward the endocardium, a tiny R‐wave is present that tends to increase in amplitude the further the electrode is away from the epicardium.
Figure 9.
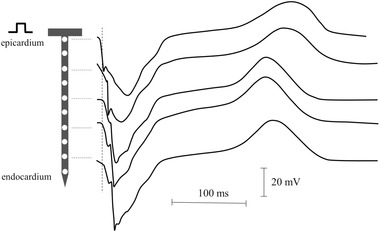
Needle electrode recordings during stimulation from the epicardium. The electrode was impaled in the left ventricle of a pig heart. Note the initial negative deflection of the unipolar electrogram at the epicardial site. Activation moves to the endocardium, which is reflected by the increase in activation time. For electrograms recorded toward the endocardium, a small R‐wave arises of which the amplitude increases slightly the further the recording site is away from the epicardium
The site beneath the epicardium activation arises has a great effect on the epicardial activation pattern, as illustrated in Figure 10. An 8 × 8 grid electrode was positioned on the epicardium of a Langendorff‐perfused pig heart. A needle electrode with 10 terminals was impaled perpendicular to the ventricular wall in the center of the grid. Panel (A) shows the isochronal epicardial map during stimulation from the upper electrode terminal of the needle, which was at the epicardium. The isochronal pattern is elliptically shaped (anisotropic) showing fast conduction in the direction perpendicular to the left anterior descending artery (LAD). At the epicardium, the fiber direction is perpendicular to the LAD. Panel (B) shows the isochronal map if stimulation was performed at the terminal of the needle electrode that was located at the endocardium. This pattern is more circular (isotropic) due to rotation of the fiber direction through the ventricular wall.
Figure 10.
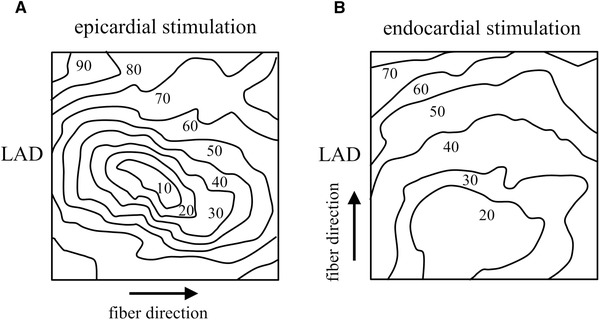
Epicardial isochronal pattern after epicardial (panel A) and endocardial (panel B) stimulation from a needle electrode in the center of the grid electrode. Electrograms were recorded with an 11 × 11 grid electrode. The map obtained during epicardial stimulation has a clear anisotropic configuration, whereas the map after endocardial stimulation is virtually isotropic. Electrode diameter: 1 mm; inter‐electrode distance: 5 mm; reference electrode: aortic root; filter setting: 0‐400 Hz
6.6. Early epicardial activation; breakthrough or focal activation
In case the origin of activation is at the epicardium, the epicardial activation map is centrifugal, although activation block in one or more directions may occur in case of cardiac disease. The unipolar electrogram at the earliest activated site is negative. During atrial fibrillation (AF), spread of epicardial activation is often centrifugal. De Groot et al showed, however, that in most cases an initial R‐wave is present, indicative for activation running toward the epicardium.27 Such observations emphasize that a centrifugal activation pattern is not sufficient for determining the site of onset of activation.
6.7. Role of epicardial fat on electrogram amplitude
Epicardial fat increases the distance between the site of activation and the recording electrode and affects electrogram morphology. The group of Zeppenfeld determined unipolar and bipolar electrogram amplitude in patients undergoing epi‐ and endocardial mapping.28 Epicardial fat was assessed by computed tomography. Sixty‐four percent of the epicardial surface of the patient's heart was covered with fat, of which the thickness was >4 mm in 25% of the adipose area. The bipolar voltage decreased in amplitude from 2.5 mV (no fat) to 0.9 mV (>4 mm fat). In contrast, unipolar amplitudes were not influenced by the epicardial fat. The authors attribute this difference to the larger field of view of the unipolar recording technique. They concluded that because epicardial fat attenuates bipolar voltage amplitude it may prevent accurate delineation of scar if bipolar voltage mapping is applied.
7. SUBSTRATE MAPPING
Substrate mapping is used to detect areas with infarction or scarring and to delineate possible pathways for re‐entry. This kind of mapping is done during sinus rhythm or pacing. Advantage of the procedure is that it can be carried out in patients in whom mapping during tachycardia is not feasible because of hemodynamic intolerance due to the frequency of the (induced) arrhythmia. Amplitude of electrograms is reduced due to structural changes or zones of activation block, decreasing the number of activated cells. Animal studies have been performed to validate the method and to assess cutoff values. Bipolar voltage criteria of 1.5 to 2.0 mV are used to distinguish healthy from impaired myocardium. Bipolar voltage amplitudes of 0.5‐1.5 mV point to diseased myocardium, <.5 mV to dense scar myocardium, 0.5 to <1.5 mV to border zone tissue and to healthy myocardium if the amplitude is >1.5 mV. The area with diseased myocardium is usually larger than that determined with gadolinium enhanced MRI, which is caused by the lower sensitivity of the noninvasive technique. Glashan et al have shown that in non‐ischemic cardiomyopathy a voltage cut‐off value to assess fibrosis is not without problems and that knowledge of wall thickness is required.29
7.1. Fractionation and late potentials
As described before, fractionated electrograms point to asynchronous activation in areas where myocardial fibers intermingle with zones consisting of in‐excitable tissue such as collagen, adipose, or ischemic myocardium. In addition, heterogeneous expression of connexins has been shown to give rise to fractionation of electrograms.30 To assess whether endo‐epicardial asynchrony contributes to electrogram fractionation, van der Does et al carried out epicardial mapping during sinus rhythm in patients undergoing cardiac surgery.31 Activation time, electrogram amplitude, RS ratio, and fractionation were assessed in the right atrial free wall of patients operated for coronary artery disease or valvular heart disease. The authors showed that during sinus rhythm, epi‐endo differences in electrogram fractionation occurred. Endo‐epicardial asynchrony was the origin of fractionation in 4% of the fractionated electrograms, but they suggested that this percentage will increase during arrhythmias.
Late potentials, deflections of myocardial activity arising after the end of the surface ECG and separated from the main local deflection by an isoelectric interval, also point to impaired myocardial tissue. They are caused by delayed activation in compromised areas and may play a role in arrhythmogenicity. Fractionated and late potentials are preferentially recorded in bipolar mode because they may be obscured by remote deflections in electrograms recorded in unipolar mode.
Although late potentials may clearly be seen after QRS, they may not always show increased delay after premature stimulation. Such areas are less likely to contribute to reentry then those that reveal decremental conduction. As shown before, such areas can be determined with DEEP mapping. It has been shown that regions with great decrement are associated with the sites of exit of re‐entrant tachycardia. Decrement stimulation has the additional advantage that late potentials that are masked by the ECG or far field electrograms may become visible because of the increase in delay.
7.2. Complex fractionated atrial electrograms as marker for the substrate of AF
Bipolar, complex fractionated atrial electrograms (CFAEs) are frequently used to detect abnormal areas in the atria of patients with AF. A frequently used marker of CFAEs is the width of the interval of the fractionated deflection. As mentioned before, a problem of the bipolar electrograms is that fractionation is dependent on the direction of the activation front. However, also for unipolar electrograms the amount of fractionation depends on the direction of the activation front. In areas where collagen fibers and myocardial fibers intermingle conduction may be asynchronous, especially if conduction is perpendicular to the fiber direction. Because of the field of view of electrodes multiple deflections will be recorded that are out of phase, which increases the interval in which deflections are present. If, however, activation in the same structure is synchronous in the various fibers, which may be the case if conduction is in the fiber direction, less fractionation will be present.
Although CFAEs are used to select areas for ablation, several studies have demonstrated that they are frequently passive phenomena and not critical for the perpetuation of AF.32 The group of Schotten recorded unipolar electrograms during AF in the left atrium of 20 patients with persistent or paroxysmal fibrillation.33 CFAEs were assessed with five semi‐automated bipolar CFAE algorithms. All CFAE assessments showed poor correlation with the complexity of the substrate. The latter was determined from conduction velocity, number of waves/breakthroughs, and electrical dissociation. In addition, CFAEs poorly correlated with fractionation index of unipolar electrograms. The previous index, however, was highly related with AF substrate complexity and a good marker for conduction block. Sohal and collaborators suggested that wide field mapping using electrodes with multiple splines, which allows simultaneous acquisition of activation over large area, would be preferential.34
7.3. Entropy analysis
Atrial electrograms during complex activation are frequently disturbed by spikes or missing samples due to unstable positioning or poor contact of electrodes. These artifacts will affect the reliability of the CFAE and non‐CFAE segmentation. Although processing techniques are available to reduce spikes, this option is often not applicable because of similarity with electrogram features. To circumvent the effect of these artifacts, sample entropy has been applied successfully.35 This procedure determines regularity of a time series by computing the negative logarithm of the conditional probability that two sequences, which are similar for a number of points, remain similar for the next point at a dissimilarity level below a certain threshold value.36 Cirugeda‐Roldán and co‐workers used this method for the analysis of noisy atrial electrograms. Electrograms free of noise and artifacts were selected by an expert and used to distinguish between CFAE and non‐CFEA electrograms. For this purpose non‐CFAE signals were created from the selected noise‐free electrograms by adding artifacts. Their results verified that sample entropy is able to distinguish between CFAE and non‐CFAE recordings even at 50% missing data or 10% of spikes.
Shannon's entropy and the Kolmogorov‐Smirnov test are similar statistical measures of complexity of a signal. These techniques evaluate the distribution of signal values within the signal histogram and provides a guide to information content. They have been successfully applied to data from AF patients in whom classification of CFAE, non CFAE, and not interpretable segments of electrograms were classified according to Nademanee criteria by four reviewers.37 Both techniques resulted in a high sensitivity and specificity in classifying uninterpretable electrograms from all other electrograms. Shannon's entropy was able to distinguish CFAE from non‐CFAE without the need for user input for threshold levels as is the case for fractional intervals.
Shannon's entropy has also been applied to differentiate bipolar electrograms recorded at pivot points of rotors from electrograms detected at peripheral regions. Pivot points are critical for rotors to maintain AF. It is expected that pivot points broaden the amplitude distribution of bipolar electrograms. Because Shannon's entropy is a measure of signal amplitude distribution, differentiation between electrograms at pivot and peripheral sites was expected to be possible. Genesan et al studied this technique in a computer model, in vitro and in vivo (AF patients).38 Their data suggest that Shannon's entropy is an objective tool to assist mapping of locally stable rotors.
8. TECHNICAL ASPECTS THAT INFLUENCE ELECTROGRAM MORPHOLOGY
8.1. Electrode size
Electrode diameter affects electrogram characteristics.39 Although the morphology of the electrograms remains similar, steepness of the signal is greater for smaller diameters of the recording electrode. This effect of the electrode diameter on the electrogram is caused by the fact that activation requires time to pass the electrode. Because the recorded signal is the mean of all activity underneath the electrode, the electrogram will widen if the electrode size increases. Although this suggests that a small diameter is preferable, one should realize that electrode noise increases with decreasing electrode diameter.40 In addition, the ionic bilayer at the electrode‐tissue interface is disturbed easily if the surface is small, which results in a greater sensitivity to movement artifacts for small diameter electrodes.
8.2. Electrogram fractionation
Jacquemet and co‐workers showed in a computer model that fractionation varied with the density of collagen strands and that fractionation increased with larger electrodes.41 Zlochiver et al demonstrated that fractionated electrograms also arise at the junction between organized rotors and surrounding tissue, where wave break results in variable conduction patterns.42
Correa de Sa et al investigated the relation between spatiotemporal variation of tissue excitation and electrode spatial resolution.43 Extracellular electrograms were calculated in a computer model of activation in a 2D sheet of excitable tissue. Temporal and spatiotemporal complexity of activation was varied and electrograms were calculated for electrodes with different length (2‐8 mm), diameter (1‐4 mm), height (0.5‐3 mm), and configuration (bipolar; inter‐electrode spacing (1‐7 mm). In simulations with tissue exhibiting temporal variation, fractionation increased with the frequency of activation (stimulation), but was independent of electrode spacing. The number of deflections (fractionation) in unipolar electrograms recorded in tissue with spatiotemporal variation (in which six lines of scar were present) increased with electrode length, diameter and height. In bipolar electrograms fractionation increased with inter‐electrode spacing.
These investigators also determined how fractionation varies with inter‐electrode spacing of various catheters in patients with AF. Results of these experiments also showed an increase in fractionation with increased spacing. Their observations are in accordance with basic concepts of bipolar electrograms, in which the number of deflections increases with the distance between the poles.
Because these electrode variables affect electrograms, Josephson and Anter suggested a standardization of recording techniques that included electrode size, inter‐electrode spacing, tissue contact, and catheter orientation.44 Iso et al showed that wave front direction and cycle length affect atrial electrogram amplitude, which, as they indicated, at least partly explains the voltage discordance during SR and AF.45
8.3. Filtering and sample frequency
Filtering and sampling frequency also influence electrogram characteristics. Filtering may attenuate respiration and movement artifacts as well as interference and far‐field components, but it affects electrogram morphology.46 Especially high‐order filters (sharp filters) that attenuate certain frequencies more steeply, may disturb morphology importantly, such as notch (60 Hz) filters. The problem with such filters is that they are prone to ringing and may generate artificial deflections in an electrogram.
8.4. Contact force and catheter position
Morphology of the electrogram and ablation size is changed by the force the catheter exerts on myocardial tissue.47 Contact pressure results in injury current that gives rise to a monophasic deflection (ST‐segment elevation), but pressure may also affect configuration and number of deflections. In addition, contact force will affect CFAE score. Electrogram parameters and initial impedance are poor predictors of contact force for radiofrequency ablation. An optimal contact force during electrogram mapping of ≥10 g is suggested, as below this value contact force has a significant influence on the electrogram and CFAE scores.
For bipolar recordings, the position of the catheter with regard to the heart wall is of importance for electrogram morphology. A catheter is usually positioned parallel to the heart wall. However, its position is not always clear and the catheter can make an angle with the wall, which will change the morphology of recorded signals. In case the catheter is parallel to the wall the bipolar electrogram recorded between the tip and the first ring electrode reveals a real bipolar electrogram, because both electrode terminals touch the ventricular wall. If the catheter is, however, perpendicular to the wall, only the tip touches the wall, the ring electrode is located in the cavity. Therefore, the ring does not record a local signal but a remote one (the cavity potential). The cavity potential is lower in amplitude and less steep than the local deflection at the tip, so that subtraction of the two results in an electrogram that tends to the unipolar morphology as recorded at the tip electrode. Filter setting will have an additional effect on electrogram morphology. The resulting bipolar electrogram virtually has a unipolar configuration. An example is illustrated in Figure 11 where endocardial electrograms of the atrium were recorded during AF with the catheter positioned along and perpendicular to the atrial wall. For both situations the unipolar and bipolar electrograms are shown together with lead II. Note that in case the catheter is parallel to the wall, the bipolar electrogram mimics the first derivative of the unipolar one, whereas in case the catheter is perpendicular to the wall, the bipolar electrogram is similar to the unipolar one. The bipolar electrogram recorded with the electrode positioned parallel to the ventricular wall shows a rotation of the amplitude from negative to positive, indicating that the wave front is changing direction.
Figure 11.
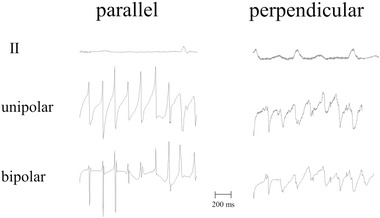
Example of unipolar and bipolar recordings of a catheter with the electrode positioned parallel (left tracing) and perpendicular to the atrial wall. Recordings were made during atrial fibrillation; the bipolar tracing is the difference between the tip and the first ring electrode. If the catheter is positioned along the wall, both terminals touch the tissue and the recording is a pure bipolar one. If the catheter is, however, positioned perpendicular to the wall, only the tip touches the wall. The ring electrode records a cavity signal, which is a remote unipolar one and lower in amplitude than the tip signal. The resulting signal approaches a unipolar recording; unipolar and bipolar electrograms are similar now. Electrode size: 2 mm × 1 mm; inter‐electrode distance: 2 mm; reference electrode: vena cava inferior; filter setting: 0.5‐200 Hz
9. CONCLUSIONS
The optimal recording procedure to assess activation times uses both unipolar and bipolar electrograms that are recorded simultaneously. The bipolar electrogram helps to detect the local component(s) in the electrogram, whereas deflections of the unipolar electrogram that correspond with the bipolar deflection(s) are preferable used to determine activation times. In addition, the morphology of unipolar electrograms can be used to select the origin of (focal) activation and/or sites with tissue discontinuity. Several aspects of electrogram recording such as filtering, contact force and catheter position should be considered with care when analyzing electrograms.
Detecting target sites for ablation with voltage mapping and/or fractionation of electrograms also have a number of pitfalls. Recording bipolar electrograms looks attractive because of the local character of this recording mode, but their amplitude is dependent on the direction of the activation front, which affects the potential map. On the other hand, unipolar recordings suffer from the fact that remote deflections that are present will affect the voltage map. However, several techniques are available to circumvent the disadvantages of the unipolar and bipolar recording technique and to avoid pitfalls associated with determination of CFAEs. In addition, high resolution automated mapping and electrogram annotating systems can be helpful to select appropriate target sites for ablation.
de Bakker JM. Electrogram recording and analyzing techniques to optimize selection of target sites for ablation of cardiac arrhythmias. Pacing Clin Electrophysiol. 2019;42:1503–1516. 10.1111/pace.13817
REFERENCES
- 1. Nakagawa H, Shah N, Matsudaira K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal” ablation. Circulation. 2001;103:699‐709. [DOI] [PubMed] [Google Scholar]
- 2. Laţcu DG, Bun SS, Casado Arroyo R, et al. Scar identification, quantification, and characterization in complex atrial tachycardia: A path to targeted ablation? Europace. 2019;21(Supplement_1):i21‐i26. [DOI] [PubMed] [Google Scholar]
- 3. Spach MS, Miller WT, III , Miller‐Jones E, Warren RB, Barr RC. Extracellular potentials related to intracellular action potentials during impulse conduction in anisotropic canine cardiac muscle. Circ Res. 1979;45:188‐204. [DOI] [PubMed] [Google Scholar]
- 4. Podziemski P, Kuklik P, van Hunnik A, Zeemering S, Maesen B, Schotten U. Far‐field effect in unipolar electrograms revisited: high‐density mapping of atrial fibrillation in humans. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:5680‐5683. [DOI] [PubMed] [Google Scholar]
- 5. Fast VG, Kleber AG. Cardiac tissue geometry as a determinant of unidirectional conduction block: Assessment of microscopic excitation spread by optical mapping in patterned cell cultures and in a computer model. Cardiovasc Res. 1995;29:697‐707. [PubMed] [Google Scholar]
- 6. Haïssaguerre M, Dartigues JF, Warin JF, Le Metayer P, Montserrat P, Salamon R. Electrogram patterns predictive of successful catheter ablation of accessory pathways. Value of unipolar recording mode. Circulation. 1991;84:188‐202. [DOI] [PubMed] [Google Scholar]
- 7. de Bakker JM, van Capelle FJ, Janse MJ, et al. Reentry as a cause of ventricular tachycardia in patients with chronic ischemic heart disease: Electrophysiologic and anatomic correlation. Circulation. 1988;77:589‐606. [DOI] [PubMed] [Google Scholar]
- 8. Ito S, Tada H, Naito S, et al. Randomized comparison of bipolar vs unipolar plus bipolar recordings during atrioventricular junction ablation: Importance and efficacy of unipolar recording. Circ J. 2007;71:874‐879. [DOI] [PubMed] [Google Scholar]
- 9. Deno DC, Balachandran R, Morgan D, Ahmad F, Masse S, Nanthakumar K. Orientation‐independent catheter‐based characterization of myocardial activation. IEEE Trans Biomed Eng. 2017;64:1067‐1077. [DOI] [PubMed] [Google Scholar]
- 10. Haldar SK, Magtibay K, Porta‐Sanchez A, et al. Resolving bipolar electrogram voltages during atrial fibrillation unsing omnipolar mapping. Circ Arrhythm Electrophysiol. 2017;10:e005018. [DOI] [PubMed] [Google Scholar]
- 11. Massé S, Magtibay K, Jackson N, et al. Resolving myocardial activation with novel omnipolar electrograms. Circ Arrhythm Electrophysiol. 2016;9:e004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coronel R, Wilms‐Schopman FJ, de Groot JR, Janse MJ, van Capelle FJ, de Bakker JM. Laplacian electrograms and the interpretation of complex ventricular activation patterns during ventricular fibrillation. J Cardiovasc Electrophysiol. 2000;11:1119‐1128. [DOI] [PubMed] [Google Scholar]
- 13. de Bakker JM, Wittkampf FH. The pathophysiologic basis of fractionated and complex electrograms and the impact of recording techniques on their detection and interpretation. Circ Arrhythm Electrophysiol. 2010;3:204‐213. [DOI] [PubMed] [Google Scholar]
- 14. Ota M, Kaneko Y, Nakajima T, et al. Detection of sequential activation of left atrium and coronary sinus musculature in the general population. J Arrhythm. 2016;32:449‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gardner PI, Ursell PC, Fenoglio JJ, Jr , Wit AL. Electrophysiologic and anatomic basis for fractionated electrograms recorded from healed myocardial infarcts. Circulation. 1985;72:596‐611. [DOI] [PubMed] [Google Scholar]
- 16. de Bakker JM, van Capelle FJ, Janse MJ, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915‐926. [DOI] [PubMed] [Google Scholar]
- 17. Cabo C, Wharton JM, Wolf PD, Ideker RE, Smith WM. Activation in unipolar cardiac electrograms: A frequency analysis. IEEE Trans Biomed Eng. 1990;37:500‐508. [DOI] [PubMed] [Google Scholar]
- 18. Anderson KP, Walker R, Ershler PR, et al. Determination of local myocardial electrical activation for activation sequence mapping. A statistical approach. Circ Res. 1991;69:898‐917. [DOI] [PubMed] [Google Scholar]
- 19. El Haddad M, Houben R, Stroobandt R, Van Heuverswyn F, Tavernier R, Duytschaever M. Novel algorithmic methods in mapping of atrial and ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014;7:463‐472. [DOI] [PubMed] [Google Scholar]
- 20. Child N, Bishop MJ, Hanson B, et al. An activation‐repolarization time metric to predict localized regions of high susceptibility to reentry. Heart Rhythm. 2015;12:1644‐1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin CA, Takigawa M, Martin R, et al. Use of novel electrogram “Lumipoint” algorithm to detect critical isthmus and abnormal potentials for ablation in ventricular tachycardia. JACC Clin Electrophysiol. 2019;5:470‐479. [DOI] [PubMed] [Google Scholar]
- 22. Takigawa M, Martin CA, Derval N, et al. Insights from atrial surface activation throughout atrial tachycardia cycle length: A new mapping tool. Heart Rhythm. 2019;2019:S1547‐5271. [DOI] [PubMed] [Google Scholar]
- 23. Jackson N, Gizurarson S, Viswanathan K, et al. Decrement evoked potential mapping: Basis of a mechanistic strategy for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2015;8:1433‐1442. [DOI] [PubMed] [Google Scholar]
- 24. Porta‐Sánchez A, Jackson N, Lukac P, et al. Multicenter study of ischemic ventricular tachycardia ablation with decrement‐evoked potential (DEEP) mapping with extra stimulus. JACC Clin Electrophysiol. 2018;4:307‐315. [DOI] [PubMed] [Google Scholar]
- 25. van Huls van Taxis CF, Wijnmaalen AP, den Uijl DW, et al. Reversed polarity of bipolar electrograms for predicting a successful ablation site in focal idiopathic right ventricular outflow tract arrhythmias. Heart Rhythm. 2011;8:665‐671. [DOI] [PubMed] [Google Scholar]
- 26. Sorgente A, Epicoco G, Ali H, et al. Negative concordance pattern in bipolar and unipolar recordings: An additional mapping criterion to localize the site of origin of focal ventricular arrhythmias. Heart Rhythm. 2016;13:519‐526. [DOI] [PubMed] [Google Scholar]
- 27. de Groot NM, Houben RP, Smeets JL, et al. Electropathological substrate of longstanding persistent atrial fibrillation in patients with structural heart disease: Epicardial breakthrough. Circulation. 2010;122:1674‐1682. [DOI] [PubMed] [Google Scholar]
- 28. van Huls van Taxis CF, Wijnmaalen AP, Piers SR, van der Geest RJ, Schalij MJ, Zeppenfeld K. Real‐time integration of MDCT‐derived coronary anatomy and epicardial fat: Impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc Imaging. 2013;6:42‐52. [DOI] [PubMed] [Google Scholar]
- 29. Glashan CA, Androulakis AFA, Tao Q, et al. Whole human heart histology to validate electroanatomical voltage mapping in patients with non‐ischaemic cardiomyopathy and ventricular tachycardia. Eur Heart J. 2018;39:2867‐2875. [DOI] [PubMed] [Google Scholar]
- 30. Prudat Y, Kucera JP. Nonlinear behaviour of conduction and block in cardiac tissue with heterogeneous expression of connexin 43. J Mol Cell Cardiol. 2014;76:46‐54. [DOI] [PubMed] [Google Scholar]
- 31. van der Does LJME, Knops P, Teuwen CP, et al. Unipolar atrial electrogram morphology from an epicardial and endocardial perspective. Heart Rhythm. 2018;15:879‐887. [DOI] [PubMed] [Google Scholar]
- 32. Rostock T, Rotter M, Sanders P, et al. High‐density activation mapping of fractionated electrograms in the atria of patients with paroxysmal atrial fibrillation. Heart Rhythm. 2006;3:27‐34. [DOI] [PubMed] [Google Scholar]
- 33. Lau DH, Maesen B, Zeemering S, et al. Indices of bipolar complex fractionated atrial electrograms correlate poorly with each other and atrial fibrillation substrate complexity. Heart Rhythm. 2015;12:1415‐1423. [DOI] [PubMed] [Google Scholar]
- 34. Sohal M, Choudhury R, Taghji P, et al. Is mapping of complex fractionated electrograms obsolete? Arrhythm Electrophysiol Rev. 2015;4:109‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cirugeda‐Roldán EM, Molina Picó A, Novák D, Cuesta‐Frau D, Kremen V. Sample entropy analysis of noisy atrial electrograms during atrial fibrillation. Comput Math Methods Med. 2018;2018:1874651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cirugeda–Roldán EM, Novak D, Kremen V, et al. Characterization of complex fractionated atrial electrograms by sample entropy: An international multi‐center study. Entropy. 2015;17:7493‐7509. [Google Scholar]
- 37. Ng J, Borodyanskiy AI, Chang ET, et al. Measuring the complexity of atrial fibrillation electrograms. J Cardiovasc Electrophysiol. 2010;21:649‐655. [DOI] [PubMed] [Google Scholar]
- 38. Ganesan AN, Kuklik P, Lau DH, et al. Bipolar electrogram Shannon entropy at sites of rotational activation: Implications for ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:48‐57. [DOI] [PubMed] [Google Scholar]
- 39. Wiley JJ, Ideker RE, Smith WM, Pollard AE. Measuring surface potential components necessary for transmembrane current computation using microfabricated arrays. Am J Physiol Heart Circ Physiol. 2005;289:H2468‐2477. [DOI] [PubMed] [Google Scholar]
- 40. Rocha PR, Schlett P, Kintzel U, et al. Electrochemical noise and impedance of au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci Adv. 2016;2:e1600516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jacquemet V, Henriquez CS. Genesis of complex fractionated atrial electrograms in zones of slow conduction: A computer model of microfibrosis. Heart Rhythm. 2009;6:803‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Correa de Sa DD, Thompson N, Stinnett‐Donnelly J, et al. Electrogram fractionation: The relationship between spatiotemporal variation of tissue excitation and electrode spatial resolution. Circ Arrhythm Electrophysiol. 2011;4:909‐916. [DOI] [PubMed] [Google Scholar]
- 44. Josephson ME, Anter E. Substrate mapping for ventricular tachycardia: Assumptions and misconceptions. JACC Clin Electrophysiol. 2015;1:341‐352. [DOI] [PubMed] [Google Scholar]
- 45. Iso K, Watanabe I, Kogawa R, et al. Wave front direction and cycle length affect left atrial electrogram amplitude. J Arrhythm. 2017;33:269‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stevenson WG, Soejima K. Recording techniques for clinical electrophysiology. J Cardiovasc Electrophysiol. 2005;16:1017‐1022. [DOI] [PubMed] [Google Scholar]
- 47. Ikeda A, Nakagawa H, Lambert H, et al. Relationship between catheter contact force and radiofrequency lesion size and incidence of steam pop in the beating canine heart: Electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode‐tissue contact force and lesion size. Circ Arrhythm Electrophysiol. 2014;7:1174‐1180. [DOI] [PubMed] [Google Scholar]


