Abstract
Excessive use of cocaine is known to induce changes in brain white and gray matter. It is unknown whether the extent of these changes is related to individual differences in vulnerability to cocaine addiction. One factor increasing vulnerability involves reduced expression of the serotonin transporter (5‐HTT). Human studies have shown that inherited 5‐HTT downregulation is associated with structural changes in the brain. These genotype‐related structural changes may contribute to risk for cocaine addiction. Here, we tested this idea by using ultrahigh‐resolution structural magnetic resonance imaging (MRI) on postmortem tissue of 5‐HTT−/− and wild‐type (5‐HTT+/+) rats with a history of long access to cocaine or sucrose (control) self‐administration. We found that 5‐HTT−/− rats, compared with wild‐type control animals, self‐administered more cocaine, but not sucrose, under long‐access conditions. Ultrahigh‐resolution structural MRI subsequently revealed that, independent of sucrose or cocaine self‐administration, 5‐HTT−/− rats had a smaller amygdala. Moreover, we found an interaction between genotype and type of reward for dorsal raphe nucleus volume. The data point to an important but differential role of the amygdala and dorsal raphe nucleus in 5‐HTT genotype–dependent vulnerability to cocaine addiction.
Keywords: cocaine self‐administration, serotonin transporter knockout rat, structural MRI
Individuals characterized by inherited serotonin‐transporter down regulation are at risk for cocaine addiction. However, the underlying mechanisms are unknown. Here, we subjected serotonin‐transporter knockout (5‐HTT−/−) and wild‐type rats to ultra high‐resolution MRI after extended access cocaine or sucrose self‐administration and found that increased cocaine intake was associated with reduced amygdala and dorsal‐raphe volume.

1. INTRODUCTION
Cocaine use remains a pressing health and societal concern. According to the “European Drug Report 2017” of the European Monitoring Centre for Drugs and Drug Addiction, 17.5 million Europeans (5.2%) have taken cocaine in their lifetime, making it the second most used illicit drug of abuse in Europe after cannabis.1 Fundamental understanding of cocaine addiction–related psychopathology is essential for improvements in the management of the disorder. It has been well established that heavy cocaine use can lead to changes in brain gray2, 3 and white matter (WM).4, 5 There are, however, substantial individual differences in these structural changes, to which various factors contribute, including predisposition.6 One factor shaping predisposition involves the short (s)‐allele of the serotonin transporter (5‐HTT)–linked polymorphic region (5‐HTTLPR).7 This polymorphism is particularly known for its association with affective disorders like depression8 but has also been linked to psychostimulant addiction.9, 10, 11 The 5‐HTTLPR s‐allele goes along with lower levels of 5‐HTT messenger RNA (mRNA) transcription,12 implying that reduced 5‐HTT expression and possibly increased synaptic levels of serotonin (5‐HT) have downstream effects on the brain that contribute to the predisposition.
Of interest, structural and functional magnetic resonance imaging (MRI) studies, which bring the advantage of brain‐wide analyses, revealed that 5‐HTTLPR s‐allele carriers display smaller gray matter (GM) volumes in the amygdala (AMY),13, 14, 15 hippocampus (Hip),13, 16, 17 orbital frontal cortex (OFC),17, 18 anterior cingulate cortex (ACC),19, 20 and insula21 compared with homozygous long (l)‐allele carriers. Additionally, diffusion tensor imaging (DTI) measurements have revealed reduced fractional anisotropy (FA), pointing towards reduced fiber density, reduced axonal diameter, or reduced myelination,22 in the uncinate fasciculus23 of healthy s‐allele carriers. Interestingly, many of these brain regions that differ between s‐ and l‐allele carriers have also been implicated in addiction, suggesting that some of the brain regions mentioned above may contribute to risk for psychostimulant addiction in s‐allele carriers.24
To investigate this further and keep control over environmental factors that may contribute to structural changes in the brain unrelated to drug exposure (eg life stress), rodent models are very helpful. 5‐HTT knockout (5‐HTT−/−) rats are a well‐established model to study the consequences of the 5‐HTTLPR polymorphism (Hariri et al25). Of particular interest for this study, 5‐HTT−/− rats show higher extracellular levels of 5‐HT in the Hip and nucleus accumbens (NAc),26 increased anxiety‐ and depression‐like behavior,27, 28 enhanced cocaine‐induced conditioned place preference (CPP), and most importantly, increased intravenous cocaine self‐administration under both short‐29 and long‐access conditions.30, 31 5‐HTT−/− rats also display an increased motivation for cocaine,28, 29 an impairment in the extinction of cocaine‐seeking behavior,29, 30 and insensitivity to punishment.30 These latter three behavioral manifestations correspond to the three criteria that have been proposed to assess addiction‐like behavior in rats.32 These findings together suggest that 5‐HTT−/− rats show higher levels of cocaine self‐administration to “self‐medicate” their negative emotional state. In support, we found that increased long‐access cocaine self‐administration in 5‐HTT−/− rats is associated with increased anxiety and with changes in corticotropin‐releasing factor (CRF) levels in the AMY. The latter is a key feature of the negative reinforcement theory of addiction.33 Therefore, we postulated that increased long‐access cocaine self‐administration in 5‐HTT−/− rats is driven by negative reinforcement.
So far, only one animal imaging study has directly investigated the relationship between reduced 5‐HTT expression, structural brain connectivity, and sensitivity to cocaine. In this study, using functional MRI and structural DTI, Van der Marel and coworkers34 did not observe structural differences between cocaine‐naive 5‐HTT−/− and 5‐HTT+/+ rats, with the exception of reduced FA values in the genu of the corpus callosum (CC) of 5‐HTT−/− rats. In response to an acute cocaine challenge, brain activity patterns were also comparable for both genotypes.34 Despite these negative findings for the effects of acute passive exposure to cocaine, chronic voluntary use to the drug may still be associated with structural changes in the brain, not least because of the distinct neural sequelae of active versus passive drug exposure (see, eg, Jacobs et al35). Because we expected that any structural differences between 5‐HTT+/+ and 5‐HTT−/− rats are more subtle than could be detected in Van der Marel's study, we here used ultrahigh‐resolution (75‐μm isotropic voxels vs 250 μm in Van der Marel's study) structural MRI on postmortem fixed brain tissue of 5‐HTT−/− and 5‐HTT+/+ rats after long access to cocaine or sucrose self‐administration, in a brain‐wide, hypothesis‐free manner. In line with previous (MRI) studies in rats and to rule out the effects of operant training, sucrose self‐administration was chosen as control.36, 37 Because rats that self‐administer sucrose undergo the exact same handlings as rats that self‐administer cocaine, such as surgery, catheter flushing, social isolation, and repeated testing, structural differences can only be attributed to differences in the type of reward. Hence, comparing structural changes in the brain between rats self‐administering sucrose and those self‐administering cocaine informs about the impact of repeated cocaine intake specifically. Since both the 5‐HTTLPR s‐allele (see above) and cocaine addiction are associated with GM volume decreases in the AMY, insula, and prefrontal areas,38, 39, 40 we hypothesized that long‐access cocaine versus sucrose self‐administration is associated with structural changes specifically in these areas.
2. MATERIALS AND METHODS
2.1. Animals
The 5‐HTT knockout rat line was generated by N‐ethyl‐N‐nitrosourea (ENU) mutagenesis as described by Smits et al41 and Homberg et al.27 Male 5‐HTT+/+ and 5‐HTT−/− rats were generated through heterozygous breeding. All rats were housed in Makrolon cages (Type III H Tecnilab‐BMI, Someren, The Netherlands) measuring 40 × 25 × 18 cm (l × w × h) in climate‐controlled rooms at 21 ± 1°C and 55 ± 15% relative humidity. Rats that underwent behavioral experiments were housed under a reversed 12‐hour day‐night cycle (lights on at 7:00 pm). Food and water were available ad libitum except during self‐administration sessions. Animals were divided into two groups: cocaine (5‐HTT+/+ [n = 11] and 5‐HTT−/− [n = 7]) or sucrose (5‐HTT+/+ [n = 9] and 5‐HTT−/− [n = 8]) self‐administration. All animal experiments were approved by the Animal Ethics Committee of Utrecht University and were conducted in accordance with Dutch laws (Wet op de Dierproeven, 1996) and European regulations (Guideline 86/609/EEC).
2.2. Surgery
Rats (PND 90‐120) that underwent sucrose and cocaine self‐administration were subjected to surgery as previously described.42, 43 The animals were anesthetized using 0.8 mL/kg im Hypnorm (0.315 mg/mL fentanyl citrate, 10 mg/mL fluanisone, VetaPharma Ltd) and 0.5 mL/kg ip Dormicum (1 mg/mL midazolam, Roche). Rats were then implanted with a single catheter (Camcaths, Ely, UK) consisting of a 22‐g stainless steel cannula attached to a nylon mesh and silastic tubing with a 0.30‐mm internal diameter. The catheter was placed in the right jugular vein and aimed towards the superior vena cava. The mesh at the end of the catheter was sutured under the skin of the dorsum. Rimadyl (50 mg/kg, Pfizer) and gentamicin (50 mg/kg) were administered subcutaneously 15 minutes prior and 24 and 48 hours after surgery. Starting 2 days after surgery, catheters were flushed daily with 0.3‐mL sterile saline containing heparin (50 IU/mL, LEO Pharma) and gentamicin (10 mg/mL, Life Technologies). The animals were allowed at least 7 days to recover from surgery.
2.3. Apparatus
Self‐administration experiments were conducted in operant‐conditioning chambers measuring 29.5 × 24 × 25 cm, l × w × h (Med Associates, St Albans City, USA). The floor of the boxes consisted of stainless steel rods over an aluminum waste pan, the left and right walls consisted of aluminum, and the back wall, top, and door at the front consisted of clear polycarbonate. The boxes were placed in sound‐attenuating cubicles, which were equipped with a ventilation fan that produced a constant background noise. Two 4.8‐cm‐wide retractable levers were placed on the right wall, 11.7 cm apart, and 6.0 cm from the grid floor. Positioned above each lever was a cue light (28 V, 100 mA), and a house light (28 V, 100 mA) was placed in the top far corner on the opposite wall. The food cup for delivering sucrose pellets was located on the right wall between the levers. Cocaine infusions were controlled by an infusion pump placed on top of the cubicles. During the cocaine self‐administration sessions, polyethylene tubing ran from the syringe placed in the infusion pump via a swivel to the cannula on the animals' back. In the operant chamber, tubing was shielded with a metal spring. The experiment was controlled and recorded by MEDState Notation using MED‐PC for Windows.43, 44
2.4. Experimental procedures
2.4.1. Long‐access cocaine and sucrose self‐administration
After recovery from surgery, rats were placed in the administration boxes and connected to the syringe pump. The session started when both the levers extended into the box and the house light was turned on, which remained on during the entire session. During the self‐administration sessions, two levers were present, an active lever and an inactive lever. The left or right position of the active and inactive levers was counterbalanced for individual animals. Pressing the active lever resulted in a reward consisting of either a 45‐mg sucrose pellet or a 0.5‐mg/kg cocaine hydrochloride infusion (volume 0.1 mg/kg), depending on the experimental group, under an FR1 schedule of reinforcement.31 Reward delivery was accompanied by illumination of the cue light for 30 seconds. During this period, active lever responding did not result in reward but was recorded as a response. Incorrect lever responding was recorded but had no programmed consequences. To prevent cocaine overdose, the maximum amount of rewards was set to 25 for the first three sessions, 30 for the next session, 50 for session 6, and 150 for the remainder of the experiment. When the maximum amount of rewards was reached, both levers retracted, prohibiting the rats from responding further. Rats were trained for 5 days a week for 6 hours a day, ie, long‐access sessions, according to Ahmed and Koob.45 In the 19th session, rats were tested under a progressive ratio (PR) schedule of reinforcement, to measure motivation for cocaine or sucrose.46 Under the PR schedule, the number of active lever presses to receive reward increases exponentially according to the following equation47: required active lever presses = (5 × e[injection number×0.2]) − 5. The session ended when the rats did not earn a reward for 30 minutes. On day 20 of testing, the rats were switched back to an FR1 schedule of reinforcement for 6 hours.31
2.4.2. Transcardial perfusion and tissue preparation
The rats were anesthetized 24 hours after self‐administration session 20 using 120 mg/kg of pentobarbital (AST pharma) injected intraperitoneally and transcardially perfused using 0.1M phosphate‐buffered saline (PBS) (pH = 7.3) followed by 4% paraformaldehyde (PFA, Sigma‐Aldrich (St. Louis, Missouri USA), pH = 7.2). Heads (brain + skull + skin + muscle) were postfixed in 4% PFA overnight at 4°C and stored in PBS at 4°C. After fixation, the skin and muscle were removed, and the brain was retained in the skull. The perfusion‐fixed skulls were inserted in a custom‐made holder and immersed in nonmagnetic and magnetic resonance (MR) invisible oil (Fomblin, Solvay Solexis) for MRI experiments (see below).
2.4.3. High‐resolution structural MRI
Structural MRI was performed using a 9.4‐T horizontal bore MR system (Varian, Palo Alto, California) equipped with a 6‐cm ID gradient insert with gradients up to 1 T/m. A custom‐made solenoid coil with an internal diameter of 2.6 cm was used for excitation and reception of the MR signal. DTI was performed using a 3‐D diffusion‐weighted spin‐echo sequence with an isotropic spatial resolution of 150 μm. The read and phase encode directions were acquired using eight‐shot echo‐planar imaging (EPI) encoding, and the second phase direction was linearly phase encoded (repetition time [TR]/echo time [TE] 500/32.4 ms, 220 × 128 × 108 matrix, field of view [FOV] 33 × 19.2 × × 16 mm3, Δ/δ 15/4 ms, b 3842 s/mm2, 60 diffusion‐weighted images in noncollinear directions and four images without diffusion weighting [b = 0], number of averages 1, total number of images 64). Subsequently, four 3‐D balanced steady‐state free precession (BSSFP) images were acquired with an isotropic spatial resolution of 75 μm (TR/TE 15.4/7.7 ms, flip angle 40°, 426 × 214 × 256 matrix, FOV 32 × 16 × 19 mm3, 12 averages, pulse angle shift 0°, 90°, 180°, and 270°). The four images were added as complex images to obtain a single BSSFP image with reduced banding artifacts in the brain, which will be referred to as an anatomical MR image. Total acquisition time was 11 hours and 15 minutes.
2.4.4. Morphometric analysis
Anatomical MR images were used to obtain brain masks using a brain extraction tool (BET2) as implemented in the FMRIB Software Library (FSL).48 The brain‐extracted images were subsequently linearly registered to one of the brain images from the control group using FSL's linear registration tool FLIRT,49, 50 followed by the nonrigid registration tool FNIRT. A template image was generated by nonrigid registration of the images of the control group to a starting template (one representative image of the control group) followed by averaging all the transformed images to the final template.
Segmentation of GM, WM, and cerebrospinal fluid (CSF) in the regions of interest (ROIs) was accomplished through thresholding. In the anatomical images, thresholding was based on signal intensity. All anatomical images were normalized by setting the median signal intensity of the motor cortex to 1. Subsequently, tissue signal intensities lower than 0.85, between 0.85 and 1.35, and higher than 1.35 were defined as GM, WM, and CSF, respectively.
ROIs were defined on a template anatomical image guided by the rat brain atlas from Paxinos and Watson.51 ROIs were selected based on the literature discussed in the introduction and included the AMY, Hip, OFC, medial prefrontal cortex (mPFC), ACC, insular cortex, caudate putamen (CPU), NAc, dorsal raphe nucleus (DRN), and CC. These ROIs were registered to the individual rat brain images, and their sizes in the individual images were obtained.
For DTI data, WM was defined as tissue with FA above 0.25 and mean diffusivity (MD) below or equal to 0.34 × 10−9 mm2/s. GM was defined as tissue with FA below or equal to 0.25 and MD below or equal to 0.34 × 10−9 mm2/s. CSF was defined as tissue with FA below or equal to 0.25 and MD above 0.34 × 10−9 mm2/s.
2.5. Statistics
All statistical analyses were performed by using IBM SPSS version 24 (IBM software). Day‐to‐day consumption of cocaine/sucrose was analyzed using a genotype (two levels) × session (19 levels) repeated‐measures analysis of variance (ANOVA), while total rewards consumed and PR breakpoint were analyzed using independent two‐sided t tests. Breakpoint is defined as the ratio at which the rats fail to complete the response requirement, which is equivalent to the number of rewards earned. All MRI variables were analyzed using multivariate ANOVAs using the Benjamini‐Hochberg correction for multiple testing on all acquired P values.52 The level of significance was set at P < 0.05 (NS = nonsignificant).
3. RESULTS
3.1. Long‐access cocaine and sucrose self‐administration
Figure 1 shows sucrose and cocaine‐taking behavior across sessions. In the sucrose cohort (Figure 1A,C,E), no significant genotype differences in self‐administration over the 6 hours–lasting sessions or PR performance were observed (self‐administration [Figure 1A]: F 18,288 = 1.426, NS; PR [Figure 1E]: t 17 = 0.101, NS). However, when all the rewards received during the 19 self‐administration sessions were pooled, 5‐HTT−/− animals consumed slightly less sucrose pellets compared with their 5‐HTT+/+ counterparts (Figure 1C: t 10.645 = −2.922, P < 0.05). In contrast, in the cocaine cohort, 5‐HTT−/− rats self‐administered more cocaine over the 6 hours–lasting sessions (Figure 1B: F 18,288 = 2.164, P < 0.01) and received more cocaine throughout the whole experiment compared with 5‐HTT+/+ rats (Figure 1D: t 11.746 = 3.271, P < 0.01). Additionally, the 5‐HTT−/− rats were also more motivated to take cocaine as measured under a PR schedule of reinforcement (Figure 1F: t 14.283 = 2.212, P < 0.05).
Figure 1.
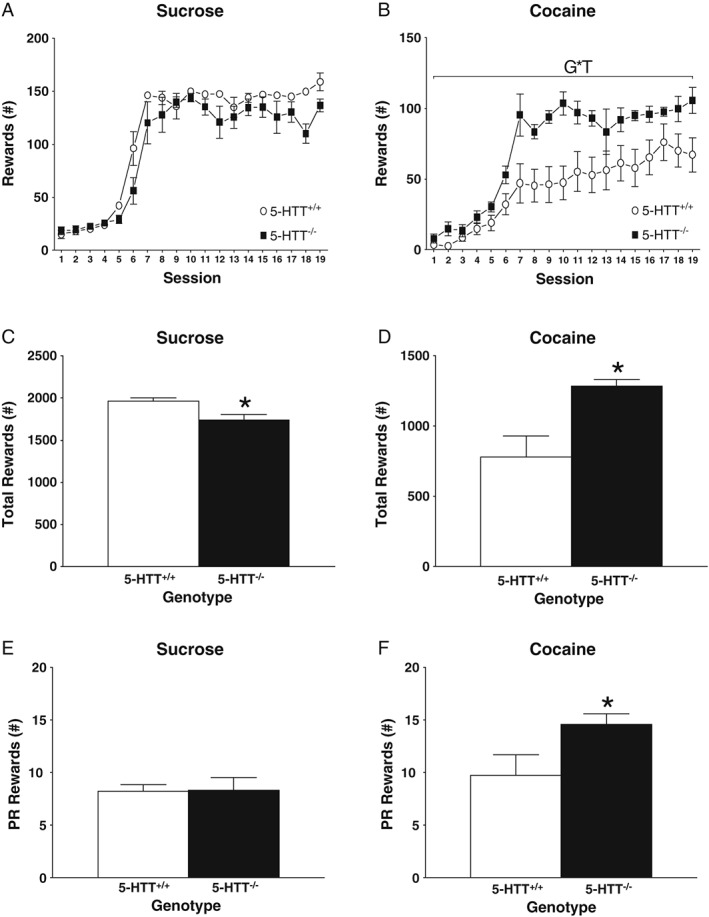
Long‐access cocaine and sucrose self‐administration in serotonin transporter (5‐HTT)−/− versus 5‐HTT+/+ rats. All data are represented as mean ± standard error of the mean (SEM). White symbols and bars represent 5‐HTT+/+ rats while black symbols and bars represent 5‐HTT−/− rats. G*T indicates a significant interaction between genotype and time using a repeated‐measures analysis of variance (ANOVA); no difference between groups is observed in day‐to‐day sucrose self‐administration A, or motivation to self‐administer sucrose E. However, cumulative intake of sucrose C, is significantly lower in 5‐HTT−/− rats. 5‐HTT−/− rats self‐administered more cocaine on a day‐to‐day basis B, had higher cumulative intake D, and show increased motivation for cocaine F. *P < 0.05 (versus 5‐HTT+/+)
3.2. Structural MRI analysis
3.2.1. Structural analysis of GM, WM, and CSF
Whole‐brain GM, WM, and CSF volume, MD, and FA values for 5‐HTT+/+ and 5‐HTT−/− rats after sucrose or cocaine self‐administration are displayed in Table 1. A genotype effect was observed for CSF and WM volume (WM: Figure 2A, F 1,31 = 4.962, P < 0.05; CSF: Figure 2B, F 1,31 = 5.481, P < 0.05). Independent‐samples t test post hoc analysis revealed that this genotype effect in the WM was caused by a significant reduction of WM volume in the 5‐HTT−/− rats in the sucrose cohort (see Figure 2A: t 15 = −2.780, P < 0.05). Post hoc analysis of the CSF volume failed to reach statistical significance in either cohort, although a trend towards significantly smaller CSF volume was observed in 5‐HTT−/− rats of the sucrose, but not cocaine group (see Figure 2B; sucrose: t 11.829 = −1.970, P = 0.073; cocaine: t 16 = 1.349, NS). Additionally, when not correcting for multiple testing, a significant genotype × treatment interaction was observed for WM volume (for statistics, see Table 1).
Table 1.
WM, GM, and CSF volume and MD and FA values of 5‐HTT+/+ and 5‐HTT−/− rats that underwent sucrose or cocaine self‐administration
| 5‐HTT+/+ | 5‐HTT−/− | Statistics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Cocaine | Sucrose | Cocaine | Genotype | Treatment | Genotype × Treatment | ||||||||||||
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | F | P | Effect Size (η p 2) | F | P | Effect Size (η p 2) | F | P | Effect Size (η p 2) | ||
| Volume,a μL | GM | 1011 | 30 | 1037 | 25 | 1015 | 24 | 1016 | 19 | 0.102 | 0.751 | 0.003 | 0.278 | 0.602 | 0.009 | 0.231 | 0.634 | 0.007 |
| WM | 421 | 17 | 366 | 8 | 362 | 12 | 369 | 12 | 4.962b | 0.033b | 0.138b | 3.593 | 0.067 | 0.104 | 6.025c | 0.020c | 0.163c | |
| CSF | 87 | 13 | 71 | 7 | 58 | 7 | 57 | 5 | 5.481b | 0.026b | 0.150b | 0.991 | 0.327 | 0.031 | 0.742 | 0.396 | 0.023 | |
| MD,d mm2/s | GM | 0.271 | 0.003 | 0.269 | 0.005 | 0.265 | 0.011 | 0.270 | 0.004 | 0.146 | 0.705 | 0.005 | 0.055 | 0.816 | 0.002 | 0.282 | 0.599 | 0.009 |
| WM | 0.258 | 0.005 | 0.253 | 0.006 | 0.252 | 0.012 | 0.255 | 0.004 | 0.118 | 0.734 | 0.004 | 0.022 | 0.882 | 0.001 | 0.274 | 0.604 | 0.009 | |
| CSF | 0.597 | 0.009 | 0.597 | 0.008 | 0.572 | 0.020 | 0.582 | 0.007 | 2.862 | 0.101 | 0.085 | 0.152 | 0.700 | 0.005 | 0.203 | 0.656 | 0.006 | |
| FA | GM | 0.177 | 0.000 | 0.178 | 0.001 | 0.177 | 0.002 | 0.178 | 0.001 | 0.252 | 0.619 | 0.008 | 1.162 | 0.289 | 0.036 | 0.005 | 0.947 | 0.000 |
| WM | 0.392 | 0.001 | 0.388 | 0.001 | 0.389 | 0.001 | 0.388 | 0.002 | 1.476 | 0.234 | 0.045 | 4.476 | 0.043 | 0.126 | 0.703 | 0.408 | 0.022 | |
| CSF | 0.132 | 0.001 | 0.132 | 0.001 | 0.136 | 0.004 | 0.135 | 0.002 | 2.228 | 0.146 | 0.067 | 0.026 | 0.873 | 0.001 | 0.080 | 0.780 | 0.003 | |
Abbreviations: CSF, cerebrospinal fluid; FA, fractional anisotropy; 5‐HTT, serotonin transporter; GM, gray matter; MD, mean diffusivity; SEM, standard error of the mean; WM, white matter.
Data acquired from anatomical data.
Effect significant after Benjamini‐Hochberg correction.
Effect significant without correction for multiple testing.
Data acquired from diffusion tensor imaging (DTI) data.
Figure 2.
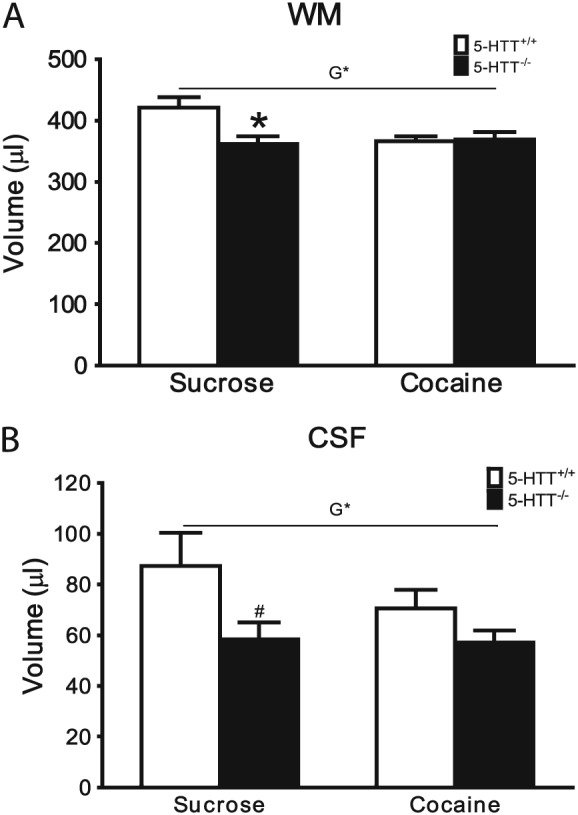
Whole‐brain differences in WM and CSF volume. All data are represented as mean ± SEM. White bars represent 5‐HTT+/+ rats while black bars represent 5‐HTT−/− rats. A significant genotype (G) effect was observed for WM A, and CSF B. Post hoc analysis revealed the effects were caused by lower WM and CSF volumes in 5‐HTT−/− rats from the control cohort. Cocaine self‐administration reduced WM volumes in 5‐HTT+/+ rats. CSF: cerebrospinal fluid; 5‐HTT: serotonin transporter; SEM: standard error of the mean; WM: white matter. * P < 0.05 (versus 5‐HTT+/+). # P < 0.1 (versus 5‐HTT+/+)
3.2.2. Structural analysis of the predefined ROIs
Volume, MD, and FA values of the 10 predefined ROIs for 5‐HTT+/+ and 5‐HTT−/− rats after sucrose or cocaine self‐administration are displayed in Table 2. Figure 3 presents the images on which these values were based. Without correction for multiple testing, CC volume was, independently of the type of reward, smaller in 5‐HTT−/− versus 5‐HTT+/+ rats, and an additional significant effect of genotype was observed in the AMY (for statistics, see Table 2). After the Benjamini‐Hochberg correction, 5‐HTT−/− rats showed a significantly lower AMY volume (Figure 4A: genotype effect, F 1,31 = 25.911, P < 0.001). Post hoc independent t testing revealed that this effect was significant in both cohorts (sucrose: t 15 = −3.910, P < 0.001; cocaine: t 16 = −3.241, P < 0.01). A significant genotype × treatment interaction was observed for DRN volume (Figure 4B: genotype × treatment interaction, F 1,31 = 11.770, P < 0.01). Post hoc independent t test analysis of this effect revealed a significant lower DRN volume in the control 5‐HTT−/− rats receiving sucrose compared with 5‐HTT+/+ rats of the same cohort (t 15 = −3.003, P < 0.01). In addition, long‐access cocaine self‐administration reduced DRN volume in 5‐HTT+/+, but not 5‐HTT−/−, rats (t 18 = 3.597, P < 0.01).
Table 2.
ROI, MD, and FA of 5‐HTT+/+ and 5‐HTT−/− rats that underwent sucrose or cocaine self‐administration
| 5‐HTT+/+ | 5‐HTT−/− | Statistics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Cocaine | Sucrose | Cocaine | Genotype | Treatment | Genotype × Treatment | ||||||||||||
| Average | SEM | Average | SEM | Average | SEM | Average | SEM | F | P | Effect Size (η p 2) | F | P | Effect Size (η p 2) | F | P | Effect Size (η p 2) | ||
| Volume,a μL | OFC | 27 | 0.8 | 27 | 0.8 | 27 | 0.6 | 27 | 0.4 | 0.430 | 0.517 | 0.014 | 0.005 | 0.945 | 0.000 | 0.003 | 0.954 | 0.000 |
| mPFC | 12 | 0.4 | 12 | 0.6 | 12 | 0.6 | 13 | 0.3 | 0.177 | 0.676 | 0.006 | 0.212 | 0.648 | 0.007 | 0.016 | 0.901 | 0.001 | |
| ACC | 21 | 0.3 | 20 | 0.7 | 19 | 0.6 | 20 | 0.3 | 1.400 | 0.246 | 0.043 | 0.127 | 0.724 | 0.004 | 2.28 | 0.141 | 0.068 | |
| CPU | 82 | 1.5 | 83 | 2.0 | 80 | 2.3 | 82 | 1.5 | 1.164 | 0.289 | 0.036 | 0.659 | 0.423 | 0.021 | 0.075 | 0.787 | 0.002 | |
| Hip | 96 | 1.5 | 97 | 2.0 | 94 | 2.6 | 96 | 1.6 | 0.343 | 0.563 | 0.011 | 0.314 | 0.579 | 0.010 | 0.07 | 0.794 | 0.002 | |
| NAc | 16 | 0.5 | 17 | 0.6 | 17 | 0.4 | 16 | 0.5 | 0.211 | 0.649 | 0.007 | 0.002 | 0.963 | 0.000 | 0.581 | 0.452 | 0.018 | |
| AMY | 27 | 0.6 | 25 | 0.4 | 24 | 0.3 | 23 | 0.5 | d25.911b | d0.000b | d0.455b | 7.057c | c0.012c | c0.185c | 0.711 | 0.405 | 0.022 | |
| DRN | 1 | 0.0 | 0.9 | 0.0 | 0.9 | 0.0 | 0.9 | 0.0 | 2.475 | 0.126 | 0.074 | 3.475 | 0.072 | 0.101 | 11.77b | 0.002b | 0.275b | |
| Insular | 46 | 1.0 | 45 | 1.1 | 44 | 1.1 | 43 | 1.2 | 3.713 | 0.063 | 0.107 | 0.415 | 0.524 | 0.013 | 0.036 | 0.852 | 0.001 | |
| CC | 37 | 0.9 | 34 | 0.8 | 33 | 0.5 | 34 | 0.9 | c5.413c | c0.027c | c0.149c | 0.308 | 0.583 | 0.010 | c4.696c | c0.038c | c0.132c | |
| MD,d mm2/s | OFC | 2.79E−04 | 1.02E−05 | 2.80E−04 | 6.37E−06 | 2.84E−04 | 7.71E−06 | 2.84E−04 | 4.66E−06 | 0.341 | 0.564 | 0.011 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 |
| mPFC | 2.76E−04 | 1.12E−05 | 2.78E−04 | 6.34E−06 | 2.81E−04 | 8.83E−06 | 2.80E−04 | 5.52E−06 | 0.194 | 0.663 | 0.006 | 0.000 | 1.000 | 0.000 | 0.047 | 0.830 | 0.002 | |
| ACC | 2.83E−04 | 9.88E−06 | 2.88E−04 | 4.69E−06 | 2.93E−04 | 6.88E−06 | 2.90E−04 | 3.50E−06 | 0.688 | 0.413 | 0.022 | 0.000 | 1.000 | 0.000 | 0.268 | 0.608 | 0.009 | |
| CPU | 2.73E−04 | 9.07E−06 | 2.73E−04 | 6.15E−06 | 2.83E−04 | 6.30E−06 | 2.77E−04 | 5.83E−06 | 1.036 | 0.317 | 0.032 | 0.165 | 0.688 | 0.005 | 0.183 | 0.671 | 0.006 | |
| Hip | 2.96E−04 | 7.09E−06 | 2.99E−04 | 3.83E−06 | 3.06E−04 | 6.30E−06 | 3.02E−04 | 2.13E−06 | 1.366 | 0.251 | 0.042 | 0.000 | 1.000 | 0.000 | 0.466 | 0.500 | 0.015 | |
| NAc | 2.70E−04 | 1.28E−05 | 2.68E−04 | 8.62E−06 | 2.73E−04 | 8.72E−06 | 2.68E−04 | 7.24E−06 | 0.000 | 1.000 | 0.000 | 0.117 | 0.734 | 0.004 | 0.000 | 1.000 | 0.000 | |
| AMY | 2.77E−04 | 1.30E−05 | 2.76E−04 | 9.33E−06 | 2.79E−04 | 9.17E−06 | 2.77E−04 | 6.65E−06 | 0.024 | 0.879 | 0.001 | 0.000 | 1.000 | 0.000 | 0.000 | 1.000 | 0.000 | |
| DRN | 2.67E−04 | 1.30E−05 | 2.72E−04 | 7.63E−06 | 2.77E−04 | 8.13E−06 | 2.73E−04 | 3.32E−06 | 0.376 | 0.544 | 0.012 | 0.000 | 1.000 | 0.000 | 0.194 | 0.663 | 0.006 | |
| Insular | 2.86E−04 | 1.06E−05 | 2.88E−04 | 6.82E−06 | 2.95E−04 | 7.26E−06 | 2.92E−04 | 5.18E−06 | 0.653 | 0.425 | 0.021 | 0.000 | 1.000 | 0.000 | 0.077 | 0.784 | 0.002 | |
| CC | 2.15E−04 | 8.35E−06 | 2.12E−04 | 6.10E−06 | 2.19E−04 | 7.00E−06 | 2.10E−04 | 3.10E−06 | 0.000 | 1.000 | 0.000 | 0.791 | 0.381 | 0.025 | 0.165 | 0.687 | 0.005 | |
| FAd | OFC | 0.218 | 0.006 | 0.218 | 0.004 | 0.218 | 0.005 | 0.221 | 0.002 | 0.059 | 0.81 | 0.002 | 0.068 | 0.796 | 0.002 | 0.074 | 0.788 | 0.002 |
| mPFC | 0.233 | 0.009 | 0.233 | 0.007 | 0.236 | 0.005 | 0.234 | 0.006 | 0.112 | 0.74 | 0.004 | 0.022 | 0.882 | 0.001 | 0.026 | 0.872 | 0.001 | |
| ACC | 0.233 | 0.009 | 0.236 | 0.006 | 0.242 | 0.005 | 0.235 | 0.005 | 0.326 | 0.572 | 0.010 | 0.068 | 0.796 | 0.002 | 0.475 | 0.496 | 0.015 | |
| CPU | 0.207 | 0.008 | 0.203 | 0.005 | 0.211 | 0.005 | 0.204 | 0.002 | 0.108 | 0.745 | 0.003 | 0.840 | 0.366 | 0.026 | 0.059 | 0.810 | 0.002 | |
| Hip | 0.236 | 0.004 | 0.235 | 0.002 | 0.234 | 0.002 | 0.235 | 0.002 | 0.228 | 0.636 | 0.007 | 0.000 | 0.989 | 0.000 | 0.162 | 0.690 | 0.005 | |
| NAc | 0.255 | 0.012 | 0.249 | 0.009 | 0.249 | 0.008 | 0.246 | 0.005 | 0.224 | 0.639 | 0.007 | 0.18 | 0.674 | 0.006 | 0.033 | 0.856 | 0.001 | |
| AMY | 0.244 | 0.012 | 0.240 | 0.008 | 0.23 | 0.008 | 0.237 | 0.006 | 0.863 | 0.360 | 0.027 | 0.031 | 0.86 | 0.001 | 0.359 | 0.553 | 0.011 | |
| DRN | 0.207 | 0.014 | 0.191 | 0.015 | 0.183 | 0.010 | 0.178 | 0.006 | 1.925 | 0.175 | 0.058 | 0.653 | 0.425 | 0.021 | 0.182 | 0.673 | 0.006 | |
| Insular | 0.209 | 0.006 | 0.209 | 0.003 | 0.207 | 0.005 | 0.202 | 0.003 | 0.984 | 0.329 | 0.031 | 0.343 | 0.563 | 0.011 | 0.242 | 0.626 | 0.008 | |
| CC | 0.587 | 0.012 | 0.587 | 0.009 | 0.596 | 0.008 | 0.584 | 0.008 | 0.101 | 0.753 | 0.003 | 0.378 | 0.543 | 0.012 | 0.400 | 0.531 | 0.013 | |
Abbreviations: ACC, anterior cingulate cortex; AMY, amygdala; CC, corpus callosum; CPU, caudate putamen; DRN, dorsal raphe nucleus; FA, fractional anisotropy; 5‐HTT, serotonin transporter; Hip, hippocampus; insular, insular cortex; MD, mean diffusivity; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; ROI, region of interest; SEM, standard error of the mean.
Data acquired from anatomical data.
Effect significant after Benjamini‐Hochberg correction.
Effect significant without correction for multiple testing.
Data acquired from diffusion tensor imaging (DTI) data.
Figure 3.
Overview of images for serotonin transporter (5‐HTT)+/+ and 5‐HTT−/− rats with a history of sucrose or cocaine self‐administration. A, Reference brains and region‐of‐interest (ROI) overlays. B, Volumetric images. C, Mean diffusivity (MD) images. D, Fractional anisotropy (FA) images
Figure 4.
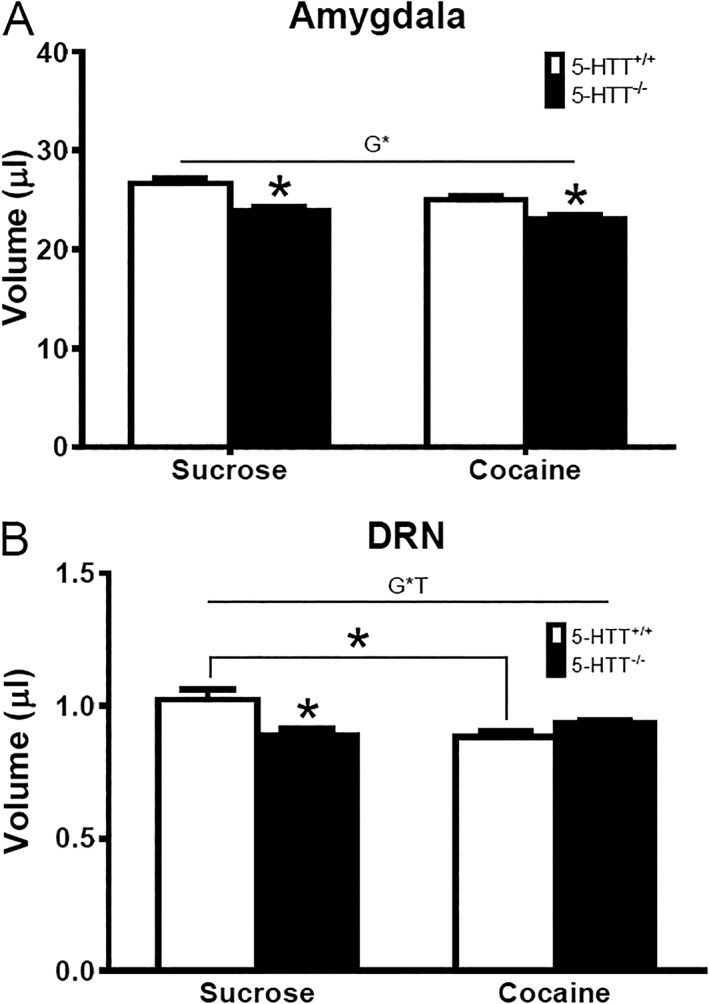
Altered AMY and DRN volume in 5‐HTT+/+ and 5‐HTT−/− rats that underwent sucrose or cocaine self‐administration. All data are represented as mean ± SEM. White bars represent 5‐HTT+/+ rats while black bars represent 5‐HTT−/− rats. A, A significant genotype (G) effect was observed for the AMY. B, A significant interaction of genotype and treatment (G*T) was observed for DRN. Post hoc analysis revealed the effect of the AMY was caused by a lower volume in 5‐HTT−/− rats from both reward cohorts. Post hoc analysis revealed a lower DRN volume in sucrose‐receiving 5‐HTT−/− rats versus sucrose‐receiving 5‐HTT+/+ rats and in 5‐HTT+/+ rats receiving cocaine versus 5‐HTT+/+ rats receiving sucrose. AMY: amygdala; DRN: dorsal raphe nucleus; 5‐HTT: serotonin transporter; SEM: standard error of the mean. * P < 0.05
4. DISCUSSION
We observed that in 5‐HTT−/−, compared with 5‐HTT+/+, rats AMY volume was reduced, regardless of reward type. However, no significant microstructure or volume changes in prefrontal cortical areas were observed. Furthermore, we found an interaction between reward type and genotype for the volume of the DRN and a significant positive correlation between DRN volume and the motivation to self‐administer cocaine, but not sucrose. These findings suggest not only that long access to cocaine leads to neurotoxicity53 but also that structural changes in response to cocaine can be influenced by 5‐HTT genetic variation.
5‐HTT−/− rats self‐administered significantly more cocaine throughout the experiment, as reflected by higher day‐to‐day self‐administration and an increase in the total amount of cocaine infusions taken compared with 5‐HTT+/+ rats. Additionally, 5‐HTT−/− animals were more motivated to take cocaine, as reflected by increased PR breakpoints. These results are in line with observations by us and others that 5‐HTT−/− rats are more vulnerable to psychostimulant self‐administration.28, 29, 54 In contrast to these findings, self‐administration of sucrose pellets did not differ between genotypes on a day‐to‐day basis, and the total amount of these pellets consumed was in fact slightly decreased in 5‐HTT−/− rats. In addition, no difference in motivation to work for sucrose was observed under the PR schedule of reinforcement. Together, these data suggest that the observed higher intake of cocaine in 5‐HTT−/− versus 5‐HTT+/+ rats is not driven by genotype differences in operant responding or general positive reinforcement mechanisms. Rather, as we replicate here our previous findings of increased cocaine intake in 5‐HTT−/− rats under long‐access conditions, which was associated with a stronger (withdrawal‐induced) negative emotional state,31 negative emotionality may drive the genotype differences in long‐access cocaine intake.
On the whole‐brain level, both WM and CSF (ventricle) volumes tended to be smaller in the 5‐HTT−/− rats after sucrose self‐administration. A recent MRI study in 5‐HTT−/− mice revealed overall reduced brain volume and reduced WM, but this was observed in female and not male mice.55 Potentially female 5‐HTT−/− rats also demonstrate larger genotype differences compared with male 5‐HTT−/− rats. We focused here on male rats, since addiction is more prevalent in males than in females. Nonetheless, investigation of sex differences would be an important asset for future research. In addition, cocaine self‐administration reduced WM volume in 5‐HTT+/+ rats, while whole GM volume did not differ between genotypes and/or treatments. WM changes have previously been observed following extended exposure to cocaine in animals56 and humans.57, 58 A decrease in WM could be indicative of reduced microstructural integrity or demyelination, potentially contributing to the emotional and affective problems seen in cocaine addicts.59, 60
When analyzing the 10 predefined ROIs, 5‐HTT−/− rats showed smaller AMY volumes, independent of sucrose or cocaine self‐administration history. As mentioned in Section 1, reduced AMY volume has also been observed in the 5‐HTTLPR s‐allele carriers.13, 14, 15 Furthermore, Ellegood et al55 demonstrated that in 5‐HTT−/− mice (both sexes combined), absolute volume was decreased in the AMY. Moreover, a recent study in rats reported that AMY volume correlated with the inability to refrain from drug seeking and taking.61 Although not measured in this study, we previously observed that 5‐HTT−/− show impaired extinction of cocaine‐seeking behavior.29, 30 Potentially, volume changes in the AMY relate to the negative emotional states that 5‐HTT−/− rats experience during withdrawal.28 In addition to this genotype effect on the AMY, a significant interaction of genotype and reward type was found for DRN volume. The DRN was significantly smaller in the control 5‐HTT−/− rats exposed to sucrose. Furthermore, cocaine self‐administration reduced DRN volume in 5‐HTT+/+ rats. Ellegood et al55 also found an effect of 5‐HTT genotype on DRN volume in mice. The DRN, where forebrain projection serotonergic neurons are located, plays a critical and complex role in reward seeking.62, 63 The DRN has also been shown to contribute to a withdrawal‐induced negative emotional state in 5‐HTT−/− rats31 and in long‐access cocaine self‐administration.64 In line with a smaller DRN, a decrease in the number of serotonergic cells in the DRN has been observed in 5‐HTT−/− mice.65 These DRN results, together with the AMY results discussed above, may support our previous finding that the DRN and AMY work in concert to cause the increased long access and compulsive cocaine intake in 5‐HTT−/− versus control rats (see also Verheij et al28). The AMY may do so because of direct structural effects of the 5‐HTT genotype, while the involvement of the DRN is dependent on the interaction between 5‐HTT genotype and cocaine. A more detailed analysis of the WM tracts between the DRN and AMY, and subsequent causal manipulations of connections between these areas, may further contribute to our understanding of increased cocaine self‐administration in 5‐HTT−/− rats.
A major strength of our study involves the high‐resolution nature of the structural MRI we conducted, allowing us to identify structural changes in areas as small as the DRN. A potential limiting factor is that while we observed absolute volume differences in the AMY and DRN, there may be no relative volume differences after correction for the reduced whole‐brain WM and CSF volume in 5‐HTT−/− rats. We did not apply such a correction because the genotype and treatment effects on whole‐brain WM, GM, and CSF volume are critical readouts of the study. Furthermore, we compared the effect of cocaine self‐administration with that of sucrose self‐administration but did not include a naive control group. We therefore cannot exclude the possibility that there are differences between experimentally naive rats and rats with a history of sucrose self‐administration, as a result of mere operant conditioning. However, we recently reported that in terms of expression of immediate early genes and plasticity‐related genes, the differences between naive control rats and rats with a history of sucrose self‐administration are modest at most.42, 44 In contrast, in these studies, profound differences were found between sucrose and cocaine self‐administering groups.42, 44 In the present study, we found that 5‐HTT−/− rats show a slight reduction in sucrose self‐administration and a strong increase in cocaine self‐administration. We are therefore confident that by using sucrose self‐administration as control, this study provides insight into which brain regions contribute to increased cocaine self‐administration in 5‐HTT−/− rats.
In conclusion, we show that increased long‐access cocaine self‐administration in 5‐HTT−/− rats is associated with decreased AMY and DRN volume, providing a neural substrate for increased vulnerability seen in individuals characterized by inherited 5‐HTT downregulation. Furthermore, our findings indicate that the DRN contributes to an increased motivation for cocaine. Because animal studies allow control over genetic and environmental factors that could influence brain structure, they can provide important contributions to understanding of the heterogeneity in structural brain differences found in cocaine addicts.6
AUTHOR CONTRIBUTIONS
PK, JRH, RD, LR, and LV were responsible for study concept and design. PK and AVT acquired animal behavior and imaging data, respectively. Data analysis and interpretation were performed by PK, AVT, and MMMV. PK drafted the manuscript and JRH, LR, RD, LV, MS, GC, and MMMV provided critical revision of the manuscript. All authors critically reviewed the content and approved the final version for publication.
ACKNOWLEDGEMENT
The work described in this paper was funded by The Netherlands Organization for Health Research and Development (ZonMw, project number 91211002 awarded to J.H.). ZonMw had no further role in the design of the study; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Karel P, Van der Toorn A, Vanderschuren L, et al. Ultrahigh‐resolution MRI reveals structural brain differences in serotonin transporter knockout rats after sucrose and cocaine self‐administration. Addiction Biology. 2020;25:e12722 10.1111/adb.12722.
Karel Peter and Van der Toorn Annette contributed equally to this work.
REFERENCES
- 1. EMCDDA . European drug report 2017: Trends and developments. Luxembourg: European Monitoring Centre for Drugs and Drug Addiction: Publications Office of the European Union; 2017. [Google Scholar]
- 2. Hall MG, Alhassoon OM, Stern MJ, et al. Gray matter abnormalities in cocaine versus methamphetamine‐dependent patients: a neuroimaging meta‐analysis. Am J Drug Alcohol Abuse. 2015;41(4):290‐299. [DOI] [PubMed] [Google Scholar]
- 3. Ide JS, Zhang S, Hu S, Sinha R, Mazure CM, Li CR. Cerebral gray matter volumes and low‐frequency fluctuation of BOLD signals in cocaine dependence: duration of use and gender difference. Drug Alcohol Depend. 2014;134:51‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51(11):890‐895. [DOI] [PubMed] [Google Scholar]
- 5. Moeller FG, Hasan KM, Steinberg JL, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine‐dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30(3):610‐617. [DOI] [PubMed] [Google Scholar]
- 6. Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta‐analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr Opin Neurobiol. 2013;23(4):615‐624. [DOI] [PubMed] [Google Scholar]
- 7. Lesch KP, Bengel D, Heils A, et al. Association of anxiety‐related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527‐1531. [DOI] [PubMed] [Google Scholar]
- 8. Bleys D, Luyten P, Soenens B, Claes S. Gene‐environment interactions between stress and 5‐HTTLPR in depression: a meta‐analytic update. J Affect Disord. 2018;226:339‐345. [DOI] [PubMed] [Google Scholar]
- 9. Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5‐HT3 receptor sensitivity predict alcohol and drug dependence. Mol Psychiatry. 2011;16(11):1139‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gerra G, Zaimovic A, Garofano L, et al. Perceived parenting behavior in the childhood of cocaine users: relationship with genotype and personality traits. Am J Med Genet. 2007;144B(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 11. Martin‐Santos R, Torrens M, Poudevida S, et al. 5‐HTTLPR polymorphism, mood disorders and MDMA use in a 3‐year follow‐up study. Addict Biol. 2010;15(1):15‐22. [DOI] [PubMed] [Google Scholar]
- 12. Heils A, Teufel A, Petri S, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621‐2624. [DOI] [PubMed] [Google Scholar]
- 13. Frodl T, Koutsouleris N, Bottlender R, et al. Reduced gray matter brain volumes are associated with variants of the serotonin transporter gene in major depression. Mol Psychiatry. 2008;13(12):1093‐1101. [DOI] [PubMed] [Google Scholar]
- 14. Kobiella A, Reimold M, Ulshofer DE, et al. How the serotonin transporter 5‐HTTLPR polymorphism influences amygdala function: the roles of in vivo serotonin transporter expression and amygdala structure. Transl Psychiatry. 2011;1(8):e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pezawas L, Meyer‐Lindenberg A, Drabant EM, et al. 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828‐834. [DOI] [PubMed] [Google Scholar]
- 16. Eker MC, Kitis O, Okur H, et al. Smaller hippocampus volume is associated with short variant of 5‐HTTLPR polymorphism in medication‐free major depressive disorder patients. Neuropsychobiology. 2011;63(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 17. Little K, Olsson CA, Whittle S, et al. Association between serotonin transporter genotype, brain structure and adolescent‐onset major depressive disorder: a longitudinal prospective study. Transl Psychiatry. 2014;4(9):e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atmaca M, Onalan E, Yildirim H, et al. Serotonin transporter gene polymorphism implicates reduced orbito‐frontal cortex in obsessive‐compulsive disorder. J Anxiety Disord. 2011;25(5):680‐685. [DOI] [PubMed] [Google Scholar]
- 19. Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci U S A. 2005;102(34):12224‐12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Selvaraj S, Godlewska BR, Norbury R, et al. Decreased regional gray matter volume in S′ allele carriers of the 5‐HTTLPR triallelic polymorphism. Mol Psychiatry. 2011;16(471):472‐473. [DOI] [PubMed] [Google Scholar]
- 21. Igata N, Kakeda S, Watanabe K, et al. Voxel‐based morphometric brain comparison between healthy subjects and major depressive disorder patients in Japanese with the s/s genotype of 5‐HTTLPR. Sci Rep. 2017;7(1):3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alexander AL, Hurley SA, Samsonov AA, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1(6):423‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pacheco J, Beevers CG, Benavides C, McGeary J, Stice E, Schnyer DM. Frontal‐limbic white matter pathway associations with the serotonin transporter gene promoter region (5‐HTTLPR) polymorphism. J Neurosci. 2009;29(19):6229‐6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward‐related learning and memory. Annu Rev Neurosci. 2006;29(1):565‐598. [DOI] [PubMed] [Google Scholar]
- 25. Hariri AR, Caspi A, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verheij MM, Karel P, Cools AR, Homberg JR. Reduced cocaine‐induced serotonin, but not dopamine and noradrenaline, release in rats with a genetic deletion of serotonin transporters. Eur Neuropsychopharmacol. 2014;24(11):1850‐1854. [DOI] [PubMed] [Google Scholar]
- 27. Homberg JR, Olivier JD, Smits BM, et al. Characterization of the serotonin transporter knockout rat: a selective change in the functioning of the serotonergic system. Neuroscience. 2007;146(4):1662‐1676. [DOI] [PubMed] [Google Scholar]
- 28. Verheij MMM, Contet C, Karel P, et al. Median and dorsal raphe serotonergic neurons control moderate versus compulsive cocaine intake. Biol Psychiatry. 2018;83(12):1024‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Homberg JR, De Boer SF, Raaso HS, et al. Adaptations in pre‐ and postsynaptic 5‐HT1A receptor function and cocaine supersensitivity in serotonin transporter knockout rats. Psychopharmacology (Berl). 2008;200(3):367‐380. [DOI] [PubMed] [Google Scholar]
- 30. Karel P, Almacellas‐Barbanoj A, Prijn J, et al. Appetitive to aversive counter‐conditioning as intervention to reduce reinstatement of reward‐seeking behavior: the role of the serotonin transporter. Addict Biol. 2018. 10.1111/adb.12596 [DOI] [PubMed] [Google Scholar]
- 31. Verheij MMM, Contet C, Karel P, et al. Median and dorsal raphe serotonergic neurons control moderate versus compulsive cocaine intake. Biol Psychiatry. 2018;83(12):1024‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deroche‐Gamonet V, Belin D, Piazza PV. Evidence for addiction‐like behavior in the rat. Science. 2004;305(5686):1014‐1017. [DOI] [PubMed] [Google Scholar]
- 33. Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van der Marel K, Homberg JR, Otte WM, Dijkhuizen RM. Functional and structural neural network characterization of serotonin transporter knockout rats. PLoS One. 2013;8(2):e57780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24(11):566‐573. [DOI] [PubMed] [Google Scholar]
- 36. Burton AC, Bissonette GB, Vazquez D, et al. Previous cocaine self‐administration disrupts reward expectancy encoding in ventral striatum. Neuropsychopharmacology. 2018;43(12):2350‐2360. 10.1038/s41386-018-0058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu H‐S, Chefer S, Lu H, et al. Dorsolateral caudate nucleus differentiates cocaine from natural reward‐associated contextual cues. Proc Natl Acad Sci. 2013;110(10):4093‐4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Crunelle CL, Kaag AM, van Wingen G, et al. Reduced frontal brain volume in non‐treatment‐seeking cocaine‐dependent individuals: exploring the role of impulsivity, depression, and smoking. Front Hum Neurosci. 2014;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Makris N, Gasic GP, Seidman LJ, et al. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44(4):729‐740. [DOI] [PubMed] [Google Scholar]
- 40. Moreno‐López L, Catena A, Fernández‐Serrano MJ, et al. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 2012;125(3):208‐214. [DOI] [PubMed] [Google Scholar]
- 41. Smits BM, Mudde JB, van de Belt J, et al. Generation of gene knockouts and mutant models in the laboratory rat by ENU‐driven target‐selected mutagenesis. Pharmacogenet Genomics. 2006;16(3):159‐169. [DOI] [PubMed] [Google Scholar]
- 42. Gao P, Limpens JH, Spijker S, Vanderschuren LJ, Voorn P. Stable immediate early gene expression patterns in medial prefrontal cortex and striatum after long‐term cocaine self‐administration. Addict Biol. 2017a;22(2):354‐368. [DOI] [PubMed] [Google Scholar]
- 43. Veeneman MM, Broekhoven MH, Damsteegt R, Vanderschuren LJ. Distinct contributions of dopamine in the dorsolateral striatum and nucleus accumbens shell to the reinforcing properties of cocaine. Neuropsychopharmacology. 2012;37(2):487‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gao P, de Munck JC, Limpens JHW, Vanderschuren LJMJ, Voorn P. A neuronal activation correlate in striatum and prefrontal cortex of prolonged cocaine intake. Brain Struct Funct. 2017b;222(8):3453‐3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298‐300. [DOI] [PubMed] [Google Scholar]
- 46. Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134(3483):943‐944. [DOI] [PubMed] [Google Scholar]
- 47. Richardson NR, Roberts DC. Progressive ratio schedules in drug self‐administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66(1):1‐11. [DOI] [PubMed] [Google Scholar]
- 48. Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62(2):782‐790. [DOI] [PubMed] [Google Scholar]
- 49. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825‐841. [DOI] [PubMed] [Google Scholar]
- 50. Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143‐156. [DOI] [PubMed] [Google Scholar]
- 51. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates in Stereotaxic Coordinates. Amsterdam; Boston: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 52. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9(7):811‐818. [DOI] [PubMed] [Google Scholar]
- 53. Pereira RB, Andrade PB, Valentao P. A comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotox Res. 2015;28(3):253‐267. [DOI] [PubMed] [Google Scholar]
- 54. Oakly AC, Brox BW, Schenk S, Ellenbroek BA. A genetic deletion of the serotonin transporter greatly enhances the reinforcing properties of MDMA in rats. Mol Psychiatry. 2014;19(5):534‐535. [DOI] [PubMed] [Google Scholar]
- 55. Ellegood J, Yee Y, Kerr TM, et al. Analysis of neuroanatomical differences in mice with genetically modified serotonin transporters assessed by structural magnetic resonance imaging. Mol Autism. 2018;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Narayana PA, Herrera JJ, Bockhorst KH, et al. Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res. 2014;221(3):220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaag AM, van Wingen GA, Caan MWA, Homberg JR, van den Brink W, Reneman L. White matter alterations in cocaine users are negatively related to the number of additionally (ab)used substances. Addict Biol. 2017;22(4):1048‐1056. [DOI] [PubMed] [Google Scholar]
- 58. Schlaepfer TE, Lancaster E, Heidbreder R, et al. Decreased frontal white‐matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9(2):147‐153. [DOI] [PubMed] [Google Scholar]
- 59. Allen M, Frank D, Glen JC, Fardo F, Callaghan MF, Rees G. Insula and somatosensory cortical myelination and iron markers underlie individual differences in empathy. Sci Rep. 2017;7(1):43316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zheng KZ, Wang HN, Liu J, et al. Incapacity to control emotion in major depression may arise from disrupted white matter integrity and OFC‐amygdala inhibition. CNS Neurosci Ther. 2018;24(11):1053‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cannella N, Cosa‐Linan A, Büchler E, Falfan‐Melgoza C, Weber‐Fahr W, Spanagel R. In vivo structural imaging in rats reveals neuroanatomical correlates of behavioral sub‐dimensions of cocaine addiction. Addict Biol. 2018;23(1):182‐195. [DOI] [PubMed] [Google Scholar]
- 62. Luo M, Li Y, Zhong W. Do dorsal raphe 5‐HT neurons encode “beneficialness”? Neurobiol Learn Mem. 2016;135:40‐49. [DOI] [PubMed] [Google Scholar]
- 63. McDevitt RA, Tiran‐Cappello A, Shen H, et al. Serotonergic versus nonserotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 2014;8(6):1857‐1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. You IJ, Wright SR, Garcia‐Garcia AL, et al. 5‐HT1A autoreceptors in the dorsal raphe nucleus convey vulnerability to compulsive cocaine seeking. Neuropsychopharmacology. 2016;41(5):1210‐1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lira A, Zhou M, Castanon N, et al. Altered depression‐related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter‐deficient mice. Biol Psychiatry. 2003;54(10):960‐971. [DOI] [PubMed] [Google Scholar]


