Abstract
Active immunotherapy of cancer aims to treat the disease by inducing effective cellular and humoral immune responses. Virus‐like particle‐based vaccines have evolved dramatically over the last few decades, greatly reducing morbidity and mortality of several infectious diseases and expectedly preventing cervical cancer caused by human papilloma virus. In contrast to these broad successes of disease prevention, therapeutic cancer vaccines remain to demonstrate clinical benefit. Yet, several preclinical and clinical trials have revealed promising results and are paving the way for medical breakthroughs. This study reviews and discusses the recent preclinical development and clinical trials in this field.
This article is categorized under:
Biology‐Inspired Nanomaterials > Protein and Virus‐Based Structures
Nanotechnology Approaches to Biology > Nanoscale Systems in Biology
Keywords: cancer, vaccine, virus like particles
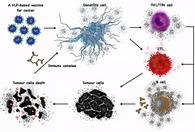
Virus‐like particles, a powerful and flexible platform for the development of cancer vaccines.
Abbreviations
- Ab
antibody
- ADCC
antibody‐dependent cellular cytotoxicity
- APC
antigen‐presenting cell
- BCR
B cell receptor
- CTL
cytotoxic T‐lymphocyte
- CTLA4
cytotoxic T‐lymphocyte‐associated antigen 4
- CuMVTT
cucumber mosaic VLP incorporating universal tetanus toxoid epitope
- DC
dendritic cell
- HBcAg
hepatitis B core antigen
- HBx
HBV X multifunctional regulatory protein
- HCC
hepatocellular carcinoma
- HER‐2 or HER‐2/neu
human epidermal growth factor receptor 2
- hMSLN
human mesothelin
- HPV
human papilloma virus
- IBDV
infectious bursal disease virus
- LN
lymph node
- MHC‐I
major histocompatibility Class I
- MHC‐II
major histocompatibility Class II
- mMSLN
murine mesothelin
- OVA
ovalbumin
- PD‐1
programmed cell death protein 1
- RHDV
rabbit hemorrhagic disease virus
- SHIV
simian‐human immunodeficiency virus
- SIV
simian immunodeficiency virus
- TACA
tumor‐associated carbohydrates antigen
- TLR
toll‐like receptor
- Treg
regulatory T cell
- TRP2
tyrosinase‐related protein
- VLP
virus‐like particle
- xCT
cystine‐glutamate exchanging transporter
1. INTRODUCTION
1.1. Virus‐like particles: A brief summary
Virus‐like particles (VLPs) constitute a powerful and flexible platform that harness the immunogenicity of viruses without compromising on safety as VLPs cannot infect nor replicate due to the absence of the viral genome (Chroboczek, Szurgot, & Szolajska, 2014). VLPs may be loaded with innate stimuli, further enhancing their immunogenicity (Storni et al., 2004; Caitlin Lemke, Krieg, & Weiner, 2016; Klimek et al., 2018). Tumor‐specific antigens may be incorporated by genetic fusion or chemical/peptide linkage allowing immunization against peptides, peptide strings or even whole proteins (Mohsen, Zha, Cabral‐Miranda, & Bachmann, 2017). Generally, VLPs have been extensively used as vaccines due to these favorable characteristics. Figure 1 and Table 1 illustrate and summarize some key characteristics of VLPs as an efficient vaccine platform. Further details of VLPs are reviewed elsewhere (Bachmann & Jennings, 2010; Garcea & Gissmann, 2004; Mohsen, Gomes, Vogel, & Bachmann, 2018; Pushko, Pumpens, & Grens, 2013; Schiller & Lowy, 2015).
Figure 1.
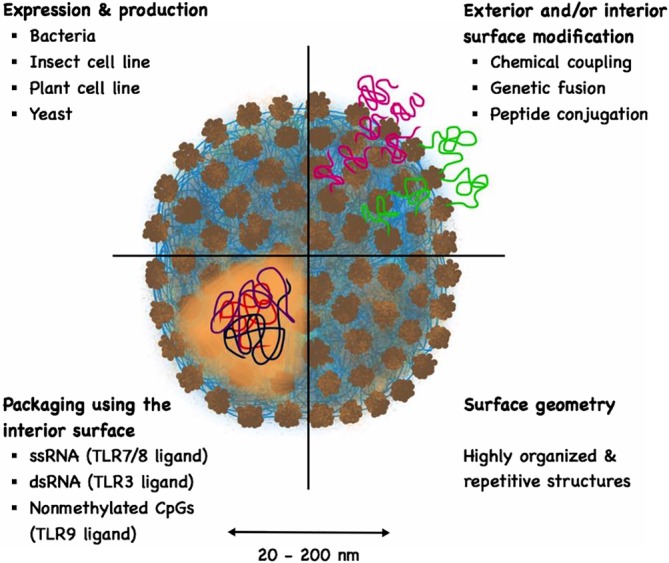
A sketch illustrating some key characteristics of virus‐like particles (VLPs) as an efficient vaccine platform
Table 1.
Key characteristics of virus‐like particles (VLPs) as an efficient vaccine platform
| Characteristics | Description | References |
|---|---|---|
| VLPs | Particles that are built‐up and self‐assembled into icosahedral or rod‐shaped structures during the expression of one or several viral‐structural proteins. Mostly, viral capsid or envelope proteins assemble into VLPs but in some cases core proteins also form VLPs. VLPs mimic the structure and symmetry of authentic viruses. They are noninfectious as they lack the proteins and genetic material for replication (replicases and nucleic acids) | Bachmann and Jennings (2010); Pumpens et al. (2009); Roldao, Mellado, Castilho, Carrondo, and Alves (2010); Sander and Lollini (2018) |
| Size | VLPs range from 20 to 200 nm, a favorable size allowing their free draining into lymph nodes | Cubas et al. (2009); Gomes, Mohsen, and Bachmann (2017) Manolova et al. (2008) |
| Expression and production | VLPs can be produced in a variety of systems including bacteria, insect or mammalian cell lines, plants or yeast | Arevalo, Wong, and Ross (2016); Santi, Huang, and Mason (2006) |
| Surface geometry | VLPs have highly organized and repetitive structures that are recognized as potent geometric pathogen‐associated structural patterns (PASP). This does not only lead to efficient cross‐linking of B cell receptors but also recruits members of the innate humoral immune system such as natural antibodies and complements, further enhancing innate and adaptive immune responses | Bachmann and Jennings (2010); Manolova et al. (2008); Rynda‐Apple, Patterson, and Douglas (2014) |
| Modifying exterior or interior surface |
The exterior or interior surface of VLPs can be functionalized and modified to display the antigens or epitopes of interest by different means:
|
Jegerlehner et al., (2002), Brune et al. (2016); Kaczmarczyk, Sitaraman, Young, Hughes, and Chatterjee (2011); Martin Caballero et al. (2012); Pomwised, Intamaso, Teintze, Young, and Pincus (2016); Pumpens (2016); Tegerstedt et al. (2005); Zeltins et al. (2017) |
| Immunostimulatory molecules | Some VLPs assemble around RNA fragments (noninfectious or replication competent) during the expression process in host cells. VLPs can also be disassembled and reassembled in the presence of different TLR‐ligands such as oligodeoxynucleotides (CpGs) (TLR‐9 ligand), polyGLU, ssRNA (TLR 7/8 ligand) or dsRNA (TLR‐3 ligand) | Dash, Federica, Ottenbrite, and Chiellini (2011); Kawano, Matsui, and Handa (2018); Sioud (2006); Storni et al. (2004); Gomes et al. (2019). |
2. VLP‐BASED VACCINES AND THE INDUCTION OF T CELL RESPONSES
T cells are key effector cells for anti‐cancer immunity. CD8+ cytotoxic T lymphocytes (CTLs) are able to directly lyse tumor cells, produce cytokines that create a pro‐inflammatory environment and activate antigen‐presenting cells (APCs) (Hadrup, Donia, & Thor Straten, 2013). CD4+ T cells may be polarized to different subtypes, such as TH1, TH2 or Tregs (Cook et al., 2012; Swain, 1995). TH1 cells produce pro‐inflammatory cytokines that assist CD8+ CTLs in killing tumor cells and help creating an environment hostile to the tumor (Knutson & Disis, 2005). Other TH cell subsets, in particular Tregs, are not desired, as they inhibit the activity of tumor killing CTLs (Facciabene, Motz, & Coukos, 2012; Y. Wang et al., 2012). Hence, induction of TH1 cells and CTLs is the goal of cancer vaccines.
VLPs displaying T cell epitopes are capable of eliciting efficient TH1 and CTL responses even though they do not carry any genetic material (Deml, Schirmbeck, Reimann, Wolf, & Wagner, 1997; Kazaks, Balmaks, Voronkova, Ose, & Pumpens, 2008; Rueda et al., 1999). While exogenous antigens usually preferably enter the major histocompatibility Class II (MHC‐II) pathway and prime CD4+ T cells, VLPs have also the ability to be cross‐presented and enter the major histocompatibility Class I (MHC‐I) pathway (Bell, Young, & Banchereau, 1999; Moron, Rueda, Sedlik, & Leclerc, 2003). This ability is owed to their particulate nature which allows the VLPs to be efficiently taken up by APCs (Figure 2) and enter the MHC‐I pathway via a transporter‐associated with antigen processing (TAP)‐independent endosomal pathway, where MHC‐I molecules are loaded within the endosome as well as a TAP‐dependent endosome to cytosol pathway (Bachmann et al., 1995; Ruedl et al., 2005; Storni & Bachmann, 2004). VLPs combined with stimuli for APCs are able to induce CTL and TH1 responses. We have, for example, shown that bacteriophage‐derived Qβ‐VLPs are able to induce CTL and TH1 responses when loaded with toll‐like receptor (TLR)‐ligands such as RNA or CpGs, whereas they fail to do so when empty or loaded with polyglutamate (Keller et al., 2010; Schwarz et al., 2005). Packaging CpGs inside VLPs' shell was found to have several advantages: (a) it ensures specific delivery of the CpGs to APCs actually presenting the peptide, (b) it enhances the stability of CpGs, (c) it reduces unfavorable side effects of administering free CpGs and (d) it augments T cell responses (Storni et al., 2004). Interestingly, TLR‐ligands and antigens can be delivered in separate VLPs without the need for physical linkage and still generate a significant CTL response (Gomes et al., 2017; Mohsen et al., 2017). Various adjuvants, in particular those that form a depot or drive additional activation pathways, provide further opportunities to enhance protective T cell responses.
Figure 2.
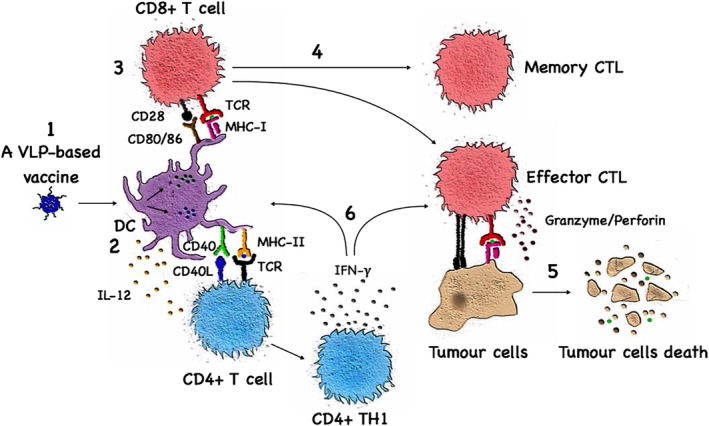
Virus‐like particle (VLP)‐based vaccines and the induction of T cell responses. Following injection, a VLP‐based vaccine is taken up by dendritic cells and macrophages (1). The phagocytosed VLPs displaying the tumor antigen will be processed and presented on both MHC‐II (2) and MHC‐I (3) for recognition by CD4+ and CD8+ T cells, respectively. Naïve CD8+ T cells will proliferate and differentiate into various types of effector and memory cytotoxic T‐lymphocyte (CTLs) (4). CTLs will initiate the killing process of tumor cells (5). Effector CD4+ TH1 cells enhance antigen presentation by antigen‐presenting cells and assist activated CTLs (6)
VLPs may be taken up locally at the injection site or freely drain to LNs where they are taken by LN resident APCs (Bachmann & Jennings, 2010; Cubas et al., 2009). Interestingly, both LN resident CD8+ and CD8− dendritic cells (DCs) are able to present VLP‐derived epitopes in association with MHC‐II molecules, while CD8+ DCs are efficient at cross‐presenting peptides on MHC‐I molecules (Keller et al., 2010). Other types of DCs, such as Langerhans and dermal DCs as well as macrophages have also been reported to cross‐present VLP‐derived antigens (Ruedl et al., 2005; Ruedl, Storni, Lechner, Bachi, & Bachmann, 2002).
We have seen a strong ability of VLPs to boost T cell responses in a homologous fashion both for Qbeta and human papilloma virus (HPV)‐E7 oligomers (Gomes, Flace, et al., 2017; Schwarz et al., 2005). However, we have also seen that passively transferred antibodies (Abs) before priming with VLPs strongly reduces subsequent cross‐priming using the Qbeta platform (Keller et al., 2010). Hence, if T cell responses upon priming are low, Abs may prevent further boosting of the T cell response. With this respect it is interesting to note that HPV VLPs are not loaded with RNA, which may limit T cell immunogenicity upon priming (Da Silva et al., 2003).
3. VLP‐BASED VACCINES AND THE INDUCTION OF B CELL RESPONSES
Antibodies may also play important roles in anti‐tumor immune responses and have been utilized for the development of effective cancer treatments (Figure 3). The effector killing of Abs can be achieved through different mechanisms such as the induction of apoptosis, abrogation of tumor‐cell signaling pathways, activating complement‐dependent cytotoxicity, regulating T cell function or by antibody‐dependent cellular cytotoxicity (ADCC; Attarwala, 2010; Snijdewint et al., 2001; W. Wang, Erbe, Hank, Morris, & Sondel, 2015; Weiner, Surana, & Wang, 2010). Antigens bound to Abs are taken up by antigen presenting cells via Fc receptors. This enhances MHC presentation and thereby modulates T cell responses. In addition, such immune‐complexes fix complement which causes activation of the APCs, further boosting T cell responses (Platzer, Stout, & Fiebiger, 2014).
Figure 3.
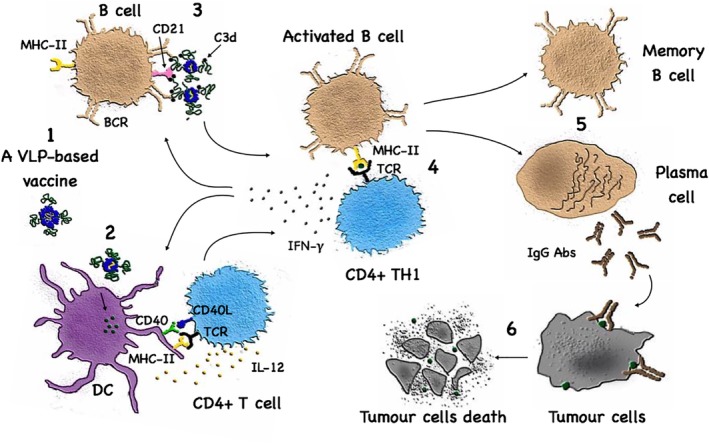
Virus‐like particle (VLP)‐based vaccines and the induction of B cell responses. The highly organized and repetitive surface geometry of a VLP‐based vaccine (1) facilitates its opsonization and phagocytosis by antigen‐presenting cells (2) as well as the engagement of CD21 on B cells (3). B cells also interact with and receive help from TH cells subsequent to uptake of VLP‐based vaccines via B cell receptor (4). This interaction between TH CD4+ T cells and B cells are essential for efficient generation of Ab‐producing plasma cells as well as for memory B cells (5). Many VLPs carry RNA packaged during production engaging TLR7/8 in B cells, promoting isotype‐switching towards protective TH1 IgG subclasses which will cause tumor cell destruction (6)
Antibodies are the crucial effector molecules induced by the majority of VLP‐based vaccines. The surface of most viruses and bacteria in nature consists of highly organized and repetitive proteins (Bachmann & Zinkernagel, 1997; Feldmann & Easten, 1971). The innate immune system has evolved to recognize such repetitive surfaces and such structures may be viewed as PASP (Bachmann & Jennings, 2011). Most notable, such repetitive patterns can effectively cross‐link B cell receptors (BCRs) and deliver strong activation signals to B cells (Bachmann et al., 1993; Chackerian, Lowy, & Schiller, 2001). This concept also holds true for peptides and proteins linked to VLPs, as up to 300 or 400 peptides can be displayed on a VLP's surface with 5–10 nm distance and thus are capable of eliciting optimal Ab responses (Jegerlehner et al., 2002).
Most antigens targeted by Abs are cell surface molecules. However, cell surface molecules expressed by the host generally induce B cell unresponsiveness (Goodnow, 1996) and conventional vaccination strategies are not able to overcome tolerance and induce Abs specific for cell surface molecules. It is therefore of key importance in this context that highly repetitive antigens as found on the viral surface (Bachmann et al., 1993) or displayed on VLPs (Chackerian et al., 2001; Spohn et al., 2007) can overcome B cell unresponsiveness and induce specific Ab responses against membrane proteins. Indeed, the membrane protein HER‐2 displayed on VLPs was also able to induce specific Abs (Palladini et al., 2018). In addition to Abs‐specific membrane proteins, intracellular proteins may also be an attractive target. Intracellular proteins are an important class of targets as many of the classical cancer testis antigens, for example, MAGE (van der Bruggen et al., 1991) or NY‐ESO‐1 (Jager et al., 1998) is in fact intracellular. Specific Abs may be able to enhance inflammatory processes in the tumor and may also enhance CD4+ and CD8+ T cell responses by modulating antigen presentation. Again, the unique immunogenicity of antigens displayed on VLPs may be a key for the success of such vaccines.
VLPs displaying self‐antigens have been used preclinically and clinically for successful induction of self‐antigen‐specific Abs that could break self‐tolerance and unresponsiveness in different chronic diseases (Bachmann & Jennings, 2011). Generally, VLPs are efficient in activating complement via the alternative and classical pathways (Barrington, Zhang, Fischer, & Carroll, 2001) facilitating subsequent activation of B cells via the engagement of the CD21/CD19 complexes (Carter & Myers, 2008). The engagement of these receptors enhances the expression of different transcription factors required for the differentiation of long‐lived plasma cells such as X‐box binding protein 1 and B lymphocyte‐induced maturation protein 1 (Gatto et al., 2005; Martins & Calame, 2008). In addition, via binding of natural IgM Abs and recruitment of C1q, B cells can bind VLPs through CD21 and deliver them into B cell follicles for deposition on follicular dendritic cells driving the germinal center reaction (Link et al., 2012). Complement‐decorated viral particles are effectively targeted to secondary lymphoid organs (Ochsenbein et al., 1999) and also stimulate T cell responses (Kopf, Abel, Gallimore, Carroll, & Bachmann, 2002). Many VLPs package RNA during production, which serves as a ligand for TLR7/8 in B cells. This drives isotype switching to more protective IgG subclasses (IgG1 in humans, IgG2a in mice) (Gomes et al., 2019; Bessa & Bachmann, 2010; Jegerlehner et al., 2007), resulting in enhanced ADCC.
4. VACCINES IN THE CONTEXT OF CHECK‐POINT INHIBITORS
Check‐point inhibitors are monoclonal Abs blocking molecules involved in inhibition of T cell activation, enhancing T cell responses and anti‐tumor protection (Figure 4). T cell activation may be inhibited through immune regulation, usually mediated by regulatory T cells (Tregs) (Tanaka & Sakaguchi, 2017). Alternatively, T cells may be blocked to become fully activated through inhibitory receptors or cytokines (Ottaviano, De Placido, & Ascierto, 2019). Ipilimumab serves as a good example for blocking Tregs by targeting the cytotoxic T‐lymphocyte‐associated antigen 4 (CTLA4) while Nivolumab blocks programmed cell death protein 1 (PD‐1), a receptor on T cells that directly blocks T cell activation (Seidel, Otsuka, & Kabashima, 2018). Inhibiting both pathways has so far shown best results in the clinic (Topalian, Taube, Anders, & Pardoll, 2016). Check‐point inhibitors can, however, only amplify preexisting T cell responses. It is therefore rather obvious that combining the vaccination approach with check‐point inhibitors may be an optimal strategy, as the vaccine induces T cell responses which are amplified in their immune attack capacity by the check‐point inhibitors. Indeed, we have recently shown in mice that VLP‐based vaccination against melanoma is much more effective, if Tregs are depleted during therapy (Mohsen, Vogel, et al., 2019).
Figure 4.
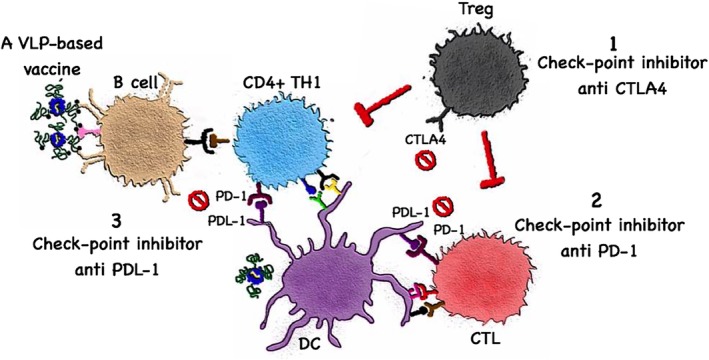
Vaccines in the context of check‐point inhibitors. Cancer immunotherapy targets immune checkpoints that regulates and inhibits the immune system. Monoclonal antibodies targeting (1) CTLA4 molecules on Tregs, for example, Ipilimumab (2) PD‐1 on T cells, for example, Nivolumab or Pembrolizumb (3) PDL‐1 on myeloid cells or/and tumor cells, for example, Atezolizumab have been approved by The food and drug administration (FDA) and are currently used in the clinics for treating different solid tumors. Combining a VLP‐based vaccine with checkpoint inhibitor is an optimal strategy to augment the immune response
5. VIRUS‐LIKE PARTICLES AS THERAPEUTIC CANCER VACCINES: PRECLINICAL AND CLINICAL STUDIES
Published preclinical and clinical studies have provided notable and convincing evidences that VLPs constitute an immunogenic template for the development of effective therapeutic cancer vaccines. The protective principle induced by VLPs may be based on T cells and/or Ab responses. In this section, we discuss some promising preclinical and clinical studies as summarized in Table 2 in different types of cancers, mainly solid tumors. The use of VLPs as a delivery system for therapeutic drugs is not discussed in this review.
Table 2.
List of preclinical studies and clinical trials using virus‐like particles (VLPs) as a vaccine template in different types of solid tumors
| VLP | Cancer type | Cancer antigen targeted | Adjuvant or combination therapy | Study phase | References |
|---|---|---|---|---|---|
| Preclinical studies | |||||
| Polyomavirus | Melanoma | OVA (model antigen), TRP2 | With or without QuilA‐saponin adjuvant | Preclinical | Brinkman et al. (2005) |
| Bacteriophage Qβ | Melanoma | PMEL17, MTC‐1, Calpastatin, ZFP518, TRP‐2, Caveolin2, Cpsf3l and Kifl8b | Anti‐CD25 | Preclinical | Mohsen, Vogel, et al. (2019) |
| Cucumber mosaic VLPs | Melanoma | LCMV‐gp33 | Microcrystalline tyrosine | Preclinical | Mohsen, Heath, et al. (2019) |
| Empty cowpea mosaic VLPs | Metastatic models | — | — | Preclinical | Lizotte et al. (2016) |
| MS2 | Breast cancer | xCT | — | Preclinical | Bolli et al. (2018) |
| AP205 | Breast cancer | HER‐2 | — | Preclinical | Palladini et al. (2018) |
| HBcAg | Hepatocellular carcinoma | MAGE‐1, MAGE‐3, AFP‐1 | — | Preclinical | H. G. Zhang et al. (2007) |
| HBcAg | Hepatocellular carcinoma | HBx | — | Preclinical | Ding et al. (2009) |
| SIV | Pancreatic cancer | Trop2 | Alone or with gemcitabine | Preclinical | Cubas, Zhang, Li, Chen, and Yao (2011) |
| SHIV | Pancreatic cancer | hMSLN | — | Preclinical | Li et al. (2008) |
| SHIV | Pancreatic cancer | mMSLN | — | Preclinical | S. Zhang et al. (2013) |
| IBDV | Cervical cancer | E7 | — | Preclinical | Martin Caballero et al. (2012) |
| RHDV | Cervical cancer | E6 | Anti‐CTLA4 or anti‐CD25 | Preclinical | Jemon et al. (2013) |
| Bacteriophage Qβ, HBcAg | Fibrosarcoma | LCMV‐gp33 | CpG type B | Preclinical | Storni et al. (2004) |
| Bacteriophage Qβ | Adenocarcinoma | TACAs (Tn) | — | Preclinical | Sungsuwan, Wu, and Huang (2017) |
| RHDV | Colorectal cancer | Topoisomerase IIα and surviving | Unmethylated CpGs | Preclinical | Donaldson et al. (2017) |
| Clinical trials | |||||
| Bacteriophage Qβ | Melanoma | — | Pembrolizumab (anti‐PD‐1) | Phase I | NCT02680184 (https://www.clinicaltrials.gov/ct2/show/NCT03596476?rank=1) |
| Bacteriophage Qβ | Malignant melanoma | — | Pembrolizumab (anti‐PD‐1) | Phase I | NCT03084640 (https://www.clinicaltrials.gov/ct2/show/NCT03084640?cond=CMP-001&rank=4) |
| Bacteriophage Qβ | Melanoma, lymph node cancer | — | Nivolumab (anti‐PD‐1) | Phase II | NCT03618641 (https://www.clinicaltrials.gov/ct2/show/NCT03618641?cond=cmp-001&rank=1) |
| Bacteriophage Qβ | Melanoma Stage II/IV | Melan‐A | CpG type A | Phase I/II | Speiser et al. (2010) |
| Bacteriophage Qβ | Melanoma Stage II/IV | Melan‐A | IFA (Montanide), topical Imiquimod +/− IFA | Phase IIa | Goldinger et al. (2012) |
| Chimaeric HPV16‐VLPs | Cervical intraepithelial neoplasia (CIN 2/3) | E7 and 16L1 | — | Phase I | Kaufmann et al. (2007) |
5.1. VLP‐based vaccines against melanoma
Brinkman et al. (2005) have used the empty polyomavirus‐like particles as a vaccine platform in a melanoma murine model. The study has evaluated the therapeutic value of the particulate structure of the major coat protein VP1 by fusing a nonself or a self‐antigen at the C terminus of VP1. Ovalbumin (OVA257–264) was used as a model of nonself‐antigen while tyrosinase‐related protein (TRP2180–188) was used as a self‐antigen. Both antigens are H2‐Kb restricted T cell epitopes. VP1‐OVA252–270 successfully formed VLPs of about 45 nm displaying the epitope inside the particle (Figure 5a‐1), while VP1‐TRP2180–188 failed to form full capsid VLPs and resulted in the formation of polyomavirus‐like‐pentamers of about 9 nm (Figure 5a‐2). The immunogenicity of VP1‐OVA252–270 VLPs was evaluated by using H2‐Kb‐OVA tetramers in the splenocytes of immunized mice. The efficacy of the VLP‐based vaccine was measured by assessing survival. VLPs and polyomavirus‐like‐pentamers of VP1‐OVA252–270 increased survival by 80–100%. In contrast, the therapeutic treatment with polyomavirus‐like‐pentamers VP1‐TRP2180–188 enhanced survival by 40%. The study indicated that both VLPs and polyomavirus‐like‐pentamers are capable of inducing CTL responses in mice (Brinkman et al., 2005). Whether increased efficacy of the OVA‐based vaccine (corresponding to a mutated epitope) compared to the TRP2‐based vaccine (corresponding to a self‐epitope) is due to increased efficacy of the “mutated” epitope or because it assembled into a full VLP remains to be seen.
Figure 5.
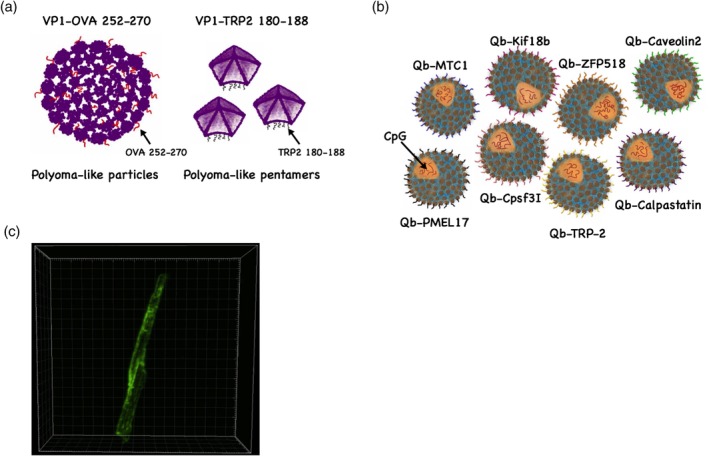
Virus‐like particle (VLP)‐based vaccines against melanoma. (a) (1) Ovalbumin (OVA257–264) nonself epitope was fused to the C terminus of VP1 major coat protein of polyomavirus forming polyoma‐like particles of ~45 nm. (2) Tyrosinase‐related protein 2 (TRP2180–188) self‐epitope was fused to VP1 major coat protein forming polyoma‐like pentamers of ~9 nm. (b) Mix multi‐target VLP‐based vaccine consisting of germline and mutated CTL epitopes coupled to Qβ‐VLPs by Cu‐free click chemistry and loaded with B‐type CpGs (Mohsen, Vogel, et al., 2019). (c) A microcrystalline tyrosine crystal decorated with CuMVTT‐p33 nanoparticles labeled with AF488 (Mohsen, Heath, et al., 2019)
In 2010, we developed a therapeutic human VLP‐based vaccine Qβ(G10)‐Melan‐A that was tested in Phase I/II study in Stage II/IV melanoma patients. The VLP‐based vaccine was designed by linking the melanoma differentiation specific antigen Melan‐A/Mart1 (The HLA‐A2 restricted epitope extended by a few amino acids) to Qβ‐VLPs which were additionally packaged with the TLR‐9 ligand G10 (a type‐A CpG) as an adjuvant for effective stimulation of DCs and CD8+ T cells (Speiser et al., 2010). Type A CpGs were used as they are the strongest inducers of type I interferons which is predominantly produced by plasmacytoid DCs (Pashine, Valiante, & Ulmer, 2005). The results of the study were promising as 63% of the patients generated specific T cell responses capable of producing high cytokine levels (IFN‐γ, TNF‐α, IL‐2), and the responses included central memory T cells. The route of administration (subcutaneous vs. intradermal) did not reveal important differences (Speiser et al., 2010). In addition to strong CD8+ T cell responses, Melan‐A‐specific CD4+ T cell responses were also induced upon vaccination (Braun et al., 2012). Subsequently, we have conducted a Phase IIa study for Stage II/IV melanoma patients using the same vaccine Qβ(G10)‐Melan‐A, formulated in different adjuvants such as Montanide (incomplete Freund's adjuvant; IFA) or topical Imiquimod and comparing different routes of administration (direct injection into the lymph node [LN], subcutaneous or intradermal). The results showed that 76% of the patients generated specific T cell responses. Using topical Imiquimod as an adjuvant with Qβ(G10)‐Melan‐A vaccine injected subcutaneous or intradermal route was the most effective at inducing central memory T cells. This study concluded that combining CpGs with topical Imiquimod could enhance CD8+ central memory T cell responses in melanoma patients (Goldinger et al., 2012). Furthermore, and most remarkably, the study demonstrated by positron emission tomography a long‐lasting immune response for more than a year in draining LNs of 87% of vaccinated individuals. This reveals a sustained immune response induced by the VLP‐based vaccine. Some patients exhibited late onset loss of Melan‐A expression by tumor cells in situ, suggesting that the strong T cell responses provoked outgrowth of antigen loss tumor cell variants, indicating that using a single peptide may not be sufficient to eliminate tumors (Goldinger et al., 2012).
CMP‐001 is the same VLP as described above and therefore is also based on the bacteriophage Qβ‐VLPs loaded with A‐type CpGs. This VLP was formerly called QβG10 and has been successfully used to treat allergies (Klimek et al., 2011) and allergic asthma (Beeh et al., 2013). The same VLP, now called CMP‐001, is being examined for the treatment of cancer. In contrast to conventional vaccines, it does not contain any tumor antigen(s). Three clinical trials are currently ongoing, testing the administration of CMP‐001 as monotherapy or in combination with anti‐PD‐1 check‐point inhibitors (Pembrolizumab or Nivolumab) in melanoma patients; NCT02680184, NCT03084640 and NCT03618641. The recently collected data from the ongoing clinical trial Ib (NCT02680184) showed a manageable toxicity profile and could reverse resistance to check‐point inhibition with significantly increased clinical benefits. The study has been extended and is expected to end in November 2019 (American Association for Cancer Research Annual Meeting, 2018). CMP‐001 is also examined in further trials including patients with NSCLC (NCT03438318), colorectal cancer (CRC) and lymphoma.
Following a similar avenue, Lizotte et al., used an empty Cowpea Mosaic VLPs (eCPMV) in a preclinical model of metastatic cancer. eCPMV is a nonenveloped plant virus that is about 30 nm in size and does not carry any nucleic acids. The study determined whether inhalation of eCPMV could induce anti‐tumor immunity in a lung metastatic B16F10 melanoma murine model. The results revealed that inhalation of eCPMV significantly reduced the tumor burden which was confirmed by assessing tyrosinase expression and metastatic‐like tumor foci in the lungs of B16F10 tumor bearing mice. Furthermore, the inhalation of eCPMV triggered the activation and accumulation of neutrophils in the lungs accompanied with an increase in neutrophil‐associated chemokines and cytokines. The study concluded that the anti‐tumor effects induced by eCPMV required neutrophils. eCPMV was also tested in the 4T1 metastatic lung murine model as well as in disseminated peritoneal ovarian cancer murine model. The results showed again that eCPMV could hinder tumor progression and enhance the survival rate. Furthermore, the authors have shown that intra‐tumoral injection of eCPMV is also effective in treating dermal B16F10 melanomas, forming central tumor necrosis (Lizotte et al., 2016).
We have recently utilized Qβ‐VLPs as a platform to develop a personalized VLP‐based vaccine. Germline and mutated CTL epitopes of B16F10 murine melanoma cell line were identified and predicted by immunopeptidomics and whole exome sequencing, respectively. Corresponding peptides were coupled to Qβ‐VLPs loaded with TLR9 ligands using the bio‐orthogonal Cu‐free click chemistry method, a key step in our novel platform. Three sets of multi‐target vaccines have been prepared, one set was based on identified germline CTL epitopes (GL‐MTV[multi target vaccine]), the second set was based on mutated CTL epitopes (Mutated‐MTV) and the last set combined both types of epitopes (Mix‐MTV) (Figure 5b). Both GL‐MTV and Mutated‐MTV induced protection; however, the best therapeutic effect was achieved by combining both germline and mutated epitopes. The Mix‐MTV could significantly hinder the progression of the aggressive transplanted B16F10 murine melanoma model by enhancement of CD8+ T cell infiltration into the tumor. Furthermore, Mix‐MTV affected the myeloid composition of the tumor by increasing the infiltration of Ly6G+ granulocytic cells and decreasing the monocytic cells characterized by Ly6C+ expression (Mohsen, Vogel, et al., 2019).
In another recent study, we have harnessed the physiological properties of the lymphatic system for better CTL responses when vaccinating with VLPs. Basically, we have formulated cucumber mosaic virus‐derived nanoparticles (CuMVTT‐VLPs) incorporating a universal tetanus toxoid epitope TT830–843 and displaying the LCMV‐gp33 peptide with the micron‐sized microcrystalline adjuvant (MCT). Our results show that CuMVTT‐p33 nanoparticles decorate the surface of the micron‐sized MCT adjuvant and form a local depot (Figure 5c). Using the stringent B16F10 murine tumor model our results showed that vaccination with this combination enhanced the specific CTL response, mediating strong anti‐tumor effects (Mohsen, Heath, et al., 2019).
5.2. VLP‐based vaccines against breast cancer
VLP‐based vaccines have also been tested in breast cancer in several preclinical studies. The human epidermal growth factor receptor 2 “HER‐2 or HER‐2/neu” (Figure 6a) has been shown to be over‐expressed in certain types of aggressive breast cancers and thus constitutes a promising vaccine target (Mitri, Constantine, & O'Regan, 2012).
Figure 6.
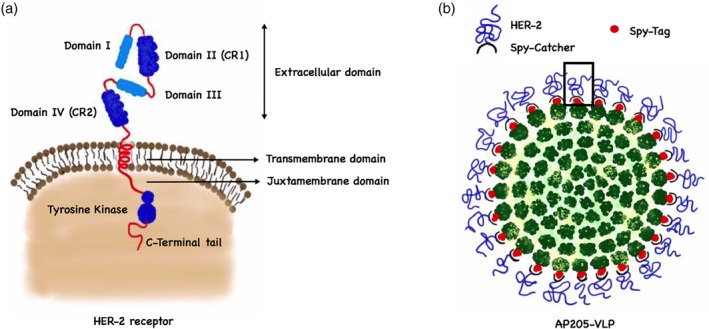
Virus‐like particle (VLP)‐based vaccines against breast cancer. (a) A schematic diagram of the structure of the HER‐2 receptor. The extracellular part consists of four domains: Domain I, Domain II (CR1), Domain III and Domain IV (CR2). The other domains are single transmembrane domain, juxtra membrane domain, tyrosine‐kinase domain and finally a C‐terminal tail. (b) SpyTag/SpyCatcher VLP‐based vaccine based on displaying the subdomains I–IV of HER‐2 combined to SyCatcher covalently attached to AP205‐VLP outer surface fused to a SpyTag part
A recent preclinical study conducted by Bolli et al. (2018) has shown that AX09‐0M6 VLPs derived from the bacteriophage MS2 can efficiently be used as a cancer vaccine platform in some aggressive forms of breast cancers, namely triple‐negative and HER‐2+ breast cancer. The study was designed to target the cysteine–glutamate exchanging transporter (xCT), a member of heterodimeric sodium‐independent transporter system that is highly specific for cysteine and glutamate (Lewerenz et al., 2013). xCT protein has been found to be over‐expressed in breast cancer stem cells (Saya, 2014). The AX09‐OM6 vaccine was designed by inserting the six extracellular domain of human xCT protein into MS2‐VLP single coat protein in the AB loop domain. The vaccine elicited a potent IgG2a Ab response directed against the inserted xCT epitope. It was also tested in mice with established 4T1 subcutaneous breast cancer tumors. The results revealed that the vaccine significantly hampered the growth of the established tumors and prevented pulmonary metastases (Bolli et al., 2018).
Palladini et al. (2018) have designed a unique VLP‐based vaccine allowing a directional and high‐density display of the full extracellular domain of HER‐2 (subdomain I–IV) on the surface of AP205‐VLPs. The developed vaccine is based on a SpyTag/SpyCatcher conjugation system where HER‐2 antigen was genetically fused to the SpyCatcher sequence which is attached to the surface of AP205‐VLPs expressing the “SpyTag” (Figure 6b). The study demonstrated that the vaccine platform could overcome B cell tolerance and induced potent anti‐HER‐2 IgG titers. The therapeutic effect of the vaccine was tested in wild type mice grafted with HER‐2 mammary carcinoma cells. The induced Ab titers could effectively hinder tumor progression. The vaccine was also successfully tested as a prophylactic vaccine in transgenic mice spontaneously developing mammary carcinoma expressing HER‐2 (Delta 16 isoform).
5.3. VLP‐based vaccines against pancreatic cancer
Development of therapeutic cancer vaccines is considered promising for the treatment of pancreatic cancer (Laheru & Jaffee, 2005). Some studies have tested peptide vaccines, or whole‐tumor vaccines targeting some key signaling proteins such as K‐RAS, vascular endothelial growth factor or epidermal growth factor receptor (Jaffee et al., 2001). Li et al. (2008) have developed a chimeric VLP‐based vaccine by incorporating human mesothelin into simian‐human immunodeficiency virus VLPs. The target epitope was chosen based on previous studies indicating a role of MSLN in tumor adhesion and dissemination (Rump et al., 2004). The results showed that vaccination with the chimeric VLP‐based vaccine substantially inhibited progression of an orthotopic pancreatic tumor in mice. The developed vaccine increased CTL activity and caused reduction in regulatory T cells (Tregs; CD4+FOXP3+) which correlated with enhanced survival. In a next step, the group has further evaluated whether the incorporation of murine mMSLN into VLPs could break tolerance to self‐mMSLN and allow to mount an effective immune response capable of controlling tumor progression. Their results showed that immunization with mMSLN‐VLP vaccine can break the tolerance towards the self‐MSLN and induce specific CD8+ T cell response leading to tumor regression in an orthotopic pancreatic cancer murine model (Li et al., 2008).
In a similar study, mTrop2 was incorporated into the surface of an enveloped simian immunodeficiency virus‐VLP. The mTrop2‐VLP vaccine was tested in a syngeneic murine pancreatic cancer model (Cubas et al., 2011). The results were promising as a significant reduction in tumor size was accompanied by an increase in the infiltration of mTrop2‐specific CD8+ T cells, CD4+ T cells as well as natural killer cells. Furthermore, immunization with mTrop‐VLP caused a decrease in Tregs and myeloid‐derived suppressor cells in the tumor microenvironment (Cubas et al., 2011). The study also showed that combining the vaccine with gemcitabine—a chemotherapeutic drug—can significantly improve the survival of tumor bearing mice.
5.4. VLP‐based vaccines against cervical cancer
HPV oncogenes are attractive targets for the development of a therapeutic vaccine. Kaufmann et al. (2007) showed the results of a clinical trial where patients with high‐grade cervical intraepithelial neoplasia (CIN 2/3) were treated with HPV16 L1E7 CVLP as a prophylactic and a therapeutic vaccine. CVLP is a chimeric VLP vaccine consisting of truncated L1 protein at the C‐terminus linked to the N‐terminal part of the E7 protein (E71–55) of HPV16. The main aim of the study was to assess the vaccine's safety and efficacy in HPV16+ patients. The results showed that CVLP has a very good safety profile and could induce high Ab titers against L1 and low Ab titers against E7 as well as CTL responses against both proteins. About 39% of the vaccinated patients showed histological improvement to (CIN 1 or normal) versus 25% in the placebo group. Furthermore, 56% of the responders were HPV16 DNA‐negative by the end of the trial.
Martin Caballero et al. (2012) have developed a preclinical vaccine model by genetically fusing the E7 protein of HPV to VP2 of the infectious bursal disease virus‐VLPs (IBDV) with T1 symmetry (i.e., 60 subunits). The generated VLPs were DNA‐free. Two chimeric VLP‐based vaccines were developed, VLP‐E7 with E7 on the surface and VLP‐E7‐B displaying the heterologous E7 sequence inside the VLP (Figure 7a). The vaccine was tested in HLA‐A2 transgenic mice engrafted with the TC‐1/A2 cervical cancer cell line expressing E7 protein. The results showed that the VLP‐E7 vaccine could elicit IFN‐γ secreting T cells against HPV T cell epitopes even without the use of an adjuvant. VLP‐E7 and VLP‐E7‐B vaccines revealed similar immunogenicity and efficacy indicating that the fusion site is not important. Furthermore, immunizing mice with VLP‐E7, 5 or 12 days post‐tumor cell line inoculation yielded 100% protection. The vaccine was also tested in more stringent situations against preexisting 200–300 mm3 tumors and showed complete eradication of the established tumors as well as enhancing the survival of the mice.
Figure 7.
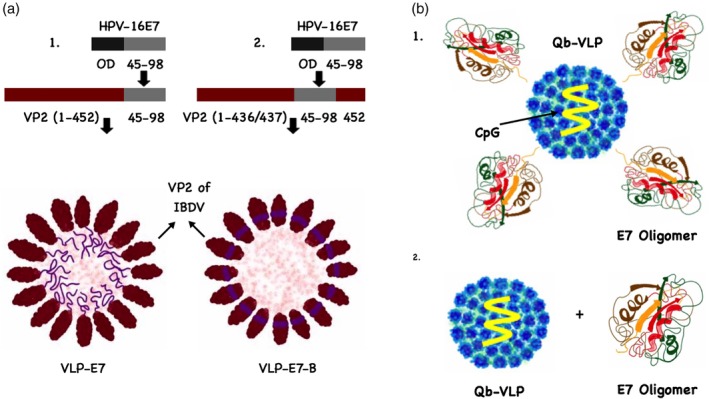
Virus‐like particle (VLP)‐based vaccines against cervical cancer. (a) A sketch illustrates human papilloma virus (HPV) VLP‐based vaccines: (1) VLP‐E7 was based on cloning the C‐terminal region of HPV‐E7 epitope “45–98” excluding the oncogenic domain OD to the C‐terminal of VP2 of IBDV‐VLP, while (2) VLP‐E7‐B was generated by inserting the HPV‐E7 epitope into the VP2 protein. (b) (1) E7 oligomers “50 nm” chemically coupled to Qβ‐VLPs “25–30 nm” using SMPH cross‐linker and loaded with nonmethylated CpGs, (2) E7 oligomers admixed with Qβ‐VLPs loaded with CpGs
Jemon et al. (2013) have used in their study a modified rabbit hemorrhagic disease virus‐VLP (RHDV‐VLP) decorated with the E748–57 peptide. The developed vaccine was tested in combination with anti‐CTLA4 or anti‐CD25 Abs to deplete Tregs in preexisting TC‐1 tumors expressing E6 and E7 in mice. The tumor burden was efficiently reduced by 50% with increased median survival of HPV tumor‐bearing mice.
Gomes, Flace, et al. (2017) have recently demonstrated that the physical linkage of antigens and adjuvants is not necessary for successful T cell activation in a therapeutic vaccine. The study has used E7 protein oligomers with a size of about 50 nm derived from HPV. E7 oligomers were chemically coupled to Qβ‐VLPs loaded with nonmethylated CpGs or simply admixed (Figure 7b). The study has shown that both formulations are capable of inducing protective CD4+ and CD8+ T cell responses as therapeutic vaccines in an HPV mouse model. The results also revealed that the physical linkage is, in contrast to T cells, necessary for generating B cell responses. The explanation for this unexpected finding was that particles of similar size drain to the same DCs, even if not physically linked.
5.5. VLP‐based vaccines against hepatocellular carcinoma
Data from H. G. Zhang et al. (2007) have shown that a chimeric VLPs can be generated using a truncated HBc‐VLP. Four different insertions of common hepatocellular carcinoma (HCC) epitopes; MAGE‐1 (278–286), MAGE‐3 (271–279), AFP1 (158–166) and AFP2 (542–550) were fused to the 3′ end of the truncated HBc‐VLPs. The developed vaccine was tested for its immunogenicity and efficacy in the B16‐pIR‐HH murine tumor model. In this study, DCs were pulsed with the generated chimaeric HBc‐VLP vaccine resulting in stronger CTL responses when compared to DCs pulsed only with peptides. The vaccine could inhibit the growth of the aggressive B16‐pIR‐HH tumor and prolonged the survival of the immunized mice (Y. Zhang et al., 2007).
Ding et al. (2009) developed a multi‐epitope peptide‐loaded VLP vaccine. This was carried out by the insertion of four HBx‐dominant CTL epitopes into the HBV core protein. HBx is the abbreviation for HBV X multifunctional regulatory protein that has been shown to participate in viral oncogenesis. The expression of this gene induces the formation of liver cancer in transgenic mouse models (Cheng, Hu, King, Jay, & Campbell, 1997; Chu, 2000; Kim, Koike, Saito, Miyamura, & Jay, 1991). The inserted HBx epitopes are namely HBx (52–60), (92–100), (115–123) and (140–148). DCs from HLA‐A*0201 transgenic mice or peripheral blood lymphocytes from patients infected with HBV who are HLA‐A2+/HBx+ were pulsed with HBx(115–123), HBx(92–100), HBx(140–148), or HBx(52–60) and CTL response was measured. The results of the study have shown that the developed multi‐epitope VLP‐based vaccine was significantly more immunogenic and induced stronger anti‐tumor protection than using a single peptide epitope.
5.6. More VLP‐based vaccines against cancer
Storni et al. (2004) have shown that peptides chemically coupled to Qβ‐VLPs or genetically fused to the hepatitis B core antigen (HBc‐Ag) and loaded with nonmethylated CpGs can induce powerful CD8+ T cell responses in mice. They have used the H2‐Db restricted T cell epitope LCMV‐gp33 as a model antigen. Both types of VLP‐based vaccines hindered the growth of fibrosarcoma tumor cell lines transfected with the relevant epitope in a challenging therapeutic model.
Tumor‐associated carbohydrate antigens (TACAs) are over‐expressed in different types of cancers and are associated with greater progression and metastasis (Monzavi‐Karbassi, Pashov, & Kieber‐Emmons, 2013; Yin & Huang, 2012). TACAs are mainly recognized by BCRs on B cells. Scientists have utilized VLPs as a vaccine template to display TACAs. Specifically, the Tn antigen “N‐acetylgalactosamine monosaccharide structure” has been chemically coupled to Qβ‐VLPs resulting in the display of about 300–400 copies of Tn antigens on the VLPs' surface (Sungsuwan et al., 2017). The results of the study indicated that Qβ‐Tn vaccine can elicit significantly higher IgG Ab titers when compared to the control group immunized with Qβ‐VLPs only without Tn antigen. Furthermore, the induced Abs could recognize tumor cells expressing Tn antigen and protected the mice from the growth of murine mammary adenocarcinoma in a therapeutic setting (Sungsuwan et al., 2017).
The therapeutic potential of VLPs has also been tested in a CRC murine model using chimeric RHDV‐VLPs containing Topoisomerase IIα (topIIα) and/or survivin epitopes derived from CRC tumor‐associated antigens. The multi‐target VLP vaccine appeared more efficacious than the mono‐target vaccine with trends in further enhancing the survival and delaying the tumor growth. These results indicate that targeting more than one epitope may reduce the tumors' capacity to evolve and escape. Interestingly, the mice which achieved complete remission after vaccination were protected from a subsequent tumor rechallenge (Donaldson et al., 2017).
6. CHALLENGES FOR DEVELOPING VLP‐BASED CANCER VACCINES
With increasing capacities in recombinant technologies, the future will likely see a large number of VLP‐based cancer vaccines entering clinical development. However, several challenges are threatening the effective development of VLP‐based vaccines. For example, the genetic fusion of epitope insertions into VLPs may result sometimes in misfolding of the particles preventing proper assembly (Sander & Lollini, 2018; Shiyu Dai & Deng, 2018). It may be challenging to achieve an optimal homogenous and high‐density display of the epitopes on the VLPs' surface when using the chemical coupling methods. These problems are accelerated by the fact that T cell epitopes usually are hydrophic and prone to cause aggregation. In addition, the selected coupled or fused epitopes may not induce optimal immune responses for protection against tumors. Finally, high reliable preclinical (murine) models are missing.
CONFLICT OF INTEREST
M.O.M. and M.F.B. own shares of DeepVax GmbH involved in developing virus‐like particles‐based vaccines for cancer. D.E.S. and A.K. have declared no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by Qatar National Research Fund (PDRA grant PDRA4‐0118‐18002) and Swiss Cancer Research (grants KFS‐4291‐08‐2017‐R and KFS‐3971‐08‐2016).
Mohsen MO, Speiser DE, Knuth A, Bachmann MF. Virus‐like particles for vaccination against cancer. WIREs Nanomed Nanobiotechnol. 2020;12:e1579 10.1002/wnan.1579
Funding information Swiss Cancer Research Foundation, Grant/Award Numbers: KFS‐3971‐08‐2016, KFS‐4291‐08‐2017‐R; Qatar National Research Fund, Grant/Award Number: PDRA4‐0118‐18002
REFERENCES
- American Association for Cancer Research Annual Meeting . (2018). Intratumoral toll‐like receptor 9 (TLR9) agonist, CMP‐001, in combination with pembrolizumab can reverse resistance to PD‐1 inhibition in a phase Ib trial in subjects with advanced melanoma. Retrieved from http://www.abstractsonline.com/pp8/#!/4562/presentation/11133
- Arevalo, M. T. , Wong, T. M. , & Ross, T. M. (2016). Expression and purification of virus‐like particles for vaccination. Journal of Visualized Experiments, 112, e54041 10.3791/54041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attarwala, H. (2010). Role of antibodies in cancer targeting. Journal of Natural Science, Biology and Medicine, 1(1), 53–56. 10.4103/0976-9668.71675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, M. F. , & Jennings, G. T. (2010). Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nature Reviews. Immunology, 10(11), 787–796. 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- Bachmann, M. F. , & Jennings, G. T. (2011). Therapeutic vaccines for chronic diseases: Successes and technical challenges. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 366(1579), 2815–2822. 10.1098/rstb.2011.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann, M. F. , Oxenius, A. , Pircher, H. , Hengartner, H. , Ashton‐Richardt, P. A. , Tonegawa, S. , & Zinkernagel, R. M. (1995). TAP1‐independent loading of class I molecules by exogenous viral proteins. European Journal of Immunology, 25(6), 1739–1743. 10.1002/eji.1830250637 [DOI] [PubMed] [Google Scholar]
- Bachmann, M. F. , Rohrer, U. H. , Kundig, T. M. , Burki, K. , Hengartner, H. , & Zinkernagel, R. M. (1993). The influence of antigen organization on B cell responsiveness. Science, 262(5138), 1448–1451. [DOI] [PubMed] [Google Scholar]
- Bachmann, M. F. , & Zinkernagel, R. M. (1997). Neutralizing antiviral B cell responses. Annual Review of Immunology, 15, 235–270. 10.1146/annurev.immunol.15.1.235 [DOI] [PubMed] [Google Scholar]
- Barrington, R. , Zhang, M. , Fischer, M. , & Carroll, M. C. (2001). The role of complement in inflammation and adaptive immunity. Immunological Reviews, 180, 5–15. [DOI] [PubMed] [Google Scholar]
- Beeh, K. M. , Kanniess, F. , Wagner, F. , Schilder, C. , Naudts, I. , Hammann‐Haenni, A. , … Renner, W. A. (2013). The novel TLR‐9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. Journal of Allergy and Clinical Immunology, 131(3), 866–874. 10.1016/j.jaci.2012.12.1561 [DOI] [PubMed] [Google Scholar]
- Bell, D. , Young, J. W. , & Banchereau, J. (1999). Dendritic cells. Advances in Immunology, 72, 255–324. [DOI] [PubMed] [Google Scholar]
- Bessa, J. , & Bachmann, M. F. (2010). T cell‐dependent and ‐independent IgA responses: Role of TLR signalling. Immunological Investigations, 39(4–5), 407–428. 10.3109/08820131003663357 [DOI] [PubMed] [Google Scholar]
- Bolli, E. , O'Rourke, J. P. , Conti, L. , Lanzardo, S. , Rolih, V. , Christen, J. M. , … Cavallo, F. (2018). A virus‐like‐particle immunotherapy targeting epitope‐specific anti‐xCT expressed on cancer stem cell inhibits the progression of metastatic cancer in vivo. Oncoimmunology, 7(3), e1408746 10.1080/2162402X.2017.1408746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, M. , Jandus, C. , Maurer, P. , Hammann‐Haenni, A. , Schwarz, K. , Bachmann, M. F. , … Romero, P. (2012). Virus‐like particles induce robust human T‐helper cell responses. European Journal of Immunology, 42(2), 330–340. 10.1002/eji.201142064 [DOI] [PubMed] [Google Scholar]
- Brinkman, M. , Walter, J. , Grein, S. , Thies, M. J. , Schulz, T. W. , Herrmann, M. , … Hess, J. (2005). Beneficial therapeutic effects with different particulate structures of murine polyomavirus VP1‐coat protein carrying self or non‐self CD8 T cell epitopes against murine melanoma. Cancer Immunology, Immunotherapy, 54(6), 611–622. 10.1007/s00262-004-0655-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune, K. D. , Leneghan, D. B. , Brian, I. J. , Ishizuka, A. S. , Bachmann, M. F. , Draper, S. J. , … Howarth, M. (2016). Plug‐and‐display: Decoration of virus‐like particles via isopeptide bonds for modular immunization. Scientific Reports, 6, 19234 10.1038/srep19234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caitlin Lemke, A. S. , Krieg, A. , & Weiner, G. (2016). Combination cancer immunotherapy using checkpoint blockade and intratumoral virus‐like particles containing CpG ODN. Cancer Research, 76(14), 1417 10.1158/1538-7445.AM2016-1417 [DOI] [Google Scholar]
- Carter, R. H. , & Myers, R. (2008). Germinal center structure and function: Lessons from CD19. Seminars in Immunology, 20(1), 43–48. 10.1016/j.smim.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian, B. , Lowy, D. R. , & Schiller, J. T. (2001). Conjugation of a self‐antigen to papillomavirus‐like particles allows for efficient induction of protective autoantibodies. Journal of Clinical Investigation, 108(3), 415–423. 10.1172/JCI11849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. , Hu, J. , King, J. , Jay, G. , & Campbell, T. C. (1997). Inhibition of hepatocellular carcinoma development in hepatitis B virus transfected mice by low dietary casein. Hepatology, 26(5), 1351–1354. 10.1002/hep.510260538 [DOI] [PubMed] [Google Scholar]
- Chroboczek, J. , Szurgot, I. , & Szolajska, E. (2014). Virus‐like particles as vaccine. Acta Biochimica Polonica, 61(3), 531–539. [PubMed] [Google Scholar]
- Chu, C. M. (2000). Natural history of chronic hepatitis B virus infection in adults with emphasis on the occurrence of cirrhosis and hepatocellular carcinoma. Journal of Gastroenterology and Hepatology, (Suppl. 15), E25–E30. [DOI] [PubMed] [Google Scholar]
- Cook, P. C. , Jones, L. H. , Jenkins, S. J. , Wynn, T. A. , Allen, J. E. , & MacDonald, A. S. (2012). Alternatively activated dendritic cells regulate CD4+ T‐cell polarization in vitro and in vivo. Proceedings of the National Academy of Sciences of the United States of America, 109(25), 9977–9982. 10.1073/pnas.1121231109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas, R. , Zhang, S. , Kwon, S. , Sevick‐Muraca, E. M. , Li, M. , Chen, C. , & Yao, Q. (2009). Virus‐like particle (VLP) lymphatic trafficking and immune response generation after immunization by different routes. Journal of Immunotherapy, 32(2), 118–128. 10.1097/CJI.0b013e31818f13c4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas, R. , Zhang, S. , Li, M. , Chen, C. , & Yao, Q. (2011). Chimeric Trop2 virus‐like particles: A potential immunotherapeutic approach against pancreatic cancer. Journal of Immunotherapy, 34(3), 251–263. 10.1097/CJI.0b013e318209ee72 [DOI] [PubMed] [Google Scholar]
- Da Silva, D. M. , Schiller, J. T. , Kast, W. M. (2003). Heterologous boosting increases immunogenicity of chimeric papillomavirus virus‐like particle vaccines. Vaccine. 21(23), 3219–3227. 10.1016/S0264-410X(03)00237-8 [DOI] [PubMed] [Google Scholar]
- Dash, M. , Federica, C. , Ottenbrite, R. M. , & Chiellini, E. (2011). A versatile semi‐synthetic polymer in biomedical applications. Progress in Polymer Science, 36(8), 981–1014. [Google Scholar]
- Deml, L. , Schirmbeck, R. , Reimann, J. , Wolf, H. , & Wagner, R. (1997). Recombinant human immunodeficiency Pr55gag virus‐like particles presenting chimeric envelope glycoproteins induce cytotoxic T‐cells and neutralizing antibodies. Virology, 235(1), 26–39. 10.1006/viro.1997.8668 [DOI] [PubMed] [Google Scholar]
- Ding, F. X. , Wang, F. , Lu, Y. M. , Li, K. , Wang, K. H. , He, X. W. , & Sun, S. H. (2009). Multiepitope peptide‐loaded virus‐like particles as a vaccine against hepatitis B virus‐related hepatocellular carcinoma. Hepatology, 49(5), 1492–1502. 10.1002/hep.22816 [DOI] [PubMed] [Google Scholar]
- Donaldson, B. , Al‐Barwani, F. , Pelham, S. J. , Young, K. , Ward, V. K. , & Young, S. L. (2017). Multi‐target chimaeric VLP as a therapeutic vaccine in a model of colorectal cancer. Journal for Immunotherapy of Cancer, 5(1), 69 10.1186/s40425-017-0270-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene, A. , Motz, G. T. , & Coukos, G. (2012). T‐regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Research, 72(9), 2162–2171. 10.1158/0008-5472.CAN-11-3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann, M. , & Easten, A. (1971). The relationship between antigenic structure and the requirement for thymus‐derived cells in the immune response. Journal of Experimental Medicine, 134(1), 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcea, R. L. , & Gissmann, L. (2004). Virus‐like particles as vaccines and vessels for the delivery of small molecules. Current Opinion in Biotechnology, 15(6), 513–517. 10.1016/j.copbio.2004.10.002 [DOI] [PubMed] [Google Scholar]
- Gatto, D. , Pfister, T. , Jegerlehner, A. , Martin, S. W. , Kopf, M. , & Bachmann, M. F. (2005). Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp‐1 and XBP‐1. Journal of Experimental Medicine, 201(6), 993–1005. 10.1084/jem.20042239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldinger, S. M. , Dummer, R. , Baumgaertner, P. , Mihic‐Probst, D. , Schwarz, K. , Hammann‐Haenni, A. , … Speiser, D. E. (2012). Nano‐particle vaccination combined with TLR‐7 and ‐9 ligands triggers memory and effector CD8(+) T‐cell responses in melanoma patients. European Journal of Immunology, 42(11), 3049–3061. 10.1002/eji.201142361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, A. C. , Flace, A. , Saudan, P. , Zabel, F. , Cabral‐Miranda, G. , Turabi, A. E. , … Bachmann, M. F. (2017). Adjusted particle size eliminates the need of linkage of antigen and adjuvants for appropriated T cell responses in virus‐like particle‐based vaccines. Frontiers in Immunology, 8, 226 10.3389/fimmu.2017.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, A. C. , Mohsen, M. , & Bachmann, M. F. (2017). Harnessing nanoparticles for immunomodulation and vaccines. Vaccines (Basel), 5(1). 10.3390/vaccines5010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, A. C. , Roesti, E. S. , El‐Turabi, A. , Bachmann, M. F. (2019). Type of RNA Packed in VLPs Impacts IgG Class Switching—Implications for an Influenza Vaccine Design. Vaccines, 7(2), 47 10.3390/vaccines7020047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodnow, C. C. (1996). Balancing immunity and tolerance: Deleting and tuning lymphocyte repertoires. Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2264–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadrup, S. , Donia, M. , & Thor Straten, P. (2013). Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenvironment, 6(2), 123–133. 10.1007/s12307-012-0127-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee, E. M. , Hruban, R. H. , Biedrzycki, B. , Laheru, D. , Schepers, K. , Sauter, P. R. , … Yeo, C. J. (2001). Novel allogeneic granulocyte‐macrophage colony‐stimulating factor‐secreting tumor vaccine for pancreatic cancer: A phase I trial of safety and immune activation. Journal of Clinical Oncology, 19(1), 145–156. 10.1200/JCO.2001.19.1.145 [DOI] [PubMed] [Google Scholar]
- Jager, E. , Chen, Y. T. , Drijfhout, J. W. , Karbach, J. , Ringhoffer, M. , Jager, D. , … Knuth, A. (1998). Simultaneous humoral and cellular immune response against cancer‐testis antigen NY‐ESO‐1: Definition of human histocompatibility leukocyte antigen (HLA)‐A2‐binding peptide epitopes. Journal of Experimental Medicine, 187(2), 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner, A. , Maurer, P. , Bessa, J. , Hinton, H. J. , Kopf, M. , & Bachmann, M. F. (2007). TLR9 signaling in B cells determines class switch recombination to IgG2a. Journal of Immunology, 178(4), 2415–2420. [DOI] [PubMed] [Google Scholar]
- Jegerlehner, A. , Storni, T. , Lipowsky, G. , Schmid, M. , Pumpens, P. , & Bachmann, M. F. (2002). Regulation of IgG antibody responses by epitope density and CD21‐mediated costimulation. European Journal of Immunology, 32(11), 3305–3314. [DOI] [PubMed] [Google Scholar]
- Jegerlehner, A. , Tissot, A. , Lechner, F. , Sebbel, P. , Erdmann, I. , Kündig, T. , … Bachmann, M.F. (2002). A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses, Vaccine, 20(25‐26), 3104–3112. 10.1016/S0264-410X(02)00266-9 [DOI] [PubMed] [Google Scholar]
- Jemon, K. , Young, V. , Wilson, M. , McKee, S. , Ward, V. , Baird, M. , … Hibma, M. (2013). An enhanced heterologous virus‐like particle for human papillomavirus type 16 tumour immunotherapy. PLoS One, 8(6), e66866 10.1371/journal.pone.0066866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk, S. J. , Sitaraman, K. , Young, H. A. , Hughes, S. H. , & Chatterjee, D. K. (2011). Protein delivery using engineered virus‐like particles. Proceedings of the National Academy of Sciences of the United States of America, 108(41), 16998–17003. 10.1073/pnas.1101874108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, A. M. , Nieland, J. D. , Jochmus, I. , Baur, S. , Friese, K. , Gabelsberger, J. , … Schneider, A. (2007). Vaccination trial with HPV16 L1E7 chimeric virus‐like particles in women suffering from high grade cervical intraepithelial neoplasia (CIN 2/3). International Journal of Cancer, 121(12), 2794–2800. 10.1002/ijc.23022 [DOI] [PubMed] [Google Scholar]
- Kawano, M. , Matsui, M. , & Handa, H. (2018). Technologies that generate and modify virus‐like particles for medical diagnostic and therapy purposes In Grumezescu A, ed. Design and development of new nanocarriers (pp. 555–594). Norwich, NY: Willian Andrew, Applied Science Publishers. [Google Scholar]
- Kazaks, A. , Balmaks, R. , Voronkova, T. , Ose, V. , & Pumpens, P. (2008). Melanoma vaccine candidates from chimeric hepatitis B core virus‐like particles carrying a tumor‐associated MAGE‐3 epitope. Biotechnology Journal, 3(11), 1429–1436. 10.1002/biot.200800160 [DOI] [PubMed] [Google Scholar]
- Keller, S. A. , Bauer, M. , Manolova, V. , Muntwiler, S. , Saudan, P. , & Bachmann, M. F. (2010). Cutting edge: Limited specialization of dendritic cell subsets for MHC class II‐associated presentation of viral particles. Journal of Immunology, 184(1), 26–29. 10.4049/jimmunol.0901540 [DOI] [PubMed] [Google Scholar]
- Kim, C. M. , Koike, K. , Saito, I. , Miyamura, T. , & Jay, G. (1991). HBx gene of hepatitis B virus induces liver cancer in transgenic mice. Nature, 351(6324), 317–320. 10.1038/351317a0 [DOI] [PubMed] [Google Scholar]
- Klimek, L. , Kundig, T. , Kramer, M. F. , Guethoff, S. , Jensen‐Jarolim, E. , Schmidt‐Weber, C. B. , … Bachmann, M. (2018). Virus‐like particles (VLP) in prophylaxis and immunotherapy of allergic diseases. Allergo Journal International, 27(8), 245–255. 10.1007/s40629-018-0074-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimek, L. , Willers, J. , Hammann‐Haenni, A. , Pfaar, O. , Stocker, H. , Mueller, P. , … Bachmann, M. F. (2011). Assessment of clinical efficacy of CYT003‐QbG10 in patients with allergic rhinoconjunctivitis: A phase IIb study. Clinical and Experimental Allergy, 41(9), 1305–1312. 10.1111/j.1365-2222.2011.03783.x [DOI] [PubMed] [Google Scholar]
- Knutson, K. L. , & Disis, M. L. (2005). Tumor antigen‐specific T helper cells in cancer immunity and immunotherapy. Cancer Immunology, Immunotherapy, 54(8), 721–728. 10.1007/s00262-004-0653-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf, M. , Abel, B. , Gallimore, A. , Carroll, M. , & Bachmann, M. F. (2002). Complement component C3 promotes T‐cell priming and lung migration to control acute influenza virus infection. Nature Medicine, 8(4), 373–378. 10.1038/nm0402-373 [DOI] [PubMed] [Google Scholar]
- Laheru, D. , & Jaffee, E. M. (2005). Immunotherapy for pancreatic cancer—Science driving clinical progress. Nature Reviews. Cancer, 5(6), 459–467. 10.1038/nrc1630 [DOI] [PubMed] [Google Scholar]
- Lewerenz, J. , Hewett, S. J. , Huang, Y. , Lambros, M. , Gout, P. W. , Kalivas, P. W. , … Maher, P. (2013). The cystine/glutamate antiporter system x(c)(−) in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxidants & Redox Signaling, 18(5), 522–555. 10.1089/ars.2011.4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Bharadwaj, U. , Zhang, R. , Zhang, S. , Mu, H. , Fisher, W. E. , … Yao, Q. (2008). Mesothelin is a malignant factor and therapeutic vaccine target for pancreatic cancer. Molecular Cancer Therapeutics, 7(2), 286–296. 10.1158/1535-7163.MCT-07-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link, A. , Zabel, F. , Schnetzler, Y. , Titz, A. , Brombacher, F. , & Bachmann, M. F. (2012). Innate Immunity Mediates Follicular Transport of Particulate but Not Soluble Protein Antigen. The Journal of Immunology, 188(8), 3724–3733. 10.4049/jimmunol.1103312 [DOI] [PubMed] [Google Scholar]
- Lizotte, P. H. , Wen, A. M. , Sheen, M. R. , Fields, J. , Rojanasopondist, P. , Steinmetz, N. F. , & Fiering, S. (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nature Nanotechnology, 11(3), 295–303. 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolova, V. , Flace, A. , Bauer, M. , Schwarz, K. , Saudan, P. , & Bachmann, M. F. (2008). Nanoparticles target distinct dendritic cell populations according to their size. European Journal of Immunology, 38(5), 1404–1413. 10.1002/eji.200737984 [DOI] [PubMed] [Google Scholar]
- Martin Caballero, J. , Garzon, A. , Gonzalez‐Cintado, L. , Kowalczyk, W. , Jimenez Torres, I. , Calderita, G. , … von Kobbe, C. (2012). Chimeric infectious bursal disease virus‐like particles as potent vaccines for eradication of established HPV‐16 E7‐dependent tumors. PLoS One, 7, 12–e52976. 10.1371/journal.pone.0052976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins, G. , & Calame, K. (2008). Regulation and functions of Blimp‐1 in T and B lymphocytes. Annual Review of Immunology, 26, 133–169. 10.1146/annurev.immunol.26.021607.090241 [DOI] [PubMed] [Google Scholar]
- Mitri, Z. , Constantine, T. , & O'Regan, R. (2012). The HER2 receptor in breast cancer: Pathophysiology, clinical use, and new advances in therapy. Chemotherapy Research and Practice, 2012, 743193 10.1155/2012/743193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen, M. O. , Gomes, A. C. , Cabral‐Miranda, G. , Krueger, C. C. , Leoratti, F. M. , Stein, J. V. , & Bachmann, M. F. (2017). Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. Journal of Controlled Release, 251, 92–100. 10.1016/j.jconrel.2017.02.031 [DOI] [PubMed] [Google Scholar]
- Mohsen, M. O. , Gomes, A. C. , Vogel, M. , & Bachmann, M. F. (2018). Interaction of viral capsid‐derived virus‐like particles (VLPs) with the innate immune system. Vaccines (Basel), 6(3). 10.3390/vaccines6030037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen, M. O. , Heath, M. D. , Cabral‐Miranda, G. , Lipp, C. , Zeltins, A. , Sande, M. , … Bachmann, M. F. (2019). Vaccination with nanoparticles combined with micro‐adjuvants protects against cancer. Journal for Immunotherapy of Cancer, 7(1), 114 10.1186/s40425-019-0587-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen, M. O. , Vogel, M. , Riether, C. , Muller, J. , Salatino, S. , Ternette, N. , … Bachmann, M. F. (2019). Targeting mutated plus germline epitopes confers pre‐clinical efficacy of an instantly formulated cancer nano‐vaccine. Frontiers in Immunology, 10(1015). 10.3389/fimmu.2019.01015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohsen, M. O. , Zha, L. , Cabral‐Miranda, G. , & Bachmann, M. F. (2017). Major findings and recent advances in virus‐like particle (VLP)‐based vaccines. Seminars in Immunology, 34, 123–132. 10.1016/j.smim.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Monzavi‐Karbassi, B. , Pashov, A. , & Kieber‐Emmons, T. (2013). Tumor‐associated glycans and immune surveillance. Vaccines (Basel), 1(2), 174–203. 10.3390/vaccines1020174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moron, V. G. , Rueda, P. , Sedlik, C. , & Leclerc, C. (2003). In vivo, dendritic cells can cross‐present virus‐like particles using an endosome‐to‐cytosol pathway. Journal of Immunology, 171(5), 2242–2250. [DOI] [PubMed] [Google Scholar]
- Ochsenbein, A. F. , Fehr, T. , Lutz, C. , Suter, M. , Brombacher, F. , Hengartner, H. , & Zinkernagel, R. M. (1999). Control of early viral and bacterial distribution and disease by natural antibodies. Science, 286(5447), 2156–2159. [DOI] [PubMed] [Google Scholar]
- Ottaviano, M. , De Placido, S. , & Ascierto, P. A. (2019). Recent success and limitations of immune checkpoint inhibitors for cancer: A lesson from melanoma. Virchows Archiv, 474, 421–432. 10.1007/s00428-019-02538-4 [DOI] [PubMed] [Google Scholar]
- Palladini, A. , Thrane, S. , Janitzek, C. M. , Pihl, J. , Clemmensen, S. B. , de Jongh, W. A. , … Sander, A. F. (2018). Virus‐like particle display of HER2 induces potent anti‐cancer responses. Oncoimmunology, 7(3), e1408749 10.1080/2162402X.2017.1408749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashine, A. , Valiante, N. M. , & Ulmer, J. B. (2005). Targeting the innate immune response with improved vaccine adjuvants. Nature Medicine, 11(Suppl. 4), S63–S68. 10.1038/nm1210 [DOI] [PubMed] [Google Scholar]
- Platzer, B. , Stout, M. , & Fiebiger, E. (2014). Antigen cross‐presentation of immune complexes. Frontiers in Immunology, 5, 140 10.3389/fimmu.2014.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomwised, R. , Intamaso, U. , Teintze, M. , Young, M. , & Pincus, S. H. (2016). Coupling peptide antigens to virus‐like particles or to protein carriers influences the Th1/Th2 polarity of the resulting immune response. Vaccines (Basel), 4(2). 10.3390/vaccines4020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpens, P. (2016). Viral nanotechnology. Philadelphia, PA: Taylor and Francis. [Google Scholar]
- Pumpens, P. , Ulrich, R. , Sasnauskas, K. , Kazaks, A. , Ose, V. , & Grens, E. (2009). Construction of novel vaccines on the basis of the virus‐like particles: Hepatitis B virus proteins as vaccine carriers In Khudyakov YE, ed. Medicinal protein engineering (pp. 205–248). Boca Raton, Fl: CRC Press. [Google Scholar]
- Pushko, P. , Pumpens, P. , & Grens, E. (2013). Development of virus‐like particle technology from small highly symmetric to large complex virus‐like particle structures. Intervirology, 56(3), 141–165. 10.1159/000346773 [DOI] [PubMed] [Google Scholar]
- Roldao, A. , Mellado, M. C. , Castilho, L. R. , Carrondo, M. J. , & Alves, P. M. (2010). Virus‐like particles in vaccine development. Expert Review of Vaccines, 9(10), 1149–1176. 10.1586/erv.10.115 [DOI] [PubMed] [Google Scholar]
- Rueda, P. , Martinez‐Torrecuadrada, J. L. , Sarraseca, J. , Sedlik, C. , del Barrio, M. , Hurtado, A. , … Casal, J. I. (1999). Engineering parvovirus‐like particles for the induction of B‐cell, CD4(+) and CTL responses. Vaccine, 18(3–4), 325–332. [DOI] [PubMed] [Google Scholar]
- Ruedl, C. , Schwarz, K. , Jegerlehner, A. , Storni, T. , Manolova, V. , & Bachmann, M. F. (2005). Virus‐like particles as carriers for T‐cell epitopes: Limited inhibition of T‐cell priming by carrier‐specific antibodies. Journal of Virology, 79(2), 717–724. 10.1128/JVI.79.2.717-724.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedl, C. , Storni, T. , Lechner, F. , Bachi, T. , & Bachmann, M. F. (2002). Cross‐presentation of virus‐like particles by skin‐derived CD8(−) dendritic cells: A dispensable role for TAP. European Journal of Immunology, 32(3), 818–825. [DOI] [PubMed] [Google Scholar]
- Rump, A. , Morikawa, Y. , Tanaka, M. , Minami, S. , Umesaki, N. , Takeuchi, M. , & Miyajima, A. (2004). Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion. Journal of Biological Chemistry, 279(10), 9190–9198. 10.1074/jbc.M312372200 [DOI] [PubMed] [Google Scholar]
- Rynda‐Apple, A. , Patterson, D. P. , & Douglas, T. (2014). Virus‐like particles as antigenic nanomaterials for inducing protective immune responses in the lung. Nanomedicine, 9(12), 1857–1868. 10.2217/nnm.14.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, A. F. , & Lollini, P. L. (2018). Virus‐like antigen display for cancer vaccine development, what is the potential? Expert Review of Vaccines, 17(4), 285–288. 10.1080/14760584.2018.1455505 [DOI] [PubMed] [Google Scholar]
- Santi, L. , Huang, Z. , & Mason, H. (2006). Virus‐like particles production in green plants. Methods, 40(1), 66–76. 10.1016/j.ymeth.2006.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saya, G. Y. H. (2014). The novel anti‐tumor therapy targeting the “functional” cancer stem cell markers. Journal of Clinical & Experimental Pharmacology, 4, 147. [Google Scholar]
- Schiller, J. T. , & Lowy, D. R. (2015). Raising expectations for subunit vaccine. Journal of Infectious Diseases, 211(9), 1373–1375. 10.1093/infdis/jiu648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, K. , Meijerink, E. , Speiser, D. E. , Tissot, A. C. , Cielens, I. , Renhof, R. , … Bachmann, M. F. (2005). Efficient homologous prime‐boost strategies for T cell vaccination based on virus‐like particles. European Journal of Immunology, 35(3), 816–821. 10.1002/eji.200425755 [DOI] [PubMed] [Google Scholar]
- Seidel, J. A. , Otsuka, A. , & Kabashima, K. (2018). Anti‐PD‐1 and anti‐CTLA‐4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Frontiers in Oncology, 8, 86 10.3389/fonc.2018.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiyu Dai, H. W. , & Deng, F. (2018). Advances and challenges in enveloped virus‐like particle (VLP)‐based vaccines. Journal of Immunological Sciences, 2(2), 36–41. 10.29245/2578-3009/2018/2.1118 [DOI] [Google Scholar]
- Sioud, M. (2006). Innate sensing of self and non‐self RNAs by toll‐like receptors. Trends in Molecular Medicine, 12(4), 167–176. 10.1016/j.molmed.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Snijdewint, F. G. , von Mensdorff‐Pouilly, S. , Karuntu‐Wanamarta, A. H. , Verstraeten, A. A. , Livingston, P. O. , Hilgers, J. , & Kenemans, P. (2001). Antibody‐dependent cell‐mediated cytotoxicity can be induced by MUC1 peptide vaccination of breast cancer patients. International Journal of Cancer, 93(1), 97–106. [DOI] [PubMed] [Google Scholar]
- Speiser, D. E. , Schwarz, K. , Baumgaertner, P. , Manolova, V. , Devevre, E. , Sterry, W. , … Bachmann, M. F. (2010). Memory and effector CD8 T‐cell responses after nanoparticle vaccination of melanoma patients. Journal of Immunotherapy, 33(8), 848–858. 10.1097/CJI.0b013e3181f1d614 [DOI] [PubMed] [Google Scholar]
- Spohn, G. , Guler, R. , Johansen, P. , Keller, I. , Jacobs, M. , Beck, M. , … Bachmann, M. F. (2007). A virus‐like particle‐based vaccine selectively targeting soluble TNF‐alpha protects from arthritis without inducing reactivation of latent tuberculosis. Journal of Immunology, 178(11), 7450–7457. [DOI] [PubMed] [Google Scholar]
- Storni, T. , & Bachmann, M. F. (2004). Loading of MHC class I and II presentation pathways by exogenous antigens: A quantitative in vivo comparison. Journal of Immunology, 172(10), 6129–6135. [DOI] [PubMed] [Google Scholar]
- Storni, T. , Ruedl, C. , Schwarz, K. , Schwendener, R. A. , Renner, W. A. , & Bachmann, M. F. (2004). Nonmethylated CG motifs packaged into virus‐like particles induce protective cytotoxic T cell responses in the absence of systemic side effects. Journal of Immunology, 172(3), 1777–1785. [DOI] [PubMed] [Google Scholar]
- Sungsuwan, S. , Wu, X. , & Huang, X. (2017). Evaluation of virus‐like particle‐based tumor‐associated carbohydrate immunogen in a mouse tumor model. Methods in Enzymology, 597, 359–376. 10.1016/bs.mie.2017.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, S. L. (1995). T‐cell subsets. Who does the polarizing? Current Biology, 5(8), 849–851. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. , & Sakaguchi, S. (2017). Regulatory T cells in cancer immunotherapy. Cell Research, 27(1), 109–118. 10.1038/cr.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegerstedt, K. , Lindencrona, J. A. , Curcio, C. , Andreasson, K. , Tullus, C. , Forni, G. , … Ramqvist, T. (2005). A single vaccination with polyomavirus VP1/VP2Her2 virus‐like particles prevents outgrowth of HER‐2/neu‐expressing tumors. Cancer Research, 65(13), 5953–5957. 10.1158/0008-5472.CAN-05-0335 [DOI] [PubMed] [Google Scholar]
- Topalian, S. L. , Taube, J. M. , Anders, R. A. , & Pardoll, D. M. (2016). Mechanism‐driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Reviews. Cancer, 16(5), 275–287. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen, P. , Traversari, C. , Chomez, P. , Lurquin, C. , De Plaen, E. , Van den Eynde, B. , … Boon, T. (1991). A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science, 254(5038), 1643–1647. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Erbe, A. K. , Hank, J. A. , Morris, Z. S. , & Sondel, P. M. (2015). NK cell‐mediated antibody‐dependent cellular cytotoxicity in cancer immunotherapy. Frontiers in Immunology, 6, 368 10.3389/fimmu.2015.00368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Ma, Y. , Fang, Y. , Wu, S. , Liu, L. , Fu, D. , & Shen, X. (2012). Regulatory T cell: A protection for tumour cells. Journal of Cellular and Molecular Medicine, 16(3), 425–436. 10.1111/j.1582-4934.2011.01437.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, L. M. , Surana, R. , & Wang, S. (2010). Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nature Reviews. Immunology, 10(5), 317–327. 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, Z. , & Huang, X. (2012). Recent development in carbohydrate based anti‐cancer vaccines. Journal of Carbohydrate Chemistry, 31(3), 143–186. 10.1080/07328303.2012.659364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltins, A. , West, J. , Zabel, F. , El Turabi, A. , Balke, I. , Haas, S. , … Bachmann, M. F. (2017). Incorporation of tetanus‐epitope into virus‐like particles achieves vaccine responses even in older recipients in models of psoriasis, Alzheimer's and cat allergy. NPJ Vaccines, 2, 30 10.1038/s41541-017-0030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. G. , Chen, H. S. , Peng, J. R. , Shang, X. Y. , Zhang, J. , Xing, Q. , … Chen, W. F. (2007). Specific CD8(+ )T cell responses to HLA‐A2 restricted MAGE‐A3 p271‐279 peptide in hepatocellular carcinoma patients without vaccination. Cancer Immunology, Immunotherapy, 56(12), 1945–1954. 10.1007/s00262-007-0338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S. , Yong, L. K. , Li, D. , Cubas, R. , Chen, C. , & Yao, Q. (2013). Mesothelin virus‐like particle immunization controls pancreatic cancer growth through CD8+ T cell induction and reduction in the frequency of CD4+ foxp3+ ICOS‐ regulatory T cells. PLoS One, 8(7), e68303 10.1371/journal.pone.0068303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Song, S. , Liu, C. , Wang, Y. , Xian, X. , He, Y. , … Sun, S. (2007). Generation of chimeric HBc proteins with epitopes in E. coli: Formation of virus‐like particles and a potent inducer of antigen‐specific cytotoxic immune response and anti‐tumor effect in vivo. Cellular Immunology, 247(1), 18–27. 10.1016/j.cellimm.2007.07.003 [DOI] [PubMed] [Google Scholar]


