Abstract
Sarcoidosis is a T‐cell driven inflammatory disease characterized by granuloma formation. Mononuclear phagocytes (MNPs)—macrophages, monocytes, and dendritic cells (DCs)—are likely critical in sarcoidosis as they initiate and maintain T cell activation and contribute to granuloma formation by cytokine production. Granulomas manifest primarily in lungs and lung‐draining lymph nodes (LLNs) but these compartments are less studied compared to blood and bronchoalveolar lavage (BAL). Sarcoidosis can present with an acute onset (usually Löfgren's syndrome (LS)) or a gradual onset (non‐LS). LS patients typically recover within 2 years while 60% of non‐LS patients maintain granulomas for up to 5 years. Here, four LS and seven non‐LS patients underwent bronchoscopy with endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA). From each patient, blood, BAL, endobronchial biopsies (EBBs), and LLN samples obtained by EBUS‐TBNA were collected and MNPs characterized using multicolor flow cytometry. Six MNP subsets were identified at varying frequencies in the anatomical compartments investigated. Importantly, monocytes and DCs were most mature with migratory potential in BAL and EBBs but not in the LLNs suggesting heterogeneity in MNPs in the compartments typically affected in sarcoidosis. Additionally, in LS patients, frequencies of DC subsets were lower or lacking in LLNs and EBBs, respectively, compared to non‐LS patients that may be related to the disease outcome. Our work provides a foundation for future investigations of MNPs in sarcoidosis to identify immune profiles of patients at risk of developing severe disease with the aim to provide early treatment to slow down disease progression.
Keywords: dendritic cell, monocyte, sarcoidosis, lymph node, Löfgren's syndrome
In‐depth phenotypic characterization of monocytes and dendritic cells in blood, lungs, and lymph nodes of sarcoidosis patients.
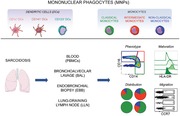
Abbreviations
- BAL
bronchoalveolar lavage
- DC
dendritic cell
- EBB
endobronchial biopsy
- EBUS‐TBNA
endobronchial ultrasound‐guided transbronchial needle aspiration
- LLN
lung‐draining lymph node
- MNP
mononuclear phagocyte
1. INTRODUCTION
Sarcoidosis is an inflammatory disease of unknown etiology affecting multiple organs with appearance of non‐necrotizing granulomas. Ninety percent of sarcoidosis patients present with granulomas in their lungs and in lung‐draining lymph nodes. The clinical presentation can be heterogeneous and diagnostic tools include chest radiography and confirmation of granulomas in biopsies from the affected tissue(s).1 Sarcoidosis can present with either an acute onset (usually Löfgren's syndrome (LS)) associated with more favorable disease outcome or a gradual onset (non‐LS). Among non‐LS patients, up to 30% maintain symptoms for up to 3 years and develop chronic, sometimes progressive, disease that requires treatment.2
Mononuclear phagocytes (MNPs): alveolar macrophages (AMs), monocytes and dendritic cells (DCs), reside in the respiratory tract to help maintain tolerance but also to initiate immune responses when encountering harmful agents. As T cells are critical in driving disease progression in sarcoidosis,3, 4 a more detailed understanding of MNPs that initially activate and modulate T cell responses could provide greater insight on sarcoidosis pathogenesis. We recently developed protocols and characterized MNPs in healthy controls and identified classical, intermediate, and non‐classical monocytes distinguished by their differential expression of CD14 and CD16 as well as CD123+ plasmacytoid DCs (PDCs) and CD1c+ and CD141+ myeloid DCs (MDCs) in the respiratory tract.5, 6 The different subsets of DCs and monocytes have common but also unique features.7 Classical monocytes circulate in blood and either extravasate into tissues or differentiate into intermediate monocytes and non‐classical monocytes.8 All monocytes can secrete proinflammatory cytokines but classical and intermediate monocytes are the most potent producers, while non‐classical monocytes mainly perform immunosurveillance in blood and tissues.9 Intermediate monocytes are expanded in the circulation of patients with infections, autoimmune, and inflammatory diseases including sarcoidosis.10, 11, 12 However, respiratory monocytes are less studied and it remains to be elucidated if intermediate monocytes also expand in the lungs of sarcoidosis patients and whether monocytes and DCs contribute to cytokine‐mediated granuloma formation similar to AMs.13 DCs, in particular CD1c+ MDCs, potently present antigen to and activate CD4 T cells that drive disease,14, 15 while CD141+ MDCs are superior at cross‐presentation to CD8 T cells.16, 17 CD123+ PDCs are potent type I interferon producers and the most common DC subset in lymphoid tissue.18, 19 MNPs from blood and BAL have been studied in sarcoidosis patients with somewhat conflicting results.20 However, a detailed description comparing anatomical compartments including lung tissue and the lung‐draining lymph nodes and distinguishing between clinical phenotypes (LS and non‐LS sarcoidosis patients) is lacking. The significance of this strategy was shown in previous studies where the elevated CD4/CD8 T cell ratio in BAL used for diagnosis could not be observed in LLN samples obtained using endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA).21, 22 Taken together, this underlines the importance of doing comparative studies in different anatomical compartments side‐by‐side within the same patient to elucidate how the immune response shapes clinical symptoms.
Here, we investigated distribution, phenotype and functional markers of monocytes and DC subsets from BAL, endobronchial biopsies (EBBs), LLNs, and blood within the same sarcoidosis patient in a cohort of LS and non‐LS sarcoidosis patients. We found that while monocytes and DCs resided in all compartments investigated, the different cell subsets displayed differential distribution and maturation status including differential chemokine receptor expression. Importantly, cells from BAL samples were not necessarily a reflection of cells obtained from endobronchial tissue or LLNs suggesting heterogeneity in distribution and function in MNPs that can result in different disease outcome in LS and non‐LS sarcoidosis patients.
2. MATERIAL AND METHODS
2.1. Study design and patient characteristics
Eleven sarcoidosis patients between the age of 30 and 58 were included in this study. All patients were referred to the University Hospital, Umeå to undergo bronchoscopy. Patients were diagnosed with sarcoidosis as defined by WASOG guidelines23 based on clinical signs, chest radiography findings, and non‐caseating granulomas in the lymph nodes and/or endobronchial biopsy (Table 1). We identified LS sarcoidosis patients based on the clinical signs (enlarged lymph nodes, erythema nodosum or periarticular arthritis) as well as blood parameters (Table 1). At time of bronchoscopy, all LS patients still had enlarged lymph nodes, as assessed by computer tomography prior to the bronchoscopy, to conduct EBUS‐TBNA. Informed consent was obtained prior to the bronchoscopy. The study was approved by the regional ethical review board in Umeå, Sweden and performed according to the declaration of Helsinki.
Table 1.
Clinical characteristics of sarcoidosis patients
| Plasma | BAL differential count (in %) | Lung function | Symptoms | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age | Sex | Smoking status | X‐ray stage | ACE (<70 E/L) | Albumin (36–45 g/L) | Macrophages | Lymphocytes | Neutrophils | CD4/CD8 ratio | VC (% of predicted) | FEV1 (% of predicted) | FEV1/FVC | DLCO SB (% of predicted) | Löfgren's syndrome | HLA‐DRB1 | Type | Duration | Medication |
| 1 | 42 | m | NS | I | 122 | 39 | 70 | 23 | 6 | 4.3 | 110 | 109 | 78.7 | 96 | No | *03,*08 | None | 0 | |
| 2 | 55 | m | Ex | II | 112 | 39 | 73 | 26 | 1 | 8 | 100 | 101 | 46.8 | 69 | No | *04,*11 | Fatigue, dry cough, dyspnoea | 1 | Salbutamol |
| 3 | 44 | m | Ex | I | n.d. | 43 | 78 | 16 | 6 | 3 | 102 | 101 | 76.3 | 104 | No | *04,*13 | Dry cough | 1 | |
| 4 | 32 | m | S | I | 50 | 38 | 77 | 21 | 2 | 5.5 | 115 | 114 | 77.8 | 103 | No | *04,*12 | Fatigue, dry cough, dyspnoea, iritis | 2 | Salbutamol, topical steroids |
| 6 | 30 | m | Ex | I | 29 | 40 | 82 | 11 | 7 | 5 | 75 | 69 | 73.5 | n.d. | No | *13,*15 | Fatigue and mild dyspnoea, iritis | 1 | Prednisolone |
| 8 | 51 | m | NS | I | 74 | 38 | 85 | 13 | 2 | 6.5 | 97 | 102 | 82.8 | 118 | No | *04,*15 | None | 0 | |
| 10 | 55 | m | NS | I | 47 | 38 | 74 | 24 | 2 | 8.1 | 111 | 108 | 74.5 | 125 | No | *03,*04 | Very mild ankle pain | 1 | |
| 5 | 41 | m | NS | I | 58 | 45 | 87 | 11 | 1 | 5 | 111 | 108 | 76.3 | 106 | Yes | *03,*13 | Mild dry cough, bilateral ankle arthritis | 1 | |
| 7 | 58 | m | Ex | I | 49 | 40 | 58 | 36 | 3 | 7 | 88 | 80 | 82.8 | n.d. | Yes | n.d. | Fatigue cough, painful and swollen ankles | 1 | |
| 9 | 39 | m | NS | I | 77 | 40 | 69 | 29 | 0 | 2.5 | 101 | 100 | 75.8 | n.d. | Yes | *04,*13 | Mild fever, fatigue, swollen ankles | 1 | |
| 11 | 49 | m | NS | I | 33 | 35 | 80 | 17 | 3 | 7.1 | 87 | 84 | 72.1 | 111 | Yes | *13,*13 | Fatigue, cough, painful and swollen ankles | 1 | |
| Median (25th–75th percentile) | 44 (39–55) | 50 (40–93) | 39 (38–40) | 77 (70–82) | 21 (13–26) | 2 (1–6) | 5.3 (4.5–6.3) | 101 (88–111) | 101 (84–108) | 76.3 (73.5–78.7) | 105 (98–116) | ||||||||
Data summary is shown as median (25th–75th percentile). m: male; NS: never smoker; Ex: ex‐smoker (considered smoke free for >1 year); S: smoker; ACE: angiotensin‐converting enzyme. Chest radiography staging defined as follows; Stage I: hilar/mediastinal lymph node infiltrates; Stage II: lymph node and lung parenchyma infiltrates; BAL: bronchoalveolar lavage; VC: vital capacity; FEV1: forced expiratory volume in one second; DLCOSB: single breath diffusing capacity of the lung for carbon monoxide; n.d.: not determined; symptoms duration 0/1/2: no symptoms/less than 1 year/more than 1 year.
2.2. Bronchoscopy
Venous blood (32 ml) was drawn from each patient in Vacutainer CPT tubes (BD) before treatment with oral midazolam (4–8 mg) and glycopyrronium (0.2–0.4 mg) i.v. 30 min prior to the bronchoscopy. Lidocaine was administered in larynx and bronchi for topical anesthesia. A Fujinon bronchoscope (FUJIFILM Corporation, Tokyo, Japan) was inserted through the mouth. First, EBBs were taken from the major carinas of the bronchial tree from one side of the lung. To detect granulomas, EBBs were fixed in paraffin and 4 μm‐thick sections were stained with hematoxylin and eosin. To avoid blood contamination, BAL with 3 × 60 ml of a saline solution was performed on the contralateral side (middle or lingual lobe). EBUS‐TBNA was performed to sample mediastinal and hilar LLNs. LLN aspirates were initially assessed for representability using on‐site cytology by transferring LLN aspirates onto glass slides followed by methanol fixation and Diff‐Quik stain using Eosin G and Thiazine.
2.3. Single cell preparations from blood, respiratory specimens, and lymph nodes
Peripheral blood mononuclear cells (PBMCs) were isolated from blood collected in CPT tubes according to the manufacturer´s protocol. BAL samples were kept on ice, filtered through a 100 μm nylon filter (Syntab) and centrifuged at 400 × g for 15 min. EBBs were processed as described earlier.6 In brief, EBBs were collected in RPMI 1640 with 2% fetal calf serum (FCS). Biopsies were washed in HBSS (Sigma–Aldrich) for 5 min and incubated with 5 mM 1,4‐DTT (Sigma–Aldrich) for 15 min. Incubations were performed on a rocker at 30 rpm at room temperature. Single biopsies were transferred to a 48‐well plate and incubated with 0.25 mg/ml Collagenase II and 0.2 mg/ml DNase (both Sigma–Aldrich) in RPMI 1640 for 60 min at 37°C. After digestion, biopsies were filtered through a 40 μm cell strainer (BD). LN aspirates were filtered through a 40 μm nylon cell strainer, centrifuged at 300 × g for 10 min. Lysis of red blood cells (RBC) was performed with 1 × RBC lysis buffer (University laboratories, Karolinska Hospital, Solna) for 5 min and subsequently centrifuged at 300 × g for 5 min. Cells were counted manually and Trypan Blue was used to assess viability.
2.4. Flow cytometry
Cell suspensions were incubated with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Life Technologies). Fc receptors were blocked using FcR block (Miltenyi) followed by staining with antibodies against surface molecules CD1c (AD5‐8E7; Miltenyi), CD3 (SP34‐2 and SK7; BD), CD11c (B‐Ly6; BD), CD14 (M5E2; BD), CD16 (3G8; Biolegend), CD19 (HIB19; Biolegend), CD20 (L27; BD), CD45 (HI30; Biolegend), CD56 (HCD56; Biolegend), CD66abce (TET2; Miltenyi), CD80 (2D10; Biolegend), CD123 (7G3; BD), CD141 (AD5‐14H12; Miltenyi), CCR7 (G043H7; Biolegend), and HLA‐DR (G46‐6; BD) for 15 min at 4°C in PBS with 2% FCS and fixed with 1% paraformaldehyde. Cells were analyzed using an LSRII flow cytometer (BD) and data were analyzed using FlowJo X software (Tree Star).
2.5. Statistical analysis
For all experiments, data are shown as median. Data were analyzed using GraphPad Prism version 6.0 (GraphPad Software). To assess statistical significance, non‐parametric Kruskal–Wallis with Dunn's test correcting for multiple comparisons and the Mann–Whitney U unpaired t‐test were used. Confidence levels lower than P < 0.05 were considered significant.
3. RESULTS
3.1. Consistent numbers of viable cells are obtained from sarcoidosis patient lymph node aspirates after EBUS‐TBNA
In this study, 11 patients with sarcoidosis were included of which 4 were clinically characterized with Löfgren's syndrome. All patients underwent bronchoscopy to sample EBBs, BAL and subsequently, the lung‐draining mediastinal and hilar lymph nodes using EBUS‐TBNA (Fig. 1A). LLNs were evaluated for granulomas on‐site (Fig. 1B) and assessed positive for 10 out of 11 patients. Presence of granuloma in EBBs was also evaluated (Fig. 1C). As a complement to immunohistochemical analysis of tissue biopsies, we recently established a protocol to digest human EBBs to single‐cell suspensions for detailed analysis of immune cell composition by flow cytometry,6 which we now implemented on EBB samples from the sarcoidosis patients. Across patients, equivalent cell numbers of single cells from all sampling sites (Fig. 1D) with overall consistent cell viability within each compartment (Fig. 1E) were obtained. Notably, LS patients had lower viability of BAL cells compared to non‐LS patients. In summary, we obtained sufficient cell numbers from the sampling compartments to perform detailed analysis by flow cytometry.
Figure 1.
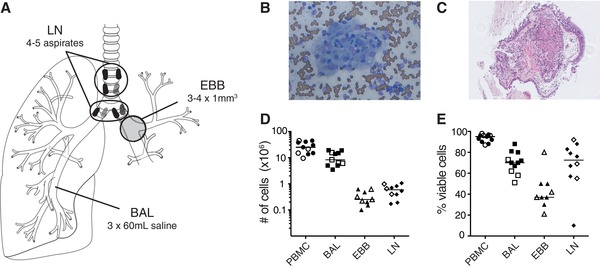
Sufficient numbers of viable cells recovered from four anatomical locations from the same sarcoidosis patient. (A) Bronchoscopy was performed on seven non‐LS and four LS sarcoidosis patients to obtain endobronchial biopsies (EBBs) from the main bronchial divisions of one lung, while bronchoalveolar lavage (BAL) was performed to retrieve aspirates from the lower airways from the contralateral side to avoid contamination due to bleeding. Subsequently, lung‐draining lymph node (LLN) aspirates were collected using endobronchial ultrasound‐guided transbronchial needle aspiration (EBUS‐TBNA) sampling the mediastinal and hilar lymph nodes. Patients provided blood samples prior to the bronchoscopy and peripheral blood mononuclear cells (PBMCs) were isolated. All samples were processed immediately after the bronchoscopy. Representative image of non‐necrotizing granuloma in (B) LLNs (Diff‐Quik staining) and (C) EBBs (hematoxylin and eosin staining) of a sarcoidosis patient. (D) Total number and (E) percentage of viable cells in single cell suspensions of PBMC (circle), BAL (square), EBBs (triangle) and LLNs (diamond) in non‐LS (filled symbols) and LS sarcoidosis patients (open symbols) were determined using Trypan Blue exclusion stain and manual counting with a Bürker counting chamber under a light microscope. One aspirate from the LLN with reduced viability (<10%) was excluded from further analysis. Lines indicate median values. n = 11 for all samples except LLNs with n = 10
3.2. Mononuclear phagocytes are more frequent in blood than in the lung mucosa and lymph nodes
All samples were processed immediately after collection and stained with a validated panel of antibodies against phenotypic and activation markers to identify and characterize MNPs. We identified live, CD45+ leukocytes in PBMCs, BAL, EBBs, and LLNs using flow cytometry and compared frequencies between the compartments (Supplementary Fig. 1 and Fig. 2A and B). In BAL samples, AMs were excluded from further analysis based on high autofluorescence to avoid mischaracterization of other MNPs (Fig. 2A, BAL sample, Supplementary Fig. 1B). MNPs were identified among the leukocytes based on their expression of HLA‐DR and lack of lineage markers (CD3, CD19, CD20, CD56, and CD66abce) (Fig. 2C) and frequencies compared between the four compartments (Fig. 2D). Taken together, our data show that total MNPs could be identified in all four compartments. However, depending on the sampling site, differences in distribution were observed with highest frequencies of MNPs in blood followed by BAL, EBB, and LLN in all sarcoidosis patients.
Figure 2.
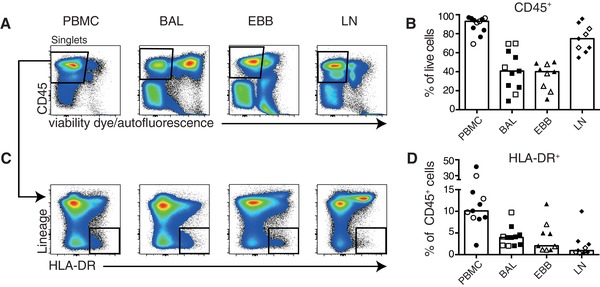
Differential distribution of mononuclear phagocytes in BAL, EBBs, LLNs, and PBMCs in sarcoidosis patients. (A) Single cell suspensions were stained with an extensive panel of antibodies against surface epitopes and flow cytometry was used to identify leukocytes and mononuclear phagocytes (MNP) in PBMCs, BAL, EBBs, and LLNs. Pseudocolor plots from BAL, EBBs, LLNs, and PBMC from one representative patient are shown to illustrate the gating strategy used. Leukocytes were identified, after excluding doublets and dead cells, by their expression of CD45 and lack of staining with a viability dye. In BAL, alveolar macrophages were excluded from further analysis based on high autofluorescence. (C) Among the live, CD45+ leukocytes, MNPs were identified by excluding lineage positive cells (CD3, CD19, CD20, CD56, and CD66abce) and gating on HLA‐DR+ cells. (B) Frequencies of CD45+, live cells and (D) of HLA‐DR+ MNPs in PBMCs (circle), BAL (square), EBBs (triangle) and LLNs (diamond) in non‐LS (filled symbols) and LS sarcoidosis patients (open symbols). n = 11 for PBMC and BAL while EBBs and LLNs are n = 9. Bar graphs show median values
3.3. Differential distribution of monocyte and DC subsets in blood, lungs, and lymph nodes in sarcoidosis patients
We next characterized six MNP subsets in blood, BAL, EBBs, and LLNs. Among live, CD45+, lineage−, HLA‐DR+ MNPs, CD123+ plasmacytoid dendritic cells (PDCs) were identified and from the CD11c+, CD123− cells, three distinct monocyte subsets were identified: CD14+CD16− classical monocytes, CD14+CD16+ intermediate monocytes and CD14− CD16+ non‐classical monocytes (Fig. 3A). In addition, two myeloid DC (MDC) subsets were identified by their expression of CD1c (CD1c+ MDC) and CD141 (CD141+ MDC) (Fig. 3A). We identified three DC and three monocyte subsets in all compartments, with the exception of intermediate and non‐classical monocytes that were not detected in EBBs (Fig. 3A), which is in line with our previous observation in healthy volunteers6 (Supplementary Table 1). Overall, the distribution of monocyte subsets in the different compartments was strikingly different (Fig. 3B) with EBBs containing only classical monocytes and BAL, unlike blood and LLNs, displaying a majority of proinflammatory intermediate monocytes (Fig. 3C). Three DC subsets were identified in varying frequencies in all the compartments (Fig. 3D). CD1c+ MDCs were the most common subset in blood, BAL, and EBBs while CD123+ PDCs were most abundant in LLNs (Fig. 3E). Taken together, our data show a higher frequency of DCs in BAL, EBBs, and LLNs than in blood suggesting a recruitment of DCs to the site of inflammation in sarcoidosis patients.
Figure 3.
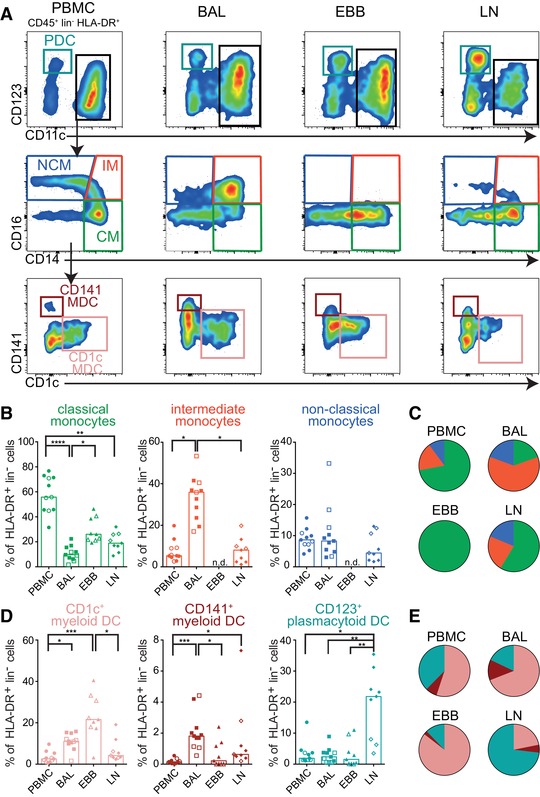
Identification of six distinct monocytes and DCs in sarcoidosis patients with differential distribution in BAL, EBBs, LLNs, and PBMCs. (A) In all compartments monocytes and dendritic cells (DCs) were identified among live, lineage (lin) negative HLA‐DR+ MNPs using flow cytometry. Plasmacytoid dendritic cells (teal) were identified based on their expression of CD123 and CD11c identified myeloid cells. From the CD11c+ cells, CD14+CD16− classical monocytes (CM, green), CD14+ CD16+ intermediate monocytes (IM, red), and CD14− CD16+ non‐classical monocytes (NCM, blue) were identified. Subsequently, myeloid dendritic cells (MDCs) were gated on in the CD14/CD16 double negative cells and identified by their expression of CD1c (coral) or CD141 (maroon), respectively. Graphs show frequencies of (B) monocyte and (D) DC subsets among live, HLA‐DR+ lineage negative cells in PBMCs (circle), BAL (square), EBBs (triangle) and LLNs (diamond) in non‐LS (filled symbols) and LS sarcoidosis patients (open symbols). n = 11 for PBMC and BAL while EBBs and LLNs are n = 9. Bars indicate median. Pie charts show the proportion of (C) monocyte and (E) DC subsets in each of the four compartments relative to each other. n.d. = not detectable. Statistical analysis was performed using the non‐parametric Kruskal–Wallis with Dunn's test for correction of multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
3.4. Lack of CD141+ MDCs in EBBs and reduced frequencies of CD123+ PDCs in LLNs of LS compared to non‐LS patients
For comparison of total monocytes and DCs, we included data from healthy controls (HC) generated in Baharom et al.,6 an earlier study of our group. The distribution of total monocytes and total DCs (three DC and monocyte subsets, respectively) in blood, BAL, and EBBs was comparable between healthy controls,6 LS and non‐LS patients (Fig. 4A). While no data is available on HC in LLNs, non‐LS, and LS patients displayed different distribution of monocytes and DCs (Fig. 4A). Non‐LS patients had more DCs than monocytes in LLNs, while LS patients had predominantly monocytes (Fig. 4A). However, of the individual subsets in LLNs, only CD123+ PDCs were significantly lower in LS patients compared to non‐LS patients (Fig. 4B). Furthermore, no CD141+ MDCs were detected in EBBs of LS patients (Fig. 4B). Taken together, LLNs displayed the most pronounced difference in MNP distribution comparing LS and non‐LS patients.
Figure 4.
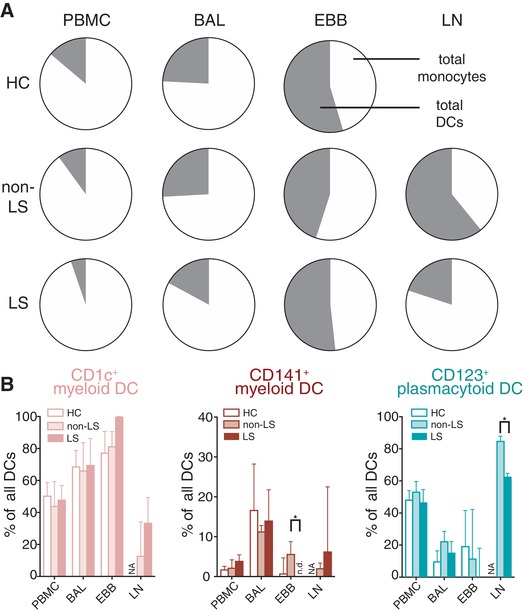
Altered DC subsets in EBBs and LLNs of LS compared to non‐LS patients. (A) Pie charts show the distribution mean frequency of total monocytes (three subsets) (white) and total DCs (three subsets) (gray) in PBMC, BAL, EBBs, and LLNs in healthy controls (n = 19, data from6), non‐LS (n = 6) and LS (n = 4, LLNs = 3) sarcoidosis patients. (B) Frequencies of CD1c+ myeloid DCs (coral), CD141+ myeloid DCs (maroon), and CD123+ plasmacytoid DCs (teal) among live, lineage negative, HLA‐DR+ MNPs in non‐LS (filled bars, n = 7) and LS (open bars, n = 4) sarcoidosis patients in blood, BAL, EBBs, and LLNs. Bar graphs show mean±sd. n.d. = not detectable; NA = not analyzed. Statistical analysis was performed using the Mann–Whitney U unpaired t‐test. *P < 0.05
3.5. Monocytes and DCs in sarcoidosis patients are more mature in BAL and EBBs compared to peripheral blood
We next assessed the maturation status of MNPs by analyzing their surface expression of HLA‐DR and CD80, which are upregulated upon MNP maturation to facilitate optimal T cell activation.24 In sarcoidosis, enhanced maturation of MNPs could be an indicator of the inflammatory processes in the different anatomical compartments. We found the most distinct differences in HLA‐DR expression on classical monocytes in all compartments; HLA‐DR expression was lowest in blood and LLNs, followed by BAL and highest in EBBs (Fig. 5A). In contrast, CD80 expression on classical monocytes was highest in cells from BAL followed by EBBs, LLNs, and blood (Fig. 5B). No differences in HLA‐DR and CD80 expression were observed on intermediate monocytes across the tissues (Fig. 5C and D) while non‐classical monocytes displayed a similar HLA‐DR and CD80 expression to the classical monocytes (Fig. 5E and F). Similar to monocytes, all DC subsets in blood and LLNs had comparable levels of HLA‐DR while DCs in BAL and EBBs had an increased expression (Fig. 5G–M). No differences were observed between non‐LS and LS sarcoidosis patients but overall, cells most mature were found in BAL and EBBs possibly reflecting a more pronounced activation of MNPs in the tissue.
Figure 5.
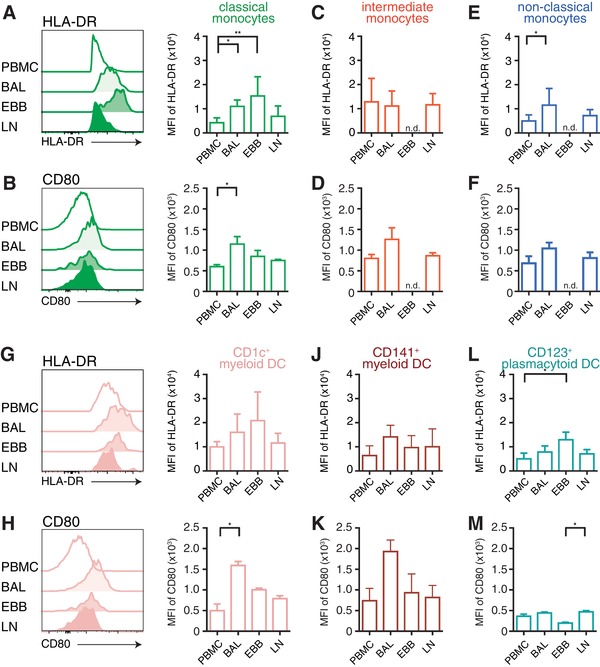
Monocytes and DCs are more mature in EBBs and BAL than in blood or LLNs. Maturation of monocytes and DCs was assessed by surface expression of HLA‐DR and CD80 using flow cytometry. Histograms from one representative non‐LS sarcoidosis patient show (A and G) HLA‐DR and (D and K) CD80 surface expression on (A and B) classical monocytes and (G and H) CD1c+ myeloid DCs. Bar graphs show mean fluorescence intensity (MFI) of HLA‐DR and CD80 of (A and B) classical monocytes, (C and D) intermediate monocytes, (E and F) non‐classical monocytes and (G and H) CD1c+ myeloid DCs, (J and K) CD141+ myeloid DCs and (L and M) plasmacytoid DCs in blood, BAL, EBBs, and LLNs in non‐LS and LS sarcoidosis patients (HLA‐DR: n = 8; CD80: n = 3). Bars show mean±sd. n.d. = not detectable. Statistical analysis was performed using the non‐parametric Kruskal–Wallis with Dunn's test for correction of multiple comparisons. *P < 0.05, **P < 0.01
3.6. Highest frequency of CCR7 expression in the respiratory mucosa on classical monocytes and in BAL on CD1c+ MDCs
Typically, antigen‐presenting cells including DCs and monocytes migrate from peripheral tissues to draining lymph nodes to present antigen to cognate T cells for the induction of adaptive immune responses. Thus, migration from blood and tissue to lymph nodes is an important aspect of MNP function. To determine the migratory potential of MNPs, we analyzed their expression of CCR7, a chemokine receptor facilitating the migration of MNPs to the draining lymph nodes. Monocytes and DCs in PBMCs did not express CCR7 (Fig. 6A–F, and Supplementary Fig. 2). Interestingly, only low frequencies of monocytes and DCs in LLNs expressed CCR7 despite its function to home cells to lymphoid tissue. Instead, the highest frequency of CCR7 expressing cells was identified in EBBs and BAL. Among monocytes, classical monocytes in EBBs displayed the highest frequency of CCR7+ cells (Fig. 6A). Intermediate and non‐classical monocytes showed no or low frequencies of CCR7+ cells in all compartments (Fig. 6B and C). A significantly higher proportion of CD1c+ and CD141+ MDCs in BAL expressed CCR7 compared to cells in EBBs (Fig. 6D and E). Taken together, our data show that in non‐LS and LS sarcoidosis patients, classical monocytes and DCs in BAL and EBBs express CCR7, suggesting that the cells have received signals to migrate to draining LNs to interact with T cells.
Figure 6.
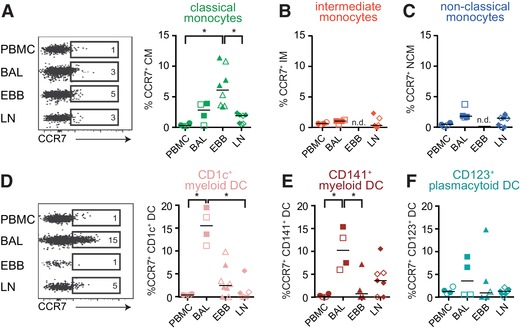
Monocytes and DCs in BAL and EBBs but not blood and LLNs express CCR7. Flow cytometry and staining for CCR7 was used to assess the prerequisites for monocytes and DCs to migrate to the lymph node. Dot plots show the expression of CCR7 in (A) classical monocytes (green), (B) intermediate monocytes (red), and (C) non‐classical monocytes (blue), and (D) CD1c myeloid DCs (coral), (E) CD141 myeloid DCs (maroon), and (F) plasmacytoid DCs (teal) in the four different compartments. Graphs show the frequency of CCR7+ cells of respective monocyte and DC populations in PBMCs (circle), BAL (square), EBBs (triangle), and LLNs (diamond); LS open symbols and non‐LS closed symbols. n = 4 in PBMC and BAL (non‐LS: n = 2, LS: n = 2) while for EBBs n = 8 (non‐LS: n = 4, LS: n = 4) and LLNs n = 7 (non‐LS: n = 4, LS: n = 3). Lines indicate the median. n.d. = not detectable. Statistical analysis was performed using the non‐parametric Kruskal–Wallis with Dunn's test for correction of multiple comparisons. *P < 0.05
4. DISCUSSION
Here, we characterized the phenotype and distribution of monocytes and DCs within the same patient from four anatomical compartments that are directly or indirectly affected by inflammation and granuloma formation during sarcoidosis. In the LS and non‐LS patients, we identified most but not all MNP subsets in the compartments investigated. However, the MNP subsets were differentially distributed and showed varying maturation status depending on their location. Cells from BAL were not necessarily a reflection of cells obtained from EBBs or LLNs, suggesting heterogeneity in distribution and function of MNPs in the compartments typically most affected by sarcoidosis. Importantly, LS and non‐LS patients had a different distribution of total monocytes and DCs in LLNs. This study highlights important differences in MNP distribution and activation across the anatomical compartments within the same patient as well as between different clinical phenotypes of sarcoidosis. Specifically, we found that distribution and activation of MNPs depend on the anatomical location sampled as well as their activation. These findings highlight that data generated from sarcoidosis patients has to be analyzed with caution and under consideration from the sampling site as the local environment might alter functional aspects of MNPs. Furthermore, we found differences in the distribution of DCs between LS and non‐LS patients that might be related to different expression of chemokine receptors resulting in altered migratory capacity. Albeit with a limited patient number, our study provide data that MNPs may be connected with sarcoidosis pathogenesis and that our findings merit further investigation in future studies.
MNPs are important in sarcoidosis both in activation and maintenance of pathogenic T cells as well as in contribution to granuloma formation by excessive TNF production by macrophages in the lungs of sarcoidosis patients.25 However, MNPs have mainly been studied in blood and BAL of sarcoidosis patients and generated heterogeneous data.26, 27, 28 This could partly be due to clinical heterogeneity among patients and limited differentiation between patients with acute and gradual disease onset. An important novelty of this study is the side‐by‐side investigation of MNP subsets in blood, BAL, EBBs, and LLNs from the same individual in both non‐LS and LS sarcoidosis patients. MNPs in BAL were different from those in EBBs with respect to distribution and maturation. Interestingly, while intermediate monocytes were the major MNP subset in BAL, this subset was lacking in EBBs, similar to previously published data on healthy volunteers.6 Despite the challenges in obtaining samples from multiple compartments from the same patient, it will be critical to, in extended studies, more fully determine the function of the different DC and monocyte populations to precisely delineate their role in sarcoidosis. Especially since environmental cues and inflammation in tissues have been shown to alter MNP phenotype and function and studies in blood cells are unlikely to represent MNP in tissue.6, 15, 29, 30, 31 Thus, additional methods, such as functional assessment of sorted cells, RNA sequencing or epigenetic analyses, in combination with correlating these data to clinical parameters as well as following the disease progression of the patients are likely required to fully understand MNP biology in sarcoidosis.
Additionally, it is necessary to compare functional aspects of MNP subsets from sarcoidosis patients with MNPs from healthy controls as indicated in the different distribution (Fig. 4 and Supplementary Table 1). Compared to our data generated from healthy volunteers,6 frequencies of CD1c+ MDCs in blood of sarcoidosis patients was decreased (Supplementary Fig. 1). This is in line with previous data from others.27 Additionally, in EBB of sarcoidosis patients, frequencies of CD1c+ MDCs and classical monocytes were increased compared to healthy controls (Supplementary Table 1), a finding that we will follow up on in future studies of larger patient cohorts.
The primary reason to perform bronchoscopy and collect EBBs in sarcoidosis patients, was to verify presence of granulomas, which limited research analysis to three to four biopsies per patient and resulted in lower cell yield (median 200,000 cells) compared to our previous study on healthy volunteers, where six to nine biopsies were processed (median 1.5 million cells6). Furthermore, viability of EBB cells was lower in sarcoidosis patients (median 37%) compared to samples from healthy volunteers (70%),6 in line with reduced viability after enzymatic digestion when fewer biopsies are processed.6 However, reduced viability could also reflect an ongoing inflammation resulting in cells that are more sensitive to processing and could cause cell maturation. Indeed, we observed 40% viable CD45+ leukocytes in EBBs of sarcoidosis patients (Fig. 2B) compared to only 10% CD45+ cells in EBBs from healthy volunteers,6 in support of inflammation‐induced leukocyte infiltration. We have previously reported that LS patients have a higher IL‐17, IL‐2, and IL‐22 production but lower IFN‐γ in BAL T cells compared to non‐LS patients.32 This elevated cytokine production may be linked to the reduced viability we observed in BAL cells from LS patients compared to non‐LS patients in the present study (Fig. 1E).
Sampling LLNs in sarcoidosis patients for analysis of MNPs has not been widely used. Only 1% of LLN cells are MNPs (Fig. 2D), and to obtain sufficient numbers of cells for analysis, a prerequisite is enlarged LLNs. Generating equivalent EBUS‐TBNA data from a suitable non‐sarcoidosis control group is challenging since normal‐size LLNs would yield too few cells. Instead, we focused on comparing MNPs in LLNs from non‐LS versus LS patients. We observed significant differences in the overall distribution of LLN monocytes versus DCs from LS and non‐LS patients (Fig. 4A) suggesting that MNPs directly or indirectly could contribute to disease outcome. The expression of chemokine receptors on MNPs is needed for optimal migration between tissues. A suitable target that could explain different distribution due to migratory behavior could be CCR2 as different haplotypes in LS and non‐LS sarcoidosis patients were described.33, 34
The different distribution of LLN MNPs in LS patients could potentially have impact on granuloma formation since pulmonary monocytes produce higher levels of granuloma‐promoting cytokines like TNF than DCs upon activation.6, 35 Additionally, we found significantly lower frequencies of LLN CD123+ PDCs in LS compared to non‐LS patients. PDCs are potent producers of type I interferons that promote Th1 skewing. IFN‐γ producing Th1 cells in lungs are associated with disease progression in non‐LS patients.36, 37, 38, 39 It remains to be determined if the low frequency of LLN PDCs in LS patients is directly associated with better disease prognosis. Furthermore, the LS patients in this study had no detectable CD141+ MDCs in lung tissue (EBB). CD141+ MDCs specialize in cross‐presentation, a process promoted by type I interferons.40 Whether the comparably low levels of type I IFN producing PDCs in LLNs of LS patients is related to the absence of CD141+ MDCs in lungs of LS and how this translates into T cell responses remains to be understood. To elucidate this, future experiments should focus on analyzing the capability of monocytes and DCs from LS and non‐LS patients to produce cytokines and to activate T cells.
Our study cohort included 11 male patients, which does not reflect the overall incidence of newly diagnosed sarcoidosis patients with respect to gender.41 The uneven gender distribution is likely a consequence of the fact that we were unable to consecutively include all newly diagnosed sarcoidosis patients over the study period, which by chance resulted in a cohort of only male patients. We investigated whether our experimental data correlated with clinical data such as duration, severity of symptoms, lung function or blood chemistry (Table 1) but could not identify statistically significant correlations in this limited cohort.
Our data highlight the importance of analyzing samples from different compartments side‐by‐side from the same individual as donor variability and the influence of clinical heterogeneity can potentially mask differences. In future and ongoing studies, functional as well as transcriptional and epigenetic analyses of highly purified cell subsets from different anatomical compartments can provide more insight into the different cell populations. A larger study cohort including both genders as well as different stages of the disease with different clinical characteristics and patients with LS must follow for correlation analyses linking MNPs and clinical phenotype. With that detailed knowledge, sarcoidosis patients with poor prognosis could potentially be identified and treated at an early stage by specifically targeting pathways involved in pathogenesis of sarcoidosis and alleviate symptoms of chronic sarcoidosis patients.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary Figure 1. Gating strategy for identification of MNP in blood, BAL, EBBs and LLNs in sarcoidosis patients.
Supplementary Figure 2. CCR7 isotype control.
Supplementary Table 1. Frequencies of mononuclear phagocytes in PBMC, BAL and EBB of healthy subjects and sarcoidosis patients.
AUTHORSHIP
R.L., A.B., A.S.S. designed the study. R.L., G.R., J.P., A.M., A.B. collected the data and/or performed the experiments. R.L., A.E., J.G., A.B., A.S.S. analyzed and interpreted the data. R.L. and A.S.S. prepared the manuscript. All authors read and approved the final manuscript.
FUNDING
This work was supported by grants to AS‐S from the Swedish Heart‐Lung Foundation, the Swedish Research Council, and Karolinska Institutet.
ACKNOWLEDGMENTS
The authors thank the staff at the Department of Medicine, Division of Respiratory Medicine and Allergy, University Hospital, Umeå for assistance in sample collection and Dr. Faezzah Baharom (National Institutes of Health, Bethesda, MD, USA) for technical advice.
Lepzien R, Rankin G, Pourazar J, et al. Mapping mononuclear phagocytes in blood, lungs and lymph nodes of sarcoidosis patients. J Leukoc Biol. 2019;105:797–807. 10.1002/JLB.5A0718-280RR
REFERENCES
- 1. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet P, Müller‐Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. [DOI] [PubMed] [Google Scholar]
- 2. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. [DOI] [PubMed] [Google Scholar]
- 3. Grunewald J, Eklund A, Hed J, Takahashi H, Wigzell H. Aspects on the alveolar accumulation of T cells in sarcoidosis. Sarcoidosis. 1992;9:142–144. [PubMed] [Google Scholar]
- 4. Muller‐Quernheim J, Prasse A, Zissel G. Pathogenesis of sarcoidosis. Presse Med. 2012;41:e275–287. [DOI] [PubMed] [Google Scholar]
- 5. Baharom F, Rankin G, Scholz S, et al. Human lung dendritic cells: Spatial distribution and phenotypic identification in endobronchial biopsies using immunohistochemistry and flow cytometry. J Vis Exp. 2017;(119):e55222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baharom F, Thomas S, Rankin G, et al. Dendritic cells and monocytes with distinct inflammatory responses reside in lung mucosa of healthy humans. J Immunol. 2016;196:4498–4509. [DOI] [PubMed] [Google Scholar]
- 7. Ziegler‐Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. [DOI] [PubMed] [Google Scholar]
- 8. Patel AA, Zhang Y, Fullerton JN, et al. The fate and lifespan of human monocyte subsets in steady state and systemic inflammation. J Exp Med. 2017;214:1913–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Idzkowska E, Eljaszewicz A, Miklasz P, Musial WJ, Tycinska AM, Moniuszko M. The role of different monocyte subsets in the pathogenesis of atherosclerosis and acute coronary syndromes. Scand J Immunol. 2015;82:163–173. [DOI] [PubMed] [Google Scholar]
- 10. Hofer TP, Zawada AM, Frankenberger M, et al. slan‐defined subsets of CD16‐positive monocytes: impact of granulomatous inflammation and M‐CSF receptor mutation. Blood. 2015;126:2601–2610. [DOI] [PubMed] [Google Scholar]
- 11. Kwissa M, Nakaya HI, Onlamoon N, et al. Dengue virus infection induces expansion of a CD14(+)CD16(+) monocyte population that stimulates plasmoblast differentiation. Cell Host Microbe. 2014;16:115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tsukamoto M, Seta N, Yoshimoto K, Suzuki K, Yamaoka K, Takeuchi T. CD14(bright)CD16+ intermediate monocytes are induced by interleukin‐10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res Ther. 2017;19(28):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muller‐Quernheim J, Pfeifer S, Mannel D, Strausz J, Ferlinz R. Lung‐restricted activation of the alveolar macrophage/monocyte system in pulmonary sarcoidosis. Am Rev Respir Dis. 1992;145:187–192. [DOI] [PubMed] [Google Scholar]
- 14. Faith A, McDonald J, Peek E, et al. Functional plasticity of human respiratory tract dendritic cells: GM‐CSF enhances T(H)2 development. J Allergy Clin Immunol. 2005;116:1136–1143. [DOI] [PubMed] [Google Scholar]
- 15. Schlitzer A, McGovern N, Teo P, et al. IRF4 transcription factor‐dependent CD11b+ dendritic cells in human and mouse control mucosal IL‐17 cytokine responses. Immunity. 2013;38:970–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bachem A, Guttler S, Hartung E, et al. Superior antigen cross‐presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jongbloed SL, Kassianos AJ, McDonald KJ, et al. Human CD141+ (BDCA‐3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross‐presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu YJ. IPC: professional type 1 interferon‐producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. [DOI] [PubMed] [Google Scholar]
- 19. Segura E, Valladeau‐Guilemond J, Donnadieu MH, Sastre‐Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zissel G, Muller‐Quernheim J. Cellular players in the immunopathogenesis of sarcoidosis. Clin Chest Med. 2015;36:549–560. [DOI] [PubMed] [Google Scholar]
- 21. Darlington P, Haugom‐Olsen H, von Sivers K, et al. T‐cell phenotypes in bronchoalveolar lavage fluid, blood and lymph nodes in pulmonary sarcoidosis–indication for an airborne antigen as the triggering factor in sarcoidosis. J Intern Med. 2012;272:465–471. [DOI] [PubMed] [Google Scholar]
- 22. Oda K, Ishimoto H, Yatera K, et al. Relationship between the ratios of CD4/CD8 T‐lymphocytes in the bronchoalveolar lavage fluid and lymph nodes in patients with sarcoidosis. Respir Investig. 2014;52:179–183. [DOI] [PubMed] [Google Scholar]
- 23. Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and other Granulomatous Disorders. Eur Respir J. 1999;14:735–737. [DOI] [PubMed] [Google Scholar]
- 24. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. [DOI] [PubMed] [Google Scholar]
- 25. Ziegenhagen MW, Benner UK, Zissel G, Zabel P, Schlaak M, Muller‐Quernheim J. Sarcoidosis: TNF‐alpha release from alveolar macrophages and serum level of sIL‐2R are prognostic markers. Am J Respir Crit Care Med. 1997;156:1586–1592. [DOI] [PubMed] [Google Scholar]
- 26. Kulakova N, Urban B, McMichael AJ, Ho LP. Functional analysis of dendritic cell‐T cell interaction in sarcoidosis. Clin Exp Immunol. 2010;159:82–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mathew S, Bauer KL, Fischoeder A, Bhardwaj N, Oliver SJ. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol. 2008;181:746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ten Berge B, Kleinjan A, Muskens F, et al. Evidence for local dendritic cell activation in pulmonary sarcoidosis. Respir Res. 2012;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desch AN, Gibbings SL, Goyal R, et al. Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung‐draining lymph nodes. Am J Respir Crit Care Med. 2016;193:614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McGovern N, Schlitzer A, Gunawan M, et al. Human dermal CD14(+) cells are a transient population of monocyte‐derived macrophages. Immunity. 2014;41:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reynolds G, Gibbon JR, Pratt AG, et al. Synovial CD4+ T‐cell‐derived GM‐CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis. 2016;75:899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaiser Y, Lepzien R, Kullberg S, Eklund A, Smed‐Sorensen A, Grunewald J. Expanded lung T‐bet+RORgammaT+ CD4+ T‐cells in sarcoidosis patients with a favourable disease phenotype. Eur Respir J. 2016;48:484–494. [DOI] [PubMed] [Google Scholar]
- 33. Spagnolo P, Renzoni EA, Wells AU, et al. C‐C chemokine receptor 2 and sarcoidosis: association with Lofgren's syndrome. Am J Respir Crit Care Med. 2003;168:1162–1166. [DOI] [PubMed] [Google Scholar]
- 34. Spagnolo P, Sato H, Grunewald J, et al. A common haplotype of the C‐C chemokine receptor 2 gene and HLA‐DRB1*0301 are independent genetic risk factors for Lofgren's syndrome. J Intern Med. 2008;264:433–441. [DOI] [PubMed] [Google Scholar]
- 35. Herrtwich L, Nanda I, Evangelou K, et al. DNA damage signaling instructs polyploid macrophage fate in granulomas. Cell. 2016;167:1264–1280. e18. [DOI] [PubMed] [Google Scholar]
- 36. Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma‐producing human CD4+ T cells. J Exp Med. 1993;178:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broos CE, Koth LL, van Nimwegen M, et al. Increased T helper 17.1 cells in sarcoidosis mediastinal lymph nodes. Eur Respir J. 2018;51(3):1–10. [DOI] [PubMed] [Google Scholar]
- 38. Rogge L, Barberis‐Maino L, Biffi M, et al. Selective expression of an interleukin‐12 receptor component by human T helper 1 cells. J Exp Med. 1997;185:825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Siegal FP, Kadowaki N, Shodell M, et al. The nature of the principal type 1 interferon‐producing cells in human blood. Science. 1999;284:1835–1837. [DOI] [PubMed] [Google Scholar]
- 40. Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross‐presentation by multiple dendritic cell subsets through a type I IFN‐dependent pathway. Blood. 2011;118:3028–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arkema EV, Grunewald J, Kullberg S, Eklund A, Askling J. Sarcoidosis incidence and prevalence: a nationwide register‐based assessment in Sweden. Eur Respir J. 2016;48:1690–1699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Gating strategy for identification of MNP in blood, BAL, EBBs and LLNs in sarcoidosis patients.
Supplementary Figure 2. CCR7 isotype control.
Supplementary Table 1. Frequencies of mononuclear phagocytes in PBMC, BAL and EBB of healthy subjects and sarcoidosis patients.


