Abstract
Background and purpose
The occurrence of intermediate uveitis, which is characterized by the presence of vitreous haze (VH), in patients with multiple sclerosis (MS) may be a sign of coexistent inflammatory central nervous system (CNS) disease activity. Using an automated algorithm to quantify VH on optical coherence tomography (OCT) scans, the aim was to investigate whether VH in MS patients is associated with signs of inflammatory CNS disease activity.
Methods
Vitreous haze was quantified on OCT macular volume scans of 290 MS patients and 85 healthy controls (HCs). The relationship between VH and clinical, retinal OCT and magnetic resonance imaging parameters of inflammatory disease activity was investigated using generalized estimating equations.
Results
Mean VH scores did not differ between patients and HCs (P = 0.629). Six patients (2.1%) showed values higher than the highest of the controls by HCs. VH scores did not differ between the different disease types or between eyes with and without a history of optic neuritis (P = 0.132). VH was not associated with inner nuclear layer volume on OCT (P = 0.233), cerebral T2 lesion load on magnetic resonance imaging (P = 0.416) or the development of new relapses (P = 0.205).
Conclusion
In this study, OCT‐based automated VH estimation did not detect increased vitreous inflammation in MS patients compared to HCs and did not find an association with CNS inflammatory burden.
Keywords: multiple sclerosis, neuro‐ophthalmology, optical coherence tomography, uveitis, vitreous haze
Introduction
A link between uveitis and multiple sclerosis (MS) exists 1. Compared to the general population, patients with uveitis are more likely to develop MS 1, 2, 3. Likewise, uveitis is more common in patients with MS. Nevertheless, the reported prevalence of uveitis in MS patients varies greatly, with numbers ranging from 0.65% to 36.7% 4. Some authors have suggested that the occurrence of uveitis in a patient with MS indicates inflammatory central nervous system (CNS) disease activity 5, 6.
Intermediate uveitis, in particular, which is characterized by inflammation of the vitreous, is thought to be associated with MS 2, 7. In intermediate uveitis, blood–ocular barrier breakdown results in the accumulation of protein‐rich fluid and inflammatory cells in the vitreous which is seen clinically as vitreous haze (VH) 8. VH is graded by indirect ophthalmoscopy 9. This method, however, has several limitations, which in turn may contribute to the aforementioned variability in reported prevalence data. Grading is subjective and data are noncontinuous with a poor sensitivity for detecting faint VH 10. Recently, a new algorithm has been developed for the objective quantification of VH on optical coherence tomography (OCT) scans 11.
It was hypothesized that this algorithm could potentially detect (subclinical) ocular inflammation reflected in increased VH in active MS patients. Therefore, our aim was to investigate whether patients with MS show higher levels of VH compared to healthy controls (HCs) and whether this VH is associated with signs of inflammatory CNS disease activity.
Methods
Study population and clinical evaluation
Subjects were recruited from the Amsterdam MS Cohort 12. All patients and HCs who had received an OCT scan at baseline assessment were eligible for inclusion (a total of 316 MS patients and 87 HCs). For the purpose of this study, subjects with ocular pathology were excluded except for patients with uveitis. However, none of the subjects had symptoms indicative of uveitis at the moment of assessment. After excluding patients due to ocular pathology (all non‐uveitic) or poor scan quality, 290 MS patients and 85 HCs in whom at least one eye was available were included in this study. A subset of subjects underwent additional OCT after 2 years of follow‐up. Clinical follow‐up of at least 1 year (including the occurence of new relapses after baseline) was available for the majority of the cohort. The inclusion and exclusion process is shown in Fig. 1.
Figure 1.
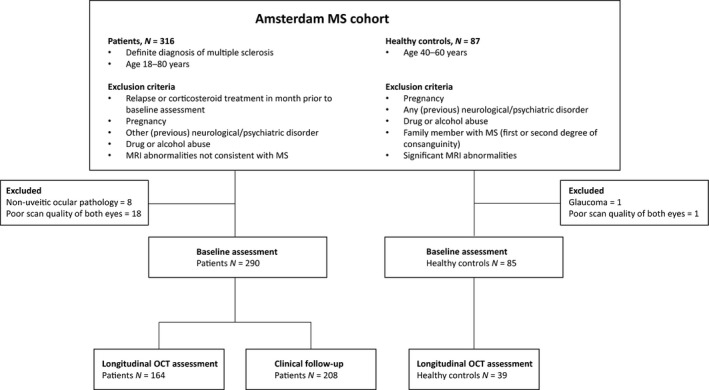
Flowchart illustrating the inclusion and exclusion of study subjects.
Clinical assessment was performed by a trained physician and included, amongst other things, history taking on the use of disease modifying therapy (DMT) and the occurrence of clinical relapses. Disease duration was defined as the time from first symptoms until assessment. The history of MS associated optic neuritis (MSON) was established according to a consensus protocol 13. The level of disability was assessed by certified physicians by means of the Extended Disability Status Scale 14.
Baseline magnetic resonance imaging (MRI) data were available for the majority of subjects (230 patients and 63 HCs). MRI was performed on a 3 T whole body scanner (GE Signa HDxt, Milwaukee, WI, USA) using an eight‐channel phased array head coil. The MRI protocol, acquisition settings and white matter lesion segmentation have been described previously 12.
This study was approved by the medical ethics committee (protocol number 2010/336) and the scientific research committee (protocol number CWO/10‐25D) of the VU University Medical Center. Written informed consent was obtained from all participants.
Optical coherence tomography and VH measurement
Optical coherence tomography scans were performed with a spectral domain OCT device (Spectralis, Heidelberg Engineering, Heidelberg, Germany; acquisition software version 1.7.1.0) with acquisition settings and segmentation as previously described (see Data S1 for more details) 15. Follow‐up scans were acquired with the automatic follow‐up function making sure the placement of the follow‐up scans was identical to the first scan.
Vitreous haze measurements were calculated from the raw images recorded by the Spectralis rather than from the contrast‐corrected, standard OCT images as described by Keane et al. 11. Raw images were exported from the Heyex software (Heidelberg Engineering) as VOL files. The measurement used in this work was obtained as the ratio of the mean intensity of all pixels of the vitreous (i.e. pixels above the segmented inner limiting membrane) and the mean values below the vitreous (Fig. 2). This ratio was calculated separately for each A‐scan in the B‐scans and then averaged. The reliability of this algorithm has recently been validated 16.
Figure 2.
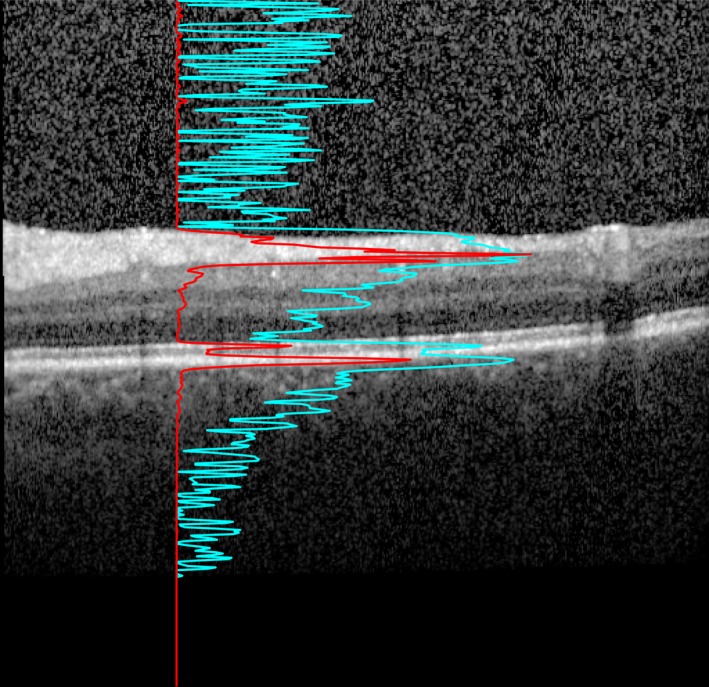
Algorithm used for measuring vitreous haze on OCT scans. An optical coherence tomography B‐scan of the macula is shown. This image is optimized for viewing on the screen, clearly rendering the retinal layers visible. The red line shows the A‐scan intensity profile in RAW format of the underlying vertical column in the image. Notice how the sharp peaks correspond to the inner segment/outer segment junction and retinal pigment epithelium – Bruch's membrane complex. This signal was used to calculate the ratio used in our measurement. The light blue line with sharper peaks in the vitreous and below the retina represents the same profile after contrast adjustment for visualization. Notice how the vitreous signal is artificially increased compared to the retinal signal.
Statistical analysis
Normality of data distribution was assessed graphically by means of histograms. Because the distribution of VH scores was skewed to the right, all VH scores were log‐transformed, which led to a normal distribution. All VH scores are reported as log VH scores. Differences in continuous variables between patients and HCs were tested using a two‐tailed t test (parametric) or Mann–Whitney U test (non‐parametric). Categorical variables were compared using a chi‐squared test. For the longitudinal analyses, annualized VH change scores were calculated. For all analyses performed on eye level (VH and retinal layer thickness), generalized estimating equations with an exchangeable correlation matrix were used to correct for intra‐subject inter‐eye dependence. Logistic regression analysis was used to test the predictive value of baseline VH scores (mean value of both eyes) on future relapses. All analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA). Statistical significance was set at P < 0.05.
Results
Vitreous haze scores and clinical measures
The baseline characteristics of the cohort are shown in Table 1. Patients were slightly older compared to HCs. As expected, the eyes of MS patients (eyes with and without a history of MSON combined) showed more atrophy of the macular ganglion cell–inner plexiform layer and peripapillary retinal nerve fiber layer but a thickening of the inner nuclear layer (INL) compared to HC eyes.
Table 1.
Baseline characteristics of the cohort
|
MS patients N = 290 |
HCs N = 85 |
P value | |
|---|---|---|---|
| Age (years), mean (±SD) | 51.5 (±10.1) | 49.3 (±8.2) | 0.039 |
| Sex (female:male) | 195:95 | 53:32 | 0.402 |
| Disease duration (years), mean (±SD) | 17.9 (±7.0) | N/a | |
| Type of MS | |||
| RRMS | 200 (69.0%) | N/a | |
| SPMS | 59 (20.3%) | ||
| PPMS | 31 (10.7%) | ||
| EDSS, median [range] | 3.5 [0–8.0] | N/a | |
| History of MSON | |||
| No MSON | 157 (54.1%) | N/a | |
| Unilateral MSON | 81 (27.9%) | ||
| Bilateral MSON | 39 (13.4%) | ||
| Unknown | 13 (4.5%) | ||
| Use of disease modifying therapy | |||
| Current | 93 (32.1%) | N/a | |
| Past | 58 (20.0%) | ||
| Never | 139 (47.9%) | ||
| Relapses in year prior to assessment | |||
| Yes | 34 (11.7%) | N/a | |
| No | 256 (88.3%) | ||
| mGCIPL thickness (μm), mean (±SD) | 77.5 (±14.3) | 92.2 (±6.0) | <0.001a |
| pRNFL thickness (μm), mean (±SD) | 84.6 (±14.4) | 95.1 (±7.9) | <0.001a |
| INL thickness (μm), mean (±SD) | 40.4 (±3.3) | 39.4 (±2.9) | 0.003a |
| Vitreous haze score, mean (±SD) | −0.96 (±0.41) | −0.93 (±0.33) | 0.629a |
EDSS, Expanded Disability Status Scale; HCs, healthy controls; INL, inner nuclear layer; mGCIPL, macular ganglion cell–inner plexiform layer; MS, multiple sclerosis; MSNON, no history of MS associated optic neuritis; MSON, MS associated optic neuritis; PPMS, primary progressive MS; pRNFL, peripapillary retinal nerve fiber layer; RRMS, relapsing–remitting MS; SPMS, secondary progressive MS.
Adjusted for age and sex.
Vitreous haze scores were negatively associated with age (β = −0.007, P = 0.001) and disease duration (β = −0.009, P = 0.004) in MS patients, meaning a higher age or longer disease duration was associated with lower VH scores. VH scores in HCs were not associated with age (β = 0.005, P = 0.129). In order to account for the effect of age, all subsequent analyses were corrected for age and sex, unless otherwise stated.
Overall, VH scores (reported as log VH scores) in MS patients ranged from −2.17 to 0.30 with a mean VH score of −0.96 (±0.41). HCs had a mean VH score of −0.93 (±0.33, range −1.76 to −0.06), which did not differ significantly from that of the MS patients (mean difference −0.03, P = 0.629) (Fig. 3a). Relapsing–remitting MS patients showed significantly higher VH scores compared to primary progressive MS patients (mean difference 0.14, P = 0.043) and secondary progressive MS patients (mean difference 0.13, P = 0.032) when age was not taken into account. However, after adjusting for age, these differences in VH score between relapsing–remitting MS patients and primary progressive MS (P = 0.201) and secondary progressive MS (P = 0.113) patients were no longer significant (Fig. 3b). Eyes with a history of MSON showed the same amount of VH compared to patient eyes without a history of optic neuritis (mean difference −0.04, P = 0.132) (Fig. 3c).
Figure 3.
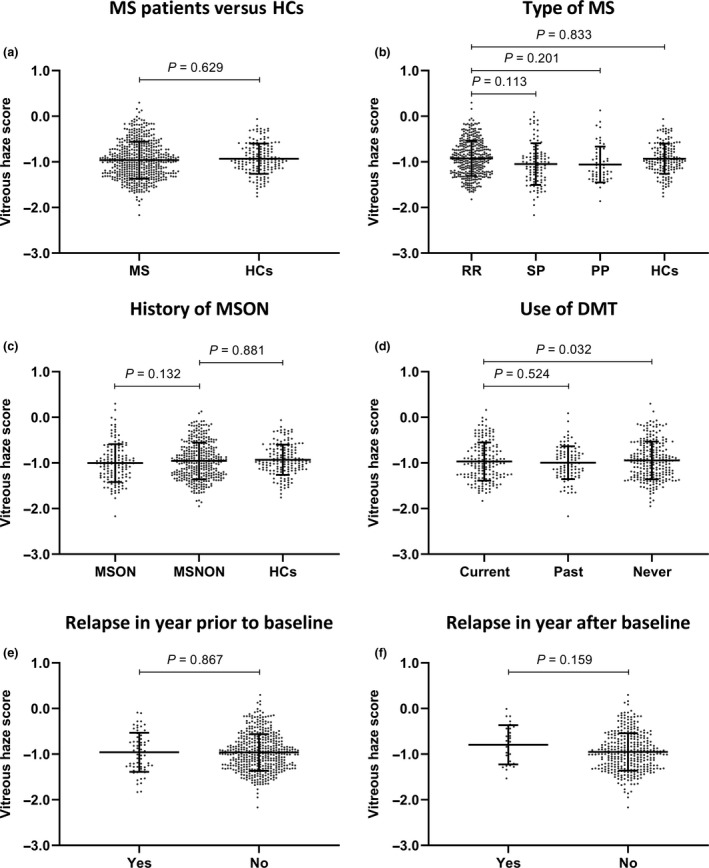
Mean vitreous haze scores (±SD) in all patients combined and HCs (a) and in the different patient subgroups (b–f). All analyses were adjusted for age and sex. The relationship between vitreous haze and use of DMT was additionally adjusted for type of MS (disease course). DMT, disease modifying therapy; HCs, healthy controls; MS, multiple sclerosis; MSNON, no history of MS associated optic neuritis; MSON, MS associated optic neuritis; PP, primary progressive; RR, relapsing–remitting; SP, secondary progressive.
Disease modifying therapy
Out of the 290 patients, 93 were using some form of DMT at the time of assessment. Beta interferons were used most often (N = 51), followed by glatiramer acetate (N = 12), natalizumab (N = 12), fingolimod (N = 11), dimethyl fumarate (N = 5), teriflunomide (N = 1) and azathioprine (N = 1). 20.0% of the patients had used one or more DMTs in the past but were not on any therapy at the moment of assessment whilst 47.9% had never used any form of DMT. After correcting for type of MS in addition to age and sex, patients who were on DMT showed significantly less VH compared to patients who had never been on DMT (mean difference 0.11, P = 0.032) (Fig. 3d).
Clinical relapses
Only a small proportion of MS patients had experienced a relapse in the year prior to baseline assessment (11.7%, Table 1). There was no significant difference in the VH score of patients who did and patients who did not experience a relapse in the year prior to baseline (mean difference 0.00, P = 0.867) (Fig. 3e). Clinical follow‐up was available in 208 patients. Of these patients, 14 (6.7%) had experienced a new relapse in the year following baseline assessment. Although these 14 patients did show a higher VH score compared to those patients who remained stable (−0.80 ± 0.43 and −0.95 ± 0.41 respectively), this was statistically not significant (P = 0.159) (Fig. 3f). VH score at baseline was not predictive of an MS relapse in the year following baseline assessment (odds ratio per 0.1 increase in VH score 1.11, P = 0.205).
Imaging parameters
Magnetic resonance imaging of the brain was available in a subset of MS patients (N = 230). In these patients, VH scores were not associated with cerebral T2 weighted lesion load (β = −0.086, P = 0.416) (Fig. 4a). Baseline macular INL thickness was available for the total cohort. Cross‐sectionally, VH was not related to macular INL thickness measured on OCT in MS patients (β = 0.003, P = 0.855, Fig. 3b) (Fig. 4b).
Figure 4.
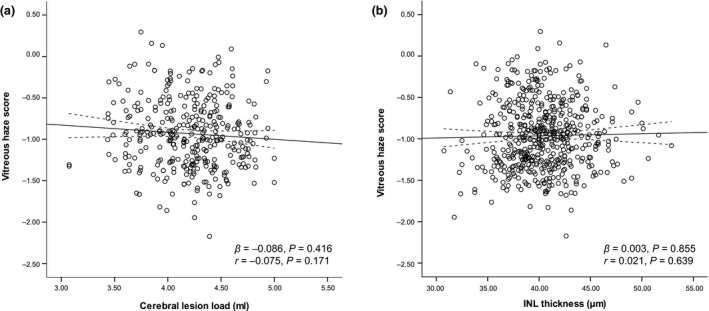
Scatter plot and fitted linear regression line (with 95% confidence interval) demonstrating the association between vitreous haze and cerebral lesion load (a) and inner nuclear layer (INL) thickness (b) in MS patients. r, Pearson correlation coefficient; β, regression coefficient.
Longitudinal changes in VH score
Longitudinal OCT data were available for 164 patients and 39 HCs. On average, these subjects had been followed up for a period of 2.19 years. During this follow‐up period, three patients experienced a clinical episode of MSON in an eye that had not been previously affected. The affected eyes of these patients were excluded from the longitudinal analyses.
Table 2 shows the annualized VH changes in MS patients and HCs. MS patients showed a small but statistically significant decline in VH score of −0.019/year (P = 0.006) whilst HCs showed no significant changes over time (−0.0026/year, P = 0.852). No difference in longitudinal VH changes was found amongst the different subgroups. Annualized changes in INL thickness were not related to annualized changes in VH scores in patients (β = 0.16, P = 0.083).
Table 2.
Annualized vitreous haze changes
| Mean annualized vitreous haze change score (95% confidence interval) | P value for subgroup analysis | |
|---|---|---|
| MS patients, overall | −0.019/year (−0.031 to −0.007) | Reference |
| Healthy controls | −0.0026/year (−0.028 to 0.023) | 0.256 |
| Type of MS | ||
| Relapsing–remitting MS | −0.014/year (−0.028 to −0.0002) | Reference |
| Secondary progressive MS | −0.021/year (−0.053 to −0.011) | 0.626 |
| Primary progressive MS | −0.044/year (−0.081 to −0.007) | 0.076 |
| History of MSON | ||
| MSON eyes | −0.016/year (−0.037 to 0.005) | Reference |
| MSNON eyes | −0.021/year (−0.036 to −0.006) | 0.850 |
| Use of disease modifying therapy | ||
| Current use | −0.007/year (−0.026 to 0.012) | Reference |
| Past | −0.027/year (−0.053 to −0.0003) | 0.208 |
| Never | −0.023/year (−0.042 to −0.005) | 0.198 |
| Relapses in the year following baseline assessment | ||
| Yes | −0.035/year (−0.079 to 0.009) | Reference |
| No | −0.018/year (−0.030 to −0.005) | 0.604 |
MS, multiple sclerosis; MSNON, no history of MS associated optic neuritis; MSON, MS associated optic neuritis.
Discussion
In this study, it was postulated that MS patients might show signs of increased ocular inflammation, reflected in increased VH, as a sign of inflammatory CNS disease activity. However, using an objective measure to quantify VH on OCT scans, no evidence of increased levels of VH in MS patients could be found compared to HCs. In addition, no association between VH and measures of CNS inflammation was found.
The absence of higher VH in the MS population might suggest that uveitis in MS might not be as common as previously thought. Studies investigating the prevalence of uveitis in MS patients have yielded a wide range of results 4, 17. Many of the smaller studies were performed in centers specialized in uveitis possibly leading to selection bias and probably an overestimation of the actual prevalence. It is noteworthy that the large, population based studies show the lowest prevalence figures of ~1% 3, 18, 19, 20. In our study, six out of 520 eyes, all in different patients, showed VH values that were higher than the highest value of the HCs (prevalence 2.1%). This percentage corresponds to the low prevalences found in the large, population based studies. It is also possible that the relatively long disease duration in our cohort of MS patients contributed to the low VH values due to a burnt‐out stage of the disease. The finding of an inverse association between disease duration and VH score further strengthens this argument. Therefore, there is a need for future studies with patients who present early with a clinically isolated syndrome, including MSON.
One difference between our study and most studies that investigate the prevalence of uveitis is that our OCT‐based approach only considers vitreous inflammation. It is therefore focused on detecting intermediate uveitis but may not detect other anatomically isolated cases of uveitis, notably anterior or posterior uveitis. However, almost all studies report intermediate uveitis to be the most common form in MS patients 18, 21, 22. One study reported posterior uveitis to be the most common anatomical subtype, although they do not provide further details and that study was conducted prior to the release of the Standardization of Uveitis Nomenclature Working Group guidelines for anatomical classification 20.
Whilst this study is the first to investigate the direct relationship between VH and signs of inflammatory CNS disease activity in MS, it expands on the association between other aspects of uveitis and increased CNS inflammatory activity is expanded. Retinal vascular abnormalities have been shown to be associated with increased MS activity. Lightman et al. reported that optic neuritis patients with retinal vasculitis (and vitreous cells) were more likely to subsequently develop MS compared to optic neuritis patients without 1. Two other studies have shown a significant association between retinal periphlebitis and inflammatory CNS disease activity 5, 6. The evidence for the association, however, is conflicting with some studies showing no differences in MS course or prognosis between patients with and without uveitis (all types of uveitis) 20, 23, and one study even finding a favorable MS prognosis in patients with uveitis 18. Since the patients in our cohort did not undergo a specialist ophthalmological examination as part of their routine assessment, it cannot be ruled out whether some patients in our cohort may have had another form of uveitis in which VH might be normal (e.g. anterior or posterior uveitis). This lack of an accompanying ophthalmological examination is an important limitation of the study and such an examination should be included in similar studies in the future. Yet it is argued that, since most of these assessments are subjective, it would be better to extend the imaging protocol to provide a more comprehensive objective assessment of ocular inflammatory status. For example, the protocol could use automated image‐based measures of anterior chamber cell count and chorioretinal lesions. The development of such markers is a major focus of our group's work. Additionally, it would be beneficial to have longitudinal MRI data, to provide a better estimation of inflammatory disease activity. Conversely, longitudinal data on INL thickness are available. INL volume changes are thought to reflect inflammatory disease activity 24. In our study, no association was found between INL thickness and VH changes.
The ability of the algorithm to objectively quantify the amount of VH on OCT scans is a strength of this study. The resulting value is continuous and allows for detection of small differences between subjects. Patients with intermediate uveitis can sometimes present with minimal complaints and establishing the presence clinically can be challenging. Another strength of this study is the large and heterogeneous cohort of MS patients.
The occurrence of uveitis in a patient with MS might cause the clinician to doubt whether the patient's current treatment is appropriate. The fact that no relationship between VH and measures of CNS inflammation (most importantly relapses) was found might provide support and reassurance for continuation of the already implemented treatment in an MS patient suffering from intermediate uveitis, who otherwise does not have any other clinical or radiological sign of disease activity. This in turn might help to avoid unnecessary switch to another form of DMT. The present study found lower VH scores in patients who were on DMT compared to those who had never been on DMT, possibly suggesting VH might serve as a biomarker for therapy efficacy. Nevertheless, further studies need to be conducted to support this claim.
In conclusion, this study found no evidence of increased ocular inflammation in MS patients compared to HCs when applying an automated algorithm to objectively measure VH on OCT scans. Moreover, there was no evidence for any association between VH and signs of inflammatory CNS disease activity. Although the clinical correlate of the OCT‐based VH measurement is limited to intermediate uveitis, the findings of the present study suggest that the link between (subclinical) uveitis and MS is less evident than previously assumed.
Disclosure of conflicts of interest
DC, GO, GM, AKD: nothing to disclose. PAK: reports speaker fees from Zeiss, Topcon, Heidelberg‐Engineering, Haag‐Streit, Novartis, Bayer and Allergan; is on the advisory board of Novartis and Bayer; has received consultancy fees from DeepMind and Optos. LJB: reports institutional support for OCT projects from TEVA. BMJU: has received personal compensation for consulting from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche and TEVA. AP: reports personal fees from Novartis, grants from Novartis, outside the submitted work; and is part of the steering committee of the OCTiMS study which is sponsored by Novartis and the VUmc received research support. DPC: has received speaker fees from Allergan and Santen.
Supporting information
Data S1. Retinal OCT protocol.
Acknowledgements
The authors would like to thank Stichting Het Remmert Adriaan Laan Fonds and the Dutch MS Research Foundation for granting D. Coric a personal travel grant. This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors. AP was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1. Lightman S, McDonald WI, Bird AC, et al Retinal venous sheathing in optic neuritis. Its significance for the pathogenesis of multiple sclerosis. Brain 1987; 110: 405–414. [DOI] [PubMed] [Google Scholar]
- 2. Gordon LK, Goldstein DA. Gender and uveitis in patients with multiple sclerosis. J Ophthalmol 2014; 2014: 565262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Biousse V, Trichet C, Bloch‐Michel E, Roullet E. Multiple sclerosis associated with uveitis in two large clinic‐based series. Neurology 1999; 52: 179–181. [DOI] [PubMed] [Google Scholar]
- 4. Olsen TG, Frederiksen J. The association between multiple sclerosis and uveitis. Surv Ophthalmol 2017; 62: 89–95. [DOI] [PubMed] [Google Scholar]
- 5. Sepulcre J, Murie‐Fernandez M, Salinas‐Alaman A, Garcia‐Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 2007; 68: 1488–1494. [DOI] [PubMed] [Google Scholar]
- 6. Tola MR, Granieri E, Casetta I, et al Retinal periphlebitis in multiple sclerosis: a marker of disease activity? Eur Neurol 1993; 33: 93–96. [DOI] [PubMed] [Google Scholar]
- 7. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura SJ, Thygeson P, Hogan MJ. Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol 1959; 47: 171–176. [DOI] [PubMed] [Google Scholar]
- 9. Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology 1985; 92: 467–471. [DOI] [PubMed] [Google Scholar]
- 10. Keane PA, Karampelas M, Sim DA, et al Objective measurement of vitreous inflammation using optical coherence tomography. Ophthalmology 2014; 121: 1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keane PA, Balaskas K, Sim DA, et al Automated analysis of vitreous inflammation using spectral‐domain optical coherence tomography. Transl Vis Sci Technol 2015; 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balk LJ, Twisk JW, Steenwijk MD, et al A dam for retrograde axonal degeneration in multiple sclerosis? J Neurol Neurosurg Psychiatry 2014; 85: 782–789. [DOI] [PubMed] [Google Scholar]
- 13. Petzold A, Wattjes MP, Costello F, et al The investigation of acute optic neuritis: a review and proposed protocol. Nat Rev Neurol 2014; 10: 447–458. [DOI] [PubMed] [Google Scholar]
- 14. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 15. Coric D, Balk LJ, Verrijp M, et al Cognitive impairment in patients with multiple sclerosis is associated with atrophy of the inner retinal layers. Mult Scler 2018; 24: 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montesano G, Way CM, Ometto G, et al Optimizing OCT acquisition parameters for assessments of vitreous haze for application in uveitis. Sci Rep 2018; 8: 1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marrie RA, Reider N, Cohen J, et al A systematic review of the incidence and prevalence of autoimmune disease in multiple sclerosis. Mult Scler 2015; 21: 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shugaiv E, Tuzun E, Kurtuncu M, et al Uveitis as a prognostic factor in multiple sclerosis. Mult Scler 2015; 21: 105–107. [DOI] [PubMed] [Google Scholar]
- 19. Langer‐Gould A, Albers KB, Van Den Eeden SK, Nelson LM. Autoimmune diseases prior to the diagnosis of multiple sclerosis: a population‐based case–control study. Mult Scler 2010; 16: 855–861. [DOI] [PubMed] [Google Scholar]
- 20. Le Scanff J, Seve P, Renoux C, Broussolle C, Confavreux C, Vukusic S. Uveitis associated with multiple sclerosis. Mult Scler 2008; 14: 415–417. [DOI] [PubMed] [Google Scholar]
- 21. Jakob E, Reuland MS, Mackensen F, et al Uveitis subtypes in a German interdisciplinary uveitis center – analysis of 1916 patients. J Rheumatol 2009; 36: 127–136. [DOI] [PubMed] [Google Scholar]
- 22. Messenger W, Hildebrandt L, Mackensen F, Suhler E, Becker M, Rosenbaum JT. Characterisation of uveitis in association with multiple sclerosis. Br J Ophthalmol 2015; 99: 205–209. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt S, Wessels L, Augustin A, Klockgether T. Patients with multiple sclerosis and concomitant uveitis/periphlebitis retinae are not distinct from those without intraocular inflammation. J Neurol Sci 2001; 187: 49–53. [DOI] [PubMed] [Google Scholar]
- 24. Knier B, Schmidt P, Aly L, et al Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain 2016; 139: 2855–2863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Retinal OCT protocol.


