Summary
Although tannins have been an important focus of studies of plant–animal interactions, traditional tannin analyses cannot differentiate between the diversity of structures present in plants. This has limited our understanding of how different mixtures of these widespread secondary metabolites contribute to variation in biological activity.
We used UPLC‐MS/MS to determine the concentration and broad composition of tannins and polyphenols in 628 eucalypt (Eucalyptus, Corymbia and Angophora) samples, and related these to three in vitro functional measures believed to influence herbivore defence: protein precipitation capacity, oxidative activity at high pH and capacity to reduce in vitro nitrogen (N) digestibility.
Protein precipitation capacity was most strongly correlated with concentrations of procyanidin subunits in proanthocyanidins (PAs), and late‐eluting ellagitannins. Capacity to reduce in vitro N digestibility was affected most by the subunit composition and mean degree of polymerisation (mDP) of PAs. Finally, concentrations of ellagitannins and prodelphinidin subunits of PAs were the strongest determinants of oxidative activity.
The results illustrate why measures of total tannins rarely correlate with animal feeding responses. However, they also confirm that the analytical techniques utilised here could allow researchers to understand how variation in tannins influence the ecology of individuals and populations of herbivores, and, ultimately, other ecosystem processes.
Keywords: Eucalyptus leaves, herbivory, hydrolysable tannins, nitrogen digestibility, oxidative activity, polyphenols, proanthocyanidins, protein precipitation capacity
Introduction
Biologists often assume that a primary role of tannins is to defend plants against herbivory. There are, however, thousands of tannin compounds displaying a diverse array of structures that often occur in complex mixtures of tens to hundreds of compounds. These structures not only influence the response to standard colorimetric assays (Schofield et al., 2001), but they also affect the biological activity of tannins, including their ability to bind proteins (Porter & Woodruffe, 1984; Jones & Palmer, 2000; Karonen et al., 2015), their pro‐ or antioxidant capacities (Barbehenn et al., 2006; Moilanen & Salminen, 2008; Moilanen et al., 2016), and, ultimately, their effects on herbivores (Ayres et al., 1997; Makkar, 2003; Mueller‐Harvey, 2006; Roslin & Salminen, 2008). Consequently, the specific tannins present in a mixture, rather than just the total tannin concentration, are important in understanding biological consequences (Barbehenn et al., 2008; Moilanen & Salminen, 2008). However, it is difficult to characterise complex tannin mixtures, and therefore to attribute specific biological consequences to tannins. Some ecologists have circumvented this problem by relating measures of herbivory to functional attributes of tannins, such as their capacity to precipitate protein (Robbins et al., 1987; e.g. McArt et al., 2009), reduce nitrogen (N) digestibility (e.g. DeGabriel et al., 2009; McArt et al., 2009) or oxidise at high pH (e.g. Appel, 1993; Steinbauer et al., 2016; Marsh et al., 2017b).
Protein binding is the primary mechanism by which tannins are traditionally thought to affect mammalian herbivores. Thus, ecologists measure the capacity of tannins to precipitate a standard amount of protein and use this as a functional measure of tannin concentrations in forage. For example, Robbins et al. (1987) were able to predict the in vivo digestible protein content of plants for mule deer (Odocoileus hemionus) and white‐tailed deer (O. virginianus) from the in vitro protein precipitation capacity and total protein concentration of plant extracts. In turn, McArt et al. (2009) demonstrated that reduced protein availability due to protein precipitation by tannins could explain differences in productivity between moose (Alces alces) living in different regions.
DeGabriel et al. (2008) developed an alternative method to assess the capacity of tannins to constrain protein digestion in mammalian herbivores. Their method measures the in vitro digestibility of plant N in the presence and absence of polyethylene glycol (PEG), a polymer that preferentially binds to tannins and releases protein (Silanikove et al., 1996; Schofield et al., 2001). Using this method, DeGabriel et al. (2009) showed that the in vitro digestible N concentration of eucalypt foliage influenced the reproductive success of female common brushtail possums (Trichosurus vulpecula). As a consequence, in vitro digestible N concentrations have been used as indicators of the nutritional quality of habitat for marsupial folivores (DeGabriel et al., 2009; Youngentob et al., 2011; Windley et al., 2016).
In contrast to mammals, there is little evidence that tannins reduce the digestibility of protein in insect herbivores (Barbehenn & Constabel, 2011). The alkaline pH in the midgut of insects can prevent tannins from forming complexes with protein (Martin et al., 1985; Appel, 1993; Barbehenn & Constabel, 2011). This is not to say that tannins are harmless to insects. Instead, the alkaline conditions may promote the oxidation of tannins and other phenolic compounds, leading to the formation of oxygen radicals and, consequently, cell damage (Appel, 1993). For example, the larvae of some tropical lepidopteran species were found to contain the oxidation products of polyphenols in their frass (Vihakas et al., 2015). Likewise, Lymantria dispar, Orgyia Leucostigma and Malacosoma distria caterpillars that fed on maple leaves with high concentrations of ellagitannins had high levels of semiquinone radicals in their midguts together with increased protein carbonyl contents that suggested increased oxidation of the proteins in the gut (Barbehenn et al., 2005, 2013).
The above examples demonstrate that measuring the activity of polyphenol mixtures has provided a better indication of their probable effects in different biological systems compared with estimates of ‘total tannins’ or ‘total phenolics’. Fortunately, new analytical techniques are now available that allow the broad characterisation of complex polyphenol mixtures in plants. For example, as part of a related study on the phylogeny of tannins in eucalypts, we used ultraperformance liquid chromatography tandem mass spectrometry (UPLC‐MS/MS, i.e. the Engström method; Engström et al., 2014, 2015; Salminen, 2018b) to measure the concentrations of a range of phenolic subgroups, including four subgroups of tannins, in leaves from 628 eucalypts representing 515 species (Marsh et al., 2017a). This provides an ideal opportunity to examine the relationship between plant polyphenol composition and traditional in vitro measures of biological activity. Knowing what groups of tannins are active also improves our ability to identify the genetic architecture of tannin production. For example, Skovmand et al. (2018) recently argued that genes responsible for variations in tannins in plants may be ‘keystone genes’ that are critical to ecosystem function.
Eucalypts are the dominant forest and woodland trees in Australia, with c. 900 species belonging to the genera Eucalyptus, Angophora and Corymbia (Bayly et al., 2013). Eucalypt foliage makes up a large proportion of the diet of four species of marsupials – the koala (Phascolarctos cinereus), greater glider (Petauroides volans), common ringtail possum (Pseudocheirus peregrinus) and common brushtail possum (Trichosurus vulpecula) (Moore et al., 2004) – and a wide variety of insect herbivores (Fox & Morrow, 1981; Paine et al., 2011). Importantly, eucalypts contain variable concentrations and types of tannins and other polyphenols, suggesting that there may also be significant variation in biological activity associated with herbivore defence (Marsh et al., 2017a).
We had several expectations about how specific tannin subgroups would influence traditional measures of in vitro biological activity (protein precipitation capacity, oxidative activity and capacity to reduce digestible N) of eucalypt samples (Table 1). However, we also understood that our hypotheses are complicated by the fact that individual tannins within the same subgroup can differ over six‐fold in both their protein precipitation (Karonen et al., 2015) and their oxidative activities (Moilanen & Salminen, 2008). Thus, differences in the types and concentrations of individual tannins between eucalypt species could influence the degree of the response to the standard assays. Nevertheless, we expected to reveal the major patterns between the tannin groups and their bioactivities, and hoped to see some of the more detailed patterns within the tannin subgroups.
Table 1.
The polyphenol constituents measured in this study, and their expected effects on biological activity.
| Constituent | Polyphenol class | Expected effect on biological activity | ||
|---|---|---|---|---|
| PPC | OA | CND | ||
| Small procyanidin | PA | + | + | |
| Medium procyanidin | PA | + | + | |
| Large procyanidin | PA | ++ | ++ | |
| Small prodelphinidin | PA | + | + | + |
| Medium prodelphinidin | PA | ++ | + | ++ |
| Large prodelphinidin | PA | +++ | + | ++ |
| % prodelphinidin | PA | + | + | + |
| mDP | PA | ++ | + | |
| Early‐eluting HHDP derivatives | HT | +++ | ||
| Late‐eluting HHDP derivatives | HT | + | ++ | |
| Early‐eluting galloyl derivatives | HT | ++ | ||
| Late‐eluting galloyl derivatives | HT | ++ | + | |
| Kaempferol derivatives | Flavonol | |||
| Quercetin derivatives | Flavonol | |||
| Myricetin derivatives | Flavonol | + | ||
| Quinic acid derivatives | Flavonoid | + | ||
A ‘+’ indicates an expected positive relationship, with ‘++’ and ‘+++’ indicating the constituents hypothesised to have the strongest effects on biological activity.
PA, proanthocyanidin; HT, hydrolysable tannin; PPC, protein precipitation capacity (mg g−1 DM pentagalloyl glucose equivalents); OA, oxidative activity (mg g−1 DM gallic acid equivalents); CND, capacity to reduce N digestibility (percentage units); mDP, mean degree of polymerisation of proanthocyanidins; HHDP, hexahydroxydiphenoyl.
We anticipated that protein precipitation capacity would be positively correlated with the concentrations of proanthocyanidins (PAs; also known as condensed tannins), their mean degree of polymerisation (mDP) and the proportion of prodelphinidin subunits in PAs (Jones et al., 1976; Porter & Woodruffe, 1984; McManus et al., 1985; Aerts et al., 1999). At a finer scale, we used the Engström method (Engström et al., 2014) to measure PA concentrations at three cone voltages (see Salminen, 2018b), which provides additional information about the size distribution of PAs. We expected that large prodelphinidin‐rich PAs in particular would contribute positively to the protein precipitation capacity of samples, assuming that these types of PAs were in sufficient concentrations in the extracts. Because most of the galloyl groups in our samples originate from monomeric ellagitannins or simple galloyl glucoses, rather than gallotannins (Marsh et al., 2017a), and because gallotannins have a better protein precipitation capacity compared with monomeric ellagitannins or simple galloyl glucoses (Haslam, 1988; Kawamoto et al., 1996; Kilkowski & Gross, 1999; Salminen & Karonen, 2011), we did not expect a strong correlation between protein precipitation capacity and galloyl‐derivative concentrations as such, unless the galloyl derivatives were the main tannins of the species.
We hypothesised that the properties of tannins that affect protein precipitation capacity would also influence their capacity to reduce in vitro N digestibility. The gut contains both exogenous protein from the diet and endogenous proteins, such as enzymes, sloughed mucosal cells and microbial protein. Tannins that precipitate any of these proteins will reduce the apparent digestibility of N. We therefore expected that the concentration, size and composition of PA molecules would strongly influence the capacity to reduce in vitro digestible N, and that there would be a positive correlation between protein precipitation capacity and capacity to reduce in vitro N digestibility in eucalypts.
In contrast to the major role that PAs may play in influencing protein precipitation capacity and capacity to reduce N digestibility, we predicted that the concentration of hexahydroxydiphenoyl (HHDP) derivatives (i.e. ellagitannins), particularly those that elute earlier during UPLC separation, would drive the oxidative activity of eucalypt samples, if they are present at sufficient concentrations compared to other oxidatively active compounds in the samples. This is because ellagitannins appear to be the class of tannins that are most oxidatively active at high pH (Barbehenn et al., 2006; Moilanen & Salminen, 2008), and those with shorter UPLC retention times tend to oxidise more readily than those with longer retention times (Salminen et al., 2011; Moilanen et al., 2013). Other polyphenol constituents, including prodelphinidin subunits of PAs and myricetin derivatives, with pyrogallol‐type substitution of the flavonoid B‐ring, may also contribute to oxidative activity to a lesser extent (Vihakas et al., 2014).
Our final prediction was that there would be a negative correlation between oxidative activity and protein precipitation capacity. This prediction was made for two reasons. First, hydrolysable tannins (HTs) with high protein precipitation capacity tend to have lower oxidative activity (Moilanen et al., 2013). And second, there is an inverse relationship between the concentrations of PAs and HTs in eucalypt leaves, probably because they compete for biosynthetic pathways (Marsh et al., 2017a). Given the expected reciprocal effects of PAs and HTs on oxidative activity and protein precipitation capacity, protein precipitation capacity may be higher in those samples dominated by PAs, while samples dominated by HTs may be more oxidatively active.
Materials and Methods
The collection of 628 leaf samples (515 eucalypt species from the allied genera Eucalyptus L'Her., Corymbia Hill & Johnson and Angophora Cav. – duplicates of species were predominantly different subspecies) from Currency Creek Arboretum, South Australia, and the measurement of the phenolic composition of these samples by UPLC‐MS/MS is described in detail in Marsh et al. (2017a). Hydrolysable tannins (HHDP and galloyl derivatives), however, were re‐integrated to determine concentrations of early‐eluting and late‐eluting derivatives for each group. We used the dimeric ellagitannin, oenothein B, as a marker to distinguish the difference between these two groups. The early‐eluting HHDP and galloyl derivatives eluted before oenothein B (0.5–2.9 min), and the late‐eluting ones were those from oenothein B onwards (2.9–6 min). Likewise, we re‐analysed the previously collected data to determine the concentration of procyanidins and prodelphinidins in each of the three size classes explained in Salminen (2018b). The smaller procyanidin and prodelphinidin oligomers were detected by cone voltages of 75 and 55 V, the medium procyanidin and prodelphinidin oligomers and polymers by cone voltages of 85 and 80 V, and the large procyanidin and prodelphinidin polymers by cone voltages of 140 and 130 V, respectively. A summary of the phenolic constituents that were measured is given in Table 1, along with their expected effects on biological activity.
Protein precipitation capacity
The protein precipitation capacity of eucalypt extracts was quantified by the radial diffusion assay (Hagerman, 1987) using BSA as the protein and pentagalloylglucose as the quantification standard. Briefly, the original, nondiluted eucalypt extract (Marsh et al., 2017a) was concentrated two‐fold via freeze‐drying and redissolving into Milli‐Q water. A 24 μl aliquot of this concentrated extract was applied to three wells punched onto a Petri dish filled with BSA‐agar gel. The Petri dishes were covered with parafilm and incubated at 30°C for 72 h to form reproducible rings with tannins and BSA. The ring area was documented with a camera on a tripod and measured by the imageJ software.
Oxidative activity
The portion of total phenolics that was easily auto‐oxidised at pH 10 was measured as both mg g−1 dry weight and as a percentage of total phenolics using the method of Salminen & Karonen (2011), calibrated with gallic acid. In short, the total phenolic content of 15× dilutions (Milli‐Q water) of the original eucalypt extracts (Marsh et al., 2017a) and the pH 10‐oxidised extracts were measured with a well‐plate reader at 730 nm. The difference in the total phenolic concentrations between these measurements revealed the level of easily oxidised phenolics in the samples (i.e. the oxidative activity in mg g−1 or in % of total phenolics).
Capacity to reduce in vitro N digestibility
A subset of leaves from each tree were freeze dried and then ground in a Foss Cyclotec 1093 mill (Foss, Höganäs, Sweden) until they passed through a 1 mm sieve. The capacity to reduce in vitro N digestibility was determined using a modified version of the method of DeGabriel et al. (2008). The method involves sequential digestion of samples of ground foliage in acid pepsin and cellulase in the presence and absence of polyethylene glycol 4000 (PEG), which binds to both HTs and PAs (Silanikove et al., 1996; Schofield et al., 2001).
For each sample, 0.8050 ± 0.0050 g leaf powder was weighed into each of four Ankom F57 fibre filter bags (Ankom Technology, Macedon, NY, USA). Two bags per sample were placed into beakers (100 bags per beaker) containing 25 ml Tris‐base buffer solution (pH 7.1) per bag with 33.33 g l−1 PEG. The remaining bags were placed into the buffer solution without PEG. Samples were incubated at 37°C for 24 h, after which they were washed thoroughly with water and dried to constant mass at 40°C. Bags were then placed into 25 ml per bag of 0.1 M HCl containing 2 g l−1 pepsin for 48 h at 37°C. Samples were removed from the pepsin solution and washed briefly, before a final incubation at 37°C for 24 h in 25 ml per bag of 100 mmol acetic acid buffer (pH 4.75) containing 6.25 g l−1 cellulase. Samples were washed thoroughly, dried at 40°C to constant mass and weighed.
After the digestion process, the N concentration was determined in 120 ± 20 mg of residue from each bag, as well as in the original ground leaf samples, using a Leco Truspec C/N analyser (Leco Corporation, Sydney, NSW, Australia). These values were used to calculate the in vitro digestibility of N in the presence and absence of PEG. Any samples with a coefficient of variation > 5% between duplicate analyses were repeated. The capacity to reduce in vitro N digestibility was calculated as the difference in N digestibility between samples incubated with and without PEG. The digestibility with PEG was used rather than total N, because the total N of a plant will never be completely digested, due to some of the N being bound in complex cell‐wall polymers.
Statistical methods
We compared the mean biological activity (protein precipitation capacity, oxidative activity and capacity to reduce in vitro N digestibility) of eucalypt phylogenetic clades (see Marsh et al., 2017a for the allocation of species to clades) using the gls function in package nlme for R (Pinheiro et al., 2017) and Pagel's λ covariance structure (R package ape; Paradis et al., 2004) to account for the phylogenetic nonindependence of samples. For all analyses, the dataset and analyses were at the individual tree level, with multiple subspecies and trees observed for some species, while other species were observed only once. Pagel's λ model is equivalent to including a within‐species error term in addition to phylogenetic between‐species term, and so is suitable for data where there are multiple observations per species. We used Tukey contrasts to determine which clades differed significantly (P < 0.05) from one another. Residuals were checked for normality and variance homogeneity in all models.
We estimated correlations between protein precipitation capacity and oxidative activity, protein precipitation capacity and capacity to reduce in vitro N digestibility, and oxidative activity and capacity to reduce in vitro N digestibility using the corphylo function in the ape package in R to take the phylogenetic relatedness of species into account. Standard errors and t‐statistics for the estimated correlations were calculated using a parametric bootstrap with 30 replicates under the null hypothesis of independence, using simple phylogenetic regression models fitted using the gls function in the ape package in R.
We also used the gls function to fit four regression models for each of the square root of protein precipitation capacity, oxidative activity and the square root of the capacity to reduce N digestibility, while taking the phylogeny into account. The square root transformation was applied to two of these three dependent variables in order to better satisfy the assumption of normally distributed errors with equal variances. The first model for each dependent variable contained the intercept only. The second model contained the total polyphenol concentration (the sum of all measured constituents). The third model contained two terms: total tannins (the sum of all constituents in the PA and HT classes; Table 1) and total flavonols (the sum of all constituents in the flavonol class; Table 1). The fourth model contained all individual constituents listed in Table 1. We tested the significance of the fourth model using likelihood ratio tests relative to the second and third models, which are both submodels of it.
To explore which constituents were most important in predicting biological activity, we performed a backward selection of all of the covariates in the fourth model outlined in the previous paragraph for each of the three transformed activities based on the significance of omitting variables as calculated using a chi‐squared likelihood ratio test with a cutoff of 0.05 for significance. So that models made biological sense, either the total concentration of prodelphinidin (sum of the concentrations of prodelphinidin from small, medium and large PAs; Table 1) was allowed in the model or one or more of the separate small, medium or large concentrations of prodelphinidin. Combinations of total prodelphinidin with prodelphinidin subgroups were not allowed. The total procyanidin (or small, medium and large), total HHDP derivatives (or early and late) and total gallic acid derivatives (or early and late) variables were treated similarly. Residuals were checked for normality and homogeneity of variances, and six outliers with high leverage were removed. These included two samples with oxidative activity and three samples with protein precipitation capacity almost double that of the next highest samples, and a sample which turned out to be an outlier in regression models of in vitro N digestibility.
Results
In vitro polyphenol‐derived biological activity
We found wide variation in the protein precipitation capacity (0–229 mg g−1 dry matter (DM) pentagalloyl glucose equivalents), oxidative activity (2–94 mg g−1 DM gallic acid equivalents, or 3–79% of total phenolics) and capacity to reduce in vitro N digestibility (0–89 percentage units) between eucalypt species (Table 2). Mean biological activity did not differ between phylogenetic clades when the relatedness of species was taken into account (Table 2).
Table 2.
The mean (range) biological activity of species belonging to different eucalypt clades.
| Phylogenetic clade | n | PPCa | OAb | CNDc |
|---|---|---|---|---|
| Angophora | 5 | 25 (9–59) | 21 (6–40) | 19 (8–44) |
| Corymbia I | 19 | 18 (0–40) | 16 (5–30) | 29 (11–45) |
| Corymbia II | 13 | 28 (5–53) | 19 (6–34) | 24 (13–38) |
| Eudesmia | 10 | 42 (6–92) | 29 (10–50) | 40 (1–89) |
| Monocalyptus | 79 | 36 (0–122) | 19 (4–56) | 25 (0–70) |
| Symphyomyrtus I | 32 | 25 (0–89) | 12 (2–51) | 10 (0–22) |
| Symphyomyrtus II | 97 | 26 (0–98) | 19 (3–48) | 11 (0–68) |
| Symphyomyrtus III | 125 | 43 (0–229) | 22 (2–86) | 9 (0–38) |
| Symphyomyrtus IV | 76 | 30 (0–113) | 22 (2–94) | 28 (0–67) |
| Symphyomyrtus V | 166 | 40 (0–201) | 20 (3–50) | 10 (0–58) |
| Pagel's λ | 0.46 | 0.77 | 0.82 | |
| F‐statistic | 0.99 | 1.65 | 1.60 | |
| P‐value | 0.453 | 0.091 | 0.103 |
Protein precipitation capacity (mg g−1 DM pentagalloyl glucose equivalents).
Oxidative activity (mg g−1 DM gallic acid equivalents).
Capacity to reduce N digestibility (percentage units).
The capacity to reduce in vitro N digestibility was not correlated with either the oxidative activity (t = −0.95, P = 0.341) or protein precipitation capacity of eucalypt leaves (t = 0.36, P = 0.719). However, there was a positive correlation between the protein precipitation capacity and oxidative activity of samples (r = 0.54, t = 3.90, P < 0.001).
Polyphenol composition and protein precipitation capacity
The model containing all of the polyphenol constituents in Table 1 explained significantly more of the variation in the square root of protein precipitation capacity compared with models that contained only the total polyphenol concentration or a combination of the total tannin and total flavonol concentrations (P < 0.001 for both model comparisons).
There were strong positive relationships between protein precipitation capacity and the concentrations of late‐eluting HHDP derivatives (Fig. 1a), procyanidin subunits from polymeric PAs (Fig. 1b), galloyl derivatives and prodelphinidin subunits from medium‐sized PAs (Table 3). Although the relationships were not as strong, the concentration of early‐eluting HHDP derivatives and the mDP of PAs were negatively correlated with protein precipitation capacity (Table 3). Pagel's lambda for the model was 0.31 (likelihood ratio χ2 = 17.3 on 1 degree of freedom, P < 0.001).
Figure 1.
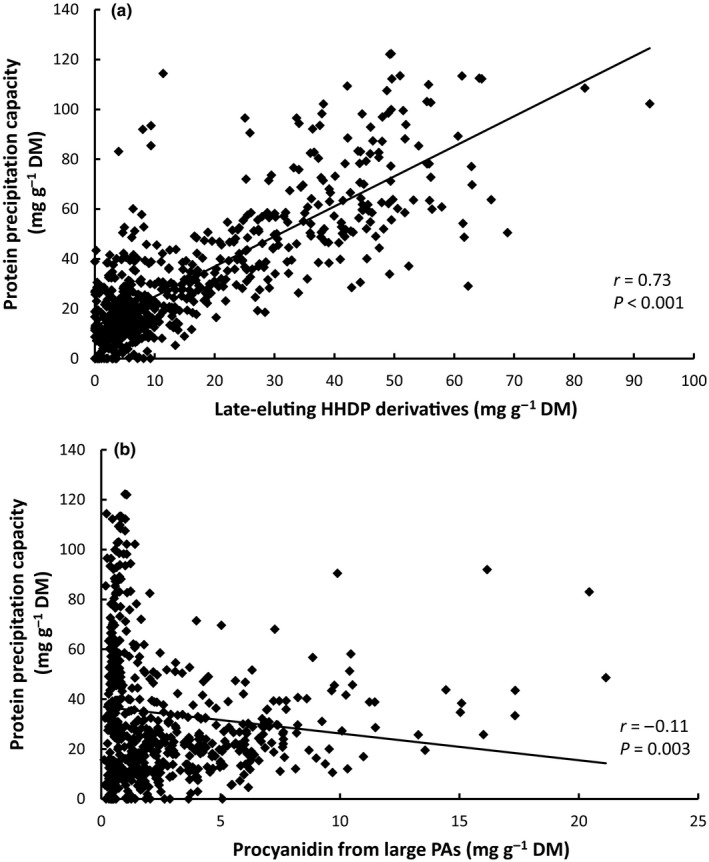
The relationship between the protein precipitation capacity of eucalypt leaves and the two polyphenol constituents that had the strongest correlation with this measurement (n = 628): (a) late‐eluting hexahydroxydiphenoyl (HHDP) derivatives, and (b) procyanidin subunits from polymeric proanthocyanidins (PAs). Note that these relationships are indicative only, because they do not take into account other covariates or phylogenetic correlations from the statistical model.
Table 3.
Final statistical model showing the phenolic constituents that had a significant effect on the square root of protein precipitation capacity.
| Model term | Parameter estimate | SE | t statistic | P‐value | Standardised coefficient |
|---|---|---|---|---|---|
| (Intercept) | 2.393 | 0.439 | 5.45 | < 0.001 | |
| Late HHDP | 0.120 | 0.006 | 18.94 | < 0.001 | 0.882 |
| Large procyanidin | 0.219 | 0.018 | 11.89 | < 0.001 | 0.298 |
| Total galloyl | 0.056 | 0.006 | 8.75 | < 0.001 | 0.235 |
| Medium prodelphinidin | 0.178 | 0.022 | 8.17 | < 0.001 | 0.209 |
| Early HHDP | −0.027 | 0.010 | −2.73 | 0.007 | −0.112 |
| mDP of PAs | −0.047 | 0.018 | −2.59 | 0.010 | −0.070 |
Degrees of freedom for all t‐statistics is 563 (n = 625).
HHDP, hexahydroxydiphenoyl derivatives; mDP of PAs, mean degree of polymerisation of proanthocyanidins.
Polyphenol composition and oxidative activity
The full model containing all measured polyphenol constituents explained significantly more variation in oxidative activity compared with either the total polyphenol concentration alone, or a combination of the total tannin and total flavonol concentrations, with P < 0.001 for both model comparisons.
The total concentration of HHDP derivatives had a very strong effect on the oxidative activity of samples (Fig. 2a), while there were also strong positive relationships between the oxidative activity of samples and the concentrations of prodelphinidin subunits from large PAs (Fig. 2b), and early‐eluting galloyl derivatives (Table 4). The mDP of PAs and the concentrations of quercetin and quinic acid derivatives were also positively correlated with oxidative activity, while the concentration of prodelphinidin subunits from medium‐sized PAs was negatively correlated (Table 4). Pagel's lambda for the model was 0.58 (likelihood ratio χ2 = 21.9 on 1 degree of freedom, P < 0.001).
Figure 2.
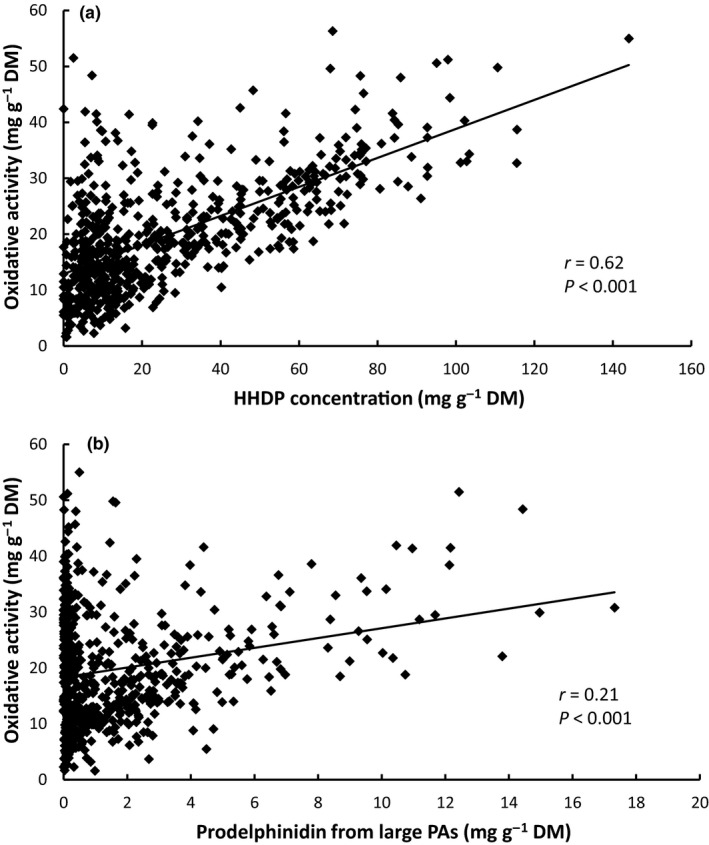
The relationship between the oxidative activity of eucalypt leaves and the two polyphenol constituents that had the strongest correlation with this measurement (n = 628): (a) hexahydroxydiphenoyl (HHDP) derivatives, and (b) prodelphinidin subunits from large proanthocyanidins (PAs). Note that these relationships are indicative only, because they do not take into account other covariates or phylogenetic correlations from the statistical model.
Table 4.
Final statistical model showing the phenolic constituents that had a significant effect on oxidative activity.
| Model term | Parameter estimate | SE | t statistic | P‐value | Standardised coefficient |
|---|---|---|---|---|---|
| (Intercept) | 6.656 | 3.344 | 1.99 | 0.047 | |
| Total HHDP | 0.303 | 0.012 | 24.78 | < 0.001 | 0.763 |
| Large prodelphinidin | 2.801 | 0.420 | 6.67 | < 0.001 | 0.737 |
| Early galloyl | 0.329 | 0.086 | 3.84 | < 0.001 | 0.113 |
| mDP of PAs | 0.235 | 0.092 | 2.56 | 0.011 | 0.079 |
| Medium prodelphinidin | −1.015 | 0.411 | −2.47 | 0.014 | −0.271 |
| Quercetin | 0.259 | 0.111 | 2.33 | 0.020 | 0.063 |
| Quinic acid | 0.273 | 0.125 | 2.19 | 0.029 | 0.067 |
Degrees of freedom for all t‐statistics is 563 (n = 626).
HHDP, hexahydroxydiphenoyl derivatives; mDP of PAs, mean degree of polymerisation of proanthocyanidins.
Polyphenol composition and capacity to reduce in vitro N digestibility
The full model containing all measured polyphenol constituents explained significantly more of the variation of the square root of the capacity to reduce in vitro N digestibility compared with models containing only the total polyphenol concentration, or a combination of the total tannin and total flavonol concentrations (P < 0.001 for both model comparisons).
There were strong positive relationships between the capacity to reduce in vitro N digestibility and the proportion of prodelphinidin in PAs (Fig. 3a), and the mDP of PAs (Fig. 3b; Table 5). The concentration of PD in small PAs had a strong negative effect on the capacity to reduce in vitro N digestibility (Table 5). There was a weaker negative relationship between the capacity to reduce in vitro N digestibility and the concentration of quercetin derivatives (Table 5). Pagel's lambda for the model was 0.66 (likelihood ratio χ2 = 72.0 on 1 degree of freedom, P < 0.001).
Figure 3.
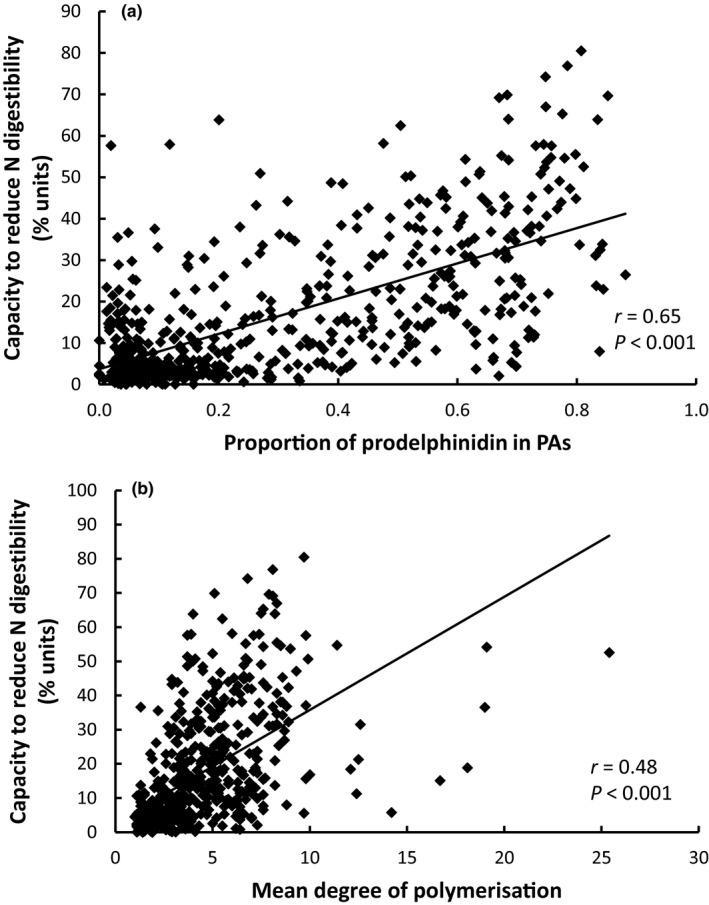
The relationship between the capacity to reduce the in vitro nitrogen (N) digestibility of eucalypt leaves and (a) the proportion of proanthocyanidins (PAs) comprising prodelphinidin, and (b) the mean degree of polymerisation (mDP) of proanthocyanidins (n = 628). Note that these relationships are indicative only, because they do not take into account other covariates or phylogenetic correlations from the statistical model.
Table 5.
Final statistical model showing the phenolic constituents that significantly influenced the square root of the capacity to reduce in vitro N digestibility.
| Model term | Parameter estimate | SE | t statistic | P‐value | Standardised coefficient |
|---|---|---|---|---|---|
| (Intercept) | 5.883 | 0.831 | 7.08 | < 0.001 | |
| % prodelphinidin | 3.244 | 0.308 | 10.54 | < 0.001 | 0.420 |
| mDP of PAs | 0.294 | 0.028 | 10.37 | < 0.001 | 0.503 |
| Small prodelphinidin | −0.217 | 0.023 | −9.35 | < 0.001 | −0.376 |
| Quercetin | −0.061 | 0.025 | −2.48 | 0.014 | −0.075 |
Degrees of freedom for all t‐statistics is 566 (n = 627).
mDP of PAs = mean degree of polymerisation of proanthocyanidins.
Discussion
The current study demonstrates that quantifying tannin subgroups in complex polyphenol mixtures provides valuable information about the biological activity of plant samples. The subgroup composition of tannins in eucalypt extracts explained significantly more of the variation in oxidative activity, protein precipitation capacity and capacity to reduce in vitro N digestibility compared with the sum of the concentrations of the measured constituents. This is not necessarily surprising, because eucalypt species with the same total polyphenol concentration can have vastly different polyphenol profiles (Marsh et al., 2017a). Nevertheless, researchers frequently (and often unsuccessfully) attempt to relate total tannin concentrations to, for example, various measures of herbivory (Ayres et al., 1997; Wang et al., 2014; Masette et al., 2015; Volf et al., 2015; Felton et al., 2018).
Different tannin subgroups influenced different types of biological activity, although not all of these correlations matched our expectations. For example, concentrations of late‐eluting HHDP derivatives (ellagitannins) were more strongly correlated with protein precipitation capacity compared with concentrations of PA subunits. It is likely, however, that high concentrations of ellagitannins relative to PAs drove this association. Likewise, the size and composition of PAs, rather than their concentration, correlated most strongly with the capacity to reduce in vitro N digestibility. As expected, however, the concentration of ellagitannins had the greatest effect on the oxidative activity of samples. This study significantly advances our understanding of structure–function relationships in natural plant tannin mixtures, and demonstrates that modern analytical techniques should be incorporated into studies examining relationships between tannins and herbivory. Below, we discuss the relationships between phenolic composition and biological activity in greater detail, and the implications for herbivores consuming foliage.
Protein precipitation by tannins has been advocated as a mechanism by which plants can defend themselves against a variety of mammalian herbivores, including against those that feed on eucalypt foliage (Marsh et al., 2003; DeGabriel et al., 2009). At equal concentrations, in a broad sense, PAs have a greater capacity than gallotannins or ellagitannins to precipitate protein, although accurate compound‐specific studies with PAs are scarce (Haslam, 1988; Kilkowski & Gross, 1999; Salminen & Karonen, 2011). The tannin profiles of many of the eucalypts that we analysed were dominated by ellagitannins, with some having concentrations > 100 mg g−1 (Fig. 2a; Marsh et al., 2017a). In this situation, higher concentrations may compensate for lower bioactivity, and can make a significant contribution to protein precipitation capacity (Johnson et al., 2014). This probably explains why the protein precipitation capacity of eucalypt extracts was most strongly correlated with the concentration of late‐eluting HHDP derivatives, and then secondarily with the concentrations of procyanidin subunits in large PAs and prodelphinidin subunits in medium‐sized PAs.
Our finding that the concentration of late‐eluting HHDP derivatives had a greater impact on protein precipitation capacity compared with early‐eluting derivatives supports previous work that individual ellagitannins can differ greatly in their capacity to precipitate protein (Salminen et al., 2011; Moilanen et al., 2013). Purified ellagitannins with greater protein precipitation capacity tend to elute later during reversed‐phase LC analyses due to their greater structural flexibility, lower water solubility and higher molecular mass (Salminen et al., 2011; Moilanen et al., 2013; Karonen et al., 2015; Engström et al., 2019). The following four structural features primarily increase the protein precipitation capacity and retention time of ellagitannins: the number of galloyl, HHDP and other functional groups attached to the central glucose core; the presence of two galloyls instead of one HHDP that is formed by C–C coupling of the two galloyls; the presence of a central glucopyranose unit instead of acyclic glucose; and the oligomerisation degree of the ellagitannin (Engström et al., 2019). Thus, separately integrating early‐ and late‐eluting ellagitannins in complex mixtures of unidentified ellagitannins could provide useful information about the proportional biological activity of these compounds. In addition, their elution profiles could give a hint of their structures that could be then verified by UV and MS spectra (Moilanen et al., 2013).
Quantifying PAs within different size classes (i.e. the Engström method; Engström et al., 2014; Salminen, 2018b) could also improve our understanding of how different mixtures of PAs affect biological activity. The statistical models for all three biological activities identified correlations between biological activity and specific size classes of PA subunits, rather than total concentrations. This suggests that we need a better understanding of the biological activities of individual PAs, and again illustrates why understanding the relationship between biological activity and tannins is so difficult in plant samples; variation in tannin composition, even within subgroups of tannins, influences biological activity.
Some of the eucalypt samples in our study possessed very high oxidative activity (up to 94 mg g−1 DM gallic acid equivalents). This is at the higher end of values that have been reported in other plant species (Vihakas et al., 2014, 2015). In theory, the oxidative capacity of polyphenols could affect plant resistance to insect herbivory (Appel, 1993), but this has yet to be demonstrated conclusively. Oxidative polyphenols may also be more effective against some insect species than against others. For example, in two species of lepidopteran larvae, Agriopis aurantiaria was much more efficient than Epirrita autumnata in metabolising pentagalloylglucose, a model hydrolysable tannin (Salminen, 2018a). Interestingly, even though A. aurantiaria appears to have a higher gut pH than E. autumnata (Kim et al., 2018), pentagalloylglucose was not more harmful to A. aurantiaria. While we learn more of plant chemistry, we should also examine the effects on herbivores, because plant chemistry alone cannot reveal the fate of the compounds in herbivores.
Eucalypts could be an ideal system in which to test hypotheses that relate oxidative activity to insect herbivory because there is wide variation in oxidative activity between eucalypt species (this study) and between individuals within species (Marsh et al., 2017b), a variety of insect herbivores feed on eucalypt foliage (Fox & Morrow, 1981; Paine et al., 2011), and herbivory by insects differs between eucalypt species and individuals (Fox & Macauley, 1977; Paine et al., 2011; Marsh et al., 2017b). If oxidative activity does deter herbivory by some insects, our results suggest that the most likely eucalypts to benefit would be those containing high concentrations of ellagitannins, as well as prodelphinidin subunits in large PAs. Caffeic acid derivatives, such as caffeoyl quinic acids, are also efficiently oxidised at high pH, and by plant oxidative enzymes (Kim et al., 2018). These compounds could be important in eucalypts as well, because quinic acid derivatives partially determined the oxidative activity of eucalypt extracts.
The fact that ellagitannin concentrations had strong effects on both protein precipitation capacity and oxidative activity probably explains why there was a positive correlation between the two biological activities. On the surface, this suggests that plants containing high concentrations of ellagitannins might be somewhat protected against both mammalian (through protein binding) and insect (through oxidation) herbivores. However, before making these sorts of assumptions, we need a better understanding of the relationship between specific in vitro activity and the in vivo effects of tannins on herbivores. This is particularly pertinent given that ellagitannin concentrations did not affect the capacity to reduce in vitro N digestibility, even though they affected protein precipitation capacity.
This is the first study to investigate the particular structural features of tannins that correlate with changes in in vitro N digestibility, which can affect habitat quality and the reproductive success of marsupial folivores (DeGabriel et al., 2009; Youngentob et al., 2011). The results suggest that PAs might be particularly important in influencing in vitro N digestibility. Despite our expectations, different tannin subgroups affected the capacity to reduce in vitro N digestibility relative to in vitro protein precipitation capacity. The capacity to reduce in vitro N digestibility was strongly positively correlated with the proportion of prodelphinidin subunits in PAs, and the mDP of PAs. Both of these factors have previously been shown to influence protein precipitation capacity generally (e.g. Jones et al., 1976; Porter & Woodruffe, 1984; Kumar & Horigome, 1986; Osborne & McNeill, 2001; Lokvam & Kursar, 2005; Huang et al., 2011; Saminathan et al., 2014), which made it surprising that there was a negative relationship between the protein precipitation capacity of eucalypt samples and the mDP of PAs. Nevertheless, this could be due to a negative correlation between the mDP of PAs and the concentration of late‐eluting HHDP derivatives (data not shown). It would be useful to know what happens to the hydrolysable tannins in samples during the digestible N assay, such as whether they dissociate from protein or hydrolyse in response to the assay conditions, because they clearly precipitate protein in the protein precipitation capacity assay.
The results of our study demonstrate that the biological activity of tannin mixtures in plants is a complex trait relying on several classes of compounds and, probably, many individual structures, as well as the specific conditions and proteins encountered after ingestion by a herbivore. Despite recent breakthroughs in identifying genes underlying some aspects of tannin structure (e.g. Liu et al., 2016), there are unlikely to be genes of large effect that explain this biological activity (Kulheim et al., 2011). Skovmand et al. (2018) argue that the genes responsible for tannin synthesis could act as keystone genes influencing many ecosystem processes, but the complexity of tannin composition in foundation trees, such as eucalypts, suggests that genes of large effect are not likely.
Conclusions
Our study shows that the tannin composition of plant extracts affects their biological activity. In particular, it is possible to elucidate the broad structural features that contribute to biological activity, even when each individual compound in a complex mixture has not been identified. This is important, because it confirms that the new analytical techniques utilised here could be a valuable tool allowing researchers to understand how the composition of a widespread group of plant secondary metabolites such as the tannins influences the ecology of both individuals and populations of herbivores. It also suggests that future studies that characterise the individual tannins in specific subgroups could provide more detailed insight into the major patterns revealed in the current work.
Author contributions
WJF, IRW and J‐PS conceived the idea. DN and IRW collected the samples, and KJM and J‐PS conducted the chemical assays. KJM, CK and RC analysed the data. KJM led the writing of the manuscript, with contributions from all other authors.
Acknowledgements
We thank the volunteers who assisted with the collection of leaf samples, and Ms Anne Koivuniemi, Ms Anni Savolainen, Ms Tiina Buss and Ms Hannah Wigley for assisting with laboratory analyses. This work was supported by grants from the Australian Research Council to KJM (DE120101263) and to WJF and IRW (DP0986142), and from the Academy of Finland to JPS (258992).
References
- Aerts R, Barry T, McNabb W. 1999. Polyphenols and agriculture: beneficial effects of proanthocyanidins in forages. Agriculture, Ecosystems and Environment 75: 1–12. [Google Scholar]
- Appel H. 1993. Phenolics in ecological interactions: the importance of oxidation. Journal of Chemical Ecology 19: 1521–1552. [DOI] [PubMed] [Google Scholar]
- Ayres M, Clausen T, MacLean S, Redman A, Reichardt P. 1997. Diversity of structure and antiherbivore activity in condensed tannins. Ecology 78: 1696–1712. [Google Scholar]
- Barbehenn R, Cheek S, Gasperut A, Lister E, Maben R. 2005. Phenolic compounds in red oak and sugar maple leaves have prooxidant activities in the midgut fluids of Malacosoma disstria and Orgyia leucostigma caterpillars. Journal of Chemical Ecology 31: 969–988. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Constabel PC. 2011. Tannins in plant–herbivore interactions. Phytochemistry 72: 1551–1565. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Jones CP, Hagerman AE, Karonen M, Salminen JP. 2006. Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: potential impact on caterpillars. Journal of Chemical Ecology 32: 2253–2267. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Niewiadomski J, Pecci C, Salminen J‐P. 2013. Physiological benefits of feeding in the spring by Lymantria dispar caterpillars on red oak and sugar maple leaves: nutrition versus oxidative stress. Chemoecology 23: 59–70. [Google Scholar]
- Barbehenn R, Weir Q, Salminen JP. 2008. Oxidation of ingested phenolics in the tree‐feeding caterpillar Orgyia leucostigma depends on foliar chemical composition. Journal of Chemical Ecology 34: 748–756. [DOI] [PubMed] [Google Scholar]
- Bayly MJ, Rigault P, Spokevicius A, Ladiges PY, Ades PK, Anderson C, Bossinger G, Merchant A, Udovicic F, Woodrow IE et al 2013. Chloroplast genome analysis of Australian eucalypts ‐ Eucalyptus, Corymbia, Angophora, Allosyncarpia and Stockwellia (Myrtaceae). Molecular Phylogenetics and Evolution 69: 704–716. [DOI] [PubMed] [Google Scholar]
- DeGabriel JL, Moore BD, Foley WJ, Johnson C. 2009. The effects of plant defensive chemistry on nutrient availability predict reproductive success in a mammal. Ecology 90: 711–719. [DOI] [PubMed] [Google Scholar]
- DeGabriel JL, Wallis IR, Moore BD, Foley WJ. 2008. A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia 156: 107–116. [DOI] [PubMed] [Google Scholar]
- Engström MT, Arvola J, Nenonen S, Virtanen VTJ, Leppä MM, Tähtinen P, Salminen JP. 2019. Structural features of hydrolyzable tannins determine their ability to form insoluble complexes with bovine serum albumin. Journal of Agricultural and Food Chemistry 67: 6798–6808. [DOI] [PubMed] [Google Scholar]
- Engström MT, Palijarvi M, Fryganas C, Grabber JH, Mueller‐Harvey I, Salminen JP. 2014. Rapid qualitative and quantitative analyses of proanthocyanidin oligomers and polymers by UPLC‐MS/MS. Journal of Agricultural and Food Chemistry 62: 3390–3399. [DOI] [PubMed] [Google Scholar]
- Engström MT, Palijarvi M, Salminen JP. 2015. Rapid fingerprint analysis of plant extracts for ellagitannins, gallic acid, and quinic acid derivatives and quercetin‐, kaempferol‐ and myricetin‐based flavonol glycosides by UPLC‐QqQ‐MS/MS. Journal of Agricultural and Food Chemistry 63: 4068–4079. [DOI] [PubMed] [Google Scholar]
- Felton AM, Wam HK, Stolter C, Mathisen KM, Wallgren M. 2018. The complexity of interacting nutritional drivers behind food selection, a review of northern cervids. Ecosphere 9: e02230. [Google Scholar]
- Fox L, Macauley B. 1977. Insect grazing on Eucalyptus in response to variation in leaf tannins and nitrogen. Oecologia 29: 145–162. [DOI] [PubMed] [Google Scholar]
- Fox LR, Morrow PA. 1981. Specialization ‐ species property or local phenomenon. Science 211: 887–893. [DOI] [PubMed] [Google Scholar]
- Hagerman AE. 1987. Radial diffusion method for determining tannin in plant extracts. Journal of Chemical Ecology 13: 437–449. [DOI] [PubMed] [Google Scholar]
- Haslam E. 1988. Plant polyphenols (syn. vegetable tannins) and chemical defense ‐ a reappraisal. Journal of Chemical Ecology 14: 1789–1805. [DOI] [PubMed] [Google Scholar]
- Huang XD, Liang JB, Tan HY, Yahya R, Long R, Ho YW. 2011. Protein‐binding affinity of Leucaena condensed tannins of differing molecular weights. Journal of Agricultural and Food Chemistry 59: 10677–10682. [DOI] [PubMed] [Google Scholar]
- Johnson MTJ, Ives AR, Ahern J, Salminen J‐P. 2014. Macroevolution of plant defenses against herbivores in the evening primroses. New Phytologist 203: 267–279. [DOI] [PubMed] [Google Scholar]
- Jones W, Broadhurst R, Lyttleton J. 1976. The condensed tannins of pasture legume species. Phytochemistry 15: 1407–1409. [Google Scholar]
- Jones R, Palmer B. 2000. In vitro digestion studies using 14C‐labelled polyethylene glycol (PEG) 4000: comparison of six tanniferous shrub legumes and the grass Panicum maximum . Animal Feed Science and Technology 85: 215–221. [Google Scholar]
- Karonen M, Oraviita M, Mueller‐Harvey I, Salminen JP, Green RJ. 2015. Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): analysis by isothermal titration calorimetry (ITC). Journal of Agricultural and Food Chemistry 63: 10647–10654. [DOI] [PubMed] [Google Scholar]
- Kawamoto H, Nakatsubo F, Murakami K. 1996. Stoichiometric studies of tannin‐protein co‐precipitation. Phytochemistry 41: 1427–1431. [DOI] [PubMed] [Google Scholar]
- Kilkowski WJ, Gross GG. 1999. Color reaction of hydrolyzable tannins with Bradford reagent, Coomassie brilliant blue. Phytochemistry 51: 363–366. [Google Scholar]
- Kim J, Palijarvi M, Karonen M, Salminen JP. 2018. Oxidatively active plant phenolics detected by UHPLC‐DAD‐MS after enzymatic and alkaline oxidation. Journal of Chemical Ecology 44: 483–496. [DOI] [PubMed] [Google Scholar]
- Kulheim C, Yeoh SH, Wallis IR, Laffan S, Moran GF, Foley WJ. 2011. The molecular basis of quantitative variation in foliar secondary metabolites in Eucalyptus globulus . New Phytologist 191: 1041–1053. [DOI] [PubMed] [Google Scholar]
- Kumar R, Horigome T. 1986. Fractionation, characterization, and protein‐precipitating capacity of the condensed tannins from Robinia pseudo acacia L. leaves. Journal of Agricultural and Food Chemistry 34: 487–489. [Google Scholar]
- Liu CG, Wang XQ, Shulaev V, Dixon RA. 2016. A role for leucoanthocyanidin reductase in the extension of proanthocyanidins. Nature Plants 2: 16182. [DOI] [PubMed] [Google Scholar]
- Lokvam J, Kursar TA. 2005. Divergence in structure and activity of phenolic defenses in young leaves of two co‐occurring Inga species. Journal of Chemical Ecology 31: 2563–2580. [DOI] [PubMed] [Google Scholar]
- Makkar HPS. 2003. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin‐rich feeds. Small Ruminant Research 49: 241–256. [Google Scholar]
- Marsh KJ, Kulheim C, Blomberg SP, Thornhill AH, Miller JT, Wallis IR, Nicolle D, Salminen J‐P, Foley WJ. 2017a. Genus‐wide variation in foliar polyphenolics in eucalypts. Phytochemistry 144: 197–207. [DOI] [PubMed] [Google Scholar]
- Marsh KJ, Wallis IR, Foley WJ. 2003. The effect of inactivating tannins on the intake of Eucalyptus foliage by a specialist Eucalyptus folivore (Pseudocheirus peregrinus) and a generalist herbivore (Trichosurus vulpecula). Australian Journal of Zoology 51: 31–42. [Google Scholar]
- Marsh KJ, Zhou W, Wigley HJ, Foley WJ. 2017b. Oxidizable phenolic concentrations do not affect development and survival of Paropsis atomaria larvae eating Eucalyptus foliage. Journal of Chemical Ecology 43: 411–421. [DOI] [PubMed] [Google Scholar]
- Martin M, Rockholm D, Martin J. 1985. Effects of surfactants, pH, and certain cations on precipitation of proteins by tannins. Journal of Chemical Ecology 11: 485–494. [DOI] [PubMed] [Google Scholar]
- Masette M, Isabirye‐Basuta G, Baranga D, Chapman CA, Rothman JM. 2015. The challenge of interpreting primate diets: mangabey foraging on Blighia unijugata fruit in relation to changing nutrient content. African Journal of Ecology 53: 259–267. [Google Scholar]
- McArt S, Spalinger D, Collins W, Schoen E, Stevenson T, Bucho M. 2009. Summer dietary nitrogen availability as a potential bottom‐up constraint on moose in south‐central Alaska. Ecology 90: 1400–1411. [DOI] [PubMed] [Google Scholar]
- McManus J, Davis K, Beart J, Gaffney S, Lilley T, Haslam E. 1985. Polyphenol Interactions. Part 1. Introduction; some observations on the reversible compexation of polyphenols with proteins and polysaccharides. Journal of the Chemical Society, Perkin Transactions 2: 1429–1438. [Google Scholar]
- Moilanen J, Karonen M, Tahtinen P, Jacquet R, Quideau S, Salminen JP. 2016. Biological activity of ellagitannins: effects as anti‐oxidants, pro‐oxidants and metal chelators. Phytochemistry 125: 65–72. [DOI] [PubMed] [Google Scholar]
- Moilanen J, Salminen JP. 2008. Ecologically neglected tannins and their biologically relevant activity: chemical structures of plant ellagitannins reveal their in vitro oxidative activity at high pH. Chemoecology 18: 73–83. [Google Scholar]
- Moilanen J, Sinkkonen J, Salminen J‐P. 2013. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 23: 165–179. [Google Scholar]
- Moore BD, Wallis IR, Marsh KJ, Foley WJ. 2004. The role of nutrition in the conservation of the marsupial folivores of eucalypt forests In: Lunney D, ed. Conservation of Australia's forest fauna. Mossman, NSW, Australia: Royal Zoological Society of New South Wales, 549–575. [Google Scholar]
- Mueller‐Harvey I. 2006. Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture 86: 2010–2037. [Google Scholar]
- Osborne N, McNeill D. 2001. Characterization of Leucaena condensed tannins by size and protein precipitation capacity. Journal of the Science of Food and Agriculture 81: 1113–1119. [Google Scholar]
- Paine TD, Steinbauer MJ, Lawson SA. 2011. Native and exotic pests of Eucalyptus: a worldwide perspective. Annual Review of Entomology 56: 181–201. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20: 289–290. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . 2017. nlme: Linear and nonlinear mixed effects models. R package v.2.1‐131 . [WWW document] URL https://CRAN.R-project.org/package=nlme [accessed 1 August 2017].
- Porter L, Woodruffe J. 1984. Haemanalysis: the relative astringency of proanthocyanidin polymers. Phytochemistry 23: 1255–1256. [Google Scholar]
- Robbins C, Hanley T, Hagerman A, Hjeljord O, Baker D, Schwartz C, Mautz W. 1987. Role of tannins in defending plants against ruminants: reduction in protein avaiability. Ecology 68: 98–107. [DOI] [PubMed] [Google Scholar]
- Roslin T, Salminen JP. 2008. Specialization pays off: contrasting effects of two types of tannins on oak specialist and generalist moth species. Oikos 117: 1560–1568. [Google Scholar]
- Salminen JP. 2018a. Metabolism of 14C‐labelled pentagalloylglucose by Epirrita autumnata and Agriopis aurantiaria (Lepidoptera: Geometridae) and implications for the nutrition of geometrid defoliators. Austral Entomology 57: 255–264. [Google Scholar]
- Salminen JP. 2018b. Two‐dimensional tannin fingerprints by liquid chromatography tandem mass spectrometry offer a new dimension to plant tannin analyses and help to visualize the tannin diversity in plants. Journal of Agricultural and Food Chemistry 66: 9162–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen JP, Karonen M. 2011. Chemical ecology of tannins and other phenolics: we need a change in approach. Functional Ecology 25: 325–338. [Google Scholar]
- Salminen JP, Karonen M, Sinkkonen J. 2011. Chemical ecology of tannins: recent developments in tannin chemistry reveal new structures and structure‐activity patterns. Chemistry–A European Journal 17: 2806–2816. [DOI] [PubMed] [Google Scholar]
- Saminathan M, Tan H, Sieo C, Abdullah N, Wong C, Abdulmalek E, Ho Y. 2014. Polymerization degrees, molecular weights and protein‐binding affinities of condensed tannin fractions from a Leucaena leucocephala hybrid. Molecules 19: 7990–8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P, Mbugua D, Pell A. 2001. Analysis of condensed tannins: a review. Animal Feed Science and Technology 91: 21–40. [Google Scholar]
- Silanikove N, Shinder D, Gilboa N, Eyal M, Nitsan Z. 1996. Binding of poly(ethylene glycol) to samples of forage plants as an assay of tannins and their negative effects on ruminal degradation. Journal of Agricultural and Food Chemistry 44: 3230–3234. [Google Scholar]
- Skovmand LH, Xu CCY, Servedio MR, Nosil P, Barrett RDH, Hendry AP. 2018. Keystone genes. Trends in Ecology and Evolution 33: 689–700. [DOI] [PubMed] [Google Scholar]
- Steinbauer MJ, Farnier K, Taylor GS, Salminen JP. 2016. Effects of eucalypt nutritional quality on the Bog gum‐Victorian metapopulation of Ctenarytaina bipartita and implications for host and range expansion. Ecological Entomology 41: 211–225. [Google Scholar]
- Vihakas M, Gomez I, Karonen M, Tahtinen P, Saaksjarvi I, Salminen JP. 2015. Phenolic compounds and their fates in tropical lepidopteran larvae: modifications In alkaline conditions. Journal of Chemical Ecology 41: 822–836. [DOI] [PubMed] [Google Scholar]
- Vihakas M, Pälijärvi M, Karonen M, Roininen H, Salminen J‐P. 2014. Rapid estimation of the oxidative activities of individual phenolics in crude plant extracts. Phytochemistry 103: 76–84. [DOI] [PubMed] [Google Scholar]
- Volf M, Hrcek J, Julkunen‐Tiitto R, Novotny V. 2015. To each its own: differential response of specialist and generalist herbivores to plant defence in willows. Journal of Animal Ecology 84: 1123–1132. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cao L, Zhang Z. 2014. Seed traits and taxonomic relationships determine the occurrence of mutualisms versus seed predation in a tropical forest rodent and seed dispersal system. Integrative Zoology 9: 309–319. [DOI] [PubMed] [Google Scholar]
- Windley HR, Barron MC, Holland EP, Starrs D, Ruscoe WA, Foley WJ. 2016. Foliar nutritional quality explains patchy browsing damage caused by an invasive mammal. PLoS ONE 11: e0155216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob KN, Wallis IR, Lindenmayer DB, Wood JT, Pope ML, Foley WJ. 2011. Foliage chemistry influences tree choice and landscape use of a gliding marsupial folivore. Journal of Chemical Ecology 37: 71–84. [DOI] [PubMed] [Google Scholar]


