Abstract
Objective:
Present rationale, guidelines, and results of ranibizumab treatment for proliferative diabetic retinopathy (PDR) in Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol S.
Design:
Post hoc analyses from a randomized clinical trial.
Participants:
Three hundred and five participants with 394 study eyes having PDR without prior PRP.
Intervention:
Post hoc analyses from a randomized clinical trial of 0.5-mg intravitreous ranibizumab versus panretinal photocoagulation (PRP) for PDR. Eyes assigned to ranibizumab (N=191) received monthly injections for 6 months unless resolution after 4 injections. After 6 months, injections could be deferred if neovascularization was stable over 3 consecutive visits (sustained stability). If neovascularization worsened, monthly treatment resumed. PRP could be initiated for failure or futility criteria.
Main outcome measures:
Neovascularization status through 2 years.
Results:
At 1 month, 19% (35 of 188) of ranibizumab-assigned eyes had complete neovascularization resolution (including fibrous proliferans) and an additional 60% (113) were improved. At 6 months, 52% (80 of 153) had neovascularization resolution, 3% (4) were still improving, 37% (56) were stable, and 8% (13) had worsened since the last visit. Among eyes with versus without resolved neovascularization at 6 months, the median (interquartile range) number of injections between 6 months and 2 years was 4 (1–7; N = 73) versus 7 (4–11; N = 67) (P<.001). Injections were deferred in 68 of 73 eyes (93%) meeting sustained stability at least once during the study; 62% (42 of 68) resumed injections within 16 weeks after deferral. At 2 years, 43% (66 of 154) had neovascularization resolution, 5% (7) were improved, 23% (36) were stable, and 27% (42) had worsened since the last visit. Only 3 eyes met criteria for failure or futility through 2 years.
Conclusions:
The DRCR Network treatment algorithm for PDR can provide excellent clinical outcomes through two years for patients initiating anti-VEGF therapy for PDR. When choosing between anti-VEGF and PRP as first-line therapy for PDR, treatment decisions should be guided by consideration of the relative advantages of each therapeutic modality and anticipated patient compliance with follow-up and treatment recommendations.
Précis:
Treatment of proliferative diabetic retinopathy with anti-vascular endothelial growth factor following the Diabetic Retinopathy Clinical Research Network treatment algorithm can provide excellent clinical outcomes through two years.
Introduction
Treatment options for proliferative diabetic retinopathy (PDR) include panretinal photocoagulation (PRP), anti-vascular endothelial growth factor (anti-VEGF) therapy, and vitrectomy.1–4 Vitrectomy is typically reserved for cases of non-clearing vitreous hemorrhage or traction retinal detachment threatening or involving the macula. Although PRP has been standard care for over 40 years,5 recent clinical trial results suggest anti-VEGF therapy is a reasonable alternative to PRP for treatment of PDR through at least 2 years, contingent on patient compliance and access to treatment.4, 6
The DRCR.net Protocol S trial demonstrated that ranibizumab therapy for PDR is non-inferior to PRP at 2 years for change in visual acuity from baseline (5-letter non-inferiority margin).4 Ranibizumab also provided several benefits over PRP through 2 years. The ranibizumab group had superior gain in visual acuity over the course of 2 years, decreased peripheral visual field loss, and fewer vitrectomies. In addition, ranibizumab-treated eyes were less likely to develop central-involved diabetic macular edema (CI-DME) causing vision impairment of 20/32 or worse. Similar 1-year outcomes were reported using aflibercept in the CLARITY randomized clinical trial.6 Based on these findings, the DRCR.net recommends considering anti-VEGF therapy for eyes with PDR, especially for eyes with co-existing CI-DME for which anti-VEGF is already indicated.7
The decision to use anti-VEGF versus PRP will vary based on the individual circumstances of each patient including anticipated compliance and access to treatment. For physicians considering the use of anti-VEGF to treat PDR, this paper clarifies the rationale and guidelines used in Protocol S. We include data from Protocol S outlining the advantages and disadvantages of anti-VEGF versus PRP in specific clinical situations.
Methods
DRCR.net Anti-VEGF Treatment Algorithm for PDR
The DRCR.net anti-VEGF treatment algorithm for PDR was based on the clinical experiences of the Network investigator group and retinopathy outcomes achieved with an analogous “defer and extend” DRCR.net anti-VEGF regimen for CI-DME.8 Protocol S evaluated whether PDR could be controlled with an initial series of consecutive 0.5-mg monthly ranibizumab injections with subsequent therapy determined based on retinal neovascularization (NV) status at each visit. The study adhered to the tenets of the Declaration of Helsinki9 and was approved by multiple institutional review boards. Study participants provided written informed consent.
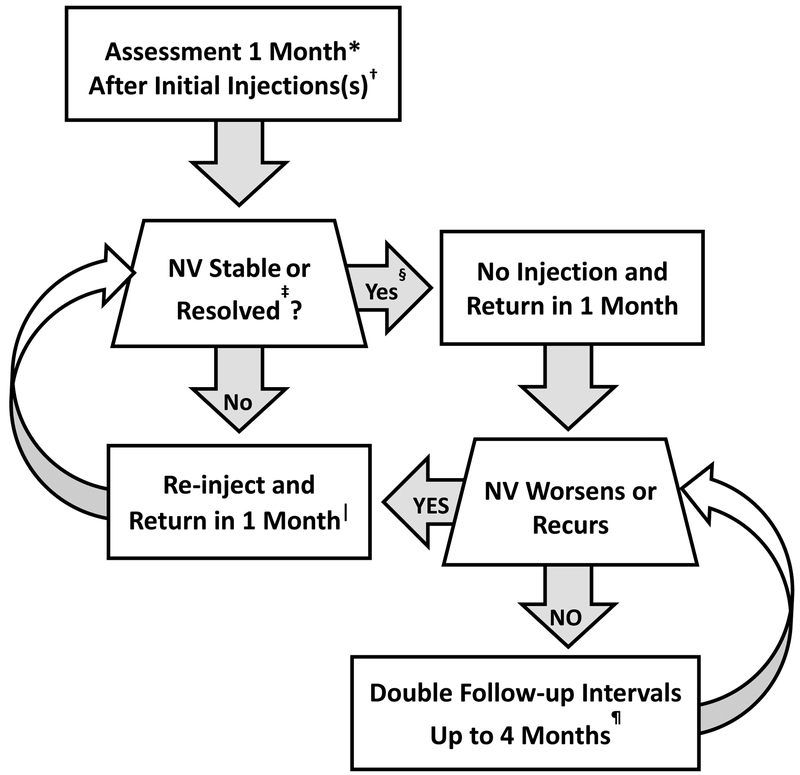
The treatment algorithm can be summarized in 3 steps. First, start with 6 monthly injections with one exception: if all NV is resolved after 4 or 5 injections, injections can be deferred. Second, after 6 months, continue injections if the NV is still worsening or improving, but defer injections if the NV is stable at the current visit and the last 2 visits (“sustained stability”). Third, resume monthly anti-VEGF if NV worsens after withholding injections. If sustained stability is again achieved, injections may once again be deferred, but this requires at least 3 consecutive injections (one given for the initial status of worsened NV and 2 more while the NV is stable). Panretinal photocoagulation is given only if NV worsens substantially despite anti-VEGF. Onset or worsening of preretinal or vitreous hemorrhage is not necessarily graded as NV worsening unless the hemorrhage precluded evaluation of NV. Further explanations are provided in Figure 1 and Table 1, which summarize the key terms used in the DRCR.net anti-VEGF treatment algorithm for PDR.
Figure 1.
Principles of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Anti-VEGF Treatment Algorithm for proliferative diabetic retinopathy.
* 4-week, not 1-month, intervals were used.
† 4 injections were required every 4 weeks initially; it is not known whether a different number of injections initially would have worked as well. DRCR.net also required 2 additional injections at months 5 and 6 unless there was complete absence of neovascularization (“resolved”).
‡ Relevant details: 1) deferral of injections due to stability occurred only when “sustained stability” criteria were met, defined as neovascularization clinically unchanged at the current visit and the and last 2 injection visits, 2) “resolved” was defined as absence of neovascularization of the iris, neovascularization of the disc, neovascularization elsewhere, and neovascularization of the angle (if the angle was assessed).
§ Injection was at investigator discretion if neovascularization status was sustained stability or resolved and was performed 15% and 23% of the time in these cases, respectively.
∥ Panretinal photocoagulation was permitted if failure criteria were met, namely, if neovascularization worsened substantially despite at least 4 monthly injections or iris neovascularization involving the angle developed.
¶ Follow-up continued every 4 weeks through the 52-week visit and did not permit extension of follow-up until after the 52-week visit. If injection was withheld due to no resolution or sustained stability at 3 consecutive visits following the week 52 visit, follow-up interval was doubled to 8 weeks and then again to 16 weeks if still no change.
Table 1.
Neovascularization (NV) assessment definitions
| Term | Definition |
|---|---|
| Resolution (or Success) | Apparent absence of NV (including fibrous proliferans) of the retina, disc, iris, and angle (if the angle was assessed) |
| Stable | Clinically unchanged NV (no improvement or worsening) from the prior visit |
| Improved | Decrease in the size of NV or diminished density of NV in any area (retina, disc, iris, or angle) compared with the prior visit |
| Sustained Stability | Stable NV at the current visit and the last 2 injection visits |
| Worsening | Recurrent or worsening NV since the last visit or presence of vitreous or preretinal hemorrhage that prevented evaluation of NV status |
| Failure | Growth of NV after at least 4 consecutive injections such that the NV is greater in extent than when treatment was initiated or definite NV worsening, following an injection, that the investigator believed was likely to lead to vision loss in the absence of PRP |
| Futility | NV persisted or recurred such that it was equal to or greater than when treatment was initiated. |
Determining the Status of NV.
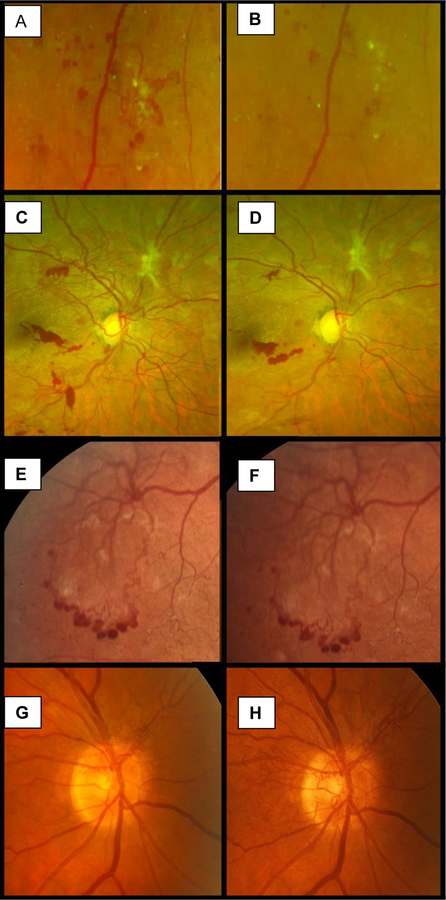
At each protocol visit, DRCR.net investigators determined the status of retinal NV by categorizing NV of the iris (NVI), NV of the disc (NVD), and NV elsewhere (NVE) as resolved (absent), present but improved, stable, or worsened compared with the last visit (Figure 2). Assessment of the angle was required only if NVI was present or if the eye met criteria for increased IOP.
Figure 2.
Paired baseline (A, C, E, G) and follow-up (B, D, F, H) fundus photographs of retinal neovascularization (NV) showing 4 types of NV change over time. Resolved: NV that fully resolved without any residual vessels or fibrosis 1 week after anti-VEGF treatment (A, B). Improved: NV decreased in extent and severity 1 week after anti-VEGF therapy but with some residual NV and fibrosis (C, D). Stable: NV that remained stable in extent and severity over a period of 1 year without treatment (E, F). Worsened: NV of the disc that worsened in extent and severity over 32 Weeks (G, H).
The methods for evaluation of NV status at each visit were left to investigator discretion based on usual clinical practice. In addition to slit-lamp and dilated fundus examinations at each visit, investigators used the following methods, individually or in combination, to assess NV status in Protocol S: extended ophthalmoscopy in 2,595 of 3,410 exams (76%), undilated examination of the iris in 904 (27%), fundus photography in 471 (14%), fluorescein angiography in 80 (2%), or ultrasound in 3 (<1%).
Extension of Follow-up Visits.
To decrease the burden of visits while minimizing risk of vision loss, follow-up intervals can be extended gradually: first to every 8 weeks, if injections are deferred two months in a row, and then to every 16 weeks if there still is no recurrence or worsening of NV. If NV recurs or worsens at any point after deferral, monthly anti-VEGF should be resumed until resolution or sustained stability over 3 consecutive injections (Figure 1). In Protocol S, extension of follow-up was not allowed prior to 1 year in order to characterize the monthly response of NV, even when injections were being withheld.
Treatment of DME in Eyes with PDR.
Treatment for DME in Protocol S was at investigator discretion; however, guidelines for DME treatment were provided and were consistent with the approach used in DRCR.net Protocol T.10 Therefore, for eyes with DME and vision loss in addition to PDR, the Protocol T DME treatment algorithm would be appropriate to follow. In such circumstances, an injection can be given if indicated for either PDR or DME, and the follow-up visit interval is the shorter of the two intervals indicated.
Results
Number of Injections
As previously reported, the median (interquartile range) number of ranibizumab injections administered for PDR or DME in the ranibizumab group was 5 (4–6) over the first 6 months, 2 (1–4) in the second 6 months, and 3 (1–6) in the second year, totaling 10 (7–14) over 2 years.4 Compliance with treatment guidelines for PDR was 97%. For eyes in the ranibizumab group that did not have vision-impairing DME at baseline, the median number of injections was 5 (4–6) over the first 6 months, 2 (1–4) in the second 6 months, and 3 (1–5) in the second year, totaling 10 (6–13) over 2 years. For eyes in the ranibizumab group with baseline vision-impairing DME, the median number of injections for PDR or DME was 5 (4–6) over the first 6 months, 4 (2–6) in the second 6 months, and 5 (2–7) in the second year, totaling 14 (10–17) over 2 years.
Resolution of NV (Treatment Success)
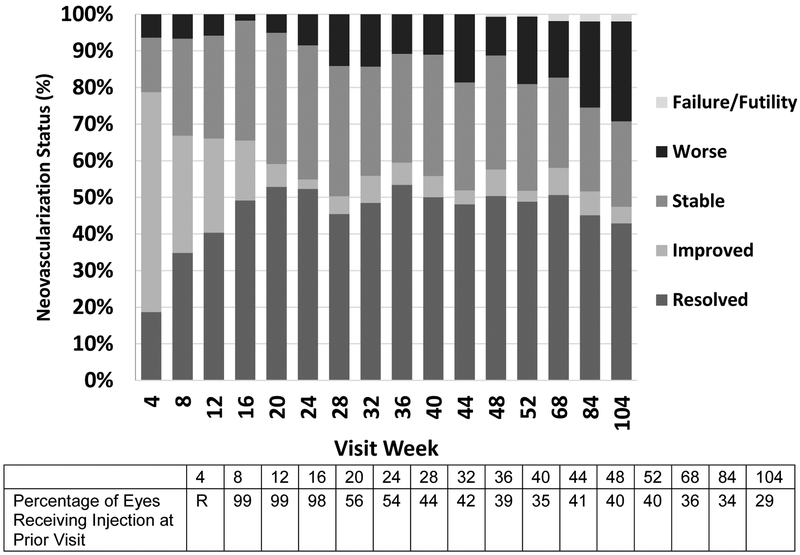
At 1 month (the first follow-up visit), 35 of 188 eyes evaluated (19%) had apparent complete resolution of NV (including the absence of fibrous proliferans) according to the treating ophthalmologist. An increasing proportion of eyes had resolution from the 2-month visit through the 6-month visit (Figure 3). At 4 months, the first time point at which injections could be deferred for success, NV had resolved in 84 of 171 eyes (49%). Among the 84 eyes with NVD based on investigator judgment at baseline, 38 (45%) had no NV at the 4-month visit, compared with 45 of 85 (53%) with only NVE at baseline. Across all follow-up visits through 2 years, NV was completely resolved at 1513 of 3410 visits (44%) when NV was assessed (Figure 3). At these visits, injections could be performed at investigator discretion and were administered 351 of 1513 times (23%). Among eyes with resolved neovascularization at 6 months, the median (interquartile range) number of subsequent injections between 6 months and 2 years was 4 (1–7; N = 73), which was fewer than eyes that were not resolved at 6 months, which received 7 (4–11; N = 67) (P<.001). Among 149 eyes with apparent resolution of NV at any time, 139 (93%) received an additional anti-VEGF injection for either PDR or PDR with concurrent DME.
Figure 3.
Status of retinal neovascularization at each visit as determined by investigators (R = randomization visit at which injection was required).
Improvement of NV
The initial 4-month period of consecutive monthly ranibizumab dosing resulted in NV improvement or resolution for the majority of eyes. At the 4-, 8-, 12- and 16-week visits, 60% (113 of 188), 32% (58 of 181), 26% (44 of 171), and 16% (28 of 171) of eyes had improved NV status compared with the prior visit. In addition, 79% (148), 67% (121), 66% (113), and 65% (112) were either resolved or improved from the prior visit (Figure 3). Starting at 24 weeks through 2 years, during which time monthly injections were not mandated and when many eyes already were resolved, rates of improvement at each protocol visit were lower and ranged from 3% to 7%. Over the same time, the rate of NV resolution or improvement at each visit ranged from 47% to 59%.
Stability of NV
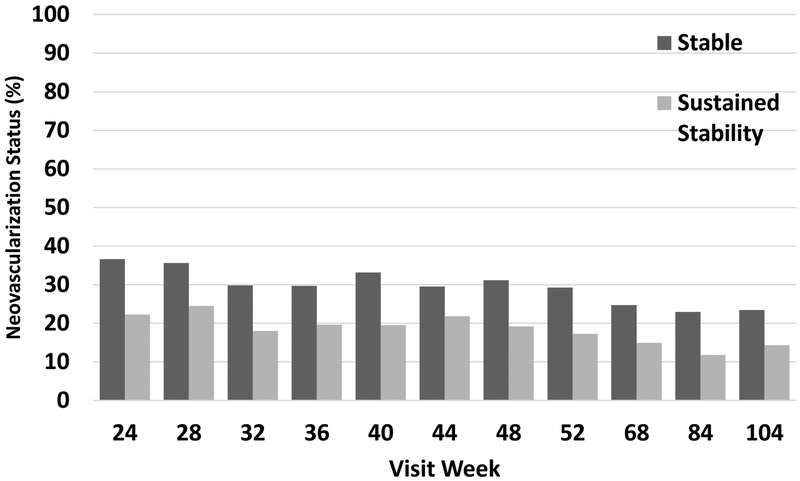
After the initial 6 months, deferral of injections was permitted at investigator discretion if the NV status achieved was “sustained stability” (i.e., became stable and remained stable after 2 additional, consecutive injections). Among 153 eyes completing the 6-month visit (the first time sustained stability criteria could be met), 56 (37%) met stability criteria compared with the last visit, including 34 (22%) achieving sustained stability compared with the last 3 consecutive injections. The distribution of stability and sustained stability at protocol visits through 2 years is presented in Figure 4. When sustained stability was achieved, injections were at investigator discretion. Investigators chose to administer treatment in 66 of 439 visits (15%) at which sustained stability was noted.
Figure 4.
Percentage of eyes with stable neovascularization or sustained stability of neovascularization (stability at 3 consecutive visits) at each visit.
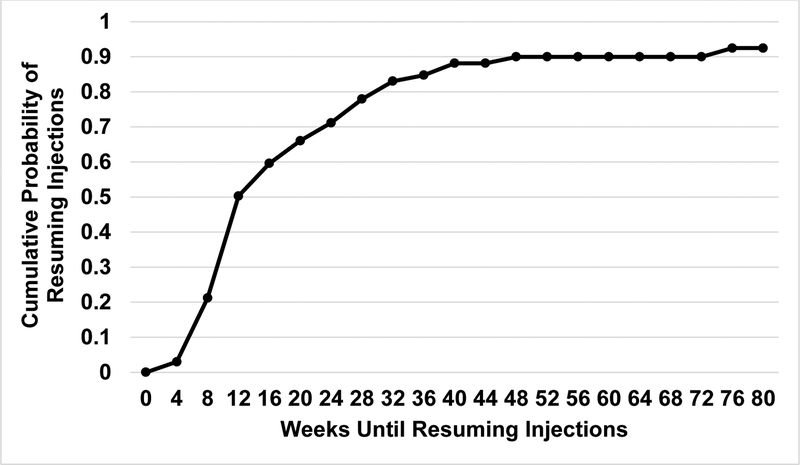
Injections were deferred in 68 of the 73 eyes (93%) meeting sustained stability at least once during the study. Among these 68 eyes, 11 (16%) did not receive another injection through 2 years and 66 (97%) deferred for at least 2 consecutive injections. Of the 68 eyes deferring at least one injection for sustained stability, 42 (62%) resumed injections by 16 weeks post-deferral (Figure 5).
Figure 5:
Cumulative probability of resuming injections after deferral due to sustained stability of neovascularization (stability at 3 consecutive visits).
Worsening NV
Rates of worsening were low (2–7%) during the first 4 months of the study when eyes were required to receive monthly ranibizumab (Figure 3). At 2 years, 42 of 154 eyes (27%) had worsened from the prior visit. However, among these 42 eyes, only 5 of 38 eyes (13%) with gradable photographs at 2 years had worsened by 2 or more levels of diabetic retinopathy severity from baseline (99% of 191 eyes had gradable photographs at baseline and 89% of 160 eyes had gradable photographs at 2 years). When eyes achieved sustained stability for which an injection was deferred, worsened NV was present at the next visit 24% of the time (83 of 353 visits).
Three of 191 eyes met failure criteria for substantial PDR worsening. Of these 3 cases, 1 eye developed worsening NV of the angle and 2 received PRP (1 for vitreous hemorrhage with vision loss and 1 for NV growth). Among eyes that received PRP at any point over 2 years, the majority (8 of 12) received PRP during vitrectomy; only 4 eyes received PRP in the clinic strictly for failure criteria or with Protocol Chair approval. Only a single eye met futility criteria for worsening PDR despite monthly anti-VEGF injections.
Discussion
While Protocol S utilized ranibizumab, results from other studies of anti-VEGF agents appear to show similar outcomes with excellent regression of NV. This includes a recent randomized trial of aflibercept for PDR treatment over 1 year in the absence of CI-DME, but not necessarily without prior PRP.6 In addition, similar findings were seen in phase 3 trials of aflibercept and ranibizumab for DME.11–14 Thus, recommendations from the DRCR.net for the clinical scenarios below are given for anti-VEGF therapy in general, with the caveat that results obtained with other agents or treatment regimens may not be identical to those obtained in Protocol S. Based on the findings from Protocol S, specific scenarios are discussed below.
PDR in the absence of central-involved DME
Eyes with PDR and no CI-DME received monotherapy with either ranibizumab, according to the PDR treatment algorithm detailed above, or PRP. Based on Protocol S results, both PRP and anti-VEGF appear to be viable therapeutic options in eyes with PDR and no DME. While anti-VEGF therapy is effective at regressing retinal NV and reducing the risk of developing CI-DME, it is not cost-effective in this cohort compared with PRP within the $50,000 to $150,000 per quality-adjusted life-year range frequently cited in the United States.15 Distinct advantages of each treatment modality may guide the clinical decision for individual eyes (see summary in Table 2).
Table 2.
Comparison of the relative advantages and disadvantages of anti-VEGF versus PRP for treatment of eyes with PDR
| Anti-VEGF | PRP | |
|---|---|---|
| Number of Procedures for PDR Treatment | Multiple injections needed for optimal results (e.g. median number of injections over 2 years was 14 in eyes with baseline DME and 10 in eyes without baseline DME). | Can often be performed in 1–2 sessions, but may require additional treatments (e.g. 45% of eyes treated with PRP needed additional PRP within 2 years of initial treatment) |
| Frequency of Follow-up Visits | Monthly or near monthly visits may be needed through the first year with subsequent follow-up periods extended if NV remains quiescent | First follow-up after PRP usually scheduled in 3–4 months with subsequent follow-up periods extended if NV remains quiescent. |
| Visual Acuity After 2 Years | Non-inferior visual acuity at 2 years to PRP (5-letter non-inferiority margin) | --- |
| Visual Acuity Over 2 Years | Average visual acuity over 2 years superior to PRP | Average visual acuity over 2 years inferior to anti-VEGF |
| Effect on Peripheral Visual Field | Non-destructive therapy with better preservation of peripheral visual field as compared with PRP | Destructive therapy that leads to defects in peripheral visual field |
| Effect on DME Status | Improves DME status and reduces rate of DME onset compared with PRP | Three-fold higher rate of central-involved DME onset compared with anti-VEGF over 2 years. In addition, some eyes develop DME after PRP which can lead to decreased vision |
| Need for Additional Procedures for PDR or DME Treatment | Few eyes receiving anti-VEGF by the DRCR.net algorithm will need PRP for persistent or worsening NV. Need for vitrectomy over 2 years is lower in anti-VEGF treated eyes. Few eyes will need additional DME treatment. | Few eyes that receive PRP will need anti-VEGF for persistent or worsening NV. Need for vitrectomy over 2 years is higher in PRP-treated eyes. Eyes may need anti-VEGF for DME onset or worsening. |
| Risk of Adverse Events Associated with Invasive Procedures | Invasive procedure with associated risks of endophthalmitis, traumatic retinal tear, or cataract | Non-invasive with no risk of endophthalmitis, and minimal risk of retinal tear, retinal detachment, or cataract |
| Risk of vitreous hemorrhage | Possibly lower rate of vitreous hemorrhage through 2 years | Possibly higher rate of vitreous hemorrhage through 2 years |
| Risk of traction retinal detachment | Possibly lower rate of retinal detachment through 2 years. Increased retinal traction is possible, but low rates of traction retinal detachment for eyes that do not have macular-threatening traction at baseline | Possibly higher rate of retinal detachment through 2 years. Increased retinal traction is possible, but low rates of traction retinal detachment for eyes that do not have macular-threatening traction at baseline |
| Risk of Glaucoma | Low rates of substantial increases in intraocular pressure, need for ocular anti-hypertensives, or glaucoma surgery | Low rates of substantial increases in intraocular pressure, need for ocular anti-hypertensives, or glaucoma surgery. Ciliochoroidal effusion and angle-closure glaucoma are very rare, but possible associated adverse events |
| Risk of Systemic Adverse Events | Theoretical higher risk of thromboembolic events and higher rates of these events in the Protocol S ranibizumab group have been reported, although increased risk has not been conclusively demonstrated in studies of intravitreal anti-VEGF for ocular conditions | No increased risk of thromboembolic or other systemic events |
| Cost Effectiveness* | Ranibizumab is cost-effective versus PRP only if DME is also present | PRP is more cost-effective than ranibizumab in eyes with PDR without DME |
| Additional Considerations | Not a good option for patients with major systemic co-morbidities in whom PDR may recur or worsen substantially if they cannot comply with monthly follow-up | Long durability of effect in regressing NV that can last decades. Excellent option for treatment of PDR for patients who have difficulty with frequent follow-ups. |
At this time, based on current drug costs and available estimates of treatment effectiveness
PDR in the presence of central-involved DME
Anti-VEGF is considered first-line therapy in most eyes with CI-DME. Ranibizumab was highly effective at treating PDR in Protocol S; aflibercept was highly effective at treating PDR in CLARITY.4, 6 Thus, the use of PRP may be deferred in eyes with PDR that otherwise are receiving anti-VEGF treatment for DME since the same anti-VEGF agent will treat both the DME and the PDR. Once anti-VEGF therapy is no longer required due to resolution or stability of DME, these eyes can be re-evaluated based on NV status to see if treatment for PDR is still necessary and whether anti-VEGF will be continued or if PRP will be used. In such situations, the use of anti-VEGF agents through 2 years can be considered cost-effective within the $50,000 to $150,000 per quality-adjusted life-year range cited in the United States when treating the worse-seeing eye.15
High-risk PDR with or without vision-impairing DME
For eyes with high-risk PDR (i.e., ≥ ETDRS level 71) that did not require vitrectomy at baseline, rates of PDR-worsening events (e.g., vitreous hemorrhage, retinal detachment, NVA, NVG) were greater compared with eyes having less than high-risk PDR, regardless of treatment.16 However, the average improvement in visual acuity over 2 years was greater with ranibizumab than PRP, and the difference appeared to be greater among eyes with high-risk PDR.17 Thus, it seems appropriate to consider ranibizumab and possibly other anti-VEGF agents as monotherapy for eyes with PDR, even when the PDR is considered high-risk PDR and prompt vitrectomy is not being planned.
Worsening PDR in eyes receiving ranibizumab for PDR
Very few eyes randomized to ranibizumab met failure criteria for substantial PDR worsening (3 of 191 eyes) and only 12 eyes received PRP, 8 of which were during vitrectomy. These results suggest that eyes being treated with ranibizumab for PDR according to the Protocol S algorithm are unlikely to need PRP for treatment of PDR. Therefore, anti-VEGF treatment can function as monotherapy for PDR, at least for the first two years if patient compliance with follow-up is good. However, NV worsening was observed at approximately 13% of visits, with more worsening later in the 2-year follow-up period. Therefore, higher rates of PRP for worsening PDR might result with less frequent dosing or longer follow-up intervals than those used in Protocol S.
Vitrectomy for PDR
Vitrectomy was permitted in Protocol S for traction retinal detachment causing visual impairment or non-clearing vitreous hemorrhage. However, investigators were asked to defer surgery for at least 8 weeks after the onset of vitreous hemorrhage. Among ranibizumab-treated versus PRP-treated eyes, there were fewer vitreous hemorrhages (27% vs 34%, P = .09), retinal detachments (6% vs 10%, P = .08), and vitrectomy procedures (4% vs 15%, P<.001).4 Eight eyes in the ranibizumab group had vitrectomy for the following reasons: non-clearing vitreous hemorrhage (6), retinal detachment (1), and vitreous hemorrhage with retinal detachment (1).16 Note that eyes with traction detachment threatening the macula or for which intraocular surgery was anticipated were excluded from enrollment in Protocol S as well as the DRCR.net Protocols I, J, and N. Thus, investigators were asked not to enroll eyes judged to be at significant risk for traction retinal detachment following anti-VEGF therapy. However, the results from these studies do not support the hypothesis that anti-VEGF given to an eye with PDR, with or without high-risk characteristics (but without macular-threatening traction at baseline), causes traction detachments more frequently than PRP.18–20
Protocol S did not compare ranibizumab versus PRP in eyes for which surgery for PDR already was planned. The safety of anti-VEGF compared with no anti-VEGF for non-clearing vitreous hemorrhage for which vitrectomy is planned is being evaluated in Protocol AB (available at http://drcrnet.jaeb.org/Studies.aspx?RecID=505, accessed 4 June 2018).
Additional considerations
Why 6 monthly injections initially?
The DRCR.net recommends 6 initial injections in order to maximize the number of eyes that attain early resolution of NV. When using the Protocol S injection algorithm, over 50% of eyes had resolution of NV by 6 months. Furthermore, an increasing proportion of eyes had resolution of NV following the 4th, 5th, and 6th injections (Figure 3). It is unknown how results would differ if injections were stopped before 6 months. It is also unknown whether withholding injections upon first NV resolution would be an effective strategy with fewer injections.
Is it safe to withhold anti-VEGF therapy after 6 months if NV is stable but present?
Protocol S data suggest that it is safe to withhold anti-VEGF therapy after 6 months once NV is stable, provided at least 3 monthly injections are given if NV worsens after withholding anti-VEGF therapy and that patients comply with the follow-up schedule to monitor NV. When withholding anti-VEGF therapy following sustained stability, there is only a small chance of visual acuity loss, need for PRP, or need for vitrectomy. In addition, this chance likely is smaller than if PRP were used to treat the PDR. Among eyes in which injections were withheld at least once, only 4 (7%) lost 15 or more letters from baseline at 2 years.
Additional clinical scenarios.
In addition to the situations discussed above, Table 3 presents several frequently asked questions in the management of eyes with PDR. Each question is accompanied by a discussion of how this issue was managed in Protocol S or how the question might be managed in clinical practice based on the experience of DRCR Network investigators with similar cases in this trial.
Table 3.
Frequently asked questions in management of eyes with PDR, management in Protocol S and DRCR.net suggestions for management in a clinical setting.
What are treatment considerations for
determining first-line therapy for eyes with PDR and
|
|
| How to manage eyes with PDR in patients with major systemic comorbidities such as myocardial infarction or stroke? | Increased risk of thromboembolic events is present in patients treated with systemic anti-VEGF at doses that are approximately 400 times those given via intraocular injection. Large or even moderate increases in risk for these events have not been demonstrated in studies of eyes receiving intravitreal anti-VEGF for ocular conditions such as AMD and DME. Nonetheless, a small increase in risk cannot be ruled out due to the small numbers of thromboembolic events that occur in these studies. Consider additional oral and/or written consent processes for patients with these conditions, especially when they have experienced recent medical events, to review the potential increased risks vs. benefits. Patients who have a more remote history of cardiac events or stroke and who are otherwise systemically stable should understand the potential risks and benefits as well, although whether there is an increased risk for a subsequent cardiac event or stroke with versus without subsequent anti-VEGF treatment remains unknown. |
| How to manage eyes being treated with anti-VEGF for PDR that develop vitreous hemorrhage? | Eyes receiving anti-VEGF for PDR that develop vitreous hemorrhage precluding evaluation of the retina should continue receiving anti-VEGF on a monthly basis until the hemorrhage clears and the retinal neovascular status can be adequately assessed. While anti-VEGF treatment is ongoing, regular evaluation with fundus examination or ultrasonography should be performed to ensure that traction retinal detachment, especially threatening the macula, is not developing or worsening. |
| How should NV status in eyes with PDR be evaluated? | Fluorescein angiography (FA) provides the most sensitive method for detecting retinal NV and assessing neovascular status. However, FA, including ultra-widefield imaging, was not routinely implemented or required in Protocol S. Dilated fundus examination was performed at all follow-up visits for the evaluation of NV. Fundus photographs were acquired at annual visits for study data purposes. Additional imaging procedures at non-annual visits were left to investigator discretion. Wide-field angiography ultimately may provide the most sensitive information regarding NV status, but its relationship to the benefits of retreatment in the absence of any other evidence of NV worsening remains unknown. The excellent results obtained in Protocol S in the absence of such imaging should be recognized. The potential utility of ultra-widefield fundus or FA imaging is being investigated in the ongoing DRCR.net Protocol AA. |
| Does anti-VEGF lead to more traction retinal detachments in eyes with PDR? | DRCR.net studies, including Protocols I, J, N, and S have not demonstrated any increased risk of traction retinal detachments in eyes with PDR that are treated with anti-VEGF. However, it is important to note that all these studies excluded eyes with pre-existing traction retinal detachment involving the macula, and that investigators may have elected not to enroll other eyes judged to be at high risk for increasing retinal traction with or without anti-VEGF therapy. |
| Are there differences in outcomes between the 0.3- and 0.5-mg doses of ranibizumab for PDR? | Since Protocol S was implemented before the FDA approval of ranibizumab 0.3-mg, and since the potential benefits of ranibizumab to treat PDR when used in a PRN regimen after 6 months in Protocol I21 utilized the 0.5-mg dose, the same dosage was chosen for Protocol S. The study does not address whether there would be a difference in outcomes between the 0.3- and 0.5-mg doses when utilized for PDR treatment. Nonetheless, we expect that similar results will be obtained with these two doses owing to the near total blockade of VEGF with the 0.3-mg dose, the similar effects seen on diabetic retinopathy in RIDE/RISE22 with monthly 0.3-mg compared with 0.5-mg ranibizumab, and the rapid regression of retinal NV that has been documented in response to a much smaller molar dose equivalent of bevacizumab.23 |
| Are there differences in outcomes between different anti-VEGF agents for treatment of PDR? | Only 0.5-mg ranibizumab was evaluated in Protocol S and only 2.0-mg aflibercept was evaluated in CLARITY.6 Therefore, it is unknown exactly how these two anti-VEGF agents would compare in terms of efficacy and safety for the treatment of PDR, nor how 1.25-mg repackaged (compounded) bevacizumab would do. Data from the DRCR.net Protocol T demonstrates that differences in efficacy can exist between anti-VEGF agents when used as treatment for DME. However, differences, if they do exist, in outcomes between anti-VEGF agents in the treatment of PDR may not be substantial given the high potency of all currently available anti-VEGF agents and documented regression of retinal NV with a bevacizumab dose as low as 1.25 mg.23 |
Conclusions
The results of Protocol S demonstrate that ranibizumab therapy over 2 years is an effective alternative to PRP for PDR treatment, warranting a detailed understanding of the DRCR Network anti-VEGF treatment algorithm for PDR. When choosing between anti-VEGF and PRP as first-line therapy, treatment decisions for individual eyes with PDR should be guided by careful consideration of the relative advantages of each treatment and anticipated patient compliance with follow-up and treatment recommendations. Application of the DRCR Network anti-VEGF treatment algorithm for PDR can provide excellent clinical outcomes through 2 years if compliance is good. Data through 5 years of follow-up are forthcoming.
Acknowledgements
A complete list of the Diabetic Retinopathy Clinical Research Network investigators who participated in this trial is available in JAMA. 2015;314(20):2137–2146.
Financial support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817. Genentech provided ranibizumab for the study and funds to DRCR.net to defray the study’s clinical site costs.
The National Institutes of Health participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study nor in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. Per the DRCR.net Industry Collaboration Guidelines (available at http://www.drcr.net), the DRCR.net had complete control over the design of the protocol, ownership of the data, and all editorial content of presentations and publications related to the protocol.
Financial Disclosures: A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Footnotes
Previous Meeting Presentations: ASRS, 2018; ARVO, 2018; Macula Society, 2018
Conflict of Interest: Authors have potential conflicts of interest to report and will list them on their conflict of interest forms returned to the journal.
References
- 1.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102:527–32. [DOI] [PubMed] [Google Scholar]
- 2.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–63. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 1981;88(7):583–600. [PubMed] [Google Scholar]
- 4.Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. JAMA. 2015;314(20):2137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976;81(4):383–96. [DOI] [PubMed] [Google Scholar]
- 6.Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389(10085):2193–203. [DOI] [PubMed] [Google Scholar]
- 7.Gross JG, Glassman AR. A novel treatment for proliferative diabetic retinopathy: Anti-vascular endothelial growth factor therapy. JAMA Ophthalmol. 2016;134(1):13–4. [DOI] [PubMed] [Google Scholar]
- 8.Diabetic Retinopathy Clinical Research Network, Writing C, Aiello LP, et al. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology. 2011;118(12):e5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. [DOI] [PubMed] [Google Scholar]
- 10.Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bressler SB, Qin H, Melia M, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol. 2013;131(8):1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122(10):2044–52. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: Results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. [DOI] [PubMed] [Google Scholar]
- 14.Bressler SB, Liu D, Glassman AR, et al. Change in Diabetic Retinopathy Through 2 Years: Secondary Analysis of a Randomized Clinical Trial Comparing Aflibercept, Bevacizumab, and Ranibizumab. JAMA Ophthalmol. 2017;135(6):558–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross EL, Hutton DW, Stein JD, et al. Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: Analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol. 2016;134(8):888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bressler SB, Beaulieu WT, Glassman AR, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology. 2017;124(4):431–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bressler S, Beaulieu WT, Glassman A, et al. Photocoagulation versus ranibizumab for proliferative diabetic retinopathy: Should baseline characteristics affect choice of treatment? Submitted 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77 e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetic Retinopathy Clinical Research Network, Googe J, Brucker AJ, et al. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31(6):1009–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diabetic Retinopathy Clinical Research N. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol. 2013;131(3):283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115(9):1447–9, 9 e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–22. [DOI] [PubMed] [Google Scholar]
- 23.Avery RL. Regression of retinal and iris neovascularization after intravitreal bevacizumab (avastin) treatment. Retina. 2006;26(3):352–7. [DOI] [PubMed] [Google Scholar]