Abstract
Background-
Macrophages are ubiquitous in all stages of atherosclerosis, exerting tremendous impact on lesion progression and plaque stability. Because macrophages in atherosclerotic plaques express angiotensin-converting enzyme (ACE), current dogma posits that local myeloid-mediated effects worsen the disease. In contrast, we previously reported that myeloid ACE overexpression augments macrophage resistance to various immune challenges, including tumors, bacterial infection and Alzheimer’s plaque deposition. Here, we sought to assess the impact of myeloid ACE on atherosclerosis.
Methods-
A mouse model in which ACE is overexpressed in myelomonocytic lineage cells, called ACE10, was generated and sequentially crossed with ApoE-deficient mice to create ACE10/10ApoE−/− (ACE10/ApoE). Control mice were ACEWT/WTApoE−/− (WT/ApoE). Atherosclerosis was induced using an atherogenic diet alone, or in combination with unilateral nephrectomy plus deoxycorticosterone acetate (DOCA) salt for eight weeks.
Results-
With an atherogenic diet alone or in combination with DOCA, the ACE10/ApoE mice showed significantly less atherosclerotic plaques compared to their WT/ApoE counterparts (p<0.01). When recipient ApoE−/− mice were reconstituted with ACE10/10 bone marrow, these mice showed significantly reduced lesion areas compared to recipients reconstituted with wild type bone marrow. Furthermore, transfer of ACE-deficient bone marrow had no impact on lesion area.
Conclusion -
Our data indicate that while myeloid ACE may not be required for atherosclerosis, enhanced ACE expression paradoxically reduced disease progression.
Keywords: angiotensin-converting enzyme, myeloid cells, atherosclerosis, hypertension, inflammation
INTRODUCTION
Cardiovascular diseases (CVD) are the leading cause of death in the western world, and atherosclerosis, an important underlying cause of myocardial infarction, cerebrovascular accidents and peripheral vascular disease, is the major cause of deaths from CVD [1]. Atherosclerosis results from multiple steps, including endothelial activation, recruitment and differentiation of immune cells, foam cell formation and death, development of fibrotic plaques, and the proliferation of smooth muscles cells [2]. These processes ultimately result in plaque rupture and thrombosis [3].
Macrophages modulate multiple aspects of atherosclerosis [2, 4, 5]. While it is generally thought that pro-inflammatory macrophages drive atherosclerosis whereas anti-inflammatory cytokines inhibit the disease process, recent data cast doubt on this oversimplification [6–8]. What is not in doubt, however, is that regardless of macrophage phenotype, failure to adequately clear lipids in the arterial wall ultimately renders macrophages active participants in the formation of foam cells, necrotic core development, and instability of the atherosclerotic plaque [2, 9]. Thus, we wondered if boosting the inflammatory response and enhancing the ability of macrophages to clear lipids may actually promote resistance to atherosclerosis.
Angiotensin converting enzyme (ACE) is a promiscuous peptidase that plays a central role in the renin angiotensin system (RAS) by converting angiotensin I into the vasoconstrictor angiotensin II [10]. Beyond its effect on blood pressure, ACE also appears to influence immune regulation. For example, ACE is required for normal myelopoiesis [11, 12]. ACE is also upregulated during monocyte-macrophage differentiation, as well as upon macrophage activation by several stimuli such as lipopolysaccharide, and infectious pathogens [13–15]. Furthermore, during certain disease processes, including sarcoidosis and several other granulomatous diseases, macrophages make significant amounts of ACE [16, 17]. Even in atherosclerosis, careful analysis has demonstrated significant ACE expression by lesional macrophages [18, 19]. In summary, observations from several different disease processes led us to question the functional role of macrophage ACE during inflammation, including that present in atherosclerosis. Is macrophage ACE, and the presumed local production of angiotensin II, deleterious to the formation of atherosclerotic lesions, or does myeloid ACE expression have a different effect?
To gain further insight into the role of ACE in myeloid cells, the ACE10 mice were generated to over-express ACE in myelomonocytic lineage cells (predominantly monocytes and macrophages) by substituting control of ACE expression from the natural ACE promoter to the c-fms promoter [20]. Unexpectedly, these mice demonstrated markedly improved resistance to tumor growth, enhanced clearance of bacterial infections, and protection from a mouse model of Alzheimer’s disease pathology [20–22]. Central to the phenotype is the enhanced ability of these macrophages to phagocytose and eliminate invading particles, including tumor cells, bacteria, and β-amyloid plaque material [23–25]. Using ApoE−/− mice and two models of accelerated atherosclerotic development, we now report that overexpression of ACE by myeloid cells protects mice from atherosclerosis. In contrast, ACE-deficient macrophages had no significant impact on atherosclerosis. Our findings add to a body of literature suggesting that myeloid expression of ACE boosts macrophage function. Further, our data indicate that in mice, an enhanced immune response increases resistance to atherosclerosis.
MATERIALS AND METHODS
Mice
Animal studies were approved by the Emory University Institutional Animal Care and Use Committee in accordance with the guidelines set forth by the NIH Guide for the Care and Use of Laboratory Animals. The ACE10 mice were engineered to produce angiotensin converting enzyme (ACE) overexpression in myelomonocytic lineage cells using the c-fms promoter [20]. After the ACE10 mice were bred to C57BL/6 for more than 10 generations, they were crossed with ApoE deficient mice (The Jackson Laboratory, Bar Harbor, ME), also bred for more than 10 generations with C57BL/6, to generate heterozygotes followed by additional breeding to generate ACE10/10ApoE−/− (ACE10/ApoE) or ACEWT/WTApoE−/− (WT/ApoE) mice. Mice were then fed a standard chow diet (Purina, Certified Rodent Chow 5001) or a high-fat Paigen’s Atherogenic Rodent Diet” (30% kcal from fat, 1.25% cholesterol, 0.5% cholic acid atherogenic diet, Research Diets, Inc). In some experiments, mice underwent further experimental manipulation by way of simultaneous unilateral nephrectomy and subcutaneous implantation of deoxycorticosterone acetate (DOCA) salt pellet to accelerate atherosclerosis [26]. Male mice between 8-12 weeks of age were used.
Blood Pressure Measurements
Systolic blood pressure was measured using a computerized, noninvasive, tail-cuff system (BP 2000 Visitech Systems). Mice acclimated to the device for a minimum of four readings before measurements were taken. Mean systolic blood pressures were calculated by averaging each set of 10 measurements taken per mouse.
Evaluation of Atherosclerotic Lesions
The aortas were pressure-perfused with 0.9% sodium chloride solution, followed by pressure fixation at approximately 100 mm Hg with a 10% formaldehyde solution. The aorta was extracted from the iliac bifurcation to the aortic root, and then placed in 4% formaldehyde. En face preparations were made by removing the descending thoracic and abdominal periaortic fat completely, followed by longitudinal dissection of the aorta. Digital images were captured, and the lesion areas were analyzed using NIH Image software.
Bone Marrow Transplantation
Bone marrow was obtained from 8-week-old ACE10/10, ACE-deficient mice (ACE−/−)[27] and ACE wild-type (ACEWT/WT) donor mice by flushing femurs and tibiae with RPMI 1640 medium. All donor mice were ApoE−/−. Nucleated cells were counted, and the bone marrow was resuspended at a concentration of 2 × 107/ml. Eight-week-old recipient ApoE−/− mice were irradiated with 1100 rads and reconstituted with either 2 × 106 ACE10/10, ACEWT/WT or ACE−/− bone marrow cells via retroorbital injection. After 8 weeks, the recipient mice were analyzed for monocyte ACE expression by flow cytometry. ACE activity was measured using the ACE-REA kit from American Laboratory Products Company, Ltd. (ALPCO, Windham, NH) as previously described [28]. ACE activity was defined as that inhibited by captopril.
In Vitro Macrophage Cultures
Thioglycollate-elicited peritoneal exudate cells were collected via peritoneal lavage 4 days after a 2-ml injection of 3% thioglycollate broth intraperitoneally (i.p.) and were cultured in tissue culture plates (1×106/ml) at 37 °C and 5% CO2 in 10% fetal calf serum RPMI 1640, 50 μM β-mercaptoethanol, 0.5 mM sodium pyruvate, 10 mM HEPES buffer, 50 units/ml penicillin, 50 μg/ml streptomycin, and 2 mM L-glutamine. Peritoneal exudate cells were allowed to adhere for 2 hours, after which nonadherent cells were washed off to achieve a >95% purity of macrophages. Cells lysates were harvested for RT-PCR.
RNA Isolation and Quantitative Real-time Polymerase Chain Reaction
Total RNA was extracted from aortas or macrophages using the RNeasy kit (Qiagen). mRNA levels were measured by amplification of cDNA using a thermocycler (Applied Biosystems, Foster City, CA), SYBR Green dye. The following primers were utilized. MCP-1 (Forward: GTCCCTGTCATGCTTCTGG, Reverse: GCGTTAACTGCATCTGGCT); Osteopontin (Forward: GTATGAGACGGGACAGCTATTTCTCCA, Reverse CTGACATAGTCCAAGCCTGGGATG), and PAI-1 (Forward: GGCTGACTTCACGAGTCTTTCA, Reverse: TTCACTTTCTGCAGCGCCT). The copy number was calculated by instrument software from standard curves of genuine templates. The copy number was normalized to 18 S rRNA.
Statistic
All data are presented as mean ± SEM, using Graphpad Prism (Graphpad Software, La Jolla, CA). Statistical significance was determined by ANOVA for multiple groups, followed by Bonferroni post-hoc analysis. In comparing only two groups, we used the unpaired Student’s t test. A p value <0.05 was considered statistically significant.
RESULTS
ACE10 Mice are Protected from Atherosclerosis
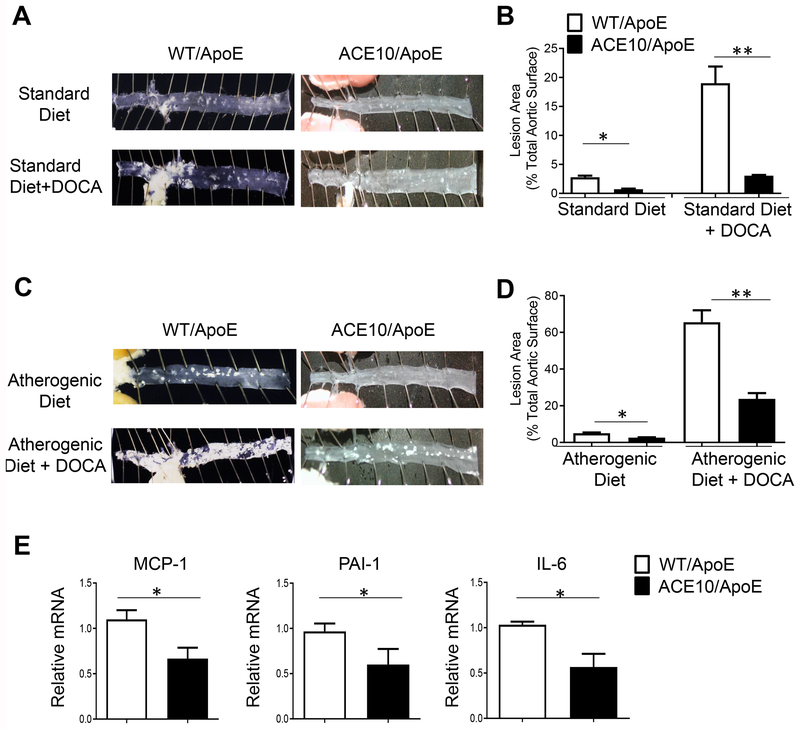
The ACE10 mice have been previously described [20]. We assessed atherosclerotic plaque formation in the ACE10/10ApoE−/− (ACE10/ApoE) and ACEWT/WTApoE−/− (WT/ApoE) mice using either the standard approach of an atherogenic diet, or an accelerated atherosclerosis model using the atherogenic diet combined with unilateral nephrectomy and DOCA salt. ACE-deficient mice were analyzed in this assay given their low blood pressure, which affects atherosclerosis [27]. After 8 weeks of either a normal diet with or without nephrectomy/DOCA salt (Fig. 1A, B) or an atherogenic diet with or without nephrectomy/DOCA salt (Fig. 1C, D), mice were euthanized, and the aortas were evaluated. Panels A and C in Fig. 1 show representative en face preparations of descending thoracic and abdominal aortas, which revealed significantly less plaque formation in the ACE10/ApoE compared to the WT/ApoE mice. Panels B and D are mean data from the analysis of the descending aorta from the four treatment groups. As expected, addition of nephrectomy/DOCA salt increased plaque formation in all groups (Fig. 1). On the standard diet, aortic lesional area was 2.8 ± 0.8% in WT/ApoE vs 0.73 ± 0.4% in ACE10/ApoE (p=0.86). With the addition of nephrectomy/DOCA salt, aortic lesional area was 19.1 ± 3.0% in WT/ApoE vs 3.1 ± 0.8% in ACE10/ApoE mice (p< 0.01). After 8 weeks of an atherogenic diet, aortic lesional area was 5.0 ± 0.7% in WT/ApoE, compared to 2.6 ± 0.5% in ACE10/ApoE mice (p<0.05). In the setting of nephrectomy/DOCA salt, lesional area was 65.5 ± 5.7% in the WT/ApoE, compared to 23.8 ± 4.6% in ACE10/ApoE (p<0.01) (Fig. 1D). Altogether, these data demonstrate protection from atherosclerosis in the ACE10/ApoE mice.
Fig. 1.
ACE10 mice are protected from atherosclerosis. A,C. Representative en face preparation of aortas after 8 weeks of standard or atherogenic diet treatment with or without nephrectomy plus deoxycorticosterone (DOCA) salt. B,D. Assessment of lesional area of WT/ApoE (white bars) and ACE10/ApoE (black bars) on standard diet or atherogenic diet with or without nephrectomy/DOCA salt. E. Assessment of vascular wall inflammation, determined by RT-PCR measurements of MCP-1, PAI-1 and IL-6 of aorta of mice treated with atherogenic diet and nephrectomy/DOCA salt for 8 weeks. n=8-10 mice per group. *p< 0.05, **p<0.01 by unpaired Student’s t test.
A key change in atherosclerosis is increased aortic wall injury, inflammation and vascular dysfunction. Thus, we compared prototypical markers of aortic wall dysfunction and inflammation between ACE10/ApoE and WT/ApoE mice that were treated with 8 weeks of atherogenic diet with DOCA salt. There was approximately 50% decrease in the RNA expression of the pro-inflammatory genes MCP-1, PAI-1 and IL-6 (Fig. 1E). These data suggest ACE10 macrophages reduce aortic wall inflammation and vascular changes.
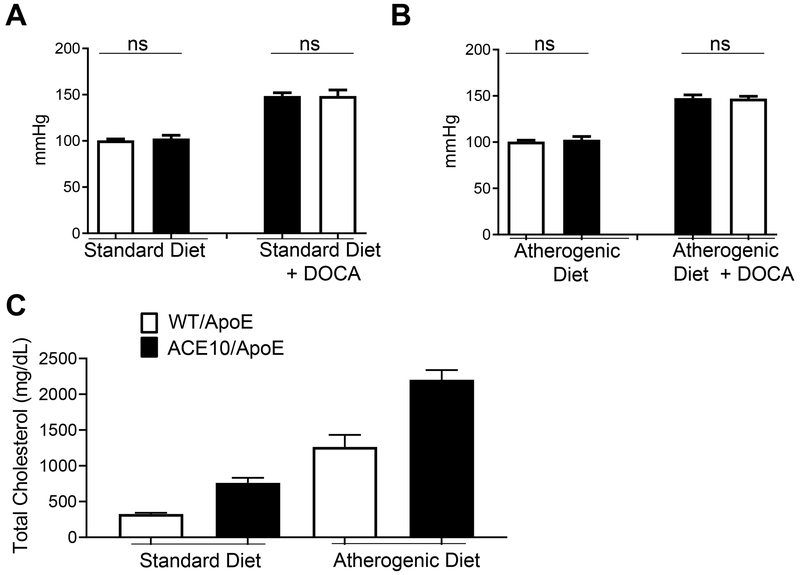
Protection from Atherosclerosis is Independent of Blood Pressure or Cholesterol Levels
Blood pressure and hemodynamic changes may differentially influence the extent of atherosclerosis in animal models. At baseline, ACE10 mice have blood pressures equivalent to wild type [29]. We measured blood pressure in the ACE10/ApoE and WT/ApoE mice and, as expected, DOCA salt induced hypertension [26], but there were no significant differences between ACE10/ApoE and WT/ApoE mice (Fig. 2A). Following the atherogenic diet, we observed no differences in blood pressure between ACE10/ApoE and WT/ApoE mice (Fig. 2B). Thus, the significant protection afforded to ApoE deficient mice by macrophage ACE overexpression appears to be independent of blood pressure differences between the two genotypes. Further, the protection from atherosclerosis is not due to ACE lowering plasma total cholesterol since the ACE10/ApoE mice had plasma total cholesterol that averaged 760 mg/dl ± 72 and 2202 mg/dl ± 134 on the standard and atherosclerotic diets respectively. This is somewhat higher than typical WT/ApoE mice which average 325 mg/dl ± 18 and 1263 mg/dl ± 170 on the standard and atherosclerotic diets (Fig. 2C).
Fig. 2.
Protection from atherosclerosis is independent of blood pressure and cholesterol lowering. A. Blood pressure measurements by tail-cuff plethysmography of WT/ApoE (white bars) and ACE10/ApoE (black bars) mice placed on standard diet with or without nephrectomy/DOCA salt for 8 weeks. B. Blood pressure measurements by tail-cuff plethysmography of ACE10/ApoE and WT/ApoE mice placed on atherogenic diet with or without nephrectomy/DOCA salt for 8 weeks. C. Total serum cholesterol measurements in WT/ApoE and ACE10/ApoE mice on standard diet and atherogenic for 8 weeks. N=5-8 mice per group. ns= not significant, by unpaired Student’s t test.
ACE10 macrophages are pro-inflammatory and anti-atherogenic
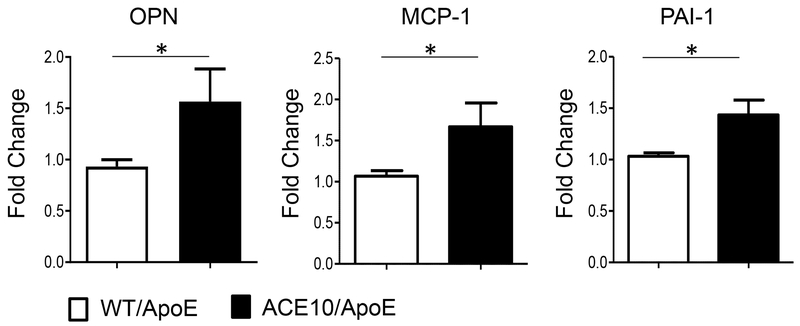
To analyze macrophage function with respect to atherosclerosis, we isolated thioglycolate-induced peritoneal macrophages after 8 weeks of an atherogenic diet and measured their inflammatory response in culture. This assay revealed elevated levels of MCP-1, PAI-1 and OPN (Fig. 3) in the ACE10/ApoE macrophages compared to WT/ApoE cells. Thus, on an ApoE-deficient mice background, ACE10 macrophages produce abundant inflammatory cytokines, similar to their behavior under normal ApoEWT/WT background.
Fig. 3.
Enhanced cytokine production in ACE10 macrophages. After eight weeks of atherogenic diet, thioglycolate was used to elicit peritoneal macrophages from atherosclerotic WT/ApoE and ACE10/ApoE. Osteopontin (OPN), monocyte chemotactic protein (MCP-1), and plasminogen activator inhibitor-1 (PAI) levels were measured by RT-PCR. Experiments were performed 3 independent times. *p<0.05, **p<0.01. n=5-6 mice per group.
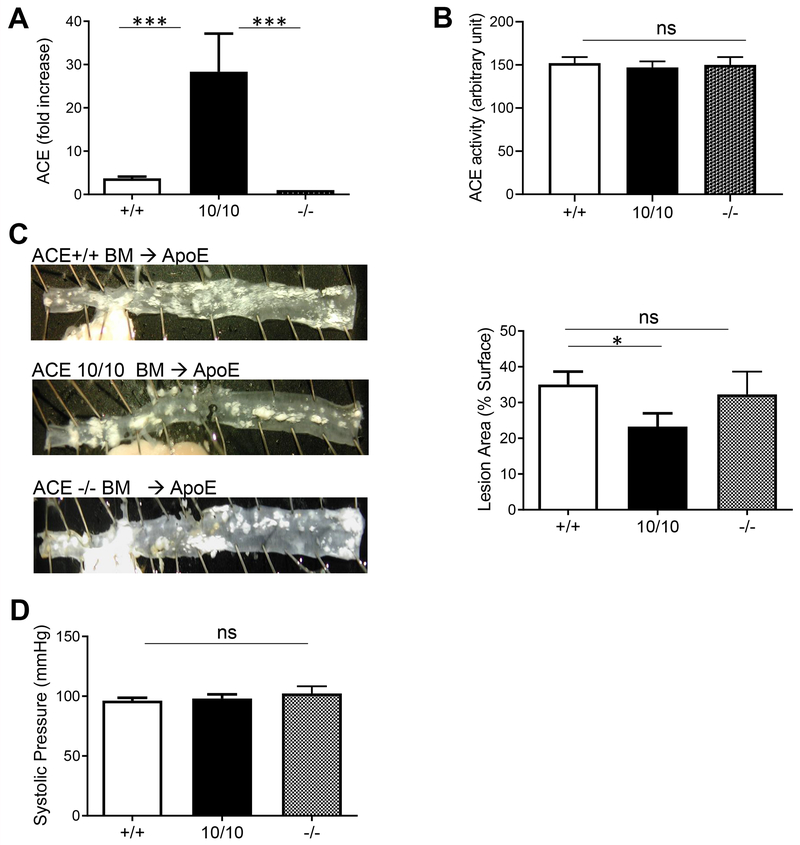
Atheroprotection by Myeloid ACE is Transferable
In the ACE10 mice, placing ACE under the control of the c-fms promoter eliminates ACE expression by vascular endothelium [20], raising the possibility that the absence of endothelial ACE explains the atheroprotection in the ACE10/ApoE mice. To address this, we transplanted bone marrow from donor ApoE−/− mice that were either ACE10/10, ACEWT/WT or ACE−/− into recipient ACEWT/WTApoE−/− mice. This approach ensures there is no difference in endothelial ACE expression. Several weeks later, ACE expression by peripheral blood monocytes was assessed to confirm successful transplantation (Fig. 4A). Plasma ACE was indistinguishable in the recipient mice regardless of the source of donor cells (Fig. 4B). After 8 weeks of the atherogenic diet, the descending aorta lesional area in recipient ApoE−/− mice receiving donor ACE10/10 was substantially lower than mice reconstituted with ACEwt/wt or ACE−/− bone morrow (23.3 ± 3% vs 36 ± 5% vs 30 ± 3.8%, p<0.05 between ACE10/10 and ACEWT/WT; Fig. 4C). Thus, the atheroprotection is independent of vascular ACE but depends on the enhanced myeloid expression of ACE. Again, this phenotype was independent of blood pressure (Fig. 4D), as this was similar between all the recipient ApoE deficient mice.
Fig. 4.
Bone marrow transfer recapitulates the atheroprotection by ACE10 cells. A. ACE expression from monocytes isolated from ApoE mice repopulated with bone marrow cells from WT (+/+), ACE10 (10/10) or ACE knockout (−/−) donor mice. Expression was measured as flow cytometric mean fluorescence intensity (MFI), and fold increase determined relative to −/−. B. Serum ACE activity of ApoE mice reconstituted with bone marrow from WT (+/+), ACE10 (10/10) or ACE knockout (−/−) donor mice C. Assessment of atherosclerotic lesional plaques on aorta. Representative en face preparation is shown on left panel. D. Blood pressure of ApoE mice reconstituted with marrow from WT (+/+), ACE10 (10/10) and ACE knockout (−/−) mice. n=5. ns= not significant, *p<0.05, *** p<0.005.
DISCUSSION
Several lines of evidence suggest that atherosclerosis is fundamentally an inflammatory disease in which macrophages play a critical role. While early studies emphasized the significance of pro-inflammatory M1 macrophages as pro-atherogenic and anti-inflammatory M2 macrophages as anti-atherogenic, recent work has shown that this M1-M2 paradigm is an oversimplification of macrophage heterogeneity in atherosclerosis and other chronic inflammatory diseases [6]. Indeed, recent analysis by single cell sequencing of macrophages in the atherosclerotic plaque revealed that pro-inflammatory macrophages entrapped fewer lipids, while anti-inflammatory macrophages were lipid-laden and formed the bulk of the pro-atherogenic foamy cells [8]. Regardless of inflammatory status, macrophages challenged with lipids in the setting of atherosclerosis ultimately lose phagocytic function, permitting accumulation of apoptotic and necrotic material, and promote the disease [30, 31], suggesting that a robust macrophage phenotype might reduce atherosclerotic progression.
Here, we report that myelomonocytic lineage cells over expressing ACE reduced the formation of atherosclerotic lesions in ApoE-deficient mice. Further, bone marrow transplantation of these cells protected recipient ApoE-deficient mice from atherosclerosis. Importantly, although the ACE10 macrophages are capable of a robust inflammatory response, they reduced vascular inflammation and remodeling, marked by equivalent or lower levels of osteopontin, PAI-1, MCP-1 and IL-6. These lines of evidence demonstrate that despite – and we posit because of – the robust inflammatory response by the ACE10, these mice are protected from atherosclerosis.
Wild type peritoneal macrophages make relatively little ACE [24]. However, in disease, WT macrophages increase ACE expression and there are several studies showing that the inflammatory cells present in all stages of atherosclerotic lesions make significant levels of ACE [19, 32, 33]. It has been suggested that this local ACE may drive local Ang II production and facilitate increased inflammation and foam cell formation [34]. However, evidence against this idea includes bone marrow transplantation experiments, both here and in the literature, showing that in mouse models of atherosclerosis bone marrow transplantation of WT bone marrow versus ACE knockout bone marrow has little or no effect on the extent of atherosclerotic plaque area [32]. In fact, we observed that transplantation of ACE overexpressing bone marrow, cells fully capable of making angiotensin II, resulted in reduced disease, not exaggerated disease. Unfortunately, the significantly lower blood pressure in the ACE knockout mice, which impacts atherosclerosis, precluded us from comparing the effect of myeloid ACE deficiency on atherosclerosis in these mice [27].
ACE10 mice were previously characterized as having an enhanced inflammatory phenotype with macrophages resembling ‘M1’ (pro-inflammatory) cells [20, 22, 35, 36]. These mice have increased resistance to tumor growth, accelerated clearance of bacterial infection, and increased removal of cerebral β-amyloid and marked decrease in amyloid plaque area in a mouse model of Alzheimer’s disease (AD) [20–23, 37]. There are obvious parallels between the effect of ACE10 macrophages on amyloid plaque development and the studies of atherosclerotic plaque reported here. In the genetic model of AD, the increased macrophage response of AD+ACE10 mice reduced the amount of deposited β-amyloid, which is a stimulus for chronic deleterious brain inflammation. In atherosclerosis, ACE10 mice protect from disease induced by vascular lipid deposition with the net result of reduced vascular wall remodeling.
Angiotensin I is an important substrate of ACE. However, it is far from the only substrate of ACE. Recent analysis indicates that ACE is highly promiscuous with hundreds of substrates [38]. Indeed, repeated careful analysis indicates that the phenotypic effect of ACE over expression on ACE10 macrophages is not due to angiotensin II or any angiotensin peptide [20, 22, 36].
Our findings raise the possibility that the observed increase in macrophage ACE in human atherosclerotic plaques is a consequence, rather than a cause, of atheroprogression. Further work is required to determine a means of enhancing macrophage ACE expression, or other means to enhance the effectiveness of macrophages in clearing the vascular insults that provoke atherosclerosis. Possibilities include either identifying the exact ACE substrate that stimulates macrophage function, or copying the intracellular pathway invoked by this substrate. In either case, it seems that an increased macrophage response might be an effective approach to reduce atherosclerotic progression.
Supplementary Material
Myeloid cells overexpressing angiotensin-converting enzyme (ACE) are protected from atherosclerosis under the ApoE background
Protection from atherosclerosis is transferable from bone marrow
ACE-deficient bone marrow had minimal impact on atherosclerosis
Acknowledgement
Sources of Funding: This work was supported by NIH P01 HL095070 (WRT), P01HL129941 (KEB), R01AI134714 (KEB) and K99HL141638 (DOD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
LITERATURE CITED
- [1].Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ, Global and regional patterns in cardiovascular mortality from 1990 to 2013, Circulation, 132 (2015) 1667–1678. [DOI] [PubMed] [Google Scholar]
- [2].Moore KJ, Sheedy FJ, Fisher EA, Macrophages in atherosclerosis: a dynamic balance, Nat Rev Immunol, 13 (2013) 709–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R, Concept of vulnerable/unstable plaque, Arterioscler Thromb Vasc Biol, 30 (2010) 1282–1292. [DOI] [PubMed] [Google Scholar]
- [4].Nahrendorf M, Swirski FK, Immunology. Neutrophil-macrophage communication in inflammation and atherosclerosis, Science, 349 (2015) 237–238. [DOI] [PubMed] [Google Scholar]
- [5].Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW, A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association, Arterioscler Thromb Vasc Biol, 15 (1995) 1512–1531. [DOI] [PubMed] [Google Scholar]
- [6].Nahrendorf M, Swirski FK, Abandoning M1/M2 for a Network Model of Macrophage Function, Circ Res, 119 (2016) 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chao LC, Soto E, Hong C, Ito A, Pei L, Chawla A, Conneely OM, Tangirala RK, Evans RM, Tontonoz P, Bone marrow NR4A expression is not a dominant factor in the development of atherosclerosis or macrophage polarization in mice, J Lipid Res, 54 (2013) 806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim K, Shim D, Lee JS, Zaitsev K, Williams JW, Kim KW, Jang MY, Seok Jang H, Yun TJ, Lee SH, Yoon WK, Prat A, Seidah NG, Choi J, Lee SP, Yoon SH, Nam JW, Seong JK, Oh GT, Randolph GJ, Artyomov MN, Cheong C, Choi JH, Transcriptome Analysis Reveals Nonfoamy Rather Than Foamy Plaque Macrophages Are Proinflammatory in Atherosclerotic Murine Models, Circ Res, 123 (2018) 1127–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kojima Y, Weissman IL, Leeper NJ, The Role of Efferocytosis in Atherosclerosis, Circulation, 135 (2017) 476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Corvol P, Williams TA, Soubrier F, Peptidyl dipeptidase A: angiotensin I-converting enzyme, Methods Enzymol, 248 (1995) 283–305. [DOI] [PubMed] [Google Scholar]
- [11].Shen XZ, Okwan-Duodu D, Blackwell WL, Ong FS, Janjulia T, Bernstein EA, Fuchs S, Alkan S, Bernstein KE, Myeloid expression of angiotensin-converting enzyme facilitates myeloid maturation and inhibits the development of myeloid-derived suppressor cells, Lab Invest, 94 (2014) 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE, Shen XZ, Angiotensin-converting enzyme is required for normal myelopoiesis, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25 (2011) 1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T, Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages, J Hypertens, 17 (1999) 537–545. [DOI] [PubMed] [Google Scholar]
- [14].Danilov SM, Sadovnikova E, Scharenborg N, Balyasnikova IV, Svinareva DA, Semikina EL, Parovichnikova EN, Savchenko VG, Adema GJ, Angiotensin-converting enzyme (CD143) is abundantly expressed by dendritic cells and discriminates human monocyte-derived dendritic cells from acute myeloid leukemia-derived dendritic cells, Experimental hematology, 31 (2003) 1301–1309. [DOI] [PubMed] [Google Scholar]
- [15].Viinikainen A, Nyman T, Fyhrquist F, Saijonmaa O, Downregulation of angiotensin converting enzyme by TNF-alpha in differentiating human macrophages, Cytokine, 18 (2002) 304–310. [DOI] [PubMed] [Google Scholar]
- [16].Baudin B, New aspects on angiotensin-converting enzyme: from gene to disease, Clin Chem Lab Med, 40 (2002) 256–265. [DOI] [PubMed] [Google Scholar]
- [17].Brice EA, Friedlander W, Bateman ED, Kirsch RE, Serum angiotensin-converting enzyme activity, concentration, and specific activity in granulomatous interstitial lung disease, tuberculosis, and COPD, Chest, 107 (1995) 706–710. [DOI] [PubMed] [Google Scholar]
- [18].Diet F, Pratt RE, Berry GJ, Momose N, Gibbons GH, Dzau VJ, Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease, Circulation, 94 (1996) 2756–2767. [DOI] [PubMed] [Google Scholar]
- [19].Ohishi M, Ueda M, Rakugi H, Naruko T, Kojima A, Okamura A, Higaki J, Ogihara T, Enhanced expression of angiotensin-converting enzyme is associated with progression of coronary atherosclerosis in humans, J Hypertens, 15 (1997) 1295–1302. [DOI] [PubMed] [Google Scholar]
- [20].Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE, Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma, Am J Pathol, 170 (2007) 2122–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bernstein KE, Koronyo Y, Salumbides BC, Sheyn J, Pelissier L, Lopes DH, Shah KH, Bernstein EA, Fuchs DT, Yu JJ, Pham M, Black KL, Shen XZ, Fuchs S, Koronyo-Hamaoui M, Angiotensin-converting enzyme overexpression in myelomonocytes prevents Alzheimer’s-like cognitive decline, The Journal of clinical investigation, 124 (2014) 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Okwan-Duodu D, Datta V, Shen XZ, Goodridge HS, Bernstein EA, Fuchs S, Liu GY, Bernstein KE, Angiotensin-converting enzyme overexpression in mouse myelomonocytic cells augments resistance to Listeria and methicillin-resistant Staphylococcus aureus, J Biol Chem, 285 (2010) 39051–39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shen XZ, Billet S, Lin C, Okwan-Duodu D, Chen X, Lukacher AE, Bernstein KE, The carboxypeptidase ACE shapes the MHC class I peptide repertoire, Nature immunology, 12 (2011) 1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bernstein KE, Khan Z, Giani JF, Cao DY, Bernstein EA, Shen XZ, Angiotensin-converting enzyme in innate and adaptive immunity, Nat Rev Nephrol, 14 (2018) 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhao T, Bernstein KE, Fang J, Shen XZ, Angiotensin-converting enzyme affects the presentation of MHC class II antigens, Laboratory investigation; a journal of technical methods and pathology, 97 (2017) 764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Weiss D, Taylor WR, Deoxycorticosterone acetate salt hypertension in apolipoprotein E−/− mice results in accelerated atherosclerosis: the role of angiotensin II, Hypertension, 51 (2008) 218–224. [DOI] [PubMed] [Google Scholar]
- [27].Esther CR Jr., Howard TE, Marino EM, Goddard JM, Capecchi MR, Bernstein KE, Mice lacking angiotensin-converting enzyme have low blood pressure, renal pathology, and reduced male fertility, Lab Invest, 74 (1996) 953–965. [PubMed] [Google Scholar]
- [28].Cole J, Quach DL, Sundaram K, Corvol P, Capecchi MR, Bernstein KE, Mice lacking endothelial angiotensin-converting enzyme have a normal blood pressure, Circ Res, 90 (2002) 87–92. [DOI] [PubMed] [Google Scholar]
- [29].Gonzalez-Villalobos RA, Janjoulia T, Fletcher NK, Giani JF, Nguyen MT, Riquier-Brison AD, Seth DM, Fuchs S, Eladari D, Picard N, Bachmann S, Delpire E, Peti-Peterdi J, Navar LG, Bernstein KE, McDonough AA, The absence of intrarenal ACE protects against hypertension, The Journal of clinical investigation, 123 (2013) 2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W, Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis, Arteriosclerosis, thrombosis, and vascular biology, 25 (2005) 1256–1261. [DOI] [PubMed] [Google Scholar]
- [31].Tabas I, Macrophage death and defective inflammation resolution in atherosclerosis, Nature reviews. Immunology, 10 (2010) 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chen X, Lu H, Zhao M, Tashiro K, Cassis LA, Daugherty A, Contributions of leukocyte angiotensin-converting enzyme to development of atherosclerosis, Arteriosclerosis, thrombosis, and vascular biology, 33 (2013) 2075–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA, Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor, Circulation, 110 (2004) 3849–3857. [DOI] [PubMed] [Google Scholar]
- [34].Ni W, Kitamoto S, Ishibashi M, Usui M, Inoue S, Hiasa K, Zhao Q, Nishida K, Takeshita A, Egashira K, Monocyte chemoattractant protein-1 is an essential inflammatory mediator in angiotensin II-induced progression of established atherosclerosis in hypercholesterolemic mice, Arteriosclerosis, thrombosis, and vascular biology, 24 (2004) 534–539. [DOI] [PubMed] [Google Scholar]
- [35].Bernstein KE, Ong FS, Blackwell WL, Shah KH, Giani JF, Gonzalez-Villalobos RA, Shen XZ, Fuchs S, Touyz RM, A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme, Pharmacol Rev, 65 (2013) 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Khan Z, Shen XZ, Bernstein EA, Giani JF, Eriguchi M, Zhao TV, Gonzalez-Villalobos RA, Fuchs S, Liu GY, Bernstein KE, Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils, Blood, 130 (2017) 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shen XZ, Lukacher AE, Billet S, Williams IR, Bernstein KE, Expression of angiotensin-converting enzyme changes major histocompatibility complex class I peptide Presentation by modifying C termini of peptide precursors, The Journal of biological chemistry, 283 (2008) 9957–9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Semis M, Gugiu GB, Bernstein EA, Bernstein KE, Kalkum M, The Plethora of Angiotensin-Converting Enzyme-Processed Peptides in Mouse Plasma, Anal Chem, 91 (2019) 6440–6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.