Abstract
Aldehyde dehydrogenase (ALDH) activity is not only a valuable marker for cancer cells with stem-like features, but also plays a vital role in drug resistance and disease progression in many tumors including melanoma. However, the precise role of ALDH activity in patient prognosis remains unclear. In this study, using the Cancer Genome Atlas (TCGA) RNA-sequencing expression data, we analyzed gene expression of ALDH isozymes in melanoma tumors to define the expression patterns and the prognostic and predictive values of these enzymes. We found that ALDH1A1 and ALDH1A3 had both higher and broader expression ranges in melanoma patients, and that ALDH1A3 expression correlated with better overall survival in metastatic melanoma. Further, stratification of the TCGA cohorts by the mutational subtypes of melanoma specifically revealed that expression of ALDH1A3 correlated with better prognosis in metastatic BRAF-mutant melanoma while expression of ALDH1A1 correlated with better prognosis in BRAF wild-type melanoma. Gene set enrichment analysis (GSEA) of these cohorts identified upregulation in oxidative phosphorylation, adipogenesis, and fatty acid metabolism signaling in ALDH1Alo patients, suggesting BRAF/MEK inhibitor resistance in that subset of patients. On the other hand, GSEA of ALDH1A3hi cohorts revealed upregulation in glycolysis, hypoxia and angiogenesis, suggesting BRAF/MEK inhibitor sensitivity in that subset of patients. Gene expression analysis using pre-treatment tumor samples supports high ALDH1A3 expression before BRAF/MEK inhibitor treatment as predictive of better treatment response in BRAF-mutant melanoma patients. Our study provides evidence that high ALDH1A3 mRNA expression is not only a prognostic marker but also a predictive marker for BRAF/MEK inhibitor treatment response in BRAF-mutant metastatic melanoma patients.
Keywords: ALDH1A1, ALDH1A3, Melanoma patient prognosis, GSEA, BRAF/MEK inhibitor response
Introduction
Aldehyde dehydrogenases (ALDHs) catalyze the oxidation of aldehydes to carboxylic acids. To date, 19 ALDH genes have been identified in the human genome, which display distinct cellular and tissue localization, physiological functions and significant roles in a wide array of diseases in humans including atherosclerosis, diabetes, chronic alcohol exposure, acute lung injury, neurodegenerative diseases and cancer [1, 2].
In coherence with these reports, high ALDH activity has been used as a relevant marker for stem cells in normal tissues [3] and cancer [4]. In leukemia and solid tumors such as breast [5], non-small cell lung, pancreatic, and brain cancers [6], ALDH activity has been associated with tumorigenesis and drug resistance. Previously, we and others have identified a subpopulation of ALDHhi tumor cells with the capacity for self-renewal, differentiation and drug resistance in human melanoma [7–9]. High ALDH activity is also shown to be directly associated with poor prognosis in ovarian cancer [10]. These reports clearly indicate that ALDH activity is not only a valuable marker, but also plays a vital role in cancer stemness, drug resistance and disease progression. However, it is unknown which of the 19 ALDH isozymes are associated with these critical roles in cancer and poor patient prognosis.
ALDH1A3 mRNA expression has been associated with worse prognosis in pancreatic cancer [11], glioblastoma [12] and breast cancer [13], whereas it has been correlated with better prognosis in non-small cell lung carcinoma [14] and non-muscle invasive bladder cancer [15]. In addition to ALDH1A3, the gene expression of another isozyme ALDH1A1 has been reportedly correlated with worse prognosis in multiple cancer types [16–18]. By using microarray and copy number qRT-PCR, we have also demonstrated that ALDH1A1 and ALDH1A3 are the predominant ALDH isozymes expressed in ALDHhi subpopulation of human melanoma tumors [7]. Recently, researchers have started analyzing publicly available RNA-sequencing expression data from the Cancer Genome Atlas (TCGA) to look at prognostic values of ALDH1 isozymes (ALDH1A1, ALDH1A2, ALDH1A3, ALDH1B1, ALDH1L1 and ALDH1L2) in breast [19], gastric [20, 21], liver [22] and ovarian [23] cancers. However, not all ALDH1 isozymes contribute to the ALDH activity detected by the ALDEFLUOR assay used to define the biology of ALDHhi and ALDHlo cells from tumors [3].
To delineate the expression levels of these ALDH isozymes and their prognostic values, we investigated their expression in metastatic melanoma patient samples using TCGA RNA-sequencing expression data. We correlated the expression of these ALDH isozymes with the clinical outcomes of patients based on their mutational subtypes. Additionally, we performed gene set enrichment analysis (GSEA) to elucidate associated gene sets and signaling pathways between the cohorts that display significant difference in disease prognosis. Lastly, we performed gene expression analysis on pre-treatment patients to investigate whether ALDH isozyme expressions correlate with responses to targeted therapy in melanoma.
Materials and Methods
The Cancer Genome Atlas (TCGA) Cutaneous Melanoma Data.
Clinical and mutational data of 329 TCGA melanoma patients were obtained from the supplementary tables of the TCGA publication [24]. Among these, 286 patients had complete pathoclinical information, and of these 42 and 244 were classified as primary and metastatic tumors, respectively. Metastatic melanoma patients were further classified based on their genomic alterations [24]: 107 of them were classified as BRAF-hotspot mutation subtype, 74 as RAS-hotspot mutation subtype, 22 as NF1-mutation subtype, 36 as triple wild-type (TWT) patients, and 5 of the patients were not assigned to any molecular subtype. Normalized RNA-seq gene expression profiles (Level 3, RSEM value) for these patients were downloaded from the cBioPortal website [25, 26]. The expression, mutational and clinical data for the patients used in this study is available as Supplementary Data.
Melanoma treatment data.
To correlate ALDH1A3hi and ALDH1A3lo expression to treatment data, we downloaded two publicly available gene expression datasets (GSE50509 and GSE99898) of BRAF-mutant melanoma patients treated with BRAF inhibitors (either vemurafenib or dabrafenib) or the combination of BRAF and MEK inhibitors (dabrafenib and trametinib). Both datasets were profiled by the Illumina HumanHT-12 V4.0 expression beadchip and normalized in log2 scale. The first dataset (GSE50509) contained 21 patients treated with BRAF inhibitors [27], whereas the second dataset (GSE99898) contained 17 patients treated with BRAF inhibitors or a combination of BRAF and MEK inhibitors [28]. One patient had no response data [28]. We defined patient responses according to the Response Evaluation Criteria In Solid Tumors (RECIST) criteria: Complete Response (CR, tumor disappearance after treatment), Partial Response (PR, tumor size reduces > 30%), Progressive Disease (PD, tumor size increases > 20%) and Stable Disease (SD, tumor size decrease less 30% and increases not more than 20%) [29].
Statistical Analysis.
The following approach was used to define an optimal cutpoint for patient classification and Cox Proportional-Hazards (PH) Modeling of Overall Survival (OS). For a given gene and cohort subset, an optimal gene expression level cutpoint associated with OS was identified by incrementing over the range of expression values and dichotomizing patients into those that were above or below the cutpoint, and the cutpoint that provided the strongest separation in OS was selected based on the logrank test. Cutpoints were assessed in increments of 1 and any that resulted in a large group imbalance (< 25% of patients in one group) were not evaluated. Furthermore, a Cox PH model was used to evaluate whether differences in OS between those above and below the cutpoint remained significant after adjusting for age, sex, and when applicable, mutational subtype. A P < 0.05 was considered statistically significant and we made no adjustments for multiple comparisons. The statistical analysis was performed in R 3.6.0 using Package ‘survival’ and Prism (version 7.0d) software (GraphPad Software, Inc, La Jolla, CA).
Gene Set Enrichment Analysis (GSEA).
GSEA version 3.0 [30] was used to determine the gene sets enriched in ALDH1A3hi versus ALDH1A3lo. We used the Hallmark [31] as the predefined gene sets, and performed 1000 permutations by gene set to determine the P-values. Gene sets with False Discovery Rate (FDR) q-value ≤ 0.1 were considered as significantly enriched.
Results and Discussion
The prognostic value of ALDH1A1 and ALDH1A3 isozymes in the molecular subtypes of melanoma
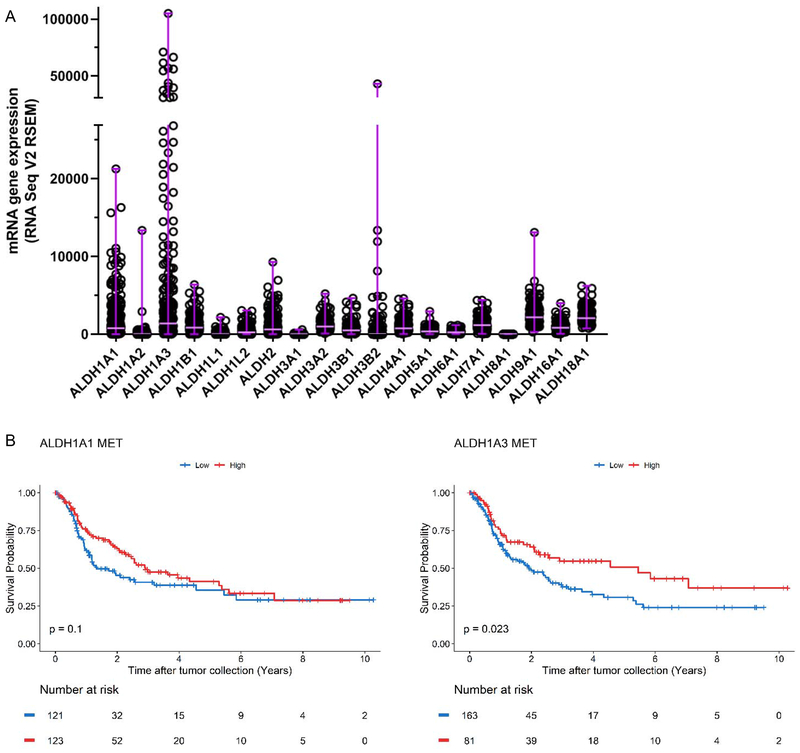
We first analyzed the expression levels of 19 ALDH enzymes from 244 metastatic melanomas reported in the TCGA cutaneous melanoma study (Figure 1A). We identified ALDH1A1 and ALDH1A3 as highly expressed isozymes with a broad range of expression in all melanoma patients and further investigated the mRNA expression levels of these two enzymes as prognostic markers using univariate Kaplan-Meier survival analysis. The patient demographic information is summarized in Table 1. This analysis revealed a correlation of high ALDH1A3 expression with a favorable OS in metastatic melanomas whereas no correlation was observed between ALDH1A1 expression and OS (Figure 1B) (log-rank test, P-value = 0.023 for ALDH1A3, P-value = 0.1 for ALDH1A1).
Figure 1: The prognostic value of ALDH1A1 and ALDH1A3 isozymes in metastatic melanoma.
(A) RNA Seq V2 RSEM values representing mRNA expression of all ALDH enzymes detected in metastatic melanoma, obtained from the SKCM TCGA provisional dataset. The median and range of each gene expression are shown using horizontal and vertical purple lines, respectively. (B) Kaplan Meier plot of ALDH1A1 (left, ALDH1A1 MET) and ALDH1A3 (right, ALDH1A3 MET) in metastatic patients. Patients above the gene expression threshold are shown in red and below in blue. The log-rank test was used to test for differences in overall survival between the two groups of each analysis.
Table 1: Overview of patient demographic information for the overall TCGA cohort and stratified by ALDH expression level.
TWT, triple wild-type for BRAF, NF1, and RAS.
| Variable | Patient Category | |||
|---|---|---|---|---|
| Overall (N=244) | P-Value | |||
| Sex (%) | Female | 94 (38.5) | ||
| Male | 150 (61.5) | |||
| Mutational Subtype (%) | ||||
| BRAF | 107 (43.9) | |||
| NF1 | 22 (9.0) | |||
| RAS | 74 (30.3) | |||
| TWT | 36 (14.8) | |||
| Unknown | 5 (2.0) | |||
| Age at sample (years) (median [IQR]) | 59.00 [48.75, 73.00] | |||
| Age at Diagnosis (years) (median [IQR]) | 55.00 [45.00, 68.25] | |||
| ALDH1A1 (median [IQR]) | 772.41 [301.53, 2429.01] | |||
| ALDH1A3 (median [IQR]) | 1382.73 [452.60, 4893.00] | |||
| AlDH1A1 - Low (N=121) | AlDH1A1 - High (N=123) | |||
| Sex (%) | ||||
| Female | 42 (34.7) | 52 (42.3) | 0.239+ | |
| Male | 79 (65.3) | 71 (57.7) | ||
| Mutational Subtype (%) | ||||
| BRAF | 45 (37.2) | 62 (50.4) | 0.171+ | |
| NF1 | 12 (9.9) | 10 (8.1) | ||
| RAS | 38 (31.4) | 36 (29.3) | ||
| TWT | 22 (18.2) | 14 (11.4) | ||
| Unknown | 4 (3.3) | 1 (0.8) | ||
| Age at sample (years) (median [IQR]) | 64.00 [54.00, 73.00] | 57.00 [46.50, 71.00] | 0.005* | |
| Age at Diagnosis (years) (median [IQR]) | 59.00 [50.00, 69.00] | 52.00 [39.50, 67.50] | 0.004* | |
| AlDH1A1 - Low (N=163) | AlDH1A1 - High (N=81) | |||
| Sex (%) | ||||
| Female | 65 (39.9) | 29 (35.8) | 0.578+ | |
| Male | 98 (60.1) | 52 (64.2) | ||
| Mutational Subtype (%) | ||||
| BRAF | 61 (37.4) | 46 (56.8) | 0.001+ | |
| NF1 | 14 (8.6) | 8 (9.9) | ||
| RAS | 52 (31.9) | 22 (27.2) | ||
| TWT | 33 (20.2) | 3 (3.7) | ||
| Unknown | 3 (1.8) | 2 (2.5) | ||
| Age at sample (years) (median [IQR]) | 61.00 [50.50, 73.00] | 58.00 [47.00, 72.00] | 0.229* | |
| Age at Diagnosis (years) (median [IQR]) | 57.00 [47.00, 69.00] | 54.00 [42.00, 66.00] | 0.141* | |
Wilcoxon Rank Sum Test ,
Fisher Exact Test
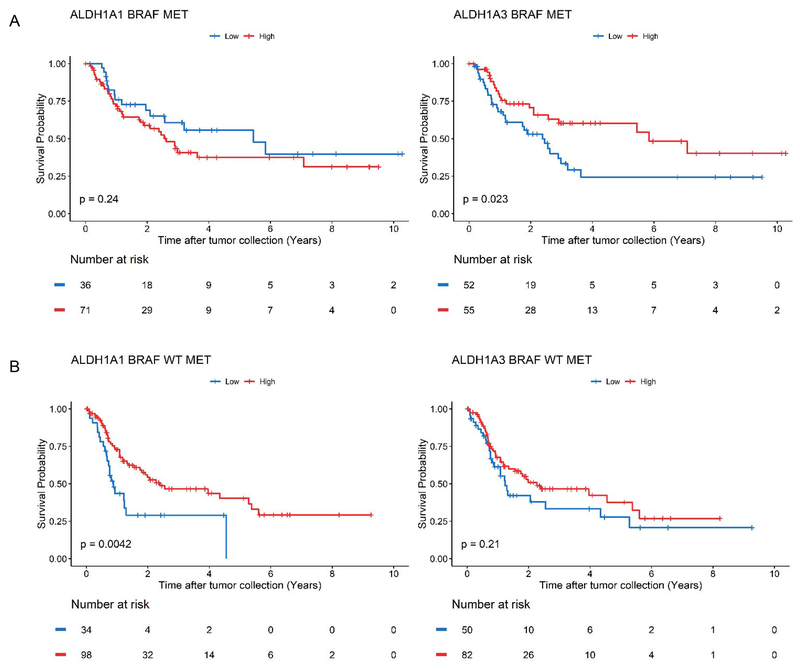
Because melanoma is uniquely classified by distinct molecular subtypes, we further stratified the patient cohort by their driver mutations for subsequent analyses: BRAF-hotspot mutation and BRAF wild-type (WT), which includes RAS-hotspot mutation, NF1-mutation, and TWT. The proportions of patients that belong to these specific subtypes, as well as their sex, age, and median mRNA expression levels of ALDH1A1 and ALDH1A3 are summarized in Table 1. We used Kaplan-Meier survival analysis to determine whether ALDH1A1 or ALDH1A3 expression correlates with disease prognosis when the patients were stratified based on their molecular subtypes. In the BRAF-hotspot cohort, high ALDH1A3 expression correlated with a favorable OS (log-rank test, P-value = 0.023) while an opposite trend was observed for ALDH1A1 expression, where high ALDH1A1 expression was associated with worse prognosis, although it was not statistically significant by log-rank test (P-value = 0.24) (Figure 2A).
Figure 2: The prognostic value of ALDH1A1 and ALDH1A3 gene expression in melanoma BRAF WT and BRAF mutant subtypes.
(A) Kaplan Meier plot of ALDH1A1 (left, ALDH1A1 BRAF MET) and ALDH1A3 (right, ALDH1A3 BRAF MET) for metastatic patients with a BRAF mutation. (B) Kaplan Meier plot of ALDH1A1 (left, ALDH1A1 BRAF WT MET) and ALDH1A3 (right, ALDH1A3 BRAF WT MET) for metastatic patients with wild-type (WT) BRAF. Patients above the gene expression threshold are shown in red and below in blue. The log-rank test was used to test for differences in overall survival between the two groups of each analysis.
On the other hand, in the BRAF WT cohort, a favorable OS was correlated with high ALDH1A1 expression but not ALDH1A3 (Figure 2B) (log-rank test, P-value = 0.0042). When the BRAF WT cohort was subsequently stratified into three genetic subtypes (RAS-hotspot mutation, NF1-mutation, and TWT), we observed a correlation of better prognosis with high ALDH1A1 expression in both the NF1 and TWT cohorts (Supplementary Figure 1A, 1B) (log-rank, P-value = 0.00036 for NF1, P-value = 0.024 for TWT) and trending correlation with ALDH1A3 expression in the RAS-hotspot cohort (Supplementary Figure 1C) (log-rank, P-value = 0.059).
To correct for potential age and sex bias, we performed multivariate analysis using a Cox PH model. We confirmed better OS to be positively correlated with ALDH1A3 in all metastatic patients and BRAF-mutant patients, even when the data were adjusted for age and sex (Table 2). Similarly, we confirmed a positive correlation of better OS with ALDH1A1 expression in BRAF WT patients, NF1 patients, and TWT patients.
Table 2: Overview of the optimal cutpoints for each cohort subset and corresponding p-values from a Cox proportional hazards model adjusted for age and sex.
When assessing the overall cohort, mutational subtype was also included as an adjustment variable. Met, metastatic melanoma; Met-BRAF-mut, metastatic melanoma with BRAF mutation; Met-BRAF WT, metastatic melanoma with wild-type (WT) BRAF; Met-NF1, metastatic melanoma with NF1 mutation; Met-RAS, metastatic melanoma with RAS mutation; Met-TWT, metastatic melanoma with triple wild-type (TWT) for BRAF, NF1, and RAS.
| Group | Variable | cutpoint | Total N | N above cutpoint | N below cutpoint | HR (95% CI) | P-Value |
|---|---|---|---|---|---|---|---|
| Overall | ALDH1A1 | 762 | 244 | 121 | 123 | 0.74 (0.51-1.08) | 0.119 |
| ALDH1A3 | 3473 | 244 | 163 | 81 | 0.62 (0.41-0.94) | 0.023 | |
| BRAF | ALDH1A1 | 533 | 107 | 36 | 71 | 1.46 (0.78-2.73) | 0.241 |
| ALDH1A3 | 2150 | 107 | 52 | 55 | 0.49 (0.28-0.88) | 0.016 | |
| BRAF WT | ALDH1A1 | 352 | 132 | 34 | 98 | 0.47 (0.27-0.82) | 0.007 |
| ALDH1A3 | 562 | 132 | 50 | 82 | 0.70 (0.43-1.63) | 0.171 | |
| NF1 | ALDH1A1 | 462 | 22 | 9 | 13 | 0.072 (0.01-0.46) | 0.006 |
| ALDH1A3 | 488 | 22 | 8 | 14 | 0.36 (0.08-1.61) | 0.181 | |
| RAS | ALDH1A1 | 354 | 74 | 19 | 55 | 0.49 (0.22-1.11) | 0.087 |
| ALDH1A3 | 1323 | 74 | 39 | 35 | 0.56 (0.28-1.12) | 0.1 | |
| TWT | ALDH1A1 | 385 | 36 | 11 | 25 | 0.34 (0.13-0.89) | 0.029 |
| ALDH1A3 | 952 | 36 | 26 | 10 | 1.42 (0.51-3.98) | 0.503 |
Although ALDH1A1 and ALDH1A3 isozymes play a similar role in retinoic acid signaling [32, 33], they have been reported to possess differential functions in breast cancer [34]. Therefore, we asked whether the high expressers and low expressers of ALDH1A1 and ALDH1A3 have common or distinct gene sets and pathways in their respective groups.
GSEA of ALDH1A3hi and ALDH1A3lo cohorts in metastatic BRAF-mutant melanoma
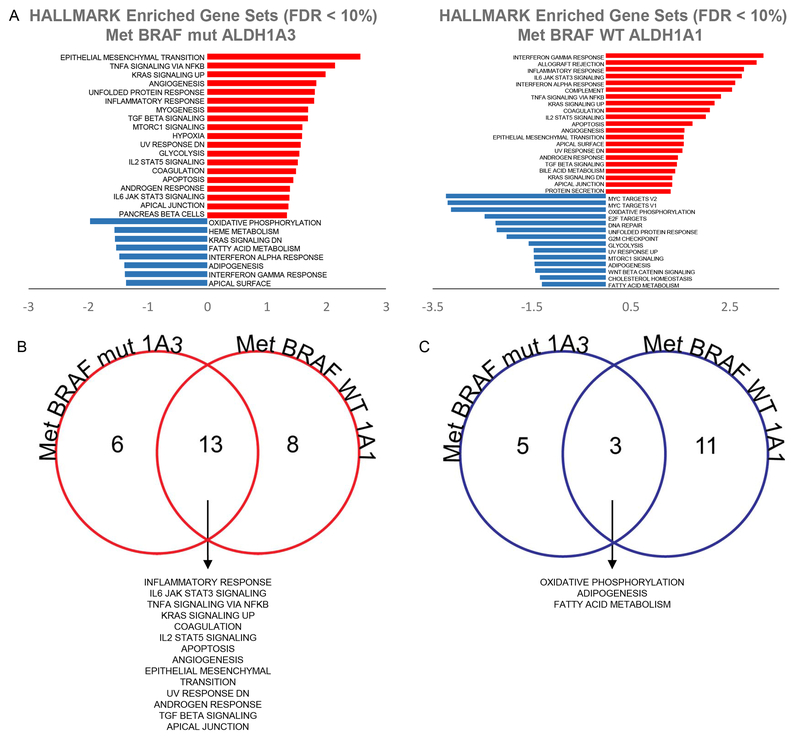
To elucidate the underlying genes and signaling pathways that contribute to better prognosis in ALDH1A3hi BRAF-mutated melanoma patients, we compared the ALDH1A3hi and ALDH1A3lo groups using GSEA. We observed 19 different pathways to be enriched in the ALDH1A3hi cohort and 8 other pathways enriched in the ALDH1A3lo cohort in gene expression profiles related to multiple hallmark gene sets (Figure 3A left, FDR q-value < 0.1).
Figure 3: GSEA of ALDH1A3hi and ALDH1A3lo cohorts in metastatic BRAF-mutant melanoma and ALDH1A1hi and ALDH1A1lo cohorts in metastatic BRAF WT melanoma.
(A) Left, Differentially enriched HALLMARK gene sets between ALDH1A3hi (in red) and ALDH1A3lo (in blue) cohorts in metastatic BRAF-mutant melanoma. Right, Differentially enriched HALLMARK gene sets between ALDH1A1hi (in red) and ALDH1A1lo (in blue) cohorts in metastatic BRAF wild-type (WT) melanoma. (B) Comparison between gene sets enriched in ALDH1A3hi metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1hi metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Shared gene sets are listed. (C) Comparison between gene sets enriched in ALDH1A3lo metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1lo metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Shared gene sets are listed. Met-BRAF-mut 1A3, metastatic melanoma with BRAF mutation with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A3; Met-BRAF WT 1A1, metastatic melanoma with WT BRAF with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A1.
The ALDH1A3hi cohorts, which revealed better prognosis (Figure 2A right), showed a significant upregulation of pathways related to a proliferative state (Figure 3B and Supplementary Figure 1A), which has been correlated with increased sensitivity towards BRAF inhibitors [35]. These included epithelial mesenchymal transition (FDR q-value = 0), hypoxia (FDR q-value = 0.0095), angiogenesis (FDR q-value = 0.0011), and glycolysis (FDR q-value = 0.0137).
It has been demonstrated that cancer cells dependent on ATP production through glycolysis are more sensitive to BRAF inhibitors [35]. Consistent with our observation in melanoma patients, ALDH1A3 has been shown to drive anaerobic glycolysis in glioma stem cells [36] and neural progenitor cells [37]. Hypoxia triggered by swift proliferation without proper development of blood vessels has also been shown to induce angiogenesis in melanoma [38, 39]. In addition, BRAF inhibitor treatments have demonstrable efficacy in inhibiting angiogenesis and hypoxia in BRAF-mutant melanomas thereby negating their effects on disease progression [40].
On the contrary, among the top pathways and gene sets significantly enriched in the ALDH1A3lo cohorts with worse prognosis (Figure 2A, right) were oxidative phosphorylation (FDR q-value = 0.001), KRAS signaling (FDR 1-value = 0.037), and fatty acid metabolism (FDR q-value = 0.0417) (Figure 3C and Supplementary Figure 1B).
These pathways and gene sets have been previously implicated in conferring resistance to BRAF- and MEK-inhibitor treatment in melanoma. For example, cancer cells that upregulate oxidative phosphorylation have been shown to be more resistant to BRAF/MEK inhibitor treatments as they rely less on glycolysis for survival and are phenotypically characterized by a slow growing state [41–44]. Activation of KRAS signaling, through KRAS amplification or the acquisition of G12 mutants, are recurrent resistance mechanisms observed in non-small cell lung cancer, and colorectal and thyroid cancers treated with BRAF/MEK inhibitors [45–49]. Fatty acid metabolism has been implicated in cancer stemness and therapeutic resistance in a number of solid tumors through overexpression of genes such as fatty acid synthase, ATP citrate lyase, and acetyl-coA carboxylase [50, 51].
GSEA of ALDH1A1hi and ALDH1A1lo cohorts in metastatic BRAF WT melanoma
Similarly, we performed GSEA comparing ALDH1A1hi and ALDH1A1lo BRAF WT melanoma patients and observed 21 different pathways enriched in the ALDH1A1hl cohort and 14 other pathways enriched in the ALDH1A1lo cohort (Figure 3A right, FDR q-value < 0.1).
The ALDH1A1hi cohorts, which revealed better prognosis (Figure 2B left), were enriched for immune-related responses, such as Interferon Gamma response, allograft rejection, IL6 JAK STAT Signaling, Interferon Alpha Response, and Complement (all FDR q-value = 0) (Figure 3B and Supplementary Figure 1A) [52].
On the other hand, among the top pathways and gene sets significantly enriched in ALDH1A1lo cohorts with worse prognosis (Figure 2A right) were MYC targets, oxidative phosphorylation, E2F Targets, DNA repair, unfolded protein response, and G2M checkpoint (all FDR q-value = 0) (Figure 3C and Supplementary Figure 1B) .
In addition to oxidative phosphorylation, upregulation of MYC target genes has been identified as a convergent pathway for BRAF/MEK inhibitor resistance in melanoma [53]. Albeit not listed in a top group, the PI3K/mTOR signaling axis is also enriched in the ALDH1A1lo cohort (FDR q-value = 0.0223) and has been reported to contribute directly to BRAF/MEK resistance as well [54, 55].
Pathways shared by cohorts with favorable or worse prognosis
To understand signatures related with better prognosis, we analyzed pathways shared by ALDH1A3hi BRAF-mutant melanoma and ALDH1A1hi BRAF WT melanoma, since both cohorts had better prognosis, despite different driver genes. Thirteen pathways were shared by the ALDH1Ahi cohorts with better prognosis (Figure 3B). The gene sets enriched in these cohorts are predominantly immune-stimulating and anti-tumor [52, 56–63], such as Inflammatory Response, IL6 JAK STAT3 Signaling, and TNFa Signaling via NFkB.
Next, to understand signatures related with worse prognosis, we analyzed pathways shared by ALDH1A3lo BRAF-mutant melanoma and ALDH1A1lo BRAF WT melanoma, since these two cohorts had worse prognosis. Three pathways were shared by the ALDH1Alo cohorts, including oxidative phosphorylation, adipogenesis, and fatty acid metabolism (Figure 3C). These pathways have been implicated as tumor-promotive and related to treatment resistance [41–43, 50, 51,59].
These results imply a tumor specific contextual role of ALDH1A1 and ALDH1A3 in melanoma, which is congruent with a report in breast cancer cells [34]. We have previously shown that both ALDH1A1 and ADH1A3 contribute to drug resistance and stem like behavior in melanoma cells [7]. Despite these findings, it is intriguing to observe the correlation with favorable prognosis. A possible explanation for this finding could be their relation to a slow proliferating or semi-quiescent stem-like state. This stem-like state has been linked to phenotypic plasticity associated with unfavorable or stressful tumor microenvironments or drug responses that force the cells to switch into a slow proliferative state to avoid cell death and exhaustion [64–67]. This is supported by the finding that many pathways such as epithelial-mesenchymal transition (EMT) and inflammatory response were upregulated in these ALDH1Ahi cohorts. Accordingly, ALDH1A1 and ALDH1A3 enzymes would be involved in ROS metabolism [68, 69], thus providing cells with survival advantage in stressful microenvironments. However, despite providing survival advantage and cancer stemness in a context-dependent manner, highly expressed ALDH1A1 and ALDH1A3 have the potential to render tumors unable to withstand hostile microenvironments due to distinct factors such as immune response, drug response, or nutrient deprivation, thus correlating with better prognosis.
Distinct pathways among cohorts with favorable or worse prognosis
By comparing distinct gene sets and pathways that are not shared by cohorts with better or worse prognosis, we found that some pathways enriched in the ALDH1A3hi BRAF-mutant cohort with better OS are also enriched in the ALDH1A1lo BRAF WT cohort with worse OS, while conversely, the pathways enriched in ALDH1A1hi BRAF WT cohort with better OS are also enriched in the ALDH1A3lo BRAF-mutant cohort with worse OS (Supplementary Figure 2). For example, unfolded protein response, MTORC signaling, and glycolysis are enriched in ALDH1A3hi BRAF-mutant and ALDH1A1lo BRAF WT cohorts, while KRAS signaling, interferon alpha response, interferon gamma response, and apical surface pathways are enriched in ALDH1A1hi BRAF WT and ALDH1A3lo BRAF-mutant cohorts.
Utilization of these pathways are likely unique within the context of a mutant or WT BRAF melanoma. Because they are shared by the cohorts with opposing OS, they may represent distinct biological phenotypes rather than being directly related with patient prognosis. For example, ALDH1A3 has been shown to drive glycolysis in stem cells [37] and cancer stem cells [36], and together with our results, these data support the general view that certain types of cancer stem cells are more dependent on glycolysis than on oxidative phosphorylation [65].
ALDH1A3 expression correlates with BRAF/MEK inhibitor treatment sensitivity in melanoma
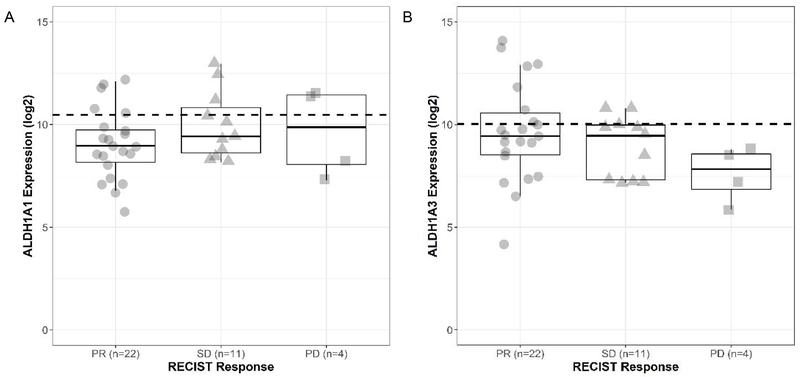
The pathways enriched in the ALDH1A3 BRAF-mutant melanoma cohort (glycolysis, hypoxia and angiogenesis) suggest a correlation between ALDH1A3 expression and BRAF inhibitor sensitivity. Therefore, to determine whether ALDH1A1 or ALDH1A3 expression levels prior to treatment effectively help predict treatment response, we tested ALDH1A1 or ALDH1A3 expression levels using two publicly available gene expression datasets of BRAF-mutant melanoma patients profiled prior to BRAF/MEK inhibitor treatment [27, 28], and correlated the levels with BRAF/MEK inhibitor treatment response defined by RECIST criteria: CR, PR, PD, and SD [29]. The patient demographic information is summarized in Table 3. We found that while there was no trend between BRAF inhibitor treatment response and ALDH1A1 levels (Figure 4A), higher ALDH1A3 levels corresponded to better outcomes (Figure 4B). For example, when ALDH1A3 expression is categorized into groups above and below the 75th percentile, PR had the highest proportion of patients at 36% (8/22), followed by SD 18% (2/11) and lastly PD 0% (0/4) in the cohort above 75th percentile. On the contrary, there was no clear trend in treatment response and patients above the 75th percentile for ALDH1A1: PR had 23% (5/22), SD had 27% (3/11) and PD had 50% (2/4) of patients above this value.
Table 3: Overview of patient demographic information for the BRAF/MEK inhibitor treatment population stratified by treatment response.
Progressive disease (PD), partial responders (PR) and stable disease (SD).
| Treatment Response | ||||
|---|---|---|---|---|
| Variable | PD (N =4) | PR (N=22) | SD (N=11) | |
| BRAF Genotype (%) | ||||
| V600E | 2 (50.0) | 13 (59.1) | 8 (72.7) | |
| V600K | 1 (25.0) | 9 (40.9) | 3 (27.3) | |
| V600R | 1 (25.0) | 0 (0.0) | 0 (0.0) | |
| Drug (%) | ||||
| Dabrafenib | 3 (75.0) | 14 (63.6) | 7 (63.6) | |
| Dabrafenib + Trametinib | 0 (0.0) | 3 (13.6) | 2 (18.2) | |
| Vemurafenib | 1 (25.0) | 5 (22.7) | 2 (18.2) | |
| Age (median [IQR]) | 47.00 [33.50, 62.25] | 57.50 [41.50, 71.00] | 60.00 [50.00, 64.00] | |
| Sex (%) | ||||
| Female | 2 (50.0) | 7 (31.8) | 1 (9.1) | |
| Male | 2 (50.0) | 15 (68.2) | 10 (90.9) | |
| ALDH1A1 (log2) (median [IQR]) | 9.87 [8.06, 11.44] | 8.97 [8.16, 9.74] | 9.42 [8.62, 10.83] | |
| ALDH1A3 (log2) (median [IQR]) | 7.84 [6.84, 8.58] | 9.44 [8.52, 10.57] | 9.45 [7.30, 9.97] | |
Figure 4: Correlation of ALDH1A expression with BRAF/MEK inhibitor treatment responses in melanoma.
(A) Log 2 ALDH1A1 expression before treatment. The horizontal dashed line represents the overall 75th percentile of ALDH1A1 expression. (B) Log 2 ALDH1A3 expression before treatment. The horizontal dashed line represents the overall 75th percentile of ALDH1A3 expression. Patients are stratified by RECIST response. ALDH1A1 levels (A) or ALDH1A3 levels (B) for partial responders (PR, n=22) are shown with circles, stable disease (SD, n=11) with triangles and non-responders with progressive disease (PD, n=4) with squares.
These results suggest that ALDH1A3 expression in BRAF-mutant melanoma patients is not only a prognostic marker but also a predictive marker of treatment response to BRAF/MEK inhibitors. It is possible that the aggressive glycolytic phenotype induced by ALDH1A3 might in turn sensitize tumor cells to BRAF/MEK inhibitor therapy, leading to a favorable prognosis and better treatment response [36]. To the best of our knowledge, no other papers have defined the prognostic and predictive role of ALDH1A3 in BRAF-mutant metastatic melanoma patients.
Common in most retrospective database studies, we were limited by the data provided by TCGA. Therefore, unmeasured clinical and biological variables may confound the effect seen between ALDH expression and patient survival. Furthermore, given there was no clear clinical expression level cutpoint, we used a data-driven method to identify where ALDH expression best separated patients based on their survival. Future work is needed to validate whether these cutpoints hold in a separate patient population. Similarly, given the exploratory nature of this work we did not correct for multiple comparisons and therefore the relationships identified here need to be confirmed in a separate patient population to examine their generalizability to the patient population at large.
In summary, we performed a systematic analysis of ALDH isozyme expressions in a large cohort of melanoma patients, and identified that the ALDH1A3hi and ALDH1A1hi cohorts were associated with better prognosis in BRAF-mutant metastatic melanoma and BRAF WT metastatic melanoma, respectively. We also identified signaling pathways and gene sets enriched in ALDH1Ahi versus ALDH1Alo groups corresponding to better or worse survival outcomes. Using an additional dataset, we could further show that ALDH1A3hi expression in BRAF-mutant melanoma patients was predictive of better responses to BRAF/MEK inhibitor therapy. Deeper investigation will be required to understand the biological and functional roles of ALDH1A3 in BRAF-mutant melanoma and ALDH1A1 in BRAF WT melanoma.
Supplementary Material
Supplementary Figure 1: The prognostic value of ALDH1A1 and ALDH1A3 gene expression in NF1, RAS, and TWT melanoma subtypes. (A) Kaplan Meier plot of: ALDH1A1 (left, ALDH1A1 NF1 MET) and ALDH1A3 (right, ALDH1A3 NF1 MET) for metastatic patients with a NF1 mutation; (B) ALDH1A1 (left, ALDH1A1 RAS MET) and ALDH1A3 (right, ALDH1A3 RAS MET) for metastatic patients with a RAS mutation; and (C) ALDH1A1 (left, ALDH1A1 TWT MET) and ALDH1A3 (right, ALDH1A3 TWT MET) for metastatic patients with triple wild-type (TWT) for BRAF, NF1, and RAS. Patients above the gene expression threshold are shown in red and below in blue. The log-rank test was used to test for differences in overall survival between the two groups of each analysis.
Supplementary Figure 2: Differentially enriched HALLMARK gene sets between ALDH high and low cohorts. (A) Comparison between gene sets enriched in ALDH1A3lo metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1lo metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Gene sets unique to each subgroup are listed. (B) Comparison between gene sets enriched in ALDH1A3lo metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1lo metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Gene sets unique to each subgroup are listed. Met-BRAF-mut 1A3, metastatic melanoma with BRAF mutation with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A3; Met-BRAF WT 1A1, metastatic melanoma with WT BRAF with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A1.
Highlights.
ALDH1A3 expression correlates with better prognosis in BRAF-mut metastatic melanoma
ALDH1A1 expression correlates with better prognosis in BRAF WT metastatic melanoma
GSEA-established BRAF inhibitor resistance pathways are enriched in ALDH1lo groups
ALDH1Alo enriched pathways include Ox Phos, Adipogenesis, and Fatty Acid Metabolism
ALDH1A3 expression can predict BRAF/MEK inhibitor response in BRAF-mutant melanoma
Acknowledgements
We thank grant support by an NIH/NCI R01CA197919 (to M. Fujita), NIH/NCI P30CA046934, Veterans Affairs Merit Review Award 5I01BX001228 (to M. Fujita), Cancer League of Colorado (to M. Fujita), Tadamitsu Cancer Research Fund (to M. Fujita), and the David F. and Margaret T. Grohne Family Foundation (to A.C. Tan). We also thank Joanne Domenico (Dermatology, UCD) for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Voulgaridou GP, et al. , DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat Res, 2011. 711(1-2): p. 13–27. [DOI] [PubMed] [Google Scholar]
- 2.Yin H, Xu L, and Porter NA, Free radical lipid peroxidation: mechanisms and analysis. Chem Rev, 2011. 111(10): p. 5944–72. [DOI] [PubMed] [Google Scholar]
- 3.Storms RW, et al. , Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A, 1999. 96(16): p. 9118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcato P, et al. , Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle, 2011. 10(9): p. 1378–84. [DOI] [PubMed] [Google Scholar]
- 5.Ginestier C, et al. , ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell stem cell, 2007. 1(5): p. 555–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreb JS, Ucar-Bilyeu DA, and Khan A, Use of retinoic acid/aldehyde dehydrogenase pathway as potential targeted therapy against cancer stem cells. Cancer Chemother Pharmacol, 2017. 79(2): p. 295–301. [DOI] [PubMed] [Google Scholar]
- 7.Luo Y, et al. , ALDH1A Isozymes are Markers of Human Melanoma Stem Cells and Potential Therapeutic Targets. Stem Cells, 2012. 30(10): p. 2100–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boonyaratanakornkit JB, et al. , Selection of tumorigenic melanoma cells using ALDH. J Invest Dermatol, 2010. 130(12): p. 2799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santini R, et al. , Hedgehog-GLI signaling drives self-renewal and tumorigenicity of human melanoma-initiating cells. Stem Cells, 2012. 30(9): p. 1808–18. [DOI] [PubMed] [Google Scholar]
- 10.Silva IA, et al. , Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res, 2011. 71(11): p. 3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong B, et al. , A subset of metastatic pancreatic ductal adenocarcinomas depends quantitatively on oncogenic Kras/Mek/Erk-induced hyperactive mTOR signalling. Gut, 2016. 65(4): p. 647–57. [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, et al. , Genome-wide DNA methylation profiling identifies ALDH1A3 promoter methylation as a prognostic predictor in G-CIMP-primary glioblastoma. Cancer Lett, 2013. 328(1): p. 120–5. [DOI] [PubMed] [Google Scholar]
- 13.Ali HR, et al. , Cancer stem cell markers in breast cancer: pathological, clinical and prognostic significance. Breast Cancer Res, 2011. 13(6): p. R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao C, et al. , Essential role of aldehyde dehydrogenase 1A3 for the maintenance of non-small cell lung cancer stem cells is associated with the STAT3 pathway. Clin Cancer Res, 2014. 20(15): p. 4154–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, et al. , HOXA9, ISL1 and ALDH1A3 methylation patterns as prognostic markers for nonmuscle invasive bladder cancer: array-based DNA methylation and expression profiling. Int J Cancer, 2013. 133(5): p. 1135–42. [DOI] [PubMed] [Google Scholar]
- 16.Alamgeer M, et al. , The prognostic significance of aldehyde dehydrogenase 1A1 (ALDH1A1) and CD133 expression in early stage non-small cell lung cancer. Thorax, 2013. 68(12): p. 1095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li XS, et al. , ALDH1A1 overexpression is associated with the progression and prognosis in gastric cancer. BMC Cancer, 2014. 14: p. 705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, et al. , ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: a systematic review and meta-analysis. BMC Cancer, 2014. 14: p. 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, et al. , Distinct prognostic values of ALDH1 isoenzymes in breast cancer. Tumour Biol, 2015. 36(4): p. 2421–6. [DOI] [PubMed] [Google Scholar]
- 20.Shen JX, et al. , Mining distinct aldehyde dehydrogenase 1 (ALDH1) isoenzymes in gastric cancer. Oncotarget, 2016. 7(18): p. 25340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li K, et al. , The prognostic roles of ALDH1 isoenzymes in gastric cancer. Onco Targets Ther, 2016. 9: p. 3405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang CK, et al. , Aldehyde dehydrogenase 1 (ALDH1) isoform expression and potential clinical implications in hepatocellular carcinoma. PLoS One, 2017. 12(8): p. e0182208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma YM and Zhao S, Prognostic values of aldehyde dehydrogenase 1 isoenzymes in ovarian cancer. Onco Targets Ther, 2016. 9: p. 1981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas, N., Genomic Classification of Cutaneous Melanoma. Cell, 2015. 161(7): p. 1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerami E, et al. , The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov, 2012. 2(5): p. 401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao J, et al. , Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal, 2013. 6(269): p. pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizos H, et al. , BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res, 2014. 20(7): p. 1965–77. [DOI] [PubMed] [Google Scholar]
- 28.Kakavand H, et al. , PD-L1 Expression and Immune Escape in Melanoma Resistance to MAPK Inhibitors. Clin Cancer Res, 2017. 23(20): p. 6054–6061. [DOI] [PubMed] [Google Scholar]
- 29.Eisenhauer EA, et al. , New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer, 2009. 45(2): p. 228–47. [DOI] [PubMed] [Google Scholar]
- 30.Subramanian A, et al. , Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A, 2005. 102(43): p. 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liberzon A, et al. , The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst, 2015. 1(6): p. 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasiliou V, Pappa A, and Estey T, Role of Human Aldehyde Dehydrogenases in Endobiotic and Xenobiotic Metabolism. Drug Metabolism Reviews, 2004. 36(2): p. 279–299. [DOI] [PubMed] [Google Scholar]
- 33.Duan J-J, et al. , ALDH1A3, a metabolic target for cancer diagnosis and therapy. International Journal of Cancer, 2016. 139(5): p. 965–975. [DOI] [PubMed] [Google Scholar]
- 34.Croker AK, et al. , Differential Functional Roles of ALDH1A1 and ALDH1A3 in Mediating Metastatic Behavior and Therapy Resistance of Human Breast Cancer Cells. International Journal of Molecular Sciences, 2017. 18(10): p. 2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardeman KN, et al. , Dependence On Glycolysis Sensitizes BRAF-mutated Melanomas For Increased Response To Targeted BRAF Inhibition. Sci Rep, 2017. 7: p. 42604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao P, et al. , Mesenchymal glioma stem cells are maintained by activated glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc Natl Acad Sci U S A, 2013. 110(21): p. 8644–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.La Rosa P, et al. , Sam68 promotes self-renewal and glycolytic metabolism in mouse neural progenitor cells by modulating Aldh1a3 pre-mRNA 3′-end processing. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rofstad EK and Danielsen T, Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. Br J Cancer, 1999. 80(11): p. 1697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rofstad EK and Danielsen T, Hypoxia-induced angiogenesis and vascular endothelial growth factor secretion in human melanoma. Br J Cancer, 1998. 77(6): p. 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bottos A, et al. , Targeting oncogenic serine/threonine-protein kinase BRAF in cancer cells inhibits angiogenesis and abrogates hypoxia. Proc Natl Acad Sci U S A, 2012. 109(6): p. E353–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roesch A, et al. , Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell, 2013. 23(6): p. 811–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang G, et al. , Targeting mitochondrial biogenesis to overcome drug resistance to MAPK inhibitors. J Clin Invest, 2016. 126(5): p. 1834–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corazao-Rozas P, et al. , Mitochondrial oxidative phosphorylation controls cancer cell’s life and death decisions upon exposure to MAPK inhibitors. Oncotarget, 2016. 7(26): p. 39473–39485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cesi G, et al. , ROS production induced by BRAF inhibitor treatment rewires metabolic processes affecting cell growth of melanoma cells. Mol Cancer, 2017. 16(1): p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bahcall M, et al. , Amplification of Wild-type <em>KRAS</em> Imparts Resistance to Crizotinib in <em>MET</em> Exon 14 Mutant Non-Small Cell Lung Cancer. Clinical Cancer Research, 2018. 24(23): p. 5963–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzawa K, et al. , Activation of KRAS Mediates Resistance to Targeted Therapy in MET Exon 14-mutant Non-small Cell Lung Cancer. Clinical Cancer Research, 2019. 25(4): p. 1248–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahronian LG, et al. , Clinical Acquired Resistance to RAF Inhibitor Combinations in <em>BRAF</em>-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discovery, 2015. 5(4): p. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sale MJ, et al. , MEK1/2 inhibitor withdrawal reverses acquired resistance driven by BRAFV600E amplification whereas KRASG13D amplification promotes EMT-chemoresistance. Nature Communications, 2019. 10(1): p. 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danysh BP, et al. , Long-term vemurafenib treatment drives inhibitor resistance through a spontaneous KRAS G12D mutation in a BRAF V600E papillary thyroid carcinoma model. Oncotarget, 2016. 7(21): p. 30907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuo C-Y and Ann DK, When fats commit crimes: fatty acid metabolism, cancer stemness and therapeutic resistance. Cancer Communications, 2018. 38(1): p. 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z and Zhang H, Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cellular and Molecular Life Sciences, 2016. 73(2): p. 377–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castro F, et al. , Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol, 2018. 9: p. 847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singleton KR, et al. , Melanoma Therapeutic Strategies that Select against Resistance by Exploiting MYC-Driven Evolutionary Convergence. Cell Rep, 2017. 21(10): p. 2796–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greger JG, et al. , Combinations of BRAF, MEK, and PI3K/mTOR inhibitors overcome acquired resistance to the BRAF inhibitor GSK2118436 dabrafenib, mediated by NRAS or MEK mutations. Mol Cancer Ther, 2012. 11(4): p. 909–20. [DOI] [PubMed] [Google Scholar]
- 55.Villanueva J, et al. , Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell, 2010. 18(6): p. 683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu Q, et al. , Prognostic significance of SOCS3 and its biological function in colorectal cancer. Gene, 2017. 627: p. 114–122. [DOI] [PubMed] [Google Scholar]
- 57.Long KB, et al. , IL6 Receptor Blockade Enhances Chemotherapy Efficacy in Pancreatic Ductal Adenocarcinoma. Mol Cancer Ther, 2017. 16(9): p. 1898–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Floros T and Tarhini AA, Anticancer Cytokines: Biology and Clinical Effects of Interferon-alpha2, Interleukin (IL)-2, IL-15, IL-21, andIL-12. Semin Oncol, 2015. 42(4): p. 539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slattery ML, et al. , JAK/STAT/SOCS-signaling pathway and colon and rectal cancer. Mol Carcinog, 2013. 52(2): p. 155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tagliaferri P, et al. , New pharmacokinetic and pharmacodynamic tools for interferon-alpha (IFN-alpha) treatment of human cancer. Cancer Immunol Immunother, 2005. 54(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferrantini M, Capone I, and Belardelli F, Interferon-alpha and cancer: mechanisms of action and new perspectives of clinical use. Biochimie, 2007. 89(6-7): p. 884–93. [DOI] [PubMed] [Google Scholar]
- 62.Afshar-Kharghan V, The role of the complement system in cancer. J Clin Invest, 2017. 127(3): p. 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bareke H and Akbuga J, Complement system’s role in cancer and its therapeutic potential in ovarian cancer. Scand J Immunol, 2018. 88(1): p. e12672. [DOI] [PubMed] [Google Scholar]
- 64.Taylor LA, et al. , High ALDH1 expression correlates with better prognosis in tumorigenic malignant melanoma. Mod Pathol, 2017. 30(5): p. 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soehngen E, et al. , Hypoxia upregulates aldehyde dehydrogenase isoform 1 (ALDH1) expression and induces functional stem cell characteristics in human glioblastoma cells. Brain Tumor Pathol, 2014. 31(4): p. 247–56. [DOI] [PubMed] [Google Scholar]
- 66.Sasaki Y, et al. , Insulin response to glucose and glucose tolerance following feeding in sheep. Br J Nutr, 1984. 52(2): p. 351–8. [DOI] [PubMed] [Google Scholar]
- 67.Moore N and Lyle S, Quiescent, Slow-Cycling Stem Cell Populations in Cancer: A Review of the Evidence and Discussion of Significance. Journal of Oncology, 2011. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pérez-Alea M, et al. , ALDH1A3 is epigenetically regulated during melanocyte transformation and is a target for melanoma treatment. Oncogene, 2017. 36: p. 5695. [DOI] [PubMed] [Google Scholar]
- 69.Allison SE, et al. , Activation of ALDH1A1 in MDA-MB-468 breast cancer cells that over-express CYP2J2 protects against paclitaxel-dependent cell death mediated by reactive oxygen species. Biochemical Pharmacology, 2017. 143: p. 79–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: The prognostic value of ALDH1A1 and ALDH1A3 gene expression in NF1, RAS, and TWT melanoma subtypes. (A) Kaplan Meier plot of: ALDH1A1 (left, ALDH1A1 NF1 MET) and ALDH1A3 (right, ALDH1A3 NF1 MET) for metastatic patients with a NF1 mutation; (B) ALDH1A1 (left, ALDH1A1 RAS MET) and ALDH1A3 (right, ALDH1A3 RAS MET) for metastatic patients with a RAS mutation; and (C) ALDH1A1 (left, ALDH1A1 TWT MET) and ALDH1A3 (right, ALDH1A3 TWT MET) for metastatic patients with triple wild-type (TWT) for BRAF, NF1, and RAS. Patients above the gene expression threshold are shown in red and below in blue. The log-rank test was used to test for differences in overall survival between the two groups of each analysis.
Supplementary Figure 2: Differentially enriched HALLMARK gene sets between ALDH high and low cohorts. (A) Comparison between gene sets enriched in ALDH1A3lo metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1lo metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Gene sets unique to each subgroup are listed. (B) Comparison between gene sets enriched in ALDH1A3lo metastatic BRAF-mutant melanoma cohort (shown in Fig. 3A, left panel) and ALDH1A1lo metastatic BRAF WT melanoma cohort (shown in Fig. 3A, right panel). Gene sets unique to each subgroup are listed. Met-BRAF-mut 1A3, metastatic melanoma with BRAF mutation with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A3; Met-BRAF WT 1A1, metastatic melanoma with WT BRAF with hi (Fig. 3B) or lo (Fig. 3C) ALDH1A1.