Abstract
Purpose
This first-in-human Phase I study investigated the safety, pharmacokinetics (PK), pharmacodynamic profile, and preliminary efficacy of CC-115, a dual inhibitor of mammalian target of rapamycin (mTOR) kinase and DNA-dependent protein kinase.
Patients and Methods
Patients with advanced solid or hematologic malignancies were enrolled in dose-finding and cohort expansion phases. In dose-finding, once-daily or twice-daily (BID) ascending oral doses of CC-115 (range: 0.5–40 mg/day) in 28-day continuous cycles identified the maximum-tolerated dose for cohort expansion in 5 specified tumor types. Twelve additional patients with mixed solid tumors participated in a bioavailability substudy.
Results
Forty-four patients were enrolled in the dose-finding cohort. Dose-limiting toxicity included thrombocytopenia, stomatitis, hyperglycemia, asthenia/fatigue, and increased transaminases. CC-115 10 mg BID was selected for cohort expansion (n=74) in which fatigue, nausea, and decreased appetite were the most frequent toxicities. Dose-proportional PK was found. CC-115 distributed to glioblastoma tissue (mean tumor/plasma concentration ratio: 0.713). Total exposure of CC-115 was similar under fasting and fed conditions. A patient with endometrial carcinoma remained in complete remission >4 years. Partial response (PR; n=2) and stable disease (SD; n=4) were reported in the bioavailability substudy; SD was reached in 53%, 22%, 21%, and 64% of patients with head and neck squamous cell carcinoma, Ewing sarcoma, glioblastoma multiforme, and castration-resistant prostate cancer, respectively. Chronic lymphocytic leukemia/small lymphocytic lymphoma showed 38% PR and 25% SD.
Conclusion
CC-115 was well-tolerated, with toxicities consistent with mTOR inhibitors. Together with biomarker inhibition and preliminary efficacy, oral CC-115 10 mg BID is a promising novel anticancer treatment.
Clinical trial registration
Keywords: CC-115, mTORC1/mTORC2, mTOR inhibitor, DNA-PK inhibitor, Phase I study
Introduction
The frequency of inappropriate activation of the mammalian target of the rapamycin (mTOR)-signaling pathway observed in many cancers via receptor tyrosine kinases and somatic mutations in specific components of the signaling pathway supports use of mTOR inhibitors as treatments.1,2 Rapamycin analogs block mTOR complex 1 (mTORC1) and, thus, cell proliferation. However, a second critical mediator of the PI3K/AKT pathway, mTOR complex 2 (mTORC2), is also activated in the process.3–5 mTORC2 has been shown to activate AKT through phosphorylation of S473, a site necessary for maximal kinase activity.5 Studies have shown that compared to single mTORC1 and mTORC2 suppression, dual inhibition leads to a higher decrease in cancer cell proliferation in preclinical studies.6,7 Thus, molecules inhibiting both mTORC1 and mTORC2 are interesting candidates for anticancer treatment.8–12 Results from a Phase I study of the dual mTORC1/mTORC2 inhibitor CC-223 demonstrated that it was tolerable, with manageable toxicity,8 and showed that CC-223 has a safety profile similar to other dual mTORC1/mTORC2 inhibitors.8,13,14
CC-115 is a dual mTORC1/mTORC2 inhibitor with favorable pharmacokinetic properties.15 In addition to inhibiting both mTORC1 and mTORC2, CC-115 also inhibits DNA-dependent protein kinase (DNA-PK).15–18 DNA-PK is a serine/threonine kinase that phosphorylates AKT in response to DNA damage, and is essential for the repair of double-strand DNA mediated by nonhomologous end-joining (NHEJ).19–22 CC-115 has been shown to inhibit the autophosphorylation of the catalytic subunit of DNA-PK, leading to the inhibition of NHEJ.22 Unless repaired, agents that break double-stranded DNA are lethal and are frequently effective anticancer drugs; thus, DNA-PK inhibitors have potential as cancer therapeutics.19,23–28 Preclinical studies demonstrated that the inhibitory activity of CC-115 is associated with potent antitumor activity in a number of solid tumor and hematopoietic cancer cell lines, along with induction of apoptosis in a subset of these cells.22 Furthermore, CC-115 induced cytotoxicity and blocked signaling pathways important for survival, proliferation, and drug resistance in chronic lymphocytic leukemia (CLL) cell lines with or without ATM/11q mutations.18
Herein, we describe the complete results for the first-in-human Phase I study evaluating CC-115 in solid and hematologic cancers.
Materials And Methods
Study Design And Patient Selection
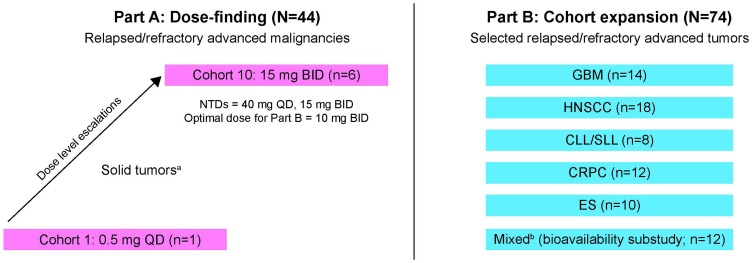
This Phase Ia/Ib study investigating CC-115 consisted of 2 parts: a sequential dose-escalation phase (Part A) to determine the maximum-tolerated dose (MTD) in unselected tumor types, followed by cohort expansion (Part B) that evaluated prespecified tumor types in parallel cohorts (Figure 1). The tumor types evaluated in Part B were selected based on results from preclinical studies18,22 and preliminary data from Part A. The primary objectives were to determine the safety and tolerability, as well as the pharmacokinetics (PK) of CC-115. Secondary objectives were to evaluate the pharmacodynamic properties of CC-115 and preliminary efficacy.
Figure 1.
Study design. In Part A, an accelerated titration design was used to establish toxicity and QD and BID dosing were evaluated. Initial cohorts of 1 patient each were administered CC-115 at dose increments of 100% in 28-day cycles until ≥grade 2 toxicity, after which a standard escalation dosing schedule with approximately 50% dose increments and 6 patients per cohort was initiated. In Part B, patients with protocol-specified tumors received CC-115 10 mg BID. aGI (25%), sarcoma (14%), biliary, breast, GYN, lung, NET, skin (7% each), CNS, renal, other (4% each), endocrine, GU, pancreas (2% each). bBreast (5 patients), ovary (2 patients), NSCLC, PEComa, CRC, thyroid, sarcoma (1 patient each).
Abbreviations: BID, twice daily; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CNS, central nervous system; CRC, colorectal cancer; CRPC, castration-resistant prostate cancer; ES, Ewing sarcoma; GBM, glioblastoma multiforme; GI, gastrointestinal; GU, genitourinary; GYN, gynecological; HNSCC, head and neck squamous cell carcinoma; NET, neuroendocrine tumor; NSCLC, non-small cell lung cancer; PEComa, perivascular epithelioid cell neoplasms; QD, once daily.
Adults with histologic or cytologic confirmation of advanced solid or hematologic malignancies, who had progressed on standard anticancer therapy, were eligible for Part A of the study. An Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 or 1 and fulfillment of prespecified laboratory criteria (absolute neutrophil count [ANC] ≥1.5 × 109/L; hemoglobin ≥9 g/dL; platelet count ≥100 × 109/L; aspartate aminotransferase and alanine aminotransferase ≤2.5 × upper limit of normal [ULN] or ≤5.0 × ULN if liver tumor was present; total bilirubin ≤1.5 × ULN or ≤2.0 × ULN if liver tumor was present; and serum creatinine ≤1.5 × ULN) were required for enrollment. Patients with symptomatic central nervous system metastases, diabetes mellitus requiring active treatment or with poorly managed hyperglycemia, significant or unstable comorbidity likely to confound evaluations, or incomplete recovery from adverse events (AEs) associated with prior anticancer treatment were excluded.
Based on preclinical studies and results from Part A, Part B enrolled up to 20 patients per tumor type into cohorts with histologically confirmed head and neck squamous cell cancer (HNSCC), glioblastoma multiforme (GBM), chronic lymphocytic leukemia or small lymphocytic lymphoma (SLL), E26 transformed specific (ETS)-positive castration-resistant prostate cancer (CRPC), or Ewing sarcoma (ES). The HNSCC cohort required ≥1 prior line of platinum-based chemotherapy. Patients with GBM were enrolled only if salvage tumor resection was planned approximately 2 weeks into therapy (to enable tumor sampling) and no prior carmustine implant (Gliadel® Wafer; Eisai Inc, Woodcliff Lake, NJ) or other localized therapy within the area of study assessment had been received. For chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), deletion of chromosome 11q22 (ATM) detected by fluorescence in situ hybridization in CLL cells was required, and hematologic criteria included ANC ≥1.0 × 109/L and platelet count ≥30 × 109/L. Castrate-resistant prostate cancer (CRPC) required confirmation ETS gene fusion or overexpression and serum testosterone <50 ng/dL. Consent was required for collection of archival tumor and paired (pretreatment and on-treatment) biopsies.
This study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Guideline E6, and all necessary local committee oversight (Supplemental Table 1). Study participants provided written informed consent before enrolling. A Safety Review Committee made decisions regarding patient welfare and dose level changes.
Drug Treatment
For Part A, patients were enrolled into once-daily (QD) or twice-daily (BID) ascending oral dose cohorts of CC-115 taken in 28-day continuous cycles in a fasting state until unacceptable toxicity, disease progression, or patient withdrawal. The starting dose in Part A was 0.5 mg QD and, using a modified accelerated design, dose increments of 100% were administered to single-patient cohorts until cycle 1 grade ≥2 toxicity was observed, at which point that cohort and subsequent cohorts were expanded to enroll 6 patients.29 Escalation proceeded in approximately 50% dose increments thereafter to determine the non-tolerated dose (NTD) and MTD. Any grade ≥3 CC-115-related AE that necessitated dose reduction during cycle 1 was considered a dose-limiting toxicity (DLT). Additional hematologic and nonhematologic DLT criteria were specified in the protocol (Supplemental Material). The NTD was determined when ≥2 of 6 evaluable patients experienced a DLT in a given cohort, after which dose escalation was stopped. The MTD was the last dose level below the NTD with ≤1 of 6 evaluable patients experiencing a DLT.
Due to the ultraviolet light-absorbing (range: 290–700 nm) properties of CC-115, patients were counseled to avoid prolonged exposure to sunlight. Caution was recommended with regard to concurrent administration of CC-115 with potent CYP3A4 and CYP2C8 inhibitors and with CYP2C8/3A4 inducers, because of potential drug–drug interactions.
Bioavailability/Food Effect Substudy
A bioavailability/food effect (BA/FE) substudy in 12 adult patients with any solid tumor except GBM was included to characterize and evaluate the PK of CC-115 administered as a single 10-mg oral dose of the tablet or capsule under fasted conditions. After a 2- to 7-day washout period, patients were administered CC-115 as a single oral dose taken with a high-fat meal to characterize the effect of food on the PK of CC-115.
Safety Evaluations
The tolerability of CC-115 was evaluated until 28 days after the last dose by monitoring clinical events and laboratory tests, including measures of thyroid and pancreatic function, serum creatine kinase, glucose, C-peptide and glycosylated hemoglobin, blood lipids, immunoglobulins, and T-cell function (CD4+ and CD8+ subsets). Fasting glucose was measured in cycle 1 (days 1, 8, 15, 22), and in subsequent cycles on days 1 and 15, as necessary. Patients self-monitored blood glucose at least once daily. Other serial safety assessments comprised left ventricular ejection fraction, ECOG PS, physical exams, and routine vital signs. Serial triplicate electrocardiograms (ECGs) were evaluated centrally with a high-resolution semi-automated on-screen caliper method. All AEs were coded with Medical Dictionary for Regulatory Activities (MedDRA) Version 16.1 (Part A) and Version 18.0 (Part B), and severity was classified using the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.30
Dose-limiting toxicities were described as a clinically relevant laboratory abnormality that is suspected to be related to CC-115, that commences within 28 days of first dose (cycle 1), and is grade ≥3, or any grade 4 laboratory abnormality that is suspected to be related to CC-115 regardless of clinical relevance; hyperglycemia that is grade 2, fasting hyperglycemia (>160 mg/dL) lasting >14 days despite optimal medical treatment, grade ≥3 fasting hyperglycemia (>250 mg/dL) lasting >4 days despite optimal medical treatment, grade 4 hyperglycemia lasting ≥12 hrs despite adequate treatment, hyperglycemia associated with diabetic ketoacidosis or nonketotic hyperosmolar coma regardless of glucose level, and hyperglycemia that necessitates dose reduction despite dose interruption and resolution to grade ≤1 hyperglycemia within 2 weeks; hematologic toxicities, including any febrile neutropenia, grade 4 neutropenia lasting >7 days, grade 4 thrombocytopenia of any duration, grade 3 thrombocytopenia with clinically significant bleeding. Grade 4 liver function tests (LFTs) were considered DLTs irrespective of underlying attribution, and grade 3 LFTs with objective radiological evidence of disease progression in the liver were not considered DLTs.
Pharmacokinetic And Pharmacodynamic Assessments, Biomarker Analyses, And Tumor Biopsies
In plasma and urine, CC-115 concentration was assayed using validated liquid chromatography/mass spectrometry assays. The lower limits of quantitation were 0.1 ng/mL (Part A) and 1.0 ng/mL (Part B) in plasma, 0.5 ng/mL in urine (Part A). Blood was collected predose and serially up to 24 hrs postdose on days 1, 15, 22 (Part A only) of cycle 1 and near the time of tumor biopsy on treatment (days 15–21). Noncompartmental analyses were performed using Phoenix WinNonlin Version 6.3 (Pharsight Corp., Mountain View, CA).
Biomarkers to evaluate target inhibition were p4E-BP1.T37/T46 (for mTORC1), pAKT.S473 (for mTORC2), and pDNA-PK.S2056 (for DNA-PK) and assessed predose, up to 5 hrs postdose on days 1 and 15 of cycle 1, and around tumor biopsy (days 15–21). During Part B, p4E-BP1 and pAKT inhibition were measured in stimulated (anti-IgD-immunoglobulin D plus lipopolysaccharide, 37°C, 15 mins) CD14+ monocytes for solid tumor cohorts, and in stimulated CD19+/CD5− lymphocytes, CD3+ lymphocytes, and CD5+/CD19+ lymphocytes for CLL using flow cytometry. Results were expressed as molecules of equivalent fluorescein.
In Part B, patients with GBM underwent mandatory salvage resection on cycle 1 between days 15 and 21 to provide biopsy samples.
Efficacy Evaluations
Solid tumors were restaged with computed tomography or magnetic resonance imaging on completion of cycles 2, 4, 6, 9, 12, every 6 cycles thereafter, and at the end of study treatment. Response by investigators was based on relevant criteria, including Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, Response Assessment for Neuro-Oncology (RANO) Working Group criteria, updated criteria of the International Workshop on Chronic Lymphocytic Leukemia (IWCLL), and the Prostate Cancer Working Group 2 (PCWG2) criteria. Patients meeting IWCLL criteria in lymph nodes, spleen, liver, and/or bone marrow, but with persistent lymphocytosis, were categorized as non-IWCLL.31–33
Statistical Analysis
This study was not powered for inferential statistical analysis. All patients taking ≥1 dose of CC-115 were included in the treated population for safety data analyses. All patients who took ≥70% of study drug in cycle 1 and had a baseline and ≥1 postbaseline tumor assessment were included in the efficacy evaluable population. Descriptive summary statistics were used for continuous variables, while frequency tabulations and percentages described discrete variables, unless otherwise specified. Best overall tumor response frequency was tabulated. Waterfall plots showed best percentage change from baseline in the sum of longest diameters of target lesions. All statistical analyses were conducted using SAS Version 9.2 or higher (SAS Institute Inc, Cary, NC).
Results
Patient Characteristics And Treatment
A total of 118 patients were enrolled at 18 sites in the United States and Europe in both the dose-finding (Part A) and expansion (Part B) cohorts. Data cutoff dates were December 2013 (Part A), April 2015 (Part B, excluding the CLL/SLL population), and June 2017 (Part B, CLL/SLL population only). Among 44 patients heavily pretreated in Part A, 57% were female, 77% were white, and 77% were ≤65 years (Table 1). Gastrointestinal cancers were most common (25%); followed by sarcomas (14%); biliary, gynecological, breast, lung, neuroendocrine, and skin cancers (7% each). In dose escalation, CC-115 was administered in 8 cohorts evaluating doses from 0.5 to 40 mg QD, then in 2 cohorts for 10 and 15 mg BID. The median treatment duration was 49 days (range: 10–907). Most patients discontinued treatment due to progressive disease (PD) (59%), withdrawal of consent (11%), AEs (7%), or other reasons (9%). One patient died from PD while on study.
Table 1.
Baseline Demographics And Clinical Characteristics
| Baseline Parameter | Dose Finding | Cohort Expansion | |||||||
|---|---|---|---|---|---|---|---|---|---|
| QD(n = 32) | BID(n = 12) | Overall (N = 44) | HNSCC (n = 18) | ES(n = 10) | BA/FE(n = 12) | GBM(n = 14) | CRPC(n = 12) | CLL/SLL (n = 8) | |
| Age, years | |||||||||
| Median (min, max) | 54 (22, 75) | 62 (42, 71) | 56 (22, 75) | 59 (27, 73) | 39 (19, 55) | 64 (41, 78) | 56 (34, 66) | 71 (55, 74) | 56 (50, 75) |
| ≤65 years, n (%) | 27 (84) | 7 (58) | 34 (77) | 15 (83) | 10 (100) | 6 (50) | 13 (93) | 3 (25) | 7 (88) |
| >65 years, n (%) | 5 (16) | 5 (42) | 10 (23) | 3 (17) | 0 | 6 (50) | 1 (7) | 9 (75) | 1 (13) |
| Sex, n (%) | |||||||||
| Male | 15 (47) | 4 (33) | 19 (43) | 13 (72) | 8 (80) | 4 (33) | 10 (71) | 12 (100) | 5 (63) |
| Female | 17 (53) | 8 (67) | 25 (57) | 5 (28) | 2 (20) | 8 (67) | 4 (29) | 0 | 3 (38) |
| Race, n (%) | |||||||||
| Asian | 7 (22) | 0 | 7 (16) | 0 | 0 | 0 | 0 | 0 | 0 |
| African American | 2 (6) | 0 | 2 (5) | 0 | 0 | 1 (8) | 1 (7) | 0 | 0 |
| White | 22 (69) | 12 (100) | 34 (77) | 18 (100) | 10 (100) | 10 (83) | 12 (86) | 12 (100) | 8 (100) |
| Other | 1 (3) | 0 | 1 (2) | 0 | 0 | 1 (8) | 1 (7) | 0 | 0 |
| BMI (kg/m2) | |||||||||
| Median (min, max) | 24.1 (17.2, 48.3) | 25.8 (18.4, 35.8) | 24.6 (17.2, 48.3) | 23.8 (14.6, 27.0) | 26.4 (16.0, 33.6) | 25.6 (21.8, 34.1) | 25.1 (21.2, 30.8) | 29.3 (23.2, 36.8) | 27.1 (21.6, 30.0) |
| ECOG PS, n (%) | |||||||||
| 0 | 17 (53) | 5 (42) | 22 (50) | 1 (6) | 6 (60) | 1 (8) | 7 (50) | 2 (17) | 6 (75) |
| 1 | 15 (47) | 7 (58) | 22 (50) | 17 (94) | 4 (40) | 11 (92) | 7 (50) | 10 (83) | 2 (25) |
| Prior anticancer regimens, n (%) | |||||||||
| ≤3 regimens | 13 (41) | 6 (50) | 19 (43) | 12 (67) | 4 (40) | 3 (25) | 13 (93) | 9 (75) | 5 (63) |
| ≥4 regimens | 16 (50) | 6 (50) | 22 (50) | 6 (33) | 6 (60) | 9 (75) | 1 (7) | 3 (25) | 3 (38) |
| Prior radiation | 18 (56) | 6 (50) | 24 (55) | 17 (94) | 9 (90) | 10 (83) | 14 (100) | 9 (75) | 0 |
Abbreviations: BA/FE, bioavailability/food effect (substudy); BID, twice daily; BMI, body mass index; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CRPC, castration-resistant prostate cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; ES, Ewing sarcoma; GBM, glioblastoma multiforme; HNSCC, head and neck squamous cell carcinoma; QD, once daily.
Among 74 patients in Part B, including 12 patients from the BA/FE sub-study, median age ranged from 39 to 71 years among the different tumor cohorts (Table 1). Median age in patients with CLL/SLL was 56 years (range: 50–75). Overall, 38% had received ≥4 prior systemic anticancer regimens. The median treatment duration in days among the cohorts at the April 2015 cutoff was 65 (HNSCC), 57 (GBM), 66 (CRPC), 54 (ES), and 93 (BA/FE), and the majority of patients (range: 42–90%) discontinued treatment due to PD. Because of PD, 2 patients from the HNSCC and BA/FE groups died during the study. The CLL/SLL population had a median treatment duration of 406 days (range: 21–1195). The majority of patients with CLL/SLL discontinued treatment secondary to PD (38%), while other reasons (25%) and intolerable AEs (13%) were also reported.
Safety And Tolerability
During Part A, 93% of patients experienced ≥1 drug-related AE (Table 2). Most common were fatigue (50%), vomiting and nausea (41% each), decreased appetite (36%), diarrhea (25%), and hyperglycemia (23%). Grade 3 toxicities were reported by 41% of patients; most frequent were fatigue and hypophosphatemia (7% each) and maculopapular rash, stomatitis, and hypertriglyceridemia (5% each). There were no drug-related grade 4 toxicities. From initially assigned dose levels, 39% of patients required dose reduction, with an average 51 days time to reduction due to an AE. Drug interruption was required for 93% of patients, with an average 56 days time to interruption due to an AE. Dose-limiting toxicities were identified in 1 patient (thrombocytopenia) at 16 mg QD, 1 patient (stomatitis) at 25 mg QD, and in 2 of 6 patients at 40 mg QD (hyperglycemia, asthenia, and fatigue). Thus, 25 mg QD was established as the MTD and 40 mg QD as the NTD. Within the 2 BID cohorts, 2 DLTs were identified at 15 mg BID (increased transaminases and stomatitis), and this was determined to be the NTD. Thus, 10 mg BID was the MTD and determined to be the most appropriate schedule for Part B, and patients received continuous CC-115 10 mg daily in 28-day cycles.
Table 2.
Common (incidence ≥10%) Drug-Related Adverse Events, By Body System Organ Class And CC-115 Exposure
| Adverse Event, n (%) | Dose Finding | Cohort Expansiona | |||||
|---|---|---|---|---|---|---|---|
| Overall (N = 44) |
HNSCC (n = 18) |
ES (n = 10) |
BA/FE (n = 12) |
GBM (n =14) |
CRPC (n = 12) |
CLL/SLL (n = 8) | |
| Patients with at least one related AE | 41 (93) | 16 (89) | 9 (90) | 11 (92) | 12 (86) | 12 (100) | 8 (100) |
| Gastrointestinal disorders | 33 (75) | <bold>10 (56)</bold> | 6 (60) | 9 (75) | 8 (57) | 9 (75) | 2 (25) |
| Diarrhea | 11 (25) | 4 (22) | 0 | 7 (58) | 3 (21) | 2 (17) | 1 (13) |
| Nausea | 18 (41) | 4 (22) | 5 (50) | 6 (50) | 4 (29) | 4 (33) | 2 (25) |
| Stomatitis | 9 (21) | 1 (6) | 2 (20) | 4 (33) | 2 (14) | 2 (17) | 1 (13) |
| Vomiting | 18 (41) | 0 | 1 (10) | 4 (33) | 1 (7) | 2 (17) | 0 |
| General disorders | 25 (57) | 12 (67) | 5 (50) | 7 (58) | 5 (36) | 9 (75) | 2 (25) |
| Fatigue | 22 (50) | 8 (44) | 3 (30) | 5 (42) | 5 (36) | 7 (58) | 0 |
| Asthenia | 2 (5) | 3 (17) | 2 (20) | 3 (25) | 0 | 1 (8) | 0 |
| Investigations | 16 (36) | 8 (44) | 4 (40) | 5 (42) | 1 (7) | 6 (50) | 2 (25) |
| Weight decreased | 7 (16) | 7 (39) | 1 (10) | 1 (8) | 0 | 1 (8) | 2 (25) |
| Metabolism and nutrition disorders | 25 (57) | 7 (39) | 7 (70) | 9 (75) | 6 (43) | 7 (58) | 4 (50) |
| Decreased appetite | 16 (36) | 5 (28) | 3 (30) | 4 (33) | 2 (14) | 6 (50) | 2 (25) |
| Hyperglycemia | 10 (23) | 4 (22) | 3 (30) | 1 (8) | 3 (21) | 4 (33) | 1 (13) |
| Nervous system disorders | 9 (21) | 2 (11) | 1 (10) | 2 (17) | 1 (7) | 2 (17) | 0 |
| Dysgeusia | 6 (14) | 0 | 0 | 1 (8) | 1 (7) | 2 (17) | 0 |
| Skin and subcutaneous tissue disorders | 18 (41) | 4 (22) | 6 (60) | 6 (50) | 6 (43) | 6 (50) | 4 (50) |
| Rash | 8 (18) | 2 (11) | 1 (10) | 2 (17) | 2 (14) | 3 (25) | 1 (13) |
| Rash maculopapular | 7 (16) | 1 (6) | 3 (30) | 1 (8) | 2 (14) | 1 (8) | 0 |
| Patients with at least one related G3 AE | 18 (41) | 4 (22) | 0 | 6 (50) | 2 (14) | 4 (33) | 3 (38) |
| Median CC-115 duration (days) | 49 | 65 | 54 | 93 | 57 | 66 | 406 |
| Median relative dose intensityb | 0.9 | 0.98 | 1.0 | 0.72 | 0.89 | 0.96 | 0.98 |
| Patients with ≥1 dose reduction | 17(39) | 14 (78) | 4 (40) | 7 (58) | 13 (93) | 8 (67) | 3 (38) |
| Patients with ≥1 dose interruption | 41 (93) | 14 (78) | 5 (50) | 12 (100) | 14 (100) | 11 (92) | 8 (100) |
Notes: aHNSCC, ES, BA/FE, GBM, and CRPC cohorts from April 2015 data cut; CLL/SLL cohort from June 2017 data cut. bRelative dose intensity is actual/expected drug exposure.
Abbreviations: AE, adverse event; BA/FE, bioavailability/food effect (substudy); CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CRPC, castration-resistant prostate cancer; ES, Ewing sarcoma; G3, grade 3; GBM, glioblastoma multiforme; HNSCC, head and neck squamous cell carcinoma.
During Part B, the majority of patients experienced ≥1 drug-related AE, and the most common were fatigue, nausea, decreased appetite, diarrhea, and hyperglycemia (Table 2). Grade 3 drug-related AEs were reported in 22%, 14%, 33%, 0%, and 50% in the HNSCC, GBM, CRPC, ES, and BA/FE cohorts, respectively. No grade 4 toxicities were reported. Overall, 40–93% of patients required ≥1 dose reduction, but less than 50% were due to AEs. Dose reductions were highest in patients with GBM (93%). The frequency of dose interruptions ranged from 50% to 100% among the tumor cohorts. Grade 3 drug-related AEs were reported in 38% of patients with CLL/SLL and 38% required dose reductions, 2% of which were due to AEs. The median time to the first dose reduction in the CLL/SLL population was 373 days.
Pharmacokinetics And Pharmacodynamics
CC-115 was rapidly absorbed, with peak plasma concentrations (Cmax) observed at 30 mins to 3 hrs after single- and multiple-dose administration, declining in a monophasic manner over the dose range evaluated (Figure 2). Exposure indices (Cmax and area under the concentration–time curve from 0 to 24 hrs [AUC24]) exhibited moderate to high interpatient variability after single- and multiple-dose administrations, with geometric coefficients of variation (CV%) ranging from approximately 23% to 149% (Table 3). Generally, CC-115 Cmax and area under the concentration–time curve from time 0 extrapolated to infinity (AUC∞) increased in a linear, dose-proportional manner after single and multiple doses ranging from 4 to 40 mg. Less than 1% was excreted in urine, suggesting CC-115 is primarily eliminated via hepatic metabolism (Table 3). CC-115 accumulation in plasma was minimal to moderate, with exposures generally increasing from approximately 20% to 60% based on ratio of geometric mean AUC24 on day 15 versus day 1. No meaningful differences were observed in drug clearance from plasma (CL/F), volume of distribution during the terminal phase after extravascular administration (Vz/F), or terminal half-life (t½) after single- or multiple-dose administrations. Mean t½ was approximately 4–8 hrs over the 8–40 mg range (Table 3). No clinically relevant differences in PK were observed across different tumor types.
Figure 2.
CC-115 pharmacokinetics. Mean (± SD) CC-115 plasma concentrations: time profiles after dosing CC-115, by dose level (Part A) at day 1 (A) and day 15 (B), and by tumor type (Part B) at day 1 (C) and day 15 (D).
Abbreviations: BID, twice daily; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; CRPC, castration-resistant prostate cancer; ES, Ewing sarcoma; GBM, glioblastoma multiforme; h, hour; HNSCC, head and neck squamous cell carcinoma; QD, once-daily; SD, standard deviation; SLL, small lymphocytic lymphoma.
Table 3.
CC-115 Pharmacokinetic Summary In The Dose-Finding Cohort (Part A)
| Parameters, Mean (CV%) | First Dose | Multiple Doses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QD | BID | QD | BID | |||||||||
| 8 mg (n = 6) | 16 mg (n = 6) | 25 mg (n = 7) | 40 mg (n = 6) | 10 mg (n = 6) | 15 mg (n = 6) | 8 mg (n = 7) | 16 mg (n = 8) | 25 mg (n = 5) | 40 mg (n = 4) | 10 mg (n = 7) | 15 mg (n = 5) | |
| Plasma | ||||||||||||
| Tmaxa, h | 1.50 (1.00–5.00) | 2.95 (1.00–3.20) | 3.00 (1.50–5.00) | 2.25 (1.00–5.00) | 2.25 (1.00–3.00) | 1.04 (1.00–23.7) | 1.50 (1.00–3.00) | 1.65 (1.00–3.00) | 1.52 (1.00–3.00) | 3.00 (3.00–3.10) | 1.42 (1.00–3.00) | 1.52 (1.50–3.10) |
| Cmax, ng/mL | 36.1 (29) | 88.7 (73) | 173 (28) | 167 (53) | 51.8 (23) | 134 (44) | 40.9 (26) | 118 (87) | 198 (40) | 244 (49) | 75.2 (45) | 162 (43) |
| AUC∞, ng × h/mL | 237 (64)b | 625 (82) | 1912 (92)c | 2,130 (45)b | 605 (61)d | 1,307 (48)b | – | – | – | – | – | – |
| AUC12, ng × h/mL | 180 (45) | 428 (86) | 1226 (51) | 1207 (41) | 311 (35) | 680 (37) | 221 (57) | 758 (130) | 1180 (58) | 1733 (40) | 383 (43)c | 1028 (56) |
| AUC24, ng × h/mL | 236 (55) | 551 (85) | 1716 (65.4) | 1833 (35)b | 622 (35) | 1,359 (37) | 286 (74) | 1095 (149) | 1573 (67) | 2650 (37) | 766 (43)c | 2056 (56) |
| CL/F, L/h | 33.7 (64.1)b | 25.6 (82) | 13.1 (92)c | 18.8 (45)b | 33.0 (61)d | 23.0 (48)b | – | – | – | – | – | – |
| Vz/F, L | 294 (57)b | 264 (104) | 149 (42)c | 201 (28.9)b | 189 (36)d | 173 (36)b | – | – | – | – | – | – |
| t1/2, h | 6.04 (36)b | 7.14 (45) | 7.90 (42.1)c | 7.42 (43)b | 3.98 (21.8)d | 5.22 (48)b | – | – | – | – | – | – |
| Urine | ||||||||||||
| Ae24, ng | 19,748 (66) | 52,491 (108) | 103,312 (79) | 136,157 (55) | 53,259 (108) | 107,283 (53) | – | – | – | – | – | – |
| CLr, mL/h | 89 (34) | 95 (47) | 60 (41) | 81 (64) | 110 (113) | 83 (93) | – | – | – | – | – | – |
| Fe, % | 0.25 (66) | 0.33 (108) | 0.41 (79) | 0.34 (55) | 0.53 (108) | 0.72 (53) | – | – | – | – | – | – |
Notes: aMedian (min, max). bn = 5. cn = 6. dn = 3.
Abbreviations: Ae24, cumulative amount of drug excreted unchanged in urine at 24 hrs; AUC, area under the concentration–time curve; AUC12, AUC from 0 to 12 hrs; AUC24, AUC from 0 to 24 hrs; AUC∞, AUC from time 0 extrapolated to infinity; BID, twice daily; CL/F, apparent clearance of drug from plasma; CLr, renal clearance of drug from plasma; Cmax, maximum plasma concentration; CV%, geometric coefficient of variation; Fe, fraction of systemically available drug excreted unchanged in urine; QD, once daily; Tmax, time to maximum plasma concentration; t1/2, terminal elimination half-life in plasma; Vz/F, apparent volume of distribution.
The geometric mean ratio of CC-115 AUC∞ and Cmax for the formulated tablet and relative to the capsule were 93% and 97%, respectively, suggesting comparable bioavailability (Supplemental Table 2). Co-administration of the tablet with food resulted in delayed CC-115 absorption (~40% decrease in Cmax and 3.5 hr delay in Tmax) but no significant impact on total CC-115 exposure with comparable AUC∞ (Supplemental Table 2). The differences in systemic exposure under fasted and fed conditions did not warrant dose adjustment, and CC-115 could be administered without regard to food intake.
Tumor biopsies from 8 GBM patients were performed approximately 3 hrs postdose with concurrent plasma samples collected 1.5 hrs postdose on days 15–21 following multiple daily oral administration of 10 mg BID CC-115. The distribution of CC-115 to tumor tissue showed a mean GBM tumor/plasma concentration ratio of 0.71 (range: 0.04–1.71) (Supplemental Table 3).
Biomarker Analysis
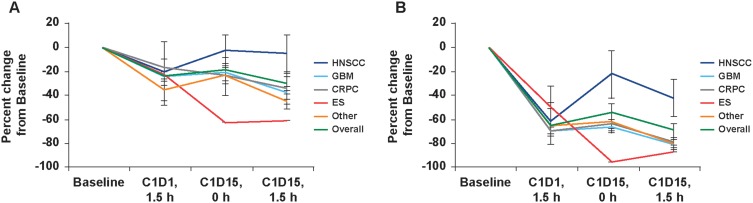
Both mTORC1 and mTORC2, as measured by changes in p4E-BP1 and pAKT expression, respectively, were inhibited in peripheral blood monocytes after repeat once-daily administration of CC-115 at doses >4 mg QD. Median biomarker in the 10 mg BID cohort after 3 hrs was 35% for p4E-BP1 and 84% for pAKT. Biomarkers showed similar kinetics, with maximal inhibition observed between 1.5 and 3 hrs and sustained for at least 5 hrs postdose. Multiple doses did not improve inhibition with QD dosing. In Part B, inhibition at 1.5 hrs was weaker for p4E-BP1 than for pAKT across all tumor types in the 10 mg BID cohort (Figure 3). Inhibition improved for both biomarkers by day 15 (median decrease of 34% and 80%, respectively). Considerable variation was observed between patients and tumor cohorts (Figure 3).
Figure 3.
Inhibition of blood TORK biomarkers for CC-115 10 mg twice-daily in cohort expansion, overall, and by tumor type. Inhibition of (A) p4EBP1 in CD14+ monocytes and (B) pAKT in CD14+ monocytes. Tumor plots represent mean ± standard error of the mean.
Abbreviations: C1D1, cycle 1 day 1; C1D15, cycle 1 day 15; CRPC, castration-resistant prostate cancer; ES, Ewing sarcoma; GBM, glioblastoma multiforme; HNSCC, head and neck squamous cell carcinoma; h, hour.
Moderate, not statistically significant, inhibition of both p4EBP1 and pAKT was observed by immunohistochemistry in paired tumor biopsies, obtained before treatment and after approximately 15 days of CC-115 treatment. The high-observed variability in paired biopsies was likely related to variability in biopsy quality and known difficulties in preparing satisfactory biopsies for analysis of labile phospho biomarkers.
Antitumor Efficacy
In the efficacy evaluable population for Part A from data extracted in December 2013 (n=39), a best response of stable disease (SD) was reported in 18 patients (46%); response was not available in 3 patients (8%) (Supplemental Table 4; Supplemental Figure 1). The overall response rate (ORR) was 5% (95% confidence interval [CI], 0.6–17.3) and the disease control rate (DCR) was 51% (95% CI, 34.8–67.6). One patient with endometrial cancer starting at 10 mg BID but reduced to 8 mg BID, showed a complete response (CR) in cycle 10 and remained tumor-free >4 years into the study. One patient with choroid melanoma taking 8 mg QD showed a partial response (PR) in cycle 2 lasting 56 days.
Based on data extracted in April 2015 for Part B, unconfirmed PR was reported in 2 of 5 patients with breast carcinoma in the BA/FE substudy. In the BA/FE substudy, SD was reported in patients with ovarian (n=2), breast (n=1), and rectal (n=1) malignancies. In the HNSCC (n=17), ES (n=9), and BA/FE (n=11) efficacy evaluable populations, best response of SD was reported for 53%, 22%, and 36% of patients, respectively (Supplemental Table 4). Of the 14 evaluable patients with GBM, 21% showed a best response of nonprogression (Supplemental Table 4). A best response of SD was reported for 7 (64%) of the 11 efficacy evaluable patients with CRPC with no objective response reported. Based on data extracted from June 2017 in the CLL/SLL population, 2 (25%) patients showed PR by IWCLL and 3 (38%) by non-IWCLL criteria (persistent lymphocytosis), as previously reported.18 Two (25%) patients showed a best response of SD by IWCLL and non-IWCLL criteria.
Median duration of SD across cohorts ranged from 109 days (95% CI, 106–111 days) in patients with ES to 345 days (95% CI, 112–345 days) in patients with CRPC. Durations of the 2 PRs in the BA/FE substudy were 85 days and 1 day. Overall median progression-free survival (PFS) ranged from 56 days (95% CI, 43.0–106.0) for ES to 345 days (95% CI, 56.0–345.0) for CRPC (Supplemental Table 4). The 6-month PFS rate ranged from 0% in ES to 58% in the CRPC cohort. The 6-month PFS rate was not estimable in the GBM cohort. Median PFS was not estimable in the CLL/SLL population; 6-month PFS was 86%.
Discussion
This study describes good tolerability and promising clinical efficacy for the first dual mTOR kinase and DNA-PK inhibitor, CC-115. Simulated PK profiles showed CC-115 plasma concentrations associated with at least 50% biomarker inhibition over a 24 hr period in stimulated monocytes with the BID regimen. The BA/FE substudy results support fasting and with food dosing and substitution of the tablet formulation in future trials.
CC-115 demonstrated acceptable tolerability; the safety profile identified in Part A was corroborated in Part B. Although differences in frequency of individual AEs cannot be reliably compared with historical data for other mTOR inhibitors,34–36 the safety profile of CC-115 was consistent with class effects reported with other mTOR inhibitors. Adverse events were managed effectively with dose modifications or supportive care, as exemplified by the very low incidence of grade 3, and no grade 4 or 5, toxicities. Hyperglycemia responded readily to oral sulphonylureas. Differences in toxicity between tumor types may be attributed to small cohort sizes, with the exception of GBM, where morbidity following salvage resection in cycle 1 was a confounding variable.
Nonclinical findings and mechanism of action considerations informed selection of the 5 specific tumor types for evaluation in the cohort expansion; no marked differences in pharmacodynamic effects were identified in peripheral blood cells or tumor biopsy tissue across these tumors. Although efficacy was modest, benefit (CR) was observed in 1 patient with endometrial carcinoma, and durable responses were observed in CLL/SLL.17,18 The distribution of CC-115 into GBM tissue was also noteworthy and provided the basis for an ongoing randomized, open-label Phase II trial with concurrent radiation therapy (INSIGhT; NCT02977780). Another Phase Ib study combining CC-115 with the nonsteroidal antiandrogen, enzalutamide, in patients with CRPC is actively recruiting (NCT02833883).
Disease control in breast, ovary, and colorectal malignancies in the BA/FE substudy suggest that efficacy may include a broader array of solid tumors than those reported for our prespecified tumor cohorts. Refractory to sorafenib/capecitabine and a subsequent AKT inhibitor, with only stable disease as best response, the patient with endometrial cancer showed inhibition of p4E-BP1 and pAKT of 51% and 85%, respectively. Her lung and nodal target lesions had completely regressed after 10 cycles and she was still in complete remission after >4 years of CC-115. Only modest efficacy in patients with endometrial cancer has been reported with other mTOR inhibitors, with response rates ranging from 0% to 22%.37 This patient’s tumor contained 2 mTOR pathway-activating mutations in phosphoinositide-3-kinase (PIK3RI) and phosphatase and tensin homolog (PTEN), along with a potentially cooperative mutation in the chromatin remodeling gene, AT-rich interaction domain 1A (ARID1A), which are common mutations reported in endometrial malignancies.38,39 Although the significance of these mutations on predicting CC-115 sensitivity remains unclear, results from recent studies may explain the relationship between these genetic abnormalities and increased sensitivity to mTOR inhibitors. Mutations in ARID1A induce aberrant activation of PI3K and phosphorylation of AKT, which is followed by activation of mTOR and subsequent dysregulation of cell division and proliferation, apoptosis, and angiogenesis.40 In endometrial biopsies, increased proliferation has been observed with concurrent loss of PTEN and ARID1A compared with either alone.41 Furthermore, preclinical studies have reported that ARID1A-deficient cancer cells have increased sensitivity to treatment with small-molecule PI3K/AKT pathway inhibitors.42
Conclusion
Results from this small cohort of patients with CLL/SLL treated with CC-115 were promising. By non-IWCLL criteria, 38% of patients with CLL/SLL had PR and 25% SD. Median ORR duration was not achieved at the June 2017 data cutoff and 6-month PFS was 86%. Preclinical studies have demonstrated that dual inhibition of DNA-PK and TORK with CC-115 induced cell death in CLL cells and suppressed proliferation.17,22 The aggregate safety and efficacy results for the dual mTOR kinase and DNA-PK inhibitor, CC-115, justify further clinical development.
Acknowledgments
We thank the patients whose findings are described in this study, together with the families who supported them. Appreciation is shown for all the investigational staff at all 15 sites; the dedication such professionals show for their patients is too infrequently acknowledged. The same is true for the very many nameless researchers at Celgene who, despite their remoteness from clinical practice, are also motivated to relieve patient suffering. We would like to acknowledge Angela Joubert James and Torsten Trowe, who were employees of Celgene at the time of the CC-115 study. Dr. Paz-Ares was funded by ISCIII: PI1401964, PIE15/00076, RTICC (R12/0036/0028) and CIBERONC (C16/12/00442), co-funded by FEDER from Regional Development European Funds (European Union). We also thank Stephanie K. Doerner, PhD, at The Lockwood Group (Stamford, CT), for providing writing and editorial assistance, funded by Celgene Corporation. This study was presented, in part, at the annual meeting of the American Society of Clinical Oncology, Chicago, IL, June 2–6, 2016.
Funding Statement
This study was supported by Celgene Corporation.
Ethics Approval And Informed Consent
This study was conducted in compliance with the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice Guideline E6, and all necessary local committee oversight (ie, Institutional Review Board or Institutional Ethics Committee). Study participants provided written informed consent before enrolling. A Safety Review Committee made decisions regarding patient welfare and dose level changes.
Data Availability
Data requests may be submitted to Celgene at www.CelgeneClinicalDataSharing.com and must include a description of the research proposal.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
CM reports grants, personal fees and is a PI/CoPI for Amgen, Astella, Astra Zeneca, Bayer, BeiGene, BMS, Genentech, Janssen, Lilly, MedImmune, MSD, Novartis, Pfizer, Roche, Sanofi, Orion, and Abbvie; grants and is a PI/CoPI for Celgene, Debiopharm, Ipsen; is a PI/CoPI for Aduro, Agios, Argenx, Astex, AVEO, Blueprint, Boehringer Ingelheim, Chugai, Clovis, Daiichi Sankyo, Eisai, Exelixis, FORMA, GSK, Incyte, H3 Biomedicine, Innate Pharma, Kura Oncology, Loxo, Merus, Nektar Therapeutics, OCTIMET, Oncoethix, Pharmamar, Pierre Fabre, Servier, Taiho, Takeda, TESARO and Xencor, during the conduct of the study. LP-A reports personal fees from Roche, Lilly, Novartis, AstraZeneca, Boehringer Ingelheim, Amgen, Pfizer, MSD, BMS, Pharmamar and Takeda, outside the submitted work. DR reports grants from Gateway for Cancer Research and research funding from Celgene, during the conduct of the study. GB reports grants from Celgene, during the conduct of the study; grants, personal fees from Bristol-Myers Squibb, Bayer, Celgene, Merck, Genentech, Novartis, Xcovery, AstraZeneca, Roche, MedImmune, Clovis Oncology, Abbvie, ARIAD, Adicet, Amgen; grants from GlaxoSmithKline, Adaptimmune, Macrogenics, Kite Pharma, Immatics, Torque, Incyte, Exelixis, and Immunocore; personal fees from Maverick Therapeutics, outside the submitted work. DCS reports grants from Celgene, during the conduct of the study; grants from Medarex, Medivation, Eli Lilly & Co, Genentech, Astellas Pharma, Medimmune, Bayer HealthCare, Seattle Genetics, Millennium Pharmaceuticals, Incyte Pharmaceuticals, Novartis Pharma, F. Hoffman-La Roche AG, ESSA Pharmaceuticals, OncoMed Pharmaceuticals, Merck, outside the submitted work. BE reports personal fees from Celgene, during the conduct of the study; grants and personal fees from Roche, Janssen, Abbvie, Gilead, and personal fees from Novartis, outside the submitted work. TC reports grants and personal fees from Celgene, during the conduct of the study; In addition, Dr Cloughesy has a patent 62/819,322 pending; has received fees for consulting services from Tocagen, Karyopharm, GW Pharma, Kiyatec, Abbvie, Boehringer Ingelheim, VBI, Dicephera, VBL, Agios, Merck, Roche, Genocea, Celgene, Puma, Lilly, BMS, Cortice, Wellcome Trust, Novocure, Novogen, Boston Biomedical, Sunovion, Human Longevity, Insys, ProNai, Pfizer, Notable labs, and Media; has contracts with UCLA for Brain tumor program, Amgen, Abbvie, DNAtrix, BMS, AstraZeneca, Kazia, Agios, Boston Biomedical, Deciphera, Tocagen, Orbus, and Karyopharm; stock options with Notable Labs and is a Board member for 501c3: Global Coalition for Adaptive Research. DHF reports that one or more patent applications related to the work disclosed in this paper, may be planned, pending, and/or granted; is as an employee of Celgene and owns Celgene stock. SL is an employee of Celgene and owns stock. HR is an employee of Celgene. HdH reports personal fees from Celgene Corporation, during the conduct of the study. KH is an employee of Celgene and owns stock. JCB reports grants from Celgene, during the conduct of the study; grants and payment made to institution for consulting services and services as PI from Genentech/Roche, Bristol Myers Squibb, Five Prime, Lilly, Merck, MedImmune, Celgene, Taiho, Marcogenics, GSK, Novartis, OncMed, LEAP, TG Therapeutics, Astra Zeneca, BI, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array, Sanofi, Agios, ARMO, Ispen, Merrimack, Oncogenex, Pieris, FORMA, Innate, Arch Oncology, Prelude Oncology, Amgen, Phoenix Bio; grants and payment made to institution for services as PI for EMD Serono, Koltan, SynDevRex, Forty Seven, AbbVie, Onyx, Takeda, Eisai, Celldex, Cytomx, Nektar, Boton Biomedical, Tarveda, Tyrogenex, Marshall Edwards, Pieris, Mersana, Calithera, Blueprint, Evelo, Merus, Jacobio, Effector, Novocare, Arrys, Tracon, Sierra, Unum Therapeutics, Vyraid, Harpoon, ADC, Pfizer, Millennium, Imclone, Acerta Pharma, Rgenix, Bellicum; grants and payment made to institution for consulting services from Cyteir, Molecular Partners, Torque, Tizona, Translational Drug Development, Seattle Genetics, Moderna Therapeutics, Tanabe Research Laboratories, Beigene, Continuun Clinical, outside the submitted work. PM, MM, AM, JN, TM, CC, and MH report no conflicts of interest in this work.
References
- 1.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664 [DOI] [PubMed] [Google Scholar]
- 2.Markman B, Serra V, Tabernero J. mTOR Inhibition beyond Rapalogs. Paris: Springer; ; 2016. [Google Scholar]
- 3.Dienstmann R, Rodon J, Serra V, Tabernero J. Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitors. Mol Cancer Ther. 2014;13(5):1021–1031. doi: 10.1158/1535-7163.MCT-13-0639 [DOI] [PubMed] [Google Scholar]
- 4.Ducker GS, Atreya CE, Simko JP, et al. Incomplete inhibition of phosphorylation of 4E-BP1 as a mechanism of primary resistance to ATP-competitive mTOR inhibitors. Oncogene. 2014;33(12):1590–1600. doi: 10.1038/onc.2013.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi: 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowling RJ, Topisirovic I, Alain T, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science (New York, NY). 2010;328(5982):1172–1176. doi: 10.1126/science.1187532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway – beyond rapalogs. Oncotarget. 2010;1(7):530–543. doi: 10.18632/oncotarget.101012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendell JC, Kelley RK, Shih KC, et al. A phase I dose-escalation study to assess safety, tolerability, pharmacokinetics, and preliminary efficacy of the dual mTORC1/mTORC2 kinase inhibitor CC-223 in patients with advanced solid tumors or multiple myeloma. Cancer. 2015;121(19):3481–3490. doi: 10.1002/cncr.29422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JO, Kim KH, Song IS, et al. Potentiation of the anticancer effects of everolimus using a dual mTORC1/2 inhibitor in hepatocellular carcinoma cells. Oncotarget. 2017;8(2):2936–2948. doi: 10.18632/oncotarget.13808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LoRusso PM. Inhibition of the PI3K/AKT/mTOR pathway in solid tumors. J Clin Oncol. 2016;34:3803–3815. doi: 10.1200/JCO.2014.59.0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musa F, Alard A, David-West G, Curtin JP, Blank SV, Schneider RJ. Dual mTORC1/2 inhibition as a novel strategy for the resensitization and treatment of platinum-resistant ovarian cancer. Mol Cancer Ther. 2016;15(7):1557–1567. doi: 10.1158/1535-7163.MCT-15-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zou Z, Chen J, Yang J, Bai X. Targeted Inhibition of Rictor/mTORC2 in cancer treatment: a new Era after rapamycin. Curr Cancer Drug Targets. 2016;16(4):288–304. [DOI] [PubMed] [Google Scholar]
- 13.Naing A, Aghajanian C, Raymond E, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZD8055 in advanced solid tumours and lymphoma. Br J Cancer. 2012;107(7):1093–1099. doi: 10.1038/bjc.2012.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Infante JR, Tabernero J, Cervantes A, et al. Abstract C252: a phase 1, dose-escalation study of MLN0128, an investigational oral mammalian target of rapamycin complex 1/2 (mTORC1/2) catalytic inhibitor, in patients (pts) with advanced non-hematologic malignancies. Mol Cancer Ther. 2013;12(11 Supplement):C252–C252. [Google Scholar]
- 15.Mortensen DS, Perrin-Ninkovic SM, Shevlin G, et al. Optimization of a series of triazole containing mammalian target of rapamycin (mTOR) kinase inhibitors and the discovery of CC-115. J Med Chem. 2015;58(14):5599–5608. doi: 10.1021/acs.jmedchem.5b00627 [DOI] [PubMed] [Google Scholar]
- 16.Lapalombella R. Two strikes against CLL. Blood. 2016;128(4):470–471. doi: 10.1182/blood-2016-06-719864 [DOI] [PubMed] [Google Scholar]
- 17.Munster PN, Mahipal A, Nemunaitis JJ, et al. Phase I trial of a dual TOR kinase and DNA-PK inhibitor (CC-115) in advanced solid and hematologic cancers. J Cancer Res Clin Oncol. 2016;34(15_suppl):2505. doi: 10.1200/JCO.2016.34.15_suppl.2505 [DOI] [Google Scholar]
- 18.Thijssen R, Ter Burg J, Garrick B, et al. Dual TORK/DNA-PK inhibition blocks critical signaling pathways in chronic lymphocytic leukemia. Blood. 2016;128(4):574–583. doi: 10.1182/blood-2016-02-700328 [DOI] [PubMed] [Google Scholar]
- 19.Dong J, Zhang T, Ren Y, et al. Inhibiting DNA-PKcs in a non-homologous end-joining pathway in response to DNA double-strand breaks. Oncotarget. 2017;8(14):22662–22673. doi: 10.18632/oncotarget.15153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16(1):20–33. doi: 10.1038/nrc.2015.2 [DOI] [PubMed] [Google Scholar]
- 21.Surucu B, Bozulic L, Hynx D, Parcellier A, Hemmings BA. In vivo analysis of protein kinase B (PKB)/Akt regulation in DNA-PKcs-null mice reveals a role for PKB/Akt in DNA damage response and tumorigenesis. J Biol Chem. 2008;283(44):30025–30033. doi: 10.1074/jbc.M803053200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuji T, Sapinoso LM, Tran T, et al. CC-115, a dual inhibitor of mTOR kinase and DNA-PK, blocks DNA damage repair pathways and selectively inhibits ATM-deficient cell growth in vitro. Oncotarget. 2017;8(43):74688–74702. doi: 10.18632/oncotarget.20342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill R, Lee PW. The DNA-dependent protein kinase (DNA-PK): more than just a case of making ends meet? Cell Cycle (Georgetown, Tex). 2010;9(17):3460–3469. doi: 10.4161/cc.9.17.13043 [DOI] [PubMed] [Google Scholar]
- 24.Shortt J, Martin BP, Newbold A, et al. Combined inhibition of PI3K-related DNA damage response kinases and mTORC1 induces apoptosis in MYC-driven B-cell lymphomas. Blood. 2013;121(15):2964–2974. doi: 10.1182/blood-2012-08-446096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stover EH, Konstantinopoulos PA, Matulonis UA, Swisher EM. Biomarkers of response and resistance to DNA repair targeted therapies. Clin Cancer Res. 2016;22(23):5651–5660. doi: 10.1158/1078-0432.CCR-16-0247 [DOI] [PubMed] [Google Scholar]
- 26.van Bussel M, Mau-Soerensen M, Damstrup L, et al. A multicenter phase I trial of the DNA-dependent protein kinase (DNA-PK) inhibitor M3814 in patients with solid tumors. J Cancer Res Clin Oncol. 2017;35(15_suppl):2556. doi: 10.1200/JCO.2017.35.15_suppl.2556 [DOI] [Google Scholar]
- 27.Yanai M, Makino H, Ping B, et al. DNA-PK inhibition by NU7441 enhances chemosensitivity to topoisomerase inhibitor in non-small cell lung carcinoma cells by blocking DNA damage repair. Yonago Acta Med. 2017;60(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Thomas HD, Batey MA, et al. Preclinical evaluation of a potent novel DNA-dependent protein kinase inhibitor NU7441. Cancer Res. 2006;66(10):5354–5362. doi: 10.1158/0008-5472.CAN-05-4275 [DOI] [PubMed] [Google Scholar]
- 29.Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–1147. doi: 10.1093/jnci/89.15.1138 [DOI] [PubMed] [Google Scholar]
- 30.CTCAE N. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009; Version 4: Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html Accessed May18, 2016.
- 31.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 32.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. doi: 10.1182/blood-2007-06-093906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541 [DOI] [PubMed] [Google Scholar]
- 34.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev. 2014;28(3):126–133. doi: 10.1016/j.trre.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 35.Sankhala K, Mita A, Kelly K, Mahalingam D, Giles F, Mita M. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4(2):135–142. doi: 10.1007/s11523-009-0107-z [DOI] [PubMed] [Google Scholar]
- 36.Wise-Draper TM, Moorthy G, Salkeni MA, et al. A Phase Ib Study of the Dual PI3K/mTOR inhibitor dactolisib (BEZ235) combined with everolimus in patients with advanced solid malignancies. Target Oncol. 2017;12(3):323–332. doi: 10.1007/s11523-017-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fleming GF. Second-line therapy for endometrial cancer: the need for better options. J Clin Oncol. 2015;33(31):3535–3540. doi: 10.1200/JCO.2015.61.7225 [DOI] [PubMed] [Google Scholar]
- 38.Chang YS, Huang HD, Yeh KT, Chang JG. Identification of novel mutations in endometrial cancer patients by whole-exome sequencing. Int J Oncol. 2017;50(5):1778–1784. doi: 10.3892/ijo.2017.3919 [DOI] [PubMed] [Google Scholar]
- 39.DeLair DF, Burke KA, Selenica P, et al. The genetic landscape of endometrial clear cell carcinomas. J Pathol. 2017;243:230–241. doi: 10.1002/path.2017.243.issue-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takeda T, Banno K, Okawa R, et al. ARID1A gene mutation in ovarian and endometrial cancers (Review). Oncol Rep. 2016;35(2):607–613. doi: 10.3892/or.2015.4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayhan A, Mao TL, Suryo Rahmanto Y, et al. Increased proliferation in atypical hyperplasia/endometrioid intraepithelial neoplasia of the endometrium with concurrent inactivation of ARID1A and PTEN tumour suppressors. J Pathol Clin Res. 2015;1(3):186–193. doi: 10.1002/cjp2.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Samartzis EP, Gutsche K, Dedes KJ, Fink D, Stucki M, Imesch P. Loss of ARID1A expression sensitizes cancer cells to PI3K- and AKT-inhibition. Oncotarget. 2014;5(14):5295–5303. doi: 10.18632/oncotarget.2092 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- CTCAE N. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009; Version 4: Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html Accessed May18, 2016.
Data Availability Statement
Data requests may be submitted to Celgene at www.CelgeneClinicalDataSharing.com and must include a description of the research proposal.